Abstract
Many carbohydrates pose advantages for tissue engineering applications due to their hydrophilicity, degradability, and availability of chemical groups for modification. For example, carboxymethylcellulose (CMC) is a water-soluble cellulose derivative that is degradable by cellulase. Though this enzyme is not synthesized by mammalian cells, cellulase and the fragments derived from CMC degradation are biocompatible. With this in mind, we created biocompatible, selectively degradable CMC-based hydrogels that are stable in routine culture, but degrade when exposed to exogenous cellulase. Solutions of CMC-methacrylate and polyethylene glycol dimethacrylate (PEG-DM) were co-crosslinked to form stable hydrogels; we found that greater CMC-methacrylate content resulted in increased gel swelling, protein diffusion and rates of degradation by cellulase, as well as decreased gel shear modulus. CMC-methacrylate/PEG-DM gels modified with the adhesive peptide RGD supported fibroblast adhesion and viability. We conclude that hydrogels based on CMC-methacrylate are suitable for bioengineering applications where selective degradability may be favorable, such as cell scaffolds or controlled release devices.
Keywords: hydrogel, natural biomaterial, carboxymethylcellulose, fibroblast adhesion
1. Introduction
Naturally-derived carbohydrate polymers have unique physical and biochemical properties advantageous for bioengineering applications. For example, agarose and alginate have enjoyed a long history of use in the molecular biology and cell encapsulation fields, respectively. Additionally, glycosaminoglycans such as heparin, chondroitin sulfate and hyaluronic acid have been selected to impart biological activity in tissue engineering scaffolds and can also function alone as active pharmaceutical agents. Cellulose derivatives such as carboxymethylcellulose (CMC) and hydroxypropylcellulose are biocompatible [1] and have been applied in drug delivery formulations [2–5] and as components of therapies for preventing postsurgical adhesions (e.g., Genzyme’s Seprafilm) [6–10]. Despite these successful applications and their very low cost, cellulose derivatives have been relatively underutilized in the bioengineering field [11–13].
Perhaps a reason why cellulose derivatives have not been selected for tissue engineering applications in particular is that they are not degradable by mammalian enzymes and therefore may not be suitable as a temporary tissue scaffold in vivo. However, we sought to take advantage of this aspect and predicted that CMC-based materials may be designed to be stable in routine mammalian cell culture, yet completely degradable when exposed to cellulase. Because cellulase and the fragments of degraded cellulose are non-toxic, hydrogels based on cellulose derivatives could pose unique advantages as selectively degradable components in an engineered cell scaffold or controlled release device. This approach would provide an alternative to existing processable materials such as those with reversible crosslinks (e.g., ionic crosslinking in alginate) or polymers that undergo phase transition when exposed to changes in temperature or an electric field.
Here we describe the synthesis and characterization of CMC-methacrylate hydrogel cell scaffolds. We present a photopolymerization crosslinking chemistry (Figure 1) to render stable CMC hydrogels with controllable degradation rates, degrees of swelling, mechanical properties, as well as the ability to support cell adhesion and growth.
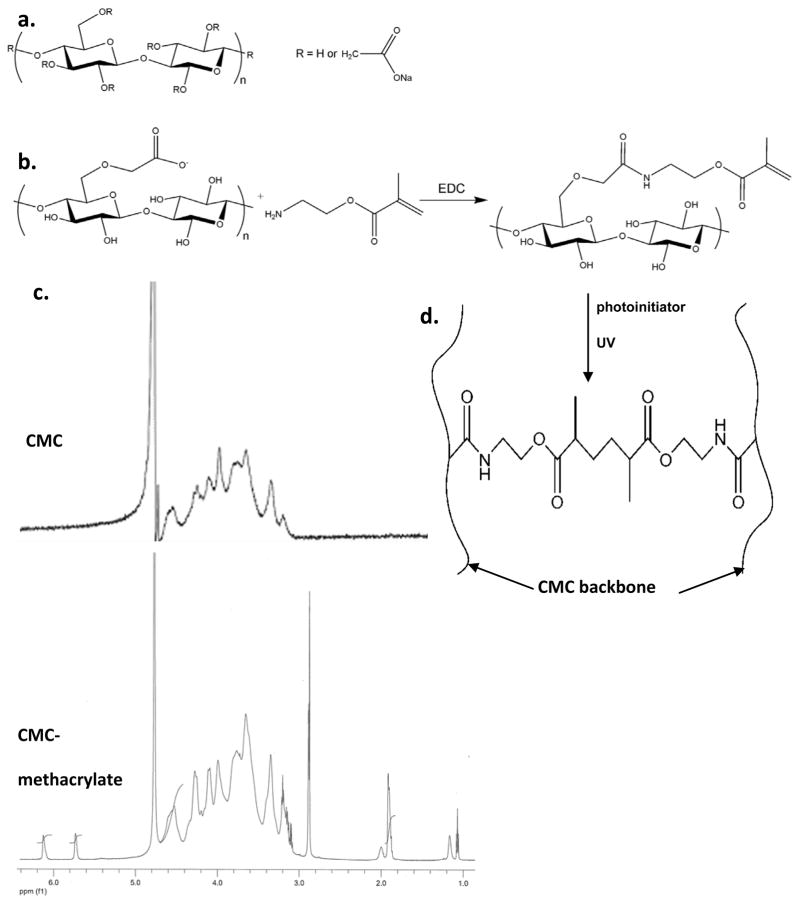
Figure 1.
