Abstract
We investigated behavioral and neurobiological mechanisms by which risk-averse advice, provided by an expert, affected risky decisions across three developmental groups [early adolescents (12–14 years), late adolescents (15–17 years), adults (18+ years)]. Using cumulative prospect theory, we modeled choice behavior during a risky-choice task. Results indicate that advice had a significantly greater impact on risky choice in both adolescent groups than in adults. Using functional magnetic resonance imaging, we investigated the neural correlates of this behavioral effect. Developmental effects on correlations between brain activity and valuation parameters were obtained in regions that can be classified into (i) cognitive control regions, such as dorsolateral prefrontal cortex (DLPFC) and ventrolateral PFC; (ii) social cognition regions, such as posterior temporoparietal junction; and (iii) reward-related regions, such as ventromedial PFC (vmPFC) and ventral striatum. Within these regions, differential effects of advice on neural correlates of valuation were observed across development. Specifically, advice increased the correlation strength between brain activity and parameters reflective of safe choice options in adolescent DLPFC and decreased correlation strength between activity and parameters reflective of risky choice options in adult vmPFC. Taken together, results indicate that, across development, distinct brain systems involved in cognitive control and valuation mediate the risk-reducing effect of advice during decision making under risk via specific enhancements and reductions of the correlation strength between brain activity and valuation parameters.
Keywords: adolescence, risk, decision making, social context, advice, DLPFC, vmPFC
INTRODUCTION
Adolescence, compared to the developmental periods of childhood and adulthood, is marked by a significant increase in potentially harmful everyday-life risky behaviors, such as experimentation with licit and illicit drugs, unsafe sexual activity as well as dangerous behavior in traffic (Boyer, 2006; Reyna and Farley, 2006; Casey et al., 2008; Rivers et al., 2008; Steinberg, 2008). Neuroimaging studies of neurocognitive development comparing adolescent to adult brain responses during monetary choice tasks have repeatedly demonstrated enhanced activation in regions associated with primary and abstract rewards, such as ventromedial prefrontal cortex (vmPFC) and ventral striatum (Ernst et al., 2005; Galvan et al., 2006). At the same time, decreased activity has been observed in adolescent, relative to adult, lateral prefrontal regions associated with cognitive control, e.g. dorsolateral PFC (DLPFC), and emotion regulation, e.g. ventrolateral PFC (Ernst et al., 2006; Casey et al., 2008; Steinberg, 2008, but see Luna et al., 2010). Drawing upon such findings, dual-system models of adolescent risk-taking suggest that the increase in risky behaviors observed during adolescence is due to an imbalance between affective/motivational compared to deliberative processes (Ernst et al., 2006; Casey et al., 2008; Steinberg, 2008; Figner et al., 2009). At the neural level, such imbalance is reflected by hyper-responsiveness of reward-related regions on the one hand (Galvan, 2010) and immature prefrontal cortex on the other (Casey et al., 2005; Galvan et al., 2006; Eshel et al., 2007).
While recent research has made considerable progress in understanding the neurobiological basis of reward and control processes in adults and adolescents, it has become increasingly apparent that a variety of contexts can modulate both reward (e.g. Tremblay and Schultz, 1999; Cromwell et al., 2005; Tobler et al., 2005; Plassmann et al., 2008; Grabenhorst and Rolls, 2009; Kalenscher et al., 2010) and cognitive control processes (e.g. Pochon et al., 2002; Erk et al., 2003; Hare et al., 2005; Small et al., 2005; Locke and Braver, 2008; Engelmann et al., 2009b). In particular, the social context in which an individual is embedded may have powerful effects on decision making pertaining to risk and reward, which may be especially true for adolescents.
A simple, but potent, form of social context occurs when an individual receives information from another person. Information can come from peers, experts or aggregated sources (e.g. popularity rankings or market reactions). Clearly, the effects of such information depend on both the source and the receiver, as well as the content and quality of the message. The current study was designed to investigate the impact of advice, which is of particular importance during the developmental period of adolescence, on reward-related and cognitive control processes. Common social settings during adolescence include interactions with peers, on the one hand, and the guidance of adult mentors on the other. Previous results have underlined that, during this developmental period, the likelihood of risky behaviors increases in the presence of peers (Gardner and Steinberg, 2005). Peers may exert their risk-enhancing influence due to a salient social reward structure provided by social cues, which can operate via approval and disapproval. Previous reports support this notion, demonstrating that adolescents show: (i) an enhanced susceptibility to peer influences (Steinberg and Monahan, 2007) and (ii) an increased sensitivity to rejection by peers (Sebastian et al., 2010). Of note, recent results demonstrate that the mere presence of peers increases risk-taking and activity in reward-related brain areas (Chein et al., 2011). Such behavioral changes within social contexts are paralleled by neurodevelopmental changes within brain circuits associated with social cognition. It has been shown that regions such as medial PFC, DLPFC and superior temporal sulcus within the temporoparietal junction (TPJ), still undergo significant developmental changes throughout adolescence (e.g. Blakemore et al., 2007; Pfeifer et al., 2007; for review, see Blakemore, 2008).
While real life increases in risky behaviors are well-documented, it has repeatedly been shown that, in laboratory settings, risk attitudes of adolescents are similar to those of adults (Byrnes et al., 1999; Loewenstein et al., 2001; van Leijenhorst et al., 2008; see also Steinberg, 2004, 2005). These seemingly disparate findings, demonstrating risky behaviors in one context and adult-like risk attitudes in another, together with increases in conformity observed during adolescence (Berns et al., 2010), may be explained by a generalized susceptibility to social cues and information during adolescence. According to this hypothesis, adolescents show greater levels of risk-seeking behaviors in the presence of peers but adjust their risk attitude to the expectations of adults in settings where this is appropriate. Understanding how adolescents respond to adult advice may have important implications for public health policies designed to mitigate risky behaviors.
The goal of the current study was to test this hypothesis by investigating the behavioral and neurobiological mechanisms through which a specific piece of information from an ‘adult expert’, influences risky decision making in three developmental groups; early (EAs, 12–14 years) and late adolescents (LAs, 15–17 years) and adults (ADs) (18–45 years). While undergoing functional magnetic resonance imaging (fMRI), participants made decisions with real financial outcomes between sure wins (SWs) and risky lotteries with relatively larger payoffs in two conditions: on half the trials, risk-averse advice from a financial expert was displayed, which participants were free to ignore. On the rest of the trials, participants made choices without advice. To assess the effect of advice on risky choice, we modeled binary decisions according to cumulative prospect theory (CPT).
We used parametric analyses to probe for brain regions involved in evaluating the two choice options (risky/safe) in order to investigate the extent to which advice influences brain correlates of valuation across the three age groups. Neurobiologically, we hypothesized that the behavioral effect of advice would be mediated by systems typically engaged during valuation in risky contexts, namely, (i) reward-related regions and (ii) prefrontal cognitive control and emotion regulation systems. Furthermore, given the social nature of the advice, we expected the developmental effects on activation patterns within regions involved in social cognition, such as TPJ.
