Abstract
Chemical investigation of a new endophytic fungus, Mycosphaerella sp. nov. strain F2140 associated with the foliage of the plant Psychotria horizontalis (Rubiaceae) in Panama, resulted in the isolation of cercosporin (1) and a new cercosporin analogue (3) as the major components. The structures of minor compounds in the extract were elucidated by detailed spectroscopic analysis as 2-(2-butyl)-3-hydroxy-6-ethyl-6-methylcyclohex-2-ene-1,5-dione (4), 3-(2-butyl)-6-ethyl-6-methyl-5-hydroxy-2-methoxy-cyclohex-2-eneone (5), and an isomer of 5 (6). To study the influence of the hydroxy groups on the anti-parasitic activity of cercosporin, compound 1 was acetylated to obtain derivative 2. The isolated compounds 1–6 were tested in vitro to determine their anti-parasitic activity against the causal agents of malaria (Plasmodium falciparum), leishmaniasis (Leishmania donovani), and Chagas disease (Trypanosoma cruzi). Also, the cytotoxicity and potential anticancer activity of these compounds were evaluated using mammalian Vero cells and MCF7 cancer cell lines, respectively. Compounds 1 and 2 displayed high potency against L. donovani (IC50 0.46 and 0.64 μM), T. cruzi (IC50 1.08 and 0.78 μM), P. falciparum (IC50 1.03 and 2.99 μM), and MCF7 cancer cell lines (IC50 4.68 and 3.56 μM). Compounds 3–6 were not active in these assays at a concentration of 10 μg/mL.
Keywords: Endophytic fungus, Mycosphaerella sp. nov, Cercosporin, Anti-parasitic activity
As part of a continuing program to identify novel compounds for the treatment of parasitic diseases, the Panamanian International Cooperative Biodiversity Group (ICBG) [1a, 1b] has been investigating new anti-parasitic agents from plants [1c, 1d], marine organisms [1e, 1f], and more recently, endophytic fungi [1g, 1h]. In the course of these latter studies, 21 fungal strains were isolated from the leaves of a neotropical shrub, Psychotria horizontalis (Rubiaceae). The extract from a new fungal species, identified as Mycosphaerella sp. nov., was selected for further purification based on initial bioactivity results. Herein, we report the isolation and structural elucidation of a new cercosporin analogue (3) along with cercosporin (1) as major metabolites, together with four new minor metabolites, 2-(2-butyl)-3-hydroxy-6-ethyl-6-methylcyclohex-2-ene-1, 5-dione (4), 3-(2-butyl)-6-ethyl-6-methyl-5-hydroxy-2-methoxy-cyclohex-2-eneone (5), and its isomer 6. The compounds were evaluated for anti-parasitic and cytotoxic activities. Although cercosporin is a well-known phytotoxin with significant cytotoxic activity, its potential anti-parasitic activities have not been previously reported.
Extracts from all the tested growth media were found to be active against T. cruzi (IG, 86–94%), P. falciparum (IG, 90–99%), and L. donovani (IG, 90.8–99.1%) (Table 1). The similar, non-selective bioactivity profile of all extracts from F2140 strain led us to the conclusion that a major compound present in all the extracts likely was responsible for the activity.
Table 1.
Biological Activity [IC50, μM]* of Compounds 1 and 2 to Tropical Parasites and MCF-7 Cell Lines
| L. donovani | P. falciparum | T. cruzi | MCF-7 | Vero cells | TW | |
|---|---|---|---|---|---|---|
| 1 | 0.46 ± 0.05 | 1.03 ± 0.03 | 1.08 ±0.03 | 4.68 ± 0.20 | 1.54±0.10 | 3.4 |
| 2 | 0.64 ± 0.05 | 2.99 ± 0.40 | 0.78 ± 0.07 | 3.56 ± 0.20 | 1.24± 0.10 | 1.9 |
The experiments were performed in duplicate. Results indicate mean ± standard error. TW, therapeutic window
TLC of extracts showed a dark brownish spot corresponding to a molecular weight of 535 amu following analysis by low resolution mass spectrometry. In addition, the MS data of the mycelial extract from potato dextrose culture showed an additional peak at 537 amu, and thus was probable an analogue of the major compound. Based on these results, the mycelial extract of potato dextrose culture was selected for further purification.
Cercosporin (1), the major component of the crude extract, was identified by comparison of its NMR and MS data with those reported previously [2a, 2b]. Acetylation of cercosporin gave a tetra-acetate (2; Fig. 1), which showed physicochemical properties consistent with the literature data [2c, 2d].
Figure 1.