CMC (a) is a derivative of cellulose that has been modified with carboxymethyl groups to render the polymer soluble in aqueous solutions. In this work, the degrees of substitution (moles carboxymethyl groups per mol of cellulose disaccharide) were 0.7 and 0.9 for 90 and 700 kDa CMC, respectively. CMC-methacrylate was synthesized by an EDC-mediated reaction between CMC and aminoethyl methacrylate (b). 1H-NMR analysis (c) indicates ~15% substitution of methacrylate groups per CMC disaccharide repeat. CMC-methacrylate was then photopolymerized to form crosslinked networks (d).
2. Results and Discussion
2.1. Impact of CMC-methacrylate molecular weight on gel synthesis
Initial studies focused on comparing the effects of CMC-methacrylate molecular weight and concentration on three key outcomes: gel transparency (to allow subsequent analysis of cells cultured in or on the gels using fluorescence microscopy), polymer solubility in aqueous solution, and polymer sterilization. Table 1 reports the compositions of CMC-methacrylate gels explored and their relative transparencies. Hydrogel transparency was judged by eye and was found to decrease with increasing CMC-methacrylate content relative to PEG-DM. While 0.5–1% (w/v) solutions of 700 kDa CMC-methacrylate formed transparent gels upon crosslinking, these solutions were too viscous to filter-sterilize. (Other sterilization methods, such as irradiation or autoclaving were avoided due to the potential for polymer degradation.) Solutions of 90 kDa CMC-methacrylate formed stable hydrogels only when copolymerized with PEG-DM and were readily filter-sterilized. Therefore, compared to 700 kDa CMC-methacrylate, 90 kDa CMC-methacrylate is more feasible for cell scaffolding applications and was selected to be the focus of the remaining characterizations.
Table 1.
Compositions of CMC-methacrylate gels and their relative transparency.
| CMC-methacrylate MW (kDa) | Total % copolymera | % CMC methacrylatea | % PEG- DMa | Transparencyb |
|---|---|---|---|---|
| 700 | 0.5 | 0.5 | 0 | +++ |
| 1.0 | 1.0 | 0 | +++ | |
| 90 | 12 | 5 | 7 | + |
| 8 | 4 | +/− | ||
| 20 | 8 | 12 | ++ | |
| 12 | 8 | + | ||
| 16 | 4 | +/− | ||
Percentages are based on weight of polymer per volume of gelation solution prior to crosslinking
Symbols note relative transparency: + transparent, +/− slightly opaque, − opaque
2.2. CMC-methacrylate:PEG-DM ratio determines hydrogel physical properties
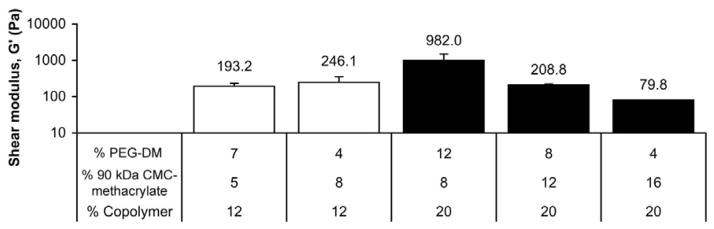
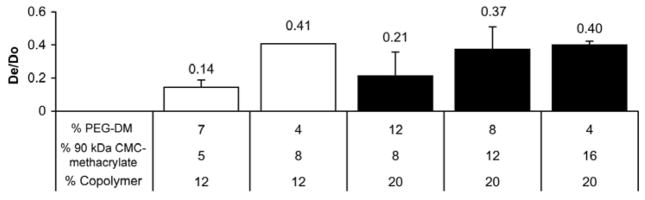
The physical characterization of CMC-methacrylate based hydrogels revealed that gels with increased CMC-methacrylate content are generally associated with decreased gel stiffness, decreased crosslink density, and increased rates of degradation in cellulase. We found that gels with higher concentrations of CMC-methacrylate were associated with lower shear moduli for 20% copolymer gels but had no significant effect for the 12% copolymer gels tested (Figure 2). Comparisons between gels of equal CMC-methacrylate or PEG-DM concentration give additional insight into the effect of each polymer on shear modulus: for gels with 8% CMC-methacrylate, increased PEG-DM content resulted in increased shear modulus, whereas for gels with 4% PEG-DM, increased CMC-methacrylate resulted in decreased shear modulus. Swelling studies indicated that both CMC-methacrylate content and total copolymer concentration impacted the effective crosslink density (Figure 3): 20% copolymer gels showed lower swelling (higher crosslink density) than 12% copolymer gels and for both copolymer densities, swelling ratios increased with increased with CMC-methacrylate content. Similarly, gels with greater CMC-methacrylate content were associated with increased effective diffusivities of BSA (Figure 4).
