Abstract
The immunostimulatory properties conferred by vaccine adjuvants require Caspase-1 for processing of IL-1β and IL-18. Caspase-1 is activated in response to a breach of the cytosolic compartment by microbes and the process is initiated by intracellular pattern recognition receptors within inflammasomes. Listeria monocytogenes is detected in the cytosol by the NLRC4, NLRP3 and AIM2 inflammasomes. NLRC4 is activated by flagellin, and L. monocytogenes evades this detector by repressing flagellin expression. We generated an L. monocytogenes strain that was forced to express flagellin in the host cell cytosol. This strain hyperactivated Caspase-1 and was preferentially cleared via NLRC4 detection in an IL-1β/IL-18 independent manner. We also created a strain of L. monocytogenes with forced expression of another NLRC4 agonist, PrgJ from the Type III secretion system of S. typhimurium. Forced expression of flagellin or PrgJ resulted in attenuation, yet both strains conferred protective immunity in mice against lethal challenge with L. monocytogenes. This work is the first demonstration of specific targeting of the Caspase-1 activation pathway to generate a safe and potent L. monocytogenes based vaccine. Moreover, the attenuated strains with embedded flagellin or PrgJ adjuvants, represent attractive vectors for vaccines aimed at eliciting T cell responses.
Introduction
Listeria monocytogenes is a ubiquitous soil dwelling saprophyte which is capable of infecting mammals when ingested through contaminated food sources. In healthy individuals, infections cause moderate gastrointestinal distress, while in individuals with weakened immune systems, infections can cause meningitis or septicemia and may be life threatening. L. monocytogenes has been extensively characterized as a pathogen because it induces a strong innate and adaptive immune response.
Although L. monocytogenes is capable of infecting many different cells, macrophages and hepatocytes are the predominant target cells and bacteria concentrate in the liver and spleen of infected mice [1–3]. L. monocytogenes expresses two receptors on its surface that, upon binding to mammalian receptors, induce bacterial uptake into intracellular vacuoles. InlA binds to E-cadherin expressed in the tight junctions of gut epithelial cells. InlB binds to Met, a receptor tyrosine kinase, on hepatocytes. Once L. monocytogenes is internalized, vacuole acidification activates Listeriolysin O, a pore-forming toxin, resulting in lysis of the vacuole and release of L. monocytogenes into the cytosol. The bacteria then express ActA to facilitate host cell actin polymerization, which permits bacterial movement within the cell and also provides the force necessary for bacterial entry into neighboring cells. This invasion strategy permits L. monocytogenes to replicate within the nutrient rich mammalian cell cytosol and propagate the infection without passing through the extracellular space where it would be exposed to complement or the humoral immune system.
Innate immune responses to L. monocytogenes occur through detection by multiple receptors, including toll like receptors (TLRs) on the cell surface and the Nod-like receptors (NLRs) in the cytosol [1, 4]. Peptidoglycan motifs are detected by TLR2, while TLR5 detects bacterial flagellin [5, 6]. Once L. monocytogenes invades the macrophage, components of the bacteria cell wall are detected by NOD1 and NOD2, which detect -D-glutamyl-meso-diaminopimelic acid and muramyl dipeptide respectively [7, 8]. Detection by the TLRs or the Nod proteins induces cytokine expression, including TNF, IL-6, IL-12, proIL-1β and proIL-18. Additionally, L. monocytogenes in the cytosol is also detected by the Nod-like receptors NLRC4 and NLRP3, as well as the Hin200-like receptor AIM2 [9–13]. NLRC4 responds to flagellin and type III secretion rod protein in the cytosol [14–17], NLRP3 responds to disruption of the lysosomal compartment [18], and AIM2 detects cytosolic DNA from lysed bacteria [10]. Upon activation, these detectors assemble to form an inflammasome, which recruits the proCaspase-1 and promotes its cleavage to the active form [19]. In turn, Caspase-1 cleaves proIL-1β and proIL-18 into their secreted forms and also induces pyroptosis, a proinflammatory form of cell death. IL-1β is a pleotropic cytokine that regulates fever, inflammation, PGE2 production, leukocyte chemotaxis, and has many other inflammatory activities [20]. IL-18 was first identified as an IFN-γ inducing factor, and is now known to have a broad array of immune stimulatory effects [21]. Thus, Caspase-1 is implicated in the generation of a strong adaptive immune response.
