Abstract
J Clin Hypertens (Greenwich). 2012; 14:388–395. ©2012 Wiley Periodicals, Inc.
As a result of the Food and Drug Administration (FDA) Modernization Act and the Best Pharmaceuticals for Children Act, the number of medications with FDA‐approved pediatric labeling has increased. To assess the success of these initiatives, we examined whether antihypertensive drugs used by children with hypertension in 2008 had FDA‐approved pediatric labeling and indications. Using a nationwide commercial insurer database, 2915 children with primary (n=2607) and secondary (n=308) hypertension were identified. Drug user rate and days of supply were calculated from pharmacy claims. Drugs were categorized based on pediatric labeling and indication and whether they were recommended for pediatric use. Antihypertensive drugs were used by 889 (34%) children with primary hypertension and 200 children (65%) with secondary hypertension. User rates were 44.3% in hypertensive children younger than 6 years, 30.9% in those 6 years to older than 12 years, and 38.1% in those 12 years to older than 18 years. Seven percent of drugs were neither labeled for pediatric use nor considered recommended for use in children. In children younger than 6 years, 29% of drugs used were not indicated for use in that age group. Despite recent legislative initiatives, many drugs used by hypertensive children still lack pediatric labeling. Additional efforts are needed to close the gap between the availability of drugs that are labeled and indicated for pediatric use and actual drug usage in children.
Drug prescribing in pediatrics has been limited by a lack of pediatric efficacy and safety information, making children “therapeutic orphans” 1 and at times forcing prescribers to make decisions without complete evidence. Although there were earlier efforts to rectify this situation, 2 little progress was made until passage of the 1997 Food and Drug Administration (FDA) Modernization Act (FDAMA), which provides incentives for manufacturers to test drugs in children in exchange for 6 additional months of market exclusivity. 3 , 4 Pediatric drug development has subsequently been further strengthened by the Best Pharmaceuticals for Children Act (BPCA) in 2002 5 and the Pediatric Research Equity Act (PREA) in 2003, 6 both of which have been reauthorized by Congress.
Antihypertensive drugs are one of the largest classes of medications affected by these initiatives, and many pediatric trials of antihypertensive agents have been conducted as a result of FDAMA‐ and BPCA‐related written requests. Although some trials have not achieved their primary objectives, 7 the overall availability of pediatric labeling for antihypertensive medications as well as pediatric dosing and safety information has increased. Additionally, clinical trial data have been disseminated through the FDA Web site and scientific publications. However, little is known about the impact of these efforts on provider practices or whether the antihypertensive drugs used by children have FDA‐approved pediatric labeling and indications.
This study investigated drugs prescribed to hypertensive children by using claims‐based data from a national commercial insurer. We sought to determine to what extent antihypertensive medications used in children are those that have FDA‐approved pediatric labeling and indications. We also analyzed the extent of off‐label drug use (ie, use of nonlabeled drugs or drugs not indicated for a particular age group) across age groups.
Methods
Data Source and Study Population
This cross‐sectional study analyzed 2008 data from Ingenix’s UnitedHealth Group Analytics Platform database (Ingenix, Eden Prairie, MN), which that year had de‐identified inpatient, outpatient, physician, and pharmacy claims data for 18.4 million persons, of which 4.1 million were children (younger than 18 years). Of those, we identified 1,449,750 children (740,344 males and 709,406 females) with continuous coverage for both medical and pharmacy benefits for the full year. As is typical of claims‐based databases, race/ethnicity data are not included.
Identification of Children with Hypertension: ETGs
Episode Treatment Groups (ETGs) software, version 5 (Ingenix), was used to identify children with hypertension and the drugs they received. ETGs organize claims into episodes and links appropriate services, including prescription drugs, into approximately 850 categories. ETGs offer a standardized methodology that can be used over a wide range of applications (eg, see Medicare Payment Advisory Commission [MedPAC] 8 ).
The ETG algorithm creates episodes in two steps: Step A identifies an “anchor record” (based on primary diagnosis of a medical encounter) that represents either a clinician’s direct evaluation of a patient’s condition, a clinician’s performance of a surgical procedure, an inpatient admission, or an emergency department visit. Step B links other records to the anchor record based on date of service, diagnosis, and procedure codes. We included children whose hypertension episode started before or in 2008, and analyzed their drug utilization in 2008. International Statistical Classification of Diseases and Related Health Problems–Ninth Revision (ICD‐9) codes used by hypertension ETGs are listed in Table I.
Table I.
