Abstract
The goal of researchers and clinicians interested in re-instituting cell based therapies for PD is to develop an effective and safe surgical approach to replace dopamine (DA) in individuals suffering from Parkinson’s disease (PD). Worldwide clinical trials involving transplantation of embryonic DA neurons into individuals with PD have been discontinued because of the often devastating post-surgical side-effect known as graft-induced dyskinesia (GID). There have been many review articles published in recent years on this subject. There has been a tendency to promote single factors in the cause of GID. In this review, we contrast the pros and cons of multiple factors that have been suggested from clinical and/or preclinical observations, as well as novel factors not yet studied that may be involved with GID. It is our intention to provide a platform that might be instrumental in examining how individual factors that correlate with GID and/or striatal pathology might interact to give rise to dysfunctional circuit remodeling and aberrant motor output.
Keywords: Grafting, Dyskinesia, Parkinson’s disease, Plasticity, Synapses, Co-Neurotransmission
Grafting Offers Unrealized Hope to Those with PD
“It's not that I'm so smart, it's just that I stay with problems longer.” -Albert Einstein
There has long been interest in whether cells of the nervous system that die due to trauma or disease could be replaced by new ones. While very early attempts at brain cell grafting were unsuccessful, Dr. Elizabeth Dunn [1], at the turn of the twentieth century, showed that brain tissue grafted from one newborn rat to another newborn rat could survive transplantation. Despite this landmark discovery, little progress was made in the area of neural transplantation over the next fifty years. However, resurgent interest in the 1970s, particularly in the field of Parkinson’s disease, led to an explosion of preclinical research. Promise from animal studies in the field led to the initiation of clinical grafting trials. A number of small open-labeled clinical trials took place throughout the 1980s and 1990s. There was a lack of consensus over the optimal grafting paradigm to employ and, accordingly, these studies were quite variable in their outcomes. However, a significant number of studies were able to show unequivocal evidence of grafted cell survival, graft-derived neurite outgrowth and behavioral recovery (e.g.: [2–8]). These encouraging findings culminated in two large double-blind placebo controlled studies preformed in the late 1990s with the aim of confirming the efficacy of fetal tissue grafting for PD.
Unfortunately, both double-blind placebo control trials reported failure to find statistical significance in their primary behavioral endpoints at the end of the blinded phase of each study [9,10]. However, a recent follow-up report from the Denver/Columbia trial [9] showed that at 2 and 4 years post-transplantation there was continued and significant symptomatic improvement [11]. Despite the generalized disappointment from these double-blind studies, clinical trials involving transplantation of embryonic mesencephalic DA neurons have demonstrated that grafted neurons can survive long-term [11], innervate the denervated striatum [12–14], release DA [15], and become functionally integrated into host neural circuits [16]. Further, it is important to appreciate that clinical data indicate that a favorable therapeutic response to transplantation appears to be dependent on specific variable including the extent of loss of nigrostriatal dopaminergic terminals at pregraft baseline and age of the recipient [e.g.: 9, 11, 51]. Thus, the clinical efficacy is perhaps best viewed as variable rather than a failure, and a primary challenge to the employment of graft therapy for PD is in understanding how to develop strategies to allow all patients to respond favorably to engraftment of replacement DA neurons.
The Unexpected Side Effect: Graft-induced Dyskinesias
More disconcerting than a lack of consistent therapeutic efficacy of grafting is an ethical challenge related to a bothersome side-effect that was noted in a subpopulation of grafted patients in both placebo control trials, and one retrospective study. The side-effect has come to be known as graft-induced dyskinesias (GID), the cause is not known, and in contrast to levodopa-induced dyskinesias (LID), these behaviors cannot be ameliorated by lowering the dose of antiparkinsonian medications like levodopa. In the remainder of this manuscript, we detail the phenomenology that is GID, contrast it to LID, and examine possible mechanisms that may underlie GID eitiology. The ethical dilemma of this unforeseen challenge relates to the Latin phrase, often attributed to the Hippocratic oath, “primum non nocere” (“above all else, do no harm”). Indeed, it was the intermittent expression of the sometimes devastating GID side-effect that was the principal reason for the discontinuation of clinical trials worldwide, although a lack of a consistent beneficial effect was a contributing factor.
The post-surgical side effect of GID was first described in the Denver/Columbia placebo control, double-blind trial [9] with an additional incidence subsequently reported in a retrospective clinical report out of Sweden [17] and the second placebo control, double blind trial in the United States [10]. In these trials, 15 to 50% of trial participants developed abnormal dyskinesia with characteristics largely unique from the common drug-induced dyskinesias. Indeed, information available suggests that GID may represent a distinct neurological entity from the common drug-induced, levodopa-induced dyskinesia (LID) [18,19].
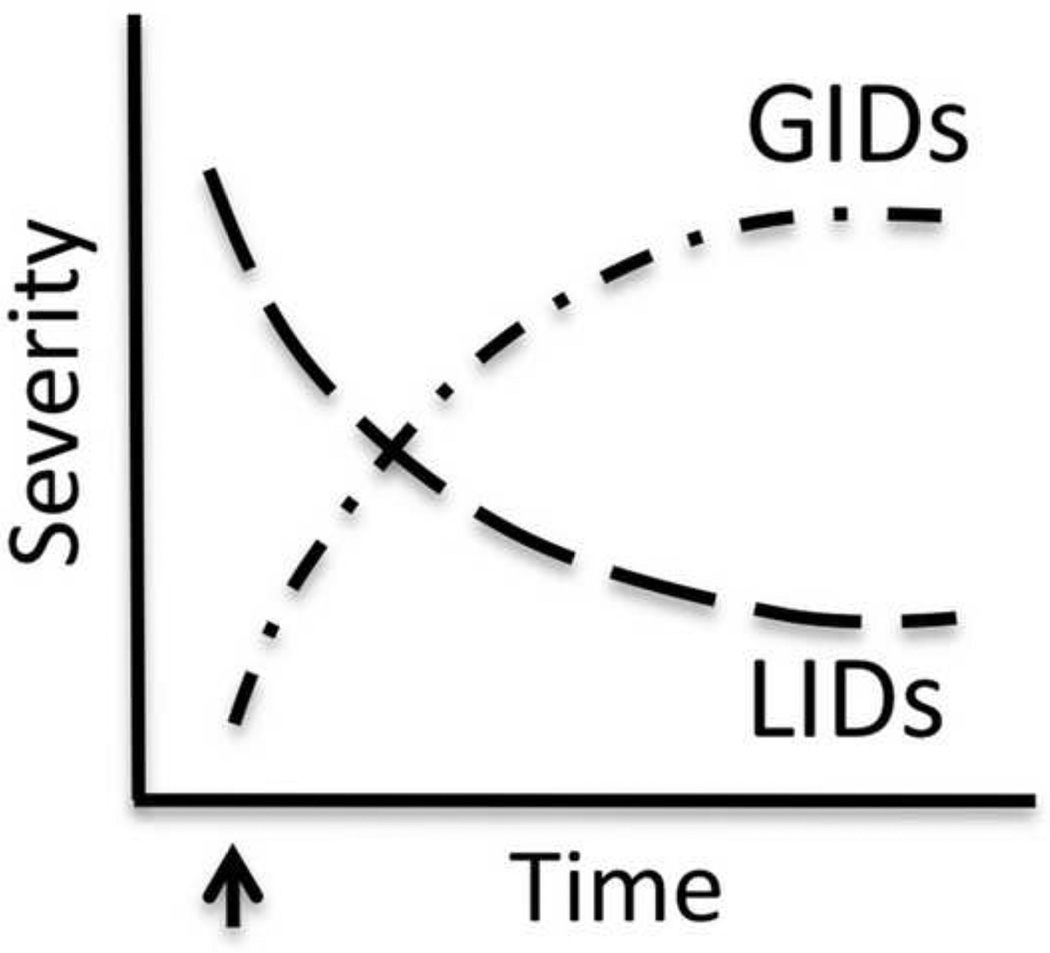
The idea that GID and LID may represent distinct neurological phenomena comes from several lines of evidence. First, the temporal expression of these two behaviors is disparate (Figure 1). In both humans [9,10,17] and animal models [19–21] the specific post-graft dyskinetic profile develops as the graft matures, and as the typical pre-graft profile of LID disappear, thus giving an inverse time-course of expression of these two behavioral phenotypes. Second, in contrast to the more widespread (e.g.: involving both upper and lower extremities), primarily dystonic and choreic appearance of drug-induced dyskinesias [22], GID bear some resemblance to biphasic drug-induced dyskinesias [18], often involving stereotypy and hyperkinesia, and localized to the upper or lower extremities [10, 17, 20]. Unlike LID, lowering the dose of levodopa does not provide relief from this troublesome side effect. In fact, GID in humans occurs primarily when plasma levodopa is low or absent. Interestingly, in both humans [9, 10, 17] and animal models [21], GID appear to be dopamine (DA) mediated despite the above mentioned paradox in patients. The pharmacology of GID will be detailed later in this review.
Figure 1. Differential time course of expression of levodopa-induced dyskinesias (LIDs) versus graft-induced dyskinesias (GID).
The arrow demarks the time of graft surgery. As the graft matures, the incidence of LID lessens and there is an emergence of GID behaviors, e.g.: [9,10,17,20,21].
Modeling Graft-induced Dyskinesias
In order to delineate the mechanisms responsible for the expression of aberrant behaviors following fetal tissue grafting in PD, research laboratories have created a rodent model of experimental GID. By utilizing rats rendered unilaterally parkinsonian via 6-hydroxydopamine (6-OHDA) delivery to the nigrostriatal pathway, researchers have found that aberrant behaviors similar to those seen in the clinic can be elicited in experimental animals following the delivery of a fetal mesencephalic tissue graft to the parkinsonian striatum.
The post-graft dyskinetic profiles expressed by animals are phenotypically similar to the GID observed in human patients. Specifically, similar to grafted patients (see [18] for review), in one of the rodent models (e.g.: the model where levodopa rather than amphetamine is used to induce the expression of experimental GID [19, 20]), experimental GID is expressed as focal, stereotypic and repetitive movements. These behaviors typically affect the upper limbs, head, mouth and tongue of the animal, and as depicted in Figure 1, these focal levodopa-elicited post-graft dyskinetic behaviors emerge as the typical, widespread pre-graft LID profiles decrease [20]. This specificity of body region and focal localization of these behaviors in grafted parkinsonian rats is comparable to the expression of GID in patients in the Denver-Columbia trial [9], whose GID were primarily restricted to the upper body, but different from those reported in the Tampa-Mount Sinai trial [10] in which GID primarily effected the extremities of the lower body. This discrepancy is more likely due to a variation in graft placement than a difference in biological mechanisms. As referenced above, another similarity to clinical GID in patients is the timing of experimental GID expression in rats. Like with human patients, these behaviors are not seen prior to grafting and develop as the grafted cells mature in the parkinsonian striatum (e.g.: [19,20,21,23,24]).
Although experimental models of GID are invaluable to our understanding of clinical GID, there are several discrepancies between the human and rodent phenomena that warrant attention. Most notably, experimental GID is elicited in animal models when plasma DA levels are elevated by pharmacological agents like levodopa or amphetamine [19–21, 23–25], while these behaviors are seen in grafted patients when patients are ‘off’ DAergic medication and plasma levodopa levels are low [9, 10, 17]. Based on the temporal and spatial expression of GID, it has been hypothesized that post-graft ‘off-medication’ dyskinesias represent a form of prolonged biphasic dyskinesia, i.e.: those that occur at the beginning and end of the levodopa cycle and tend to be expressed as focal, stereotypic and repetitive movements [26]. In Figure 2 we pose the question of whether the difference in amount of tissue typically grafted into patients with PD versus rats with experimental parkinsonism might impact how pharmacological agents interact with the grafted striatum to produce clinical versus experimental GID behaviors, respectively. Briefly, because of the relative small amount of tissue that has generally been grafted in the rat brain, it is possible that there is need for a DA agonist to “push” the extracellular levels of DA up into the concentration range that would induce a biphasic-like dyskinesia and give rise to expression of the experimental GID phenotype. In contrast, in PD patients, the relatively larger quantity of tissue that has generally been grafted may, as levodopa concentrations are waning or absent, reduce striatal DA concentrations into the concentration range that provokes a biphasic-like dyskinesia phenotype (more detail is given in Figure 2). Importantly, experimental GID can be observed in grafted rats while off levodopa, however, they occur at such random intervals as to make their systematic evaluation impossible.
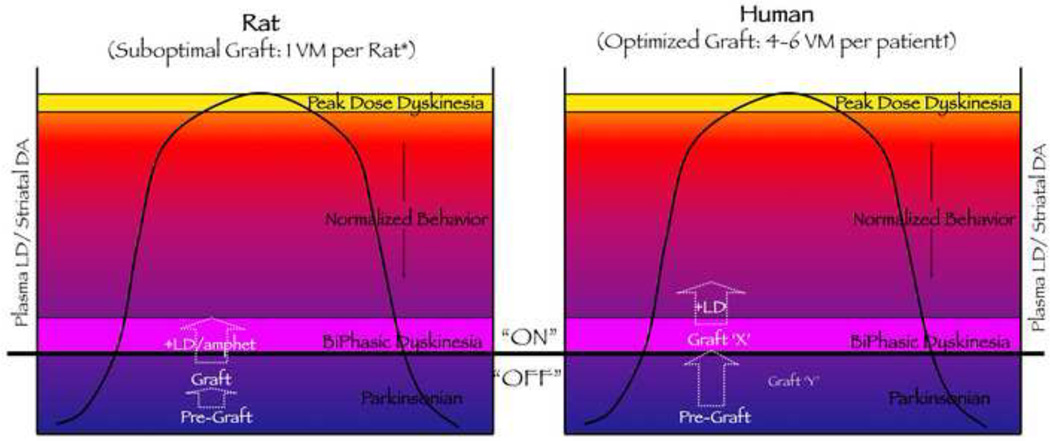
Figure 2. Modeling Graft-induced Dyskinesia: Does Graft Size Matter?
