Abstract
The goal of this study was to develop a 66Ga-based positron emission tomography (PET) tracer for non-invasive imaging of CD105 expression during tumor angiogenesis, a hallmark of cancer. 66Ga was produced using a cyclotron with natZn or isotopically enriched 66Zn targets. TRC105, a chimeric anti-CD105 monoclonal antibody, was conjugated to 2-S-(4-isothiocyanatobenzyl)-1, 4, 7-triazacyclononane-1, 4, 7-triacetic acid (p-SCN-Bn-NOTA) and labeled with 66Ga. No difference in CD105 binding affinity or specificity was observed between TRC105 and NOTATRC105 based on flow cytometry analysis. Reactivity of 66Ga for NOTA, corrected to the end of bombardment, was between 74 and 222 GBq/μmol for both target enrichments with < 2 ppb of cold gallium. 66Ga-labeling was achieved with > 80% radiochemical yield. Serial PET imaging revealed that the murine breast cancer 4T1 tumor uptake of 66Ga-NOTA-TRC105 was 5.9 ± 1.6, 8.5 ± 0.6, and 9.0 ± 0.6 %ID/g at 4, 20, and 36 h post-injection, respectively (n = 4). At the last time point, tumor uptake was higher than all organs which gave excellent tumor contrast with a tumor/muscle ratio of 10.1 ± 1.1. Biodistribution data as measured by gamma counting were consistent with the PET findings. Blocking experiment, control studies with 66Ga-NOTA-cetuximab, as well as ex vivo histology all confirmed the in vivo target specificity of 66Ga-NOTA-TRC105. Successful PET imaging with high specific activity 66Ga (> 700 GBq/μmol has been achieved) as the radiolabel opens many new possibilities for future PET research with antibodies or other targeting ligands.
Keywords: 66Ga, Positron emission tomography (PET), Tumor angiogenesis, CD105/Endoglin, TRC105, Breast cancer
INTRODUCTION
The radionuclide 66Ga (t1/2 = 9.3 h, 56.5% β+, 43.5% electron capture) is a useful surrogate for 67Ga (t1/2 = 78.3 h) and 68Ga (t1/2 = 68.3 min), which are used for single photon emission computed tomography (SPECT) and positron emission tomography (PET) imaging, respectively.1-3 Modern preclinical PET scanners capably cope with both fast positrons and prompt gamma emissions that are characteristic of many non-standard radionuclides, including 68Ga and 66Ga.4, 5 The relatively long half-life of 66Ga makes it a more practical radiolabel for peptides, proteins, and antibodies, whose slower in vivo kinetics are poorly matched by the much shorter half-life of 68Ga.6 Labeling chemistry with radiogallium has been well studied because of the popularity of 68Ga from 68Ge/68Ga generators, as well as its production in very high specific activities relative to other radiometals (e.g. 64Cu, 89Zr, etc.).
Past work with 66Ga has been limited, due to the relatively low specific activities achieved using previously reported methods, as well as the high energy gammas and positrons characteristic of its decay. Lewis et al. irradiated natural and enriched zinc targets to produce 66Ga which was purified by cation exchange chromatography and solvent extraction, with the latter separation method been used in most 66Ga-based studies to date.7 However, the final reactivity of 66Ga did not exceed 4.6 GBq/μmol of chelant, which was more than 100 fold below the theoretical limit. Subsequently, this method has been used to produce 66Ga-DOTA-D-Phe1-Tyr3-octreotide6 (DOTA denotes 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid) and 66Ga-deferoxamine-folate8 for PET imaging applications, as well as to label chitosan complexes with 66Ga for radiotherapy after intratumoral injection.9 Direct production and separation of 66Ga-citrate has been accomplished using anion exchange chromatography.10 However, this strategy affords few prospects for radiolabeling of targeting ligands.
Recently, we have produced high specific activity 66Ga from natZn(p,n) and 66Zn(p,n) with a cyclotron using proton irradiations between 7 and 16 MeV,11 which has resulted in specific activity of > 70 GBq/μmol for common bifunctional chelators, many fold higher that the reported literature values (< 4.6 GBq/μmol). In this study, we investigated the characteristics of 66Ga-based immunoPET of tumor angiogenesis, a hallmark of solid tumor progression.12 The protein target we have chosen for this study was CD105, also called “endoglin”, a 180 kDa disulphide-linked homodimeric transmembrane protein.13-16 Considered as the “gold standard” marker of proliferating endothelium, CD105 modulates TGF-β receptor signaling through serine/threonine kinases, mainly modifying the phosphorylation of Smad proteins.14
Clinically, high CD105 expression on tumor vascular endothelial cells correlates with poor prognosis in more than 10 solid tumor types.14, 15 To date, molecular imaging of CD105 expression has been understudied and most literature reports on CD105 imaging were based on labeling anti-CD105 antibodies.17-24 TRC105, a human/murine chimeric IgG1 monoclonal antibody (mAb) which binds to both human and murine CD105 (with a dissociation constant of ~ 30 picomolar for human CD105 and within the nanomolar range for murine CD105), has been evaluated in a multicenter Phase 1 first-in-human dose-escalation trial in the United States.25 Multiple Phase 2 clinical trials are underway in patients with various solid tumor types (e.g. breast, prostate, bladder, ovarian, and liver cancer). In this work, we conjugated TRC105 with NOTA (1, 4, 7-triazacyclononane-1, 4, 7-triacetic acid) for 66Ga-labeling and investigated 66Ga-NOTA-TRC105 for immunoPET of CD105 expression during tumor angiogenesis in a murine breast cancer model.
