Abstract
Photosystem biogenesis in the thylakoid membrane is a highly complicated process that requires the coordinated assembly of nucleus-encoded and chloroplast-encoded protein subunits as well as the insertion of hundreds of cofactors, such as chromophores (chlorophylls, carotenoids) and iron-sulfur clusters. The molecular details of the assembly process and the identity and functions of the auxiliary factors involved in it are only poorly understood. In this work, we have characterized the chloroplast genome-encoded ycf4 (for hypothetical chloroplast reading frame no. 4) gene, previously shown to encode a protein involved in photosystem I (PSI) biogenesis in the unicellular green alga Chlamydomonas reinhardtii. Using stable transformation of the chloroplast genome, we have generated ycf4 knockout plants in the higher plant tobacco (Nicotiana tabacum). Although these mutants are severely affected in their photosynthetic performance, they are capable of photoautotrophic growth, demonstrating that, different from Chlamydomonas, the ycf4 gene product is not essential for photosynthesis. We further show that ycf4 knockout plants are specifically deficient in PSI accumulation. Unaltered expression of plastid-encoded PSI genes and biochemical analyses suggest a posttranslational action of the Ycf4 protein in the PSI assembly process. With increasing leaf age, the contents of Ycf4 and Y3IP1, another auxiliary factor involved in PSI assembly, decrease strongly, whereas PSI contents remain constant, suggesting that PSI is highly stable and that its biogenesis is restricted to young leaves.
The light reactions of photosynthesis are performed by a highly complex macromolecular machinery that resides in the thylakoid membrane. In photosynthetic eukaryotes, the thylakoids are encapsulated in a dedicated organelle, the chloroplast. Over the past years, remarkable progress has been made with resolving the composition and three-dimensional structure of the major thylakoidal protein complexes involved in photosynthetic electron transfer and ATP synthesis (for review, see Nelson and Ben-Shem, 2004; Nelson and Yocum, 2006). In contrast, we still know very little about the biogenesis of these big multiprotein complexes. Their assembly requires the coordinated synthesis of many protein subunits, some of which are encoded in the chloroplast genome and others in the nuclear genome. These proteins need to be inserted into the thylakoid membrane in a sequential order (Ossenbühl et al., 2004; Rokka et al., 2005). Moreover, hundreds of cofactors, such as chlorophylls, carotenoids, quinones, and iron-sulfur clusters, need to find their correct place in the complexes. How the cell accomplishes the daunting task of assembling the photosynthetic complexes in the thylakoid membrane is still largely a mystery.
PSI, the plastocyanin-ferredoxin oxidoreductase of the photosynthetic electron transport chain, is one of the largest multiprotein complexes known to reside in biological membranes. In photosynthetic eukaryotes, PSI is composed of 15 protein subunits (PsaA–PsaL and PsaN–PsaP). Five of these subunits (PsaA–PsaC, PsaI, and PsaJ) are encoded in the chloroplast genome of higher plants; the others are encoded by nuclear genes and posttranslationally imported into the chloroplast compartment. Four subunits (PsaG, PsaH, PsaN, and PsaO) represent evolutionarily new acquisitions in photosynthetic eukaryotes, whereas one subunit (PsaM) found in cyanobacterial PSI was lost and is not present in eukaryotic PSI complexes (Amunts et al., 2007, 2010; Busch and Hippler, 2011; Schöttler et al., 2011). In addition to the 15 subunits constituting the catalytically active PSI core complex, at least four stably bound light-harvesting complex proteins forming the PSI antenna (LhcA1–LhcA4) are associated with each monomeric PSI unit. PSI also harbors a huge number of cofactors, including at least 173 chlorophylls, two phylloquinones, three iron-sulfur clusters, and 15 carotenoids (Amunts et al., 2010).
PSI complex assembly is only poorly understood. One reason for this is that assembly intermediates cannot be readily identified, presumably because the assembly process occurs very fast (Ozawa et al., 2010). Also, assembly intermediates cannot be easily resolved by molecular mass-based separation techniques (such as gradient centrifugation or native electrophoresis), because PSI biogenesis begins with the formation of the large PsaA/PsaB reaction center heterodimer, which accounts for almost half the total molecular mass of PSI. Afterward, beginning with the three extrinsic subunits of the so-called stromal ridge involved in ferredoxin binding (PsaC, PsaD, and PsaE), only low-molecular-mass subunits are added to the reaction center, so that a resolution of the different assembly intermediates is much more challenging than in the case of other photosynthetic complexes (Schöttler et al., 2011).
In recent years, forward and reverse genetics approaches have provided a promising entry point into the study of PSI assembly. The analysis of mutants deficient in PSI accumulation has led to the discovery of several proteins that are required for efficient PSI biogenesis without being part of the active PSI complex (Boudreau et al., 1997; Ruf et al., 1997; Göhre et al., 2006; Stöckel et al., 2006; Albus et al., 2010). The molecular defects in these mutants fall into at least three different categories: (1) defects in cofactor synthesis; (2) defects in cofactor insertion; and (3) defects in protein subunit incorporation. As phylloquinone is a cofactor required only in PSI, mutants in phylloquinone biosynthesis show a specific deficiency in PSI accumulation (Lohmann et al., 2006). An example for a gene involved in cofactor insertion is Hcf101, which encodes a scaffold protein for [4Fe-4S] cluster assembly (Stöckel and Oelmüller, 2004; Schwenkert et al., 2010). A few proteins have been implicated in the assembly of the PSI protein subunits. ALBINO3 (ALB3) mediates the membrane insertion of the two large reaction center proteins, PsaA and PsaB (Göhre et al., 2006). It is not just involved in PSI biogenesis but presumably also in the assembly of all other protein complexes of the photosynthetic electron transport chain (Ossenbühl et al., 2004; Pasch et al., 2005; Schöttler et al., 2011). In contrast, PALE YELLOW GREEN7 (PYG7; Stöckel et al., 2006) acts in a PSI-specific manner. It contains three tetratricopeptide repeat domains, structural motifs known to mediate protein-protein interactions. Like ALB3, PYG7 is encoded in the nuclear genome.
Surprisingly, two other factors that have been implicated in PSI biogenesis are encoded in the chloroplast (plastid) genome. Knockout of the open reading frame ycf3 (for hypothetical chloroplast reading frame) in the higher plant tobacco (Nicotiana tabacum; Ruf et al., 1997) and the unicellular green alga Chlamydomonas reinhardtii (Boudreau et al., 1997) led to a complete and specific loss of PSI. Ycf3 was shown to act in PSI biogenesis at the posttranslational level (Boudreau et al., 1997; Ruf et al., 1997), and, like PYG7, it is a tetratricopeptide repeat protein. In Chlamydomonas, Ycf3 interacts with the PSI subunits PsaA and PsaD (Naver et al., 2001) and thus may chaperone the correct association of these proteins with each other and/or other subunits in the stromal ridge of the complex. Interestingly, transplastomic tobacco plants with reduced Ycf3 expression displayed a nearly proportional reduction in PSI accumulation (Petersen et al., 2011), possibly indicating a rate-limiting role of Ycf3 in PSI biogenesis. A search for protein interaction partners of Ycf3 in tobacco led to the discovery of a novel (nucleus-encoded) assembly factor, designated Y3IP1 (for Ycf3-interacting protein 1; Albus et al., 2010).
Reverse genetics in Chlamydomonas has provided evidence for a second plastid genome-encoded factor involved in PSI biogenesis: Ycf4 (Boudreau et al., 1997). Loss of ycf4 gene function resulted in the complete loss of PSI activity and, hence, loss of autotrophic growth. Consistent with this, earlier work in the cyanobacterium Synechocystis sp. PCC 6803 had shown that inactivation of the ycf4 homolog leads to an increased PSII-to-PSI ratio (Wilde et al., 1995). In Chlamydomonas, the thylakoid membrane-intrinsic Ycf4 protein was detected in complexes with the PSI subunits PsaA to PsaF and the opsin-related eyespot protein COP2 (Ozawa et al., 2009). The COP2 protein may be unlikely to function in PSI biogenesis, because silencing of the Cop2 gene by RNA interference did not affect PSI accumulation in the alga (Ozawa et al., 2009). Interestingly, Ycf4 was also identified as a protein component of the eyespot in Chlamydomonas chloroplasts (Schmidt et al., 2006), possibly suggesting a second function of Ycf4 (in association with COP2) in the eyespot. Because embryophytes do not have eyespots, the data raise questions about the validity of the findings in Chlamydomonas for higher plants.
Here, we have performed a functional analysis of the open reading frame ycf4 in the plastid genome of tobacco plants. Using chloroplast transformation, we have generated stable knockout mutants for ycf4. We show that the Ycf4 protein is specifically involved in PSI assembly and is present in protein complexes that are associated with the thylakoid membrane. However, unlike in Chlamydomonas, Ycf4 is not essential for photosynthesis in tobacco, and the ycf4 knockout mutants are capable of assembling sufficient amounts of PSI to allow for slow autotrophic growth.