METHODS
Participants
Twenty-four AD (ages 18–45 years, 15 females, mean age: 23.62, s.d.: 5.17), 15 EA (ages 12–14 years, eight females, mean age: 13.76, s.d.: 0.62) and 24 LA (ages 15–17 years, 17 females, mean age: 16.13, s.d.: 0.88) participants took part in the current study, which was approved by the Emory University Institutional Review Board. One participant from the EA group was excluded from further analyses because choices were not variable enough to allow for extraction of decision parameters (the subject exclusively chose SWs throughout the experiment). All participants were right-handed, reported good health with no history of psychiatric and neurological disorders and gave written informed consent. Prior to the actual experiment, adolescent participants were instructed in the nature of probabilities and had to successfully demonstrate this knowledge by correctly ordering a series of pie-charts which represented probabilities of winning a lottery and were equivalent to stimuli used in the experiment.
Experimental task
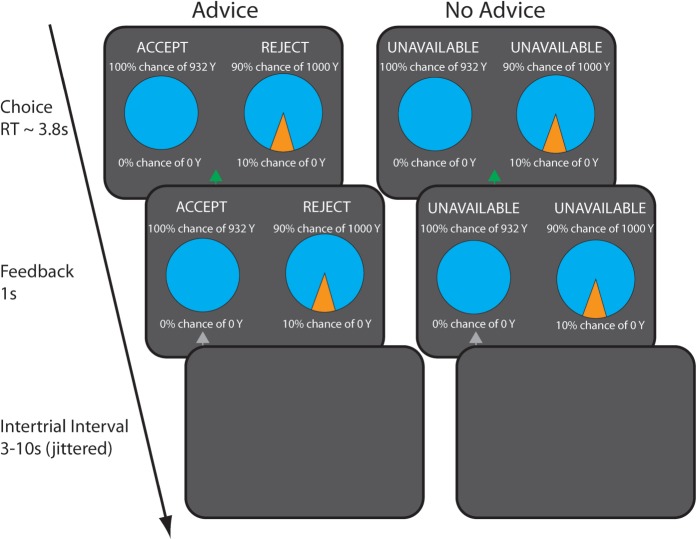
The experimental task was described in detail previously (Engelmann et al., 2009a). Briefly, we employed a certainty equivalent (CE) task, in which subjects made choices between a sure win (SW) and lotteries providing ex ante probabilities (0.01, 0.1, 0.2, 0.37, 0.8, 0.9, and 0.99) of winning a comparatively higher payoff (Figure 1). While the amount the individual received by winning the lottery was constant across trials (1000 U of the experimental currency), the value of the SW was adjusted according to the decisions made by the subject via staircase algorithms.
Fig. 1.
Task schematic and timing of fMRI design. On each trial, participants were asked to choose between a sure win and a lottery, either in the presence of advice from an expert (ADVICE) or in its absence (NO ADVICE). Risk-averse advice from the expert economist was provided on half the trials by way of placing the words ‘ACCEPT’ above the option that the expert would choose and ‘REJECT’ above the option that the expert would not choose. In the NO ADVICE condition, the expert’s advice was hidden by placing the words ‘UNAVAILABLE’ above both options. The probability of the lottery varied across seven probability conditions ranging from 1% to 99% and the amount of the sure win varied based on decision weights estimated in a behavioral pre-scanning session using the PEST procedure. The self-paced decision period was followed by a 1 s feedback period, which provided confirmatory information about which option was chosen by the participant. Finally, a jittered and optimized intertrial interval that varied between 3 and 10 s was presented.
To obtain baseline risk attitudes and optimize the range of offers presented during scanning for each subject, we employed an iterative staircase procedure (Parameter Estimation by Sequential Testing, e.g. Cho et al., 1994) during a behavioral pre-scanning session (see Engelmann et al., 2009a for a detailed description of the algorithm). The goal of this pre-experiment was to obtain baseline CEs for all lotteries employed in the main experiment for each subject. Knowledge of these baseline parameters allowed for more efficient sampling of choices around the indifference point, which improves subjectwise estimation of behavioral parameters due to ensuring varied responses, as well as providing meaningful choice scenarios. For instance, if a subject’s indifference point or CE, for a lottery offering 1000 monetary units (MU) with a probability of 80% (and 0 MU with a probability of 20%) lies at 750 MU, an offer range of 200–500 MU would not provide interesting choices, because the subject is expected to choose the lottery in every instance, except in the case of error. Offering SWs closely above and below this indifference point, however, produces meaningful decisions that are likely to vary depending on whether the SW amount offered is above or below the estimated indifference point. Offer magnitudes presented to subjects in the main experiment were then randomly selected from the interval [CE − (0.4 × CE), CE + (0.4 × CE)], as outlined in detail in Engelmann et al. (2009a).
Inside the scanner, subjects made choices between lotteries and SWs with an expert economist providing his suggestions during half of the trials. In order to make the economist trustworthy, participants were informed of the economist’s credentials and achievements, as well as his preferred decision strategy, in detailed instructions. The expert’s suggestions followed approximately a satisfying rule, which were, in part, consistent with those of a decision maker trying to maximize his probability of winning at least 200 MU. Specifically, if a SW of >200 MU was offered, the expert advice was always to choose the SW over the lottery, where as SW magnitudes <200 MU resulted in advice recommending the lottery, except when lotteries offered winning probabilities ≤10%, in which case a utility maximizing strategy was adopted (see Figure 2A for an illustration of the advice strategy). Suggestions were displayed at the top of the screen via placing the word ‘ACCEPT’ above the recommended option and ‘REJECT’ above the option not recommended (see Figure 1). In the other half of the trials, the word ‘UNAVAILABLE’ was displayed above both options, to indicate that the economist’s recommendations were not provided on that trial. Participants were instructed to pay attention to and consider the expert’s recommendations, but to make choices based on which option they considered most attractive.
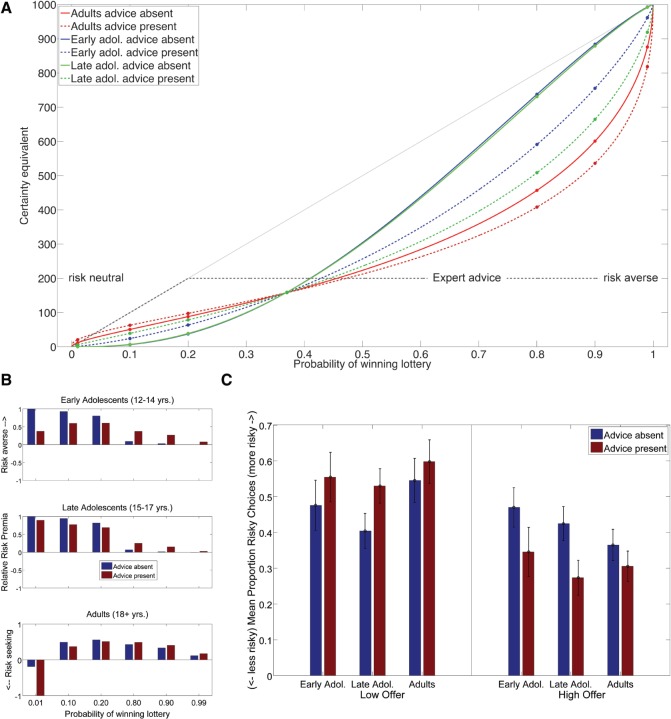
Fig. 2.