This diagram is used to consider whether it is it possible that the unique post-graft dyskinetic behaviors noted in either parkinsonian rats or humans are differentially responsive to dopamine replacement therapy by virtue of graft size. In considering this, it is noteworthy that in the Denver/Columbia clinical trial after transplantation, the increase in overall putamenal PET 18F-DOPA signal in the GID-expressing patient group was twice that of the GID-negative group at 12 months (P < 0.03) and almost three times larger at 24 months (P < 0.005) [27]. Despite the 18F-DOPA signal being 3 times higher in the GID expressing patients, it was still below the level of the normal striatum by about 20% [27]. It is interesting to speculate whether the GID-expressing patient group could be represented by Graft ‘X’ and the GID-negative group represented by Graft ‘Y’ (both Graft ‘X’ and ‘Y’ appear in the right hand panel of graph). If one compares the amount of tissue that has been grafted into the parkinsonian rat in models examining GID-like behavior, there is substantially less tissue proportionately grafted in the rodent models compared to most human trials. Rat Model: [20]: 1 ventral mesencephalon (VM) with postmortem TH+ cell number = 2,800; [21]: < 1 VM with postmortem TH+ cell number = 280 (small grafts) or 17,408 (large grafts). Human Grafting: [9]: noodles; postmortem TH+ cell number = 62,507; [10]: 1–4 VM per patient with 70,000–120,000 TH+ cells per side; [12,13]: 6 VM per patient; postmortem TH+ cell number= 210,067; [17]: 6.3 ± 2.8 VM per patient, TH+ number not available. Thus, is it possible that the smaller number of grafted DA neurons in the rat brain requires a DA agonist to “push” the levels of DA into the biphasic dyskinesia range to elicit expression of GID? The case presented here is undoubtedly an oversimplification in that it does not account for potential differences in DA neuron biology between rodents and humans, the degree of DA neuron survival or neurite outgrowth, and the relative striatal volume influenced by grafts in the two situations. If one just looks at the number of DA neurons grafted per volume of the striatum between rat and human, such calculations remain equivocal in answering this question. For example, the human putamen (common clinical grafting site) can be estimated to be approximately 100× larger than the rat striatum [169–171, 174–175], and there are approximately 10× more DA neurons in the human SN compared to the rat SN [166–168, 172–173]. Based on such estimates grafting 1 ventral mesencephalon into a rat striatum would result in approximately 10,000 DA cells /0.06 cm3 or 166,666 DA cells/cm3, which was the paradigm done by [20, 21] and one in which LD or amphetamine is apparently necessary to “push” striatal DA to levels that induce reliable experimental GID. In contrast, grafting 6 VM into the human striatum/putamen as was done by [17] would result in approximately 600,000 DA cells/ 6.25 cm3 or 96,000 DA cells/cm3, a scenario where clinical GID are expressed “OFF” LD. In these two example comparisons the density of DA neurons/cm3 in the human striatum/putamen would be approximately 60% of that found in the rat striatum (assuming equal degree of DA neuron survival). However, this comparison would be accurate only if the grafted cells spread uniformly through the entire “striatum”. It is well documented that grafted DA neurons remain largely confined within and immediately surrounding the injection site [e.g.; 20,21,10,13], with the bolus of grafted neurons giving rise to neurites that extend into the target striatum, varying in density and distance. Thus, factors including neurite outgrowth and degree of regional DA replacement need to be considered when pondering the question above, which is suggested by the behavioral and pharmacological differences associated with the model and the patient. Abbreviations: 1) “ON” and “OFF” refer to the relationship with the intake of levodopa medication, i.e.; on-medication and off-medication. 2) The Y-axis label “Plasma LD/Striatal DA” refers to the plasma concentration of levodopa (LD) and the likely corresponding striatal concentration of DA, with lowest levels represented at the X–Y axis intercept, and higher levels at the top of the Y-axis. 3) +LD or +LD/amphet refers to administration of the drug LD or amphetamine to grafted subjects.
While there are these discrepancies in post-graft dyskinesias between patients and rodent models, in this review, we will refer to dyskinetic behaviors in the rat that fulfill the characteristics of “not seen prior to grafting, or worsening/emerging in the presence of a mesencephalic graft” as “experimental GID”. As appropriate, we will further define whether we are referring to these post-graft dyskinesias occurring in patients, or an animal model that requires induction by a DA agonist. It is important to point out that in the model where experimental GID is elicited by levodopa (e.g.: [19,20,25]), it is inappropriate to refer to these as LID without additional qualifiers. Specifically, the time course of expression is inverse for expression of these post-graft experimental GID phenotypes elicited by levodopa compared to the typical pre-graft LID. Further, these levodopa-elicited experimental GID behaviors show differential regulation of the transcription factor fosB/ΔfosB from the pre-graft LID profiles, and the outward phenotype is different [20].
Solving the Mystery of Graft-induced Dyskinesias: Theories and Hypotheses
It has been 10 years since GID were first reported in the literature. In the past decade numerous theories have been proposed and hypotheses tested in an attempt to better understand possible mechanisms of GID. Broadly speaking, there are two sources of factors that could underlie GID development: 1) the graft itself, and 2) the host brain. Within these two categories, multiple factors have been considered to be of interest based principally on correlation with clinical findings and/or strong biological rationale.
-
“The Graft”:
-
◦
Pattern of Reinnervation/Degree of DA Replacement
-
◦
Preparation of Tissue (stored versus fresh; solid versus suspension)
-
◦
Non-DA cells/Serotonin cells
-
◦
-
“The Host”:
-
◦
Immune Response
-
◦
Age of Graft Recipient
-
◦
Disease Severity
-
◦
Pre-graft Levodopa History (e.g.: “priming”):
-
◦
Abnormal Target Plasticity
-
◦
Indeed, several of these as well as other factors have been evaluated in clinical studies and/or tested in animal models, with some appearing to be more promising for their involvement in GID than others. However, it is likely that no single factor in isolation will be found to be responsible for GID development. It is beyond the scope of this review to do an extensive recitation of what is known about all factors considered of interest in GID, and numerous reviews have already provided such discussion. In this review, we attempt to provide comparison of studies exploring some of the factors that we consider the most provocative of those bulleted above. We also introduce new ideas regarding how these mechanisms may interact to alter neuronal circuitry in the parkinsonian, grafted striatum to produce the unwanted behavioral output known as GID. Significantly, clinical grafting in PD patients is now being re-visited in a European trial entitled “Transeuro” (http://www.transeuro.org.uk, December 2011). “Transeuro” is hoping to develop an effective and safe method to treat individuals with PD using fetal cell based therapy, guided by what is known about potential factors underlying the lack of favorable outcome. So what do we know about potential causes of GID that can help guide future trials of this still promising therapy?
Pattern of Reinnervation and Graft-induced Dyskinesias
One initial hypothesis proposed that GID were the result of an excessive reinnervation of the striatum [9]. However, post-mortem analyses failed to report overgrowth of dopaminergic tyrosine hydroxylase positive (TH+) fiber reinnervation or supranormal levels of DA function measured with 18fluorodopa (18F-DOPA) positron emission tomography (PET) [10,12,13,27]. More probable is the suggestion that GID is the result of concentrated ‘hot spots’ of DA activity against a background of significant DA depletion. Evidence of focal areas of increased 18F-DOPA uptake was reported in grafted patients expressing clinical GID [27]. This hypothesis is supported by findings in the parkinsonian rat. Specifically, more focal grafts consisting of an estimated ¾ of an embryonic ventral mesencephalon placed into 2 separate striatal sites [21] or one full ventral mesencephalon placed into a single striatal site [20] induced experimental GID in rats. In contrast, when this same amount of tissue (i.e.: one ventral mesencephalon) was placed into 6 sites distributed across the striatum, resulting in uniform widespread DA cell distribution, experimental GID was significantly reduced [20].
Clinical data also supports the hypothesis that incomplete or patchy reinnervation seems to plays a role in GID, and that the degree of partial reinnervation may be a critical variable. Specifically, it is interesting that in the Denver/Columbia clinical trial, the increase in overall putamen PET 18F-DOPA signal in the GID-expressing patient group was twice that of patients not expressing GID at 12 months (P < 0.03) and almost three times larger at 24 months (P < 0.005) [27]. Despite the 18F-DOPA signal being 3 times higher in the GID expressing patients, it was still below the level of the normal striatum by about 20% [27]. Thus, it has been suggested that, in the patients expressing GID behaviors, the levels of DA replacement by the grafted cells, while incomplete, is perhaps in the range of that required to induce biphasic dyskinesia (e.g.: [26,27]). Again, the interaction of graft size and relative restoration of striatal DA concentration as it relates to the various behavioral outputs including biphasic dyskinesias or GID is presented in Figure 2.
Beyond the fact that grafted cells are releasing DA into the parkinsonian striatum at levels which are below normal and in patchy areas, there are more complex considerations of how and whether these grafted cells are able to re-establish physiological circuits in an adult, diseased brain. In the following paragraphs we consider how the cellular composition of a graft and the environment into which it is placed might impact the establishment of graft-host circuits.
Cellular Composition and Graft-induced Dyskinesias
The cellular composition of fetal tissue grafts has been suggested as a possible contributing factor to the appearance of GID as well as the limited benefit observed in grafted patients. For example, depending on the dissection protocol employed, fetal mesencephalic grafts can contain numerous cell types including glial cells, DAergic (A8, A9 and A10 cell groups), γ-aminobutyric acid (GABA)ergic, and serotonergic neurons. Recent studies have suggested that the proportion of A9 DAergic progenitors (destined for a substantia nigra (SN) fate) grafted is positively correlated with motor recovery benefit in a rodent model [28]. This is likely due to the fact that these neurons, in contrast to A10 cells (destined to become ventral tegmental area (VTA) neurons), have the unique ability to innervate and establish functional synapses with neurons native to the target structure in which they are grafted, i.e.: the striatum. The impact of differential proportions of A9 and A10 neurons on GID-type behaviors has not been directly investigated. While it could be hypothesized that the reversal of motor disability might benefit more from grafting A9 cells, this cell type may be more prone to producing post-graft dyskinetic behaviors. Rationale for this concept is expanded in the section below entitled “Dopamine Depletion as a Modifier of Graft Outcome” but in brief, the attempted formation of synaptic contacts between A9 neurons and striatal target neurons (i.e.: medium spiny neurons (MSNs)) that have an abnormal morphology, which is known to occur in the PD brain, could result in abnormal circuitry and the expression of GID.
An additional cellular component that has been proposed to impact graft functional outcome, specifically GID behavior, is the proportion of serotonergic to dopaminergic progenitors within the graft. Further, sprouting of host 5-hydroxytryptamine (5HT, serotonin) neurons into the grafted, parkinsonian striatum is also considered a risk factor that may influence this side effect. An abundance of recent data suggests a mechanistic role for 5HT neurons in the expression of typical LID in non-grafted subjects, although not without caveats (e.g.: see [29] for conflicting results). However, data suggesting a role of 5HT in post-graft GID is less conclusive and strongly mixed. Recent findings supporting or refuting the involvement of 5HT terminals in GID behaviors from both clinical and preclinical studies are contrasted in Table 1.
Table 1. Serotonin and Graft-induced Dyskinesias (GID).