L. monocytogenes infection induces an adaptive immune response that is largely mediated by T lymphocytes [1, 4, 22]. Bacterially derived proteins are presented on both MHCI and MHCII, leading to activation of CD4 and CD8 T cell responses. For this reason, there has been a great deal of research into using L. monocytogenes as a vaccine vector [23–25]. Many of the most prevalent diseases, including malaria, tuberculosis, HIV, HCV, and cancer, have failed to respond to traditional vaccine strategies which induce a humoral immune response. Vaccination with L. monocytogenes expressing a heterologous antigen confers durable immunity against the antigen, and vaccination with L. monocytogenes expressing a tumor derived self-antigen can induce regression of metastases [25]. Furthermore, since L. monocytogenes does not induce a significant antibody response, it can be used for repeated boosting against a single antigen or to immunize against multiple different antigens [22]. Several attenuated strains have been developed to serve as vaccine vectors, including the ΔactA mutant ([26]. L. monocytogenes based vaccines have been tested in multiple clinical trials for ovarian cancer, mesothelioma, and hepatocellular carcinoma, as well as HCV (www.clinicaltrials.gov).
Our prior work suggested that Caspase-1 activation by L. monocytogenes was submaximal. L. monocytogenes lacking the flgK gene, which is required for flagellin assembly into a polymerized flagella, hypersecret flagellin monomers into the extracellular space [9] . This results in increased Caspase-1 activation and preferential clearance in vivo [9]. However, the utility of ΔflgK mutants as vaccine strains is limited because flagellin expression in L. monocytogenes is inhibited by the transcriptional repressor MogR at 37°C [27]. We hypothesized that sustained flagellin expression in the mammalian cytosol would further increase Caspase-1 activation, and that this could result in a superior vaccine vector. In this work, we describe two Caspase-1 hyperactivating L. monocytogenes strains and characterize their effect upon the innate immune system. We demonstrate that they are preferentially cleared from WT mice, and that this clearance is dependent upon NLRC4 detection but not IL-1β/IL-18. Finally, we demonstrate that these strains are attenuated yet confer protective immunity against WT L. monocytogenes.
Materials and Methods
Bacterial Strains
L. monocytogenes with the OVA construct (LM-OVA) was developed by Dr. Hao Shen [28] and is on the 10403s background. LM ΔactA OVA and LM ΔLLO strain were a gift from Dr. Dan Portnoy (University of California, Berkeley, CA). The LMΔactA OVA strain was generated by Dr. Chris Wilson [29]. The Forced FlaA and Forced PrgJ strains were constructed with the pPL2 integration vector [30] and integrated in the bacterial chromosome. Listeria were cultured in Brain Heart Infusion broth (BHI) with streptomycin or streptomycin plus chloramphenicol.
Mice
C57BL/6 mice were obtained from The Jackson Laboratory. Caspase-1−/− and NLRC4−/− mice were a generous gift from Dr. Vishva Dixit (Genentech, South San Francisco, CA) [31, 32]. TLR5−/− mice were a gift from Dr. Shizuo Akira (Osaka University, Japan) and crossed to obtain NLRC4/TLR5DKO mice. IL-1β/IL-18DKO mice were a gift from Mark Wewers (Ohio State University) [33]. All knockout mice were either derived from C57BL/6 embryonic stem cells or backcrossed a minimum of eight times onto the C57BL/6 background. All mice were housed under specific pathogen free conditions and with approval of the Institutional Animal Car and Use Committee at the Institute for Systems Biology.
Mammalian cell culture
Bone marrow derived macrophages (BMM) were cultured in L cell media consisting of 50% DMEM, 30% L929 cell supernatant, 20% fetal bovine serum, 1% L-glutamine 1% penicillin/streptomycin for 6 days before infection. One day before infection, cells were seeded at appropriated density in 80% DMEM, 20% FBS, 10% L929 cell supernatant without antibiotics. Dendritic cells (DC) were cultured in 80% RPMI, 20% fetal bovine serum, 1% L-glutamine, 1% penicillin/streptomycin, and 10ng/ml rGM-CSF.