Differential effect of advice on risk attitude across different age groups. (A) Certainty equivalents (CEs) as a function of the probability of winning the lottery, presence of advice and age group. Certainty equivalents are plotted as dots overlayed onto scaled probability weighting functions for each lottery, advice condition and age group. Results from behavioral choice model are shown with adult probability weighting function shown in red, early adolescents shown in blue and late adolescents shown in green. The satisficing advice strategy employed by the expert is illustrated by the dotted gray line. The effect of expert advice on probability weighting functions is shown in dotted lines. Probability weighting curves in the absence of advice of both adolescent groups differed from adults in curvature, but not from each other. Advice had a differential effect on adolescents compared to adults. Specifically, it increased curvature of early and late adolescents w(p) relative to adults, making them significantly more risk averse for gambles with high probabilities. (B) In order to illustrate age differences in risk attitude, certainty equivalents from (A) are plotted as relative risk premia (RRP) for each age group and advice condition. RRP values >1 reflect risk aversion for a specific lottery, RRPs of zero reflect risk neutrality and RRPs smaller than one reflect risk-seeking attitudes. Relative to adults, adolescents are more risk averse for small probability lotteries and more risk neutral for high probability lotteries. (C) Model-free analysis of choice proportions. Proportion of risky choices for low (x < 200) and high (x > 200) sure win amounts. For late adolescents, the presence of advice significantly increased the amount of risky choices for low sure win amounts and decreased risky choices for high sure win amounts. Similar trends were observed across all age groups, but did not reach significance for early adolescents and adults.
Econometric choice model
We employed non-linear least squares regression to estimate each participant’s probability weighting and value functions from binary decisions using a model grounded in CPT (e.g. Bruhin et al., 2010). According to CPT, an individual’s estimate of the value of a given lottery depends on two functions that assign subjective weights to (i) monetary outcome magnitudes via the value function, v(x), as well as (ii) outcome probabilities via the probability weighting function, w(p). The value function was modeled as a power function in the domain of gains only:
| (1) |
The probability weighting function, w(p), was modeled using a modified version of Prelec’s (1998) two-parameter compound invariant form with additional parameters to allow for estimating the effect of advice on probability weighting, such that
| (2) |
According to Gonzalez and Wu (1999), the parameters of the probability weighting function have intuituve psychological interpretations, with the parameter β, which largely governs the elevation of w(p), representing optimism, where as the parameter α, which largely governs the curvature of w(p), represents deviations from rationality. Given that the goal of the current study was to investigate irrational behavior in adolescents, we were particularly interested in the effect of advice on the curvature of probability weighting, which is why we loaded the effect of advice onto the parameter α via the parameter δ, which captures the effect of advice. A learning parameter (λ) was included to allow for the possibility of memory and learning effects, such that expert advice affected decision making across all trials, including trials without advice (Klucharev et al., 2008).
We calculated the difference (Φ) in value between the lottery and the SW as the main determinant of our behavioral decision-making model:
| (3) |
Finally, the probability of choosing the lottery (Pl) was estimated via the logit or softmax function:
| (4) |
To obtain robust fits for each individual subject, ideal starting parameters were selected by iterating through a range of potential values and selecting the set of values that minimized least squares residuals. Parameters estimated via the above model provided estimates of the curvature of the value function, v(x) and the shape of the probability weighting function, w(p), for each subject. From these values, CEs were obtained as follows:
| (5) |
A lottery’s CE entails two factors that can affect risk attitude, namely, (i) the curvature of the value function, v(x), reflective of diminishing sensitivity to changes in value (see Supplementary Figure S2D) and (ii) the shape of the probability weighting function, w(p), reflective of probability distortions that indicate departures from rationality and level of optimism (see Supplementary Figure S2A–C). Because CEs reflect all risk-related parameters obtained via CPT, behavioral results are reported based on CEs and these were employed as parametric modulators in neuroimaging analyses.
Representative agent model to assess age effects on choice parameters
A representative agent model was employed to assess the differential effect of advice on the curvature of the probability weighting function across age groups (AD, EA, and LA). The representative agent analysis employed the same approach as outlined for individual subjects, except that decisions from subjects within a given age group were combined and treated as one representative agent that reflects the typical behavior of a developmental group. To this end, we extended equation 2 by adding additional parameters to model (i) group differences in the curvature of w(p) (αg1, αg2) and (ii) differential effects of the advice on the curvature of w(p) across age groups (ig1, ig2).
| (6) |
Additionally, the probability of choosing the lottery (Pl) was estimated via the logit or softmax function:
| (7) |
where μ represents a behavioral error parameter (Fechner, 1966; see also Andersen et al., 2010), which was allowed to vary across age groups, and ϕ is the difference between the subjective values of the lottery and SW as outlined above. Since inclusion of additional parameters can lead to model overspecification, we compared two full models that contained all parameters of interest, but had different error term specifications, against a number of reduced models, in which parameters and parameter combinations were systematically left out (see Supplementary Figure S1 and Supplementary Table S1 for results from model comparison). The best-fitting model is outlined in equations (5) and (6) and results from this model are reported below.
Functional magnetic resonance imaging
Neuroimaging data were collected using a 3T Siemens Magnetron Trio whole body scanner (Siemens Medical Systems, Erlangen, Germany). A three-dimensional, high-resolution anatomical data set was acquired using Siemens’ magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (TR of 2300 ms, TE of 3.93 ms, TI of 1100 ms, 1 mm isotropic voxels and a 256 mm FOV). Functional data consisted of 35 axial slices that were sampled with a thickness of 3 mm and encompassing a field of view of 192 mm with an inplane resolution of 64 × 64 (T2* weighted, TR = 2500 ms, TE = 31 ms). The task was presented with Presentation software (Neurobehavioral Systems, Albany, CA, USA) and visual stimuli were projected onto a frosted glass screen, which the subject viewed through an angled mirror mounted to the head coil. Inhomogeneities in the magnetic field introduced by the participant were minimized with a standard two-dimensional head shimming protocol before each run and the anatomical data acquisition. Each participant completed four runs with 56 trials each, whose length depended on participants’ decision time.