EXPERIMENTAL SECTION
Reagents
natZn foils (0.25 mm thick) and natZnCl2, both of 99.999% purity, were purchased from Sigma Aldrich (St. Louis, MO). Isotopically enriched 66Zn (98.58%) was acquired from Isoflex (San Francisco). TRC105 was provided by TRACON Pharmaceuticals, Inc. (San Diego, CA). Cetuximab (a human/murine chimeric IgG1 mAb that binds to human epidermal growth factor receptor [EGFR] but does not cross-react with murine EGFR26) was from Bristol-Myers Squibb Company (Princeton, NJ). AlexaFluor488- and Cy3-labeled secondary antibodies were purchased from Jackson Immunoresearch Laboratories, Inc. (West Grove, CA). 2-S-(4-isothiocyanatobenzyl)-1, 4, 7-triazacyclononane-1, 4, 7-triacetic acid (abbreviated as p-SCN-Bn-NOTA) and Chelex 100 resin (50-100 mesh) were purchased from Macrocyclics, Inc. (Dallas, TX) and Sigma-Aldrich, respectively. Cation exchange resin (AG50W-X4, 100-200 mesh) was acquired from Bio-Rad (Hercules, CA). Trace-metals grade HCl was purchased from VWR (Radnor, PA). Water and all buffers were of Millipore grade and pre-treated with Chelex 100 resin to ensure that the aqueous solution was heavy metal-free. PD-10 desalting columns were purchased from GE Healthcare (Piscataway, NJ). All other chemicals were purchased from Thermo Fisher Scientific (Fair Lawn, NJ).
Cell Lines and Animal Model
4T1 murine breast cancer, MCF-7 human breast cancer, and human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured as described previously and used for in vitro and in vivo experiments when they reached ~80% confluence.23 All animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. The 4T1 tumor model was established as previously described and mice were used for in vivo experiments when the diameter of tumors reached 5-8 mm (typically 1-2 weeks after inoculation).23
Production of 66Ga
Targets of natZn or 66Zn were electrodeposited from 0.05 N hydrochloric acid solution onto gold or silver target backings with dimensions matched to the cyclotron beam of a General Electric (Waukesha, WI) PETtrace cyclotron.11 Targets were irradiated with 20 - 30 μA of 13 MeV protons for between 1 and 3 h, dissolved in concentrated HCl, and purified by cation exchange chromatography with successive additions of 10 N and 7 N HCl to recover the Zn target material and elute trace contaminant metals. The product was collected in 4 N HCl, evaporated to dryness, and redissolved in 0.1 N HCl prior to buffering. If the target of natZn was used, 68Ga was allowed to decay overnight before target processing commenced. In this case, the only radioisotopic contaminant was 67Ga, present as < 5% of the radioactivity at 16 h after the end of bombardment (EoB). On the other hand, radioisotopic purity of 66Ga produced from the 66Zn target exceeded 99.9%.
NOTA Conjugation and 66Ga-Labeling
NOTA conjugation to mAbs (TRC105 and cetuximab) was carried out as previously described.27, 28 Briefly, TRC105 or cetuximab was mixed with p-SCN-Bn-NOTA at pH 9.0 with a molar ratio of 1:25 and the resulting NOTA-TRC105 or NOTA-cetuximab was purified with size exclusion chromatography. For radiolabeling, 74-111 MBq of 66Ga-acetate (pH 5.5) was prepared from 0.1 N HCl solution by addition of 0.25 M NH4OAc solution (pH 7.2), which was then added to a solution of NOTA-TRC105 or NOTA-cetuximab with 25 μg of NOTA-mAb conjugate per 37 MBq of 66Ga. The reaction mixture was incubated for 30 min at 37 °C with constant shaking. 66Ga-NOTA-TRC105 and 66Ga-NOTA-cetuximab were purified by size exclusion chromatography using normal saline buffered with 0.25 M NH4OAc (pH 7.2) as the mobile phase. The radioactive fractions containing 66Ga-NOTATRC105 or 66Ga-NOTA-cetuximab were collected and passed through a 0.2 μm syringe filter prior to injection into tumor-bearing mice.
Flow Cytometry
The immunoreactivity of TRC105 and NOTA-TRC105 to HUVECs (high CD105 expression20, 29) and MCF-7 cells (CD105-negative20) were evaluated by fluorescence-activated cell sorting (FACS) analysis, as described previously.20, 30 FACS studies were carried out using a BD FACSCalibur 4-color analysis cytometer (Becton-Dickinson, San Jose, CA), which is equipped with 488nm and 633nm lasers and FlowJo analysis software (Tree Star, Inc., Ashland, OR).
Imaging and Biodistribution Studies
PET and CT (with a voxel size of 210×210×210 μm3) scans at various time points post-injection (p.i.) were performed using an Inveon microPET/microCT rodent model scanner (Siemens Medical Solutions USA, Inc.) as described previously,23, 30 with a 2.8 nanosecond coincidence time window. The images were reconstructed using a maximum a posteriori (MAP) algorithm, with no attenuation or scatter correction. For each PET scan, three-dimensional regions-of-interest (ROIs) were drawn over the 4T1 tumor and major organs with vendor software (Inveon Research Workplace [IRW]) on decay-corrected whole-body images. Assuming a tissue density of 1 g/mL, the ROIs were converted to MBq/g using a conversion factor pre-determined using a 20 mL centrifuge tube filled with ~20 MBq of 66GaCl3 as a phantom, and divided by the total administered radioactivity to obtain an image ROI-derived percentage injected dose per gram of tissue (%ID/g).
Four additional 4T1 tumor-bearing mice were each injected with 2 mg of unlabeled TRC105 at 2 h before 66Ga-NOTA-TRC105 administration to evaluate the CD105 specificity of 66Ga-NOTA-TRC105 in vivo (i.e. blocking experiment). Biodistribution studies were carried out to validate quantitative tracer uptake values derived from ROI analysis of PET scans against radioactivity distribution measured ex vivo. At 36 h p.i., mice were euthanized and blood, 4T1 tumor, and major organs/tissues were collected and wet-weighed. The radioactivity in each tissue was measured using a gamma-counter (Perkin Elmer) and presented as %ID/g (mean ± SD). The 4T1 tumor, liver, and spleen (i.e. tissues with significant uptake of 66Ga-NOTA-TRC105) were also frozen for histological analysis.
