Abstract
The transfer of fatty acids across biological membranes is a largely uncharacterized process, although it is essential at membranes of several higher plant organelles like chloroplasts, peroxisomes, or the endoplasmic reticulum. Here, we analyzed loss-of-function mutants of the unicellular cyanobacterium Synechocystis sp. PCC 6803 as a model system to circumvent redundancy problems encountered in eukaryotic organisms. Cells deficient in the only cytoplasmic Synechocystis acyl-acyl carrier protein synthetase (SynAas) were highly resistant to externally provided α-linolenic acid, whereas wild-type cells bleached upon this treatment. Bleaching of wild-type cells was accompanied by a continuous increase of α-linolenic acid in total lipids, whereas no such accumulation could be observed in SynAas-deficient cells (Δsynaas). When SynAas was disrupted in the tocopherol-deficient, α-linolenic acid-hypersensitive Synechocystis mutant Δslr1736, double mutant cells displayed the same resistance phenotype as Δsynaas. Moreover, heterologous expression of SynAas in yeast (Saccharomyces cerevisiae) mutants lacking the major yeast fatty acid import protein Fat1p (Δfat1) led to the restoration of wild-type sensitivity against exogenous α-linolenic acid of the otherwise resistant Δfat1 mutant, indicating that SynAas is functionally equivalent to Fat1p. In addition, liposome assays provided direct evidence for the ability of purified SynAas protein to mediate α-[14C]linolenic acid retrieval from preloaded liposome membranes via the synthesis of [14C]linolenoyl-acyl carrier protein. Taken together, our data show that an acyl-activating enzyme like SynAas is necessary and sufficient to mediate the transfer of fatty acids across a biological membrane.
Fatty acids have many functions in plants, for example, as structural components of phospholipids and galactolipids, as storage reserves in triacyl glycerols, or as precursors for signaling molecules and plant hormones. As essential components of biological membranes, they enable the subcellular compartmentalization of plant cells and ensure the vital separation of biochemical reactions and pathways that require different chemical environments.
The transmembrane transport of fatty acids has been characterized in detail in unicellular organisms (Black and DiRusso, 2003). In gram-negative bacteria like Escherichia coli, two components contribute to the uptake of fatty acids from the external medium. One is the integral membrane protein FadL, which is localized in the outer membrane of the E. coli envelope and exhibits typical transport protein characteristics. As a consequence, FadL-defective mutants do not show any uptake of exogenous long-chain fatty acids (Black et al., 1985). The other component, FadD, displays acyl-CoA synthetase activity and is associated with the inner membrane of the cell envelope. Both FadL and FadD are necessary and act in concert in a process termed vectorial acylation, because mutant cells lacking either activity are unable to grow on medium containing long-chain fatty acids as the sole carbon source (Nunn and Simons, 1978).
In yeast (Saccharomyces cerevisiae), a similar vectorial acylation system consisting of a transmembrane protein and acyl-CoA synthetases exists. Here, both the membrane protein Fat1p and the acyl-activating enzymes Faa1p and Faa4p are associated with the plasma membrane. Mutants lacking either Fat1p or any Faa1 or Faa4 activity are unable to grow on long-chain fatty acids when endogenous fatty acid synthesis is inhibited through the addition of cerulenin (Faergeman et al., 1997, 2001). Hence, although Fat1p harbors an acyl-activating domain (Obermeyer et al., 2007), the presence of at least one of the acyl-CoA synthetases is essential for the import of long-chain fatty acids into yeast cells in addition to a functional Fat1p.
In higher plants, fatty acid biosynthesis is exclusively located in chloroplasts, indicating the necessity of fatty acid export from chloroplasts destined for the assembly of phospholipids derived from the “eukaryotic pathway” in the endoplasmic reticulum (Gardiner et al., 1982). However, although knowledge about the mechanisms involved in the transfer of lipids from the chloroplast to the endoplasmic reticulum and vice versa has greatly advanced in recent years (Benning, 2008), understanding of fatty acid transport across the chloroplast membrane is still limited. Tracer experiments applying [18O]acetate or [14C]acetate to spinach (Spinacia oleracea) or pea (Pisum sativum) leaves (Pollard and Ohlrogge, 1999; Koo et al., 2004) provided initial experimental verification for a model first outlined by Shine et al. (1976). This model involves thioesterase-mediated cleavage of acyl-acyl carrier protein (ACP) at the inner envelope membrane, transfer of the free fatty acids to the outer envelope membrane, and acyl-activating enzyme activity at the outer envelope membrane. Moreover, incubation of isolated chloroplasts with radiolabeled fatty acids revealed that chloroplasts are also able to take up exogenously supplied fatty acids and elongate and incorporate them into lipids (Thompson et al., 1986; Koo et al., 2005). Koo et al. (2005) provided evidence that the acyl-activating enzymes AAE15 and AAE16 are essential in that process. Their study showed that isolated Arabidopsis (Arabidopsis thaliana) chloroplasts activated exogenously applied laureate very rapidly through esterification to ACP and that this reaction is dependent mostly on AAE15.
In both E. coli and yeast, acyl-activating enzymes are involved in transmembrane fatty acid transport. Because the large superfamily of 63 acyl-activating enzymes in Arabidopsis suggested a possible obstruction of mutant analyses through functional redundancy (Schnurr et al., 2002; Shockey et al., 2003), we employed the more simple unicellular cyanobacterium Synechocystis sp. PCC 6803 as a model. Previous studies had revealed the presence of only a single protein in Synechocystis homologous to higher plant acyl-activating enzymes (Kaczmarzyk and Fulda, 2010). These studies also established that the Synechocystis acyl-acyl carrier protein synthetase (SynAas) specifically uses ACP as a cosubstrate to recycle free fatty acids occurring naturally in Synechocystis cells (Kaczmarzyk and Fulda, 2010). Here, we provide evidence that SynAas is also involved in the transfer of free fatty acids across membranes by vectorial acylation and discuss an analogous mechanism for fatty acid transfer across the chloroplast envelope.
RESULTS
We used the deduced amino acid sequence of Arabidopsis LACS9 to search for homologous proteins in the cyanobacterium Synechocystis sp. PCC 6803. Analogous to Kaczmarzyk and Fulda (2010), we identified Slr1609 as the only homologous protein through the BLASTP function of the CyanoBase Web site (http://genome.kazusa.or.jp/cyanobase). Slr1609 had been named Aas (SynAas throughout this paper). In order to gain additional insight into SynAas function in Synechocystis metabolism, we generated knockout mutants (Δsynaas) through homologous recombination (Supplemental Fig. S1) and analyzed cell growth and viability in various conditions.
