Abstract
This study examined whether middle-aged adults exposed to poverty in childhood or current financial hardship have detectable brain differences from those who have not experienced such adversity. Structural magnetic resonance imaging (MRI) was conducted as one aspect of the Personality and Total Health (PATH) through life study: a large longitudinal community survey measuring the health and well-being of three cohorts from south-eastern Australia. This analysis considers data from 431 middle-aged adults in the aged 44–48 years at the time of the interview. Volumetric segmentation was performed with the Freesurfer image analysis suite. Data on socio-demographic circumstances, mental health and cognitive performance were collected through the survey interview. Results showed that, after controlling for well-established risk factors for atrophy, adults who reported financial hardship had smaller left and right hippocampal and amygdalar volumes than those who did not report hardship. In contrast, there was no reliable association between hardship and intra-cranial volume or between childhood poverty and any of the volumetric measures. Financial hardship may be considered a potent stressor and the observed results are consistent with the view that hardship influences hippocampal and amygdalar volumes through hypothalamic–pituitary–adrenal axis function and other stress-related pathways.
Keywords: hippocampus, amygdale, socio-economic position, financial hardship, hypothalamic–pituitary–adrenal axis, depression
INTRODUCTION
The effects of hardship, socio-economic disadvantage and deprivation during both childhood and adulthood on mental functions are well-established. On average, children from disadvantaged socio-economic backgrounds have poorer language (Noble et al., 2005), executive (Ardila et al., 2005), memory (Farah et al., 2006) and attention skills (Mezzacappa, 2004). Adults who endured difficult socio-economic conditions during their childhood have a higher risk of cognitive impairment (Zhang et al., 2008) and poorer physical health (Painter et al., 2005). Current socio-economic disadvantage and particularly the experience of deprivation, is associated with increased risk of psychiatric disorders (Weich and Lewis, 1998; Butterworth et al., 2009). Individuals who experienced deprivation and/or social disadvantage during their adult life have lower cognitive abilities and higher risk of dementia (Basta et al., 2008; Nguyen et al., 2008; Gao et al., 2009).
Despite such evidence linking socio-economic disadvantage across the life course with mental function, relatively little research has examined the influence of the socio-economic environment on brain development and how this impacts on brain function to create vulnerabilities to mental and cognitive disorders (Raizada and Kishiyama, 2010). In animals, sensory, social and dietary deprivation has been associated with decreased axonal complexity and connectivity particularly in the hippocampus, amygdala, corpus callosum and medial pre-frontal cortex (Andrade et al., 1996; Innocenti, 2007; McEwen, 2008b). Such findings have been demonstrated in very young, pubescent and adult animals with effects detected with relatively short exposures. For instance, chronic stress initiated by physical restraint for 21 days in 8 week-old rats (puberty = 50–60 days, life expectancy 2–3 years) produced significant dendritic retraction in the medial pre-frontal cortex. In humans such effects cannot be investigated systematically, however, extreme visual sensory deprivation (blindness before 2 years of age) is associated with decreased grey matter volume and white matter connectivity (Noppeney et al., 2005). Early social deprivation in children reared in orphanages was found to be associated with cerebral white matter microstructural abnormalities and correlated with inattention and hyperactivity scores (Govindan et al., 2009). Poor nutrition, particularly the absence of certain micronutrients and starvation, in early and late life was found to be related to greater brain atrophy, lower intra-cranial volumes (ICV), larger white matter hyperintensities and more cerebral abnormalities (Hulshoff Pol et al., 2000; Casella et al., 2005; Vogiatzoglou et al., 2008).
While the pathophysiological mechanisms mediating the effect of hardship and deprivation on brain structure and cognition are unclear, both factors, particularly when enduring, can be conceptualized as sources of chronic stress which influence neuroendocrine function (e.g. Brunner et al., 2002; Dowd et al., 2009). Thus, the neurobiological system likely to be involved is the limbic system and other related structures through their involvement in the modulation of the hypothalamus–pituitary–adrenal (HPA) axis and autonomic cardiovascular reactivity in response to stress. HPA function is well understood: detection of threat is mediated by the medula, the amygdala and other cerebral structures (pre-frontal cortex, cingulate, hippocampus). Threats and perceived threats lead to production of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) in the hypothalamus. These hormones trigger the secretion of adrenocorticotropin hormone (ACTH) in the pituitary, which leads to the production of glucocorticoid in the adrenal cortex. In addition, based on input from limbic structures, the autonomic nervous system also modulates cardiovascular and visceral responses to stress via the vagal nerve and stimulates the production of catecholamines in the adrenal medulla through increased sympathetic activation (see McEwen, 2008a; Dedovic et al., 2009; Ulrich-Lai and Herman, 2009; Pruessner et al., 2010 for a review).