Dose-Escalation Study
The effect of NOTA-TRC105 dose on the biodistribution of 66Ga-NOTA-TRC105 in 4T1 tumor-bearing mice was investigated. Four groups of mice (three mice per group) each received ~4 MBq of 66Ga-NOTA-TRC105, which contained escalating NOTATRC105 doses of 0.5, 5, 25, and 100 μg, respectively. At 36 hours p.i., mice were euthanized for biodistribution studies as described above.
Histology
Frozen tissue slices of 5 μm thickness were fixed with cold acetone for 10 min and dried in the air for 30 min. After rinsing with phosphate-buffered saline and blocking with 10% donkey serum for 30 min at room temperature, the slices were incubated with TRC105 (2 μg/mL) for 1 h at 4 °C and visualized using AlexaFluor488-labeled goat anti-human IgG. The tissue slices were also stained for endothelial marker CD31 as described previously.31, 32 All images were acquired with a Nikon Eclipse Ti microscope.
Statistical Analysis
Quantitative data were expressed as mean ± SD. Means were compared using Student’s t-test. P values < 0.05 were considered statistically significant.
RESULTS
Flow Cytometry Analysis
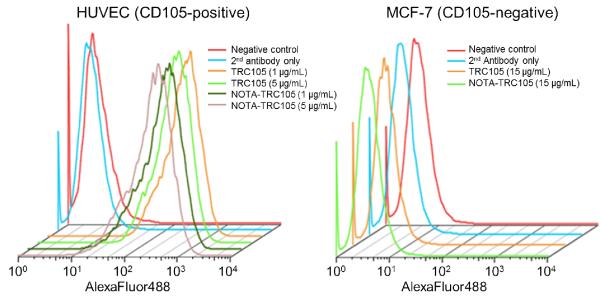
Based on FACS analysis of HUVECs which express high level of CD105, there was no observable difference between TRC105 and NOTA-TRC105 at 1 μg/mL or 5 μg/mL concentrations (Figure 1). The binding to HUVECs was antigen specific, as neither TRC105 nor NOTA-TRC105 bound to CD105-negative MCF-7 cells, even at a much higher concentration of 15 μg/mL (Figure 1). Taken together, FACS analysis indicated that NOTA conjugation did not alter the antigen binding affinity or specificity of TRC105.
Figure 1.
Flow cytometry analysis of TRC105 and NOTA-TRC105 in HUVECs (CD105-positive) and MCF-7 cells (CD105-negative) at several non-antigen-saturating concentrations.
66Ga Production and Radiolabeling
Specific activity of 66Ga for NOTA, measured by titration with various concentrations of NOTA and corrected to EoB, was between 74 and 222 GBq/μmol for both target enrichments. Inductively coupled plasma mass spectrometry (ICP-MS) detected < 2 ppb of cold gallium and < 20 ppb of iron in the final 66Ga solutions. 66Ga-labeling, including purification using PD-10 columns, took 70 ± 10 min (n = 7). The decay-corrected radiochemical yield was 80.2 ± 4.1 %, based on 25 μg of antibody conjugate (NOTA-TRC105 or NOTA-cetuximab) per 37 MBq of 66Ga, with radiochemical purity of > 95%. The ratio of 66Ga activity to antibody mass was ~1.2 GBq/mg of antibody for both 66Ga-NOTA-TRC105 and 66Ga-NOTA-cetuximab, assuming complete recovery of the NOTA-mAb conjugates after size exclusion chromatography.
Small Animal PET Imaging
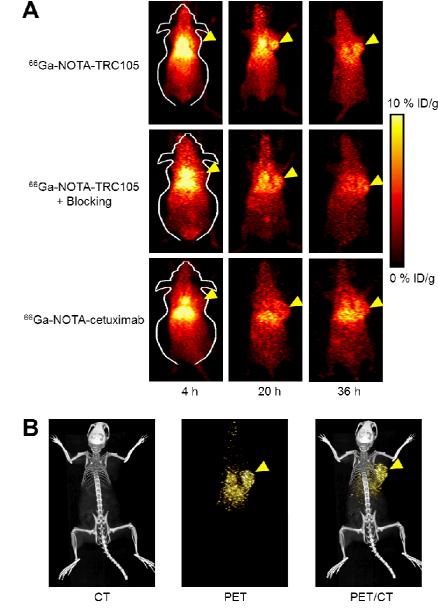
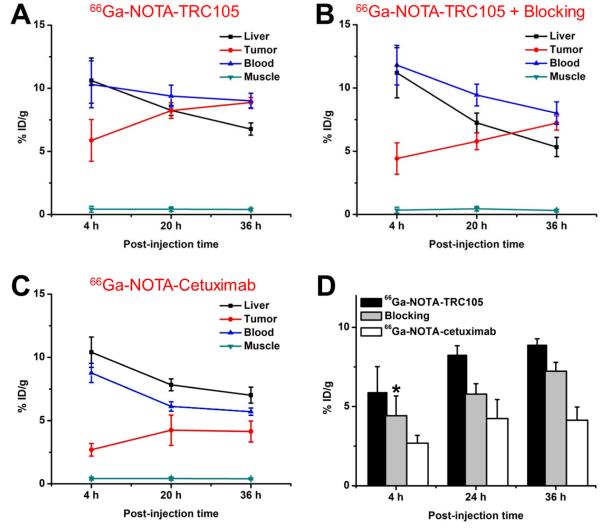
Based on the half-life of 66Ga (9.3 h), 4, 20, and 36 h p.i. were chosen as the time points for serial PET scans after intravenous tracer injection. The coronal slices that contained the 4T1 tumors are shown in Figure 2A and representative PET/CT fused images of a mouse at 20 h p.i. of 66Ga-NOTA-TRC105 are shown in Figure 2B. The quantitative data obtained from ROI analysis are shown in Figure 3. Because of the superb stability of the 66Ga-NOTA complex, liver uptake of 66Ga-NOTA-TRC105 in 4T1 tumor-bearing mice was significantly lower at all time points examined than that observed for 64Cu-DOTA-TRC105 in our previous study (> 25.0 %ID/g at 4 h p.i, which may reflect a certain degree of 64Cutranschelation hence elevated liver uptake of radioactivity).20 Meanwhile, blood pool activity was prominent at early time points (due to long circulation half-life of the antibody conjugate) and gradually declined thereafter. The liver uptake of 66Ga-NOTA-TRC105 was 10.6 ± 1.8, 8.2 ± 0.4, and 6.8 ± 0.5 %ID/g at 4, 20, and 36 h p.i. respectively, whereas the radioactivity in the blood was 10.3 ± 1.9, 9.4 ± 0.9, and 9.0 ± 0.6 %ID/g at 4, 20, and 36 h p.i. respectively (n = 4; Figure 3A). Importantly, tumor uptake of 66Ga-NOTA-TRC105 was clearly visible as early as 4 h p.i. and plateaued at around 20 h p.i. (5.9 ± 1.6, 8.5 ± 0.6, and 9.0 ± 0.6 %ID/g at 4, 20, and 36 h p.i. respectively; n = 4; Figure 3A&D).