Δsynaas Cells Are Resistant to α-Linolenic Acid Treatment
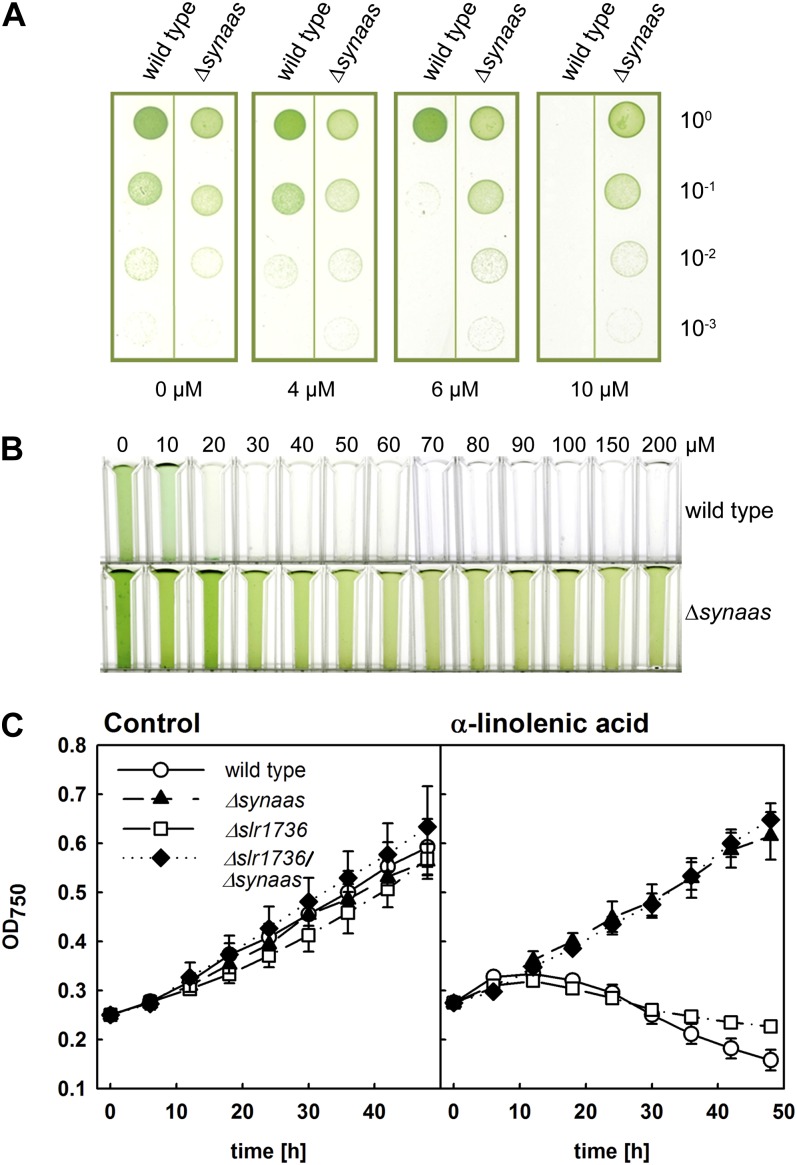
On regular BG11 medium, fully segregated Δsynaas mutants did not display any phenotypic deviation from wild-type cells (Fig. 1A), indicating that SynAas function is not essential under the conditions used. Because previous studies had shown that exogenous α-linolenic acid, once taken up into the cell, has a detrimental effect on cells (Maeda et al., 2005; Kunz et al., 2009), we tested the cell growth and viability of Δsynaas when exposed to increasing concentrations in the growth medium. Interestingly, Synechocystis wild-type cells were impaired in growth both on plates and in liquid culture supplemented with α-linolenic acid (Fig. 1). Whereas plate drop assays showed that wild-type cells when diluted from a pregrown culture on control medium simply would not grow in the presence of 10 µm α-linolenic acid or above, the phenotype in liquid culture was much more dramatic. Here, an initially green culture diluted to an optical density at 750 nm (OD750) of 0.25 (compare 0 µm α-linolenic acid in Fig. 1B) would completely bleach within 24 h when challenged with 20 µm α-linolenic acid or above (Fig. 1B). Moreover, when monitoring the growth of wild-type α-linolenic acid-challenged cultures as optical density, cell growth rapidly ceased and cells died (Fig. 1C). In contrast, Δsynaas mutant cells were unaffected on plates, showed resistance in liquid culture to very high concentrations of α-linolenic acid of up to 200 µm, and were able to maintain growth rates comparable to control conditions at 40 µm α-linolenic acid (Fig. 1, B and C). These data show that high concentrations of α-linolenic acid in the external medium have a detrimental effect on Synechocystis cells and that this effect is dependent on the intracellular, SynAas-dependent activation of fatty acids.
Figure 1.
A, α-Linolenic acid-resistant growth of Δsynaas. Growth assays of Synechocystis wild-type and Δsynaas suspension culture dilution series pregrown in control solution (100, OD750 = 0.05) are shown. Five microliters of suspension culture was spotted on plates supplemented with α-linolenic acid as indicated and incubated at constant light for 7 d. B, Aliquots of Synechocystis wild-type and Δsynaas liquid culture adjusted to OD750 = 0.25, supplemented with α-linolenic acid as indicated, and incubated at constant light for 24 h. C, Growth curves of wild-type and mutant cells in the presence or absence of 40 µm α-linolenic acid. Suspension cultures were adjusted to OD750 = 0.25, and OD750 was monitored for 48 h in the absence (left panel) or presence (right panel) of α-linolenic acid.
Photosynthesis Is Impaired in Synechocystis Wild-Type Cells But Not Δsynaas Cells
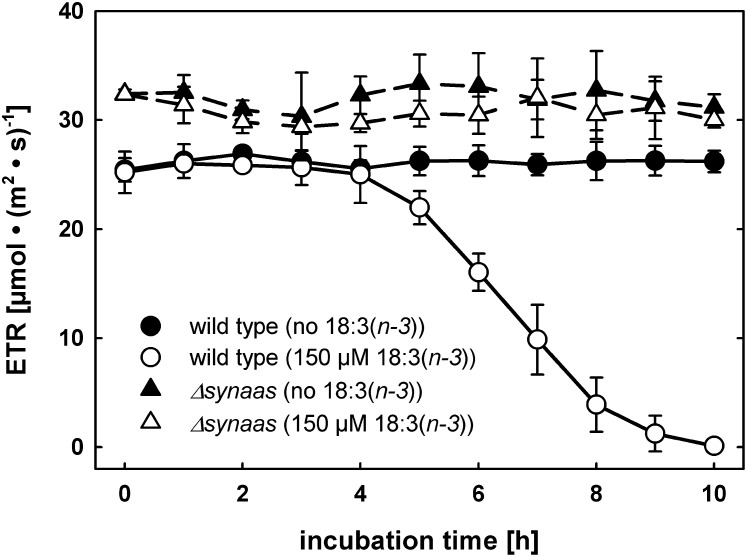
Because α-linolenic acid-challenged wild-type cells bleached completely after 24 h of incubation, we investigated the impact of exogenously fed α-linolenic acid on photosynthesis in wild-type and Δsynaas cells after shorter periods of time using chlorophyll fluorescence. We monitored the photosynthetic electron transport rate (ETR) as a sensitive parameter for intactness of the photosynthetic apparatus. The ETR in wild-type cells started to steeply decrease after 5 h of incubation with 150 µm α-linolenic acid, until virtually no photosynthetic electron transport was detectable after 9 to 10 h of incubation (Fig. 2). However, the wild-type cell culture did not visibly bleach in these conditions as after 24 h of α-linolenic acid treatment (Fig. 1B). In contrast, photosynthesis in Δsynaas cells remained unchanged in response to α-linolenic acid incubation (Fig. 2). In addition, control treatment with only the α-linolenic acid solvent (ethanol) had no effect on ETR in wild-type or Δsynaas cells (Fig. 2).
Figure 2.
Toxic effect of α-linolenic acid on photosynthesis. The photosynthetic ETR of Synechocystis wild-type and Δsynaas cells is shown after different periods of incubation with 150 µm α-linolenic acid (white symbols) or in control conditions (black symbols). Error bars indicate se (n = 3).
Subcellular Compartmentation Is Lost in Synechocystis Cells in Response to α-Linolenic Acid Treatment
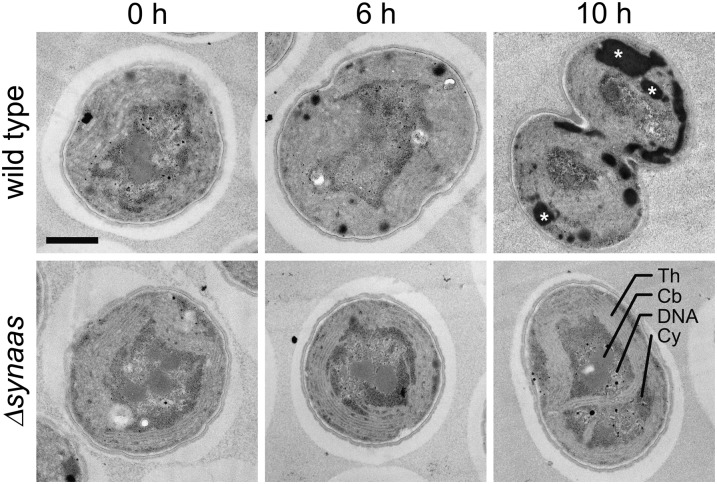
We analyzed the impact of α-linolenic acid treatment on cell structure and integrity in wild-type and Δsynaas cells using electron microscopy. Samples taken after 0, 6, and 10 h of incubation with 150 µm α-linolenic acid showed a gradual change in cell structure with the duration of α-linolenic acid treatment in wild-type cells. Here, an accumulation of electron-dense particles could be observed after 6 h (Fig. 3). In electron microscopy, lipophilic structures often appear as dark regions (Neiss, 1983). Hence, the detected electron-dense particles could be assigned either to collapsed membrane material or the accumulation of free fatty acids and lipids. These particles increased massively in size and number after 10 h of incubation, and the subcellular compartmentation into peripheral thylakoid membranes and central carboxysome and DNA was almost completely lost (Fig. 3), congruent with the observed lack of photosynthetic electron transport in these cells (Fig. 2). In contrast, Δsynaas cells did not show any alteration of their ultrastructural appearance after 6 or 10 h of incubation (Fig. 3).