Diagnosis Codes Used to Define Episode Treatment Groups (ETGs)
| ETGs used | 0278 and 0279, malignant hypertension with and without comorbidity; 0280 and 0281, benign hypertension with and without comorbidity |
| International Statistical Classification of Diseases and Related Health Problems–Ninth Revision (ICD‐9) codes used to define hypertension | 401, 401.1, 401.9, 402, 402.01, 402.1, 402.11, 402.9, 402.91, 403, 403.01, 403.1, 403.11, 403.9, 403.91, 404, 404.01, 404.02, 404.03, 404.1, 404.11, 404.12, 404.13, 404.9, 404.91, 404.92, 404.93, 405, 405.01, 405.09, 405.1, 405.11, 405.19, 405.9, 405.91, 405.99 |
| Primary vs secondary hypertension | Patients were defined as having primary hypertension if he or she had the ICD‐9 code for primary hypertension (401, 401.1, and 401.9), while all other hypertensive children were considered to have secondary hypertension |
Metrics
We classified the drugs in the database according to the following levels: therapeutic category (eg, cardiovascular), drug class (eg, calcium channel antagonist), and drug (eg, amlodipine).
We defined two metrics to capture use and measure both the prevalence of users and the quantity of drug per user: Drug user rate was the percentage of children with hypertension who used a prescription drug, and days of supply was the average number of days that the patient had access to the drug through a prescription.
Pediatric Labeling and Indication
To determine the pediatric labeling, indication, and age‐appropriate considerations for each drug, we relied on the FDA report Pediatric Labeling Changes through December 24, 2009 9 (henceforth referred to as the “FDA Report”), which provides the date of pediatric labeling for each drug and specific information on whether the drug is indicated for pediatric use as well as age‐specific indication. We determined whether each drug used by our population was labeled and indicated. We also determined whether the labeling was available before or after passage of the FDAMA, as pre‐FDAMA labels generally provide fewer details on age‐specific indications than post‐FDAMA labels. Drugs that received pediatric labeling after 2008 were categorized as labeled, because our primary focus was on drugs that still require pediatric labeling.
In the case of antihypertensive medications, pediatric dose recommendations for many drugs that lack FDA labeling are contained in the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents (the Fourth Report).10 Therefore, we subclassified unlabeled drugs according to whether the Fourth Report contained a pediatric dose recommendation for that compound. The 6 categories of drugs based on these criteria are listed in Table II.
Table II.
Categories of Drugs Based on Availability of Labeling, Indication, and Clinical Dose Recommendation
| Labeled pre–Food and Drug Administration Modernization Act (FDAMA), indicated | Drug that has a label created prior to FDAMA and the label provides pediatric prescribing information |
| Labeled pre‐FDAMA, not indicated | Drug that has a label created prior to FDAMA but the label does not provide pediatric prescribing information |
| Labeled post‐FDAMA, indicated | Drug that has a label created after FDAMA and the label states that the drug is indicated for pediatric use |
| Labeled post‐FDAMA, not indicated | Drug that has a label created after FDAMA but the label does not state that the drug is indicated for pediatric use |
| Not labeled with dose recommendations | Drug whose label lacks any pediatric information but the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents (Fourth Report) provides pediatric dose recommendations |
| Not labeled without dose recommendations | Drug whose label lacks pediatric information and the Fourth Report does not provide pediatric dose recommendations |
Data Analysis
We calculated the percentage of drugs among the 6 categories defined above by counting users of each drug, weighing each drug by the number of users. By design, a child who uses two drugs was counted twice.
Because some antihypertensive drugs are specifically not FDA‐labeled for use in children younger than 6, we reported prevalence rates and user rates for three age groups (0 to <6 years, 6 to <12 years, and 12 to <18 years) based on the child’s age on June 30, 2008. In order to determine whether there were any age‐related patterns (for example, for children <6 relative to children aged 6 to <12 and 12 to <18) in terms of drug use across different drug classes or individual drugs, we used chi‐square or Fisher's exact test (as appropriate) to test the equality of proportions in drug user rates across the 3 age groups. When the overall test was significant (at P<.05), we further conducted pair‐wise the chi‐square or Fisher's exact tests across age groups, adjusting the P values for multiple testing.
Results
Prevalence
Of the approximately 1.4 million children in our study population, 2607 were diagnosed based on the recorded ICD‐9 code as having primary hypertension and another 308 as having secondary hypertension (Table III). The prevalence rate for primary hypertension (0.18%) was 9 times that of secondary hypertension (0.02%). The combined prevalence rate was 0.20%. Combined prevalence rates were higher for males (0.26%) than for females (0.15%).
Table III.