In vitro Infections
Cells were plated in 96 well culture plates at 5*104 cells/well. The following day, cells were stimulated with 50ng/ml ultrapure LPS (List Biological Labs) for 3 hours at 37°C to induce proIL-1β expression when Caspase-1 activation was examined. Cells were then infected with L. monocytogenes at a multiplicity of infection of 10 followed by centrifugation at 1000rpm (250×g) for 5 minutes to promote contact between the bacteria and the mammalian cells. One hour later, 15ug/ml gentamicin was added to kill all extracellular bacteria. Supernatants were harvest after 4 hours total infection, and cytokines were assessed by ELISA. For the TNF ELISA, cells were not stimulated with LPS. Student’s T Test was used to assess significance.
Western Blot
WT BMM were seeded at 1*10^6 cells/well into a 6 well culture dish, and overnight cultures of Listeria were grown in 2ml BHI at 37°C shaking. The following day, macrophages were stimulated with 50ng/ml LPS for 3 hours at 37°C and bacteria were diluted 1/10 and grown 3 hours at 37°C shaking to log phase. Macrophages were then washed twice with PBS and infected at MOI 10 in serum free media followed by centrifugation at 1000rpm (250×g) for 5 minutes. After 1 hour of infection, 15ug/ml gentamicin was added to kill extracellular bacteria. After four hours of infection, supernatants were harvested and cells were lysed in 1% Triton X-100. Proteins were precipitated from the supernatants with trichloroacetic acid and combined with bacterial lysates before being run on a 4–20% gradient cell and transferred to polyvinlyidene difluoride membrane. Blots were then probed with rabbit anti- Caspase-1 p10 specific antibody (Santa Crux Biotechnology, Cat. Sc-514) at 1:1000 overnight followed by donkey anti-rabbit secondary antibody for 1 hour. 10 second exposure is shown.
Mouse infections
For the primary survival assays, naïve WT mice were infected with 1*106 cfu/head i.v. For the secondary challenge, the mice that survived primary challenge with Forced FlaA or Forced PrgJ, or naïve mice, were infected with 1*106 WT L. monocytogenes and monitored for survival. For competitive index experiments, mice were infected i.v. with 5*105 WT and 5*105 Forced FlaA or Forced PrgJ L. monocytogenes. Mice were sacrificed after two days and liver and spleen homogenates were generated and plated onto BHI/streptomycin and BHI/streptomycin/chloramphenicol by 1:4 dilutional plating in replicate 10ul spots. Bacterial recovery from each plate was assessed, and relative recovery of WT and Forced FlaA/PrgJ strain was calculated by subtracting the bacteria recovered on BHI/strep/chlor (Forced FlaA/PrgJ) from BHI/strep (WT and Forced FlaA/PrgJ). Student’s T Test was used to assess significance.
Intracellular Bacterial Survival
WT BMM were seeded into 24 well dishes. Overnight cultures of L. monocytogenes were started as previously described. BMM were stimulated with 50ng/ml LPS for 3 hours, then infected with log phase L. monocytogenes at MOI 1 for 30 minutes. Gentamicin was added for 1 hour, then cells were washed 3× with PBS and media was replaced. At the indicated times, supernatant was removed from the well and BMM were lysed in Triton X-100. Bacteria from lysates were enumerated by 1:4 dilutional plating of lysates were plated onto BHI/strep plates in 10ul spots. Plates were incubated at 37°C overnight and cfu were counted.