RESULTS
Targeted Inactivation of the ycf4 Gene in the Tobacco Plastid Genome
The ycf4 gene is part of a gene cluster in the large single-copy region of the tobacco plastid genome, which comprises the psaI gene (encoding a small nonessential subunit of PSI; Schöttler et al., 2011) upstream of ycf4 and the genes ycf10 (encoding a nonessential membrane protein possibly involved in inorganic carbon uptake into the chloroplast; Rolland et al., 1997) and petA (encoding the cytochrome f subunit of the cytochrome b6f complex [Cyt bf]) downstream of ycf4 (Fig. 1A). To construct a knockout allele for ycf4, the corresponding region was cloned from the tobacco plastid DNA, and most of the ycf4 reading frame was deleted from the cloned fragment and replaced with a chimeric aadA cassette (Fig. 1A). The aadA gene serves as selectable marker for the isolation of chloroplast-transformed (transplastomic) cells (Goldschmidt-Clermont, 1991; Svab and Maliga, 1993) and encodes the enzyme aminoglycoside 3″-adenylyltransferase, which confers resistance to the aminoglycoside-type antibiotics spectinomycin and streptomycin. The aadA cassette was inserted in the same transcriptional orientation as ycf4 (Fig. 1A) to avoid the generation of antisense RNA that potentially could interfere with the expression of the neighboring genes.
Figure 1.
Targeted inactivation of the plastid genome-encoded open reading frame ycf4 by stable transformation of the chloroplast genome. A, Physical maps of the ycf4-containing region of the tobacco plastid genome and of the transformed plastid genome in Nt-Δycf4 transplastomic lines. All genes shown are transcribed from left to right. Restriction sites relevant for cloning and/or RFLP analysis of transplastomic plants are indicated. Hybridization probes used for Southern-blot and northern-blot analyses are denoted by horizontal bars. The expected sizes of hybridizing fragments in RFLP analysis of wild-type and transplastomic plants are indicated in kb. In Nt-Δycf4 transplastomic plants, most of the ycf4 coding region is deleted and replaced with the selectable marker gene aadA. The expression of aadA is driven by a chimeric rRNA operon promoter (Prrn) and the 3′ untranslated region from the psbA gene (Svab and Maliga, 1993). B, RFLP analysis of transplastomic lines carrying the ycf4 knockout allele. Total cellular DNA was digested with XhoI and HindIII and hybridized to a radioactively labeled probe detecting the accD region of the plastid genome, which flanks the transgene insertion site. Fragment sizes are given in kb. The size difference of 0.7 kb corresponds to the difference between the wild-type ycf4 gene and the mutant allele disrupted with the aadA selectable marker cassette (compare with A). C, Analysis of ycf4 mRNA accumulation (left panel) and analysis of the expression of petA, an essential gene for Cyt bf function located downstream of ycf4 (right panel), by northern blotting. As a control, RNA from a previously generated knockout line for ycf3, a plastid gene encoding an essential factor for PSI assembly (Ruf et al., 1997), was also loaded. Sizes of the RNA marker bands are given in kb. The absence of ycf4 transcripts from the Nt-Δycf4 transplastomic lines confirms the loss of ycf4 function from the plastid genome. As expected, expression of the aadA selectable marker gene cassette from the strong constitutive Prrn promoter causes increased accumulation of petA transcripts, due to read-through transcription (Zoubenko et al., 1994; Wurbs et al., 2007). To control for equal loading, the ethidium bromide (EtBr)-stained agarose gels photographed prior to blotting are also shown. WT, Wild type. D, Absence of detectable Ycf4 protein from Nt-Δycf4 transplastomic plants. To reveal the detection limit of the anti-Ycf4 antibody, a dilution series of thylakoid proteins from wild-type plants was loaded (20 µg chlorophyll = 100%), and the amount of loaded extract from the knockout plant was increased up to 1,000%. To control for loading, a membrane stained with Ponceau red (P.) is shown below the blot.
The ycf4 knockout vector was used to biolistically bombard tobacco leaves followed by selection for spectinomycin-resistant cell lines on a plant regeneration medium. Several independent spectinomycin-resistant clones were obtained and passed through three additional rounds of regeneration under stringent antibiotic selection to eliminate residual wild-type copies of the plastid genome and purify homoplasmic transplastomic clones (Bock, 2001; Maliga and Bock, 2011). Successful transformation of the plastid genome and faithful integration of the transforming DNA by homologous recombination was confirmed by DNA gel-blot analysis (Fig. 1B). As often seen in RFLP analyses of plastid transformants, faint hybridization signals corresponding in size to the wild-type DNA were detected in addition to strong signals for the transgenic plastid genome (Fig. 1B). These signals are not normally indicative of heteroplasmy (i.e. low-level presence of residual copies of the wild-type plastid genome) but rather correspond to so-called promiscuous DNA: chloroplast DNA pieces that have integrated into the nuclear genome (Ayliffe et al., 1998; Hager et al., 1999; Ruf et al., 2000; Bock and Timmis, 2008). To confirm homoplasmy of the ycf4 knockout plants, two independently generated transplastomic lines were selected (subsequently referred to as Δycf4-1 and Δycf4-2) and analyzed by northern blotting (Fig. 1C). Hybridization of RNA gel blots to a ycf4-specific probe revealed the complete loss of ycf4 expression (Fig. 1C), as expected. To confirm that the knockout of ycf4 does not impair the expression of adjacent genes (Fig. 1A), petA mRNA accumulation was analyzed. petA was chosen because, unlike psaI and ycf10 (whose knockout does not result in a mutant phenotype; Rolland et al., 1997; Schöttler et al., 2011; S. Ruf, K. Krech, D. Bednarczyk, M.A. Schöttler, and R. Bock, unpublished data), it encodes an essential protein for photosynthesis. Northern-blot analysis using a petA-specific probe revealed similar petA transcript patterns in the wild type and the Δycf4 mutants. The major transcript species accumulating in the wild type (more than 4 kb) corresponds to the tetracistronic mRNA covering all genes of the operon from psaI to petA (Fig. 1, A and C). The same tetracistronic transcript is also detected by the ycf4-specific probe in the wild type (Fig. 1C). The Δycf4 mutants accumulated even more petA transcripts than the wild type (Fig. 1C), which is most probably due to read-through transcription from the upstream aadA gene (which is driven by the strong ribosomal operon promoter Prrn; Svab and Maliga, 1993). Similar accumulation of read-through transcripts has been observed previously in many transplastomic studies (Zoubenko et al., 1994; Ruf et al., 1997).
To confirm the homoplasmy of the transplastomic Δycf4 mutants, seeds were harvested and germination assays were performed on spectinomycin-containing synthetic medium. The progeny of Δycf4 mutant plants turned out to be uniformly resistant to spectinomycin (Supplemental Fig. S1). This lack of phenotypic segregation in the next generation provides strong genetic evidence of homoplasmy (Bock, 2001; Maliga, 2004; Ruf et al., 2007).
To be able to analyze the expression of ycf4 at the protein level, we generated specific antibodies against Ycf4-derived specific peptide sequences. With these antibodies, the Ycf4 protein could be readily detected in thylakoids of wild-type tobacco plants, demonstrating that the protein is membrane associated (Fig. 1D). Complete absence of a hybridization signal from western blots with thylakoid proteins of the Δycf4 lines confirmed the specificity of the antibody and also provided additional evidence of homoplasmy of our transplastomic ycf4 knockout lines (Fig. 1D).
To ultimately prove homoplasmy and the lack of expression of the promiscuous ycf4-like sequences presumably present in the nucleus (Fig. 1B), we conducted quantitative real-time (qRT) PCR experiments. Whereas in the wild type ycf4 transcripts were detected at high sensitivity (even in a 1:100 cDNA dilution), no specific amplification was detectable with cDNA from the transplastomic Δycf4 mutants (Supplemental Fig. S2), thus confirming the absence of ycf4 gene product from our knockout lines.
Phenotype of Transplastomic ycf4 Knockout Plants
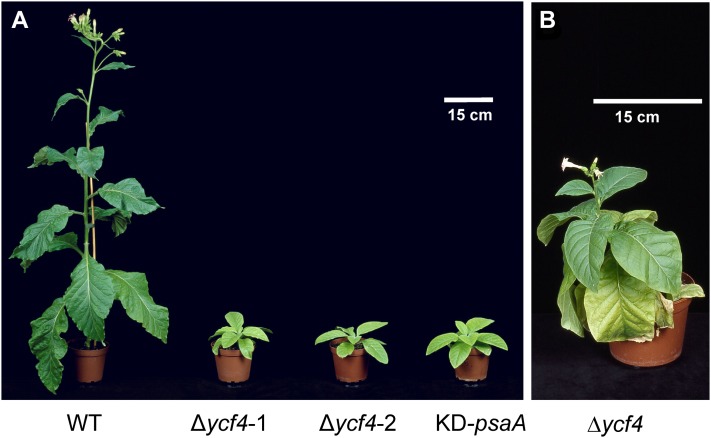
The successful generation of stable ycf4 knockout plants enabled us to analyze the phenotypic consequence of the loss of ycf4 gene function. To this end, mutant and wild-type plants were grown side-by-side in a controlled-environment chamber. The Δycf4 plants proved to be extremely sensitive to light and were unable to grow at light intensities higher than 80 µE m−2 s−1. However, under low-light conditions (40–50 µE m−2 s−1), Δycf4 mutant plants grew photoautotrophically. Although their growth and development were severely retarded (Fig. 2A), the plants eventually reached the reproductive stage and set a small number of flowers (Fig. 2B).
Figure 2.
Phenotypes of Δycf4 transplastomic tobacco lines. A, Wild-type (WT) and Δycf4 transplastomic knockout plants are shown after 8 weeks of growth in soil under low-light conditions (40–50 µE m−2 s−1). For comparison, a PSI-deficient mutant generated by knockdown of the gene encoding the reaction center subunit PsaA (KD-psaA; for details, see text) is also shown. B, After extended growth under low-light conditions (for 16 weeks), Δycf4 transplastomic lines reached the reproductive stage but developed only very few flowers.