fMRI data analysis
fMRI preprocessing
Initial preprocessing of the data was conducted using Analysis of Functional Neuroimages (AFNI; http://afni.nimh.nih.gov/afni). Data underwent slice-time acquisition correction using Fourier interpolation. The functional data were then spatially aligned to the volume acquired closest to each subject’s anatomical image. After motion correction, anatomical and mean functional data sets were co-registered using a localized Pearson correlation cost function. Alignment was then confirmed visually for all subjects. Individual gray matter tissue probability maps were computed from anatomical data sets and spatially warped to standard MNI space using the VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm) running in SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Normalization to standard MNI space was conducted in SPM5 by applying the transformation matrix obtained from normalizing the anatomical data set to the functional data using quintic interpolation. Using AFNI, functional data then underwent spatial smoothing using an isotropic Gaussian kernel (full width at half maximum = 6 mm). Finally, intensity normalization was conducted separately for each session by scaling each voxel’s signal intensity to its own session-specific mean of 100.
fMRI analysis
Functional magnetic resonance imaging data were analyzed using a standard regression model at the single-subject level implemented in AFNI. We created subject-specific design matrices containing the following 12 regressors of interest: (i) two regressors in the presence of advice and two regressors in the absence of advice encoding the choice of the subject in terms of whether expert advice was followed or ignored (choice-related regressors were not further analyzed in the current report, but were included in order to control for the effects of following or ignoring advice) and (ii) two additional regressors in each condition in the form of parametric modulators reflective of (a) offer magnitude (om) for the SW on each trial and (b) CEs, which represent the subjective value of the lottery by incorporating subjectwise parameters estimated by the econometric model, including subjectively weighted probability, w(p), and the inverse of the value function. Parametric modulators therefore reflect each subject’s level of risk attitude. We orthogonalized regressors reflecting parametric modulation by CE relative to regressors reflecting parametric modulation by om within each condition (Andrade et al., 1999), using a Classical Gram–Schmidt orthogonalization procedure implemented in Matlab. This procedure generated new regressors for CE within each condition whose variance is not explained by om and thereby allowed us to probe for neural correlates of CE that are independent of om. First-level statistical models included additional regressors of no interest for each run to model slow signal drifts (constant and polynomial terms) to account for residual head motion (roll, pitch, yaw and displacement in superior, left and posterior directions) and one regressor to model the 1 s feedback period presented after each decision.
Second-level analyses
On any given trial, subjects faced a binary choice between a lottery that yielded a large payout (1000 MU) and a SW of a smaller amount that was adjusted to each participants risk attitude and changed on every trial (<1000 MU). Participants’ decisions therefore reflected an estimate of whether the subjective value of the SW was greater or smaller than that of a lottery, which could yield a higher payoff, but involved risk. Our behavioral model assumes that participants’ choices are based on a comparison between the value of the SW and the value of the lottery. Given that (i) the ‘probability’ of the SW is constant and (ii) the ‘payoff magnitude’ of each lottery is constant, the choice situation that subjects faced in the CE paradigm employed in the current study can be reduced to two parameters that participants compared during decision making, namely, (i) the magnitude of the SW, reflective of the certain payoff in any given choice scenario and (ii) the CE of the lottery, reflective of the subjective level of risk subjects required to obtain the maximum payoff amount (1000 MU). Our imaging analyses followed the same logic, in that we probed for neural correlates of valuation parameters involved in the decision process, reflective of (i) the payoff magnitude of the sure option and (ii) the CE of the lottery based on subjectwise parameters estimated by our behavioral model. In the context of the current experiment, neural correlates of valuation parameters are therefore related to choice behavior as follows: a significant correlation with offer magnitude is reflective of regions processing the safe choice option, which can vary only in offer magnitude, where as a significant correlation with CEs is reflective of regions processing the risky choice option, as the lottery varies only in terms of probability. The current investigation focused on the effects of age on the strength of the correlations between brain activity and parameters reflective of valuation (offer magnitude, CE).
All t-maps were thresholded at a cluster-level corrected α of 0.05, which was determined via Monte-Carlo simulations using the AFNI program 3dClustSim (cluster extent k = 39, voxel-level α = 0.005, uncorrected). Exploratory fMRI results using an uncorrected threshold (cluster extent k = 10, voxel-level α = 0.005, uncorrected) are reported in Supplementary Data.
Brain responses showing developmental effects
The goal of our analysis approach was to isolate regions showing developmental effects on correlations between neural activation patterns and valuation parameters. To this end, specific contrasts were conducted probing for linear (EA > LA > AD) age effects on neural correlates of valuation (CE, om) in the presence and absence of advice. We performed region of interest (ROI) analyses on activation clusters for two reasons: (i) in order to illustrate developmental effects on correlation strength between brain activity and valuation parameters, we regressed beta coefficients extracted from activation clusters against age using robust regression. Of note, these results are reported for illustrative purposes only, as the discrete ROI selection variable (age group) is not independent of the continuous predictor variable (age) and (ii) to probe for developmental differences in the effect of advice on neural correlates related to valuation, we performed ROI analyses on activation clusters.
Effect of advice on neural activation patterns across age
To investigate the differential impact of advice on valuation across different age groups, beta coefficients reflective of the slope of the valuation parameter of interest for each subject were extracted from activation clusters that showed significant ‘age’ effects on neural correlates of valuation in whole brain analyses (cluster-level corrected α = 0.05) and these were subsequently submitted to planned pairwise comparisons contrasting presence with absence of advice within each of the three age groups. Given that the selection criterion for activation clusters (increased valuation ‘across’ age group in each of the advice conditions) was independent of subsequent analyses comparing the effect of advice ‘within’ each age group on mean parameters extracted from voxels, a significance threshold of α = 0.05 was adopted for planned comparisons in ROI analyses.
RESULTS
Behavior
Response times
Mean response times were obtained within each condition for each subject and entered into separate mixed ANOVA with Offer Magnitude (<200, ≥200) and Presence of Advice (present, absent) as within-subjects factors and age group (EAs/LAs, ADs) as between subjects factor (see Table 1). The factor Offer Magnitude was included to investigate the possibility of differential effects of risk-neutral (<200) and risk-averse (≥200) advice strategies adopted by the expert on RT (see also confirmatory analyses reported in SM). A significant main effect of advice was obtained [F(1,59) = 4.4, P = 0.04, η2 = 0.07], which indicates that choices were faster in the presence (3.377) than in the absence (3.645) of advice. Furthermore, a significant interaction between offer magnitude and advice was obtained [F(1,59) = 5.352, P = 0.024, η2 = 0.08], indicating that subjects chose significantly faster when the advice strategy was risk neutral than when it was risk averse. Post hoc paired sample t-tests indicate a significant effect of advice on response times when subjects considered low offers [t(61) = −3.86, P < 0.001], such that response times were decreased significantly by the advice, but no effect during high offers [t(61) = −0.15, P = 0.88]. Of note, analyses of mean response times did not yield significant age effects or interactions with the factor age group, indicating no systematic differences in mean response times across age groups.
Table 1.