Figure 3.
Impact of α-linolenic acid on Synechocystis cell structure. Electron micrographs of individual wild-type and Δsynaas cells are shown after different periods of α-linolenic acid incubation (0, 6, and 10 h). Wild-type cells accumulate dark, lipophilic substances with increasing incubation time (white asterisks in 10-h panel) and display a disintegration of thylakoid membranes and subcellular structure in general. Th, Thylakoid membrane; Cb, carboxysome; DNA, nucleosome; Cy, cytoplasm. Bar = 0.5 µm.
Wild-Type But Not Δsynaas Cells Accumulate α-Linolenic Acid in Short- and Long-Term Incubation Experiments
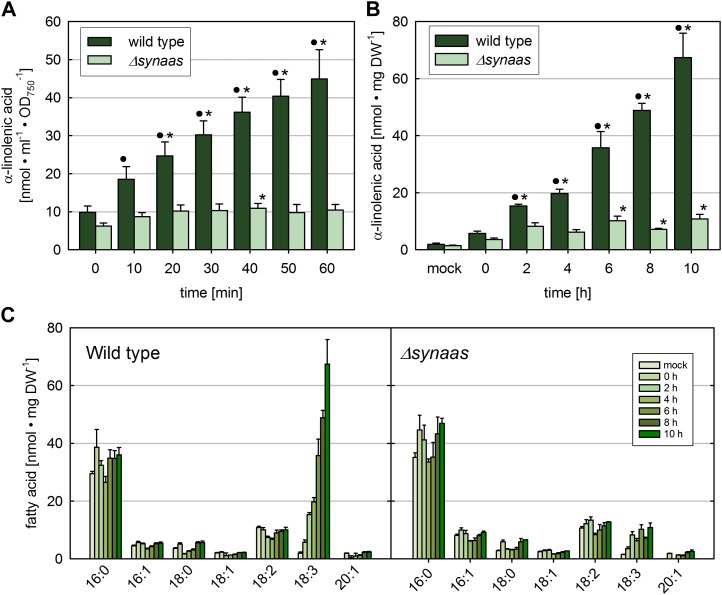
In order to test whether exogenously fed α-linolenic acid accumulates in Synechocystis cells, we exposed wild-type and Δsynaas cells to moderate concentrations (33 µm) of radiolabeled α-[14C]linolenic acid for up to 60 min and measured cell-associated radioactivity after intensive washing. Data show that even immediately after the addition of α-[14C]linolenic acid, a basal level of radioactivity remained attached to the cells and could not be washed off from either wild-type or Δsynaas cells (Fig. 4A, time 0). However, while this basal level remained constant in Δsynaas cells after 10 min of incubation and was significantly less than in wild-type cells at any time point, cell-associated radiolabel constantly increased in wild-type cells, indicating continuous uptake and incorporation (Fig. 4A).
Figure 4.
Accumulation of α-linolenic acid in Synechocystis cells. A, Short-term accumulation of 14C-lableled α-linolenic acid (33 µm) in wild-type and Δsynaas cells. B, Long-term incubation of α-linolenic acid. Wild-type and Δsynaas cells were incubated with 150 µm α-linolenic acid. Circles and asterisks indicate significant (P ≤ 0.01) differences compared with Δsynaas at the same time point or with time 0, respectively. Error bars indicate se (n = 3). C, Fatty acid profile of total lipids in wild-type and Δsynaas cells upon long-term incubation with α-linolenic acid. Only the α-linolenic acid concentration in wild-type cells is substantially increased over time, and other fatty acids remain unaltered. DW, Dry weight. [See online article for color version of this figure.]
Moreover, when determining the fatty acid composition in total lipid extracts of Synechocystis cells treated for up to 10 h with α-linolenic acid (150 µm), we found that the α-linolenic acid concentration strongly increased in wild-type cells to values up to 67.4 ± 8.6 nmol mg−1 dry weight, whereas the concentration in untreated cells was 1.9 ± 0.4 nmol mg−1 dry weight (Fig. 4B). In contrast, no continuous increase of α-linolenic acid in total lipids could be detected in Δsynaas cells. There is, however, as with the radiolabeled α-linolenic acid treatment, an initial leap to concentrations significantly above the level in untreated cells that during the further course of experiments remained constant (Fig. 4, B and C). This initial increase of α-linolenic acid in Δsynaas cells probably results from free fatty acids that are adhering to the cell surface or have been integrated into the plasma membrane and not been washed off during wash steps after incubation.
The increase in α-linolenic acid in wild-type cells over the course of 10 h was specific for this fatty acid, because the other fatty acids did not display any change in levels (Fig. 4C).
Exogenous α-Linolenic Acid Accumulates in Lipids and as Free Fatty Acid
In order to assess the distribution of α-linolenic acid absorbed from the external medium among cellular lipid species, we applied thin-layer chromatography (TLC). Lipids were extracted from cell samples after 0, 6, and 10 h of α-linolenic acid incubation and separated by TLC. A substantial increase of free α-linolenic acid is visible in the wild-type strain after 6 and 10 h of incubation (Fig. 5), indicating that free fatty acids constitute a major component of the electron-dense accumulations apparent in the electron micrographs (Fig. 5). Additionally, there is an increase in monogalactosyl diacylglycerol (MGDG), sulfoquinovosyl diacylglycerol (SQDG), and phosphatidyl glycerol (PG) over time. When applying α-[14C]linolenic acid for incubation times up to 60 min, radiolabel could clearly be detected in major lipid classes after 20 min (Supplemental Fig. S2), showing that α-linolenic acid is incorporated into most of the predominant lipid species of Synechocystis (Wada and Murata, 1998). We suspect that the additional band, appearing right above MGDG (Fig. 5), represents an MGDG with two 18:3 fatty acid residues, which would lead to a more lipophilic molecule than the common MGDG with 16:0 fatty acids at the sn-2 position (Wada and Murata, 1990). An unknown very polar molecule, running underneath PG, is also increasing over time. However, we could not clarify its identity with our standards. In contrast to the findings in the wild type, there was no increase in any of the lipid species in the Δsynaas mutant (Fig. 5).
Figure 5.
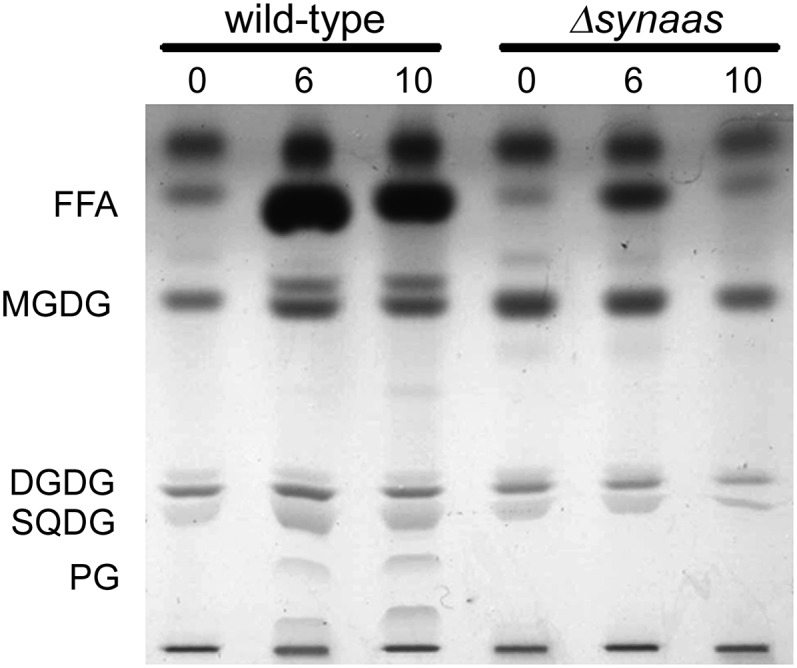
TLC of total lipids. Total lipids were extracted from wild-type and Δsynaas cells after 0, 6, and 10 h of α-linolenic acid incubation. A clear increase in free α-linolenic acid (FFA), MGDG, SQDG, and PG can be observed in wild-type cells. DGDG, Digalactosyl diacylglycerol.