Prevalence of Hypertension by Age, Sex, and Diagnosis, 2008
| Age | Primary | Secondary | ||||
|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |
| Children with hypertension, No. | ||||||
| Total | 2607 | 1709 | 898 | 308 | 189 | 119 |
| 0 to <6 y | 191 | 120 | 71 | 39 | 23 | 16 |
| 6 to <12 y | 426 | 254 | 172 | 62 | 39 | 23 |
| 12 to <18 y | 1990 | 1335 | 655 | 207 | 127 | 80 |
| Prevalence, % | ||||||
| Total | 0.18 | 0.23 | 0.13 | 0.02 | 0.03 | 0.02 |
| 0 to <6 | 0.04 | 0.05 | 0.03 | 0.01 | 0.01 | 0.01 |
| 6 to <12 | 0.09 | 0.10 | 0.07 | 0.01 | 0.02 | 0.01 |
| 12 to <18 | 0.39 | 0.51 | 0.26 | 0.04 | 0.05 | 0.03 |
Drug User Rate by Drug Class
Table IV presents drug user rates by age group (0 to <6, 6 to <12, and 12 to <18 years), drug class, and diagnosis. Drug use was lower among children with primary hypertension (889 users or 34.1%) than among those with secondary hypertension (200 users or 64.9%) (data not shown). The percentages of hypertensive children who used prescription drugs were similar for both sexes: 37.4% for males vs 37.3% for females with either primary or secondary hypertension (data not shown). For all hypertensive children combined, a similar pattern was observed across ages: the user rate was significantly lower in the middle group (30.9%) than in the youngest group (44.3%) and the oldest group (38.1%).
Table IV.
Percentage of Hypertensive Children Who Used a Prescription Drug by Age and Drug Class, 2008
| Therapeutic Category Drug Class | Percentage | P Value | |||||
|---|---|---|---|---|---|---|---|
| Total, % | 0 to <6 y, % | 6 to <12 y, % | 12 to <18 y, % | 0 to <6 vs 6 to <12 | 0 to <6 vs 12 to <18 | 6 to <12 vs 12 to <18 | |
| All drugs | 37.4 | 44.3 | 30.9 | 38.1 | .001 | .01 | |
| Cardiovascular agents | 34.1 | 36.5 | 28.9 | 35.0 | .03 | ||
| Angiotensin‐converting enzyme inhibitors | 17.6 | 19.6 | 15.6 | 17.8 | |||
| Angiotensin II receptor antagonists | 2.6 | 0.9 | 1.6 | 3.0 | |||
| Antihypertensive combinationa | 2.8 | 0.9 | 0.6 | 3.6 | .001 | ||
| β‐Adrenergic antagonists without ISAb | 6.7 | 3.0 | 5.1 | 7.4 | .04 | ||
| Calcium channel antagonists | 9.0 | 15.2 | 8.2 | 8.6 | .012 | .003 | |
| Centrally acting antiadrenergic agents | 3.8 | 3.5 | 4.9 | 3.6 | |||
| Electrolyte‐caloric‐water balance agents | 6.5 | 11.3 | 6.6 | 6.0 | .005 | ||
| Loop diuretics | 1.7 | 7.8 | 2.3 | 0.9 | .001 | <.0001 | .04 |
| Thiazides and related agents | 3.7 | 3.0 | 3.5 | 3.8 | |||
Abbreviation: ISA, intrinsic sympathomimetic activity. Drug classes included in this Table met the minimum threshold of a 1.0% user rate. P values are based on pair‐wise chi‐square test adjusting for multiplicity, unless otherwise indicated. aBased on Fisher exact test adjusting for multiplicity.
The test for equality of proportions found that the user rates were significantly different across age groups for the cardiovascular agents and 3 drug classes under it. A similar pattern was seen in the user rates for the electrolyte‐caloric‐water balance agents and its class, loop diuretics. A subsequent pair‐wise test (adjusting for multiplicity) found that compared with the oldest group, the youngest group used significantly less β‐adrenergic antagonists without intrinsic sympathomimetic activity and significantly more calcium channel antagonists.
Pediatric Labeling and Indications by Drug
As drug labeling and indication are defined at the drug level (not the drug class level), Table V disaggregates the drug user rate and the distribution of days supply by drug. Approximately 90% of the supply days across the two diagnoses were for cardiovascular agents and 10% for electrolyte‐caloric‐water balance agents (diuretics). Within the former, the two drug classes with the most days of supply were angiotensin‐converting enzyme (ACE) inhibitors (38.8%) and calcium channel antagonists (17.8%). Among the ACE inhibitors, the two drugs with the most days of supply were lisinopril (19.8%) and enalapril (16.1%).
Table V.