Our finding that ycf4 knockout plants can grow autotrophically is in stark contrast to the situation in the unicellular green alga Chlamydomonas, where ycf4 mutant strains have been reported to be incapable of photoautotrophic growth (Boudreau et al., 1997). As work in Chlamydomonas had revealed an involvement of the Ycf4 gene product in PSI biogenesis, we also wanted to compare the phenotype of our Δycf4 plants with a mutant that is deficient in PSI accumulation. To this end, we selected a tobacco mutant that, under autotrophic growth conditions, has only 10% of the PSI levels of the wild type (on a leaf area basis) due to reduced translation initiation efficiency of the psaA mRNA encoding one of the two reaction center proteins of PSI (D. Bednarczyk, R. Bock, and M.A. Schöttler, unpublished data). Interestingly, the phenotype of this mutant (referred to as KD-psaA for knockdown of psaA) was very similar to that of the Δycf4 plants (Fig. 2A). Both mutants showed comparably slow growth and severe pigment deficiency, suggesting that their photosynthetic performance is similarly strongly affected.
Photosynthesis in ycf4 Knockout Plants
To characterize the mutant phenotype of the Δycf4 plants in more detail, several parameters related to the efficiency of the light reactions of photosynthesis were measured (Fig. 3). To minimize secondary effects from carbon starvation and/or photooxidative damage, all parameters were measured not only in plants grown autotrophically in soil but also in plants raised on Suc-containing synthetic medium in sterile containers (mixotrophic growth conditions; Fig. 3). As expected from the pale-green phenotype of the Δycf4 mutant plants, their chlorophyll content was significantly lower than in wild-type plants. Also, the chlorophyll a/b ratio was reduced in the mutants, possibly indicating a deficiency in the (mainly chlorophyll a-containing) photosystem core(s) relative to the antennae (Fig. 3). The maximum quantum efficiency of PSII was also significantly reduced in the Δycf4 plants, supporting a defect in the photosynthetic apparatus in the thylakoid membrane. To test if the defect can be attributed to a specific complex, we next determined the contents of the components of the photosynthetic electron transport chain (PSII, Cyt bf, plastocyanin, and PSI; Fig. 3) by spectroscopic methods (Schöttler et al., 2007a, 2007b). These analyses revealed that the Δycf4 mutants displayed a specific reduction in PSI contents (Fig. 3). PSI levels in plants raised under mixotrophic conditions reached only approximately 15% to 20% of wild-type levels. Plants grown in soil were even more strongly affected and had less than 10% of the wild-type levels of PSI. Under mixotrophic conditions, none of the other components of the electron transport chain were significantly affected by the loss of ycf4 function, whereas under autotrophic conditions, the Cyt bf was strongly decreased (Fig. 3). Importantly, this reduction was also seen in the KD-psaA control plants, clearly indicating that the lowered Cyt bf content is an indirect consequence of a severe PSI deficiency. Whether this loss of Cyt bf is triggered by a carbon starvation signal (released in the mutants upon autotrophic growth but suppressed by the sugar in the medium upon mixotrophic growth) or rather by increased oxidative stress under our autotrophic growth conditions remains to be investigated.
Figure 3.
Analysis of chlorophyll contents and various photosynthetic parameters in transplastomic Δycf4 knockout mutants and wild-type (WT) as well as KD-psaA control plants. Data sets are shown for plants grown under 40 to 50 μE m−2 s−1 in either synthetic Suc-containing medium (mixotrophic conditions) or soil (autotrophic conditions) for 6 weeks. For each plant line, three or more different plants were measured, and data were subjected to one-way ANOVA using a pairwise multiple comparison procedure (Holm-Sidak method) in SigmaPlot. Highly significant differences are indicated by asterisks (P < 0.05). Error bars represent sd. Photosynthetic complexes were quantified from difference absorbance measurements of cytochrome b559 (PSII), Cyt bf, plastocyanin, and P700 (PSI) in isolated thylakoids.
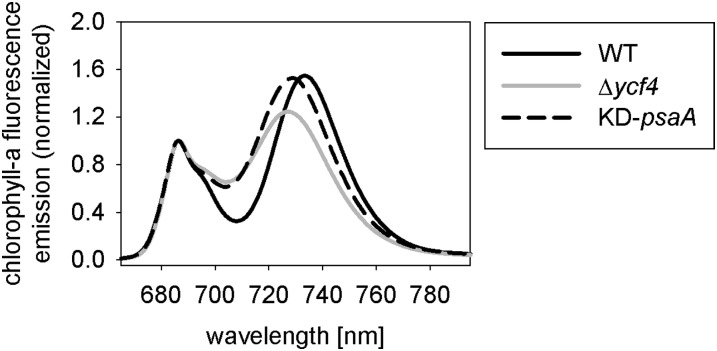
To further analyze the photosynthesis-deficient phenotype of the Δycf4 plants, chlorophyll a fluorescence emission measurements at 77 K were conducted. At this low temperature, the characteristic long-wavelength emission from PSI-LHCI can be readily analyzed and compared with the shorter wavelength fluorescence emission originating from PSII (Krause and Weis, 1991). Recording of 77 K chlorophyll a fluorescence emission spectra of wild-type plants and the Δycf4 mutants revealed that the maximum fluorescence emission from PSI (peaking at 733 nm in the wild type) was significantly shifted to shorter wavelengths in the Δycf4 mutant plants, and a very similar shift was also seen in the PSI-deficient KD-psaA mutant (Fig. 4). This suggests an altered functional organization of PSI (Jensen et al., 2004; Schöttler et al., 2007b) and indicates that a substantial proportion of the PSI antenna is not connected to PSI reaction centers (Stöckel et al., 2006). Taken together, the results from our spectroscopic analyses (Figs. 3 and 4) support a specific function of the Ycf4 protein in the biogenesis of PSI cores.
Figure 4.
The 77 K chlorophyll a fluorescence emission measurements. The fluorescence emission signals were normalized to the PSII emission maximum at 685-nm wavelength. The PSI emission signal peaks at 733 nm in the wild type (WT). The blue-shifted wavelength of the PSI emission maximum in the Δycf4 knockout mutants and the KD-psaA control plants indicates that PSI antenna organization is altered and suggests that a significant proportion of PSI antenna proteins are not attached to PSI core complexes.
Accumulation of Thylakoid Protein Complexes in Δycf4 Plants
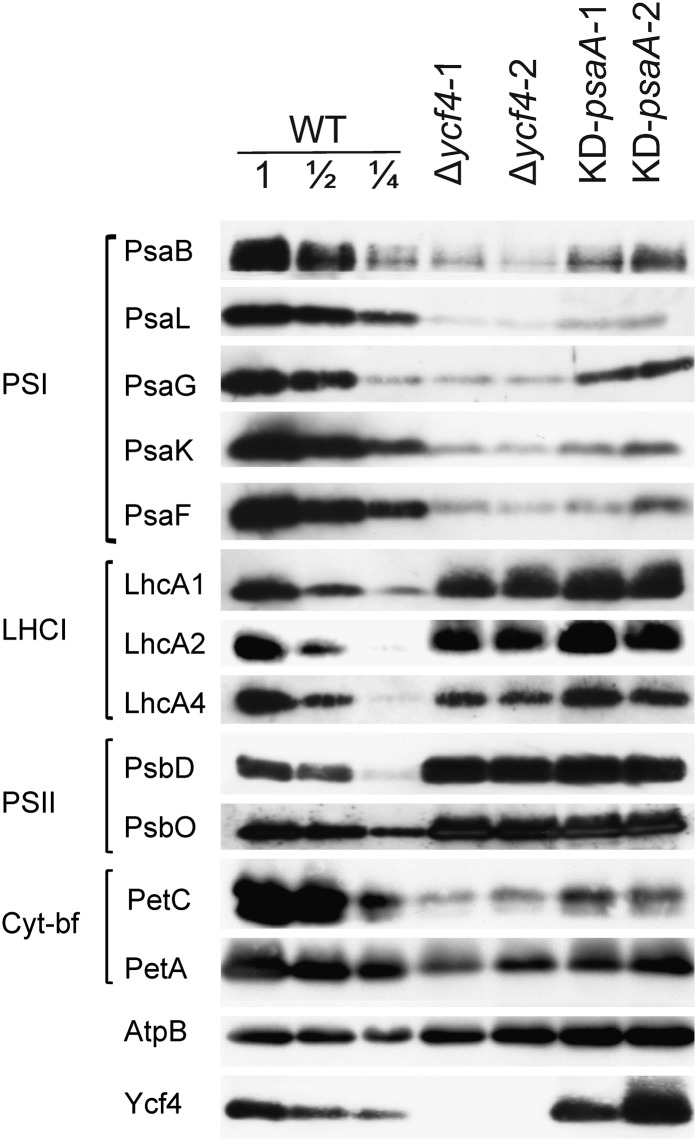
To confirm the PSI deficiency in Δycf4 mutant plants at the molecular level, thylakoids were isolated from soil-grown plants and analyzed immunobiochemically using a set of specific antibodies raised against diagnostic subunits of all four multiprotein complexes in the thylakoid membrane (PSII, Cyt bf, PSI, and ATP synthase). Five subunits of PSI were investigated: the reaction center protein PsaB, the plastocyanin-docking protein PsaF, and the three small subunits PsaG, PsaK, and PsaL. At least the reaction center protein PsaB is diagnostic of PSI complex accumulation in that no PSI can assemble in thylakoid membranes of psaB knockout mutants (Schaffner et al., 1995). This holds true also for the tested subunits of the other thylakoidal protein complexes: PsbD and PsbO (diagnostic of PSII), PetA and PetC (diagnostic of Cyt bf), and AtpB (diagnostic of the ATP synthase).