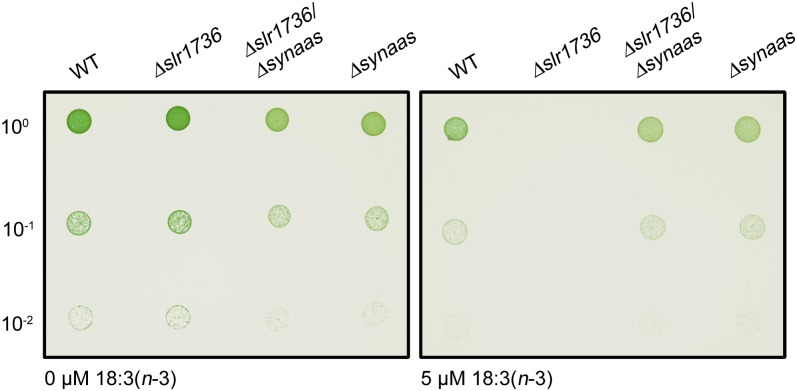
The α-Linolenic Acid Hypersensitivity of Tocopherol-Deficient Δslr1736 Cells Is Reverted to Resistance of Δsynaas Cells in Δslr1736/Δsynaas Double Mutants
For cyanobacteria, it has been shown that α-tocopherol is essential for diminishing lipid peroxidation caused through α-linolenic acid, because α-tocopherol-free Δslr1736 mutants are hypersensitive to treatment with exogenous α-linolenic acid (Maeda et al., 2005). By generating and analyzing the cell growth of a Δslr1736/Δsynaas double mutant in the presence or absence of α-linolenic acid, we intended to determine whether α-linolenic acid enters Synechocystis cells. Plate drop assays showed that all cell lines grew equally well on control medium but that α-tocopherol-free Δslr1736 cells were unable to grow on plates containing as little as 5 µm α-linolenic acid, a concentration that did not affect the growth of Synechocystis wild-type cells (Figs. 1A and 6; Supplemental Fig. S4). In addition, Δslr1736 growth in liquid culture was as severely impaired in the presence of α-linolenic acid as wild-type cells (Fig. 1C). In contrast, the Δslr1736/Δsynaas double mutant grew not only at concentrations that were still tolerated by wild-type cells but also at much higher concentrations that are tolerated by Δsynaas mutants only (Figs. 1C and 6; Supplemental Fig. S3). These data strongly indicate that exogenous α-linolenic acid is not executing its detrimental effect in Δslr1736/Δsynaas double mutants and Δsynaas single mutants because it is not entering these cells.
Figure 6.
Hypersensitivity phenotype of Δslr1736 and phenotype rescue in the Δslr1736/Δsynaas double mutant. Five microliters (100, OD750 = 0.05) of wild-type (WT), Δslr1736, Δslr1736/Δsynaas, and Δsynaas suspension cultures pregrown in low-light conditions (15 µE m−2 s−1) was spotted on plates supplemented with α-linolenic acid as indicated and incubated at high-light conditions (300 µE m−2 s−1) for 7 d.
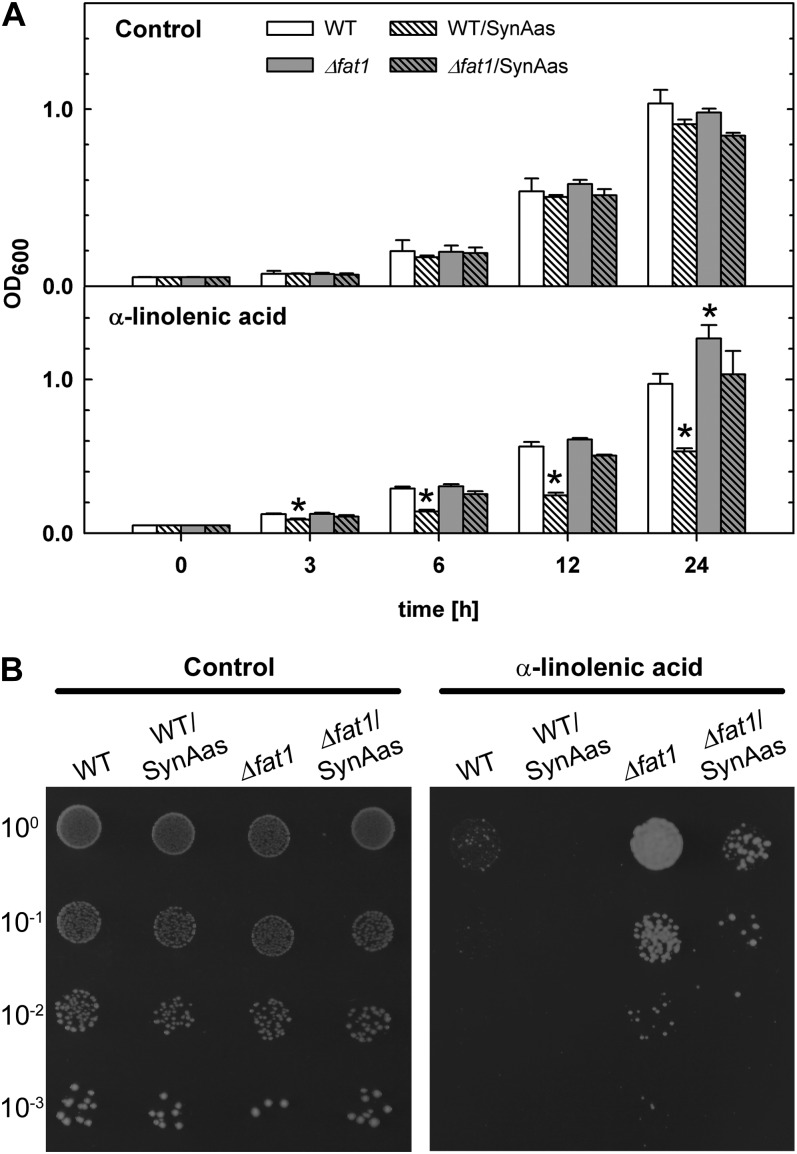
Overexpression of SynAas in Yeast Wild-Type or Δfat1 Mutant Cells Leads to Increased α-Linolenic Acid Sensitivity
The components involved in fatty acid import in yeast have been identified as Fat1p, Faa1p, and Faa4p, where Fat1p is the essential integral membrane transport protein. Postulating that fatty acid import-defective yeast mutants may also be resistant to the toxic effects of exogenous α-linolenic acid, we analyzed single mutant growth in the presence of high external concentrations of α-linolenic acid. Wild-type yeast cells challenged with 3.6 mm α-linolenic acid in drop assay experiments were unable to grow in the absence of any additional carbon source (data not shown); however, reduced growth of wild-type cells compared with control plates could be observed when Glc was added at a low concentration (0.55 mm; Fig. 7B). In contrast, Δfat1 single mutant cells showed α-linolenic acid-resistant growth on the same plates (Fig. 7B). Because yeast cell growth in liquid culture measured as optical density at 600 nm (OD600) could more reliably be characterized at a Glc concentration of 5.5 mm in the medium, we determined the effect of α-linolenic acid on wild-type and Δfat1 cells at moderate Glc concentrations (Fig. 7A). Under these conditions, wild-type cell growth in the presence of 3.6 mm α-linolenic acid is only weakly inhibited compared with control conditions and does not differ from Δfat1 growth (Fig. 7A). However, overexpression of a SynAas-GFP fusion protein in the Δfat1 mutant and wild-type background led to a significant depression of cell growth (Fig. 7A), which was even more evident in the presence of low Glc in the medium (Fig. 7B). Here, the residual growth of SynAas-GFP-expressing wild-type cells observed in liquid culture (Fig. 7A) was completely abolished in SynAas-GFP-overexpressing cells (Fig. 7B). These data support the conclusion that the GFP-tagged SynAas protein can functionally complement Fat1p-mediated α-linolenic acid uptake and that an acyl-activating enzyme alone is sufficient to mediate the import of fatty acids from the external medium into the cell.
Figure 7.
Increased sensitivity to α-linolenic acid by overexpression of SynAas in yeast. A, Wild-type (WT), wild-type/SynAas, Δfat1, and Δfat1/SynAas cultures were diluted to OD600 = 0.05 and grown in liquid YNB medium supplemented with 5.5 mm Glc, 1% tergitol, and 3.6 mm α-linolenic acid. Ethanol was used as a control. Asterisks indicate significantly (P ≤ 0.01) different values compared with the wild type. Error bars indicate se (n = 4–5). B, Wild-type, wild-type/SynAas, Δfat1, and Δfat1/SynAas cells were diluted to OD600 = 0.05 and spotted on YNB plates supplemented with 0.55 mm Glc, 1% tergitol, and 3.6 mm α-linolenic acid. Ethanol was used as a control.