Figure 2.
The shear modulus (G′) was determined for gels synthesized from various amounts of CMC-methacrylate (90 kDa) and PEG-DM with a total copolymer % (% CMC-methacrylate plus % PEG-DM) of 12 or 20 (n = 3–4).
Figure 3.
Hydrogel swelling ratios indicate that gels with increased % copolymer or increased PEG-DM content are associated with gels of increased crosslinking density. All pair-wise differences between gel types are statistically significant except between 16:4% CMC-methacrylate:% PEG-DM and either 5:7 or 12:8 (n = 3).
Figure 4.
BSA release rates were determined in order to calculate the effective diffusion coefficient, De, for each gel type. De was normalized by the diffusion coefficient of BSA in water at 37 °C (Do), 9.14 × 10−7 cm2/s [14]. Differences are significant between 5:7% CMC-methacrylate:% PEG-DM and 8:4, 12:8 or 16:4 gels (n = 4–5).
Disparities in polymer structure and location of crosslinking sites may account for how these polymers differentially impact gel properties. For example, the polymers have distinct differences in crosslinking site density and location: CMC-methacrylate has reactive sites along its backbone and likely lacks methacrylates at the termini, whereas PEG-DM has reactive sites only at the polymer termini. A rough comparison of polymer molecular weight between crosslinking sites indicates that CMC-methacrylate has ~4× more polymer between crosslinking sites. These findings indicate that CMC-methacrylate content in copolymer gels may serve to decrease the effective crosslink density to yield softer and more hydrated gels while keeping total polymer concentration constant.
2.3. CMC-methacrylate content impacts enzymatic degradation of copolymer gels
Studies of hydrogel degradation in cellulase revealed three significant findings. First, the ratio of CMC-methacrylate to PEG-DM can be selected to yield gels that are either completely degradable or only partially degradable. For example, Figure 5a contrasts the weight loss of two gels with equal copolymer density (16%) but disparate ratios of % CMC-methacrylate: % PEG-DM (6:10 and 10:6). In this case, gels with greater CMC-methacrylate content degrade completely within a day, whereas the weight loss of gels with greater PEG-DM content leveled off to about 50% of their original weight in the same time period. All of the gel compositions presented in Table 1 yielded gels that degraded completely (100% weight loss) within 24 h in 0.02 U/mL cellulase. These results suggest that there may be some critical CMC-methacrylate:PEG-DM ratio above which the CMC-methacrylate plays an integral role in the gel structure and yields gels that can degrade completely; below the critical ratio results in sufficient nondegradable PEG-PEG crosslinks that allow the gel to remain intact even after the CMC-methacrylate has completely degraded. For example, 5:7 and 8:12 gels are composed of ~40% CMC-methacrylate by dry weight (~60% PEG-DM) and are completely degradable, whereas 6:10 gels are composed of 37.5% CMC-methacrylate by dry weight and are only partially degradable.