Percentage of Hypertensive Children Who Used a Prescription Drug, Their Days of Supply, and Labeling‐Indication Status by Drug and Diagnosis, 2008
| Therapeutic Category Drug Class Drug Name | User Rate, % | Total Days’ Supply, % | Pediatric Labeling | Label Indicated for Pediatric Use | Pediatric Dosing Recommendation | Age Indication | |
|---|---|---|---|---|---|---|---|
| Primary Hypertension | Secondary Hypertension | ||||||
| All drugs | 34.1 | 64.9 | 100 | ||||
| Cardiovascular agents | 30.6 | 63.6 | 89.4 | ||||
| α‐ and β‐Adrenergic antagonists | 0.6 | 3.6 | 1.9 | ||||
| Labetalol HCL | 0.6 | 3.6 | 1.9 | No | NA | Yes | NA |
| Angiotensin‐converting enzyme inhibitors | 14.8 | 40.9 | 38.8 | ||||
| Lisinopril | 8.2 | 22.4 | 19.8 | Yes (post) | Yes | Yes | 6–16 y |
| Enalapril maleate | 5.8 | 17.9 | 16.1 | Yes (post) | Yes | Yes | 1 mo–16 y |
| Captopril | 0.5 | 1.6 | 1.3 | No | NA | Yes | NA |
| Ramipril | 0.2 | 0.6 | 0.7 | No | NA | No | NA |
| Quinapril | 0.2 | 0.3 | 0.4 | No | NA | Yes | NA |
| Angiotensin II receptor antagonists | 2.0 | 7.1 | 5.3 | ||||
| Losartan potassium | 1.0 | 6.2 | 3.2 | Yes (post) | Yes | Yes | 6–16 y |
| Valsartan | 0.5 | 0.3 | 0.8 | Yes (post) | Yes | No | 6–16 y |
| Olmesartan medoxomil | 0.2 | 0.3 | 0.5 | No | NA | No | NA |
| Candesartan cilexetil | 0.3 | 0.0 | 0.5 | Yes (post) | Yes | No | 1 to <17 y |
| Antihypertensive combination | 2.9 | 2.3 | 4.2 | ||||
| Amlodipine/benazepril | 0.5 | 0.3 | 0.6 | No | NA | No | NA |
| Lisinopril/hydrochlorothiazide | 1.5 | 1.0 | 1.9 | No | NA | No | NA |
| Losartan potassium/hydrochlorothiazide | 0.2 | 0.3 | 0.6 | No | NA | No | NA |
| Bisoprolol/hydrochlorothiazide | 0.2 | 0.3 | 0.3 | No | NA | Yes | NA |
| β‐Adrenergic antagonists without ISA | 6.4 | 9.4 | 12.1 | ||||
| Atenolol | 3.2 | 4.9 | 7.0 | No | NA | Yes | NA |
| Metoprolol succinate sustained‐release | 1.0 | 1.3 | 1.9 | Yes (post) | Noa | No | NA |
| Propranolol HCL | 1.5 | 1.3 | 1.6 | Yes (pre) | Yes | Yes | N/A |
| Metoprolol tartrate | 0.5 | 1.6 | 0.7 | No | NA | Yes | NA |
| Propranolol HCL long‐acting | 0.2 | 0.3 | 0.4 | Yes (pre) | Yes | Yes | N/A |
| Nadolol | 0.2 | 0.0 | 0.3 | No | NA | No | NA |
| Calcium channel antagonists | 7.2 | 24.7 | 17.8 | ||||
| Amlodipine besylate | 5.4 | 20.1 | 13.2 | Yes (post) | Yes | Yes | 6–17 y |
| Nifedipine sustained‐release | 1.0 | 4.2 | 2.4 | No | NA | Yes | NA |
| Diltiazem HCL sustained‐release | 0.2 | 0.0 | 0.8 | No | NA | No | NA |
| Verapamil HCL sustained‐release | 0.3 | 0.0 | 0.4 | No | NA | No | NA |
| Centrally acting antiadrenergic agentsb | 3.5 | 6.5 | 8.0 | ||||
| Clonidine HCL, oral | 2.4 | 4.5 | 5.3 | Yes (Pre) | Yes | Yes | N/A |
| Guanfacine HCL | 0.8 | 0.3 | 1.4 | Yes (post) | Yes | No | ≥12 y |
| Clonidine HCL, transdermal | 0.5 | 2.6 | 1.3 | Yes (pre) | Yes | Yes | N/A |
| Vasodilating agents | 0.2 | 2.3 | 0.9 | ||||
| Hydralazine HCL | 0.2 | 1.0 | 0.6 | Yes (pre) | Yes | Yes | N/A |
| Minoxidil | 0.1 | 1.6 | 0.3 | Yes (pre) | Yes | Yes | N/A |
| Electrolyte‐caloric‐water balance agents | 6.0 | 10.7 | 9.9 | ||||
| Combination diuretics | 0.7 | 0.3 | 0.8 | ||||