Figure 2.
Serial PET imaging of 4T1 tumor-bearing mice. (A) Coronal PET images at 4, 20, and 36 h post-injection of 66Ga-NOTA-TRC105, 2 mg of TRC105 before 66Ga-NOTA-TRC105 (i.e. blocking), or 66Ga-NOTA-cetuximab. (B) Representative PET/CT images of 66Ga-NOTATRC105 in 4T1 tumor-bearing mice at 20 h post-injection. Tumors are indicated by arrowheads.
Figure 3.
Quantitative analysis of the PET data. (A) Time-activity curves of the liver, tumor, blood, and muscle upon intravenous injection of 66Ga-NOTA-TRC105 into 4T1 tumor-bearing mice (n = 4). (B) Time-activity curves of the liver, tumor, blood, and muscle upon intravenous injection of 66Ga-NOTA-TRC105, after a blocking dose of TRC105, into 4T1 tumor-bearing mice (n = 4). (C) Time-activity curves of the liver, tumor, blood, and muscle upon intravenous injection of 66Ga-NOTA-cetuximab into 4T1 tumor-bearing mice (n = 4). (D) Comparison of tracer uptake in the 4T1 tumor among the three groups. P values are < 0.05 or < 0.01 in all cases when compared with the 66Ga-NOTA-TRC105 group, except one case where P > 0.05 (denoted with “*”).
Administration of a blocking dose of TRC105 at 2 h before 66Ga-NOTA-TRC105 injection reduced the tumor uptake to 4.4 ± 1.2, 5.8 ± 0.6, and 7.2 ± 0.6 %ID/g at 4, 20, and 36 h p.i. respectively (n = 4; Figure 2A, 3B&D). Statistically significant differences were achieved at 20 and 36 h p.i. (P < 0.05) when compared with the tumor uptake in mice injected with 66Ga-NOTA-TRC105 alone, which indicated CD105 specificity of the tracer in vivo. Liver uptake of 66Ga-NOTA-TRC105 in the blocking group (11.2 ± 2.0, 7.2 ± 0.8, and 5.3 ± 0.7 %ID/g at 4, 20, and 36 h p.i. respectively; n = 4) was comparable to that of mice injected with 66Ga-NOTATRC105 alone. A similar trend was also observed for radioactivity in the blood (11.8 ± 1.6, 9.4 ± 0.9, and 8.0 ± 0.9 %ID/g at 4, 20, and 36 h p.i. respectively for the blocking group; n = 4; Figure3B). Overall, tracer uptake in all major organs was similar between the two groups yet the 4T1 tumor uptake was significantly higher in mice injected with 66Ga-NOTA-TRC105 alone than in the blocking group, confirming CD105 specificity of the tracer in vivo.
To further investigate CD105 specificity of 66Ga-NOTA-TRC105, 66Ga-NOTA-cetuximab was used as an isotype-matched control. Both TRC105 and cetuximab are human/murine chimeric IgG1 mAbs. Since cetuximab does not cross-react with murine tissues, it serves as a suitable control for evaluating tracer uptake in the tumor due to passive targeting alone (i.e. the enhanced permeability and retention [EPR] effect33). As can be seen in Figure 2A, 3C&D, 4T1 tumor uptake of 66Ga-NOTA-cetuximab was 2.7 ± 0.5, 4.2 ± 1.2, and 4.1 ± 0.8 %ID/g at 4, 20, and 36 h p.i. respectively (n = 4), significantly lower than that of 66Ga-NOTA-TRC105 at all time points examined (P < 0.05 at 4 h p.i. and P < 0.01 at 20 and 36 h p.i.) and confirmed CD105 specificity of 66Ga-NOTA-TRC105 in vivo.