Striatal 5HT fibers, derived from brainstem host projections and/or donor graft tissue, have been postulated to play a role in GID. Evidence supporting or negating this hypothesis is presented in this table. While there is a great deal of data to suggest that following levodopa administration there is non-regulated DA overflow produced from the striatal serotonergic hyperinnervation within the parkinsonian striatum that causes typical LID behavior in non-grafted subjects, data for the role for 5HT in GID is less certain. However, if we are to accept that 5HT neurons are a causative factor in off-time GID in patients, the question that needs to be considered is: “When the patient is off levodopa, what is the source of excess DA production from serotonin neurons?” Abbreviations: positron emission tomography, PET; single photon emission computed tomography, SPECT; 11C-labeled 3-amino-4-(2-dimethylaminomethylphenylsulfanyl) benzonitrile, 11C-DASB; ventral mesencephalon, VM.
| Evidence Supporting a Role for 5HT neurons |
Citation | Evidence Against a Role for 5HT neurons |
Citation |
|---|---|---|---|
| PET and SPECT evidence shows elevated 5HT/ DA transporter ratio in one PD patient that developed GID; the 5-HT1A agonist/ DA antagonist molecule, given at small, repeated doses suppressed this behavior | [90] | In a rat model, 5-HT cells within a graft were shown to be neither detrimental nor beneficial for functional effects of DA-rich transplants; however, in absence of sufficient numbers of DA neurons, transplanted 5-HT neurons were able to induce dyskinetic behaviors following levodopa (i.e.: LIDs) | [123] |
| 11C-DASB PET imaging of sertonergic innervation shows evidence of excessive serotonergic innervation in the grafted striatum of two patients that exhibited major motor recovery and later developed GID; the 5-HT1A agonist/ DA antagonist molecule, give at small, repeated doses suppressed this behavior | [91] | Evidence demonstrates that in each of 5 PD patients grafted with human fetal VM tissue there were abundant serotonin neurons but that none of them were observed to have GID | [124,125] |
| In a rat model, transplant-induced AIMs noted following amphetamine administration were seen to primarily depend on a pharmacological activation of dopamine receptors; and serotonin neurons within either the grafts or the host brain played a negligible role | [24] |
Serotonin Neural Circuits and Graft-induced Dyskinesias
While the “jury is still out” on the importance of 5HT neurons in GID, it is worth considering that if these neurons do promote this behavior, how might this occur? Both nigral DA and raphe 5HT neurons contain aromatic amino acid decarboxylase (AADC) and vesicular monoamine transporter 2 (VMAT2) and thus both of these neuronal populations can produce and release DA. According to the current dogma, the mechanism by which 5HT neurons produce dyskinesias is related to their capacity to synthesize, store and release DA from systemically administered levodopa treatment (reviewed in [30]). While 5HT neurons can release DA as a so-called “false transmitter,” the release is non-regulated because of a lack of DA autoreceptors. Thus, DA release from 5HT terminals is non-regulated and non-physiological, which has long been associated with dyskinetic side effects of long-term levodopa [31–33]. Interestingly, two recent reports reveal that in the non-grafted parkinsonian rat [34] and non-human primate [35] dyskinogenic levodopa treatment results in increased numbers of serotonergic varicosities beyond that noted after the destruction of the nigrostriatal pathway. Thus, it is reasonable to assume that an increased density of 5HT fibers, which are releasing increased amounts of non-regulated DA, could lead to dyskinetic behaviors.
However, there is an additional mechanism by which 5HT neurons might be influencing striatal circuitry and expression of dyskinetic behavior, perhaps both LID and GID. Interestingly, and perhaps of greater significance than the appearance of increased axonal sprouting, is the recent report of a significant increase in 5HT synapses per se in the dyskinetic rat brain [34]. This is of interest because under normal conditions 5HT terminals within the striatum have a very low incidence (†6 – *18%) of actual synapse formation [34*,36–38, 39†]. Thus, most of the neurotransmitter release in the striatum from 5HT varicosities presumably occurs through autocrine mechanisms. The finding of an increase from ≤18% to 46% incidence of 5HT terminals forming synaptic specializations in parkinsonian rats expressing LID [34] is striking and potentially of significance in understanding the role of the 5HT system in dyskinesias.
A New Layer of Complexity: Serotonin/Glutamate Co-Transmission
While the role of 5HT hyperinnervation producing excess DA makes intuitive sense with regard to the production of dyskinetic behavior, the involvement of striatal 5HT axons in this behavior may be more complex than the relatively simple notion of enhanced non-regulated DA release. Specifically, an unexpected observation was made in recent years that a majority of central 5HT neurons express the vesicular glutamate transporter 3 (vGLUT3) [40–44]. Interestingly, vGLUT3 is expressed primarily in neurons thought to release other conventional neurotransmitters, such as 5HT, acetylcholine, and GABA, in a classical manner [45,46]. Indeed, in the adult rat or mouse raphe nuclei, approximately 80% of 5HT neurons, which are the neurons that hyperinnervate the parkinsonian striatum, express vglut3 mRNA and axonal vGLUT3 protein [42,47]. Interestingly, triple label immunofluorescence studies have shown that axon terminals that are positive for vGLUT3 and 5HT are generally not positive for the serotonin transporter (SERT, the 5HT reuptake transporter protein).
Little is yet known about the functional significance of vGLUT3 co-expression in 5HT neurons and it is certainly not clear whether it plays a role in GID (or LID). However, there is compelling evidence that suggests that it may warrant investigation. First, vGLUT proteins are unambiguous markers of glutamate neurotransmission [45,46]. Accordingly, both in vitro, using electrophysiological cell culture technique [48], and in vivo, using an optogenetic murine model [49], it has been demonstrated that the co-release of glutamate from 5HT neurons can result in fast α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)-receptor mediated depolarizing currents. This colocalization of 5HT and glutamate in the axons that hyperinnervate the parkinsonian striatum implies that there would be an enhanced fast excitatory signaling component at these synapses viewed as being strictly modulatory. Because of the important role of glutamatergic signaling in striatal plasticity, these findings add a new layer of complexity that has untapped potential in understanding the dynamics of 5HT hyperinnervation in dyskinesia development and expression.
In addition to vesicular packaging of glutamate that is involved with fast excitatory currents, vGLUT3 in 5HT terminals has another important role. As reviewed by [46], a major functional property of the glutamatergic co-phenotype is increased filling of synaptic vesicles with the ‘primary’ neurotransmitter, in this case 5HT. This phenomenon is known as “vesicular synergy”. However, if vesicular synergy is to be considered important in the development of dyskinesias, it would be necessary for DA to be included in the enhanced vesicular loading in 5HT neurotransmitter vesicles. Current data supports the idea that in 5HT neurons the same pre-synaptic vesicles contain both vGLUT and VMAT2 [45], suggesting that vesicular synergy would likely involve enhanced DA loading in striatal 5HT terminals within the parkinsonian striatum in individuals treated with levodopa. However, this hypothesis remains untested.
In addition, both excitatory post-synaptic signaling (from glutamate co-release) and the size of the readily releasable pool of transmitter (e.g.: DA and/ or 5HT) are heavily dependent on the number of synapses formed by a neuron [45]. Keeping in mind that in the dyskinogenic parkinsonian rat striatum there is a 2.5-fold increase in the number of synapses formed by 5HT terminals [34], it would be reasonable to suggest that dyskinesias might be linked to enhanced post-synaptic glutamatergic signaling as well as enhanced DA release, with both events coming from the 5HT terminal fields. The incidence of 5HT synapses from grafted 5HT cells and their potential correlation to GID has not been examined.
A recent report by Dupre and colleagues [50] presents an intriguing, potential bridge in the gap between serotonergic and glutamatergic mechanisms in the expression of LID [30]. Their study demonstrates that striatal vesicular glutamate release is directly tied to 5-hydroxytrypamine 1A receptor (5HT1AR)-mediated suppression of LID. Specifically, these investigators found that stimulation of striatal 5HT1AR reduces local levodopa-induced glutamate efflux in the parkinsonian striatum while concomitantly diminishing the expression of LID [50]. The assumption in this study is that the reduced glutamate efflux is tied to cortical glutamate axons, which may or may not be the case. It is interesting to consider that accompanying striatal 5HT hyperinnervation and hyper-synapse formation in the vGLUT3 expressing raphe 5HT terminals there may be enhanced packaging and subsequent non-regulated release of DA and enhanced glutamate co-release. Such phenomena would be expected to exert powerful modulation of circuit activity within the grafted striatum.
Target Environment and Graft Dysfunction
Regardless of the cellular composition of grafts, the status of the host target neurons will undoubtedly impact the ability of grafted cells to reconnect and remodel the diseased brain. This assumption is supported by findings from clinical trials showing graft efficacy to be dependent on the patient’s age [9] and/or disease severity [10], and to vary amongst grafted individuals despite similar levels of graft survival and 18F-DOPA uptake [10]. Recent studies have attempted to determine how the PD modified striatal environment may be impacting graft efficacy and the development of GID. In the following paragraph, we will examine how disease severity as it relates to pathology of striatal target neurons and the host immune environment might interact with grafted embryonic neurons to alter synaptic circuitry and produce post-graft dyskinesia behaviors. Table 2 summarizes clinical and preclinical observations related to various factors involved in striatal pathology and how they might impact graft dysfunction. We provide additional detail for some of these factors in the following 4 sections.
Table 2. Target Pathology and Graft Dysfunction.
It is not an unexpected finding that grafting new DA neurons into a target wrought with pathology will result in suboptimal therapeutic outcome. Examples of how specific pathologies associated with PD have been shown to influence DA graft efficacy are outlined in this table.
| Evidence | Citation | Potential Mechanism |
|---|---|---|
| Preventing loss of dendritic spines on striatal medium spiny neurons (MSNs) moderates GID in parkinsonian rats | [25] | Striatal DA depletion results in loss of dendritic spines on striatal MSNs* (e.g.: [53,56]), which are normal input sites for nigral DA afferents and glutamatergic inputs originating in the cerebral cortex and thalamus. In parkinsonian striatum, grafted nigral DA neurons make atypical contacts on dendritic shafts and the loss of typical spinous contacts correlates with abnormal dyskinetic behaviors post-grafting [19] |
| Preventing loss of dendritic spines on striatal medium spiny neurons (MSNs) enhances reversal of motor dysfunction in parkinsonian rats | [25] | Per the above described pathology*, enhancing normal input sites is predicted to enable the formation of a typical pattern of synaptic connectivity giving rise to enhanced behavioral output following DA terminal replacement |
| Long-term levodopa in parkinsonian rats decreases the ability of grafted DA neurons to reverse motor deficits | [126] | Chronic levodopa results in a vast array of neurochemical and electrophysiological alterations within the parkinsonian striatum† e.g.: [29,35,133,134] |
| Long-term levodopa increases amphetamine-induced post-graft dyskinesias in grafted parkinsonian rats | [127] | Per the above described pathology† |
| PD patients with the best functional outcome after DA neuron grafting exhibited significantly less DA denervation in areas outside the grafted areas preoperatively compared to patients that showed lesser functional recovery | [17,51] | Less severe DA depletion coincides with less severe striatal pathology ††; e.g.: [53, 128] |
| Survival, axonal outgrowth and functional benefit of grafted DA neurons are critically dependent on the severity of damage to the host DA system | [52,129] | Per the above described pathology††; also, sparing pathology of non-striatal forebrain areas innervated by midbrain DA systems may provide additional benefit to striatal grafting paradigms |
| Advanced age decreases efficacy of grafted DA neurons in patients with PD and parkinsonian rats | [9,130] | Advancing age is a definitive risk factor for pathology within the striatum; e.g.: [131,132] |
Dopamine Depletion as a Modifier of Graft Outcome
One potentially important finding from clinical trials has been the observation that GID seems to correlate strongly with a patient’s pre-surgical extent of DA depletion [17,51]. Similarly, animal studies show that a DA graft in the context of less severe DA depletion is also more therapeutically effective [52]. This finding seems to implicate the severity of striatal DA loss as a modifier of graft integration. It has been known for several decades that the loss of striatal DA results in significant morphological alterations of MSNs, the primary target neuron for DA axons. Specifically, in advanced PD, there is a marked atrophy of dendrites and spines of these neurons [53–55]. A similar pathology is observed in mice, rats, and non-human primates with severe DA depletion (e.g.: [25,56–58]). In the striatum, dendritic spines of MSNs are the primary site of afferent input for nigral DA neurons, and glutamate input from the cortex and thalamus. The majority of nigral DA neurons primarily synapse onto the neck of dendritic spines where DA is thought to modulate glutamate signaling, which is transmitted to the MSN through excitatory synapses onto the head of the same dendritic spines [59] (Figure 3a). This morphological arrangement of synaptic inputs between DA and glutamate in the striatum is critical for normal motor behavior and is disrupted in the parkinsonian brain. It is reasonable to suggest that the loss of MSN spines that accompanies severe DA depletion is creating an environment that fosters inappropriate connectivity between host MSNs and grafted cells due to lack of normal contact sites.
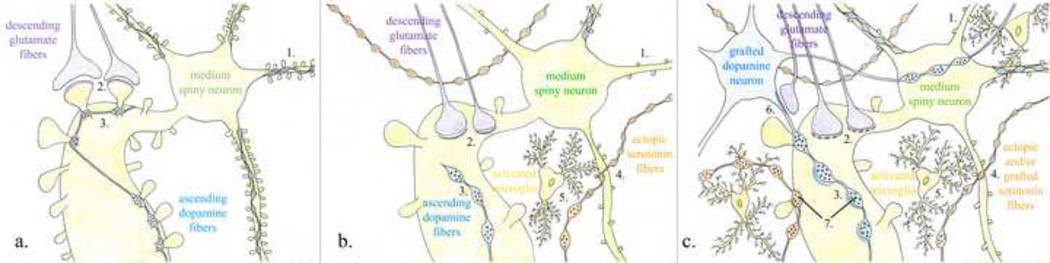
Figure 3. Schematic Depiction of the Connectivity in the (a) Healthy, (b) Parkinsonian and (c) Dopamine-grafted Parkinsonian Striatum.