| Triamterene/hydrochlorothiazide | 0.5 | 0.0 | 0.5 | No | NA | Yes | NA |
| Loop diuretics | 1.3 | 4.5 | 1.8 | ||||
| Furosemidec | 1.3 | 4.5 | 1.8 | No | NA | Yes | NA |
| Potassium‐sparing diuretics | 0.8 | 3.2 | 1.7 | ||||
| Spironolactone | 0.7 | 2.6 | 1.1 | No | NA | Yes | NA |
| Amiloride | 0.2 | 0.6 | 0.6 | No | NA | Yes | NA |
| Thiazides and related agents | 3.5 | 5.2 | 5.6 | ||||
| Hydrochlorothiazide | 3.0 | 3.9 | 4.6 | Yes (pre) | Yes | Yes | N/A |
| Chlorothiazide | 0.4 | 1.0 | 0.6 | No | NA | No | NA |
Abbreviations: HCL, hydrochloride; ISA, intrinsic sympathomimetic activity; NA, not applicable (because no labeling or not indicated); N/A, not available (pre–Food and Drug Administration Modernization Act [FDAMA] drugs); Pre, approved pediatric labeling occurred prior to FDAMA (1997); post: approved pediatric labeling occurred after FDAMA (1997). Reported days of supply may not sum to totals because drugs with low supply day counts are not reported. aLabeled post‐FDAMA; however, because the drug did not meet its primary end point in a study of 144 pediatric hypertensive patients aged 6 to 16 years, 9 we classified it as labeled but not indicated for children. bCertain antihypertensive drugs can also be used to treat other conditions (eg, guanfacine and clonidine for use in children with attention‐deficit hyperactivity disorder). cDrug labeled only for treatment of edema, but may be used as add‐on therapy in children with resistant hypertension, particularly in children with renal disease.
Among the 35 drugs used by our population, 15 had pediatric indications or dose recommendations based on the FDA Report 9 (8 drugs) or the Fourth Report 10 (7 drugs), and 20 did not have pediatric indications or dose recommendations (Table V).
Figure 1 summarizes the labeling‐indication status of the drugs, weighted by the number of users. Seventy‐two percent of the drugs had FDA‐approved pediatric label information and 28% did not. The 72% of drugs with FDA‐approved pediatric label information can be subcategorized as: 17% labeled pre‐FDAMA and indicated, 0% labeled pre‐FDAMA but not indicated, 53% labeled post‐FDAMA and indicated, and 2% labeled post‐FDAMA but not indicated for children. The 28% of drugs without FDA‐approved pediatric label information can be subcategorized as: 21% with recommended dose available and 7% without. Weighting drugs used by days of supply (instead of by number of users) yielded similar results.
Figure FIGURE.
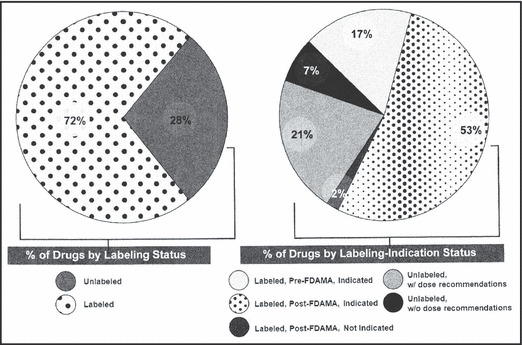
Pediatric labeling and indication for antihypertensive drugs weighted by the number of users, 2008. Pediatric labeling may or may not include labeling for children younger than 6 years. FDAMA indicates Food and Drug Administration Modernization Act.
Table VI presents the prescription patterns by age group with a focus on age‐specific indications. For the youngest group, 26.3% of drugs were both labeled and indicated for children of any age. This figure was significantly higher than the figures for the middle group (20.5%) and oldest group (11.9%). About one sixth of drugs were labeled and indicated pre‐FDAMA.
Table VI.