Biodistribution Studies
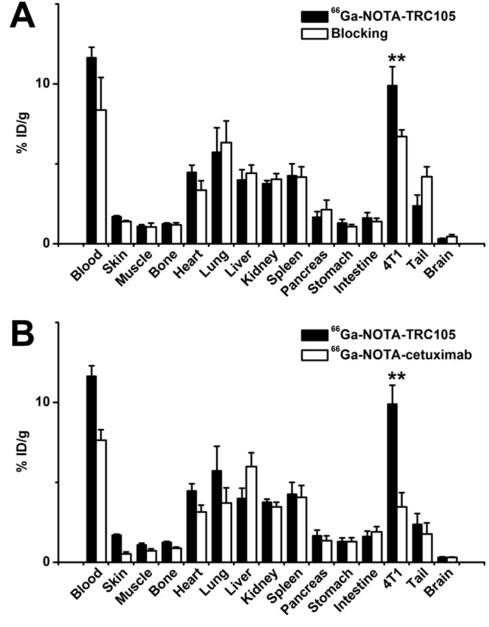
All mice were euthanized after the last PET scans at 36 h p.i. for biodistribution studies to validate the in vivo PET data. Besides the 4T1 tumor, blood also had significant radioactivity at 36 h p.i., which was expected for a radiolabeled antibody (Figure 4A). When compared with all major organs in mice, the uptake of 66Ga-NOTA-TRC105 in the 4T1 tumor was higher, which provided good tumor contrast with a tumor/muscle ratio of 10.1 ± 1.1 at 36 h p.i. (n = 4). Pre-injection of a blocking dose of TRC105 led to a significant decrease in 4T1 tumor uptake (P < 0.01; n = 4; Figure 4A) with similar uptake in the blood and liver, corroborating PET results.
Figure 4.
Biodistribution studies in 4T1 tumor-bearing mice. (A) Biodistribution of 66Ga-NOTATRC105 at 36 h post-injection, with or without a pre-injected blocking dose of TRC105 (n = 4). (B) Biodistribution of 66Ga-NOTA-TRC105 and 66Ga-NOTA-cetuximab at 36 h post-injection (n = 4). **: P < 0.01.
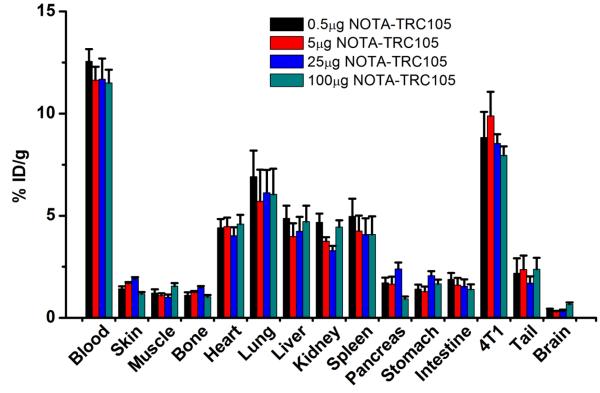
A comparison of the biodistribution data between the two tracers at 36 h p.i. revealed that the uptake of 66Ga-NOTA-cetuximab was similar to 66Ga-NOTA-TRC105 in most organs except the 4T1 tumor and blood (Figure 4B), which again indicated specific tumor targeting of 66Ga-NOTA-TRC105 (P < 0.01 when comparing the 4T1 tumor uptake between the two tracers). Dose-escalation study revealed that there was no significant difference in the 4T1 tumor uptake of 66Ga-NOTA-TRC105, at doses ranging from 0.5 μg to 100 μg of NOTA-TRC105 per mouse (Figure 5). At 36 h p.i., tracer uptake in the 4T1 tumor was 8.8 ± 1.2, 9.9 ± 1.2, 8.5 ± 0.5, and 8.0 ± 0.5 %ID/g for 0.5, 5, 25, and 100 μg of NOTA-TRC105 per mouse, respectively. Overall, quantitative data from ex vivo biodistribution studies and in vivo PET scans were in good agreement, confirming that ROI analysis of non-invasive PET scans accurately reflected tracer distribution in vivo.
Figure 5.
The effect of NOTA-TRC105 dose on the biodistribution of 66Ga-NOTA-TRC105 in 4T1 tumor-bearing mice (n = 3 per group).
Histology
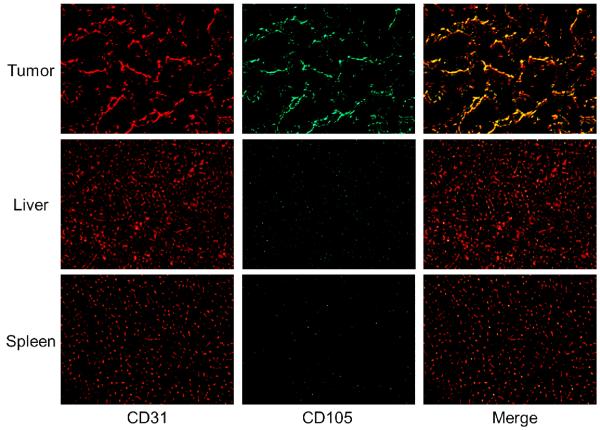
Immunofluorescence staining revealed that CD105 expression in the 4T1 tumor was primarily located on the tumor vasculature, as evidenced by excellent co-localization of CD105 and CD31 staining (Figure 6). Since tumor vasculature is generally more actively proliferating in the peripheral region than in the center of the tumor, uptake of 66Ga-NOTA-TRC105 in the 4T1 tumor was rather heterogeneous (i.e. higher uptake in the peripheral region and lower uptake in the tumor center).
Figure 6.
Immunofluorescence CD105/CD31 staining of the 4T1 tumor, liver, and spleen tissue sections. CD31 staining was shown in red and CD105 staining was displayed in green. All images were acquired under the same condition and displayed at the same scale. Magnification: 200×.
CD105 staining of mouse liver and spleen both gave very low signal, indicating that these tissues do not express CD105. Therefore, uptake of 66Ga-NOTA-TRC105 in the liver/spleen was largely unrelated to CD105 binding and more likely attributed to non-specific capture by the reticuloendothelial system and hepatic clearance of the antibody-based tracer. Taken together, histological findings corroborated the in vivo data of 66Ga-NOTA-TRC105, warranting further investigation and application of this tracer.