These diagrams are oversimplifications of particular aspects of striatal circuitry that may be relevant to understanding GID behaviors. Studies on which these diagrams are based are detailed in the body of this review. Numbers is parenthesis ‘()’ in the legend refer to numbers in the figures; numbers in brackets ‘[ ]’ refer to citations. (a) In the healthy striatum, descending glutamate fibers (purple) from the cerebral cortex and thalamus make asymmetric contacts (2) preferentially onto the head of numerous dendritic spines (1) of the resident MSNs. This input is modulated by ascending DA fibers (blue) from the substantia nigra pars compacta (3) that make symmetric en passant contacts preferentially onto the necks of those same spines. Additionally there are a small number of 5HT fibers from the raphe nucleus (not depicted) that project to the striatum, although these typically do not form synapses with the MSNs. (b) In the parkinsonian striatum, following severe dopamine depletion, there is a significant loss of dendritic spines (1) of the MSNs of the indirect pathway in rodents [56,57], and direct and indirect pathways in non-human primates [58]. With the loss of these critical input sites both glutamatergic (purple) (2) and remaining DAergic (blue) (3) fibers show an increase in the number of atypical contacts onto dendrites rather than onto their normal spinous targets [19]. Additionally, there is an increase in the number of ectopic serotonergic fibers (orange) (4) [e.g.: 34]; and in parkinsonian rats treated with dyskinogenic levodopa there is a larger proportion of these making actual synaptic contacts onto the MSN (not depicted) [e.g.: 34]. Finally, there is an increase in inflammation in the parkinsonian striatum depicted here with the presence of activated microglia (yellow) (5). (c) In the dopamine-grafted parkinsonian striatum, despite some restoration of DAergic tone, current data suggests that there remains a significant loss of dendritic spines (1) on MSNs. In addition to making atypical contacts onto dendrites, glutamate (purple) (2) and DA (blue) (3) fibers show additional changes in their specialization and symmetry, with glutamate fibers making more perforated contacts (2) [19], which is generally associated with synaptic plasticity, and DA fibers making more asymmetrical contacts (3), which are atypical for DA fibers, and is classically associated with excitatory synapses. There is also presence of excitatory synapses onto the grafted cells themselves (6) [19]. There continues to be an increase in the presence of 5HT fibers (orange), potentially from both the raphe nucleus as well as from serotonergic cells within the grafts themselves (4). Since all grafted PD patients to date have undergone long-term levodopa treatment, there should be a marked increased incidence of synapses (darkened orange lines) between these ectopic 5HT fibers and MSNs [34]. Additionally, pre-synaptic vesicles in 5HT terminal boutons co-express vGLUT3 protein (7) [40–44], indicative of co-transmission of glutamate at these monoaminergic synapses. Similarly, following striatal DA depletion in the rat, there is re-expression of vGLUT2 protein (7) [68]; however, whether this occurs in grafted DA neurons is unknown. Research evidence suggests that much of this atypical connectivity may be modulated by the increased inflammatory response observed in the grafted striatum, depicted here to be influenced by activated microglia (yellow) (5). ‘Perforated synapses’ are depicted with the dashed, thickened post-synaptic arcs opposite the glutamate terminals; ‘Asymmetric synapses’ are depicted with thickened post-synaptic arcs opposite glutamate, DA and 5HT terminals. (7) While VMAT2 and vGLUT proteins likely occur in the same synaptic vesicles of monoaminergic neurons the occurrence of glutamate as a minor vesicular transmitter in the DA and 5HT terminals is depicted in this schematic as a single dark glutamate vesicle, indicating its relative minor presence.
Indeed, ultrastructural analyses have documented aberrant synaptology in the parkinsonian brain between graft and host cells with the grafted DA neurons making more atypical contacts onto dendritic shafts and fewer normal contacts onto spines themselves [60–64]. This shift in post-synaptic target and alterations in other synaptic features has recently been shown to correlate with the abnormal behaviors in the rat model, including drug-induced experimental GID and the suboptimal behavioral efficacy observed following grafting [19]. While such data is a good first step toward understanding the functional impact of remodeled circuits in the parkinsonian striatum, there is currently very limited data on this topic. In addition, there is very limited understanding of the multitude of changes that likely occur and/or specific factors that influence the remodeling of circuits within the parkinsonian and grafted striatum. Without such understanding, scientists will not know how to achieve physiological remodeling within the PD brain, or whether it will ever be possible to do so.
An Additional Layer of Complexity: Dopamine/Glutamate Co-Transmission
There have been several reports suggesting that the dynamic changes of the DAergic pre-synaptic ‘compartment’ determines susceptibility to dyskinetic behaviors in rats, particularly LID (e.g.: [65–67]). However, we suggest here that the complexity of pre-synaptic DA release and its involvement with dyskinesias, either LID or GID, has perhaps been underestimated, and that there is an important interplay between pre- and post-synaptic compartments. Similar to the discussion above about the co-localization of the vGLUT3 glutamate transporter in 5HT neurons in the section entitled “A New Layer of Complexity: Serotonin/Glutamate Co-Transmission”, midbrain DA neurons can also use fast glutamatergic transmission to communicate with their post-synaptic targets. Indeed, molecular and biochemical evidence demonstrate that DA neurons also co-localize a glutamate transporter, specifically vGLUT2 [68–74]. Similar to 5HT neurons, there are several studies that provide unequivocal evidence that midbrain DA neurons can co-release glutamate with DA and that this co-release of vesicular glutamate from DA terminals results in the generation of fast excitatory current via ionotropic glutamate receptors (e.g.: [71,75–78]). This contrasts the slower modulatory action of synaptic DA acting on G-protein coupled DA receptors. These findings suggest the interesting possibility that such co-release could lead to the encoding of parallel signals that operate on different timelines (e.g.: fast excitatory transmission via ionotropic glutamate receptors versus slower G-protein coupled modulatory transmission via DA receptors).
Whether there is any functional significance of the glutamatergic co-localization in DA neurons in the development of dyskinetic behaviors is unknown. However, there is accumulating evidence that suggests a critical assessment of the possible involvement of glutamate release from DA terminals in the pathophysiology of dyskinesias is warranted. It is important to point out that most evidence for the co-release of glutamate with DA, and the generation of fast excitatory current has been found in the normal adult rat in the mesolimbic DA system, but not the dorsal striatal DA system [71,78]. That being said, there importantly appears to be a dynamic regulation of vGLUT2 expression and function in DA neurons of the striatum with PD-relevant factors including age and DA itself being apparent dynamic regulators of this co-transmission. Specifically, Mendez and colleagues [70], using single-cell reverse transcriptase polymerase chain reaction (RT-PCR), showed that VGluT2 is the only vGLUT expressed in VTA/SN DA neurons in vivo, that its expression decreases with age and is negatively regulated through a contact-dependent signal provided by GABA and DA neurons. Similar findings of an age-associated decrease in the amplitude of fast excitatory currents in cultured midbrain DA neurons also has been reported [79]. Of relevance to PD, a recent study revealed that there is an induction of vGLUT2 expression in TH+ neurons of the striatum under pathological conditions (i.e.: following striatal DA depletion) [68]. Moreover, the functional properties of midbrain DA-glutamate co-release can be regulated by chronic application of glial cell-line derived neurotrophic factor (GDNF) [79]. Thus, the glutamatergic phenotype of striatal mesencephalic DA neurons is dynamically regulated by age, pathology and trophic factors; these are all relevant factors for consideration in PD research.
Target Neuron Pathology Alters Dopamine Circuitry in the Grafted Brain
On the basis of ultrastructural parameters, a concept was formulated that symmetric (Gray Type II) synapses are inhibitory and asymmetric (Gray Type I) synapses are excitatory (reviewed in [80]). In the normal adult striatum, nigral DA afferent axons that preferentially synapse onto the neck of dendritic spines of MSNs establish symmetric (presumed inhibitory) synaptic connections 97% of the time [19]. Work in our lab using immunoelectron microscopic methods has revealed that when embryonic DA neurons are grafted into the striatum of parkinsonian rats and they develop the experimental GID phenotype, the TH+ terminals show an increased incidence of asymmetric (presumed excitatory) post-synaptic specializations. The incidence of asymmetric (Gray Type I) TH+ profiles in the striatum of rats expressing experimental GID phenotype increases approximately 10-fold from 3% to 38% [19]. It remains to be determined whether the increase in asymmetric profiles, which are traditionally associated with the excitatory neurotransmitter glutamate, are created by reintroduction of vGLUT2 that occurs in the parkinsonian striatum [68], or reflects the fact that the grafted DA neurons, being embryonic likely express vGLUT2 [70]. Further, it is hypothesized that the loss of dendritic spines on MSNs predisposes the new DA terminals from grafts to make contacts onto the dendritic shaft (e.g.: [19,60]; discussed above in “Dopamine Depletion as a Modifier of Graft Outcome”), which further predisposes the new synapses to establish a new functional phenotype (i.e.: change from symmetric to asymmetric per [19]).
While recent optogenetic data demonstrates that the activation of DA axon terminals that originate in the VTA and co-localize vGLUT2 induces AMPA receptor mediated fast synaptic responses of MSNs of the nucleus accumbens [71,78], it remains to be determined whether similar glutamatergic receptor activation occurs in the striatum of a parkinsonian subject in which there is re-introduction of vGLUT2 co-expression [68]. Similarly, it remains to be determined whether the asymmetric TH+ synapses within the grafted striatum [19] mediate any functional excitatory response or contain AMPA or other glutamatergic receptors in the post-synaptic density. Because striatal AMPA receptors are strongly implicated in the pathophysiology of LID [81–83], elucidating whether such phenomenon occur in the parkinsonian striatum could be highly instructive. Whereas it is clear that AMPA receptor signaling in the striatum is linked to LID, it has been assumed that the source of aberrant glutamatergic signaling derives exclusively from corticostriatal or thalamostriatal axons. Indeed this may be the case. However, understanding whether there is contribution from the nigrostriatal DA and glutamate co-phenotype would provide a new level of insight into the pathophysiology of LID and/or GID. While the pharmacological characterization of LID is well established for clinical and experimental LID, there is a large paucity of pharmacological data in experimental GID in rats and in clinical GID in PD patients (Table 3).
Table 3. The Pharmacology of LID and GID.
In this table, what is known about the pharmacology of LID (i.e.: the widespread typical dyskinesias noted in the absence of DA cell grafting) is contrasted with what is known about the pharmacology of GID (i.e. the largely focal dyskinesias that develop in the presence of a DA cell graft). + indicates that there is evidence that the neurotransmitter, receptor, or second messenger system plays a role in LID or GID; Nt = not tested and indicates that there is no evidence that the neurotransmitter, receptor, or second messenger system plays a role in LID or GID. This table contains examples of citations for each system listed; because it is beyond the scope of this article, it is not meant to serve as a comprehensive source.
| Neurotransmitter/ Receptor |
Levodopa Dyskinesia |
Example Citations |
Post-Graft Dyskinesia |
Example Citations |
|---|---|---|---|---|
| D1R | + | [136–138] | + | [89] |
| D2R | + | [138,139] | + | [89–91] |
| D3R | + | [138,140–142] | Nt | - |
| Second messengers: Ras MEK 1/2 ERK1 mTOR |
+ + + + |
[144] [143] [144] [145] |
Nt Nt Nt Nt |
- - - - |
| Opioid Receptors | + | [146–148] | Nt | - |
| Endocannabinoids | + | [149–151] | Nt | - |
| 5-HT | + | [135,152–155] | + | [89, 90,91,98] |
| Adenosine Receptors | + | [156, 157] | Nt | - |
| Nicotinic Receptors | + | [158–160] | Nt | - |
| Muscarinic Receptors | + | [161] | Nt | - |
| NMDA Receptors | + | [162,163] | Nt | - |
| AMPA Receptors | + | [162,164,165] | Nt | - |
Finally, in addition to potential post-synaptic excitatory receptor plasticity, the potential role of vGLUT2 proteins in grafted DA neurons would also be anticipated to result in “vesicular synergy” ([46]; introduced above in “A New Layer of Complexity: Serotonin/Glutamate Co-Transmission”) and enhanced local release of the primary transmitter (i.e.: DA). If vesicular synergy does occur in grafted DA neurons, this could contribute to focal “hot-spot” activation of striatal circuits, which is considered as a potential causative factor in GID behaviors. Additionally, as demonstrated by Soderstrom and colleagues [19], in parkinsonian rats that express experimental GID, there is a complex array of excitatory circuits in the grafted striatum [19] (Figure 3c). Specifically, in the vicinity of the graft, there is an increase in the density of perforated, asymmetric, non-TH+ synapses contacting dendritic shafts (rather than spine heads) of local target neurons. Perforated synapses are ultrastructural landmarks of long-term potentiation (LTP) [84], which is characterized by a long-lasting enhancement of synaptic efficacy. There is also the presence of asymmetric, excitatory synapses contacting the grafted TH+ cells, which presumably would increase the excitatory synaptic drive of these cells. In addition, the grafted TH+ cells themselves form asymmetric (presumably excitatory) synapses onto dendrites of local target neurons. Thus, we hypothesize that this dramatic synaptic reorganization could produce experimental GID by producing significant alterations in the functional output of striatal circuitry. Interestingly, this ‘rewiring’ of striatal circuity correlates significantly with the severity of experimental GID in rats [19].
Neuroplasticity, the Immune System, and Graft-induced Dyskinesias
Although factor(s) that could lead to dysfunctional circuit remodeling and aberrant motor output are likely numerous, insight into some of these may be garnered from examining the timing of GID expression in clinical trials. For example, in one study [10] patients showed improvements in parkinsonian motor deficits with no abnormal behaviors reported for 6 months followed by the appearance of clinical GID and a cessation of behavioral improvement. It is possible that in this 6-month period something adverse occurred to the grafts themselves or to their synaptic contacts with the host neurons. One potential factor that could explain this delay is the host immune response to grafting. Despite historically being regarded as an immuno-privileged site, there is a moderate degree of immune activation that occurs in the central nervous system following grafting. This occurs primarily because of the necessity of using non-genetically identical donor tissue, or allografts.
While animal studies suggest an extremely low immune response when genetically identical donor tissue is used, this is not feasible in human grafting studies and, therefore, allogeneic grafts are often used in conjunction with immunosuppression regimes. In two clinical grafting trails for PD, the appearance of GID appeared to coincide with the withdrawal of immunosuppression [10,17]. This observation, coupled with our recent finding that graft-induced dyskinesias fail to develop in grafted rats receiving donor tissue from rats of the same inbred strain [19] but occur with increased frequency in allografted rats with elevated immune responses, suggests a possible role for the host immune response in the development of dyskinesias following grafting.