In an isolated, acute situation, this cascade of responses raises blood pressure and heart rate, redirects blood flow to essential organs, increases alertness and leads to fast adaptive responses that increase the odds of avoiding the threat or of successfully confronting it and is, therefore, beneficial to the organism. However, when stress becomes chronic, normal physiological responses can have deleterious effects. For example, a study investigating stress reactivity to social stress (public speaking) found that women who had a history of childhood abuse and suffering from major depression had a 6-fold increased ACTH response as well as increased cortisol and heart rate responses (Kirschbaum et al., 1993). However, because the stress response of women with a history of abuse but no depression was not different from controls it was unclear whether this effect was due to variation in resilience across individuals and/or depressive pathology related or unrelated to childhood abuse. Another study clarified this question and showed that women with a history of childhood abuse with and without depression showed an abnormal response to stressors at a level thought to be associated with increased risk (Heim et al., 2002). Similar effects were also reported in maltreated non-depressed men (Carpenter et al., 2007) but are known to differ between males and females (Young and Korszun, 2010; see also, Heim et al., 2008 for a review).
Sustained cerebral exposure to glucocorticoids has been shown to lead to dendritic retraction, and neuronal death in the hippocampus, cingulate and medial pre-frontal cortex as well as decreased neurogenesis in the hippocampus, while in the amygdala neuronal hypertrophy is observed, at least in a first stage (see Radley and Morrison, 2005; McLaughlin et al., 2009; Ulrich-Lai and Herman, 2009 for a review). In rats, chronic stress exposure has been found to produce hippocampal shrinkage and in humans PTSD and depression have been associated with hippocampal atrophy (McEwen, 2000; Roozendaal et al., 2009). In ageing individuals, higher cortisol levels are associated with smaller hippocampal volumes and poorer memory function (Raskind et al., 1994). The picture is less clear for the amygdala. As mentioned above, sustained stress can initially lead to amygdala hypertrophy, however, it appears that high corticosteroid exposure and depressive illness ultimately lead to amygdala atrophy (Brown et al., 2008; Desai et al., 2009). Thus, it is possible that amygdala volume is modulated by the level and length of exposure to stressors. However, it is also possible that individuals with larger amygdala are protected against some of the effects of chronic stress, thus any causal relationship must be considered with care. Total brain atrophy and smaller corpus callosum surface have been reported in PTSD and therefore might also be present in other forms of chronic stress. Finally, the neurobiological changes produced by chronic stress have been shown to be associated with enhanced excitability of the HPA axis (Akana et al., 1992) which is likely to compound the effects of pre-existing stressors and further harm the brain (see Pruessner et al., 2010 for a review of neuroimaging findings). Recent research also found an association between stress and increased amyloid plaque, one of the hallmark of Alzheimer’s disease (Dong and Csernansky, 2009) which is consistent with findings discussed above showing associations between deprivation, low SES and increased risk of dementia.
In summary, based on the proximal and distal effects of chronic stress reviewed above it would be expected that hardship would be associated with specific brain changes, particularly in the hippocampus and amygdala. The aim of this study was to use data from a community-based survey of middle-aged adults to assess whether individuals exposed to poverty in childhood or current hardship (being excluded from minimally accepted ways of life due to a lack of resources) present with detectable brain differences from those who did not experience such adversity. It was hypothesized that hardship would be associated with hippocampal and amygdala atrophy even after controlling for an extensive range of previously identified correlates of atrophy. If smaller medial temporal lobe volume occurs in the context of smaller ICV, this may be best explained as a generalized effect reflecting compromised early brain development in childhood. Alternatively, if the experience of poverty and hardship is associated with medial temporal lobe atrophy in the absence of ICV differences, the results would more likely be a consequence of sustained exposure to environmental stressors and the corresponding HPA axis response.
METHODS
Study design
The data used for this analysis are drawn from the Personality and Total Health (PATH) through life study. This is a large longitudinal community survey measuring the health and well-being of three cohorts, born in 1975–79, 1956–60 and 1937–41 and originally residing in Canberra and the neighbouring town of Queanbeyan in south-eastern Australia. The sampling frame was the electoral roll (registration on the electoral roll is compulsory for Australian citizens). It is planned to follow-up each cohort every 4 years over a 20-year period.
This analysis considers data from the middle-aged cohort. The current data are largely drawn from the second wave interview, conducted in 2004, when respondents were in the age group of 44–48 years. However, some retrospective information on childhood experiences collected during the Wave 1 interview (conducted in 2000) is also used in analysis. The Wave 1 response rate was 64.6% and the follow-up rate at Wave 2 was 93.0%. At Wave 2 there were 2354 respondents.
A randomly selected subsample of 656 participants was offered an MRI scan, which 503 accepted and 431 eventually completed. There were no differences in age, sex and years of education between those who had an MRI scan and those who did not (P > 0.05). One scan was lost due to a technical fault, giving a total number of 430 scans. The reasons for not undergoing an MRI scan after having initially agreed, included subsequent withdrawal of consent, medical conditions precluding MRI and claustrophobia or other anxiety about the procedure.
All participants provided written informed consent. The Human Research Ethics Committee of The Australian National University approved the study protocol. Further details of the survey including the sampling procedure and the MRI substudy are reported elsewhere (Jorm et al., 2003; Cherbuin et al., 2009).