Mean response times and s.d. as a function of age group, advice and offer size
| Advice present |
Advice absent |
|||
|---|---|---|---|---|
| Low offer | High offer | Low offer | High offer | |
| Early adolescents | 3.367 (0.448) | 3.636 (0.443) | 3.749 (0.502) | 4.035 (0.392) |
| Late adolescents | 3.470 (0.342) | 3.570 (0.338) | 3.968 (0.383) | 3.335 (0.299) |
| Adults | 2.906 (0.342) | 3.310 (0.338) | 3.404 (0.383) | 3.377 (0.299) |
Differential influence of expert advice on financial decisions across development
The estimation of the econometric model via non-linear least squares provided parameter estimates reflecting (i) the curvature of the probability weighting function, α, and potential group differences in this parameter and (ii) the effect of advice for each group, and potential group differences in the effect of advice on the curvature of w(p). To display the effect of advice on valuation across age groups, CEs, reflective of the indifference point between the SW and the lottery, were obtained as outlined above. CEs for lotteries employed in the current study are shown as a function of age group and advice condition in Figure 2A. In order to further illustrate the effect of advice on risk attitude, relative risk premia [RRP = (ev − ce)/|ce|] are shown in Figure 2B for illustrative purposes. RRP were calculated from CEs estimated via the representative agent model and can be interpreted as follows: RRP > 0 is reflective of risk aversion, RRP = 0 indicates risk neutrality and RRP < 0 risk seeking behavior. Finally, we show the differential effect of advice on proportions of risky choices for low (x<200 MU) and high (X≥200) offers across age groups in Figure 2C (see Supplementary Material for further details on model-free confirmatory analyses).
Our behavioral results indicate risk aversion across all age groups, as illustrated by CEs below the unity line (Figure 2A, Table 2, see also Supplementary Figure S2A–D), where unity is reflective of risk neutrality. Importantly, the expert’s advice significantly influenced choice behavior across all age groups, but differentially so. AD group-level parameter estimates (see Table 2) are consistent with an ‘inverted’ S-shape probability weighting function (αad = 0.564, Figure 2A and Supplementary Figure S2C). Adolescents, on the other hand, showed S-shaped probability weighting functions, signified by curvature parameters >1 (αea = 1.197, αla = 1.195, Supplementary Figures S2B and S2C). Such curvatures produce CEs that are reflective of risk-aversion for low probability lotteries and risk neutrality for high probability lotteries. Probability weighting functions of EAs and LAs differed significantly from ADs, but not from each other (EA vs LA = −0.002, EA vs AD = −0.624, LA vs AD = −0.622]. As illustrated via RRP in Figure 2B, these differences indicate that, compared to ADs, both adolescent groups were significantly more risk-averse for low probability lotteries, while more risk-neutral decisions were observed for high probability lotteries.
Table 2.
Results from behavioral choice model
| Parameter name | Parameter estimate | s.e. | t-value | P-value |
|---|---|---|---|---|
| Early adolescents (12–14 years, EA) | ||||
| μ | 0.44095 | 0.07265 | −4.515 | <0.0001**** |
| α | 1.19672 | 0.12189 | 5.119 | <0.0001**** |
| α × advice | −0.27493 | 0.13557 | −2.028 | 0.04259* |
| Late adolescents (15–17 years, LA) | ||||
| μ | 0.58512 | 0.05711 | −3.219 | 0.00129** |
| α | 1.19477 | 0.07422 | 8.38 | <0.0001**** |
| α × advice | −0.4223 | 0.08367 | −5.047 | <0.0001**** |
| Adults (18+ years, AD) | ||||
| μ | 0.76892 | 0.1287 | 5.974 | <0.0001**** |
| α | 0.57283 | 0.02541 | 22.546 | <0.0001**** |
| α × advice | −0.08976 | 0.03262 | −2.752 | 0.00594** |
| Parameters held constant across groups | ||||
| β | 0.54503 | 0.0343 | 15.89 | <0.0001**** |
| γ | 0.29481 | 0.01966 | 14.997 | <0.0001**** |
| λ | −0.06229 | 0.01265 | −4.923 | <0.0001**** |
| Group differences in baseline curvature of w(p) in the absence of advice | ||||
| EA vs LA | −0.001973 | 0.136268 | −0.014 | 0.98845 |
| EA vs AD | −0.623911 | 0.12189 | −5.119 | <0.0001**** |
| LA vs AD | −0.621947 | 0.074219 | −8.38 | <0.0001**** |
| Group differences in the effect of advice on the curvature of w(p) | ||||
| EA vs LA | −0.147353 | 0.151344 | −0.974 | 0.33026 |
| EA vs AD | 0.274945 | 0.135576 | 2.028 | 0.04258* |
| LA vs AD | 0.422306 | 0.083667 | 5.047 | <0.0001**** |
Parameter estimates with s.e., t and P value from non-linear logistic regression are shown. Parameter estimates from behavioral choice model including all age groups. μ: temperature parameter or behavioral error term, α: curvature of w(p); α × advice: effect of advice on curvature of w(p) in; β: intercept of w(p), γ: curvature of value function, v(x), λ: effect of learning on curvature.
Significance codes: <0.0001**** 0.001*** 0.01** 0.05*.
As demonstrated in our previous experiment (Engelmann et al., 2009a), the presence of the expert’s advice led to a significant reduction in risk-taking behavior across all developmental groups. The effect of advice on w(p) is such that participants in all groups show increased overweighting of low probabilities and reduced overweighting of large probabilities after receiving the advice relative to its absence (Supplementary Figure S2A–C). Furthermore, the effect of advice on CEs shows an interesting developmental trajectory, indicating that CEs became significantly more AD-like with increasing age during the presence of advice. RRP, shown in Figure 2B, indicate that all participants became more risk-averse for high probabilities in the presence of advice. While adolescents exhibited small increases in risk neutrality for lotteries with outcome probabilities <37% in the presence of advice, the most striking effect of the advice on AD behavior was a large increase in risk-seeking choices for low probability gambles. Importantly, advice had a significantly greater effect on EAs and LAs compared to ADs, as reflected by significant interaction terms comparing the effect of the advice across groups. The effect of advice on the curvature of w(p) was greater for both adolescent groups compared to ADs [EA vs AD = 0.275; LA vs AD = 0.422] but did not differ for both adolescent groups [EA vs LA = −0.147]. Of note, similar trends were observed in model-free analyses of choice proportions as shown in Figure 2C (see Supplementary Materials).
It has to be noted that the type of advice provided by the expert depends in part on baseline CEs estimated via the PEST procedure. Given that we observed significant differences in CEs across developmental groups, it is possible that behavioral effects outlined below are partially due to differences in the advice strategy adopted by the expert. We have investigated this possibility by comparing group differences in the relative frequency of risk averse and risk neutral advice provided by the expert for each lottery and across age groups. Results form these analyses demonstrate no significant group differences in the relative frequency of risk-averse advice (see Supplementary Figure S4), indicating that behavioral results are not driven by group differences in the advice strategy of the expert.