Figure 5.
The degradation rate of 90 kDa CMC-methacrylate/PEG-DM gels was determined by weighing the gels during incubation in cellulase. (a) Representative percent weight loss vs time data for 16% copolymer gels with varying CMC composition during incubation in 0.02 U/mL cellulase. (b) Degradation rates in 0.02 U/mL cellulase for gels of varying CMC-methacrylate and PEG-DM content. Differences are statistically significant between 5:7% CMC-methacrylate:% PEG-DM and 8:4, 12:8 or 16:4 gels (n = 4). (c) Degradation rates for 12% copolymer gels in response to 0.02–2.0 U/ml cellulase (n = 3–4). No degradation was measured for CMC-methacrylate gels incubated in buffer alone.
Second, CMC-methacrylate content impacts gel degradation rate: gels with increased concentrations of CMC-methacrylate were associated with increased degradation rates (Figure 5b). Lastly, gel weight did not change in buffer solutions lacking cellulase (negative control) and degradation rates correlate with cellulase concentration (Figure 5c), indicating that the degradation over the examined timeframe (~days) proceeded primarily by an enzymatically-mediated reaction rather than, for example, hydrolysis or polymer dissolution.
Taken together, the degradation results suggest that the activity of cellulase is effective for degrading CMC-methacrylate, but not PEG-DM. Moreover, as CMC-methacrylate content is increased, CMC plays a larger role in the gel structure, and the gel becomes more susceptible to enzymatic attack. Recent reports of photocrosslinked alginate hydrogels synthesized by a very similar chemistry for cartilage tissue engineering describe that aminoethyl-methacrylate linkages in this system are hydrolytically degradable with complete gel degradation occurring after several weeks in culture [15,16]. While our studies herein were limited to short term analyses (~days), it is likely that slow hydrolytic degradation may occur in the CMC-methacrylate system as well. Thus, the tunable degradation of these gels may provide advantages for bioengineering applications where tailored spatial or temporal degradation may be favorable.
2.4. CMC-methacrylate/PEG-DM gels support cell adhesion and viability
To determine whether the materials are suitable as cell scaffolds, we quantified the adhesion and viability of a model cell type, Balb/3T3 fibroblasts, on RGD-modified 12:8% CMC-methacrylate/% PEG-DM hydrogels (Figure 6). We selected this composition as a representative gel type that is transparent and fully degradable.
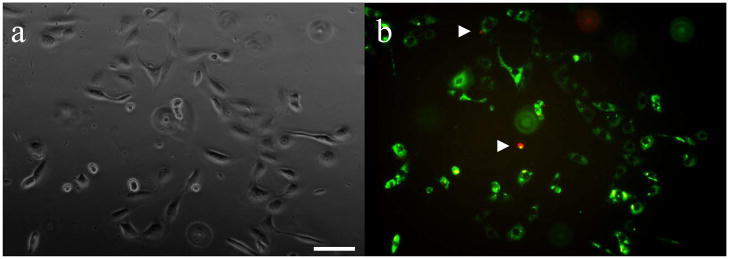
Figure 6.
The morphology and viability of fibroblasts cultured for 24 h on RGD-modified 12:8% CMC-methacrylate/% PEG-DM gel substrates. Fibroblasts adherent to the gel surface imaged under (a) phase contrast to view cell morphology and (b) fluorescence microscopy to analyze the viability of cells stained with calcein (green) to label live cells and ethidium homodimer-1 (red; arrowheads) to label dead cells. Scale, 100 μm.
After one day in culture, we found that 82.7 ± 4.4% of attached cells were viable. The cells were well spread and demonstrate a typical fibroblastic-type morphology. These results demonstrate that the CMC-methacrylate based gels support fibroblast adhesion and viability, and preliminary studies indicate that Balb/3T3 cells remain viable following incorporation into the polymer solution and encapsulation within the gels during crosslinking (data not shown); therefore future work in our laboratory focuses on applying these materials as selectively degradable cell adhesive scaffolds for in vitro cell assays.
3. Experimental Section
3.1. Materials
CMC sodium salt (90 and 700 kDa; Figure 1a) and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Aminoethyl methacrylamide hydrochloride (AEM), N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide (EDC), and cellulase from Aspergillus niger (1.31 U/mg) were purchased from Fisher Scientific (Pittsburg, PA).