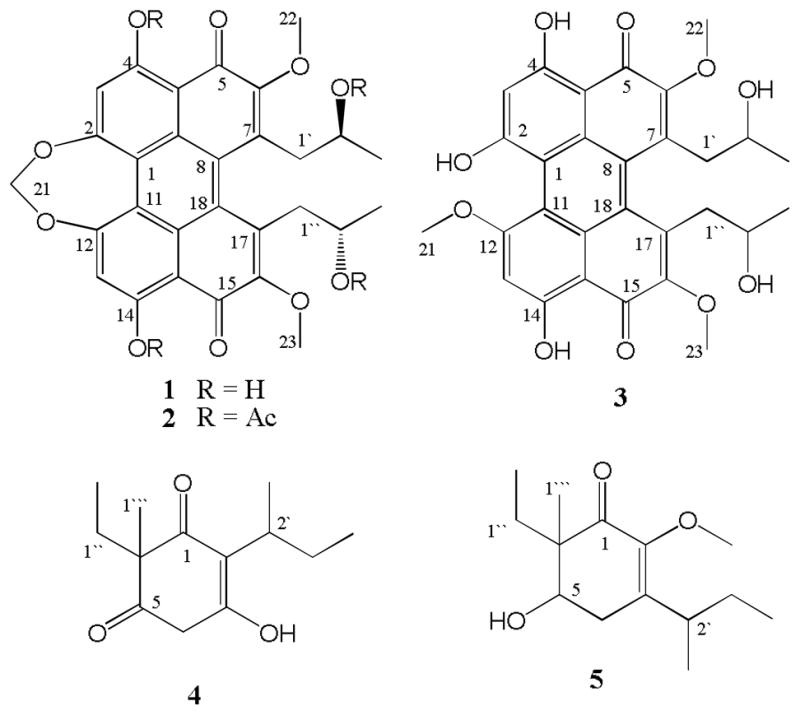
Chemical Structures of the Isolated Metabolites (1, 3–5) and one Derivative (2).
The molecular formula of compound 3 was established as C29H28O10 on the basis of its APCI-HR-MS (m/z 537.1758 [M + H]+). The 1H and 13C NMR signals of 3 were closely similar to those of 1. However, 3 displayed twice the number of NMR signals, suggesting the lack of structural symmetry in 3. Also, the NMR signals corresponding to the methylene group [δH 5.75 (2H, s, H-21); δC 92.6] of the dioxepane ring in compound 1 was replaced by the signals for a methoxy group [δH 4.27 (3H, s, OMe); δC 57.9] in compound 3, indicating a ring-opened analogue of cercosporin with a methoxy group at one terminus and a hydroxy group at the other. The position of the methoxy group in 3 was confirmed by HMBC correlations from δH 4.27 to δC 163.3 (C-12).
Compound 4 was obtained as colorless oil with a specific rotation ([α] 26D) of −2.6°. The molecular formula was determined to be C13H20O3 based on the APCI-HR-MS data [M+H]+ at m/z 225.1471. The 13C spectrum of 4 confirmed the presence of 13 carbon resonances, with their multiplicities determined from a DEPT spectrum as four methyl [δC 9.2, 12.5, 18.3, and 18.8], three methylene [δC 27.4, 28.1, and 55.9], one methine [δC 33.1] and five quaternary carbons [δC 50.4, 152.7, 161.6, 207.4, and 209.5]. The HSQC spectrum identified the corresponding 1H signals for methyl [δH 0.71 (t, J = 7.5 Hz, H-2″), 0.84 (t, J = 7.5 Hz, H-4′), 1.26 (d, J = 7.2 Hz, H-1′), and 1.11 (s, H-1‴)], methylene [δH 1.71 (m, H-1″), 1.69 (dq, J = 7.5, 15.0 Hz, H-3′), and 4.64 (s, H-4)], and methine [δH 2.85 (m, H-2′)] groups. Analysis of the COSY spectrum suggested the presence of ethyl and 2-butanyl moieties. In the HMBC spectrum, H-2′ [δH 2.85] of the sec-butyl group showed long-range correlations to C-1 [δC 207.4], C-2 [δC 161.6] and C-3 [δC 152.7] indicating that the 2-butyl group was attached to a double bond (C-2, see Figure 3). HMBC correlations from the methyl group [δH 1.11] to C-1″ [δC 28.0], C-6 [δC 50.4], C-1 [δC 207.4], and C-5 [δC 209.5] confirmed that the methyl and ethyl groups were attached to C-6. Finally, the HMBC correlations of H-4 with C-3, C-2 and C-5 enabled formulation of a six-membered cyclic diketoenol structure for 4. The absolute configuration of C-6 position was not determined due to the low amount of compound isolated. Thus, the structure of 4 was determined to be 2-(2-butyl)-3-hydroxy-6-ethyl-6-methylcyclohex-2-ene-1, 5-dione- (4).