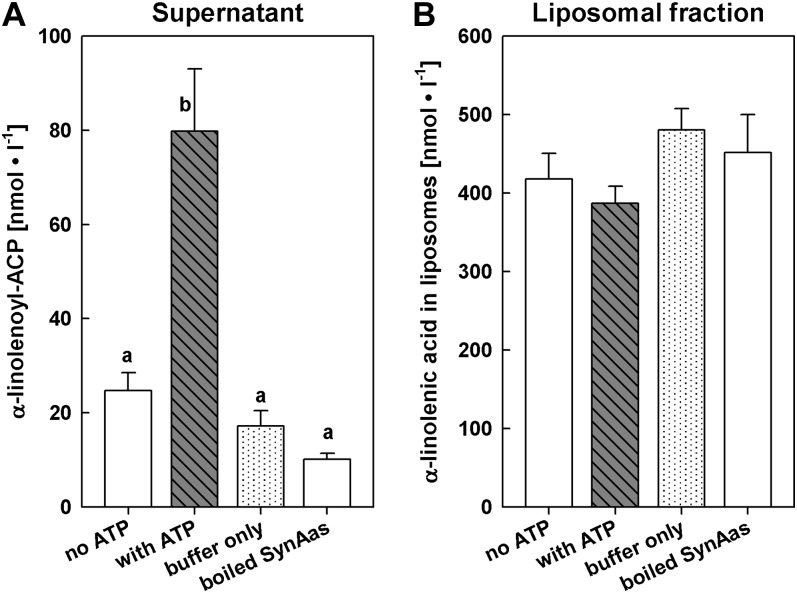
SynAas Can Retrieve and Activate α-Linolenic Acid from Artificial Liposome Membranes
In order to analyze the ability of SynAas to retrieve and activate fatty acids from artificial membranes, we labeled large unilamellar vesicles (0.2 µm) with α-[14C]linolenic acid and incubated these liposomes with purified SynAas. When α-linolenoyl-ACP was determined as water-soluble radioactivity in the supernatant of 14C-labeled liposomes, substantial synthesis of α-linolenoyl-ACP was detected in assay conditions containing SynAas, ACP, ATP, and Mg2+ (Fig. 8A). A low (about four times reduced) level of radioactivity was observed in the absence of either ATP (with SynAas and ACP present; Fig. 8A, no ATP) or SynAas and ACP (with ATP present; Fig. 8A, buffer only) or when adding boiling-inactivated SynAas to otherwise complete assay medium (Fig. 8A, boiled SynAas). Complementary, the 14C label was lowest in assay conditions providing all components and was approximately equally high when carried out without either ATP or SynAas and ACP or with boiling-inactivated SynAas when liposome-associated radioactivity was determined from the liposome pellet after assay performance (Fig. 8B). This indicates that α-[14C]linolenic acid could be retrieved and activated from liposome membranes through the action of SynAas.
Figure 8.
Free fatty acids in membranes can serve as substrate for SynAas. Liposomes loaded with α-[14C]linolenic acid were incubated for 15 min with 10 µg of purified SynAas, 15 µm ACP, and cofactors. Liposomes and supernatant were separated by ultracentrifugation, and radioactivity in supernatant and liposomal fractions was measured. An acyl-ACP concentration of 10 nmol L−1 in the assay supernatant (A) corresponds to an amount of 1 pmol α-linolenoyl-ACP produced µg−1 SynAas. Different letters indicate significantly different values (P ≤ 0.01). Error bars indicate se (n = 6).
DISCUSSION
Although polyunsaturated fatty acids are part of all plant membranes, they are toxic to the cell in higher concentrations. In plants, the accumulation of α-linolenic acid within the cell is detrimental to the integrity of chloroplasts by unfolding of grana stacks and subsequent changes in the thylakoid membrane ultrastructure (Siegenthaler, 1972; Okamoto et al., 1977; Kunz et al., 2009). Here, we used the cytotoxic effect of intracellularly accumulating α-linolenic acid as a tool to demonstrate the involvement of an acyl-activating enzyme in fatty acid translocation across biological membranes.
Loss of Function of the Acyl-Activating Enzyme Protects the Cell from the Toxic Effect of α-Linolenic Acid
Analyses of Synechocystis wild-type cells challenged with α-linolenic acid all revealed negative effects on cell viability detected as impaired growth and bleaching (Fig. 1), reduced photosynthetic electron transport (Fig. 2), disintegration of membranes and subcellular organization, and agglomeration of large lipophilic structures inside cells (Fig. 3). Simultaneous with this negative impact on wild-type cells, the content of α-linolenic acid in total lipids and as free fatty acid steadily increased with the duration of treatment (Figs. 4, B and C, and 5). The apparently continuing, ATP-consuming activation of α-linolenic acid in the presence of deteriorating photosynthesis might be fueled for a limited period of time through the breakdown of storage reserves like glycogen.
Because a negative impact of α-linolenic acid on ultrastructure and photosynthetic electron transport has also been described for isolated chloroplasts (Siegenthaler, 1972; Okamoto et al., 1977; Golbeck et al., 1980; Kunz et al., 2009), it appears reasonable that the accumulation of excess amounts of α-linolenic acid within Synechocystis cells is the major cause for the observed toxicity. No accumulation of α-linolenic acid could be observed in Δsynaas mutant cells, either in short- or long-term experiments (Figs. 4 and 5). Consequently, α-linolenic acid-resistant growth could be observed in the nonaccumulating Δsynaas mutant cells (Figs. 1 and 4). The exact mechanism of α-linolenic acid toxicity to cells remains unclear. Although our study did not aim at exploring the physiological basis for the observed toxicity in Synechocystis and yeast wild-type cells, we adopt its occurrence as an argument for strongly reduced or absent α-linolenic acid uptake into mutant cells.
In fact, our analyses of Synechocystis wild-type cells showed strongly elevated levels of α-linolenic acid in lipids and as free fatty acid (Fig. 5). Because it had been shown previously that hydrogen atoms adjacent to olefinic bonds such as those found in polyunsaturated fatty acids are susceptible to oxidative attack (Singh et al., 2002), we speculated that the observed detrimental effects of α-linolenic acid might partly be caused by lipid peroxidation. Intracellular lipid peroxidation results in secondary effects like protein modification and degradation and DNA damage (McIntyre et al., 1999), which would fit well with the observed general reduction in cell viability of α-linolenic acid-accumulating wild-type cells. In order to protect polyunsaturated fatty acid acyl chains from oxidative damage, cyanobacteria, algae, and higher plants have evolved a scavenger mechanism employing α-tocopherol as antioxidant (Hunter and Cahoon, 2007; Maeda and DellaPenna, 2007). α-Tocopherol is a lipophilic compound that has the unique ability to prevent oxidative damage to polyunsaturated fatty acid acyl chains through the interception of fatty acid peroxyl radicals and hence interruption of the chain reaction process inherent to lipid peroxidation (Schneider, 2005). Moreover, recent studies on Synechocystis mutants suggest that α-tocopherol is also important for the repair of photodamaged PSII by protecting the de novo biosynthesis of high-turnover PSII components like D1 (Inoue et al., 2011). The tocopherol-deficient Synechocystis mutant Δslr1736 is highly susceptible to treatment with α-linolenic acid (Maeda et al., 2005). In agreement with the findings described by Maeda et al. (2005), we could also demonstrate that low concentrations of α-linolenic acid (5 µm) in high-light conditions (300 µE) are toxic for Δslr1736 single mutant cells (Fig. 6). Interestingly, the Δslr1736 hypersensitivity phenotype could be completely reverted to Δsynaas resistance by generating a Δsynaas/Δslr1736 double knockout (Fig. 6; Supplemental Fig. S3). Apparently, α-linolenic acid incorporated from the external medium leads to lipid peroxidation within Synechocystis cells during high-light exposure that cannot be prevented in the Δslr1736 single mutant because of the absence of tocopherols. In wild-type cells, α-tocopherol is able to efficiently protect cells at 5 µm α-linolenic acid but evidently is insufficient for cells to tolerate an external α-linolenic acid concentration of 10 µm and above. In contrast, Δsynaas/Δslr1736 double mutants are as resistant as Δsynaas single mutants to α-linolenic acid of 10 µm and above (Figs. 1C and 6; Supplemental Fig. S3). Because α-tocopherol exhibits its antioxidant function inside cells, the absence of α-linolenic acid-sensitive growth in both Δsynaas/Δslr1736 and Δsynaas mutants can only be explained by a strongly reduced or even absent uptake of the exogenously supplied α-linolenic acid, which is well supported by the α-linolenic acid-nonaccumulating phenotype of Δsynaas cells (Fig. 4).