3.2. Synthesis of CMC-methacrylate
To manufacture crosslinked CMC-based hydrogels, CMC was first modified with photocrosslinkable methacrylate groups (Figure 1b). Solutions of 0.5% CMC in 50 mM sodium bicarbonate buffer (pH 8.5) were prepared and then dry AEM was added at a 2-molar excess relative to the number of CMC carboxylate groups. The pH was readjusted to 8.5 using 1 M NaOH. Dry EDC (equimolar to AEM) was then added to the CMC-AEM solution. The reaction was stirred in the dark for 2 h at room temperature and then the AEM and EDC reactants were added again as above and allowed to react for an additional 2 h.
The CMC-methacrylate product was purified by precipitation and dialysis. For 700 kDa CMC-methacrylate, the product was precipitated in acetone, collected on a glass stir rod and redissolved in deionized water. For 90 kDa CMC-methacrylate, the product was precipitated in acetone, centrifuged, and the precipitate pellet was redissolved in deionized water. The CMC-methacrylate solution was dialyzed against deionized water for at least 24 h in the dark. The CMC-methacrylate was lyophilized and stored desiccated in the dark at 4 °C.
The modification of CMC with methacrylate groups was verified by 1H-NMR (Varian Inova-600 mHz spectrometer) in D2O: 1.92 (AEM CH3), 3.1–4.5 (CMC), 5.73 and 6.13 (AEM =CH) (Figure 1c). Peak integration suggests that the degree of methacrylate substitution is ~15% relative to the glucose disaccharide repeat in CMC.
3.3. Synthesis of PEG-DM
The properties of CMC-methacrylate hydrogels were modified by the addition of polyethylene glycol-dimethacrylate (PEG-DM) to the gelation solution. The methacrylate groups of CMC-methacrylate and PEG-DM crosslink together during the photocrosslinking reaction. PEG-DM (10 g batch) was prepared using the following method. Adsorbed water was removed from PEG (3400 MW) using azeotropic distillation in toluene (4 mL/g PEG) and then the PEG was concentrated with a rotary evaporator and dried overnight under vacuum. Under an N2 or Ar purge, dry pyridine (3 mL/g PEG) was added to dissolve the PEG and the solution was cooled to ~0 °C in an ice bath. Methacrylic anhydride (4 molar excess to the moles of PEG) was added slowly with a syringe while mixing the PEG/pyridine solution. The reaction was mixed in the ice bath for ~10 min and then removed from the bath and allowed to react for ~48 h in the dark at room temperature. Working in minimal light, the reaction was diluted ~10–15× with dichloromethane and washed twice with 1 M HCl. (Acid neutralizes the pyridine and basic reaction byproducts.) The DCM/PEG-DM solution was then dried with sodium sulfate, filtered, concentrated with a rotary evaporator to ~50 mL, precipitated in ice-cold ether, filtered, and dried overnight under vacuum in the dark at room temperature. The PEG-DM product was stored desiccated and in the dark at −20 °C.
Percent methacrylation of PEG-DM was determined by 1H-NMR (JEOL ECX 400MHz spectrometer; UMBC NMR Facility) in deuterated dichloromethane: 1.9 (methacrylate CH3), 3.4–3.6 (PEG), 4.2–4.3 (methacrylate CH2), 5.5 and 6.0 (methacrylate =CH on PEG-DM), 5.65 and 6.2 (unreacted methacrylic acid =CH). Peak integration indicates that the degree of methacrylate substitution is ~70% relative to the PEG terminal hydroxyl groups.
3.4. Photocrosslinking of CMC hydrogels
CMC-methacrylate gels and CMC-methacrylate/PEG-DM copolymer gels were synthesized by photo-induced free radical crosslinking (Figure 1d). Hydrogel compositions studied in this work are listed in Table 1. CMC-methacrylate and PEG-DM (if used) were dissolved in phosphate-buffered saline (PBS) containing the photoinitiator Irgacure 2959 (0.05%; Ciba, Basel, Switzerland). The gelation solutions were then exposed to ultraviolet (UV) light (365 nm, 1–5 min; UVP XX15BLB lamp).