Compound 5 was obtained as colorless oil with a specific rotation ([α] 26D) of +60.2. The molecular formula was determined as C14H24O3 by APCI-HR-MS in positive ion mode, displaying a protonated molecular ion peak at m/z 241.1786 [M+H]+. The formula was consistent with the number of protons and carbons observed in the NMR spectra. In addition to a methoxy functional group [δH 3.60 (s, OMe), δC 59.9], a methyl [δH 1.14 (s, H-1‴), δC 17.7], an ethyl [δH 0.84 (t, J = 7.5 Hz, H-2″), δC 7.9; δH 1.64 (t, J = 7.5 Hz, H-1″), δC 22.7], and a 2-butyl [δH 1.04 (d, J = 7.0 Hz, H-1′),δC 18.6; δH 3.00 (m, H-2′), δC 34.8; δH 1.41 (m, H-3′), δC 27.1; δH 0.88 (t, J = 7.5 Hz, H-4′), δC 12.5] group were identified from the NMR data. The remaining six 13C NMR signals [δC 198.0 (C-1), 147.3 (C-2), 146.3 (C-3), 73.1 (C-5), 50.9 (C-6) and 28.8 (C-4)] belonged to a cyclic skeleton similar to 4. The positions of the functional groups in 5 were confirmed by analysis of HMBC correlations from methylene [δH 2.48, H-4], methyl [δH 1.14 H-1‴], and methoxy [δH 3.60, OMe] protons. The sec-butyl group was now attached to C-3 as indicated by the correlation of H-2′ with C-2 and C-3, and H2-4 with C-2′, C-2, C-3 and C-5. The position of the OMe was determined by the correlation of this three proton resonance with C-2 (see Figure 2). Thus, the structure of 5 was determined to be 3-(2-butyl)-6-ethyl-6-methyl-5-hydroxy-2-methoxy-cyclohex-2-eneone.
Figure 2.
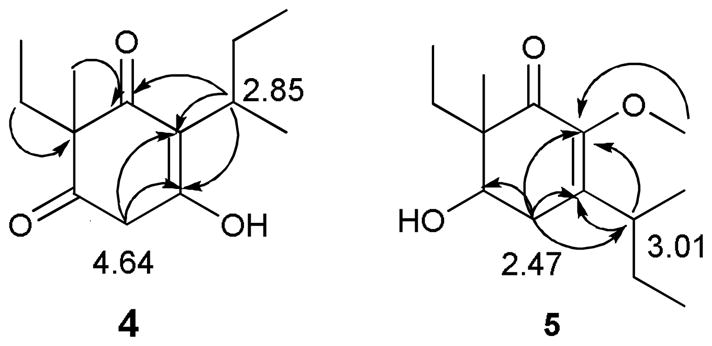
Key HMBC correlations for placing substituents on compounds 4 and 5
Compound 6 was obtained as a colorless oil with a specific rotation ([α] 26D) of +15.7. Its molecular formula was determined to be C14H24O3 based on the mass spectrum (APCI-HR-MS) displaying an [M+H]+ at m/z 241.1757. Compound 6 showed very similar NMR signals to 5 and these were confirmed by further 2D NMR analysis. On the basis of these data, compound 6 was identified as a diastereomer of 3-(2-butyl)-6-ethyl-6-methyl-5-hydroxy-2-methoxy-cyclohex-2-eneone
Cercosporin belongs to the perylenequinone-class of metabolites [3] which are of polyketide origin [4] and are produced by fungi within the genus Cercospora [2b]. It is a photosensitizing toxin that produces highly reactive superoxide anions as well as singlet oxygen upon irradiation. This causes peroxidation of membrane fatty acids, leading to rupture of plasma membrane in plants and cell death [5].
The majority of the thousands of species currently circumscribed as Cercospora lack a known sexual stage, but in a small number of species a Cercospora anamorph (asexual stage) has been connected to a Mycosphaerella teleomorph (sexual stage) [6]. Isolate F2140 did not produce sexual or asexual structures in culture, and phylogenetic analyses placed it in a previously unknown clade of endophytic Mycosphaerella from diverse hosts for which anamorphs are not presently known [7a, 7b]. Given our observation of cercosporin production by this isolate we anticipate that F2140 represents a clade of Mycosphaerella characterized as Cercospora when anamorphic.
Among the extracts from the different growth media, the one obtained from the potato dextrose culture was found to be the most active in our anti-parasitic assays. Recently, perylenequinones including cercosporin have been evaluated for toxicity against a broad spectrum of cancer cell lines and for protein kinase C inhibition activity [8]. However, previous studies do not appear to describe the activity of cercosporin against parasitic diseases such as malaria, leishmaniasis and Chagas. The in vitro assay results suggest that cercosporin is highly active against P. falciparum (IC50 1.03 μM), L. donovani (IC50 0.46 μM), and T. cruzi (IC50 1.08 μM). This bioactivity profile of cercosporin showed that it was not selective between the assayed parasites. Except in T. cruzi assay (Table 1), the acetylated cercosporin showed lower activity compared to the parent compound. However, the bioactivity of cercosporin varied only slightly when it was acetylated. It is possible that the hydroxy groups do not have a marked effect on the bioactivity of cercosporin, or that the esters are labile and removed by esterases upon entering cells. Because the bioassays were performed in the dark, any possible phototoxic effect of cercosporin on the parasites was minimized. Compound 1 and 2 showed lower cytotoxicity to mammalian Vero cells (1.54 and 1.24 μM) compared to their anti-leishmanial activity, thus giving a modest therapeutic window of 3.4 and 1.9, respectively (Table 1). Compound 3, identified as a seven-membered dioxepane ring-opened analogue of cercosporin, showed a significant reduction in activity in all of these biological assays (IC50>10 μg/mL), indicating the importance of the methelenedioxy functionality to the biological properties of compound 1. A similar reduction activity was also observed with some of the anti-leishmanial alkaloids isolated in our lab [9]. The minor compounds 4–6 did not show significant activity (IC50>10 μg/mL) in any of the biological assays.