Taken together, these results suggest that the uptake mechanism in Synechocystis is strictly coupled to fatty acid activation. This adds a function in fatty acid uptake for SynAas to its role in internal fatty acid recycling demonstrated by Kaczmarzyk and Fulda (2010).
Consequently, fatty acids can only enter the cell by activation with ACP. Because wild-type cells constantly import fatty acids under the imposed experimental conditions (Fig. 4), there must be a fast recycling of ACP. In order to maintain the free ACP pool, these activated fatty acids need to be rapidly integrated into lipids, as demonstrated by the increase in MGDG, SQDG, and PG in wild-type cells (Fig. 5; Supplemental Fig. S2). However, the simultaneous rise in free α-linolenic acid in wild-type cells (Fig. 5) also suggests that a substantial amount α-linolenic acid is cleaved off from lipids. Free fatty acids accumulating in lipophilic droplets as observed in electron micrographs (Fig. 3) might be inaccessible for further activation by SynAas. Hence, lipophilic droplet formation may counteract a futile cycle of repeated fatty acid activation, integration into lipids, and phospholipase-mediated cleavage, thereby enabling continuous influx of external fatty acids (Fig. 4). Imposing a distorted fatty acid composition on Synechocystis membrane lipids might lead to the necessity of remodeling these lipids to maintain favorable physicochemical membrane properties (Okazaki et al., 2006) and may also contribute to the observed α-linolenic acid toxicity. The rise in intracellular free fatty acids could be the result of increased phospholipase activity as a reaction to the elevated α-linolenic acid content in polar lipids. Previous studies have revealed the natural occurrence of free fatty acids in Synechocystis in the apparent absence of an acyl-ACP thioesterase (Kaczmarzyk and Fulda, 2010). Thus, lipase activity appears as a reasonable explanation for free fatty acids inside Synechocystis cells. A functional β-oxidation pathway appears to be missing in cyanobacteria. Therefore, incorporation of fatty acids into lipids is the only destination for moderate amounts of imported fatty acids. Excess fatty acids are apparently secreted into the surrounding medium, because Δsynaas alone and in combination with other genetic modifications was recently shown to cause fatty acid enrichment in the culture medium (Kaczmarzyk and Fulda, 2010; Liu et al., 2011). In this context, the discovery of the fatty acid resistance phenotype in Δsynaas may prove useful for the production of biofuels in approaches aimed at engineering organisms that accumulate high concentrations of fatty acids in the culture medium.
SynAas Mediates Fatty Acid Import When Heterologously Expressed in Yeast
In order to further examine the involvement of acyl-activating enzymes in the import of fatty acids, we tested the yeast mutant Δfat1 upon treatment with α-linolenic acid. Fat1p, as the postulated fatty acid transporter, acts in concert with Faa1p and Faa4p in vectorial acylation in yeast (Obermeyer et al., 2007). Analysis of the Δfat1 mutant had revealed that the import of oleate or the fatty acid analogon BODIPY-3823 is strongly reduced (Faergeman et al., 1997). However, earlier studies also revealed that the two acyl-activating enzymes Faa1p and Faa4p are essential for the uptake of fatty acids (Knoll et al., 1995). Here, we describe a new phenotype of the Δfat1 mutant, which displays α-linolenic acid-resistant growth on plates containing high concentrations of α-linolenic acid, whereas yeast wild-type cells are highly sensitive under these conditions (Fig. 7). We propose that, as in Synechocystis, the reduced uptake of toxic α-linolenic acid is the major cause for the resistance phenotype.
In our studies, we focused on Fat1p because it is the only acyl-activating enzyme with several membrane spans, characteristics of a classical transport protein, and has also been proven to have a major impact on fatty acid import in yeast (Faergeman et al., 1997). Overexpression of the cyanobacterial SynAas protein fused to GFP in the Δfat1 mutant led to an increased sensitivity when grown in medium containing 3.6 mm α-linolenic acid and 5.5 mm Glc (Fig. 7A). More strikingly, α-linolenic acid hypersensitivity was imposed in wild-type cells overexpressing SynAas-GFP upon α-linolenic acid treatment (Fig. 7). We postulate that in addition to the functional, endogenous import mechanism of wild-type cells, SynAas mediates the increased import of toxic fatty acids, which causes the observed growth retardation. A generally stronger α-linolenic acid sensitivity in SynAas-expressing wild-type and Δfat1 mutants cells could be observed by reducing the Glc content in the medium to 0.55 mm (Fig. 7B). This effect probably reflects the fact that the uptake of fatty acids becomes less important the more Glc is provided as a carbon source and implies that the native yeast fatty acid import mechanism may be down-regulated at the transcriptional or posttranscriptional level in the presence of sufficient quantities of Glc.
The recovery of the Δfat1/SynAas α-linolenic acid-sensitive phenotype and the hypersensitive phenotype of the wild-type/SynAas cells suggests that the acyl-activating enzyme from Synechocystis is functionally equivalent to Fat1p and further strengthens our assumption that SynAas is able to mediate fatty acid import without the help of any additional transport protein.
Fatty Acids Embedded in Membranes Serve as a Substrate for the Acyl-Activating Enzyme
It had been demonstrated previously that uncharged fatty acids can rapidly flip-flop between the exoplasmic face and the cytoplasmic face of membranes without the help of proteins (Kamp and Hamilton, 1992). In order to generate an influx of fatty acids across membranes, a mechanism needs to be present that removes fatty acids from the membrane at the cytosolic side. Flipping of free fatty acids from one leaflet to the other is reversible; however, when fatty acids are removed on one side, a continuous, transmembrane flux of fatty acids would be created. Such a mechanism can be driven by an intracellular acyl-activating enzyme, which retrieves the lipohilic free fatty acid from the membrane and converts it to the water-soluble acyl-ACP or acyl-CoA ester. This so-called vectorial acylation has been discussed and investigated in great detail for yeast and E. coli as the main mediator of cellular fatty acid uptake (Black and DiRusso, 2003, 2007). For this mechanism to work properly, it would be beneficial if the acyl-activating enzyme was membrane associated. Although SynAas does not contain any apparent membrane domains according to various prediction programs, it has been purified from the membrane fraction of heterologous expression systems in this study and previous reports (Kaczmarzyk and Fulda, 2010). Moreover, SynAas has been identified in a proteomics study of Synechocystis plasma membrane proteins (Pisareva et al., 2007), indicating that it is probably membrane associated in vivo. In order to analyze whether an acyl-activating enzyme can process free fatty acids from artificial membranes, we established an acyl-ACP synthetase assay where the fatty acid substrate is embedded in an artificial liposome membrane. Using this assay, we could show that the recombinant acyl-activating enzyme from Synechocystis is able to highly increase the concentration of water-soluble α-linolenoyl-ACP to 79.8 ± 13.3 nmol L−1, whereas without ATP in the assay, the concentration remains at 24.7 ± 3.8 nmol L−1 in the supernatant (Fig. 8). Concomitantly, the amount of membrane-embedded free fatty acids decreases from 417.8 ± 33.0 to 387.0 ± 21.7 nmol L−1 in an ATP-dependent manner (Fig. 8). From these results, it is evident that fatty acids embedded in artificial liposome membranes can serve as a substrate for an acyl-activating enzyme like SynAas and hence can be transferred across a biological membrane solely by the action of such an enzyme.