Immunobiochemical analysis of thylakoid proteins from two Δycf4 lines and the KD-psaA control mutant revealed a strong reduction in PSI amounts compared with wild-type tobacco plants (Fig. 5). The reduction was slightly stronger in the Δycf4 plants than in the KD-psaA plants, supporting the quantitative data of our spectroscopic measurements (Fig. 3). As the thylakoids were isolated from soil-grown plants, the western blots also showed the reduction in the Cyt bf complex secondarily occurring under autotrophic conditions (Fig. 3). Importantly, this reduction was also seen in the KD-psaA control plants, indicating that it represents a secondary consequence of strongly decreased PSI levels. In the Δycf4 lines and the KD-psaA mutant, the diagnostic subunits of PSII and the ATP synthase accumulated to similar or slightly elevated levels compared with the wild type (Fig. 5), confirming that Ycf4 is specifically required for PSI biogenesis and is unlikely to be directly involved in the biogenesis of the other complexes of the photosynthetic electron transport chain.
Figure 5.
Immunoblot analysis of diagnostic components of the multiprotein complexes in the thylakoid membrane in transplastomic Δycf4 knockout mutants, wild-type plants (WT), and KD-psaA control plants grown under autotrophic conditions. Isolated thylakoid proteins were separated by SDS-PAGE, blotted, and probed with antibodies against the proteins indicated on the left side of each panel. Equal amounts of chlorophyll were loaded. For quantitative assessment of protein accumulation in the mutants, a dilution series of the wild-type sample (100%, 50%, and 25%) was loaded. A Ycf4-specific antibody generated in this study was used to assess the accumulation of the ycf4 gene product.
To confirm the preliminary conclusion from our spectroscopic data (Fig. 4) that the Ycf4 protein specifically functions in the biogenesis of PSI cores, we also investigated the accumulation of the PSI antenna proteins LhcA1, LhcA2, and LhcA4 using specific antibodies. LhcA proteins have been shown to accumulate independently of the PSI reaction center (Stöckel and Oelmüller, 2004; Stöckel et al., 2006). In contrast to all PSI core subunits tested, accumulation of the three proteins of the PSI antenna (LHCI) was unaffected in the transplastomic Δycf4 lines (and, as expected, also in the KD-psaA control mutant; Fig. 5), suggesting that the function of Ycf4 in PSI biogenesis is restricted to the assembly of the core complex.
Interestingly, accumulation of the Ycf4 protein was not reduced in the psaA knockdown mutants (KD-psaA; Fig. 5). This shows that Ycf4 accumulates in the thylakoid membrane independently of PSI, a finding that is well compatible with an auxiliary role of Ycf4 in PSI biogenesis.
Faithful Expression of Plastid PSI Genes in ycf4 Knockout Plants
We next wanted to test whether Ycf4 controls PSI accumulation at the posttranslational level (i.e. at the level of complex assembly or stability). To this end, we sought to exclude the alternative possibilities that the Ycf4 protein is involved in the transcription of PSI genes, the accumulation of stable PSI mRNAs, and/or the translation of PSI subunits. As Ycf4 is encoded in the plastid genome and the protein therefore cannot be present in the nucleocytosolic compartment, only the PSI genes in the plastid genome are relevant to any possible function of Ycf4 in transcription, RNA stability, or translation. The tobacco plastid genome encodes three essential subunits of PSI: PsaA, PsaB (the two reaction center proteins of PSI encoded by the psaA/B operon and translated from a large polycistronic transcript; Meng et al., 1988), and PsaC (an essential iron-sulfur cluster-containing PSI protein; Takahashi et al., 1991). The other two small plastid-encoded PSI subunits, PsaJ and PsaI, are nonessential for PSI accumulation, and their loss has only very minor effects on PSI accumulation, which cannot account for the strong photosynthetic phenotype observed here (Schöttler et al., 2007b, 2011). We additionally included the plastid ycf3 gene in our analyses, because the Ycf3 protein has previously been shown to be an essential chloroplast genome-encoded assembly factor for PSI (Ruf et al., 1997; Albus et al., 2010).
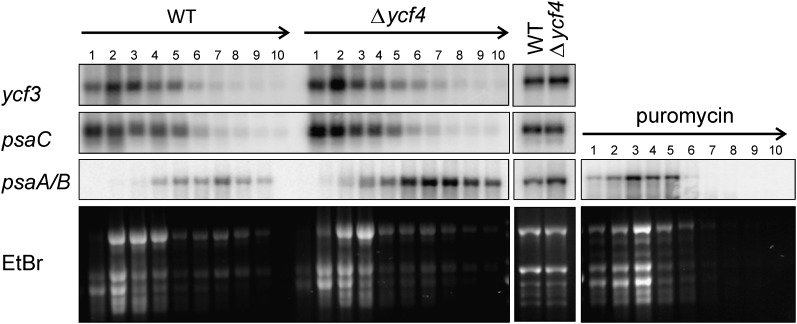
We compared transcript patterns and accumulation levels for these four essential plastid PSI-related genes in Δycf4 lines and wild-type plants. To test for a possible involvement of Ycf4 in the translation of plastid PSI mRNAs, the ribosome association of the psaA/B, psaC, and ycf3 mRNAs in Δycf4 plants and wild-type plants was also comparatively assessed by polysome-loading experiments. When the transcripts were investigated by northern blotting, none of them showed any reduction in RNA abundance or alteration in RNA processing patterns (Fig. 6), indicating that Ycf4 acts neither at the transcriptional level nor at the level of mRNA maturation or stability. Interestingly, the psaA/B mRNA level was found to be significantly higher in the Δycf4 mutant than in the wild type. This could indicate that the plant responds to the PSI deficiency by transcriptionally up-regulating the operon comprising the genes for the two PSI reaction center subunits. This transcriptional up-regulation was also clearly seen when ribosome-associated mRNAs were analyzed (Fig. 6). Polysomes are complexes of mRNAs covered with translating ribosomes, and their migration into continuous Suc gradients upon ultracentrifugation correlates with the number of ribosomes bound to the mRNA molecule (in that intensely translated mRNAs are loaded with many ribosomes and migrate deeper into the gradient than weakly translated mRNAs). For all the PSI-related plastid mRNAs analyzed, the polysome profiles (i.e. the mRNA distribution across the Suc gradient) in wild-type and Δycf4 mutant plants were very similar (Fig. 6), indicating that PSI mRNA translation proceeds at comparable efficiency. These data suggest that Ycf4 also does not act as a translation factor for plastid genome-encoded PSI mRNAs.
Figure 6.
Unaltered gene expression of all essential plastid-encoded PSI genes in Δycf4 knockout mutants. Translational efficiency for the essential plastid genome-encoded PSI-related genes psaA/B, psaC, and ycf3 was analyzed by polysome-loading assays. Collected fractions from the Suc density gradients are numbered from top to bottom. Equal aliquots of extracted RNAs from all fractions were separated by denaturing agarose gel electrophoresis, blotted, and hybridized to gene-specific radiolabeled probes. The arrows at the top indicate the gradient in Suc density (from low to high). As a control, a sample was treated with the antibiotic puromycin to cause the dissociation of ribosomes from the mRNAs. Ribosome distribution in the gradients is revealed by ethidium bromide (EtBr) staining of the agarose gels prior to blotting. PSI transcript patterns and mRNA accumulation levels were also determined (middle panel) for the three essential plastid genome-encoded PSI subunits (psaA/B and psaC) and the ycf3 gene encoding an essential PSI assembly factor (Ruf et al., 1997). To confirm equal loading, an ethidium bromide-stained agarose gels is also shown. WT, Wild type.
Taken together, our analyses of PSI gene transcription and translation strongly suggest that the PSI deficiency in Δycf4 mutants is not caused by a defect in plastid gene expression. Instead, the data are consistent with a function of Ycf4 at the posttranslational level, either in PSI assembly or PSI stability.