MRI acquisition
MRI data were acquired on a 1.5 Tesla Gyroscan scanner (ACS-NT, Philips Medical Systems, Best, The Netherlands). T1-weighted 3D structural MRI images were acquired in coronal plane using Fast Field Echo (FFE) sequence. About mid-way through this study, for reasons outside the researchers’ control, the original scanner (scanner A) was replaced with a similar Philips scanner (scanner B). The scanning parameters were kept essentially the same. The first 164 subjects were scanned on scanner A with TR = 8.84 ms, TE = 3.55 ms, a flip angle of 8°, matrix size = 256 × 256, slices 160 and the field of view (FOV) 256 × 256 mm. Slices were contiguous with slice thickness of 1.5 mm. For the remaining 268 subjects scanned on scanner B, the TR = 8.93 ms, TE = 3.57 ms values were slightly different in order to improve image quality, but all other parameters were exactly the same. No significant differences were observed between scanners (Cherbuin et al., 2009).
Image analysis
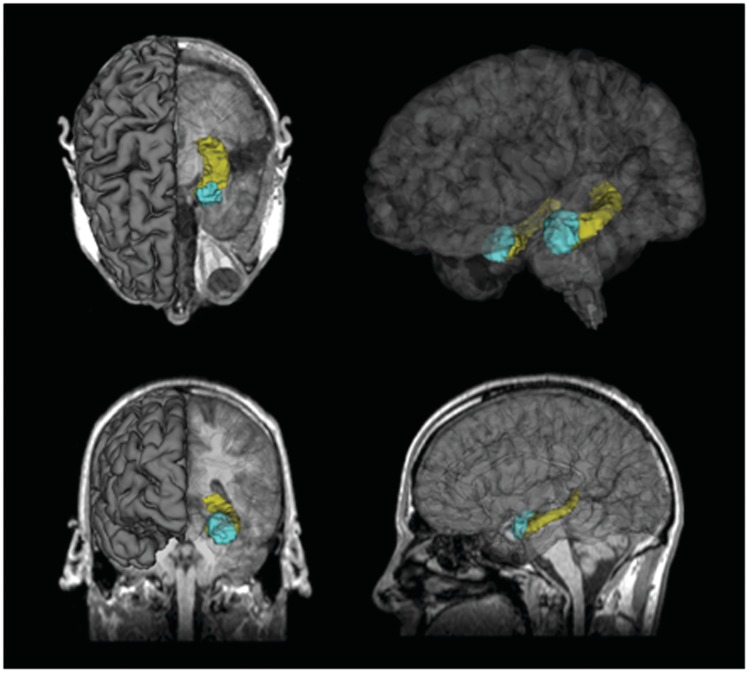
Volumetric segmentation was performed with the Freesurfer image analysis suite (Figure 1), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). This processing includes motion correction, removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Ségonne et al., 2004), automated Talairach transformation and segmentation of the subcortical white matter and deep grey matter volumetric structures (including for the present investigation hippocampus, amygdala, total grey and white matter for each hemisphere, as well as a measure of ICV; Fischl et al., 2002, 2004). The validity and reliability of the Freesurfer package has been assessed in a number of recent studies and was found to be very good (Tae et al., 2008; Cherbuin et al., 2009). The scans of 27 participants were excluded from the sample due to poor scan quality, low signal-to-noise ratio, or movement artefacts which did not allow for normal processing with the standard Freesurfer pipeline, leaving 403 cases for MRI analysis. Each segmented volume was inspected slice by slice and reprocessed with additional parameters if errors were detected.
Fig. 1.
Three-dimensional model of the segmented hippocampus (yellow) and amygdala (blue) using Freesurfer and displayed in Slicer (www.slicer.org).
Other measures
Participants completed the majority of the PATH questionnaire on a hand-held computer, with the interviewer administering physical and cognitive tests. Only measures used in the current analysis are described.
The key independent measure in this analysis assessed the experience of financial hardship. Four dichotomous hardship items that assessed lack of basic goods and opportunities due to limited financial resources—(over the past year have the following happened because you were short of money: pawned or sold something; went without meals, unable to heat home, asked for help from welfare/community organizations;—see Butterworth et al., 2009). We also considered a fifth item which assessed whether respondents had to—go without things they really needed because they were short of money—over the past 12 months. We focus on those who reported ‘yes’ often. An overall measure representing any experience of hardship (any of the five items) was constructed for this analysis (0 hardships reported vs 1 or more). A dichotomous measure of childhood poverty (yes or no) was based on a single item from Wave 1 which asked respondents whether they had ‘grown up in poverty’. Other measures of contemporaneous (Wave 2) socio-economic status included: labour force status (employed, unemployed or not participating in the labour force), educational attainment (years of full-time education corresponding with highest level of educational attainment) and low-skilled occupational background (those employed in elementary clerical and labouring jobs).