Results
Generation of L. monocytogenes strains that over express NLRC4 agonists
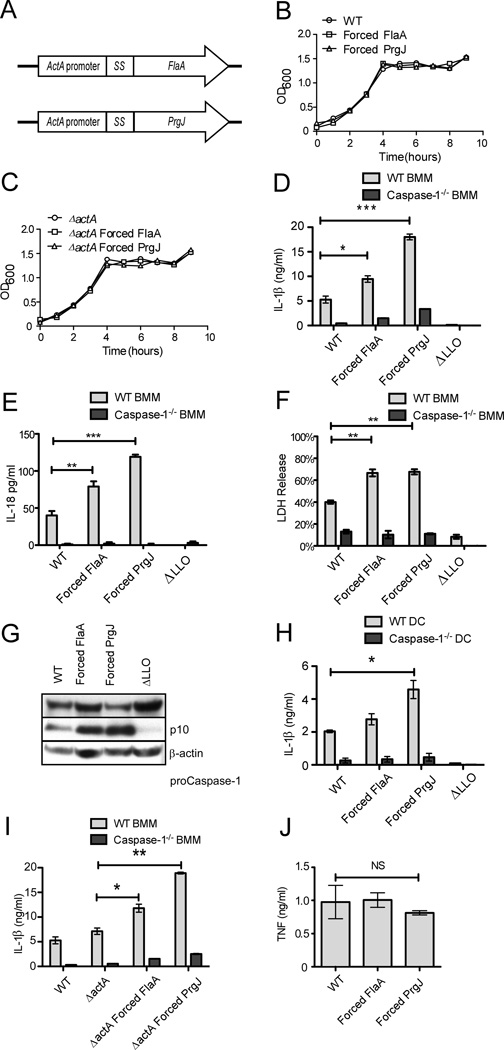
To generate a strain that would hyperactivate Caspase-1 in the macrophage cytosol, we placed either L. monocytogenes flagellin (flaA) or the S. typhimurium SPI1 T3SS rod protein (prgJ) under control of the L. monocytogenes actA promoter, and integrated these constructs into the chromosome. ActA expression is controlled by the transcription factor PrfA, which is active in the cytosol of mammalian cells [34]. The introduced flaA expression construct is therefore not regulated by MogR, which represses the endogenous flaA gene. These strains were named “Forced FlaA” and “Forced PrgJ” (Figure 1A). L. monocytogenes FlaA activates both TLR5 and NLRC4 [6, 9]. PrgJ does not activate TLR5, but induces a stronger NLRC4 response than L. monocytogenes FlaA ([17] and unpublished observations). The InlA secretion signal was included at the amino terminus of FlaA or PrgJ to ensure export from the cytosol of the bacterium through the Sec secretion pathway. [34]We hypothesized that introduction of the NLRC4 agonist would cause the strains to be avirulent, which would permit their use as attenuated vaccine vectors. To ensure that we would have an attenuated strain, we also generated strains on the ΔactA background, which cannot express the ActA protein that promotes cell to cell spread (the actA promoter within our constructs remains unaffected). The engineered strains displayed no growth defects in broth (Figure 1B, C).
Figure 1.
Forced FlaA and Forced PrgJ L. monocytogenes induced enhanced Caspase-1 activation and IL-1β secretion. A) L. monocytogenes FlaA or S. typhimurium PrgJ was placed under control of the actA promoter and fused to the secretion signal from InlA. Constructs were integrated into the L. monocytogenes chromosome of either LM OVA or LM ΔactA OVA via pPL2 integration. B, C) Strains were characterized for growth in broth. D,E,F) WT or Caspase-1−/− BMM were infected with the indicated strains of L. monocytogenes at MOI 10 and supernatants were assessed after four hours by ELISA for (D) IL-1β secretion or (E) IL- 18 secretion, and (F) cytotoxicity was assessed by LDH release . G) Caspase-1 processing was assessed from WT BMM infected with the indicated strains of L. monocytogenes (MOI 10). Proteins precipitated from supernatants were combined with cell lysates and run on western blot and probed for the active (p10) subunit of Caspase-1. H) WT or Caspase-1−/− DCs were infected with L. monocytogenes and IL-1β secretion was assessed. I) WT or Caspase-1−/− BMM were infected with the Forced FlaA/PrgJ on the ΔactA background and IL-1β secretion was assessed. J) TNF secretion from WT BMM infected with the indicated strains of L. monocytogenes was assessed after 4 hours total infection (these cells were not stimulated with LPS). Mean +/− standard deviation is displayed; data are representative of three experiments. *p<0.5; **p<0.01; ***p<0.001; NS – not significant.