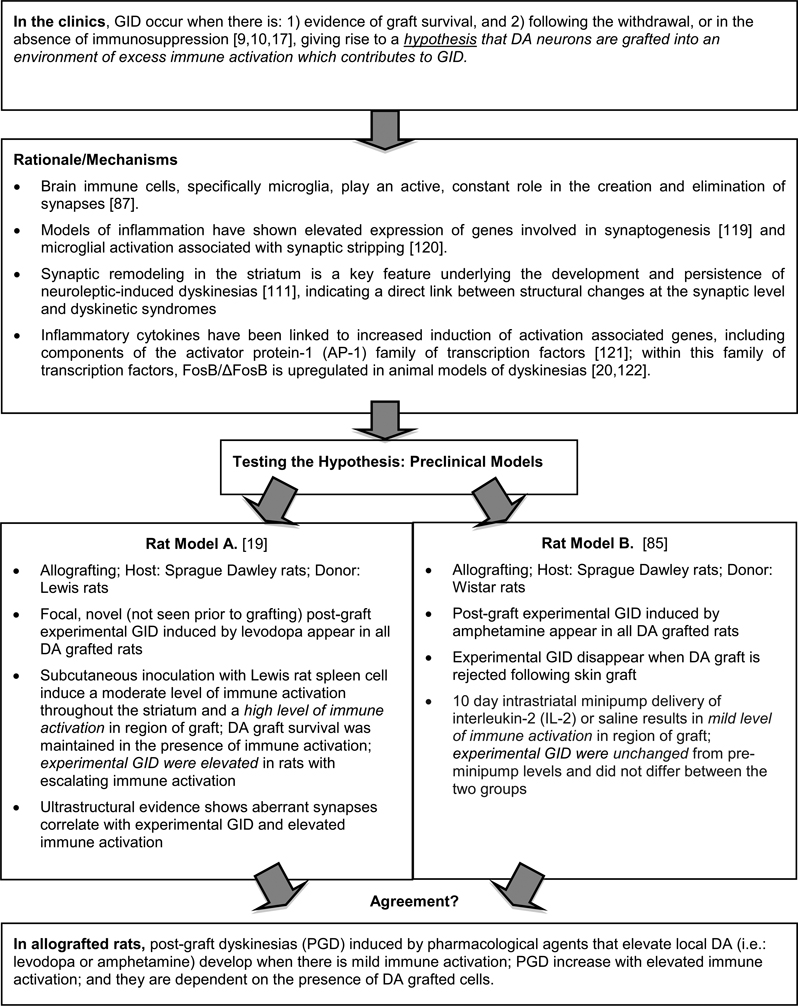
Two parallel preclinical studies [19,85] have used varying experimental protocols in attempts to determine whether the host immune response might play a role in experimental GID expression. In Figure 4 we put forth rationale for the possible involvement of immune factors in modulating striatal circuitry and compare the results of these two aforementioned studies. The collective data from these 2 studies in allografted rats with experimental GID induced by pharmacological agents that elevate local DA (i.e.: levodopa or amphetamine) seems to suggest that: 1) these behaviors develop when there is mild immune activation; 2) they increase in severity with elevated immune activation; and 3) they are dependent on the presence of DA grafted cells, presumably to act as the source of the formation of aberrant synaptic connections. Indeed, when grafted DA neurons are completely rejected, experimental GID in those rats disappear [85].
Figure 4.
Immune Activation and Graft-induced Dyskinesias.
Footnote; Model A: Experimental GID occurred only in dopamine-grafted rats, and not in cell-free, sham-grafted rats, despite the presence of equal numbers of MHC class II+ cells in sham and G21 rats, suggesting that the impact of host immune factors is more likely to be related to graft–host synaptic organization than to a direct action on host signaling pathways.
In contrast to the long held belief that glial cells in the brain play passive roles in structural support or immune surveillance, there is a large body of evidence that demonstrates an active role of glial cells in synaptic plasticity in health and disease (e.g.: [86,87]). Recent data suggests that even in the healthy brain, quiescent microglia appear to regulate the dynamics of plasticity at individual synapses as they explore the local environment to pursue immune surveillance roles [87]. In addition, the concept of tripartite synapse (presynapse, postsynapse, and astrocytes) was introduced approximately a decade ago in which synaptically associated astrocytes were proposed to exchange information with neuronal synaptic elements at glutamatergic synapses, even impacting synaptic strength (reviewed in (e.g.: [86]). Recent ultrastructural data has revealed that there is a significant expansion of the astrocytic presence in striatal tripartite synapses in the parkinsonian brain, providing further evidence that both neuronal and glial elements are involved in the pathophysiology of PD [86]. Thus, continued examination of host immune cell function in post-graft motor dysfunction in PD patients should be considered a high priority. Understanding whether dysfunctional remodeling and/or experimental GID can be eliminated by immunosuppressive therapies will be an important next step in preclinical studies. It would also be useful to understand which specific components (microglia, compliment factors, specific cytokines, etc.) of the immune system play an important role in the dynamic regulation of the phenotype of synapses in the parkinsonian, grafted striatum. Based on what is known about some immune/glial factors and what has been hypothesized as mechanisms underlying GID, it is possible that some glial factors may enhance synaptic remodeling that leads to GID (e.g.: GDNF, [79]) while others may play a role in preventing this phenomenon (e.g.: interleukin-2 (IL-2), [88]).
Validating Circuit Components of Graft-Induced Dyskinesias with Pharmacology
Primarily what has been studied in both experimental models of GID in rats and in clinical GID in PD patients are the responses of these behavioral phenotypes to drugs that impact synaptic DA and/or or 5HT content. To assess the role of DA and 5HT in the expression of experimental GID induced by amphetamine (AMPH), the selective DA or 5HT uptake inhibitors, GBR 12909 and fluvoxamine, respectively, were administered either alone or in combination to rats that previously received intra-striatal graft tissue derived from the embryonic ventral mesencephalon and the effects compared to AMPH administration [21]. Although systemic administration of fluvoxamine alone did not produce experimental GID, systemic administration GBR 12909 alone produced low levels of limb and orolingual experimental GID. Co-administration of GBR 12909 and fluvoxamine produced levels of limb and orolingual experimental GID comparable to that observed following AMPH administration. In contrast, axial and locomotor experimental GID induced by administration of the GBR 12909 alone or by co-administration of the DA uptake inhibitor plus the 5HT inhibitor did not differ qualitatively or quantitatively from that induced by systemic AMPH administration [21]. These findings suggest a role for an increase in extracellular DA concentration in the expression of experimental GID. With regard to the role of 5HT in the expression of GID, the findings suggest an interaction between the 5HT and DA neurotransmitter systems since 5HT uptake inhibition increased GID only when extracellular DA levels were increased. Moreover, these findings are consistent with the notion of the release of DA from 5HT neurons as a so-called “false neurotransmitter” discussed above in the section entitled “Serotonin Neural Circuits and Graft-induced Dyskinesias”. Unfortunately, despite the fact that AMPH, which stimulates the release of DA, 5HT, and norepinephrine (NE), is often used to evoke abnormal involuntary movements in experimental models of GID, the role of NE in the expression of GID has not yet been directly assessed (e.g.: via the administration of the potent and selective inhibitor of NE reuptake, nisoxetine [89]).
In grafted PD patients, the role of 5HT has also been examined to test the hypothesis that clinical GID are caused by dysregulated DA release from graft-derived striatal serotonergic terminals, discussed above in the section “Serotonin Neural Circuits and Graft-induced Dyskinesias”. In recent reports by Politis and colleagues [90,91] three PD patients who had received intra-striatal implants of DA-rich fetal mesencephalic tissue received repeated systemic administration of buspirone (Buspar), a 5HT1AR partial agonist (IC50 = 35 nm) that displays dopamine D2 receptor (D2R) antagonist (IC50 = 250 nm) properties [92] and is metabolized to the potent α2-adrenergic receptor antagonist, 1-(2-pyrimidinyl-piperazine) [93–95]. Repeated administration of buspirone attenuated clinical GID severity in these patients [90,91]. Although several other possibilities exist including the activation of postsynaptic 5HT1AR, it was proposed that the buspirone alleviated GID behaviors by blocking the release of DA from serotonergic neurons by activating inhibitory 5HT autoreceptors (per mechanisms described in [96,97]). This clinical finding suggests that excess DA release from ectopic 5HT terminals may indeed contribute to GID; however, it is counter to what seems to be evidenced from preclinical studies [19,29,98].
In addition to the aforementioned studies that have examined the contribution of pre-synaptic monoamine release, the role of DA and 5HT receptors in the expression of experimental GID in rats has been examined. Lane and colleagues [98] reported that systemic administration of the selective D2R antagonist, raclopride [99], and the selective dopamine D1 receptor (D1R) antagonist, SCH23390 [100], suppressed AMPH-induced experimental GID. This finding suggests that activation of D1R and D2R play a role in the induction of experimental GID in the rat model [98]. Moreover, these authors found that systemic administration of the selective 5HT1AR agonist, 8-OH-DPAT, reduced the severity of AMPH-induced experimental GID. However, rats that received a 6-OHDA-lesion plus a DA graft and were subjected to 5, 7-dihydroxytryptamine (5, 7 DHT) lesions to dramatically decrease the serotonergic innervation of the striatum, exhibited a level of AMPH-induced experimental GID comparable to those animals without the 5, 7 DHT lesion [98]. This finding indicates that host raphe-striatal 5HT system does not appear to play a role in the expression of experimental GID.
It is important to appreciate that continued pharmacological studies in subjects with an altered striatal synaptology will advance our understanding of GID and how to effectively treat them. Future pharmacological studies that rely on procedures that provide for better spatial and temporal control of drug administration have the ability to directly test current hypotheses regarding the neural substrates of GID and may allow for the development of new hypotheses. One rational approach is to use selective compounds to test the hypothesis that the pattern of synaptic connections formed between transplanted neurons and host neurons, although dynamic and in a state of flux, may underlie both behavioral recovery as well as GID [19,25,60,62]. For instance, as first introduced above in the sections “Dopamine Depletion as a Modifier of Graft Outcome” and “Target Neuron Pathology Alters Dopamine Circuitry in the Grafted Brain”, in animals that receive rat embryonic ventral mesencephalic grafts following a 6-OHDA lesion of the nigrostriatal pathway and showed complete behavioral recovery, the axons of the grafted neurons formed symmetric (Gray Type II, presumably inhibitory) synapses onto spine necks and dendritic shafts of host MSNs [60], a pattern of synaptic input observed in untreated rats [59]. However, in these same animals DAergic fibers of the transplanted neurons formed dense “baskets” of fibers with frequent symmetric synaptic contacts that surrounded the perikarya of the host large aspiny cholinergic interneurons, a phenomenon never seen in the normal striatum [60]. Since DA has an inhibitory effect on striatal cholinergic interneurons [101] and striatal cholinergic interneurons have an excitatory effect on MSNs [102], this pattern of abnormal synaptic connectivity suggests a mechanism by which DA could potently dampen the activity of MSNs. Studies that explore the role of activation and/or blockade of striatal muscarinic M1-like and/or M2-like receptors, which are expressed on the cell surface of both MSN and the large aspiny cholinergic interneurons [103–105] in GID would be useful in determining whether or not this pattern of abnormal synaptic connectivity is related to the induction or expression of GID.
As detailed in the section above “Target Neuron Pathology Alters Dopamine Circuitry in the Grafted Brain”, Soderstrom and colleagues [19] demonstrated that in DA grafted rats expressing experimental levodopa-induced GID, GID severity was positively correlated with the proportion of asymmetric (Gray Type I, presumably excitatory) synapses contacting grafted cells. Importantly, most, but not all, asymmetric synapses are glutamatergic and, thus, in most, but not all cases, the terminal bouton exhibits immunoreactivity for glutamate [106–108]. In addition, the post-synaptic density, one of the defining features of the asymmetric (Gray Type I) synapse, is best viewed as a complex assembly of hundreds of structural and signaling proteins associated with the post-synaptic membrane including the ionotropic glutamate receptors (i.e.: AMPA and N-methyl-D-aspartate (NMDA)) [109,110]. Thus, studies that explore the role of activation and/or blockade of striatal AMPA and/or NMDA receptors in the induction and expression of GID would be of great interest.
Future Directions: What Pieces of the Puzzle are Missing?
There is good correlative data now available to suggest that several specific mechanisms are likely to play a role in the development of abnormal motor movements following engraftment of embryonic DA neurons into the parkinsonian striatum. As detailed in this review, these mechanistic factors include (but are likely not limited to) striatal 5HT fibers, co-localization of vesicular glutamate transporters in monoaminergic neurons, unknown component(s) of the immune system, and striatal target neuron pathology. In the above paragraphs, we presented a discussion of some of the primary data that is suggestive of particular mechanisms and what is still missing to determine the unequivocal role of these factors in clinical expression of GID. However, there are numerous other factors that have not yet been considered. In this final section of the manuscript, we present a few pieces of the scientific puzzle that are still missing and may warrant further investigation in the quest for understanding why abnormal behavioral profiles develop upon attempted brain remodeling during embryonic DA neuron grafting in PD.