Socio-demographic characteristics included in the analyses were age, gender and partner status (married or de facto relationship vs other). A range of covariates, previously shown to be associated with atrophy of medial temporal lobe structures were included in analyses including current (the Alcohol Use Disorders Identification Test; Saunders et al., 1993) and previous (Anstey et al., 2006) hazardous or harmful alcohol consumption (Wrase et al., 2008), experience of trauma (e.g. rape, sexual molestation and physical assault; Brewin et al., 2002) or childhood adversity (including neglect, physical abuse and sexual abuse; Vythilingam et al., 2002; Rosenman and Rodgers, 2006; Woon et al., 2010) and conditions such as diabetes (based on reported use of medication), stroke, hypertension (no evidence, borderline or definite), heart disease and stroke (Korf et al., 2004; Gold et al., 2007). We also included a measure of self-rated health (excellent or very good vs other). The scale score from the Patient Health Questionnaire (PHQ; Spitzer et al., 1999) was used as a measure of depression symptoms. Various domains of cognitive functioning were also evaluated. As the focus of the current study is on cognitive functioning broadly defined, a composite measure was constructed which represented the mean standardized score across measures of episodic memory (assessed by the immediate recall task from the first trial of the California Verbal Learning Test; Delis et al., 1987), working memory (the Digits Backwards subtest of the Wechsler Memory Scale; Wechsler, 1945) and processing speed (the Symbol-Digit Modalities Test; Smith, 1982). Finally, current use of anti-depressive medication was assessed.
Statistical analysis
As a preliminary analysis, a series of linear regression models assessed whether the relationship between each measure of socio-economic circumstance and mental functioning (depression and cognition) was evident in the restricted MRI sample after controlling for age and sex. The primary focus of this study, however, was analysis of the volumetric measures and an initial regression model tested whether the hardship measures were associated with overall ICV. In the key analyses, measures of left and right hippocampus and amygdala volumes (adjusted for/divided by ICV) were regressed on financial hardship, childhood poverty and the range of covariates described above. Aside from the variables critical to the hypotheses tested, non-significant terms were eliminated from models in a step-wise procedure to arrive at the most parsimonious final model for each dependent measure. Because of the low prevalence of hardship in the sample and concern to ensure the comparability of the hardship and the control groups, the analyses were rerun using a subsample of ‘control’ respondents who were selected to closely match the respondents in the hardship group (matching variables were sex, partner status, labour-force status, depression medication, experience of traumatic life events, depression symptoms, cognitive performance and ICV). Further sensitivity analyses examined whether the inclusion of an interaction between current hardship and childhood poverty, or the interaction between sex and financial hardship, improved overall model fit.
Missing data for most of the items examined in this analysis were minimal. One measure (hypertension) had missing data for 2.5% of respondents. Missing data for all other scales/items were <1% of cases. Missing data were imputed using a multivariate regression approach.
RESULTS
Table 1 presents data on the characteristics of the 403 respondents in the MRI substudy and contrasts those who did and did not report hardship. Overall, the sample represents a relatively advantaged population, with very low levels of financial hardship and unemployment, skilled occupational backgrounds and high levels of educational attainment. Compared to the control group, those who did experience hardship were more likely to be unemployed or not participating in the workforce, to have previously consumed alcohol at hazardous/harmful levels, to have experienced rape or physical assault, to currently experience depression, to be less likely to have a partner and to have lower levels of cognitive performance.
Table 1.
Characteristics of the sample
| Characteristics | Overall | Hardship | No hardship | P-value |
|---|---|---|---|---|
| N | 403 | 19 | 384 | |
| Sex (men) (%) | 44.2 | 47.4 | 44.0 | 0.77 |
| Age (years) (mean, s.e.) | 46.7 (0.07) | 46.8 (0.28) | 46.7 (0.07) | 0.76 |
| Partnered (married or de facto) (%) | 81.4 | 63.2 | 82.3 | 0.04 |
| Experienced financial hardship (%) | 3.7 | 100 | 0.0 | |
| Grew up in poverty (%) | 12.4 | 21.1 | 12.0 | 0.24 |
| Educational attainment (mean years) | 14.7 (0.11) | 14.2 (0.66) | 14.7 (0.11) | 0.40 |
| Low skilled occupational background (%) | 5.0 | 5.3 | 5.0 | 0.95 |
| Unemployed (%) | 1.7 | 5.3 | 1.6 | 0.000 |
| Not participating in labour force (%) | 7.0 | 31.6 | 5.7 | |
| Hypertensive (borderline or definite) (%) | 38.5 | 52.6 | 37.8 | 0.08 |
| Heart disease (%) | 2.7 | 5.3 | 2.6 | 0.49 |
| Diabetes (%) | 2.0 | 5.3 | 1.8 | 0.29 |
| Stroke (%) | 0.7 | 0.0 | 0.8 | 0.70 |
| Current hazardous/harmful alcohol consumption (%) | 5.7 | 21.1 | 5.4 | 0.35 |
| Previous history of hazardous/harmful alcohol consumption (%) | 26.3 | 47.4 | 25.3 | 0.03 |
| Lifetime experience of: | ||||
| Rape (%) | 6.5 | 21.1 | 5.7 | 0.008 |
| Sexual molestation (%) | 15.4 | 14.8 | 15.4 | 0.18 |
| Physical attack/assault (%) | 9.7 | 31.6 | 8.6 | 0.001 |
| Childhood adversity: | ||||
| Neglect (%) | 2.5 | 5.6 | 2.3 | 0.43 |
| Physical abuse (%) | 8.2 | 15.8 | 7.8 | 0.22 |
| Sexual abuse (%) | 1.2 | 1.3 | 1.2 | 0.62 |
| Depression—from PHQ (%) | 10.0 | 33.3 | 8.9 | 0.001 |
| Taking depression medication (%) | 6.5 | 15.8 | 6.0 | 0.09 |
| Cognition | 2.08 (.31) | 1.11 (1.44) | 2.24 (.32) | 0.023 |
The preliminary series of analyses, controlling for age and sex, confirmed that socio-economic position was related to mental function in this sample (Table 2). The experience of financial hardship and not participating in the workforce were associated with greater depression symptoms and poorer cognitive functioning. Reported poverty in childhood and low-skilled occupational background were each associated with greater depression symptoms but not with cognitive functioning. Similar results were obtained for cognitive functioning when controlling for depressive symptoms (a potential mediator of the relationship between socio-economic position and cognitive functioning).