Overall, the results presented here suggest that cercosporin produced by the new fungal strain Mycosphaerella sp. nov is responsible for the antiparasitic and cytotoxic activities. The results also provide further insights into the structure- activity relationship of cercosporin against the tropical parasites which could be useful in designing more potent and selective agents against these diseases.
Experimental
Isolation and identification of fungal strain
The host plant P. horizontalis was collected from the tropical forest at Barro Colorado Natural Monument, Panama and was identified by Dr. Alicia Ibáñez, a voucher specimen (B1704) was deposited at the Smithsonian Tropical Research Institute, Panama. The fungal strain was isolated on malt extract agar from a surface-sterilized, healthy, mature leaf in August 2005 following methods outlined in Arnold and Lutzoni [10]. Because the strain did not sporulate in culture, phylogenetic analyses of molecular sequence data were used to verify its taxonomic placement.
Briefly, total genomic DNA was extracted from fresh mycelium following Arnold and Lutzoni [10]. The nuclear ribosomal internal transcribed spacers and 5.8s gene (nrITS) were amplified as a single fragment and sequenced bidirectionally at the Genomics and Technology Core at The University of Arizona using primers ITS1F and ITS4 [11] (for PCR protocols see Hoffman and Arnold [12] and Higgins et al. [13]). Forward and reverse reads were assembled and evaluated by phred and phrap [14a,14b] with automation provided by Mesquite, http://mesquiteproject.org., and manual editing in Sequencher v4.5 (Gene Codes Corporation, Ann Arbor, MI). The consensus sequence was submitted to a BLAST search of the NCBI GenBank database for preliminary identification at higher taxonomic levels and to establish appropriate taxon sampling for phylogenetic analysis. BLAST matches suggested placement in Mycosphaerella (Mycosphaerellaceae, Capnodiales, Dothideomycetes, and Ascomycota). To overcome widely recognized limitations associated with BLAST-based taxonomy, including potential misidentification of sequences, relatively limited representations of endophytic fungi, and the uncertain validity of taxonomic placement based on the non-evolutionary BLAST algorithm, were verified placement using phylogenetic analyses. Briefly, maximum likelihood analyses of two complementary data sets, the first consisting of 100 sequences of top hits obtained from GenBank, and the second consisting of 34 sequences of closely related strains and eight strains obtained in broader surveys of endophyte diversity [7a,7b] confirmed placement in Mycosphaerella and provided high bootstrap support for recognition of this strain as a novel species relative to known diversity in this highly species-rich, and often geographically specialized, genus. Therefore, the strain was characterized as a new species of Mycosphaerella (Mycosphaerella sp. nov., Arnold et al. unpub.) [7c]. A voucher specimen was deposited as plugs of agar in sterile water at the Smithsonian Tropical Research Institute, Panama (accession F2140), and at the Robert L. Gilbertson Mycological Herbarium, The University of Arizona (accession F2140/TK1648).
Cultivation and extraction procedures
F2140 was cultured on slants of potato dextrose agar (PDA) at 25°C for 7 days. Agar plugs (2×2 cm) then were transferred to four different media (700 mL each; malt extract, potato dextrose, Czapek Dox, and V8) and incubated at 28°C for 15 d on a rotary shaker (120 rpm). Suspensions from each medium were vacuum-filtered and the filtrate successively partitioned between hexane, chloroform, and ethyl acetate to obtain 29.6, 32.1, 37.4 mg (malt extract), 54.4, 129.4, 67.0 mg (potato dextrose), 5.9, 34.7, 28.3 mg (Czapek Dox) and 10.3, 9.4, 8.1 mg (V8) of extract, respectively. Erlenmeyer flasks containing each medium without inocula were treated in the same way as controls. Mycelial mass collected by filtration from each medium was homogenized using a polytron and extracted with EtOAc to produce 149.7, 1511.5, 226.1, and 81.1 mg of extract from each medium, respectively. A total of 16 extracts, obtained as described above, were evaluated for their anti- parasitic activity at a concentration of 10 μg/mL.