3.5. Rheological characterization of hydrogel shear modulus
We characterized the hydrogel shear modulus with standard rheology tests. The gels were synthesized by the method described above with the exception that the gelation solutions were crosslinked in a standard 12-well tissue culture plate (0.5 mL solution per well). The hydrogels were swollen in PBS overnight and then cut to 25-mm diameter discs using an Ateco cookie cutter (August Thomsen Corp., Glen Cove, NY). The hydrogel shear modulus was measured using an AR 2000ex rheometer (TA Instruments, New Castle, DE) with 25-mm parallel plate geometry set to 25 °C and an oscillatory frequency of 1.0 rad/s, fixed stress of 50 Pa, and normal force of less than 0.5 N.
3.6. Swelling ratio
To determine the hydrogels’ relative degree of crosslinking, we measured the swelling ratio based on mass for each gel composition [17]. Lower swelling ratio values are associated with gels of higher degrees of crosslinking. The hydrogels (0.1 mL) were synthesized in 8-mm diameter, 2-mm deep rubber molds (perfusion chambers; Sigma-Aldrich). The gels were swollen in 1–2 mL PBS overnight and the swollen mass was determined (Ms). The dry weight (Md) of each sample was determined after lyophilization. The swelling ratio was calculated as Ms/Md.
3.7. Release of BSA
Trends in relative degrees of crosslinking observed from the swelling ratio measurements were complimented by comparing the effective diffusivity (De) of bovine serum albumin (BSA) for each gel composition [18]. Lower De values are associated with gels of higher degrees of crosslinking. To carry out the BSA release studies, 1% BSA was dissolved in the gelation solution and gels were crosslinked in 8-mm diameter, 2-mm deep molds. The gels (0.1 mL) were then transferred to 15 mL of 37 °C PBS containing 0.1% of the preservative methylparaben. The solutions were mixed during incubation at 37 °C. At time points up to 8 h, 1-mL samples were collected and replaced with fresh 37 °C PBS. The sample BSA concentrations were determined with a standard Bradford-type protein assay (Bio-Rad, Hercules, CA). The experiment was designed such that the volume of the release solutions was ~300× the volume of the gels to fit the assumptions of an infinite slab, an infinite open system, and near-zero boundary conditions (>20× greater fluid volume than that of the release matrix) [18,19]. Knowing the mass of BSA released at each time-point, De was calculated using previously described methods [18–20]. Briefly, BSA-loaded CMC-methacrylate based gels are homogeneous matrix controlled release systems, which can be described by Fick’s law of diffusion where 2δ is the hydrogel thickness. Using the appropriate boundary conditions and simplifying for short release times (fractional release or mass released at time t divided by the mass released at time infinity, Mt/M∞ < 0.6), Fick’s law gives
Thus, De was calculated from the slope of the BSA release data plotted as Mt/M∞ versus t1/2. M∞ was determined in separate experiments carried out for several days to confirm complete BSA release.
3.8. In vitro enzymatic degradation
Hydrogel degradation rates were measured during incubation in cellulase solutions. Hydrogels were crosslinked in 8-mm diameter, 2-mm deep molds and equilibrated overnight at room temperature in citrate-phosphate buffer (0.1 M citric acid, 0.2 M Na2HPO4·7H2O, pH 5.0). Cellulase from A. niger is active from pH 3–8 with a maximum activity at pH 5.0 [21]. The pH of maximum activity was chosen for these experiments to ensure enzymatic stability over the course of the experiment. The gels (0.1 mL) were removed from the buffer, carefully blotted to remove excess liquid and the gel masses were determined. Solutions of citrate-phosphate buffer containing cellulase (0.02–20 U/mL) were added to each gel and incubated at 37 °C with mixing. Every 1–2 h, the cellulase solution was removed; the gels were blotted, weighed, and returned to fresh 37 °C cellulase solution. The half-life of cellulase exceeds the longest incubation time (i.e., 2 h) used in these experiments [21]. Negative controls consisted of gels incubated in citrate-phosphate buffer alone (no enzyme).