Table 2.
Association between measures of socio-economic position and depression symptoms (PHQ depression score) and cognitive function, reporting regression β-coefficients and standard errors from a series of regression models controlling for age and sex
| Depression symptoms | Cognitive functioning | |
|---|---|---|
| Coefficient (and standard error) | Coefficient (and standard error) | |
| Experience of financial hardship | 3.60 (0.86)*** | −3.31 (1.47)* |
| Grew-up in poverty | 1.49 (0.56)** | 1.78 (0.95) |
| Labour-force status (employed) | ||
| Unemployed | −0.66 (1.42) | −0.93 (2.38) |
| Not participating in the labour force | 1.64 (0.74)* | −3.00 (1.24)** |
| Low-skilled occupational background | 1.99 (0.86)* | −2.25 (1.44) |
***P < 0.001; **P < 0.01; *P < 0.05.
Analysis of simple and multivariate linear regression models demonstrated that neither the experience of financial hardship (coefficient from full multivariate model = 21 670, s.e. = 30 037, n.s.) nor reported childhood poverty (coefficient = 11 630, s.e. = 19 136, n.s.) were associated with overall ICV. Table 3 presents mean volumes and results of a series of multivariate linear regression models examining predictors of left and right hippocampus and amygdala volume (adjusted for ICV). The experience of current financial hardship was associated with smaller volume in each of the medial temporal lobe structures after controlling for covariates. There was no evidence that reported childhood poverty was associated with hippocampal or amygdalar volume. Overall, these models accounted for between 8% and 11% of variance in adjusted volume.
Table 3.
Mean volume (in mm3 and standard error) and regression results (β-coefficient and s.e.) for series of models examining association of hardship and childhood poverty with volume of medial-temporal lobe structures, including covariatesa
| Financial hardship |
||||
|---|---|---|---|---|
| No | Yes | Coefficient | P-value | |
| Full sample | ||||
| (n = 384) | (n = 19) | |||
| Left hippocampusa | 3691 (18.9) | 3619 (92.2) | −9.9 (5.00) | 0.049 |
| Right hippocampusb | 3981 (19.4) | 3850 (85.2) | −12.7 (5.04) | 0.012 |
| Left amygdalac | 1462 (11.1) | 1341 (47.3) | −7.6 (2.76) | 0.007 |
| Right amygdalad | 1628 (11.2) | 1527 (43.2) | −7.9 (2.59) | 0.003 |
| Matched control group | ||||
| (n = 42) | (n = 19) | |||
| Left hippocampusa | 3756 (50.1) | 3619 (92.2) | −11.4 (6.55) | 0.087 |
| Right hippocampusb | 4057 (58.1) | 3850 (85.2) | −14.4 (6.22) | 0.025 |
| Left amygdalac | 1492 (37.7) | 1341 (47.3) | −6.19 (3.08) | 0.050 |
| Right amygdalad | 1664 (35.7) | 1527 (43.2) | −6.39 (2.81) | 0.027 |
| Childhood poverty |
||||
|---|---|---|---|---|
| No | Yes | Coefficient | P-value | |
| (n = 353) | (n = 50) | |||
| Left Hippocampus a | 3690 (20.1) | 3674 (48.4) | −2.9 (3.18) | 0.362 |
| Right Hippocampus b | 3968 (20.3) | 4017 (52.4) | 2.7 (3.23) | 0.401 |
| Left Amygdala c | 1453 (11.6) | 1476 (30.4) | 1.4 (1.78) | 0.429 |
| Right Amygdala d | 1620 (11.8) | 1650 (26.7) | 1.8 (1.72) | 0.289 |
Covariates in final models are:
asex, years of education, labour-force status, diabetes, experience of physical assault, experienced physical abuse as child, depression and cognition.
bSex, years of education, diabetes, depression and cognition.
cSex, labour-force status, reported heart disease, neglect during childhood, experienced physical abuse as child, depression and cognition.
dSex, diabetes, stroke, experience of sexual molestation, experience of physical assault, depression and cognition.