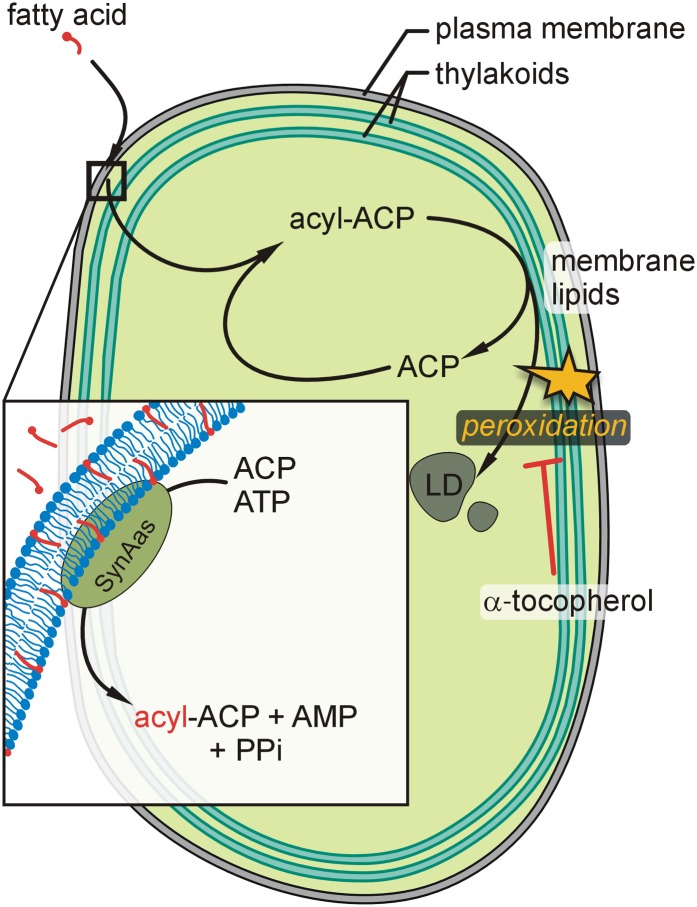
A Model for Fatty Acid Translocation across Membranes
Through analyses of Synechocystis wild-type and mutant cells, complementation of a yeast fatty acid transporter mutant, and liposome experiments, we provide direct evidence for a model in which fatty acids can cross a membrane bilayer by first integrating into the membrane according to their physicochemical properties and subsequent retrieval through the action of SynAas at the cytosolic side (Fig. 9). Although this model has been derived from studying cellular fatty acid uptake in a prokaryotic organism, it might be speculated that an analogous mechanism is operating at endomembranes of eukaryotic cells. Transferring this model to fatty acid export from chloroplasts of higher plants would indicate that fatty acids synthesized as acyl-ACP esters inside chloroplasts would have to be released as free fatty acids into the inner envelope membrane by the action of a stroma-localized thioesterase such as FatA1 or FatB1 (Jones et al., 1995). In the intermembrane space, an acyl-activating enzyme would activate the free fatty acid through esterification to CoA, providing fast export rates by vectorial acylation. The acyl moiety might then either be directly incorporated into phosphatidylcholine via acyl editing, as suggested recently (Tjellström et al., 2012), or transferred by a second vectorial acylation step to the cytosol for incorporation into endoplasmic reticulum-derived lipids. The identity of many of the molecular components involved in this process in higher plants is still elusive. However, the Synechocystis and yeast mutant phenotypes reported here may prove to be useful in future research aiming to demonstrate the putative involvement of candidate proteins in fatty acid export from chloroplasts.
Figure 9.
Model for SynAas-mediated fatty acid membrane translocation in Synechocystis cells. Free fatty acids like α-linolenic acid integrate into the plasma membrane and are retrieved from the membrane phase by SynAas action through simultaneous activation to acyl-ACP (inset). Synechocystis wild-type cells accumulate an excess of α-linolenic acid in cell lipids when exposed to elevated concentrations in the external medium, which leads to lipid peroxidation. In regular growth conditions, lipid peroxidation can largely be prevented through the presence of α-tocopherol. LD, Lipophilic droplets (as depicted in Fig. 3); PPi, inorganic pyrophosphate. [See online article for color version of this figure.]
MATERIALS AND METHODS
Strains and Growth Conditions
Synechocystis sp. PCC 6803 wild-type and mutant strains were grown under constant light conditions at 45 µE m−2 s−1 and 30°C. Liquid cultures were grown in BG11 medium (Rippka et al., 1979), and mutant strains were cultivated in the presence of antibiotics for selection (25 µg mL−1 kanamycin or 25 µg mL−1 spectinomycin). Yeast (Saccharomyces cerevisiae) wild-type and mutant strains were cultivated in liquid synthetic defined medium (0.67% yeast nitrogen base, 2% Glc, and amino acids with uracil for the wild type and without uracil for transformation selection) at 29°C.
Generation of Synechocystis sp. PCC 6803 Knockout Mutants
Synechocystis sp. PCC 6803 mutants were generated as follows. A total of 710 bp of the open reading frame of SynAas was amplified from wild-type genomic DNA via primers SynAas_s (5′-CTAGATGGCGAAACCATTGAC-3′) and SynAas_as (5′-ATGAGAGTTTCCAGTCTGCCC-3′). The PCR product was ligated into pGEM-T Easy (Promega) to yield SynAas_pGEM-T. The HincII-liberated kanamycin cassette from pUC4_Kan (GenBank accession no. X06404) was inserted into SmaI-digested SynAas_pGEM-T, yielding a kanamycin resistance cassette flanked by two equal halves of the SynAas-specific sequence (pGEM-T-Δsynaas_Kan). Transformation of Synechocystis sp. PCC 6803 with pGEM-T-Δsynaas_Kan resulted in the Δsynaas mutant strain.
SLR1736 knockout cells were created as described before (Savidge et al., 2002): the SLR1736 open reading frame was amplified from genomic DNA using primers with added NdeI and BamHI (underlined) restriction sites: SLR1736_s (5′-TATTCATATGGCAACTATCCAAGCTTTTTG-3′) and SLR1736_as (5′-GGATCCTAATTGAAGAAGATACTAAATAGTTC-3′). Digestion of pGEM-T with EcoRV and NdeI offered suitable ligation sites for the NdeI- and BamHI-digested PCR product. pGEM-T-slr1736 was digested with MfeI and ligated with the EcoRI-digested spectinomycin resistance cassette from PUC4_S (pUC 4 with an inserted spectinomycin cassette). Transformation of Synechocystis sp. PCC 6803 and Δsynaas mutant cells with the pGEM-T-Δslr1736_Spec vector resulted in Δslr1736 and Δsynaas/Δslr1736 double mutant strains, respectively.
Generation of Yeast Complementation Strains
The coding sequence of SynAas was amplified using the primers SynAas_CDS_s (5′-CTGTGGGAATCCCTCTACGA-3′) and SynAas_CDS_as (5′-AAACATTTCGTCAATTAAATGTTG-3′) without the stop codon from Synechocystis sp. PCC 6803 genomic DNA. By using the vector pENTR/D-TOPO (Invitrogen), the coding sequence of SynAas was inserted into the yeast expression vector pDR-GW-eGFP (Loqué et al., 2007), which allows for the synthesis of a C-terminal eGFP fusion protein via Gateway cloning (Invitrogen). Transformation of pDR-GW-SynAas-eGFP into the yeast wild-type strain BY4741 and Δfat1(YBR041w) (Euroscarf) yielded the strains wild type/SynAas and Δfat1/SynAas.
Lipid Composition Analysis
Liquid cultures of Synechocystis sp. PCC 6803 wild-type and Δsynaas cells were adjusted to OD750 = 0.25 and incubated with 150 µm α-linolenic acid (Sigma-Aldrich) for 10 h at 45 µE m−2 s−1 and 30°C. Samples (15 mL) were taken every 2 h. Cells were pelleted at 10,000g and subsequently washed two times in BG11 (10 mL) supplemented with bovine serum albumin (1%, w/v) and one time in BG11 (10 mL). Lipids and fatty acids were extracted with dichloromethane:methanol (2:1, v/v) as described by Von Elert and Stampfl (2000). Analyses were performed on an Agilent 6890N gas chromatography system equipped with a flame ionization detector and helium as carrier gas in a capillary gas chromatography column (J&W DB-225, 30 m, i.d. of 0.25 mm, film thickness of 0.25 µm). Fatty acid methyl esters were identified by comparison of retention times with fatty acid methyl ester standards (Sigma-Aldrich). Quantification of the fatty acids was done by comparison with internal standards (C17:0 and C23:0 methyl esters).
TLC
Synechocystis wild-type and Δsynaas suspension culture solutions were adjusted to OD750 = 0.25 and incubated with 150 µm α-linolenic acid. Lipids were extracted from 50 mL of suspension culture at the time points indicated according to Bligh and Dyer (1959). Using the Linomat 5 (CAMAG, Berlin, Germany), 30 µL of the lipid extract was applied to a 20- × 10-cm high-performance TLC Silica Gel 60 plate (Merck), which was prewashed twice with chloroform:methanol (1:1, v/v) and air dried for 30 min. Lipids were separated using acetone:toluene:water (91:30:8, v/v/v) as solvent. For detection of lipid bands, the TLC plate was dipped into a phosphoric acid-copper sulfate reagent [15.6 g of CuSO4(H2O)5 and 9.4 mL of H3PO4 (85%, w/v) in 100 mL of water] and charred at 180°C for 10 min (Yao and Rastetter, 1985). Lipids were identified by comparison with standards of MGDG, SQDG, digalactosyl diacylglycerol, and PG purchased from Lipid Products, Ltd.