Association of the Ycf4 Protein with High-Mr Complexes
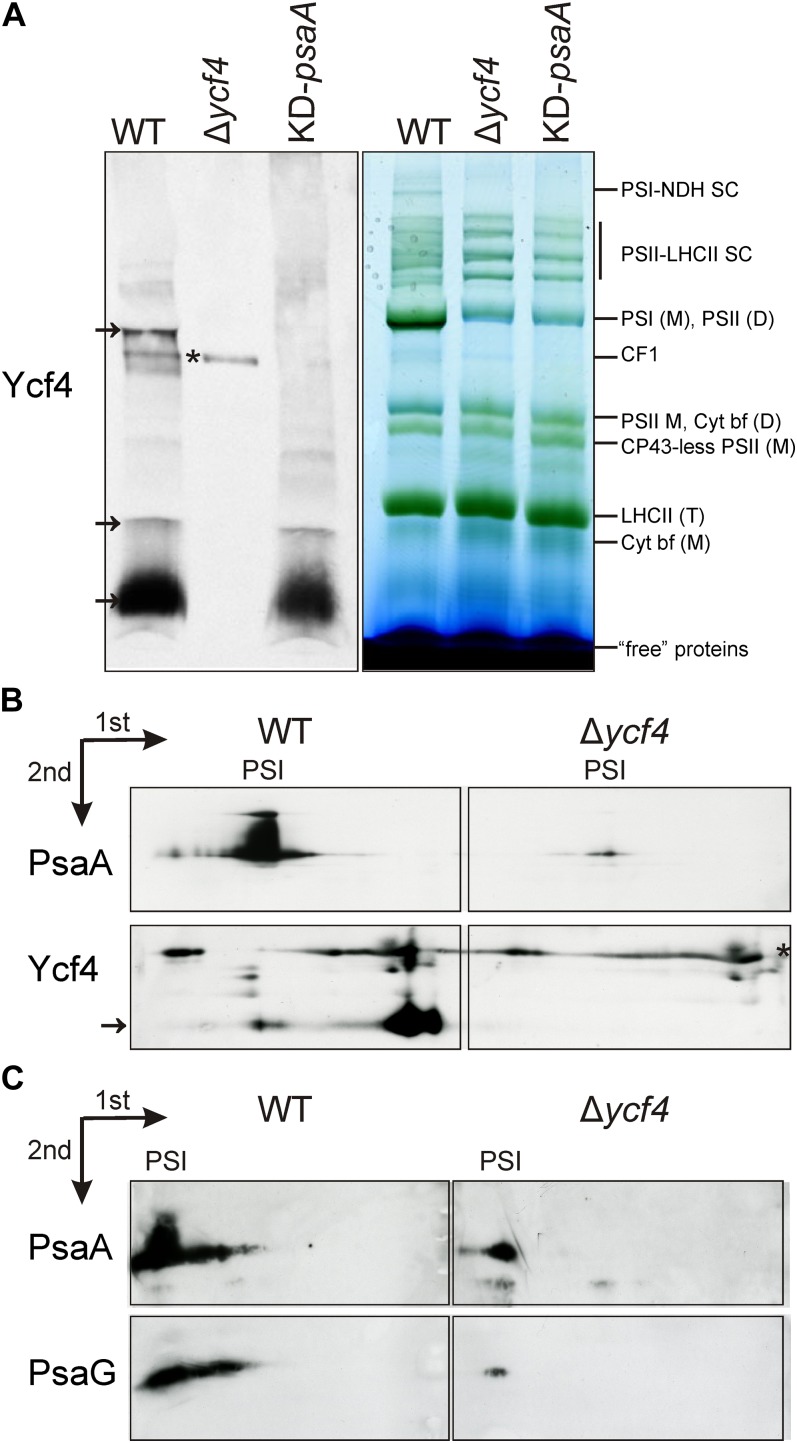
The successful generation of a sensitive Ycf4-specific antibody enabled us to assess the possible association of the Ycf4 protein with higher Mr complexes in the thylakoid membrane. To this end, we separated thylakoidal protein complexes by blue-native (BN) PAGE, blotted the gels, and hybridized the membranes to our anti-Ycf4 antibodies. In these experiments, the bulk of Ycf4 protein migrated with the non-complex-associated fraction (“free” proteins; Fig. 7A). A smaller fraction was associated with at least two different high-Mr complexes, one of which correlated in migration with PSI. This raises the possibility that a subfraction of Ycf4 is associated with PSI particles, either because it is involved in late steps of PSI assembly or because it helps in stabilizing the assembled complex.
Figure 7.
Detection of Ycf4-containing high-Mr complexes by BN gel electrophoresis. A, One-dimensional BN gel electrophoretic separation of thylakoidal protein complexes. Transplastomic Δycf4 knockout mutants, wild-type plants (WT), and KD-psaA control plants were analyzed. Arrows denote Ycf4-containing fractions. The asterisk indicates a nonspecific immunological cross-reaction (as evidenced by the presence of the signal in the Δycf4 knockout mutants). D, Dimer; M, monomer; SC, supercomplex; T, trimer. B, Two-dimensional electrophoretic analysis of PSI complexes and Ycf4-containing complexes in wild-type and Δycf4 transplastomic knockout plants. The first dimension was BN gel electrophoresis, and the second dimension was SDS-PAGE (see “Materials and Methods”). Nonspecific cross-reactions are indicated by the asterisk, and the Ycf4 protein is indicated by an arrow. C, Two-dimensional electrophoretic analysis of residual PSI complexes accumulating in the Δycf4 knockout mutant. The gels were blotted, and the blots were probed with antibodies against the PSI reaction center protein PsaA and the small PSI subunit PsaG. The identical electrophoretic migration of the PSI complexes from wild-type and mutant plants and the stable association of the small G-subunit with the complex also in the Δycf4 knockout suggest that the composition of the PSI complexes in the mutant is unaltered compared with the wild type.
To confirm this result and supply additional evidence for the association of Ycf4 with PSI particles, we performed two-dimensional gel electrophoresis experiments followed by western blotting and immunological detection of the Ycf4 protein and the PSI reaction center protein PsaA as a diagnostic subunit of PSI complexes. These analyses confirmed the comigration of a subfraction of the Ycf4 protein with PSI (Fig. 7B), thus providing further evidence for a physical interaction of Ycf4 with PSI complexes. Moreover, identical electrophoretic migration of the PSI complexes from wild-type and mutant plants and stable association of the small G-subunit with the complex also in the transplastomic Δycf4 knockout confirmed that the composition of the PSI complexes in the mutant is unaltered compared with the wild type (Fig. 7C).
Differential Accumulation of PSI and Its Assembly Factors during Plant Development
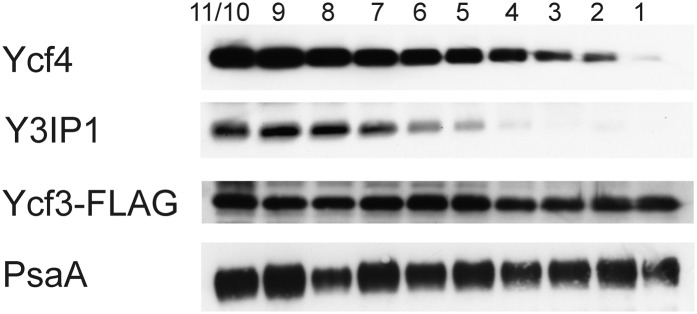
The sensitive detection of the Ycf4 protein by our specific antibody enabled us to investigate the relationship between Ycf4 protein accumulation and PSI accumulation during leaf ontogenesis. To conduct a more systematic analysis of PSI assembly during development, we also included two previously identified PSI assembly factors: the chloroplast genome-encoded Ycf3 (Ruf et al., 1997) and its nucleus-encoded interaction partner Y3IP1 (Albus et al., 2010). As PSI accumulation is strictly dependent on Ycf3 and Y3IP1 function, comparison of Ycf3 and Y3IP1 protein accumulation with PSI subunit accumulation may also provide information about the turnover rate of the PSI complex. For Ycf3, we had previously constructed an epitope-tagged transplastomic line expressing a Ycf3 protein with a C-terminal FLAG epitope (Albus et al., 2010). The use of this line enabled us to follow the fate of Ycf3 during development. Because for Y3IP1 no specific antibodies or tagged lines were available, we generated specific antibodies in rabbits. Testing of the antibodies in the wild type and Y3IP1 knockdown lines (Albus et al., 2010) confirmed their specificity and revealed that they detect the Y3IP1 protein at high sensitivity (Fig. 8).
Figure 8.
Developmental regulation of PSI assembly factors. Transplastomic Ycf3-FLAG lines (Albus et al., 2010) were used to follow the fate of three assembly factors for PSI: the plastid genome-encoded proteins Ycf3 and Ycf4 and the nuclear genome-encoded interaction partner of Ycf3, Y3IP1 (Schöttler et al., 2011). A developmental series of leaves from a tobacco plant prior to flowering is shown. Leaves are numbered from bottom to top. The two youngest leaves (nos. 10 and 11) had to be combined due to their small size. The assembly factors were detected with specific antibodies (for details, see text). For comparison, PSI accumulation was followed with an antibody against the essential reaction center subunit PsaA.
In order to analyze Ycf3, Y3IP1, and Ycf4 expression in relation to PSI accumulation during plant development, we compared the accumulation of all three proteins with that of the PSI reaction center subunit PsaA in a developmental series of leaves harvested from 12-week-old tobacco plants. In these plants, leaf 1 represents the oldest leaf and leaves 10 and 11 (which had to be combined due to their small size; Fig. 8) represent the youngest leaves at the top of the plant. Analysis of PsaA accumulation revealed comparably high amounts of PSI over the entire developmental series analyzed, well in line with previous observations (Schöttler et al., 2007b). A similar constitutive accumulation was seen for Ycf3. Interestingly, Y3IP1, the interaction partner of Ycf3, exhibited a very different pattern. Its accumulation was highest in very young leaves, continuously declined with leaf age, and was undetectable in older leaves (Fig. 8). As Y3IP1 is essential for PSI accumulation (Albus et al., 2010), this suggests that PSI biogenesis ceases in old leaves. As PSI levels remain unaltered, this in turn indicates a very high stability of the PSI complex. The Ycf4 protein showed a similar age-dependent decline in protein accumulation as Y3IP1 (Fig. 8). This indicates that Ycf4 is not required for PSI stability and suggests that Ycf4 acts as an assembly factor for PSI.