Isolation of compounds
Mycelial extract from potato dextrose (100 mg from 1.5 g) was chromatographed on a GRACE Econosphere NP-HPLC column (250 × 10 mm) using CHCl3-MeOH (97:3) as eluents for 40 min at a flow rate of 1.5 mL/min to yield two major compounds: cercosporin (1) (47 mg, tR 17.2 min), and a new isomer of cercosporin (3) (20 mg, tR 21.3 min). Following Kuyama et al. [2c], 10 mg of cercosporin was acetylated in pyridine using acetic anhydride to obtain a tetra-acetyl derivative. The remainder of the extract (1.4 g) was fractionated over a silica column (70–230 mesh, Merck) using hexanes- CHCl3, CHCl3-MeOH mixtures and MeOH as eluents to give three subfractions (A-C). Subfraction A (20 mg) was further purified on an Xterra C18 HPLC column (250 × 10 mm) using 35% aqueous MeCN for 60 min at a flow rate of 1.5 mL/min to obtain a mixture (5.6 mg, tR 44.6 min) and the new compound (4) (2.0 mg, tR 46.7min). The mixture of compounds was successfully separated using a normal phase HPLC column chromatography (GRACE Econosphere, 250 × 10 mm) using an isocratic solvent system containing hexanes-CHCl3 (4:6) for 30 min at a flow rate of 1.0 mL/min to give two new compounds (5) (2.0 mg, tR 46.7min), and (6) (0.9 mg, tR 52.5 min), respectively.
General experimental procedures
Optical rotations were measured with a Rudolf Research Analytical Autopol III 6971 automatic polarimeter. NMR spectra were recorded on a Bruker spectrometer with standard pulse sequences operating at 300 MHz in 1H NMR and 75 MHz in 13C NMR using TMS as an internal chemical shift reference. Mass spectra were acquired on a JEOL LC-mate mass spectrometer. TLC was performed on precoated silica gel plates (Kieselgel 60, F254, 20 × 20 cm, 0.25 mm thick, Merck). Spots were detected by staining with a solution of p-anisaldehyde-sulfuric acid in methanol followed by heating. Reverse phase HPLC was performed on a Waters 600 model system with a photodiode array UV detector 2996. Normal phase HPLC was performed on a Waters 1515 model system with a dual wavelength UV detector model 2487.
Bioassays
Leishmania bioassay
Axenically grown (cell free) amastigotes of L. donovani (LD-1S/MHOM/SD/00-strain 1S), the species responsible for the visceral and lethal forms of leishmaniasis, were used to assess parasite growth and survival. Samples were tested in duplicate at 10 μg/mL. The results were expressed as a percentage of growth inhibition (IG) compared to controls. Samples that showed above 70% IG were considered active and were then assayed at four different concentrations (0.08, 0.4, 2, and 10 μg/mL) to determine IC50 values. Amphotericin B was used as a positive control with the typical IC50 response of 0.08–0.13 μM [1h,15].
Chagas’ disease bioassays
T. cruzi bioassays were performed using a colorimetric method, and the inhibition of parasite growth was assessed by the expression of the reporter gene for beta-galactosidase (β-Gal) in the recombinant Tulahuen clone C4 of T. cruzi. Assays were performed in duplicate on the amastigote, the intracellular form of the parasite infecting African green monkey kidney (Vero) cells, exposed during 120 h to different concentrations (10, 2 and 0.4 μg/mL) of the test compounds at 37°C under an atmosphere of 5% CO2/95% air. The resulting color from the cleavage of chlorophenol red-β-D-galactoside (CPRG) by β-Gal expressed by the parasite was measured at 570 nm. The concentration that inhibited 50% expression of β-Gal (IC50) was calculated by log regression of the obtained optical density values, and compared with the untreated controls. Nifurtimox was used as a positive control (IC50 10–16 μM) [16,17].
Malaria bioassays
Antiplasmodial activity was evaluated using a fluorometric method based on the detection of parasite DNA with the fluorochrome PicoGreen using a chloroquine-resistant strain (Indochina W2) of P. falciparum. The sample was considered active if it inhibited >70% of the growth of the parasites as compared to their untreated controls at 10 μg/ml. The IC50 was calculated by normal regression of the resulting inhibition percentages at 0.08, 0.4, 2, and 10 μg/mL. The parasites were maintained in vitro by a modification of the method of Trager and Jensen [18a]. Chloroquine was used as a positive control (IC50 80–100 nM) [1g,18b].
Cancer bioassays
Cytotoxic activity against MCF-7 human breast cancer cells was performed following the standard protocol of the National Cancer Institute [19]. Adriamycin was used as a positive control (IC50 0.02–0.3 nM).
Mammalian Cell Cytotoxicity bioassay
Vero cells, derived from kidneys of the African green monkey, were adhered to 96-well plates and examined for reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO). After treatment with the test compound for 4 h of incubation at 37°C, cell viability was evaluated in an ELISA reader at 570 nm [1g,17, and 20].