Together, these findings indicate that, relative to ADs: (i) adolescents were significantly more risk-averse for low probabilities and more risk-seeking for high probabilities and (ii) the presence of advice led to significantly greater risk-averse behavior in both adolescent groups relative to ADs. Importantly, the effect of advice followed a clear developmental trajectory, such that adolescents’ choice behavior approached AD baseline risk-attitudes in the presence of advice. Taken together, these results indicate significantly more AD-like behavior in both adolescent groups in the presence of advice, as reflected by a significant change in the shape of w(p) leading to more risk-averse CEs (see Supplementary Data for confirmatory evidence using an independent analysis approach).
fMRI results
Developmental effects on brain regions involved in valuation
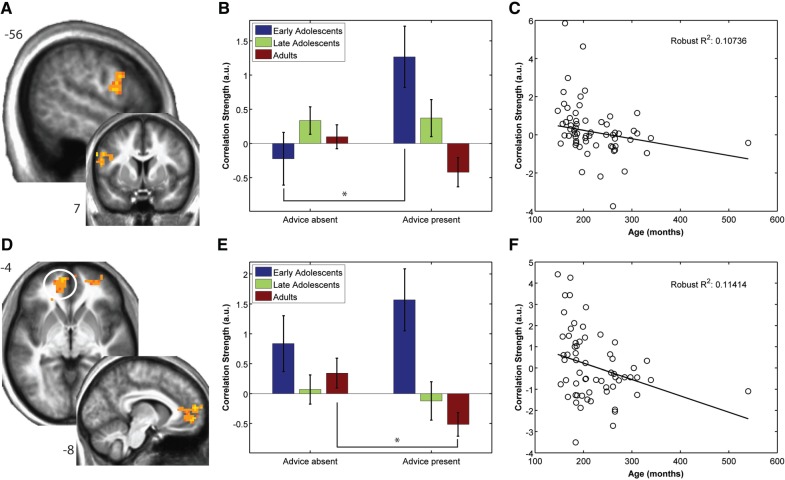
In order to investigate the extent to which the behavioral effects of age on risky choice are reflected by brain correlates of valuation, we probed for brain regions showing differential activation patterns related to valuation parameters as a function of age group. Significant group differences in the extent to which activity correlated with ‘offer magnitude’ was observed in left DLPFC (x = −54, y = 7, z = 23, Figure 3A) and right posterior TPJ (Supplementary Figure S5). Further significant age effects on correlations between brain activity and ‘CEs’ were observed in vmPFC (x = −11, y = 53, z = 7, Figure 3D) and ventrolateral PFC (x = 30, y = 53, z = 0, see Supplementary Table S2), as well as bilateral caudate nucleus (see Supplementary Figure S6). Robust regression analyses regressing parameter estimates against age confirm these results in DLPFC and vmPFC [DLPFC: slope = −0.0044, t(60) = −2.28, P = 0.027, R2 = 0.107, Figure 3C; vmPFC: slope = −0.0077, t(60) = −2.46, P = 0.017, R2 = 0.114, Figure 3F]. Additional developmental effects on brain activation patterns related to valuation and advice are reported in Supplementary Tables S3–S5.
Fig. 3.
Valuation regions showing developmental effects in the presence of advice. Developmental effects on neural correlates of valuation during the presence of advice. (A) Voxels in left DLPFC that show significant linear decreases in correlation strength with offer magnitude as a function of increasing age (cluster size corrected P < 0.05). (B) Bar plots show a significant effect of age group on correlation strength with the safe valuation parameter in the presence of advice, but not its absence. ROI analyses investigating the effect of advice indicate a significant increase in correlation strength in the presence of advice in early adolescents. (C) Illustrative results from robust regression analysis showing decreasing correlation strength with the safe valuation parameter as a function of age in the presence of advice. (D) Voxels in vmPFC that show significant linear decreases in correlation strength with certainty equivalents as a function of increasing age (cluster size corrected P < 0.05). (E) Bar plots showing significant effects of age group on correlation strength with the risky valuation parameter in the presence of advice, but not its absence. ROI analyses investigating the effect of advice indicate a significant decrease in correlation strength in the presence of advice in adults. (F) Illustrative results from robust regression analysis showing decreasing correlation strength with the risky valuation parameter as a function of age in the presence of advice. Each point represents a participant. Asterisk denotes a significant effect of advice on ROI activity, P < 0.05, all error bars represent s.e.
Advice influences activation patterns differentially across development
Our findings indicate significant developmental effects on neural correlates of valuation in the presence of advice in DLPFC and vmPFC. That these regions did not exhibit differential effects of age on valuation in the absence of advice suggests that advice differentially modulated their activity as a function of age. To test the involvement of those regions in mediating the behavioral effect of advice within specific age groups, we performed ROI analyses by extracting subjectwise beta estimates during the absence and presence of advice for each age group. These were entered into planned pairwise comparisons to test whether the presence of advice significantly affected valuation-related parametric effects for a given age group within these regions.
In EAs, advice significantly increased parametric effects related to ‘offer magnitude’ in left DLPFC [t(13) = 2.5319, P = 0.025, Figure 3B]. Advice also had a significant effect on parametric effects of ‘CEs’ in ADs. Specifically, decreases in correlation strength between CEs and brain activity were obtained in vmPFC [t(23) = −2.28, P = 0.032, Figure 3E].
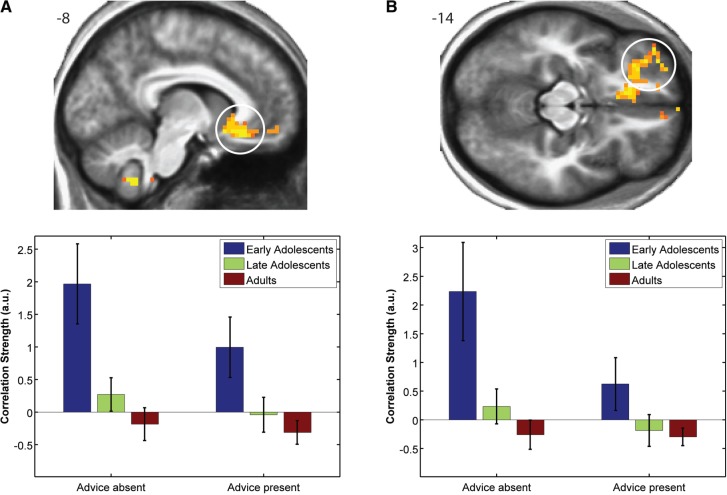
Developmental effects in the absence of advice
Significant developmental effects on neural correlates of valuation in the absence of advice were observed in vmPFC (x = −8, y = 26, z = −10, Figure 4A) and left vlPFC (x = −26, y = 44, z = −15, Figure 4B). Further significant correlations with CEs were observed in parahippocampal gyrus and medial orbitofrontal cortex (mOFC, see Supplementary Table S2). No effects of advice were observed in these regions, as activation patterns followed the same trend in both the presence and absence of advice (see Figure 4, bottom panel), suggesting a developmental effect on generalized valuation-related signals during choice within these regions.
Fig. 4.