Forced FlaA and Forced PrgJ hyperactivate Caspase-1
Macrophages were infected with the engineered L. monocytogenes strains and inflammasome activation was characterized (Figure 1D–G). Both Forced FlaA and Forced PrgJ induced greater IL-1β secretion through Caspase-1 than WT L. monocytogenes, with Forced PrgJ being much more potent. As expected, ΔLLO mutants did not induce IL-1β secretion. These results were also observed in the secretion of IL-18 (Figure 1E). Since Caspase-1 activation can also induce pyroptosis, we measured LDH release from infected cells as a proxy for cell death (Figure 1F). Both Forced FlaA and Forced PrgJ induced greater Caspase-1 dependent cell death than WT L. monocytogenes. Increased Caspase-1 processing was observed by detection of the p10 fragment on western blot from macrophages infected with either the Forced FlaA or the Forced PrgJ strains (Figure 1G). As was seen in macrophages, dendritic cells also secreted more Caspase-1 dependent IL-1β in response to both strains, reaching statistical significance for the Forced PrgJ strain (Figure 1H). Similar results were observed on the ΔactA background; both Forced FlaA and Forced PrgJ induced more IL-1β secretion than WT L. monocytogenes, with Forced PrgJ being more potent (Figure 1I). These results verify that our forced constructs activate the inflammasome in vitro to induce greater Caspase-1 processing and IL-1β secretion than WT L. monocytogenes. This activation was specific for Caspase-1 dependent cytokine release, as TNF secretion was unaffected (Figure 1J).
Forced FlaA and Forced PrgJ L. monocytogenes are attenuated in vivo
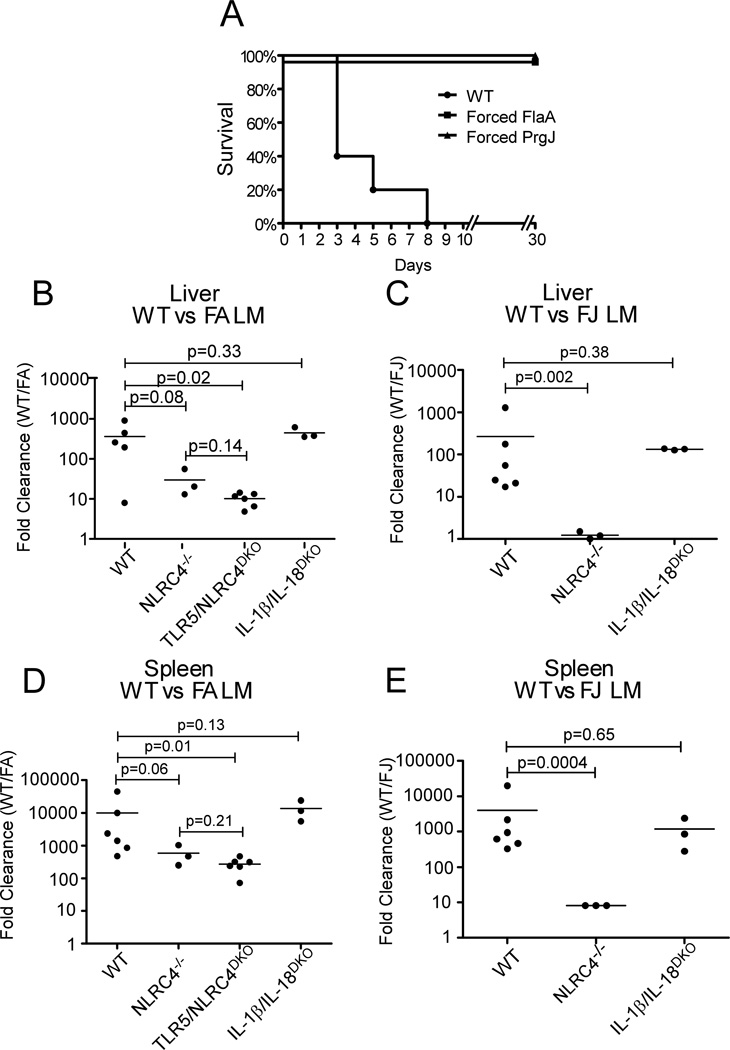
To determine if these strains were attenuated, the Forced FlaA and Forced PrgJ strains were injected into WT mice at a high dose (1*106 CFU/mouse) and survival was assessed. Mice infected with WT L. monocytogenes succumbed to infection by Day 8, whereas mice infected with Forced FlaA or Forced PrgJ showed no signs of illness and survived the infection (Figure 2A). To study the in vivo phenotype of the engineered strains in a more quantitative manner, a competitive index assay was used to evaluate clearance of the engineered L. monocytogenes from liver and spleen (Figure 2B–E). WT and engineered L. monocytogenes were injected into the tail vein of mice in a 1:1 ratio. Two days later, bacteria counts from liver and spleen homogenates were determined and the two bacteria differentiated based on differential antibiotic resistance. A clearance ratio was calculated by dividing the number of WT L. monocytogenes recovered by the number of engineered