DISCUSSION
PET has been widely used in clinical oncology for cancer staging and monitoring the therapeutic response.34-37 The goal of this study was to evaluate the in vivo performance of 66Ga-NOTATRC105 for PET imaging of tumor angiogenesis. 66Ga is suited for this purpose since its decay characteristics matches the in vivo kinetics of the labeled mAb. In addition, the stability of NOTA as a chelator for radiogallium has been well documented in the literature.1 As confirmed by the in vivo study, the stability of 66Ga-NOTA-TRC105 (indicated by blood radioactivity and liver capture) was clearly higher than that previously reported for 64Cu-DOTA-TRC105.20
Although 66Ga has higher positron energy (Emax of 4.15 MeV) than other commonly used PET isotopes (e.g. Emax of 1.90 MeV for 68Ga), high energy positrons (which can lead to a longer positron range) and co-emitted gammas (which can affect image reconstruction and quantitation) have only a small effect on the PET image quality and quantitation accuracy (Figure 2, 4), further enabling researchers to consider future use of the versatile radiometal for PET. It was reported that for glass capillaries using filtered back projection, the full widths at half maximum for 68Ga PET using the Inveon microPET scanner was 2.46 mm (which had a mean positron range of 3.48 mm in water), while the full widths at half maximum for 18F PET was 1.81 mm (which had a mean positron range of 0.62 mm in water).38 To ensure that the quantitative results obtained from ROI analysis of the PET scans in our study were accurate, a solution of 66Ga with known amount of radioactivity was used as the phantom to correlate PET measurement to the true radioactivity concentration.
66Zn is a more economical cyclotron target than the feedstocks used to produce other radiometals commonly used for PET applications (e.g. 64Ni for 64Cu), and we have produced 66Ga with reactivities for DOTA and NOTA approaching 740 GBq/μmol (i.e. 20 Ci/μmol) using 66Zn targets. 68Ga has been used to label a wide variety of compounds, which sets the stage for future investigation of these compounds with the longer-lived 66Ga. For example, many tracers (some are currently in clinical investigation) can only be imaged with PET within a few hours after injection, due to the short 68.3 min half-life of 68Ga. The use of 66Ga can allow for PET imaging at later time points to evaluate the long term fate of these tracers, which may provide more biological insights. Besides the significantly higher specific activity of 66Ga than many other radiometals such as 89Zr, the high energy positron emission (although not ideal for PET imaging) also makes 66Ga a desirable isotope for Cerenkov luminescence imaging, a research topic that is under active investigation.39, 40
Compared with other PET tracers (e.g. peptide-based),41-43 labeled antibodies are desirable agents for imaging of tumor angiogenesis due to their high affinity for the targets and long circulation half-lives, as the target mass is usually low (only a few percentage of the total tumor mass). One major challenge in radiolabeling of antibodies is the minimization of interference with their antigen binding affinity and specificity. The complementarity-determining region (CDR) of TRC105 each contains only one lysine residue,16 among a total of ~1400 amino acid residues and ~70 lysines in the whole antibody. Therefore, the possibility of NOTA conjugation at the lysine residue within the CDR is extremely low. Indeed, FACS analysis confirmed that NOTA-TRC105 maintained high avidity to its target at several non-antigen-saturating conditions (Figure 1) and did not bind to CD105-negative cells. Based on the specific activity of 66Ga, it was calculated that there were 0.8 ~ 2.4 66Ga per TRC105.
The imaging target of our study, CD105, is primarily present on the tumor neovasculature. Even though the 4T1 tumor cells are CD105-negative per se (the fluorescence signal for CD105 staining of the tumor is predominantly on the tumor vasculature but not on the 4T1 cells; Figure 6), they grow rapidly when inoculated into mice and contain a highly angiogenic tumor vasculature with prominent CD105 expression, which allowed for noninvasive PET imaging. The lack of CD105 expression on the tumor cells is one reason why uptake of 66Ga-NOTA-TRC105 in the 4T1 tumor is not as high as that observed for certain other antibody-based tracers,32, 44 which target tumor cells rather than the tumor vasculature. In solid tumors, typically there are significantly fewer tumor vascular endothelial cells than tumor cells.
Partly due to the excellent stability of the 66Ga-NOTA complex, blood radioactivity of 66Ga-NOTA-TRC105 was prominent even at late time points (~10 %ID/g at 20 and 36 h p.i.), which could lead to appreciable tumor uptake attributed to the EPR effect.33 Therefore, both the 66Ga-NOTA-TRC105 and the blocking group exhibited an increasing trend of tumor uptake over time. In future studies, radiolabeled antibody fragments of TRC105 (e.g. F(ab’)2) may exhibit less EPR effect-based tumor uptake and give better tumor/blood ratio. 66Ga-NOTA-cetuximab had significantly lower blood radioactivity level at late time points (~5%ID/g), which resulted in lower tumor uptake based on the EPR effect alone, as well as the absence of an increasing trend in the tumor uptake level. The blood radioactivity difference of isotype-matched IgG1 mAbs (e.g. TRC105 and cetuximab) may be attributed to many factors, including antigen binding or lack thereof, different interactions with the neonatal Fc receptor (FcRn) which binds to the CH2-CH3 hinge regions in the constant region (Fc) of IgG antibodies (notably the amino acid sequences in the Fc region may be different even for isotype-matched antibodies),45, 46 variable glycosylation patterns in the Fc regions, etc.
CONCLUSION
We report the use of 66Ga as a radiolabel for immunoPET imaging of CD105, a protein overexpressed on actively proliferating tumor endothelial cells. CD105-specific tumor targeting was achieved with 66Ga-NOTA-TRC105, which also exhibited appreciable tumor uptake attributed to the EPR effect. Successful PET imaging with 66Ga as the radiolabel, which can be produced with high reactivities (up to 740 GBq/μmol for NOTA), opens many possibilities for more detailed clinical/preclinical investigations of a wide variety of PET agents that were previously limited by the short half-life of 68Ga. In addition, the suitable half-life also makes 66Ga a desirable isotope for immunoPET, which has been a vibrant research field over the last several decades.
ACKNOWLEDGEMENTS
This work is supported, in part, by the University of Wisconsin Carbone Cancer Center, the Department of Defense (W81XWH-11-1-0644), the National Center for Advancing Translational Sciences (NCATS) grant 9U54TR000021, the Elsa U. Pardee Foundation, and the NIH through the UW Radiological Sciences Training Program 5 T32 CA009206-32.