Age‐Specific Pediatric Labeling and Indication, Antihypertensive Drugs, Weighted by the Number of Users, 2008
| Labeling/Indication Status | No. | Percentage | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total, % | 0 to <6 y | 6 to <12 y | 12 to <18 y | Total, % | 0 to <6 y, % | 6 to <12 y, % | 12 to <18 y, % | 0 to <6 vs 6 to <12 | 0 to <6 vs 12 to <18 | 6 to <12 vs 12 to <18 | |
| All | 1462 | 133 | 224 | 1105 | 100.0 | 100.0 | 100.0 | 100.0 | |||
| Labeled post‐FDAMA, indicated (for children 1–17 y) | 212 | 35 | 46 | 131 | 14.5 | 26.3 | 20.5 | 11.9 | <.0001 | .002 | |
| Labeled pre‐FDAMA (age‐indication not available) | 248 | 16 | 42 | 190 | 17.0 | 12.0 | 18.8 | 17.2 | |||
| Not labeled | 408 | 44 | 55 | 309 | 27.9 | 33.1 | 24.6 | 28.0 | |||
| Labeled post‐FDAMA, not indicated (for children) | 30 | 0 | 2 | 28 | 2.1 | 0.0 | 0.9 | 2.5 | |||
| Labeled post‐FDAMA, indicated (not for children <6 y) | 564 | 38 | 79 | 447 | 38.6 | 28.6 | 35.3 | 40.5 | .020 | ||
Abbreviation: FDAMA, Food and Drug Administration Modernization Act. P values are based on pair‐wise chi‐square test adjusting for multiplicity, unless otherwise indicated.
One quarter to one third of drugs were not labeled for pediatric use. The youngest age group used disproportionally more unlabeled drugs (33.1%) than the middle group (24.6%) and the oldest group (28.0%). Of particular interest was that 28.6% of the drugs used by the youngest age group were not specifically indicated for use in children younger than 6 years.
Of interest are 5 drugs (lisinopril, losartan, valsartan, amlodipine, and guanfacine) that were labeled post‐FDAMA and indicated for children but specifically not FDA‐labeled for use in the youngest age group. While the percentage of use of these drugs by the youngest group was significantly lower (28.6%) than in the oldest age group (40.5%), it is notable that drugs without pediatric labeling are being used in such young patients. For amlodipine, the percentage used was significantly higher in the youngest age group (24.8%) than the middle group (14.3%) and the oldest group (12.4%) (data not shown).
Discussion
The intent of the pediatric provisions of the FDAMA, BPCA, and the PREA is to ensure that safe and effective drugs are available for use in children. This claims‐based analysis of pediatric use of antihypertensive drugs indicates that although most drugs being used are both labeled and indicated for children, there are two sources of concern. First, 7% of prescribed antihypertensive drugs had neither FDA‐approved pediatric label information nor dosing recommendations in the Fourth Report, thus resulting in continued off‐label prescribing. It may be appropriate to focus future research on these agents. Second, 28.6% of drugs used by children younger than 6 years did not have FDA‐approved indications for use in that age group, suggesting a need for greater efforts at drug development for young patients and also a need for better dissemination of existing age‐specific indication information.
Both the successes and potential shortcomings of the FDAMA were recognized shortly after its passage. 4 While successful in prompting studies of medications that still had patent protection remaining, off‐patent medications were not included in the pediatric provisions of the FDAMA, with the result that only newer medications underwent industry‐sponsored trials. The BPCA was intended to address this shortcoming by charging the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) with determining which off‐patent medications warranted pediatric study, then referring this list to the FDA for issuance of written requests to manufacturers. If such written requests were declined, the NICHD could then sponsor research to conduct appropriate pediatric studies. To date, only one antihypertensive medication, intravenous sodium nitroprusside, which is used only in the operating room or the intensive care unit, has been studied by this mechanism (ClinTrials.gov studies NCT00621816 and NCT00135668). 11 Hence, there is still a need for studies of oral antihypertensives without patent protection. Indeed, our data demonstrate that many hypertensive children are still using such medications.
To date, only two antihypertensive medications have been studied in children younger than 6 years, 12 , 13 and neither medication has received FDA‐approved pediatric labeling or an indication for use in this age group. Although the pediatric provisions of the FDAMA and related legislation do not exclude children younger than 6 years, in practice, nearly all of the studies conducted in response to FDAMA‐related written requests have been limited to hypertensive children 6 and older. This situation is reflected in our data, which demonstrate that a majority of the drugs used by children younger than 6 years did not have pediatric labeling or were labeled but without an indication for their age group. Indeed, another recent study also found that the availability of labeled and indicated medications for children increased with age. 14 The fact that there are fewer labeled medications for the youngest children is unfortunate, as most young hypertensive children will have secondary forms of hypertension that may require multiple medications to achieve adequate blood pressure control, as evidenced by the participants in the valsartan and candesartan trials mentioned earlier. 12 , 13
Several unique aspects of this study merit comment. We found the prevalence of hypertension based on recorded ICD‐9 code to be 0.2% in the claims database, which is substantially lower than prevalence estimates based on recent screening studies 15 and which is also well below the 0.9% prevalence recently reported by Hansen and colleagues using data from an electronic medical record in an academic‐based medical system. 16 However, if one excludes children with only elevated blood pressure (ICD‐9 code 796.2), the 0.9% prevalence rate reported by Hansen falls to 0.56%. The difference between this figure and our estimate of 0.2% could be the result of several factors, including the broader group of providers included in our database, the presence of a more diverse range of insurance types in the Hansen study (eg, Medicaid), and the use of the ETG algorithm, which only takes into consideration the primary diagnosis code. Including children with a hypertension diagnosis in the first 3 positions on a claim, we still found a low prevalence rate (0.24%). Similar to the children identified by the ETG algorithm, most of the additional 0.04% of children represented had primary hypertension.