Compound 3
Dark red powder.
[α]D26: −879 (c 0.045, CHCl3).
1HNMR (300 MHz, CDCl3): δ 0.61 (d, J = 6.2 Hz, 3H, H-3′), 0.65 (d, J = 6.2 Hz, 3H, H-3″), 2.79 (dd, J = 12.7, 6.0 Hz, 1H, H-1a″), 2.85 (dd, J = 12.7, 6.0 Hz, 1H, H-1a′), 3.40 (m, 1H, H-2″), 3.43 (m, 1H, H-2′), 3.51 (dd, J = 12.7, 1.9 Hz, 1H, H-1b″), 3.53 (dd, J = 12.7, 2.7 Hz, 1H, H-1b′), 4.19 (s, 3H, H-23), 4.20 (s, 3H, H-22), 4.27 (s, 3H, H-21), 6.86 (s, 1H, H-13), 6.91 (s, 1H, H-3).
13CNMR (75 MHz, CDCl3): δ 23.6 (C-3″), 23.8 (C-3′), 42.2 (C-1″), 42.4 (C-1′), 58.1 (C-21), 61.6 (C-22, C-23), 68.5 (C-2′, C-2″), 101.9 (C-3), 106.3 (C-10), 107.1 (C-20), 107.9 (C-13), 112.7 (C-1), 113.5 (C-11), 128.2 (C-9), 129.5 (C-19), 131.6 (C-17/7), 134.5 (C-8), 136.2 (C-18), 152.9 (C-6), 153.2 (C-16), 161.5 (C-12), 163.3 (C-2), 169.5 (C-4), 170.0 (C-14), 180.5 (C-5), 181.8 (C-15). APCI-HR-MS m/z 537.1758 [M+H]+ (calcd for C29H29O10, 537.1761).
2-(2-butyl)-6-ethyl-3-hydroxy-6-methylcyclohex-2-ene- 1, 5-dione (4)
Colorless oil.
[α]D26: −2.6 (c 0.07, CHCl3).
1HNMR (300 MHz, CDCl3): δ 0.71 (t, J = 7.4 Hz, 3H, H-2″), 0.84 (t, J = 7.4 Hz, 3H, H-4′), 1.11 (s, 3H, H-1‴), 1.26 (d, J = 7.1 Hz, 3H, H-1′), 1.69 (m, 2H, H-1″), 1.71 (m, 2H, H-3′), 2.85 (m, 1H, H-2′), 4.64 (s, 2H, H-4).
13CNMR (75 MHz, CDCl3): δ 9.2 (C-2″), 12.5 (C-4′), 18.3 (C-1′), 18.8 (C-1‴), 27.4 (C-3′), 28.1 (C-1″), 33.1 (C-2′), 50.4 (C-6), 55.9 (C-4), 152.7 (C-3), 161.6 (C-2), 207.4 (C-1), 209.5 (C-5).
APCI-HR-MS m/z 225.1471 [M+H]+ (calcd for C13H21O3, 225.1491);
3-(2-butyl)-6-ethyl-5-hydroxy-2-methoxy-6-methyl-cyclohex-2-enone (5)
Colorless oil.
[α]D26: +60.2 (c 0.04, CHCl3).
1HNMR (300 MHz, CDCl3): δ 0.84 (t, J = 7.7 Hz, 3H, H-2″), 0.88 (t, J = 7.4 Hz, 3H, H-4′), 1.04 (d, J = 7 Hz, 3H, H-1′), 1.14 (s, 3H, H-1‴), 1.41 (m, 2H, H-3′), 1.64 (m, 2H, H-1″), 2.39 (dd, J = 18.1, 8.2 Hz, 1H, H-4a), 2.56 (dd, J = 18.1, 5.2 Hz, 1H, H-4b), 3.00 (m, 1H, H-2′), 3.60 (s, 3H, OMe), 3.88 (t, J = 8.2, 5.2 Hz, 1H, H-5) 13CNMR (75 MHz, CDCl3): δ 7.9 (C-2″), 12.5 (C-4′), 17.7 (C-1‴), 18.6 (C-1′), 22.7 (C-1″), 27.1 (C-3′), 28.8 (C-4), 34.8 (C-2′), 50.9 (C-6), 59.9 (OMe), 73.1 (C-5), 146.3 (C-3), 147.3 (C-2), 198.0 (C-1).
APCI-HR-MS m/z 241.1786 [M+H]+ (calcd for C14H25O3, 241.1804);
Compound 6
Colorless oil.
[α]D26: +15.7 (c 0.07, CHCl3).