REFERENCES
- (1).Velikyan I. Positron emitting 68Ga-based imaging agents: chemistry and diversity. Med. Chem. 2011;7:345–79. doi: 10.2174/157340611796799195. [DOI] [PubMed] [Google Scholar]
- (2).Even-Sapir E, Israel O. Gallium-67 scintigraphy: a cornerstone in functional imaging of lymphoma. Eur. J. Nucl. Med. Mol. Imaging. 2003;30(Suppl 1):S65–81. doi: 10.1007/s00259-003-1164-7. [DOI] [PubMed] [Google Scholar]
- (3).Severin GW, Knutson LD, Voytas PA, George EA. Half-life of 66Ga. Phys. Rev. C. 2010;82:067301. [Google Scholar]
- (4).Laforest R, Rowland DJ, Welch MJ. MicroPET imaging with nonconventional isotopes. IEEE Trans. Nucl. Sci. 2002;49:2119–2126. [Google Scholar]
- (5).Graham MC, Pentlow KS, MAwlawi O, Finn RD, Daghighian F, Larson SM. An investigationof the physical characteristics of 66Ga an an isotope for PET imaging and quantification. Med. Phys. 1997;24:317–327. doi: 10.1118/1.597924. [DOI] [PubMed] [Google Scholar]
- (6).Ugur O, Kothari PJ, Finn RD, Zanzonico P, Ruan S, Guenther I, Maecke HR, Larson SM. Ga-66 labeled somatostatin analogue DOTA-DPhe1-Tyr3-octreotide as a potential agent for positron emission tomography imaging and receptor mediated internal radiotherapy of somatostatin receptor positive tumors. Nucl. Med. Biol. 2002;29:147–57. doi: 10.1016/s0969-8051(01)00290-6. [DOI] [PubMed] [Google Scholar]
- (7).Lewis MR, Reichert DE, Laforest R, Margenau WH, Shefer RE, Klinkowstein RE, Hughey BJ, Welch MJ. Production and purification of gallium-66 for preparation of tumor-targeting radiopharmaceuticals. Nucl. Med. Biol. 2002;29:701–6. doi: 10.1016/s0969-8051(02)00330-x. [DOI] [PubMed] [Google Scholar]
- (8).Mathias CJ, Lewis MR, Reichert DE, Laforest R, Sharp TL, Lewis JS, Yang Z-F, Waters DJ, Snyder PW, Low PS, Welch MJ, Green MA. Preparation of 66Ga- and 68Ga-labeled Ga(III)-deferoxamine-folate as potential folate-receptor-targeted PET radiopharmaceuticals. Nucl. Med. Biol. 2003;30:725–731. doi: 10.1016/s0969-8051(03)00080-5. [DOI] [PubMed] [Google Scholar]
- (9).Akhlaghi M, Pourjavadi A. Preparation and primary evaluation of 66Ga-DTPA-chitosan in fibrosarcoma bearing mice. Nuckleonika. 2011;56:41–47. [Google Scholar]
- (10).El-Azony KM, Ferieg K, Saleh ZA. Direct separation of 67Ga citrate from zinc and copper target materials by anion exchange. Appl. Radiat. Isot. 2003;59:329–331. doi: 10.1016/s0969-8043(03)00203-3. [DOI] [PubMed] [Google Scholar]
- (11).Engle JW, Lopez-Rodriguez V, Gaspar-Carcamo RE, Valdovinos HF, Valle-Gonzalez M, Trejo-Ballado F, Severin GW, Barnhart TE, Nickles RJ, Avila-Rodriguez MA. Very high specific activity 66/68Ga from zinc targets for PET. Appl. Radiat. Isot. 2012 doi: 10.1016/j.apradiso.2012.03.030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- (13).Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003;22:6557–63. doi: 10.1038/sj.onc.1206813. [DOI] [PubMed] [Google Scholar]
- (14).Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin. Cancer Res. 2008;14:1931–7. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- (15).Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc. Res. 2010;86:12–9. doi: 10.1093/cvr/cvp332. [DOI] [PubMed] [Google Scholar]
- (16).Seon BK, Haba A, Matsuno F, Takahashi N, Tsujie M, She X, Harada N, Uneda S, Tsujie T, Toi H, Tsai H, Haruta Y. Endoglin-targeted cancer therapy. Curr. Drug Deliv. 2011;8:135–43. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bredow S, Lewin M, Hofmann B, Marecos E, Weissleder R. Imaging of tumour neovasculature by targeting the TGF-beta binding receptor endoglin. Eur. J. Cancer. 2000;36:675–81. doi: 10.1016/s0959-8049(99)00335-4. [DOI] [PubMed] [Google Scholar]
- (18).Fonsatti E, Jekunen AP, Kairemo KJ, Coral S, Snellman M, Nicotra MR, Natali PG, Altomonte M, Maio M. Endoglin is a suitable target for efficient imaging of solid tumors: in vivo evidence in a canine mammary carcinoma model. Clin. Cancer Res. 2000;6:2037–43. [PubMed] [Google Scholar]
- (19).Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin. Cancer Res. 2007;13:323–30. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- (20).Hong H, Yang Y, Zhang Y, Engle JW, Barnhart TE, Nickles RJ, Leigh BR, Cai W. Positron emission tomography imaging of CD105 expression during tumor angiogenesis. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:1335–43. doi: 10.1007/s00259-011-1765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Zhang Y, Hong H, Cai W. PET tracers based on Zirconium-89. Curr. Radiopharm. 2011;4:131–139. doi: 10.2174/1874471011104020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yang Y, Zhang Y, Hong H, Liu G, Leigh BR, Cai W. In vivo near-infrared fluorescence imaging of CD105 expression. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:2066–76. doi: 10.1007/s00259-011-1886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hong H, Severin GW, Yang Y, Engle JW, Zhang Y, Barnhart TE, Liu G, Leigh BR, Nickles RJ, Cai W. Positron emission tomography imaging of CD105 expression with 89Zr-Df-TRC105. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:138–148. doi: 10.1007/s00259-011-1930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Deshpande N, Ren Y, Foygel K, Rosenberg J, Willmann JK. Tumor Angiogenic Marker Expression Levels during Tumor Growth: Longitudinal Assessment with Molecularly Targeted Microbubbles and US Imaging. Radiology. 2011;258:804–11. doi: 10.1148/radiol.10101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Mendelson DS, Gordon MS, Rosen LS, Hurwitz H, Wong MK, Adams BJ, Alvarez D, Seon BK, Theuer CP, Leigh BR. Phase I study of TRC105 (anti-CD105 [endoglin] antibody) therapy in patients with advanced refractory cancer. J. Clin. Oncol. 2010;28:15s. [Google Scholar]