L. monocytogenes. Thus, a clearance ratio of 100 indicates that the engineered strain is cleared 100 fold more than WT L. monocytogenes. In livers of WT mice, engineered L. monocytogenes are cleared at an average rate 263 (Forced PrgJ) or 365 (Forced FlaA) times greater than WT L. monocytogenes (Figure 2B, C). In the spleen, the difference was even greater, with Forced PrgJ being cleared at an average of 4019 times WT and Forced FlaA being cleared at 9998 times WT L. monocytogenes (Figure 2D, E). The difference in clearance of both strains in the spleen versus the liver likely reflects the differences in the cellular makeup of the two organs. In the spleen, L. monocytogenes will preferentially infect macrophages; in the liver, the L. monocytogenes will infect both resident macrophages (Kupffer cells) and hepatocytes. L. monocytogenes in hepatocytes will be protected from inflammasome-mediated clearance, as hepatocytes do not express Caspase-1.
Figure 2.
Forced FlaA and Forced PrgJ L. monocytogenes are attenuated during primary infection and are preferentially cleared from liver and spleen. A, B. Naïve WT mice (A) or Caspase-1−/− mice (B) were infected with a high dose (1*106/head) of L. monocytogenes and survival was monitored for 30 days. N=5 mice per group. C,D,E,F. Mice were infected with 5*105 WT and 5*105 Forced FlaA or Forced PrgJ L. monocytogenes, and clearance of each L. monocytogenes strain from liver and spleen was calculated by plating organ homogenates onto BHI plates with selective antibiotics. N=5 mice for WT and TLR5/NLRC4DKO. N=3 mice for NLRC4−/− and IL-1β/IL-18DKO.
NLRC4 mediates clearance independent of IL-1β/IL-18
We next compared clearance of the engineered strains in mice lacking relevant components of the innate immune system. NLRC4−/− mice exhibited reduced clearance ratios, indicating that NLRC4-mediated detection was responsible for the majority of the enhanced clearance of engineered L. monocytogenes (Figure 2B–E). Since flagellin is detected through both NLRC4 and TLR5, the clearance of Forced FlaA L. monocytogenes could also mediated by TLR5. TLR5/NLRC4DKO mice were infected with WT and Forced FlaA L. monocytogenes. There was no statistically significant difference between the clearance ratio for NLRC4−/− and TLR5/ NLRC4DKO mice, indicating that TLR5 does not play a major role (Figure 2B, D). Interestingly, the NLRC4 mediated clearance was more profound for Forced PrgJ than Forced FlaA L. monocytogenes. This parallels the in vitro observations that the Forced PrgJ construct more potently activated Caspase-1 than the Forced FlaA construct (Figure 1D–I).