So what is missing? One important piece of the puzzle that is missing is a more extensive characterization of the pharmacology of GID. As can be appreciated in the section “Validating Circuit Components of Graft-Induced Dyskinesias with Pharmacology” there have been some important initial studies done on the pharmacology of GID in the rat model and in PD patients. However, the extent of pharmacological classification of this phenomenon is extremely limited compared to what has been accomplished for non-graft associated drug-induced/levodopa-induced dyskinesias. A comparison of what is known about the pharmacology of GID versus what is known about the pharmacology of LID is presented in Table 3.
One important entity that is missing in the quest for understanding causative mechanisms of GID is a non-human primate (NHP) model of this side effect. There are currently no published reports of GID in a NHP model of parkinsonism. Doubtless, the establishment of off-medication dyskinesias following DA terminal replacement in a NHP model would be valuable. While the rodent model of drug-induced LID has strong face and predictive validity relative to the corresponding disorder in primates, the rodent model of experimental GID has the notable weakness that these behaviors are reliably quantifiable only after administration of drugs that elevate the striatal DA concentration. While this may or may not negate its validity (see Figure 2), an animal model of off-time GID would be ideal. Based on size and phylogenetic similarities between NHP and humans, it could be predicted that the NHP may be a more suitable preclinical model. Comparisons between a NHP model and the rodent models of experimental GID would additionally allow for validation studies between these models and ultimately patients. Validation of the rodent model of GID, in spite of the fact that these experimental GID behaviors need to be elicited by drugs like AMPH or levodopa, would provide a less expensive, more widely available model for preclinical testing.
Another piece of the puzzle that is missing is the examination of post-mortem striatum from patients that had suffered with GID. Post-mortem analyses detailing the patterns of integration of DA fibers, and a characterization at the ultrastructural level of the extent of DA and non-DA synaptic remodeling that occurs would be instructive not only for understanding mechanisms of circuit plasticity in GID but for validating experimental findings in models of GID. While there is indication that specific structural modifications of post-synaptic specializations correlate with dyskinetic behaviors in rodent models [19,111], examination of this type of synaptic reorganization in striatal circuitry in the patients expressing GID are lacking.
Examinations of extrastriatal pathology are another missing puzzle piece. To date all mechanisms that have been described as relevant to GID have been principally restricted to the striatum (caudate/putamen). It is well known in parallel research (i.e.: drug abuse) that behavioral sensitization involving midbrain DA systems involve functional and molecular alterations throughout basal ganglia nuclei (reviewed in [112]). Specifically, it known that ‘initiation’ of mesolimbic DA sensitization phenomena during chronic psychomotor stimulant administration involves temporally and anatomically distinct processes from ‘expression’, which is defined as the enduring neural alterations arising from the initiation process that directly mediate the augmented behavioral response [112]. It is conceivable that, similar to the mesolimbic DA system, the sight of the dyskinesia ‘initiation’ process within nigrostriatal DA system might be the locus of the DA cells, specifically the SN pars compacta. If these systems are similar, it is further conceivable that neuronal events associated with ‘expression’ of dyskinesia may be distributed among the interconnected nuclei that are part of, or in communication with the basal ganglia motor system including globus pallidus (internal or external segments), subthalamic nuclei, prefrontal cortex, or supplementary motor cortex. Thus, to comprehensively impact our understanding of dyskinetic DA sensitization behaviors in PD, it will likely be necessary to examine alterations not only in the striatum, but also outside of this location.
Along these same lines, additional neurotransmitter systems such as cholinergic and noradrenergic systems have not been considered as factors contributing to GID. For example, in addition to 5HT neurons expressing vGLUT3, striatal cholinergic interneurons have also recently been shown to mediate fast vGLUT3-dependent glutamate transmission via NMDA-type receptor activation [113]. Despite representing a small percentage of total neurons in the striatum (1–3%), cholinergic interneurons have been shown to significantly influence the level of cocaine-induced sensitization [114]. This suggests another level of complexity of co-transmitter release regulating the excitability of striatal MSN activity. Whether cholinergic-vGLUT3 system influences striatal circuits causing the sensitization phenomena of dyskinesias, in general, and GID, specifically, is unclear.
Finally, we propose that one important aspect of GID research is the lack of a cohesive account of how the various phenomenological events interact to produce abnormal signaling and GID behaviors. As with drug-induced, non-graft associated dyskinesias (e.g.: typical LID) there is a large variety of phenomena that are documented to be associated with LID (reviewed in [115]). However, there remains no general consensus of which mechanism is “the mechanism” that “causes” expression of LID. This statement is supported by the fact that no effective clinical treatment is currently available to prevent or significantly abate LID expression in PD patients. Thus, ultimately it will be important as investigations worldwide proceed to consider that GID (and probably LID) are centered in a collection of interconnected biochemical, molecular and structural changes that occur throughout the basal ganglia.
Summary
While there is valid disappointment that DA neuron grafting in PD is not yet successful, ‘surprise’ at the fact that it has not worked is perhaps less valid. Perhaps it would have been more surprising if functional restoration had been found following engraftment of new embryonic cells into the adult, aged brain; one lacking molecular signals for re-establishment appropriate synaptic connections and plagued with neurochemical and neuroanatomical remodeling that are hallmarks of PD pathology. We suggest that it is instructive to consider that we have largely uncovered conditions in which grafting of embryonic DA neurons do not work. There are, of course, exceptions to the idea that grafting does not work in PD. Thus, as the field moves forward, the focus of basic and clinical researchers will be to figure out under what circumstances the parkinsonian brain can be successfully remodeled with grafts of new cells.
In closing, we also would like to provide the perspective that transplantation of DA-producing cells into the brain to treat PD is still in a period of relative infancy. Indeed, the path to clinical success for grafting in PD is not unlike the path taken during the development of other organ transplant protocols. As one example, the first functional kidney transplant in a dog took place in 1902, with active research and lack of clinical use for 52 years, from 1902 thru 1954. It was not until 1954 at Brigham Hospital in Boston, that Dr. Joseph Murray performed the first successful human kidney transplant when a living donor gave a kidney to his identical twin ([116]; December 2011, US Department of Health and Human Services: http://www.organdonor.gov). While this was considered the first successful human kidney transplant, it was not until 1976 that real success was gained by the introduction of the immunosuppressant cyclosporine. Further modifications have continued to improve efficacy and survival over the past 30+ years. For DA neuron transplantation in PD, the first functional grafts in parkinsonian rats were reported in 1979 [117]. The first report of DA neuron grafting into patients with PD was in 1989 [118]. In the 22 years since the first DA neuron graft in a patient was reported, the field has made astonishing progress in understanding what works and what does not. Given the progress and promise of grafting for PD, it would be tragic to consider that we may be giving up too soon.
Acknowledgements
Supported by National Institute of Neurological Disorders and Stroke (Udall Center of Excellence: P50NS058830 (KSC); R01NS045132 (KSC)); and The Michael J. Fox Foundation (KSC). We would also like to thank Dr. Timothy J. Collier for his constructive comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dunn EH. Primary and secondary findings in a series of attempts to transplant cerebral cortex in the albino rat. J Comp Neurol. 1917;27:565–582. [Google Scholar]
- 2.Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- 3.Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- 4.Lindvall O, Sawle G, Widner H, Rothwell JC, Björklund A, Brooks D, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann Neurol. 1994;35:172–180. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- 5.Peschanski M, Defer G, N’Guyen JP, Ricolfi F, Monfort JC, Remy P, et al. Bilateral motor improvement and alteration of L-dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain. 1994;117:487–499. doi: 10.1093/brain/117.3.487. [DOI] [PubMed] [Google Scholar]
- 6.Wenning GK, Odin P, Morrish P, Rehncrona S, Widner H, Brundin P, et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Ann Neurol. 1997;42:95–107. doi: 10.1002/ana.410420115. [DOI] [PubMed] [Google Scholar]
- 7.Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, et al. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain. 1999;122:1121–1132. doi: 10.1093/brain/122.6.1121. [DOI] [PubMed] [Google Scholar]
- 8.Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain. 2000;123:1380–1390. doi: 10.1093/brain/123.7.1380. [DOI] [PubMed] [Google Scholar]
- 9.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 10.Olanow CW, Goetz CG, Kordower JH, Stoessl A, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, et al. Dopamine cell implantation in Parkinson's disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med. 2010;51:7–15. doi: 10.2967/jnumed.109.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 13.Kordower JH, Rosenstein JM, Collier TJ, Burke MA, Chen EY, Li JM, et al. Functional fetal nigral grafts in a patient with Parkinson’s disease: chemoanatomic, ultrastructural, and metabolic studies. J Comp Neurol. 1996;370:203–230. doi: 10.1002/(SICI)1096-9861(19960624)370:2<203::AID-CNE6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Kordower JH, Freeman TB, Chen EY, Mufson EJ, Sanberg PR, Hauser RA, et al. Fetal nigral grafts survive and mediate clinical benefit in a patient with Parkinson's disease. Mov Disord. 1998;13:383–393. doi: 10.1002/mds.870130303. [DOI] [PubMed] [Google Scholar]
- 15.Piccini P, Brooks DJ, Björklund A, Gunn RN, Grasby PM, Rimoldi O, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 16.Piccini P, Lindvall O, Björklund A, Brundin P, Hagell P, Ceravolo R, et al. Delayed recovery of movement-related cortical function in Parkinson’s disease after striatal dopaminergic grafts. Ann Neurol. 2000;48:689–695. [PubMed] [Google Scholar]
- 17.Hagell P, Piccini P, Björklund A, Brundin P, Rehncrona S, Widner H, et al. Dyskinesias following neural transplantation in Parkinson’s disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 18.Hagell P, Cenci MA. Dyskinesias and dopamine cell replacement in Parkinson’s disease: a clinical perspective. Brain Res Bull. 2005;68:4–15. doi: 10.1016/j.brainresbull.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Soderstrom KE, Meredith G, Freeman TB, McGuire SO, Collier TJ, Sortwell CE, et al. The synaptic impact of the host immune response in a parkinsonian allograft rat model: Influence on graft-derived aberrant behaviors. Neurobiol Dis. 2008;32:229–242. doi: 10.1016/j.nbd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, et al. Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol Dis. 2006;21:165–180. doi: 10.1016/j.nbd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Lane EL, Winkler C, Brundin P, Cenci MA. The impact of graft size on the development of dyskinesia following intrastriatal grafting of embryonic dopamine neurons in the rat. Neurobiol Dis. 2006;22:334–345. doi: 10.1016/j.nbd.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Fahn S. The spectrum of levodopa-induced dyskinesias. Ann Neurol. 2000;47:S2–S9. discussion S9-11. [PubMed] [Google Scholar]
- 23.Carlsson T, Winkler C, Lundblad M, Cenci MA, Björklund A, Kirik D. Graft placement and uneven pattern of reinnervation in the striatum is important for development of graft-induced dyskinesia. Neurobiol Dis. 2006;21:657–668. doi: 10.1016/j.nbd.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Lane EL, Brundin P, Cenci MA. Amphetamine-induced abnormal movements occur independently of both transplant- and host-derived serotonin innervation following neural grafting in a rat model of Parkinson’s disease. Neurobiol Dis. 2009;35:42–51. doi: 10.1016/j.nbd.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Soderstrom KE, O’Malley JA, Levine ND, Sortwell CE, Collier TJ, Steece-Collier K. Impact of dendritic spine preservation in medium spiny neurons on dopamine graft efficacy and the expression of dyskinesias in parkinsonian rats. Eur J Neurosci. 2010;31:478–490. doi: 10.1111/j.1460-9568.2010.07077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olanow CW, Gracies JM, Goetz CG, Stoessl AJ, Freeman T, Kordower JH, et al. Clinical pattern and risk factors for dyskinesias following fetal nigral transplantation in Parkinson’s disease: a double blind video-based analysis. Mov Disord. 2009;24:336–343. doi: 10.1002/mds.22208. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol. 2002;52:628–634. doi: 10.1002/ana.10359. [DOI] [PubMed] [Google Scholar]