DISCUSSION
In this work, we have investigated the function of the open reading frame ycf4 in the plastid genome of the higher plant tobacco. Previous work in the unicellular alga Chlamydomonas had revealed that the Ycf4 gene product is essential for PSI biogenesis and photosynthetic activity (Boudreau et al., 1997). Our data demonstrate that, in the seed plant tobacco, the Ycf4 protein is neither essential for PSI biogenesis nor for photoautotrophic growth. Although the Δycf4 transplastomic mutants generated in this work had reduced levels of PSI and their growth was severely retarded, they assembled sufficient amounts of functional PSI complexes to enable autotrophic growth in soil. This points to mechanistic differences in PSI biogenesis between algae and higher plants and may indicate that higher plants have evolved additional auxiliary functions (proteins?) that can partially replace Ycf4 in PSI assembly. The identity of these additional accessory factors and their possible interplay with Ycf4 in one of the Ycf4-containing high-Mr complexes (Fig. 7) remains to be investigated. It is noteworthy in this respect that the ycf4 gene, although otherwise well conserved in the green lineage, has been lost from the chloroplast genome in the legume species Lathyrus odoratus and separately in three other groups of legumes (Magee et al., 2010). A nuclear copy of ycf4 could not be identified in Lathyrus (Magee et al., 2010), making it unlikely that a functional gene copy was transferred to the nuclear genome. Although this conclusion still awaits ultimate confirmation from whole-genome sequencing, it lends support to the idea that Ycf4 function can be replaced by other factor(s) acting in PSI assembly.
The study of algal strains expressing tagged and point-mutated versions of the Ycf4 protein has provided some evidence for a function of Ycf4 in the PSI assembly process (Onishi and Takahashi, 2009; Ozawa et al., 2009) but did not directly exclude a function in the translation of plastid genome-encoded PSI mRNAs. Performing polysome-loading analyses, we have shown here that, at least in higher plants, Ycf4 is unlikely to be involved in the synthesis of PSI subunits (Fig. 6). This suggests a posttranslational cause of the PSI deficiency in Δycf4 mutants, well compatible with a function of Ycf4 in PSI assembly and/or stabilization. At the moment, we can only speculate which specific steps of PSI biogenesis are supported by Ycf4. In Chlamydomonas, Ycf4 was found to be part of a PSI assembly intermediate complex comprising the three stromal ridge subunits and PsaF (Ozawa et al., 2009). Therefore, a role in either the formation of the stromal ridge or the insertion of PsaF seems conceivable (Ozawa et al., 2010). However, in our two-dimensional gel electrophoresis experiments, whereas most of the Ycf4 protein was found to be present as free protein, a significant subfraction of Ycf4 comigrated with the mature PSI complex (Fig. 7B). This may suggest a role rather late in PSI biogenesis, for example, in the attachment of the small peripheral subunits and/or the antenna proteins. A somewhat different function of Ycf4 in higher plants from the proposed function in Chlamydomonas would also be compatible with the different phenotypic effects of ycf4 inactivation in Chlamydomonas and tobacco.
Our analysis of Ycf4 expression during development supports a function of Ycf4 in PSI assembly rather than stability. Whereas PSI is still present in high amounts in mature and old leaves, the level of Ycf4 (and also that of Y3IP1; Fig. 8) declines continuously. The high requirement for PSI synthesis in young developing leaves is expected to coincide with a high demand for the PSI assembly factors Ycf3, Ycf4, and Y3IP1, thus potentially explaining their high abundance in very young leaf tissue (Fig. 8). The strong decline in Ycf4 and Y3IP1 accumulation during leaf development indicates that PSI biogenesis is restricted to young leaves, suggesting that PSI is highly stable. The evolutionary optimization of both the efficiency of the electron transfer reactions in PSI and the stability of the PSI core complex may have allowed plants to keep PSI turnover at a minimum and avoid the costly resynthesis of this gigantic pigment-protein complex. The accumulation of high amounts of PSI in the virtual absence of Y3IP1 and Ycf4 in old leaves furthermore suggests that the function of these factors is confined to the PSI assembly process and seems to exclude an important function in PSI stability.
The lack of coregulation of Ycf3 with its interaction partner Y3IP1 and with Ycf4 is somewhat surprising. Whether there is a continued demand for Ycf3 function in old leaves and in the absence of the other PSI assembly factors remains to be determined. One possibility could be that some components of the PSI complexes in mature and old leaves suffer photooxidative damage and need to be exchanged by newly synthesized molecules through the action of Ycf3 in a Y3IP1- and Ycf4-independent fashion. Indications for photoinhibition of the PSI acceptor side (Kudoh and Sonoike, 2002; Scheller and Haldrup, 2005; Sonoike, 2011) and a repair cycle replacing the stromal ridge subunits, which are most sensitive to oxidative damage, have recently been reported (Oh et al., 2009). Interestingly, Ycf3 has been specifically implicated in the assembly of PsaD into PSI (Naver et al., 2001) and therefore might also play an important role in the repair of the stromal ridge.
In sum, our work establishes the chloroplast-encoded Ycf4 protein as an important, but nonessential, factor for PSI assembly of higher plants. Together with previously identified assembly factors (Ruf et al., 1997; Stöckel et al., 2006; Albus et al., 2010; Schöttler et al., 2011), we now know at least a small set of proteins that are specifically involved in PSI biogenesis. The big challenge for the future will be to determine the relationship between these factors and reveal the mechanistic details of the assembly process.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum ‘Petit Havana’) plants were aseptically grown by germinating surface-sterilized seeds on agar-solidified Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) with 30 g L−1 Suc. Homoplasmic transplastomic lines were rooted and propagated on the same medium. Rooted homoplasmic plants were transferred to soil and grown under low-light conditions (40–50 µE m–2 s–1) in the greenhouse (day temperature, 20°C; night temperature, 18°C). For photosynthetic measurements with plants grown on Suc-containing synthetic medium, the sterile containers were kept in a controlled-environment chamber, and similar growth temperatures and light intensity as for autotrophic growth were chosen, except that the day temperature was 25°C and the night temperature was 20°C. Daylength was set to 16 h of light.
Cloning Procedures
To construct the chloroplast transformation plasmid pΔycf4, the cloning vector pBluescript II SK+ was digested with EcoRV and HincII and religated to eliminate the restriction site for ClaI. Subsequently, the ycf4-containing region of the tobacco plastid genome was cloned into the ClaI(−) pBluescript II SK+ vector as a 3,251-bp BamHI fragment (nucleotide positions 60,864–64,115 in the tobacco ptDNA; Yukawa et al., 2005; Fig. 1A). The resulting plasmid was digested with ClaI and treated with Klenow DNA polymerase to fill in the overhanging ends. An aadA cassette (Svab and Maliga, 1993) was ligated into the blunted ClaI site as an Ecl136II/DraI fragment generating the final plastid transformation vector pΔycf4.
Transformation of Tobacco Chloroplasts
Plastid transformation was carried out using the biolistic protocol (Svab and Maliga, 1993). Briefly, young leaves from tobacco plants grown under aseptic conditions were bombarded with plasmid DNA-coated 0.6-μm gold particles using a biolistic gun (PDS1000He; Bio-Rad). Primary spectinomycin-resistant lines were selected on regeneration medium containing spectinomycin (500 mg L−1; Svab and Maliga, 1993). Plastid transformation was preliminarily confirmed by double resistance tests on regeneration medium with both spectinomycin and streptomycin (500 mg L−1 each; Bock, 2001). Several independent transplastomic lines were subjected to three additional rounds of regeneration on spectinomycin-containing MS medium to eliminate residual wild-type plastome copies and obtain homoplasmic tissue. Homoplasmy was confirmed by inheritance assays (Bock, 2001), in which seeds were germinated on MS medium containing spectinomycin (500 mg L−1).
Isolation of Nucleic Acids and Gel-Blot Analyses
Total DNA from tobacco leaf samples was isolated by a cetyltrimethylammoniumbromide-based method (Doyle and Doyle, 1990). Total cellular RNA was extracted using the peqGOLD TriFast reagent (Peqlab). For Southern-blot analysis, samples of 5 µg of total DNA were digested with the restriction enzymes XhoI and HindIII (Fig. 1), separated by agarose gel electrophoresis on 1% agarose gels, and transferred onto Hybond nylon membranes (GE Healthcare) by capillary blotting. Total cellular RNA samples were electrophoresed on formaldehyde-containing 1% agarose gels and blotted onto Hybond nylon membranes. Hybridizations were performed at 65°C using standard protocols. Hybridization probes were purified by agarose gel electrophoresis after extraction of the DNA fragments of interest from excised gel slices using the GFX PCR (DNA and Gel Band Purification) kit (GE Healthcare). A 943-bp XhoI/BglII restriction fragment containing the 3′ part of the accD coding region was used as an RFLP probe to verify plastid transformation and assess homoplasmy. A ClaI restriction fragment (Fig. 1A) was used as a hybridization probe for the detection of ycf4 transcripts in northern-blot analyses, and a petA-specific probe was prepared by PCR amplification using total DNA as a template and the gene-specific primers PpetA5 (5′-GCGACTGGGCGTATTGTATGTGC-3′) and PpetA3 (5′-CGCCCTCGGAAACAAGAAGTTCTG-3′). [α-32P]dCTP-labeled probes were generated by random priming (Multiprime DNA labeling system; GE Healthcare). Hybridization signals were analyzed using a Typhoon Trio+ variable mode imager (GE Healthcare).
cDNA Synthesis and qRT-PCR
For cDNA synthesis, total plant RNA was isolated using the NucleoSpin RNA Plant kit (Macherey-Nagel) following the instructions of the supplier. To fully remove contaminating DNA, a second DNase digest was done using rDNase (Macherey-Nagel). cDNA synthesis was performed with random hexamer primer (3 µg per reaction) and the SuperScript III reverse transcriptase enzyme (Life Technologies) following the instructions of the manufacturer. qRT-PCR assays were performed according to standard protocols using SYBR Green to monitor the amplification process. ycf4 was amplified with primers Pycf4-5 (5′-GGCGATCAGAACATATATGGATAG-3′) and Pycf4-3 (5′-CCAACTAATAAGAAGCCTAATGAACC-3′), atpA with primers PatpA5 (5′-TTCTACCGTGAGAGGAGCTGATTGG-3′) and PatpA3 (5′-GCCTTTGCACAATTTGCTTCTGATC-3′), and EF1-α with primers Pef1-5 (5′-TGAGATGCACCACGAAGCTC-3′) and Pef1-3 (5′-CCAACATTGTCACCAGGAAGTG-3′).