A number of sensitivity analyses were conducted. Respondents who reported financial hardship were matched with a subsample of 42 similar respondents who had not experienced hardship. The matching procedure was effective: repeating the series of comparisons presented in Table 1 confirmed that there were no significant differences between the two groups on any of the reported characteristics apart from the experience of hardship. Analysis of adjusted volumetric measures using the matched sample confirmed that hardship was associated with smaller hippocampus and amygdala volume (though the effect on left hippocampus was only marginally significant; Table 3). The current results were also consistent with those from analyses in which all covariates were included and analyses which used the actual measures of hippocampus and amygdala volume as the dependent variable and included ICV as a covariate (vs analysis of volume adjusted for ICV). However, again in these last two analyses, the effect of hardship on left hippocampus volume was of marginal significance (P = 0.057 and P = 0.091, respectively). Finally, the results were similar when cases with missing data were excluded from the analysis rather than using data imputation.
The cognitive measures were only significant predictors in the analysis of right amygdalar volume (0.18, s.e. = 0.088, P = 0.047) and marginally significant for left amygdalar volume (.16, s.e. = 0.093, P = 0.089). The measure of depression was not a significant independent predictor in any of the analyses. There was also no evidence in any of these models that the inclusion of the interaction term between current financial hardship and childhood poverty (i.e. entrenched disadvantage) or the interaction between sex and financial hardship improved model fit.
DISCUSSION
This study examined the consequences in adulthood of recent and early life socio-economic adversity. Our analyses focused on a retrospective measure of childhood poverty and 12-month prevalence of financial hardship. The initial analysis sought to verify the well-established association between socio-economic adversity and mental function (both cognition and depression) in this cohort of middle-aged adults from a community survey. Consistent with expectations, the results showed that financial hardship was associated with higher levels of current depression symptoms and poorer cognitive performance. The effects of childhood poverty were more modest and restricted to an association with depression. The primary and unique aim of this article, however, was to extend this behavioural analysis and examine whether the effects of adverse socio-economic circumstances were observable in measures of global intra-cranial or more specific medial-temporal lobe volumes. The critical findings were that middle-aged adults who reported current financial hardship had smaller left and right hippocampal and amygdalar volumes than those who did not report hardship after controlling for a comprehensive range of well-established risk factors. This was the case in the full sample and also in an analysis using a control group more closely matched to those respondents who experienced financial hardship.
The observed pattern of results is consistent with the view that hardship can be conceptualized as a potent stressor and that the association between hardship and smaller hippocampal and amygdalar volumes reflects the impact of HPA function and corticosteroid exposure. The notion of hardship as a source of severe stress is supported by the well-established association between hardship and depression and consistent with past research demonstrating smaller hippocampal and amygdalar volumes in depression (Kronmuller et al., 2009; Rigucci et al., 2009). Further, the hypothesized effect of hardship on the brain is consistent with recent results in rats showing that repeated experimental stressors led to detectable proteomic expression changes in the adult hippocampus which are associated with neuroplasticity, metabolism and apoptosis pathways. Confidence in the hypothesized biological consequences of the experience of hardship is enhanced by finding that hardship was only associated with the volume of medial-temporal lobe structure but not with overall ICV, suggesting the results were not due to more generalized early developmental differences.
The measure of financial hardship examined in this study focused on difficulties satisfying basic requirements of daily living due to limited financial resources. These types of hardship measures are strongly associated with depression and psychological distress (Lewis et al., 1998; Weich and Lewis, 1998; Lorant et al., 2007; Butterworth et al., 2009) and have been found to mediate the relationship between other measures of socio-economic position and depression (Lewis et al., 1998). Experiencing difficulties satisfying basic requirements may impact on mental function because it is such a fundamental concern and likely to be associated with ongoing worry and stress and feelings of hopelessness, lack of control and demoralization (Brown, 2002). The current study, therefore, provides biological evidence to support the hypothesized pathway between hardship and mental function. It is interesting, however, that the depression and cognitive measures were generally not independently associated with hippocampal/amydgdala volume. The fact that hardship was more strongly associated with right hippocampal volume than with other medial-temporal lobe structures warrants further investigation to examine if this reflects a localized effect. Previous findings have also reported asymmetrical effects (Kronmuller et al., 2009) and investigations in animals have found that gene expression is different in the left and right hippocampi which may modulate their susceptibility to stressor (Samara et al., 2009).