1HNMR (300 MHz, CDCl3): δ 0.84 (t, J = 7.3 Hz, 3H, H-2″), 0.85 (t, J = 7.3 Hz, 3H, H-4′), 1.05 (d, J = 7.0 Hz, 3H, H-1′), 1.14 (s, 3H, H-1‴), 1.41 (m, 2H, H-3′), 1.65 (m, 2H, H-1″), 2.35 (dd, J = 18.2, 7.6 Hz, 1H, H-4a), 2.45 (dd, J = 18.2, 5.1 Hz, 1H, H-4b), 3.01 (m, 1H, H-2′), 3.60 (s, 3H, OMe), 3.89 (t, J = 5.4 Hz, 1H, H-5). 13CNMR (75 MHz, CDCl3): δ 6.8 (C-2″), 11.3 (C-4′), 16.9 (C-1‴), 17.3 (C-1′), 21.7 (C-1′), 26.4 (C-3′), 27.9 (C-4), 33.6 (C-2′), 49.6 (C-6), 58.9 (OMe), 71.9 (C-5), 144.9 (C-3), 146.2 (C-2), 197.1 (C-1). APCI -HR-MS m/z 241.1757 [M+H]+ 241.1804).
Acknowledgments
This work was supported by US NIH grant for the International Cooperative Biodiversity Groups program (ICBG–Panama; 2 U01 TW006634-06). We thank Malkanthi Gunatilaka and Douglas Mahana for assistance with DNA sequencing, and the College of Agriculture and Life Sciences at the University of Arizona for logistical and financial support. We express our thanks to the personnel of Panama’s Autoridad Nacional del Ambiente for facilitating this research.
References
- 1.(a) Kursar TA, Caballero-George CG, Capson TL, Cubilla-Rios L, Gerwick WH, Ibanez A, Linington RG, McPhail KL, Ortega-Barria E, Romero LI, Solis PN, Coley PD. Securing economic benefits and promoting conservation through bioprospecting. Bioscience. 2006;56:1005–1012. [Google Scholar]; (b) Kursar TA, Caballero-George CC, Capson TL, Cubilla-Rios L, Gerwick WH, Heller MV, Ibanez A, Linington RG, McPhail KL, Ortega-Barria E, Romero LI, Coley PD. Linking bioprospecting with sustainable development and conservation: the Panama case. Biodiversity and Conservation. 2007;16:2789–2800. [Google Scholar]; (c) Jimenez-Romero C, Torres-Mendoza D, Ureña-Gonzales LD, Ortega-Barria E, McPhail KL, Gerwick WH, Cubilla-Rios L. Hydroxy-alkenylresorcinols fromStylogyne turbacensis. Journal of Natural Products. 2007;70:1249–1252. doi: 10.1021/np070081d. [DOI] [PubMed] [Google Scholar]; (d) Montenegro H, Gonzalez J, Ortega-Barria E, Cubilla-Rios L. Antiprotozoal activity of flavonoid glycosides isolated fromClimedia sericea and Mosquitoxylon jamaicense. Pharmaceutical Biology. 2007;45:376–380. [Google Scholar]; (e) Linington RG, Clark BR, Trimble EE, Almanza A, Ureña LD, Kyle DE, Gerwick WH. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. Journal of Natural Products. 2007;72:14–17. doi: 10.1021/np8003529. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Medina RA, Goeger DE, Hills P, Mooberry SL, Huang N, Romero LI, Ortega-Barria E, Gerwick WH, McPhail KL. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium. Leptolyngbya sp Journal of American Chemical Society. 2008;130:6324–6325. doi: 10.1021/ja801383f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Jimenez-Romero C, Ortega-Barria E, Arnold AE, Cubilla-Rios L. Activity against. Plasmodium falciparum of lactones isolated from the endophytic fungus Xylaria sp. Pharmaceutical Biology. 2008;46:700–703. [Google Scholar]; (h) Martinez-Luis S, Della-Tonga G, Coley PD, Kursar TA, Gerwick WH, Cubilla-Rios L. Antileishmanial constituents of the Panamanian endophytic fungusEdenia sp. Journal of Natural Products. 2008;71:2011–2014. doi: 10.1021/np800472q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Kuyama S, Tamura T. Cercosporin. A pigment of cercosporina kikuchii matsumoto et tomoyasu i Cultivation of fungus, isolation and purification of pigment. Journal of American Chemical Society. 1957;79:5725–5726. [Google Scholar]; (b) Assante G, Locci R, Camarda L, Merlini L, Nasini G. Screening of the genus. Cercospora for secondary metabolites. Phytochemistry. 1977;16:243–247. [Google Scholar]; (c) Kuyama S, Tamura T. Cercosporin. A pigment of cercosporina kikuchii matsumoto et tomoyasu ii Physical and chemical properties of cercosporin and its derivatives. Jouranl of American Chemical Society. 1957;79:5726–5729. [Google Scholar]; (d) Yamazaki S, Ogawa T. The chemistry and stereochemistry of cercosporin. Agricultural and Biological Chemistry. 1972;36:1707–1718. [Google Scholar]
- 3.Bringmann G, Gunther C, Ochse M, Schupp O, Tasler S. Axially chiral biaryls, a multi-facetted class of stereochemically, biosynthetically, and pharmacologically intriguing secondary metabolites. Progress in Chemistry of Organic Natural Products. 2001;82:1–249. doi: 10.1007/978-3-7091-6227-9_1. [DOI] [PubMed] [Google Scholar]
- 4.Daub ME. Cercosporin, a photosensitizing toxin from Cercospora species. Phytopathology. 1982;72:370–374. [Google Scholar]