Polysome-Loading Assays
Isolation of polysomes and RNA extraction from polysomal gradient fractions were performed as described previously (Kahlau and Bock, 2008; Rogalski et al., 2008). Gradient fractionation was carried out using the Auto Densi-Flow (Labconco) and the Pharmacia LKB RediFrac fraction collector (GE Healthcare). RNA pellets were dissolved in 30 µL of sterile water, and 5-µL aliquots were used for northern-blot analyses. As a control, 4 µg of total RNA from wild-type and mutant plants was analyzed.
Isolation of Thylakoids and Immunoblotting
Thylakoid proteins from wild-type and transplastomic plants were isolated from total leaf material using published procedures (Machold et al., 1979; Schöttler et al., 2004). For western blotting, samples were normalized to chlorophyll, electrophoretically separated on SDS-polyacrylamide gels (Laemmli, 1970), and transferred to Hybond-P polyvinylidene difluoride membranes (GE Healthcare) using standard protocols. Immunoblot detection was performed with specific antibodies using the ECL PLUS system (GE Healthcare). Polyclonal antibodies against PsaL, PsaG, PsaA, PsaB, PsaF, PsaK, PsbD, PsbO, PetC, and AtpB (produced in rabbits) were purchased from Agrisera. Goat anti-rabbit IgG (H + L)-horseradish peroxidase conjugate (Bio-Rad) was used as a secondary antibody. The Ycf3-FLAG protein (Albus et al., 2010) was detected with an affinity-purified anti-FLAG M2 monoclonal antibody (Agilent Technologies) and anti-mouse IgG peroxidase conjugated (Sigma). The chemiluminescence signal was visualized by exposure to Kodak Biomax XAR film (Sigma-Aldrich).
Generation and Affinity Purification of Antibodies against Ycf4 and Y3IP1
Polyclonal antibodies against Ycf4 were produced in rabbits using the synthetic peptide sequences CVGSGYDRFDRKEGI-amide and CTDENLTPREIEQKA-amide and LPH as the carrier protein. Specific antibodies were enriched by purification of serum samples with antigen-coupled HiTrap NHS-activated HP columns following the instructions of the supplier (GE Healthcare; Antibody Purification Handbook).
For the generation of Y3IP1 antibodies, a 172-amino acid sequence representing the hydrophilic part of the mature tobacco Y3IP1 protein was chosen as an epitope. The respective coding sequence was amplified from tobacco cDNA with the primers 5′-TTCCATGGGGAAAGAAGAAGACAGTGCAACC-3′ and 5′-TTCTCGAGACCCAAAGCTGGAGGAACATC-3′, introducing the restriction sites NcoI and XhoI, respectively. The resulting PCR product was cloned in frame into the vector pET-28a(+) (Novagen) via the NcoI and XhoI restriction sites. The resulting plasmid encoding the C-terminally His6-tagged Y3IP1 fragment was introduced into Escherichia coli BL21 cells. After isopropylthio-β-galactoside induction, the overexpressed protein was isolated in a two-step purification by nickel-nitrilotriacetic acid agarose (Qiagen) and subsequent separation of the eluate by SDS-PAGE and recovery of the purified protein from the gel. To this end, the excised gel slice containing the Y3IP1 protein fragment was macerated in 50 mm Tris-HCl, 150 mm NaCl, and 0.1 mm EDTA, pH 7.5, and incubated overnight at 30°C. The protein-containing buffer was then filtered (Whatman filter; 0.45-µm cutoff) to remove the gel matrix, concentrated by ultrafiltration (Microcon YM-3; Millipore), tested for identity and purity by tandem mass spectrometry, and subsequently used for the immunization of rabbits (BioGenes).
BN Gel Electrophoresis
One- and two-dimensional BN gels were prepared according to published procedures (Dietzel et al., 2011) with the sole exception that the gels were run in the PROTEAN II ξ Cell gel system (Bio-Rad). For immunoblot analysis of thylakoid membrane proteins, one-dimensional BN gels were transferred onto polyvinylidene difluoride membranes (GE Healthcare) and destained according to published protocols (Wittig et al., 2006). For two-dimensional BN-PAGE, the second dimension was performed as in standard SDS-PAGE (Laemmli, 1970).
Pigment Analysis and Photosynthesis Physiology
Chlorophyll contents were determined in 80% (v/v) acetone (Porra et al., 1989). The 77 K chlorophyll a fluorescence emission spectra of isolated thylakoids (equivalent to 10 µg chlorophyll mL−1) were determined using a Jasco F-6500 fluorimeter. Chlorophyll a fluorescence was excited at 430-nm wavelength (10-nm spectral bandwidth), and fluorescence emission was determined with a spectral bandwidth of 1 nm in a wavelength range from 660 to 800 nm.
Chlorophyll fluorescence was recorded with a pulse amplitude-modulated fluorimeter (Dual-PAM-100; Heinz Walz) on intact wild-type and mutant plants at room temperature. Plants were dark adapted for 1 h prior to measurement of maximum quantum efficiency of PSII. The contents of PSII, Cyt bf, plastocyanin, and PSI were determined in thylakoids prepared as described previously (Schöttler et al., 2004). PSI was quantified from P700 difference absorption signals at 830 to 870 nm in solubilized thylakoids using the Dual-PAM instrument (Schöttler et al., 2007a, 2007b). Contents of PSII and Cyt bf were determined from difference absorption measurements of cytochrome b559 and Cyt bf, respectively. Measurement procedures and data deconvolution methods have been described in detail previously (Kirchhoff et al., 2002; Schöttler et al., 2007b).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Confirmation of homoplasmy and uniparental maternal inheritance of the ycf4 knockout allele.
Supplemental Figure S2. qRT-PCR assays to confirm the absence of detectable ycf4 transcripts in Δycf4 knockout plants.
Supplementary Material
Acknowledgments
We are grateful to Steffi Seeger (Max-Planck-Institut für Molekulare Pflanzenphysiologie) for help with tissue culture and plant transformation.
Glossary
- qRT
quantitative real-time
- Cyt bf
cytochrome b6f complex
- BN
blue-native
- MS
Murashige and Skoog
References
- Albus C, Ruf S, Schöttler MA, Lein W, Kehr J, Bock R. (2010) Y3IP1, a nucleus-encoded thylakoid protein, cooperates with the plastid-encoded Ycf3 protein in photosystem I assembly of tobacco and Arabidopsis. Plant Cell 22: 2838–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts A, Drory O, Nelson N. (2007) The structure of a plant photosystem I supercomplex at 3.4 A resolution. Nature 447: 58–63 [DOI] [PubMed] [Google Scholar]
- Amunts A, Toporik H, Borovikova A, Nelson N. (2010) Structure determination and improved model of plant photosystem I. J Biol Chem 285: 3478–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayliffe MA, Scott NS, Timmis JN. (1998) Analysis of plastid DNA-like sequences within the nuclear genomes of higher plants. Mol Biol Evol 15: 738–745 [DOI] [PubMed] [Google Scholar]
- Bock R. (2001) Transgenic plastids in basic research and plant biotechnology. J Mol Biol 312: 425–438 [DOI] [PubMed] [Google Scholar]
- Bock R, Timmis JN. (2008) Reconstructing evolution: gene transfer from plastids to the nucleus. Bioessays 30: 556–566 [DOI] [PubMed] [Google Scholar]
- Boudreau E, Takahashi Y, Lemieux C, Turmel M, Rochaix J-D. (1997) The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. EMBO J 16: 6095–6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A, Hippler M. (2011) The structure and function of eukaryotic photosystem I. Biochim Biophys Acta 1807: 864–877 [DOI] [PubMed] [Google Scholar]
- Dietzel L, Bräutigam K, Steiner S, Schüffler K, Lepetit B, Grimm B, Schöttler MA, Pfannschmidt T. (2011) Photosystem II supercomplex remodeling serves as an entry mechanism for state transitions in Arabidopsis. Plant Cell 23: 2964–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15 [Google Scholar]
- Göhre V, Ossenbühl F, Crèvecoeur M, Eichacker LA, Rochaix J-D. (2006) One of two alb3 proteins is essential for the assembly of the photosystems and for cell survival in Chlamydomonas. Plant Cell 18: 1454–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M. (1991) Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker of site-directed transformation of Chlamydomonas. Nucleic Acids Res 19: 4083–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager M, Biehler K, Illerhaus J, Ruf S, Bock R. (1999) Targeted inactivation of the smallest plastid genome-encoded open reading frame reveals a novel and essential subunit of the cytochrome b(6)f complex. EMBO J 18: 5834–5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PE, Haldrup A, Zhang S, Scheller HV. (2004) The PSI-O subunit of plant photosystem I is involved in balancing the excitation pressure between the two photosystems. J Biol Chem 279: 24212–24217 [DOI] [PubMed] [Google Scholar]
- Kahlau S, Bock R. (2008) Plastid transcriptomics and translatomics of tomato fruit development and chloroplast-to-chromoplast differentiation: chromoplast gene expression largely serves the production of a single protein. Plant Cell 20: 856–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H, Mukherjee U, Galla HJ. (2002) Molecular architecture of the thylakoid membrane: lipid diffusion space for plastoquinone. Biochemistry 41: 4872–4882 [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42: 313–349 [Google Scholar]