The fact that the hypertension prevalence in our data was one twentieth of the rate reported by Din‐Dzietham and colleagues 15 may reflect the conservatism of current consensus guidelines, which recommend that a child be documented to have elevated blood pressure on multiple occasions before a diagnosis of hypertension is made.10 On the other hand, the case could be made that perhaps our data support Hansen’s finding that underdiagnosis of hypertension in children and adolescents is common, and may add weight to recent calls for revision of criteria for diagnosis of hypertension in the young. 17
Not all children diagnosed with hypertension used antihypertensive medications. For children with primary hypertension, this finding is not unexpected, because lifestyle modification is currently recommended as the first step in the management of uncomplicated hypertension. 10 However, a substantial percentage of children with secondary hypertension were also not receiving antihypertensive medications. It is possible that some of them were still undergoing evaluation in 2008, were receiving treatment of the underlying cause of the hypertension, or had not yet received an antihypertensive prescription. Unfortunately, we do not have the clinical data needed to answer this question.
Study Limitations
Limitations of this study include the relatively small sample size of children actually receiving antihypertensive medications and the lack of data on Medicaid recipients, which may limit the generalizability of our conclusions. However, we believe that this analysis also has significant strengths, the most notable of which is the claims‐based approach’s ability to conduct detailed analyses of actual drug utilization and an accurate accounting of drugs being used for direct treatment of hypertension. Additionally, the use of claims‐based data from a national commercial insurer provides information from all regions of the country and a wide span of American pediatric providers from community settings and academic centers, including both primary physicians and specialist pediatricians. Relative to survey‐based data, claims‐based data of prescription drug use can be obtained for many more children at substantially lower costs. Further, claims‐based data have more precision in terms of specific drug, dosage, and date of prescription than survey‐based data, allowing for more precise analyses.
Another limitation of the study is lack of clinical information such as actual blood pressure measurements, blood pressure measurement technique, and assessment of clinical efficacy and safety of the antihypertensive medications prescribed. Without such information, we cannot confirm the diagnosis of hypertension among the children represented in the claims database, which could have influenced the calculation of the prevalence of hypertension, and we cannot assess any harm resulting from prescription of medications without FDA‐approved pediatric labeling. However, our intent was not to conduct a clinical study, but rather to examine patterns of drug utilization in the expectation that questions would be generated for future study. As noted above, we believe the analysis of insurance claims data adds unique strengths to the study and permits us to answer questions that could not be addressed by a clinical study.
An obvious question raised by these results is why prescribers continue to utilize medications without FDA‐approved labeling or pediatric indications. Plausibly, pediatricians have grown accustomed to off‐label medication use for children. Additionally, while it is true that the evidence for safety is greater for drugs that have gone through labeling changes based on clinical trial data, the relationship between labeling and safety can be subtle. On the one hand, a drug that is “Labeled/Indicated” may be updated with safety concerns based on an improved knowledge base after it was originally labeled (ie, black box warning). On the other hand, one cannot infer that “Not Labeled” prescription drugs are unsafe. Some unlabeled (often off‐patent) drugs may have been used in children for years without significant adverse events, making continued use of such agents acceptable to prescribers.
Additionally, in reviewing the number of drugs with pediatric labeling, pediatric indications, and/or pediatric dosing recommendations, it is clear that a prescriber’s choice of agents might be significantly limited if only agents with labeling, indications, and dose recommendations were used. As highlighted by our data, this issue would be even more significant when prescribing antihypertensive agents for children younger than 6 years. We would speculate that prescribers continue to use unlabeled agents for specific clinical reasons, for example, intolerance of labeled agents or the need to use combination therapy. 18
Substantial efforts have been made to disseminate the data derived by FDAMA‐related studies, including publication of trial results in scientific journals, and publication of FDA analyses of trial data on the Internet. 19 Additionally, the most recent consensus guidelines (Fourth Report) for high blood pressure treatment in childhood recommend that prescribers only utilize drugs with pediatric efficacy and safety data, either in literature or in the label.10 Yet, this study shows that hypertensive children continue to receive medications that are neither labeled nor indicated for use in their age group.