There are a number of limitations which must be considered when interpreting the current findings. Firstly, our data were cross-sectional and although we posit a theoretic model in which hardship has biological consequences on brain structure, it may be that respondents with smaller medial temporal lobe structures are selected into financial hardship. It may be that effects attributed to hardship may reflect that hardship is an effective marker of underlying social and economic disadvantage. So, for example, hardship in midlife may be associated with childhood adversity and social selection, life long behaviours (poor diet, limited exercise, etc.), poor social support, a lack of marketable job skills, under-employment and/or low income. Better data are needed to differentiate the volumetric correlates of short-term vs persistent and sustained hardship (Lynch et al., 1997). The lack of association between poverty in childhood and the volumetric measures may reflect that this measure, based on retrospective report of childhood circumstances, lacks precision.
In conclusion, the current study has shown the association between financial hardship and poorer mental function is paralleled by an association between hardship and medial temporal lobe volume. These findings suggest that hardship may have a significant impact on an individual brain as well as observable effects on mental function. Importantly, evidence of neurological correlates of hardship suggests the adverse behavioural effects of financial deprivation do not simply reflect a mood-induced reporting bias. There remains a need to consider these effects longitudinally to contrast persistent and short-term hardship and examine whether the medial temporal lobe effects can be overcome by a more positive social environment. This sort of evidence is critical to ascertain whether social interventions aimed at reducing poverty can be considered as a tertiary intervention or whether interventions need to be preventative in nature.
Conflict of Interest
None declared
Acknowledgments
The authors are grateful to Anthony Jorm, Helen Christensen, Bryan Rodgers, Patricia Jacomb, Karen Maxwell, Andrew Janke and the PATH interviewers. National Health and Medical Research Council (NHMRC) of Australia (Grant No. 973302, 179805, 157125, and 350833); National Facility of the National Computational Infrastructure; the Australian Rotary Health Research Fund; Australian Brewers Foundation; NHMRC Research Fellowship (No. 525410 to P.B., No. 471501 to N.C., No. 366756 to K.A.).
REFERENCES
- Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131(1):57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Castanheira-Vale AJ, Paz-Dias PG, Madeira MD, Paula-Barbosa MM. The dendritic trees of neurons from the hippocampal formation of protein-deprived adult rats. A quantitative Golgi study. Experimental Brain Research. 1996;109(3):419–33. doi: 10.1007/BF00229626. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Jorm AF, Reglade-Meslin C, Maller J, Kumar R, von Sanden C. Weekly alcohol consumption, brain atrophy, and white matter hyperintensities in a community-based sample aged 60 to 64 years. Psychosomatic Medicine. 2006;68:778–85. doi: 10.1097/01.psy.0000237779.56500.af. [DOI] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Matute E, Guajardo S. The influence of the parents' educational level on the development of executive functions. Developmental Neuropsychology. 2005;28(1):539–60. doi: 10.1207/s15326942dn2801_5. [DOI] [PubMed] [Google Scholar]
- Basta NE, Matthews FE, Chatfield MD, Brayne C. Community-level socio-economic status and cognitive and functional impairment in the older population. European Journal of Public Health. 2008;18(1):48–54. doi: 10.1093/eurpub/ckm076. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Rose S, Andrews B, et al. Brief screening instrument for post-traumatic stress disorder. British Journal of Psychiatry. 2002;181:158–62. doi: 10.1017/s0007125000161896. [DOI] [PubMed] [Google Scholar]
- Brown ES, Woolston DJ, Frol AB. Amygdala volume in patients receiving chronic corticosteroid therapy. Biological Psychiatry. 2008;63(7):705–9. doi: 10.1016/j.biopsych.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW. Social roles, context and evolution in the origins of depression. Journal of Health and Social Behavior. 2002;43:255–76. [PubMed] [Google Scholar]
- Brunner EJ, Hemingway H, Walker BR, et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;19:2659–65. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- Butterworth P, Rodgers B, Windsor TD. Financial hardship, socio-economic position and depression: results from the PATH Through Life Survey. Social Science & Medicine. 2009;69(2):229–38. doi: 10.1016/j.socscimed.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–7. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella EB, Valente M, de Navarro JM, Kok F. Vitamin B12 deficiency in infancy as a cause of developmental regression. Brain and Development. 2005;27(8):592–4. doi: 10.1016/j.braindev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Anstey KJ, Reglade-Meslin C, Sachdev PS. In vivo hippocampal measurement and memory: a comparison of manual tracing and automated segmentation in a large community-based sample. PLoS One. 2009;4(4):e5265. doi: 10.1371/journal.pone.0005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47(3):864–71. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. San Antonio: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- Desai S, Khanani S, Shad MU, Brown ES. Attenuation of amygdala atrophy with lamotrigine in patients receiving corticosteroid therapy. Journal of Clinical Psychopharmacology. 2009;29(3):284–7. doi: 10.1097/JCP.0b013e3181a3e2a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Csernansky JG. Effects of Stress and Stress Hormones on Amyloid-\beta Protein and Plaque Deposition. Journal of Alzheimer's Disease. 2009;18:459–69. doi: 10.3233/JAD-2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. International Journal of Epidemiology. 2009;38:1297–309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Research. 2006;1110(1):166–74. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;31:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23:S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Gao X, Scott T, Falcon LM, Wilde PE, Tucker KL. Food insecurity and cognitive function in Puerto Rican adults. American Journal of Clinical Nutrition. 2009;89(4):1197–203. doi: 10.3945/ajcn.2008.26941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Sweat V, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–9. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS) Cerebral Cortex. 2010;20:561–69. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15(3):117–25. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Hoek HW, Susser E, et al. Prenatal exposure to famine and brain morphology in schizophrenia. American Journal of Psychiatry. 2000;157(7):1170–2. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. Subcortical regulation of cortical development: some effects of early, selective deprivations. Progress in Brain Research. 2007;164:23–37. doi: 10.1016/S0079-6123(07)64002-3. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Korten AE, Christensen H, Jacomb PA, Rodgers B, Parslow RA. Association of obesity with anxiety, depression and emotional well-being: a community survey. Australian and New Zealand Journal of Public Health. 2003;27:434–40. doi: 10.1111/j.1467-842x.2003.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Korf ESC, White LR, Scheltens P, Launer LJ. Midlife BOLD pressure and the risk of hippocampal atrophy: the Honolulu Asia aging study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- Kronmuller KT, Schroder J, Kohler S, et al. Hippocampal volume in first episode and recurrent depression. Psychiatry Research. 2009;174(1):62–6. doi: 10.1016/j.pscychresns.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Lewis G, Bebbington P, Brugha T, et al. Socioeconomic status, standard of living, and neurotic disorder. Lancet. 1998;352:605–9. doi: 10.1016/S0140-6736(98)04494-8. [DOI] [PubMed] [Google Scholar]
- Lorant V, Croux C, Weich S, Deliege D, Mackenbach J, Ansseau M. Depression and socio-economic risk factors: 7-year longitudinal population study. British Journal of Psychiatry. 2007;190:293–8. doi: 10.1192/bjp.bp.105.020040. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. New England Journal of Medicine. 1997;337(26):1889–95. doi: 10.1056/NEJM199712253372606. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biological Psychiatry. 2000;48(8):721–31. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McEwen B. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008a;583:174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008b;57(Suppl. 2):S11–15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Molecular Neurobiology. 2009;40:166–82. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development. 2004;75(5):1373–86. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Couture MC, Alvarado BE, Zunzunegui MV. Life course socioeconomic disadvantage and cognitive function among the elderly population of seven capitals in Latin America and the Caribbean. Journal of Aging Health. 2008;20(3):347–62. doi: 10.1177/0898264308315430. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8(1):74–-87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Friston KJ, Ashburner J, Frackowiak R, Price CJ. Early visual deprivation induces structural plasticity in gray and white matter. Current Biology. 2005;15(13):R488–90. doi: 10.1016/j.cub.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reproductive Toxicology. 2005;20(3):345–52. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35(1):179–91. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Research Reviews. 2005;4(2):271–87. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Raizada RS, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Frontiers in Human Neuroscience. 2010;4:3. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Wilkinson CW. Hypothalamic-pituitary-adrenal axis regulation and human aging. Annals of the New York Academy of Sciences. 1994;746:327–35. doi: 10.1111/j.1749-6632.1994.tb39251.x. [DOI] [PubMed] [Google Scholar]
- Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: The contribution of neuroimaging studies. World Journal of Biological Psychiatry. 2009;9:1–16. doi: 10.1080/15622970903131571. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10(6):423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosenman S, Rodgers B. Childhood adversity and adult personality. Australian and New Zealand Journal of Psychiatry. 2006;40:482–90. doi: 10.1080/j.1440-1614.2006.01826.x. [DOI] [PubMed] [Google Scholar]
- Samara A, Vougas K, Papadopoulou A, et al. Proteomics reveal rat hippocampal lateral asymmetry. Hippocampus. 2009;17:17. doi: 10.1002/hipo.20727. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test (SDMT) Manual. LA: Western Psychological Services; 1982. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD - The PHQ primary care study. Journal of the American Medical Association. 1999;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–81. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826–32. doi: 10.1212/01.wnl.0000325581.26991.f2. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry. 2002;159:2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. A standardized memory scale for clinical use. Journal of Psychology. 1945;19:87–95. [Google Scholar]
- Weich S, Lewis G. Poverty, unemployment, and common mental disorders: population based cohort study. British Medical Journal. 1998;317:115–9. doi: 10.1136/bmj.317.7151.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:1181–8. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, et al. Amygdala volume associated with alcohol abuse repalse and craving. American Journal of Psychiatry. 2008;68:1179–84. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Young E, Korszun A. Sex, trauma, stress hormones and depression. Molecular Psychiatry. 2010;15(1):23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gu D, Hayward MD. Early life influences on cognitive impairment among oldest old Chinese. Journal of Gerontology series B: Psychological Sciences Social Sciences. 2008;63(1):S25–33. doi: 10.1093/geronb/63.1.s25. [DOI] [PubMed] [Google Scholar]