Valuation regions showing developmental effects in the absence of advice. Voxels showing significant developmental effects on neural correlates of valuation in the absence of advice in vmPFC (A) and left vlPFC (B) (cluster size corrected P < 0.05). Bar plots illustrate significant age group effects during the absence of advice in corresponding regions. No effects of advice were observed in these regions, as activation patterns followed the same trend in both the presence and absence of advice. Error bars represent s.e.
DISCUSSION
Previous experiments investigating adolescent decision making under risk have demonstrated adult-like risk-attitudes in laboratory settings on one hand (Steinberg, 2004, 2008; Boyer, 2006; Reyna and Farley, 2006; van Leijenhorst et al., 2008) and risk-seeking attitudes in the presence of peers on the other (Gardner and Steinberg, 2005). We hypothesized that these seemingly opposing results are suggestive of a generalized sensitivity to the demands of social context that can explain commonly observed increases in conformity during adolescence (e.g. Berns et al., 2010). This hypothesis suggests that arousing advice provided by peers and real-life settings can lead to enhanced risk-taking, while risk-averse advice commonly provided by adults can lead to decreased risk-taking. We therefore investigated developmental effects of a specific risk-averse social context, provided by advice from an expert economist, on risky decision making across three age groups.
Behavioral results indicate, in agreement with our hypothesis, that advice had the greatest impact on risk-taking of EAs and LAs compared to ADs. Neuroimaging results demonstrate that the effect of advice was mediated by modulating activity in DLPFC. The effect of advice within this brain region was such that it underlined the salience of safe choice options in adolescents by specifically enhancing the correlation strength between brain activity and valuation parameters reflective of safe choice options. Taken together with prior research implicating DLPFC in behavioral control and suppression of emotional impulses (Knoch et al., 2006; Delgado et al., 2008a; Hare et al., 2009), these results are suggestive of advice modulating relevant cognitive and affective processes involved in decision making by influencing activity in lateral prefrontal cortex, a region that is known to undergo significant structural and functional developmental changes during adolescence. Our results, together with previous reports on adolescent decision making (e.g. Gardner and Steinberg, 2005; Berns et al., 2010), therefore suggest that risk attitude during adolescence is influenced by demands made by a particular social context.
Employing a behavioral model grounded in CPT, we observed that advice had a significant impact on risk-taking across all age groups, such that its presence produced more risk-averse behavior. Advice significantly decreased risk-taking for high-probability gambles in both adolescent groups compared to ADs, such that adolescents showed a significant change in the curvature of w(p), changing from an S-shaped curve previously observed in adolescents (Harbaugh et al., 2002), to an inverted S-shape commonly observed in adults (e.g. Tversky and Kahneman, 1992; Gonzalez and Wu, 1999), which produced risk-averse CEs. These results demonstrate that expert advice significantly reduced risk-taking in both adolescent groups relative to ADs. Indeed, in the presence of advice, more adult-like risk attitudes were observed in adolescents, suggesting that risk-averse advice can significantly affect adolescent behavior, when it comes from an adult.
Functional magnetic resonance imaging results support our main hypothesis that advice can have a significantly greater modulatory impact on adolescent, compared to adult brain systems relevant for integrating the advice in the decision process. Developmental differences in the recruitment of neural valuation mechanisms were obtained mainly in the presence of advice in regions implicated in cognitive control, behavioral inhibition and emotion regulation, such as dorsolateral and ventrolateral PFC (Delgado et al., 2008b; Koechlin et al., 2003), reward-processing, such as vmPFC and medial OFC (e.g., Grabenhorst and Rolls, 2011), as well as social cognition, such as posterior TPJ (e.g., Saxe and Kanwisher, 2003). Importantly, the effect of advice was specific in adolescents, such that it led to an increase in correlation strength of BOLD with risk-averse valuation parameters. Given the risk-reducing effect of the advice on behavior in adolescents, combined with a specific modulatory impact on neural correlates of valuation, we conjecture that expert advice operates by enhancing inhibitory processes in adolescents, leading to a more deliberative decision strategy.
A number of previous observations support this conjecture. Previous research has demonstrated delayed development within prefrontal systems implicated in cognitive control and behavioral inhibition relative to neural systems involved in affective processing (Casey et al., 2005; Blakemore, 2008; Galvan, 2010). Functionally, such delayed development is commonly reflected by an increased engagement of cognitive control regions, such as DLPFC, with age in a variatey of cognitive and social choice tasks (e.g. Crone et al., 2006; Guroglu et al., 2011; van den Bos et al., 2011). Interestingly, a similar developmental pattern has been observed in TPJ, such that increased recruitment is observed with age in the context of social tasks (e.g. Guroglu et al., 2011; van den Bos et al., 2011; see also Blakemore, 2008). This developmental pattern has been interpreted to reflect a growing ability to engage cognitive and inhibitory control (Bunge and Wright, 2007; Crone, 2009), as well as social cognition (Blakemore, 2008; but see Poldrack, 2010). Our results support and extend this notion by demonstrating that social context generated by an adult advisor can lead to the expression of adult-like behavior in adolescents via enhancing activation patterns within cognitive control regions in the context of a task that requires both mentalizing and suppression of prepotent behavior (Engelmann et al., 2009a). Finally, a number of recent fMRI studies investigated the influence of advice on neural correlates related to valuation and learning (Behrens et al., 2008; Biele et al., 2011; Klucharev et al., 2008). Biele et al., (2011) examined the effect of advice on outcome-related signals in the context of a reward-based decision-making task. Results demonstrated modulations of BOLD activity after following advice in a network of regions including the septal area, caudate nucleus and vmPFC. Here we show that advice can also modulate value-related signals in vmPFC and DLPFC during the choice period, but that the extent of such modulation depends on developmental stage. A neuroimaging study by Klucharev et al. (2008) investigated expert influences on memory of and attitude toward products. Their results demonstrated that a single pairing of a picture depicting a product with a photograph depicting a celebrity perceived as an expert for a given product category enhances: (i) subsequent recall and (ii) positive attitude toward products (Klucharev et al., 2008). Interestingly, the attitude enhancing effect of celebrities judged to be experts in a given product category was mediated by elevated activity in caudate nucleus. Results from the current investigation confirm and extend these findings by demonstrating developmental differences in brain signals reflective of subjective value during choice in bilateral caudate nucleus (see Supplementary Figure S6).
A few limitations should be mentioned. We employed an adult expert as our treatment across all age groups, which may confound age status with expertise in the current investigation. This was done because an actual person was chosen to function as advisor to create a naturalistic experimental setting and, additionally, his long list of accomplishments in the fields of economics and finance was presented to participants in detailed instructions to provide trustworthy advice in the treatment condition (see Engelmann et al., 2009a for further details). Since such accomplishments could not have been accumulated by an adolescent advisor, an adult expert was used across all age groups. Finally, age groups were not matched by gender in the current investigation.