- Kudoh H, Sonoike K. (2002) Irreversible damage to photosystem I by chilling in the light: cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 215: 541–548 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lohmann A, Schöttler MA, Bréhélin C, Kessler F, Bock R, Cahoon EB, Dörmann P. (2006) Deficiency in phylloquinone (vitamin K1) methylation affects prenyl quinone distribution, photosystem I abundance, and anthocyanin accumulation in the Arabidopsis AtmenG mutant. J Biol Chem 281: 40461–40472 [DOI] [PubMed] [Google Scholar]
- Machold O, Simpson DJ, Moller BL. (1979) Chlorophyll-proteins of thylakoids from wild-type and mutants of barley (Hordeum vulgare L.). Carlsberg Res Commun 44: 235–254 [Google Scholar]
- Magee AM, Aspinall S, Rice DW, Cusack BP, Sémon M, Perry AS, Stefanović S, Milbourne D, Barth S, Palmer JD, et al. (2010) Localized hypermutation and associated gene losses in legume chloroplast genomes. Genome Res 20: 1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P. (2004) Plastid transformation in higher plants. Annu Rev Plant Biol 55: 289–313 [DOI] [PubMed] [Google Scholar]
- Maliga P, Bock R. (2011) Plastid biotechnology: food, fuel, and medicine for the 21st century. Plant Physiol 155: 1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng BY, Tanaka M, Wakasugi T, Ohme M, Shinozaki K, Sugiura M. (1988) Cotranscription of the genes encoding two P700 chlorophyll a apoproteins with the gene for ribosomal protein CS14: determination of the transcriptional initiation site by in vitro capping. Curr Genet 14: 395–400 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Naver H, Boudreau E, Rochaix J-D. (2001) Functional studies of Ycf3: its role in assembly of photosystem I and interactions with some of its subunits. Plant Cell 13: 2731–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N, Ben-Shem A. (2004) The complex architecture of oxygenic photosynthesis. Nat Rev Mol Cell Biol 5: 971–982 [DOI] [PubMed] [Google Scholar]
- Nelson N, Yocum CF. (2006) Structure and function of photosystems I and II. Annu Rev Plant Biol 57: 521–565 [DOI] [PubMed] [Google Scholar]
- Oh M-H, Safarova RB, Eu Y-J, Zulfugarov IS, Kim J-H, Hwang HJ, Lee CB, Lee C-H. (2009) Loss of peripheral polypeptides in the stromal side of photosystem I by light-chilling in cucumber leaves. Photochem Photobiol Sci 8: 535–541 [DOI] [PubMed] [Google Scholar]
- Onishi T, Takahashi Y. (2009) Effects of site-directed mutations in the chloroplast-encoded Ycf4 gene on PSI complex assembly in the green alga Chlamydomonas reinhardtii. Plant Cell Physiol 50: 1750–1760 [DOI] [PubMed] [Google Scholar]
- Ossenbühl F, Göhre V, Meurer J, Krieger-Liszkay A, Rochaix J-D, Eichacker LA. (2004) Efficient assembly of photosystem II in Chlamydomonas reinhardtii requires Alb3.1p, a homolog of Arabidopsis ALBINO3. Plant Cell 16: 1790–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S-I, Nield J, Terao A, Stauber EJ, Hippler M, Koike H, Rochaix J-D, Takahashi Y. (2009) Biochemical and structural studies of the large Ycf4-photosystem I assembly complex of the green alga Chlamydomonas reinhardtii. Plant Cell 21: 2424–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S-I, Onishi T, Takahashi Y. (2010) Identification and characterization of an assembly intermediate subcomplex of photosystem I in the green alga Chlamydomonas reinhardtii. J Biol Chem 285: 20072–20079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasch JC, Nickelsen J, Schünemann D. (2005) The yeast split-ubiquitin system to study chloroplast membrane protein interactions. Appl Microbiol Biotechnol 69: 440–447 [DOI] [PubMed] [Google Scholar]
- Petersen K, Schöttler MA, Karcher D, Thiele W, Bock R. (2011) Elimination of a group II intron from a plastid gene causes a mutant phenotype. Nucleic Acids Res 39: 5181–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Rogalski M, Schöttler MA, Thiele W, Schulze WX, Bock R. (2008) Rpl33, a nonessential plastid-encoded ribosomal protein in tobacco, is required under cold stress conditions. Plant Cell 20: 2221–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokka A, Suorsa M, Saleem A, Battchikova N, Aro E-M. (2005) Synthesis and assembly of thylakoid protein complexes: multiple assembly steps of photosystem II. Biochem J 388: 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland N, Dorne A-J, Amoroso G, Sültemeyer DF, Joyard J, Rochaix J-D. (1997) Disruption of the plastid ycf10 open reading frame affects uptake of inorganic carbon in the chloroplast of Chlamydomonas. EMBO J 16: 6713–6726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf S, Biehler K, Bock R. (2000) A small chloroplast-encoded protein as a novel architectural component of the light-harvesting antenna. J Cell Biol 149: 369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf S, Karcher D, Bock R. (2007) Determining the transgene containment level provided by chloroplast transformation. Proc Natl Acad Sci USA 104: 6998–7002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf S, Kössel H, Bock R. (1997) Targeted inactivation of a tobacco intron-containing open reading frame reveals a novel chloroplast-encoded photosystem I-related gene. J Cell Biol 139: 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner C, Laasch H, Hagemann R. (1995) Detection of point mutations in chloroplast genes of Antirrhinum majus L. I. Identification of a point mutation in the psaB gene of a photosystem I plastome mutant. Mol Gen Genet 249: 533–544 [DOI] [PubMed] [Google Scholar]
- Scheller HV, Haldrup A. (2005) Photoinhibition of photosystem I. Planta 221: 5–8 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Gessner G, Luff M, Heiland I, Wagner V, Kaminski M, Geimer S, Eitzinger N, Reissenweber T, Voytsekh O, et al. (2006) Proteomic analysis of the eyespot of Chlamydomonas reinhardtii provides novel insights into its components and tactic movements. Plant Cell 18: 1908–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttler MA, Albus CA, Bock R. (2011) Photosystem I: its biogenesis and function in higher plants. J Plant Physiol 168: 1452–1461 [DOI] [PubMed] [Google Scholar]
- Schöttler MA, Flügel C, Thiele W, Bock R. (2007a) Knock-out of the plastid-encoded PetL subunit results in reduced stability and accelerated leaf age-dependent loss of the cytochrome b6f complex. J Biol Chem 282: 976–985 [DOI] [PubMed] [Google Scholar]
- Schöttler MA, Flügel C, Thiele W, Stegemann S, Bock R. (2007b) The plastome-encoded PsaJ subunit is required for efficient photosystem I excitation, but not for plastocyanin oxidation in tobacco. Biochem J 403: 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttler MA, Kirchhoff H, Weis E. (2004) The role of plastocyanin in the adjustment of the photosynthetic electron transport to the carbon metabolism in tobacco. Plant Physiol 136: 4265–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkert S, Netz DJ, Frazzon J, Pierik AJ, Bill E, Gross J, Lill R, Meurer J. (2010) Chloroplast HCF101 is a scaffold protein for [4Fe-4S] cluster assembly. Biochem J 425: 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoike K. (2011) Photoinhibition of photosystem I. Physiol Plant 142: 56–64 [DOI] [PubMed] [Google Scholar]
- Stöckel J, Bennewitz S, Hein P, Oelmüller R. (2006) The evolutionarily conserved tetratrico peptide repeat protein pale yellow green7 is required for photosystem I accumulation in Arabidopsis and copurifies with the complex. Plant Physiol 141: 870–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckel J, Oelmüller R. (2004) A novel protein for photosystem I biogenesis. J Biol Chem 279: 10243–10251 [DOI] [PubMed] [Google Scholar]
- Svab Z, Maliga P. (1993) High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA 90: 913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Goldschmidt-Clermont M, Soen S-Y, Franzén LG, Rochaix J-D. (1991) Directed chloroplast transformation in Chlamydomonas reinhardtii: insertional inactivation of the psaC gene encoding the iron sulfur protein destabilizes photosystem I. EMBO J 10: 2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A, Härtel H, Hübschmann T, Hoffmann P, Shestakov SV, Börner T. (1995) Inactivation of a Synechocystis sp strain PCC 6803 gene with homology to conserved chloroplast open reading frame 184 increases the photosystem II-to-photosystem I ratio. Plant Cell 7: 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig I, Braun H-P, Schägger H. (2006) Blue native PAGE. Nat Protoc 1: 418–428 [DOI] [PubMed] [Google Scholar]
- Wurbs D, Ruf S, Bock R. (2007) Contained metabolic engineering in tomatoes by expression of carotenoid biosynthesis genes from the plastid genome. Plant J 49: 276–288 [DOI] [PubMed] [Google Scholar]
- Yukawa M, Tsudzuki T, Sugiura M. (2005) The 2005 version of the chloroplast DNA sequence from tobacco (Nicotiana tabacum). Plant Mol Biol Rep 23: 359–365 [Google Scholar]
- Zoubenko OV, Allison LA, Svab Z, Maliga P. (1994) Efficient targeting of foreign genes into the tobacco plastid genome. Nucleic Acids Res 22: 3819–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.