3.9. Gel biocompatibility and support of mammalian cell adhesion
To determine whether the CMC-methacrylate based gels have potential for cell culture and tissue engineering applications, fibroblasts were cultured on hydrogels covalently modified with the fibronectin-derived peptide, RGD. Before crosslinking, the CMC-methacrylate and PEG-DM were sterilized in separate procedures: dilute solutions of CMC-methacrylate (90 kDa) were sterilized by dissolving in deionized water, filtering through a syringe filter for viscous solutions (0.8/0.2 μm Supor Acrodisc PF, Pall Gelman, Ann Arbor, MI) into sterile rubber-capped serum vials and dried in a DuraDry/DuraStop μP stoppering tray lyophilizer (FTS Systems, Stone Ridge, NY). Concentrated solutions of PEG-DM in PBS were similarly filter-sterilized and stored as a sterile solution in the dark at 4 °C.
Hydrogels were rendered cell-adhesive by incorporating RGD peptides into the gel structure using previously reported methods [22,23]. Briefly, acryloyl-PEG-RGDS was synthesized by mixing RGDS (Fisher) and acryloyl-PEG-N-hydroxysuccinimide (acryloyl-PEG-NHS; 3400 Da; Nektar Therapeutics, Huntsville, AL) at a 1:1 molar ratio in 50 mM sodium bicarbonate buffer (pH 8.5). Acryloyl-PEG-NHS was roughly 10 mg/ml in the reaction. The reaction was mixed in the dark at room temperature for 2 h, and then dialyzed against deionized water for 24 h before lyophilization. The reaction was confirmed to proceed to ~100% with MALDI-TOF mass spectrometry (Bruker Daltonics; UMBC Mass Spectrometry Facility). Acryloyl-PEG-RGD was added to the PEG-DM solutions prior to sterilization.
Sterile gelation solutions of 12:8% CMC-methacrylate:% PEG-DM were prepared with 3.0 μM acryloyl-PEG-RGD and 0.05% Irgacure 2959 in PBS. To prepare a cell culture substrate, gelation solution (0.8–1.0 mL) was pipetted onto a 12-mm glass coverslip, smoothed with a syringe needle bent to a 90° angle so that the coverslip was completely coated with gelation solution and then crosslinked under UV light. Each gel/coverslip assembly (4 total per gel type) was transferred to a 12-well plate and seeded with Balb/3T3 fibroblasts (ATCC, Manassas, VA) at 25,000 cells/well in high glucose Dulbecco’s Modified Eagle’s Medium (Hyclone, Logan, UT) with 10% bovine calf serum, 40 mM L-glutamine (ATCC), 100 u/mL penicillin (MP Biomedicals, Solon, OH), and 100 μg/mL streptomycin (MP Biomedicals). After 24 h in culture, the cells were stained with the LIVE/DEAD viability/cytotoxicity assay (Invitrogen, Carlsbad, CA) and imaged (3 fields of view per sample) using an Olympus IX-81 microscope. Cell densities were quantified from phase contrast images. Balb/3T3 fibroblasts have a doubling time of ~1 d [24], so we chose to analyze cell density at 24 h to minimize the influence of proliferation on cell density measurements.
3.10. Statistical analysis
We performed ANOVA and pair-wise Student’s t-tests to determine the statistical significance of the differences between results. A significance level of p < 0.05 was used as the cutoff (i.e., p values are reported only for cases in which p < 0.05).
4. Conclusions
In conclusion, we report a simple method for synthesizing a selectively degradable hydrogel system that presents unique advantages for bioengineering applications requiring soft hydrogels with tunable physical and biochemical properties. We found that greater CMC-methacrylate content in the copolymer gels increases swelling, diffusion of a model protein and the rate of enzymatic degradation while decreasing gel shear modulus. Moreover, the CMC-methacrylate based gels supported cell viability and adhesion, which suggests that these uniquely processable gels may be suited to tissue engineering applications where selective degradability may be required.
Acknowledgments
This work was funded by the UMBC College of Engineering and Information Technology and the Henry Luce Foundation; CMC 1H-NMR analysis was carried out at the University of Georgia Complex Carbohydrate Research Center, which is supported in part by the Department of Energy-funded (DE-FG09-93ER-20097) Center for Plant and Microbial Complex Carbohydrates.
Footnotes
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Contributor Information
Robert Reeves, Email: reeves1@umbc.edu.
Andreia Ribeiro, Email: andreia1@umbc.edu.
Leonard Lombardo, Email: llom1@umbc.edu.
Richard Boyer, Email: richboy1@umbc.edu.