Taken together, the current pattern of results provides evidence demonstrating that, across development, different brain systems mediate the risk-reducing effect of advice during decision making under risk. Specifically, advice modulated activity in vmPFC in adults, while in adolescents, advice modulated brain responses related to valuation in DLPFC. Based on these results we propose that the risk-reducing effect of the social context on behavior in adolescents operates via valuation-specific enhancements of inhibitory and cognitive control processes that lead to a more deliberate decision strategy. To our knowledge, this is the first neurobiological study of the mechanisms whereby advice from an authority figure affects risky choices differentially across development. Despite previous reports questioning the efficacy of interventions for reducing dangerous behaviors among adolescents (Steinberg, 2008), our results indicate that advice from an authority figure or more specifically risk-averse advice, can effectively reduce risk-seeking choices in adolescents. However, because during key moments in adolescent life, advice is likely provided by peers, an important goal of future investigations is to examine whether such intervention methods would maintain their efficacy within social contexts that favor risk-seeking behavior, such as the presence of peers.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
FUNDING
This research was supported by grants from NIDA (grants R01 DA20116 and R01 DA016434 to G.S.B.). J.B.E. gratefully acknowledges support from the Mercator Foundation Switzerland, the NCCR Affective Sciences, the Neurochoice project of SystemsX and the research priority program at the University of Zurich ‘Foundations of Human Social Behavior’.
Supplementary Material
ACKNOWLEDGEMENTS
We are thankful to Thomas Epper, Charles Efferson and Tony Williams for helpful discussions and Todd A. Hare for helpful comments on an earlier version of this manuscript. We would also like to thank the reviewers for their insightful comments.
REFERENCES
- Andersen S, Harrison GW, Lau MI, Rutstroem EE. Behavioral econometrics for psychologists. Journal of Economic Psychology. 2010;31:553–76. [Google Scholar]
- Andrade A, Paradis AL, Rouquette S, Poline JB. Ambiguous results in functional neuroimaging data analysis due to covariate correlation. Neuroimage. 1999;10(4):483–6. doi: 10.1006/nimg.1999.0479. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS. Associative learning of social value. Nature. 2008;456(7219):245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Capra CM, Moore S, Noussair C. Neural mechanisms of the influence of popularity on adolescent ratings of music. Neuroimage. 2010;49(3):2687–96. doi: 10.1016/j.neuroimage.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biele G, Rieskamp J, Krugel LK, Heekeren HR. The neural basis of following advice. PLoS biology. 2011;9(6):e1001089. doi: 10.1371/journal.pbio.1001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9(4):267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2(2):130–9. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer TW. The development of risk-taking: a multi-perspective review. Developmental Review. 2006;26(3):291–345. [Google Scholar]
- Bruhin A, Fehr-Duda H, Epper T. Risk and rationality: uncovering heterogeneity in probability distortion. Econometrica. 2010;78(4):1375–412. [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Current Opinion in Neurobiology. 2007;17(2):243–50. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: a meta-analysis. Psychological Bulletin. 1999;125:367–83. [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15(2):239–44. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Developmental Science, Peer influence on risk taking. 2010;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. doi:10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Luce RD, von Winterfeld D. Tests of assumptions about the joint receipt of gambles in rank- and sign-dependent utility theory. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:931–43. [Google Scholar]
- Cromwell HC, Hassani OK, Schultz W. Relative reward processing in primate striatum. Experimental Brain Research. 2005;162(4):520–5. doi: 10.1007/s00221-005-2223-z. [DOI] [PubMed] [Google Scholar]
- Crone EA. Executive functions in adolescence: inferences from brain and behavior. Developmental Science. 2009;12(6):825–30. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9315–20. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nature Neuroscience. 2008a;11(8):880–1. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008b;59(5):829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Capra CM, Noussair C, Berns GS. Expert financial advice neurobiologically “Offloads” financial decision-making under risk. PLoS One. 2009a;4(3):e4957. doi: 10.1371/journal.pone.0004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Frontiers in Human Neuroscience. 2009b;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Kiefer M, Grothe J, Wunderlich AP, Spitzer M, Walter H. Emotional context modulates subsequent memory effect. Neuroimage. 2003;18(2):439–47. doi: 10.1016/s1053-8119(02)00015-0. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–9. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechner G. Elements of Psychophysics. Vol. 1. New York: Holt, Rinehart and Winston; 1966. (originally published 1860) [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the columbia card task. Journal of Experimental Psychology Learning. 2009;35(3):709–30. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41(4):625–35. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Wu G. On the shape of the probability weighting function. Cognitive Psychology. 1999;38:129–66. doi: 10.1006/cogp.1998.0710. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Different representations of relative and absolute subjective value in the human brain. Neuroimage. 2009;48(1):258–68. doi: 10.1016/j.neuroimage.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Guroglu B, van den Bos W, van Dijk E, Rombouts SA, Crone EA. Dissociable brain networks involved in development of fairness considerations: understanding intentionality behind unfairness. Neuroimage. 2011;57(2):634–41. doi: 10.1016/j.neuroimage.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Harbaugh WT, Krause K, Vesterlund L. Risk attitudes of children and adults: choices of small and large probability gains and losses. Experimental Economics. 2002;5:53–84. [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57(6):624–32. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Kalenscher T, Tobler PN, Huijbers W, Daselaar SM, Pennartz CM. Neural signatures of intransitive preferences. Frontiers in Human Neuroscience. 2010;4:49. doi: 10.3389/fnhum.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucharev V, Smidts A, Fernández G. Brain mechanisms of persuasion: how “expert power” modulates memory and attitudes. Social Cognitive and Affective Neuroscience. 2008;3(4):353–66. doi: 10.1093/scan/nsn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314(5800):829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science (New York, N.Y.) 2003;302(5648):1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cognitive, Affective & Behavioral Neuroscience. 2008;8(1):99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychological Bulletin. 2001;127(2):267–86. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition. 2010;72(1):101–13. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19(8):1323–37. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(3):1050–4. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, et al. The neural system that bridges reward and cognition in humans: an fMRI study. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(8):5669–74. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Interpreting developmental changes in neuroimaging signals. Human Brain Mapping. 2010;31(6):872–8. doi: 10.1002/hbm.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelec D. The probability weighting function. Econometrica. 1998;66:497–527. [Google Scholar]
- Reyna VF, Farley F. Risk and rationality in adolescent decision-making: implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Rivers S, Reyna VF, Mills B. Risk taking under the influence: a fuzzy-trace theory of emotion in adolescence. Developmental Review. 2008;28:107–44. doi: 10.1016/j.dr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain Cognition. 2010;72(1):134–45. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cerebral Cortex. 2005;15(12):1855–65. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–8. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Science. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Developmental Psychology. 2007;43(6):1531–43. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. Neuroimage. 2004;21(3):1045–54. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. Journal of Risk and Uncertainty. 1992;5:297–323. [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SA, Crone EA. Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychology Science. 2011;22(1):60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the cake gambling task: age and gender analyses of probability estimation and reward evaluation. Developmental Neuropsychology. 2008;33(2):179–96. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.