- 28.Grealish S, Jönsson ME, Li M, Kirik D, Björklund A, Thompson LH. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson’s disease. Brain. 2010;133:482–495. doi: 10.1093/brain/awp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nevalainen N, Af Bjerkén S, Lundblad M, Gerhardt GA, Strömberg I. Dopamine release from serotonergic nerve fibers is reduced in L-DOPA-induced dyskinesia. J Neurochem. 2011;118:12–23. doi: 10.1111/j.1471-4159.2011.07292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huot P, Brotchie JM. 5-HT(1A) receptor stimulation and L-DOPA-induced dyskinesia in Parkinson’s disease: bridging the gap between serotonergic and glutamatergic mechanisms. Exp Neurol. 2011;231:195–198. doi: 10.1016/j.expneurol.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Obeso JA, Luquin MR, Vaamonde J, Grandas F, Martinez Lage JM. Continuous dopaminergic stimulation in Parkinson’s disease. Can J Neurol Sci. 1987;14:488–492. doi: 10.1017/s0317167100037963. [DOI] [PubMed] [Google Scholar]
- 32.Sage JI, McHale DM, Sonsalla P, Vitagliano D, Heikkila RE. Continuous levodopa infusions to treat complex dystonia in Parkinson’s disease. Neurology. 1989;39:888–891. doi: 10.1212/wnl.39.7.888. [DOI] [PubMed] [Google Scholar]
- 33.Sage JI, Mark MH. The rationale for continuous dopaminergic stimulation in patients with Parkinson’s disease. Neurology. 1992;42:23–8. discussion 57-60. [PubMed] [Google Scholar]
- 34.Rylander D, Parent M, O'Sullivan SS, Dovero S, Lees AJ, Bezard E, et al. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol. 2010;68:619–628. doi: 10.1002/ana.22097. [DOI] [PubMed] [Google Scholar]
- 35.Zeng BY, Iravani MM, Jackson MJ, Rose S, Parent A, Jenner P. Morphological changes in serotonergic neurites in the striatum and globus pallidus in levodopa primed MPTP treated common marmosets with dyskinesia. Neurobiol Dis. 2010;40:599–607. doi: 10.1016/j.nbd.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Arluison M, de la Manche IS. High resolution radioautographic study of the serotonin innervation of the rat corpus striatum after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1980;5:229–240. doi: 10.1016/0306-4522(80)90100-1. [DOI] [PubMed] [Google Scholar]
- 37.Corvaja N, Doucet G, Bolam JP. Ultrastructure and synaptic targets of the raphe-nigral projection in the rat. Neuroscience. 1993;55:417–427. doi: 10.1016/0306-4522(93)90510-m. [DOI] [PubMed] [Google Scholar]
- 38.Descarries L, Soghomonian JJ, Garcia S, Doucet G, Bruno JP. Ultrastructural analysis of the serotonin hyperinnervation in adult rat neostriatum following neonatal dopamine denervation with 6-hydroxydopamine. Brain Res. 1992;569:1–13. doi: 10.1016/0006-8993(92)90363-e. [DOI] [PubMed] [Google Scholar]
- 39.Soghomonian JJ, Descarries L, Watkins KC. Serotonin innervation in the adult rat neostriatum. II. Ultrastructural features: a radioautographic and immunocytochemical study. Brain Res. 1989;481:67–86. doi: 10.1016/0006-8993(89)90486-1. [DOI] [PubMed] [Google Scholar]
- 40.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci USA. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schäfer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 43.Takamori S, Malherbe P, Broger C, Jahn R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep. 2002;3:798–803. doi: 10.1093/embo-reports/kvf159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, et al. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weston MC, Nehring RB, Wojcik SM, Rosenmund C. Interplay between VGLUT isoforms and endophilin A1 regulates neurotransmitter release and short-term plasticity. Neuron. 2011;69:1147–1159. doi: 10.1016/j.neuron.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 46.El Mestikawy S, Wallén-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- 47.Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, et al. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Com Neurol. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- 48.Johnson MD. Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron. 1994;12:433–442. doi: 10.1016/0896-6273(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 49.Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, et al. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- 50.Dupre KB, Ostock CY, Eskow Jaunarajs KL, Button T, Savage LM, Wolf W, et al. Local modulation of striatal glutamate efflux by serotonin 1A receptor stimulation in dyskinetic, hemiparkinsonian rats. Exp Neurol. 2011;229:288–299. doi: 10.1016/j.expneurol.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piccini P, Pavese N, Hagell P, Reimer J, Björklund A, Oertel WH, et al. Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease. Brain. 2005;128:2977–2986. doi: 10.1093/brain/awh649. [DOI] [PubMed] [Google Scholar]
- 52.Kirik D, Winkler C, Björklund A. Growth and functional efficacy of intrastriatal nigral transplants depend on the extent of nigrostriatal degeneration. J Neurosci. 2001;21:2889–2896. doi: 10.1523/JNEUROSCI.21-08-02889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson's disease. Brain Res. 1988;455:148–152. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- 54.Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, et al. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
- 55.Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, et al. Evidence of a breakdown of corticostriatal connections in Parkinson's disease. Neuroscience. 2005;132:741–754. doi: 10.1016/j.neuroscience.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 57.Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience. 2007;149:457–464. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol. 2009;215:220–227. doi: 10.1016/j.expneurol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- 60.Freund TF, Bolam JP, Björklund A, Stenevi U, Dunnett SB, Powell JF, et al. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: a tyrosine hydroxylase immunocytochemical study. J Neurosci. 1985;5:603–616. doi: 10.1523/JNEUROSCI.05-03-00603.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahalik TJ, Finger TE, Stromberg I, Olson L. Substantia nigra transplants into denervated striatum of the rat: ultrastructure of graft and host interconnections. J Comp Neurol. 1985;240:60–70. doi: 10.1002/cne.902400105. [DOI] [PubMed] [Google Scholar]
- 62.Bolam JP, Freund TF, Björklund A, Dunnett SB, Smith AD. Synaptic input and local output of dopaminergic neurons in grafts that functionally reinnervate the host neostriatum. Exp Brain Res. 1987;68:131–146. doi: 10.1007/BF00255240. [DOI] [PubMed] [Google Scholar]
- 63.Clarke DJ, Dunnett SB, Isacson O, Sirinathsinghji DJ, Björklund A. Striatal grafts in rats with unilateral neurostriatal lesions--I. Ultrastructural evidence of afferent synaptic inputs from the host nigrostriatal pathway. Neuroscience. 1988;24:791–801. doi: 10.1016/0306-4522(88)90067-x. [DOI] [PubMed] [Google Scholar]
- 64.Triarhou LC, Brundin P, Doucet G, Norton J, Björklund A, Ghetti B. Intrastriatal implants of mesencephalic cell suspensions in weaver mutant mice: ultrastructural relationships of dopaminergic dendrites and axons issued from the graft. Exp Brain Res. 1990;79:3–17. doi: 10.1007/BF00228869. [DOI] [PubMed] [Google Scholar]
- 65.Ulusoy A, Sahin G, Kirik D. Presynaptic dopaminergic compartment determines the susceptibility to L-DOPA-induced dyskinesia in rats. Proc Natl Acad Sci USA. 2010;107:13159–13164. doi: 10.1073/pnas.1003432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lundblad M, af Bjerkén S, Cenci MA, Permerleau F, Gerhardt GA, Strömberg I. Chronic intermittent L-DOPA treatment induces changes in dopamine release. J Neurochem. 2009;108:998–1008. doi: 10.1111/j.1471-4159.2008.05848.x. [DOI] [PubMed] [Google Scholar]
- 67.de la Fuente-Fernández R, Schulzer M, Mak E, Calne DB, Stoessl AJ. Presynaptic mechanism of motor fluctuations in Parkinson’s disease: a probabilistic model. Brain. 2004;127:888–899. doi: 10.1093/brain/awh102. [DOI] [PubMed] [Google Scholar]
- 68.Dal Bo G, Bérubé-Carrière N, Mendez JA, Leo D, Riad M, Descarries L, et al. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 2008;156:59–70. doi: 10.1016/j.neuroscience.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 69.Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88:1398–1405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- 70.Mendez JA, Bourque MJ, Dal Bo G, Bordeau ML, Danik M, Williams S, et al. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci. 2008;28:6309–6318. doi: 10.1523/JNEUROSCI.1331-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- 74.Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, et al. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- 75.Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, et al. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Joyce MP, Rayport S. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience. 2000;99:445–456. doi: 10.1016/s0306-4522(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 77.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, et al. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 80.Klemann CJ, Roubos EW. The gray area between synapse structure and function-Gray’s synapse types I and II revisited. Synapse. 2011;65:1222–1230. doi: 10.1002/syn.20962. [DOI] [PubMed] [Google Scholar]
- 81.Kobylecki C, Cenci MA, Crossman AR, Ravenscroft P. Calcium-permeable AMPA receptors are involved in the induction and expression of l-DOPA-induced dyskinesia in Parkinson’s disease. J Neurochem. 2010;114:499–511. doi: 10.1111/j.1471-4159.2010.06776.x. [DOI] [PubMed] [Google Scholar]
- 82.Ouattara B, Hoyer D, Grégoire L, Morissette M, Gasparini F, Gomez-Mancilla B, et al. Changes of AMPA receptors in MPTP monkeys with levodopa-induced dyskinesias. Neuroscience. 2010;167:1160–1167. doi: 10.1016/j.neuroscience.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 83.Silverdale MA, Kobylecki C, Hallett PJ, Li Q, Dunah AW, Ravenscroft P, et al. Synaptic recruitment of AMPA glutamate receptor subunits in levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate. Synapse. 2010;64:177–180. doi: 10.1002/syn.20739. [DOI] [PubMed] [Google Scholar]
- 84.Geinisman Y, deToledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991;566:77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- 85.Lane EL, Soulet D, Vercammen L, Cenci MA, Brundin P. Neuroinflammation in the generation of post-transplantation dyskinesia in Parkinson’s disease. Neurobiol Dis. 2008;32:220–228. doi: 10.1016/j.nbd.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 86.Villalba RM, Smith Y. Neuroglial plasticity at striatal glutamatergic synapses in Parkinson’s disease. Front Syst Neurosci. 2011;5:68. doi: 10.3389/fnsys.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tremblay MÈ, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tancredi V, Zona C, Velotti F, Eusebi F, Santoni A. Interleukin-2 suppresses established long-term potentiation and inhibits its induction in the rat hippocampus. Brain Res. 1990;525:149–151. doi: 10.1016/0006-8993(90)91331-a. [DOI] [PubMed] [Google Scholar]
- 89.Fuller RW, Snoddy HD, Molloy BB. Blockade of amine depletion by nisoxetine in comparison to other uptake inhibitors. Psychopharmacol Commun. 1975;1:455–464. [PubMed] [Google Scholar]
- 90.Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med. 2010;2:38ra46. doi: 10.1126/scitranslmed.3000976. [DOI] [PubMed] [Google Scholar]
- 91.Politis M, Oertel WH, Wu K, Quinn NP, Pogarell O, Brooks DJ, et al. Graft-induced dyskinesias in Parkinson’s disease: High striatal serotonin/dopamine transporter ratio. Mov Disord. 2011;26:1997–2003. doi: 10.1002/mds.23743. [DOI] [PubMed] [Google Scholar]
- 92.Taylor DP. Buspirone, a new approach to the treatment of anxiety. FASEB J. 1988;2:2445–2452. doi: 10.1096/fasebj.2.9.2836252. [DOI] [PubMed] [Google Scholar]
- 93.Caccia S, Conti I, Viganó G, Garattini S. 1-(2-Pyrimidinyl)-piperazine as active metabolite of buspirone in man and rat. Pharmacology. 1986;33:46–51. doi: 10.1159/000138199. [DOI] [PubMed] [Google Scholar]
- 94.Engberg G. A metabolite of buspirone increases locus coeruleus activity via alpha 2-receptor blockade. J Neural Transm. 1989;76:91–98. doi: 10.1007/BF01578749. [DOI] [PubMed] [Google Scholar]
- 95.Golbert A, Rivet JM, Cistarelli L, Melon C, Millan MJ. Alpha2-adrenergic receptor blockade markedly potentiates duloxetine- and fluoxetine-induced increases in noradrenaline, dopamine and serotonin levels in the frontal cortex of freely-moving rats. J Neurochem. 1997;69:2616–2619. doi: 10.1046/j.1471-4159.1997.69062616.x. [DOI] [PubMed] [Google Scholar]
- 96.Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- 97.Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Lane EL, Brundin P, Cenci MA. Amphetamine-induced abnormal movements occur independently of both transplant- and host-derived serotonin innervation following neural grafting in a rat model of Parkinson’s disease. Neurobiol Dis. 2009;35:42–51. doi: 10.1016/j.nbd.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 99.Köhler C, Hall H, Ogren SO, Gawell L. Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem Pharmacol. 1985;34:2251–2259. doi: 10.1016/0006-2952(85)90778-6. [DOI] [PubMed] [Google Scholar]
- 100.Billard W, Ruperto V, Crosby G, Iorio LC, Barnett A. Characterization of the binding of 3H-SCH 23390, a selective D-1 receptor antagonist ligand, in rat striatum. Life Sci. 1984;35:1885–1893. doi: 10.1016/0024-3205(84)90540-x. [DOI] [PubMed] [Google Scholar]
- 101.Lehmann J, Langer SZ. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience. 1983;10:1105–1120. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- 102.Bernardi G, Floris V, Marciani MG, Morocutti C, Stanzione P. The action of acetylcholine and L-glutamatic acid on rat caudate neurons. Brain Res. 1976;114:134–138. doi: 10.1016/0006-8993(76)91014-3. [DOI] [PubMed] [Google Scholar]
- 103.Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Elbert PJ, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 105.Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–1024. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 106.Kozell B, Meshul K. Alterations in nerve terminal glutamate immunoreactivity in the nucleus accumbens and ventral tegmental area following single and repeated doses of cocaine. Psychopharmcology (Berl) 2003;165:337–345. doi: 10.1007/s00213-002-1296-7. [DOI] [PubMed] [Google Scholar]
- 107.Kozell LB, Meshul CK. Nerve terminal glutamate immunoreactivity in the rat nucleus accumbens and ventral tegmental area after a short withdrawal from cocaine. Synapse. 2004;51:224–232. doi: 10.1002/syn.10304. [DOI] [PubMed] [Google Scholar]
- 108.Pinard R, Benfares J, Lanoir J. Electron microscopic study of GABA-immunoreactive neuronal processes in the superficial gray layer of the rat superior colliculus: their relationships with degenerating retinal nerve endings. J Neurocytol. 1991;20:262–276. doi: 10.1007/BF01235544. [DOI] [PubMed] [Google Scholar]