- 5.Daub ME. Peroxidation of tobacco membrane lipids by the photosensitizing toxin, cercosporin. Plant Physiology. 1982;69:1361–1364. doi: 10.1104/pp.69.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodwin SB, Dunkle LD, Zismann VL. Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology. 2001;91:648–658. doi: 10.1094/PHYTO.2001.91.7.648. [DOI] [PubMed] [Google Scholar]
- 7.(a) Arnold AE, Henk DA, Eells RA, Lutzoni F, Vilgalys R. Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia. 2007;99:185–206. doi: 10.3852/mycologia.99.2.185. [DOI] [PubMed] [Google Scholar]; (b) Arnold AE, Miadlikowska J, Higgins KL, Sarvate SD, Gugger P, Way A, Hofstetter V, Kauff F, Lutzoni F. Hyperdiverse fungal endophytes and endolichenic fungi elucidate the evolution of major ecological modes in the Ascomycota. Systematic Biology. 2009;58:283–297. doi: 10.1093/sysbio/syp001. [DOI] [PubMed] [Google Scholar]; (c) Arnold AE, Gunatilaka MK, Varughese T, Moreno E, Coley PD, Kursar TA, Cubilla LC. A novel species of Mycosphaerella endophytic in foliage of Psychotria horizontalis (Rubiaceae) in Panama. 2011. (In preparation) To be submitted to Fungal Diversity. [Google Scholar]
- 8.Morgan BJ, Dey S, Johnson SW, Kozlowski MC. Total synthesis of cercosporin and new photodynamic perylenequinones: Inhibition of the protein kinase C regulatory domain. Journal of American Chemical Society. 2009;131:9413–9425. doi: 10.1021/ja902324j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correa JE, Rios CH, Castillo AR, Romero LI, Ortega-Barrira E, Coley PD, Kursar TA, Heller MH, Gerwick WH, Cubilla-Rios L. Minor alkaloids from Guatteria dumentorum with antileishmanial activity. Planta Medica. 2006;72:270–272. doi: 10.1055/s-2005-916179. [DOI] [PubMed] [Google Scholar]
- 10.Arnold AE, Lutzoni F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology. 2007;88:541–549. doi: 10.1890/05-1459. [DOI] [PubMed] [Google Scholar]
- 11.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. San Diego: Academic Press; 1990. pp. 222–315. [Google Scholar]
- 12.Hoffman M, Arnold AE. Geography and host identity interact to shape communities of endophytic fungi in cupressaceous trees. Mycological Research. 2008;112:331–344. doi: 10.1016/j.mycres.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Higgins KL, Arnold AE, Miadlikowska J, Sarvate SD, Lutzoni F. Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Molecular Phylogenetics and Evolution. 2007;42:543–555. doi: 10.1016/j.ympev.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 14.(a) Ewing B, Hillier L, Wendl M, Green P. Base–calling of automated sequencer traces using. phred I Accuracy assessment. Genome Research. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]; (b) Ewing B, Green P. Base-calling of automated sequencer traces using Phred. II. Error probabilities. Genome Research. 1998;8:186–194. [PubMed] [Google Scholar]
- 15.Calderon A, Romero LI, Ortega-Barria E, Brun R, Correa MA, Gupta MP. Evaluation of larvicidal and in vitro antiparasitic activities of plants in a biodiversity plot in the Altos de Campana National Park, Panama. Pharmaceutical Biology. 2006;44:1–16. [Google Scholar]
- 16.Buckner FS, Verlinde CLMJ, La Flamme AC, Van Voorhis WC. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrobial Agents and Chemotherapy. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez LA, Capitan Z, Romero LI, Ortega-Barría E, Gerwick WH, Cubilla-Rios L. Bio-Assay guided isolation of germacranes with anti-protozoan activity from Magnolia sororum. Natural Product Communications. 2007;2:1065–1198. [Google Scholar]
- 18.(a) Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]; (b) Corbett Y, Herrera L, Gonzalez J, Cubilla L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barria E. A Novel DNA-Based Microfluorimetric Method to Evaluate Antimalarial Drug Activity. American Journal of Tropical Medicine and Hygiene. 2004;70:119–124. [PubMed] [Google Scholar]
- 19.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. Journal of National Cancer Institute. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 20.Van de Loosdrecht AA, Beelen RHJ, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MM. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. Journal of Immunological Methods. 1994;174:311–320. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]