Conclusions
The present study is a status report on the efforts to improve the availability of safe and effective antihypertensive medications for children. As data from the FDA and the scientific literature indicate, substantial progress has been made. More antihypertensive medications have been granted pediatric labeling and many clinical trial results have been published. Despite this progress, however, many hypertensive children are receiving antihypertensive medications that lack pediatric labeling or are not indicated for their age group. To fulfill the mandate of FDAMA, BPCA, and related legislation, a multipronged approach is needed. This could include performance of clinical trials focusing on the drugs still in use that lack pediatric labeling and an educational campaign to increase provider awareness of available label information and of the continued gaps in labeling, especially with respect to drugs used in the youngest hypertensive children. The results of such efforts would help ensure that children receive safe, effective, and age‐appropriate medications.
Sources of Funding: This study was performed by the Lewin Group, Inc, with funding by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Acknowledgments
Acknowledgements and disclosures: We appreciate the comments received from Jaclyn Marshall, MS, Carol Simon, PhD, Anjali Jain, MD , and Anne Zajicek, MD, PharmD , and the programming and analysis by Drew Braucht, Soumita Lahiri, and Nikolay Manolov . Pete Welch, Yang: Employees of The Lewin Group, which is a subsidiary of Ingenix, which is a subsidiary of UnitedHealth Group. Flynn: Consultant to the Lewin Group. Taylor‐Zapata: Employee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
- 1. Shirkey H. Therapeutic orphans. J Pediatr. 1968;72:119–120. [DOI] [PubMed] [Google Scholar]
- 2. Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290:905–911. [DOI] [PubMed] [Google Scholar]
- 3. Wells TG. Trials of antihypertensive therapies in children. Blood Press Monit. 1999;4:189–192. [PubMed] [Google Scholar]
- 4. Flynn JT. Successes and shortcomings of the Food and Drug Modernization Act. Am J Hypertens. 2003;16:889–891. [DOI] [PubMed] [Google Scholar]
- 5. Public Law 107‐109: Best Pharmaceuticals for Children Act, Public Law 107‐109: Best Pharmaceuticals for Children Act, (2002). http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM049874.pdf. Accessed April 3, 2012.
- 6. Public Law No: 108‐155: Pediatric Research Equity Act of 2003, (2003). http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM077853.pdf. Accessed April 3, 2012.
- 7. Benjamin DK Jr, Smith PB, Jadhav P, et al. Pediatric antihypertensive trial failures: analysis of end points and dose range. Hypertension. 2008;51:834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MedPAC . Report to the Congress: Improving Incentives in the Medicare Program . 2009 June 15. http://www.medpac.gov/documents/Jun09_EntireReport.pdf. Accessed April 3, 2012.
- 9. United States Food and Drug Administration . New Pediatric Labeling Information Database. http://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase. Accessed April 3, 2012.
- 10. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 11. Clintrial.gov, National Institutes of Health . Search results of: http://clinicaltrials.gov/ct2/results?term=nitroprusside. Accessed April 3, 2012.
- 12. Flynn JT, Meyers KE, Neto JP, et al. Efficacy and safety of the angiotensin receptor blocker valsartan in children with hypertension aged 1 to 5 years. Hypertension. 2008;52:222–228. [DOI] [PubMed] [Google Scholar]
- 13. Schaefer F, van de Walle J, Zurowska A, et al. Efficacy, safety and pharmacokinetics of candesartan cilexetil in hypertensive children from 1 to less than 6 years of age. J Hypertens. 2010;28:1083–1090. [DOI] [PubMed] [Google Scholar]
- 14. Bazzano AT, Mangione‐Smith R, Schonlau M, et al. Off‐label prescribing to children in the United States outpatient setting. Acad Pediatr. 2009;9:81–88. [DOI] [PubMed] [Google Scholar]
- 15. Din‐Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. [DOI] [PubMed] [Google Scholar]
- 16. Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874–879. [DOI] [PubMed] [Google Scholar]
- 17. Flynn JT, Falkner BE. Should the current approach to the evaluation and treatment of high blood pressure in children be changed? J Pediatr. 2009;155:157–158. [DOI] [PubMed] [Google Scholar]
- 18. Lande MB, Flynn JT. Treatment of hypertension in children and adolescents. Pediatr Nephrol. 2009;24:1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. United States Food and Drug Administration (FDA) . Medical, Statistical, and Clinical Pharmacology Reviews of Pediatric Studies Conducted under Section 505A and 505B of the Federal Food, Drug, and Cosmetic Act (the Act), as amended by the FDA Amendments Act of 2007 (FDAAA). http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm049872.htm. Accessed April 3, 2012.