To dissect the contribution of cytokine production versus pyroptosis in mediating enhanced clearance of engineered L. monocytogenes, we compared the clearance ratios in WT and IL-1β/IL-18DKO mice (Figure 2B–E). No difference was observed, indicating that the cytokines are not contributing to bacterial clearance. Since we observe increased pyroptosis in vitro (Fig. 1F), we hypothesize that Caspase-1-dependent pyroptosis could be responsible for clearing Forced FlaA/PrgJ L. monocytogenes in vivo. This hypothesis is supported by our recent finding that flagellin expressing Salmonella typhimurium are cleared in a cytokine independent manner through pyroptosis; as bacteria activate Caspase-1, infected cells lyse via pyroptosis, releasing bacteria from their replicative niche in the cytosol into the extracellular space where they are exposed to additional immune defense mechanisms [35].
Engineered strains have a minor replication defect
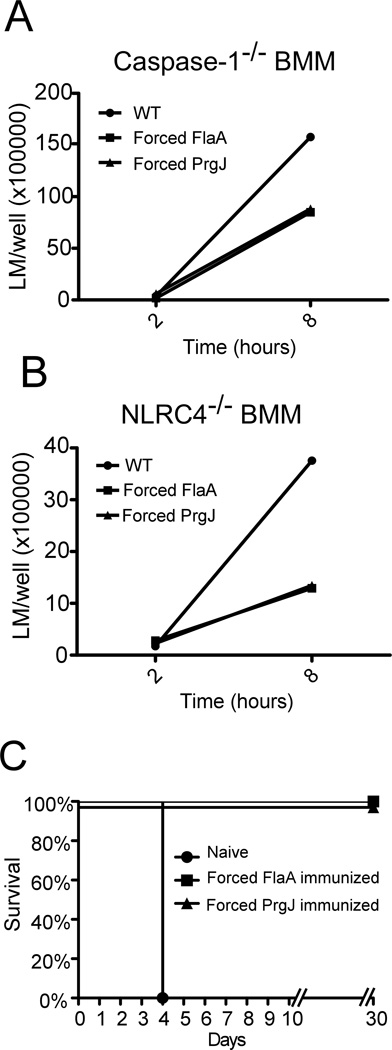
Although a significant amount of the bacterial clearance was NLRC4 dependent for Forced FlaA, and the vast majority of the clearance was NLRC4 dependent for Forced PrgJ, there was also an NLRC4 independent effect. Although the engineered strains grow normally in broth, the PrfA regulon is not activated in BHI, and expression of the constructs may cause an intracellular growth defect. To evaluate growth in the absence of inflammasome activation, bacterial replication was enumerated from Caspase-1−/− and NLRC4−/− macrophages (Figure 3A, B). There was no difference in bacterial entry into the cell, but a 2–3-fold replication defect was observed at 8 hours. This slight replication defect could account for the NLRC4-independent reduced recovery observed in vivo.
Figure 3.
Forced FlaA and Forced PrgJ L. monocytogenes have intracellular growth defect but confer protective immunity in vivo. A,B. Caspase-1−/− (A) or NLRC4−/− (B) BMM were infected with L. monocytogenes and intracellular bacterial replication was calculated by plating cell lysates onto BHI plates at the indicated times after infection. Data are representative of three experiments. B. Naïve mice or mice immunized with Forced FlaA or Forced PrgJ were infected with a lethal challenge of WT L. monocytogenes (1*106/head) and survival was monitored up to 30 days. N=5 mice per group.
Forced FlaA and Forced PrgJ confer protective immunity
To assess whether the engineered L. monocytogenes could confer immunity against a lethal challenge with WT L. monocytogenes, the mice that survived the initial challenge (Figure 2A) were rechallenged after 30 days with 1*106 WT L. monocytogenes (Figure 3C). While all naïve mice succumbed on day 4, both the Forced FlaA and the Forced PrgJ immunized mice survived challenge. Thus, despite attenuation, the engineered L. monocytogenes mutants are able to elicit a protective immune response.
Concluding Remarks
We present here the first demonstration of a live vaccine vector that is engineered to specifically enhance Caspase-1 activity. The resulting strains are attenuated and confer protective immunity. Notably, the cytokines IL-1β and IL-18 are not required for attenuation; however they may still promote development of a robust adaptive immune response. These strains can also be used as vaccine vectors for the delivery of heterologous antigens. We further hypothesize that the introduction of Forced FlaA/PrgJ into the ΔactA background could provide adjuvant effects. Forced cytosolic delivery of flagellin represents a novel strategy to enhancing efficacy while maintaining safety of L. monocytogenes based vaccines.