References
- 1.Miyamoto T, Takahashi S, Ito H, Inagaki H, Noishiki Y. Tissue biocompatibility of cellulose and its derivatives. J Biomed Mater Res. 1989;23:125–133. doi: 10.1002/jbm.820230110. [DOI] [PubMed] [Google Scholar]
- 2.Barbucci R, Leone G, Vecchiullo A. Novel carboxymethylcellulose-based microporous hydrogels suitable for drug delivery. J Biomater Sci Polym Ed. 2004;15:607–619. doi: 10.1163/156856204323046870. [DOI] [PubMed] [Google Scholar]
- 3.Pal K, Banthia AK, Majumdar DK. Development of carboxymethyl cellulose acrylate for various biomedical applications. Biomed Mater. 2006;1:85–91. doi: 10.1088/1748-6041/1/2/006. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez R, Alvarez-Lorenzo C, Concheiro A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J Control Release. 2003;86:253–265. doi: 10.1016/s0168-3659(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 5.Tas C, Ozkan Y, Savaser A, Baykara T. In vitro release studies of chlorpheniramine maleate from gels prepared by different cellulose derivatives. Farmaco. 2003;58:605–611. doi: 10.1016/S0014-827X(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Yeo Y, Highley CB, Bellas E, Benitez CA, Kohane DS. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials. 2007;28:975–983. doi: 10.1016/j.biomaterials.2006.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach RE, Burns JW, Dawe EJ, SmithBarbour MD, Diamond MP. Reduction of postsurgical adhesion formation in the rabbit uterine horn model with use of hyaluronate/carboxymethylcellulose gel. Fertil Steril. 1998;69:415–418. doi: 10.1016/s0015-0282(97)00573-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Nho YC, Lim YM, Son TI. Prevention of surgical adhesions with barriers of carboxymethylcellulose and poly(ethylene glycol) hydrogels synthesized by irradiation. J Appl Polym Sci. 2005;96:1138–1145. [Google Scholar]
- 9.Liu LS, Berg RA. Adhesion barriers of carboxymethylcellulose and polyethylene oxide composite gels. J Biomed Mater Res. 2002;63:326–332. doi: 10.1002/jbm.10211. [DOI] [PubMed] [Google Scholar]
- 10.Tate MC, Shear DA, Hoffman SW, Stein DG, LaPlaca MC. Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials. 2001;22:1113–1123. doi: 10.1016/s0142-9612(00)00348-3. [DOI] [PubMed] [Google Scholar]
- 11.Coviello T, Matricardi P, Marianecci C, Alhaique F. Polysaccharide hydrogels for modified release formulations. J Control Release. 2007;119:5–24. doi: 10.1016/j.jconrel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 13.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Han JH, Krochta JM, Hsieh YL, Kurth MJ. Mechanism and characteristics of protein release from lactitol-based cross-linked hydrogel. J Agric Food Chem. 2000;48:5658–5665. doi: 10.1021/jf0002239. [DOI] [PubMed] [Google Scholar]
- 15.Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Jeon O, Powell C, Ahmed SM, Alsberg E. Biodegradable, Photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng A. 2010 doi: 10.1089/ten.TEA.2010.0096. in press. [DOI] [PubMed] [Google Scholar]
- 17.Leach JB, Bivens KA, Patrick CW, Jr, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: Natural biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 18.Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Crank J. The Mathematics of Diffusion. Oxford University Press; Oxford, UK: 1975. [Google Scholar]
- 20.Narasimhan B, Mallapragada SK, Peppas NA. Release kinetics, data interpretation. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Wiley Interscience; Hoboken, NJ, USA: 1999. pp. 921–935. [Google Scholar]
- 21.Siddiqui KS, Azhar MJ, Rashid MH, Rajoka MI. Stability and identification of active-site residues of carboxymethylcellulases from Aspergillus niger and Cellulomonas biazotea. Folia Microbiol. 1997;42:312–318. doi: 10.1007/BF02816941. [DOI] [PubMed] [Google Scholar]
- 22.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Leach JB, Bivens KA, Collins CN, Schmidt CE. Development of photocrosslinkable hyaluronic acid-polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res A. 2004;70:74–82. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- 24.Carrino D, Gershman H. Division of BALB/c mouse 3T3 and simian virus 40-transformed 3T3 cells in cellular aggregates. Proc Natl Acad Sci USA. 1977;74:3874–3878. doi: 10.1073/pnas.74.9.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]