- 109.Baron MK, Boeckers TM, Vaida B, Faham S, Gingery M, Sawaya MR, et al. An architectural framework that may lie at the core of the postsynaptic density. Science. 2006;311:531–535. doi: 10.1126/science.1118995. [DOI] [PubMed] [Google Scholar]
- 110.Li KW, Jimenez CR. Synaptic proteomics: current status and quantitative applications. Expert Rev Proteomics. 2008;5:353–360. doi: 10.1586/14789450.5.2.353. [DOI] [PubMed] [Google Scholar]
- 111.Meredith GE, De Souza IE, Hyde TM, Tipper G, Wong ML, Egan MF. Persistent alterations in dendrites, spines, and dynorphinergic synapses in the nucleus accumbens shell of rats with neuroleptic-induced dyskinesias. J Neurosci. 2000;20:7798–7806. doi: 10.1523/JNEUROSCI.20-20-07798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 113.Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, et al. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cenci MA, Ohlin KE. Rodent models of treatment-induced motor complications in Parkinson’s disease. Parkinsonism Relat Disorder. 2009;15:S13–S17. doi: 10.1016/S1353-8020(09)70828-4. [DOI] [PubMed] [Google Scholar]
- 116.Klintmalm GB. The history of organ transplantation in the Baylor Health Care System. Proc (Bayl Univ Med Cent) 2004;17:23–34. doi: 10.1080/08998280.2004.11927954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pertlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204:643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- 118.Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch Neurol. 1989;46:615–631. doi: 10.1001/archneur.1989.00520420033021. [DOI] [PubMed] [Google Scholar]
- 119.Yukhananov R, Kissin I. Persistent changes in spinal cord gene expression after recovery from inflammatory hyperalgesia: a preliminary study on pain memory. BMC Neurosci. 2008;9:32. doi: 10.1186/1471-2202-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rasmussen S, Wang Y, Kivisäkk P, Bronson RT, Meyer N, Imitola J, et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing--remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–2829. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- 121.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 122.Cenci MA. Transcription factors involved in the pathogenesis of L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Amino Acids. 2002;23:105–109. doi: 10.1007/s00726-001-0116-4. [DOI] [PubMed] [Google Scholar]
- 123.García J, Carlsson T, Döbrössy M, Nikkhah G, Winkler C. Impact of dopamine to serotonin cell ratio in transplants on behavioral recovery and L-DOPA-induced dyskinesia. Neurobiol Dis. 2011;43:576–587. doi: 10.1016/j.nbd.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 124.Cooper O, Astradsson A, Hallet P, Robertson H, Mendez I, Isacson O. Lack of functional relevance of isolated cell damage in transplants of Parkinson’s disease patients. J Neurol. 2009;256:310–316. doi: 10.1007/s00415-009-5242-z. [DOI] [PubMed] [Google Scholar]
- 125.Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steece-Collier K, Soderstrom KE, Collier TJ, Sortwell CE, Maries-Lad E. Effect of levodopa priming on dopamine neuron transplant efficacy and induction of abnormal involuntary movement in parkinsonian rats. J Comp Neurol. 2009;515:15–30. doi: 10.1002/cne.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lane EL, Vercammen L, Cenci MA, Brundin P. Priming for L-DOPA-induced abnormal involuntary movements increases the severity of amphetamine-induced dyskinesia in grafted rats. Exp Neurol. 2009;219:355–358. doi: 10.1016/j.expneurol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 128.Paillé V, Picconi B, Bagetta V, Ghiglieri V, Sgobio C, Di Filippo M, et al. Distinct levels of dopamine denervation differentially alter striatal synaptic plasticity and NMDA receptor subunit composition. J Neurosci. 2010;30:14182–14193. doi: 10.1523/JNEUROSCI.2149-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Breysse N, Carlsson T, Winkler C, Björklund A, Kirik D. The functional impact of the intrastriatal dopamine neuron grafts in parkinsonian rats is reduced with advancing disease. J Neurosci. 2007;27:5849–5856. doi: 10.1523/JNEUROSCI.0626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Collier TJ, Sortwell CE, Daley BF. Diminished viability, growth, and behavioral efficacy of fetal dopamine neuron grafts in aging rats with long-term dopamine depletion: an argument for neurotrophic supplementation. J Neurosci. 1999;19:5563–5573. doi: 10.1523/JNEUROSCI.19-13-05563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, et al. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de la Fuente-Fernández R, Schulzer M, Kuramoto L, Cragg J, Ramachandiran N, Au WL, et al. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol. 2011;69:803–810. doi: 10.1002/ana.22284. [DOI] [PubMed] [Google Scholar]
- 133.Glavan G. Intermittent L-DOPA treatment differentially alters synaptotagmin 4 and 7 gene expression in the striatum of hemiparkinsonian rats. Brain Res. 2008;1236:216–224. doi: 10.1016/j.brainres.2008.07.071. [DOI] [PubMed] [Google Scholar]
- 134.Belujon P, Lodge DJ, Grace AA. Aberrant striatal plasticity is specifically associated with dyskinesia following levodopa treatment. Mov Disord. 2010;25:1568–1576. doi: 10.1002/mds.23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carlsson T, Carta M, Winkler C, Björklund A, Kirik D. Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. J Neurosci. 2007;27:8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grondin R, Bédard PJ, Britton DR, Shiosaki K. Potential therapeutic use of the selective dopamine D1 receptor agonist, A-86929: an acute study in parkinsonian levodopa-primed monkeys. Neurology. 1997;49:421–426. doi: 10.1212/wnl.49.2.421. [DOI] [PubMed] [Google Scholar]
- 137.Gomez-Mancilla B, Bédard PJ. Effect of D1 and D2 agonists and antagonists on dyskinesias produced by L-dopa in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys. J Pharmacol Exp Ther. 1991;259:409–413. [PubMed] [Google Scholar]
- 138.Monville C, Torres EM, Dunnett SB. Validation of the l-dopa-induced dyskinesia in the 6-OHDA model and evaluation of the effects of selective dopamine receptor agonists and antagonists. Brain Res Bull. 2005;68:16–23. doi: 10.1016/j.brainresbull.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 139.Blanchet P, Bédard PJ, Britton DR, Kababian JW. Differential effect of selective D-1 and D-2 dopamine receptor agonists on levodopa-induced dyskinesia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-exposed monkeys. J Pharmacol Exp Ther. 1993;267:275–279. [PubMed] [Google Scholar]
- 140.Kumar R, Riddle L, Griffen SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009;56:944–955. doi: 10.1016/j.neuropharm.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mela F, Millan MJ, Brocco M, Morari M. The selective D(3) receptor antagonist, S33084, improves parkinsonian-like motor dysfunction but does not affect L-DOPA-induced dyskinesia in 6-hydroxydopamine hemi-lesioned rats. Neuropharmacology. 2010;58:528–536. doi: 10.1016/j.neuropharm.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 142.Riddle LR, Kumar R, Griffin SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 dopamine receptor selective agonist/partial agonist PG01042 on L-dopa dependent animal involuntary movements in rats. Neuropharmacology. 2011;60:284–294. doi: 10.1016/j.neuropharm.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, et al. Critical involvement of cAMP/DARP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schuster S, Nadjar A, Guo JT, Li Q, Ittrich C, Hengerer B, Bezard E. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of L-DOPA-induced abnormal involuntary movements in experimental Parkinson's disease. J Neurosci. 2008;28:4311–4316. doi: 10.1523/JNEUROSCI.4720-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;80:ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- 146.Fox S, Silverdale M, Kellet M, Davies R, Steiger M, Fletcher N, et al. Non-subtype-selective opioid receptor antagonism in treatment of levodopa-induced motor complications in Parkinson’s disease. Mov Disord. 2004;19:554–560. doi: 10.1002/mds.10693. [DOI] [PubMed] [Google Scholar]
- 147.Koprich JB, Fox SH, Johnston TH, Goodman A, Le Bourdonnec B, Dolle RE, et al. The selective mu-opioid receptor antagonist ADL5510 reduces levodopa-induced dyskinesia without affecting antiparkinsonian action in MPTP-lesioned macaque model of Parkinson’s disease. Mov Disord. 2011;26:1225–1233. doi: 10.1002/mds.23631. [DOI] [PubMed] [Google Scholar]
- 148.Ikeda K, Yoshikawa S, Kurokawa T, Yuzawa N, Nakao K, Mochizuki H. TRK-820, a selective kappa opioid receptor agonist, could effectively ameliorate L-DOPA-induced dyskinesia symptoms in a rat model of Parkinson’s disease. Eur J Pharmacol. 2009;620:42–48. doi: 10.1016/j.ejphar.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 149.Cao X, Liang L, Hadcock JR, Iredale PA, Griffith DA, Menniti FS, et al. Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther. 2007;323:318–326. doi: 10.1124/jpet.107.125666. [DOI] [PubMed] [Google Scholar]
- 150.Morgese MG, Cassano T, Cuomo V, Giuffida A. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson’s disease: role of CB(1) and TRPV1 receptors. Exp Neurol. 2007;208:110–119. doi: 10.1016/j.expneurol.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology. 2001;57:2108–2111. doi: 10.1212/wnl.57.11.2108. [DOI] [PubMed] [Google Scholar]
- 152.Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- 153.Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 154.Olanow CW, Damier P, Goetz CG, Mueller T, Nutt J, Rascol O, et al. Multicenter, open-label, trial of sarizotan in Parkinson disease patients with levodopa-induced dyskinesias (the SPLENDID Study) Clin Neuropharmacol. 2004;27:58–62. doi: 10.1097/00002826-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 155.Vanover KE, Betz AJ, Weber SM, Bibbiani F, Kielaite A, Weiner DM, et al. A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav. 2008;90:540–544. doi: 10.1016/j.pbb.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lundblad M, Vaudano E, Cenci MA. Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. J Neurochem. 2003;84:1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- 157.Xiao D, Bastia E, Xu YH, Benn CL, Cha JH, Peterson TS, et al. Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J Neurosci. 2006;26:13548–13555. doi: 10.1523/JNEUROSCI.3554-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bordia T, Campos C, Huang L, Quick M. Continuous and intermittent nicotine treatment reduces L-3,4-dihyroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327:239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- 159.Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-DOPA-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Quik M, Cox H, Parameswaren N, O’Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol. 2007;62:588–596. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- 161.Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci USA. 2011;108:840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bibbiani F, Oh JD, Kielaite A, Collins MA, Smith C, Chase TN. Combined blockade of AMPA and NMDA glutamate receptors reduces levodopa-induced motor complications in animal models of PD. Exp Neurol. 2005;196:422–429. doi: 10.1016/j.expneurol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 163.Papa SM, Chase TN. Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol. 1996;39:574–578. doi: 10.1002/ana.410390505. [DOI] [PubMed] [Google Scholar]
- 164.Kobylecki C, Cenci MA, Crossman AR, Ravenscroft P. Calcium-permeable AMPA receptors are involved in the induction and expression of l-DOPA-induced dyskinesia in Parkinson’s disease. J Neurochem. 2010;114:499–511. doi: 10.1111/j.1471-4159.2010.06776.x. [DOI] [PubMed] [Google Scholar]
- 165.Konitsiotis S, Blanchet PJ, Verhagen L, Lamers E, Chase TN. AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology. 2000;54:1589–1595. doi: 10.1212/wnl.54.8.1589. [DOI] [PubMed] [Google Scholar]
- 166.Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM. Comparison of the basal ganglia in rats, marmosets,macaques, baboons, and humans: volume andneuronal number for the output, internal relay, and striatal modulating nuclei. J Comp Neurol. 2002;445(3):238–255. doi: 10.1002/cne.10165. [DOI] [PubMed] [Google Scholar]
- 167.Spieles-Engemann AL, Behbehani MM, Collier TJ, Wohlgenant SL, Steece-Collier K, Paumier K, Daley BF, Gombash S, Madhavan L, Mandybur GT, Lipton JW, Terpstra BT, Sortwell CE. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis. 2010;39(1):105–115. doi: 10.1016/j.nbd.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oorschot DE. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol. 1996;366(4):580–599. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 169.Andersson C, Hamer RM, Lawler CP, Mailman RB, Lieberman JA. Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2002;27(2):143–151. doi: 10.1016/S0893-133X(02)00287-7. [DOI] [PubMed] [Google Scholar]
- 170.Hsu JW, Lee LC, Chen RF, Yen CT, Chen YS, Tsai ML. Striatal volume changes in a rat model of childhood attention-deficit/hyperactivity disorder. Psychiatry Res. 2010;179(3):338–341. doi: 10.1016/j.psychres.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 171.Roberts RC, Force M, Kung L. Dopaminergic synapses in the matrix of the ventrolateral striatum after chronic haloperidol treatment. Synapse. 2002;45(2):78–85. doi: 10.1002/syn.10081. [DOI] [PubMed] [Google Scholar]
- 172.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999;122(Pt 8):1421–1436. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]
- 173.Ma SY, Ciliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ, Kordower JH, Mash DC, Levey AI, Mufson EJ. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J Comp Neurol. 1999;409(1):25–37. doi: 10.1002/(sici)1096-9861(19990621)409:1<25::aid-cne3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 174.Yin D, Valles FE, Fiandaca MS, Forsayeth J, Larson P, Starr P, Bankiewicz KS. Striatal volume differences between non-human and human primates. J Neurosci Methods. 2009;176(2):200–205. doi: 10.1016/j.jneumeth.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Raz N, Torres IJ, Acker JD. Age, gender, and hemispheric differences in human striatum: a quantitative review and new data from in vivo MRI morphometry. Neurobiol Learn Mem. 1995;63(2):133–142. doi: 10.1006/nlme.1995.1013. [DOI] [PubMed] [Google Scholar]