Acknowledgements
We wish to thank David Rodriguez and the vivarium staff for their excellent management of the mouse colony, and Garrett Zipp for technical assistance.
This work was supported by NIH grant: R01 AI052286, RO1 AI32972 and R37 AI25032
References
- 1.Pamer EG. Immune responses to Listeria monocytogenes. Nature Reviews Immunology. 2004;4(10):812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 2.Vazquez-Boland JA, et al. Listeria Pathogenesis and Molecular Virulence Determinants. Clinical Microbiology Reviews. 2001;14(3):584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizarro-Cerda J, Cossart P. Subversion of cellular functions by Listeria monocytogenes. J Pathol. 2006;208(2):215–223. doi: 10.1002/path.1888. [DOI] [PubMed] [Google Scholar]
- 4.Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect. 2007;9(10):1208–1215. doi: 10.10110/2/076/j.micinf.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flo TH, et al. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164(4):2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178(4):2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 8.Opitz B, et al. Listeria monocytogenes activated p38 MAPK and induced IL-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J Immunol. 2006;176(1):484–490. doi: 10.4049/jimmunol.176.1.484. [DOI] [PubMed] [Google Scholar]
- 9.Warren SE, et al. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180(11):7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren SE, et al. Cutting Edge: Cytosolic Bacterial DNA Activates the Inflammasome via Aim2. The Journal of Immunology. 2010;185(2):818–821. doi: 10.4049/jimmunol.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauer JD, et al. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7(5):412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchiya K, et al. Involvement of Absent in Melanoma 2 in Inflammasome Activation in Macrophages Infected with Listeria monocytogenes. The Journal of Immunology. 2010;185(2):1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 Inflammasomes in Caspase-1 Activation by Listeria monocytogenes. Journal of Clinical Immunology. 2010 doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunology. 2006;7(6):569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 15.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nature Immunology. 2006;7(6):576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 16.Sun YH, Rolan HG, Tsolis RM. Injection of Flagellin into the Host Cell Cytosol by Salmonella enterica Serotype Typhimurium. Journal of Biological Chemistry. 2007;282(47):33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- 17.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107(7):3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin C, Flavell RA. Molecular Mechanism of NLRP3 Inflammasome Activation. Journal of Clinical Immunology. 2010 doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 19.Martinon F, Tschopp J. Inflammatory CaspasesLinking an Intracellular Innate Immune System to Autoinflammatory Diseases. Cell. 2004;117(5):561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello CA. Biology of interleukin 1. FASEB J. 1988;2(2):108–115. [PubMed] [Google Scholar]
- 21.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 22.Edelson BT, Unanue ER. Immunity to Listeria infection. Curr Opin Immunol. 2000;12(4):425–431. doi: 10.1016/s0952-7915(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 23.Bruhn K, Craft N, Miller J. Listeria as a vaccine vector. Microbes and Infection. 2007;9(10):1226–1235. doi: 10.1016/j.micinf.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Shen H, et al. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci U S A. 1995;92(9):3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brockstedt DG. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proceedings of the National Academy of Sciences. 2004;101(38):13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darji A, et al. Induction of immune responses by attenuated isogenic mutant strains of Listeria monocytogenes. Vaccine. 2003;21(Suppl 2):S102–S109. doi: 10.1016/s0264-410x(03)00208-1. [DOI] [PubMed] [Google Scholar]
- 27.Shen A, Higgins DE. The MogR transcriptional repressor regulates nonhierarchal expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathog. 2006;2(4):e30. doi: 10.1371/journal.ppat.0020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pope C, et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166(5):3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 29.Way SS, et al. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178(7):4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauer P, et al. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184(15):4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamaillard M, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4(7):702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 32.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 33.Raupach B, et al. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74(8):4922–4926. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scortti M, et al. The PrfA virulence regulon. Microbes and Infection. 2007;9(10):1196–1207. doi: 10.1016/j.micinf.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]