- (26).Goldberg RM. Cetuximab. Nat. Rev. Drug Discov. 2005;(Suppl):S10–1. doi: 10.1038/nrd1728. [DOI] [PubMed] [Google Scholar]
- (27).Zhang Y, Hong H, Engle JW, Bean J, Yang Y, Leigh BR, Barnhart TE, Cai W. Positron Emission Tomography Imaging of CD105 Expression with a 64Cu-Labeled Monoclonal Antibody: NOTA Is Superior to DOTA. PLoS One. 2011;6:e28005. doi: 10.1371/journal.pone.0028005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zhang Y, Hong H, Engle JW, Yang Y, Barnhart TE, Cai W. Positron emission tomography and near-infrared fluorescence imaging of vascular endothelial growth factor with dual-labeled bevacizumab. Am. J. Nucl. Med. Mol. Imaging. 2012;2:1–13. [PMC free article] [PubMed] [Google Scholar]
- (29).Takahashi N, Haba A, Matsuno F, Seon BK. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res. 2001;61:7846–54. [PubMed] [Google Scholar]
- (30).Zhang Y, Hong H, Engle JW, Yang Y, Theuer CP, Barnhart TE, Cai W. Positron Emission Tomography and Optical Imaging of Tumor CD105 Expression with a Dual-Labeled Monoclonal Antibody. Mol. Pharm. 2012;9:645–53. doi: 10.1021/mp200592m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, Chen X. PET of vascular endothelial growth factor receptor expression. J. Nucl. Med. 2006;47:2048–2056. [PubMed] [Google Scholar]
- (32).Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. In vitro and in vivo characterization of 64Cu-labeled AbegrinTM, a humanized monoclonal antibody against integrin αvβ3. Cancer Res. 2006;66:9673–81. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]
- (33).Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics. A review. J. Control. Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- (34).Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J. Nucl. Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- (35).Eary JF, Hawkins DS, Rodler ET, Conrad EUI. 18F-FDG PET in sarcoma treatment response imaging. Am. J. Nucl. Med. Mol. Imaging. 2011;1:47–53. [PMC free article] [PubMed] [Google Scholar]
- (36).Vach W, Høilund-Carlsen PF, Fischer BM, Gerke O, Weber W. How to study optimal timing of PET/CT for monitoring of cancer treatment. Am. J. Nucl. Med. Mol. Imaging. 2011;1:54–62. [PMC free article] [PubMed] [Google Scholar]
- (37).Grassi I, Nanni C, Allegri V, Morigi JJ, Montini GC, Castellucci P, Fanti S. The clinical use of PET with 11C-acetate. Am. J. Nucl. Med. Mol. Imaging. 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- (38).Disselhorst JA, Brom M, Laverman P, Slump CH, Boerman OC, Oyen WJ, Gotthardt M, Visser EP. Image-quality assessment for several positron emitters using the NEMA NU 4-2008 standards in the Siemens Inveon small-animal PET scanner. J. Nucl. Med. 2010;51:610–7. doi: 10.2967/jnumed.109.068858. [DOI] [PubMed] [Google Scholar]
- (39).Ruggiero A, Holland JP, Lewis JS, Grimm J. Cerenkov luminescence imaging of medical isotopes. J. Nucl. Med. 2010;51:1123–30. doi: 10.2967/jnumed.110.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Thorek DLJ, Robertson R, Bacchus WA, Hahn J, Rothberg J, Beattie BJ, Grimm J. Cerenkov imaging - a new modality for molecular imaging. Am. J. Nucl. Med. Mol. Imaging. 2012;2:163–173. [PMC free article] [PubMed] [Google Scholar]
- (41).Alauddin MM. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging. 2012;2:55–76. [PMC free article] [PubMed] [Google Scholar]
- (42).Hao G, Hajibeigi A, De León-Rodríguez LM, Öz OK, Sun X. Peptoid-based PET imaging of vascular endothelial growth factor receptor (VEGFR) expression. Am. J. Nucl. Med. Mol. Imaging. 2011;1:65–75. [PMC free article] [PubMed] [Google Scholar]
- (43).Wang RE, Niu Y, Wu H, Amin MN, Cai J. Development of NGR peptide-based agents for tumor imaging. Am. J. Nucl. Med. Mol. Imaging. 2011;1:36–46. [PMC free article] [PubMed] [Google Scholar]
- (44).Cai W, Ebrahimnejad A, Chen K, Cao Q, Li ZB, Tice DA, Chen X. Quantitative radioimmunoPET imaging of EphA2 in tumor-bearing mice. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:2024–36. doi: 10.1007/s00259-007-0503-5. [DOI] [PubMed] [Google Scholar]
- (45).Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- (46).Jefferis R, Lefranc MP. Human immunoglobulin allotypes: possible implications for immunogenicity. MAbs. 2009;1:332–8. doi: 10.4161/mabs.1.4.9122. [DOI] [PMC free article] [PubMed] [Google Scholar]