Short-Term Incorporation of 1-14C-Labeled α-Linolenic Acid
Synechocystis sp. PCC 6803 wild-type and Δsynaas cells were adjusted to OD750 = 3.0 in 300 µL and incubated with 33 µm (final concentration) α-linolenic acid in a 1:10 dilution with [1-14C]linolenic acid (Hartmann Analytic) at 30°C in the light. Samples (60 µL) were collected every 10 min and spotted on Millipore 45-µm membrane filters. Cells were washed with 10-mL of ice-cold BG11 medium supplemented with 33 µm unlabeled α-linolenic acid. Radioactivity in the retained cells was determined in a Beckmann scintillation counter.
Determination of ETR in Synechocystis Cells
Synechocystis sp. PCC 6803 wild-type and Δsynaas cells were adjusted to OD750 = 0.25 and incubated with 150 µm α-linolenic acid (constant light of 45 µE m−2 s−1, 30°C). Mock-treated cells were incubated with 0.1% ethanol. The photosynthetic ETR was measured every 60 min using a WATER-PAM chlorophyll fluorometer (Walz). For measurements, cultures were diluted 1:10 in BG11 medium in a final volume of 2 mL. Light curves were recorded with the manufacturer’s standard settings with increasing photosynthetic active radiation (PAR; 380–710 nm) from 0 to 1,650 (photon flux density in µmol photons m−2 s−1). The equation for the calculation of ETR is as follows: ETR = yield × PAR × 0.5 × 0.84. Graphs display the ETR at PAR = 200.
Electron Microscopy
Wild-type and Δsynaas Synechocystis sp. PCC 6803 cultures were grown to OD750 = 0.25 and incubated with 150 µm α-linolenic acid, and samples were pelleted at the indicated time points. Cell pellets were fixed with 2% osmium tetroxide and 2.5% glutaraldehyde, dehydrated in an ethanol series, and embedded in Epon 812 resin. Ultrathin sections were prepared using an ultramicrotome (UC7; Leica). The sections were stained with lead citrate and uranyl acetate and viewed with a Phillips CM 10 apparatus.
Growth Assays on Plates and Liquid Culture
For the Synechocystis sp. PCC 6803 drop test, liquid cultures of Synechocystis sp. PCC 6803 wild type and the corresponding mutants were adjusted to OD750 = 0.05, and 5 µL was spotted in four dilutions on BG11 agar plates supplemented with 0.1% ethanol and 0 to 10 µm linolenic acid. Photographs were taken after 7 d of growth at constant light (45 µE m−2 s−1) and 30°C. For the drop test of the tocopherol-deficient mutant, wild-type, Δslr1736, Δsynaas/Δslr1736, and Δsynaas cultures were grown under low-light conditions (15 µE m−2 s−1), diluted to the indicated OD750, shifted to high-light conditions (300 µE m−2 s−1; Maeda et al., 2005), and grown for 7 d. For the determination of growth rates in the presence and absence of α-linolenic acid, cultures were adjusted to OD750 = 0.25. Culture volumes of 25 mL were supplemented with α-linolenic acid (40 µm final concentration) or ethanol as a control. Cultures were incubated at 30°C and medium-light conditions (100 μE m−2 s−1) for 48 h. OD750 was measured every 6 h.
For the yeast drop test, liquid cultures of yeast wild-type and indicated mutant strains were diluted to OD600 = 0.05, and 2 µL was dropped on synthetic dextrose plates containing 1% tergitol (added to increase α-linolenic acid solubility), 3.6 mm (0.1%, w/v) α-linolenic acid, and 0.55 mm (0.01%, w/v) or 5.5 mm (0.1%, w/v) Glc in the indicated combinations. Plants were grown for 5 d at 30°C. For the OD600 measurements, synthetic defined medium was prepared containing 0.67% yeast nitrogen base, 1% tergitol, 3.6 mm (0.1%, w/v) α-linolenic acid, and 5.5 mm (0.1%, w/v) Glc in the indicated combinations. The culture was continuously shaken at 29°C, and OD600 was measured at the indicated time points.
Purification of SynAas from Synechocystis and ACP from Escherichia coli
For amplification of the SLR1609 coding sequence from Synechocystis, the primers SLR1609cds fw (5′-CACCAAAATGTCTGACAGTGGCCATGGCGC-3′) and SLR1609cds rev (5′-AAACATTTCGTCAATTAAATGTTG-3′) and Synechocystis genomic DNA as template were used. Via the vector pENTR-D-TOPO (Invitrogen), the SLR1609 coding sequence was transferred to the yeast expression vector pYES-DEST52 (Invitrogen) to produce SLR1609 with a C-terminal 6× His tag. For amplification of the ACP coding sequence from E. coli genomic DNA, the following primer combination was used: ACP_fw (5′-CACCATGAGCACTATCGAAGAACGCG-3′) and ACP_rev (5′-CGCCTGGTGGCCGTTGATGTAATCAATG-3′). After subcloning into pENTR-D-TOPO (Invitrogen), the coding sequence was transferred to the E. coli expression vector pDEST17 (Invitrogen). For protein expression, the plasmids was transformed into the yeast (SynAas) strain INVSc1 from Invitrogen or E. coli (ACP) expression strain BLR(DE3)pLys (Merck). Protein expression was induced through the addition of 2% Gal (yeast) or 1 mm isopropylthio-β-galactoside (E. coli). Protein purification was carried out using nickel-nitrilotriacetic acid agarose (Qiagen) according to the manufacturer’s instructions with modifications. Concentrations and purity of the proteins were confirmed by SDS-PAGE (Supplemental Fig. S5).
Liposome Assay
Liposomes were prepared as described previously (Takei et al., 1998) with some modifications. Five milligrams of phosphatidylcholine was dissolved in chloroform, dried under nitrogen, and rehydrated in 0.3 m Suc solution for 1 h. The liposomes formed were suspended in 25 mm Tris-HCl buffer, pH 8, and standardized to 200-nm size using lipid extruder (Avanti Polar Lipids). Liposomes were pelleted at 160,000g in a Beckmann ultracentrifuge. The liposomes were incubated with 500 µL of Tris-HCl, pH 8, containing 2 µm [1-14C]linolenic acid (Hartmann Analytic) for 10 min at 30°C followed by three wash steps with Tris-HCl, pH 8. The [1-14C]linolenic acid-loaded liposomes were incubated with 400 µL of acyl-ACP synthetase assay according to Kaczmarzyk and Fulda (2010) for 15 min at 30°C. Liposomes and supernatant were separated by centrifugation at 16,000g and counted separately in a Beckmann scintillation counter.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Confirmation of the inserted kanamycin and spectinomycin cassette and full segregation of the Δslr1736, Δslr1609, and Δslr1736/Δslr1609 mutant.
Supplemental Figure S2. Autoradiography of total lipid extracts from α-[14C]linolenic acid-treated wild-type and Δsynaas cells separated by thin-layer chromatography.
Supplemental Figure S3. Synechocystis wild type and mutant growth assay on plates at higher α-linolenic acid concentrations.
Supplemental Figure S4. α-Tocopherol measurement spectrograms.
Supplemental Figure S5. Purification of 6× histidin-tagged SynAas from Synechocystis sp. PCC 6803 and ACP from E. coli using Ni-NTA affinity chromatography.
Supplemental Materials and Methods S1: Tocopherol measurement.
Supplementary Material
Acknowledgments
We thank Martin Fulda for valuable discussion on SynAas function and physiology. Katja Preuss is gratefully acknowledged for help with lipid analysis and Karin Buchmann for help with electron microscopy.
Glossary
- OD750
optical density at 750 nm
- ETR
electron transport rate
- TLC
thin-layer chromatography
- MGDG
monogalactosyl diacylglycerol
- SQDG
sulfoquinovosyl diacylglycerol
- PG
phosphatidyl glycerol
- OD600
optical density at 600 nm
- PAR
photosynthetic active radiation
References
- Benning C. (2008) A role for lipid trafficking in chloroplast biogenesis. Prog Lipid Res 47: 381–389 [DOI] [PubMed] [Google Scholar]
- Black PN, DiRusso CC. (2003) Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol Mol Biol Rev 67: 454–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PN, DiRusso CC. (2007) Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta 1771: 286–298 [DOI] [PubMed] [Google Scholar]
- Black PN, Kianian SF, DiRusso CC, Nunn WD. (1985) Long-chain fatty acid transport in Escherichia coli: cloning, mapping, and expression of the fadL gene. J Biol Chem 260: 1780–1789 [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Faergeman NJ, Black PN, Zhao XD, Knudsen J, DiRusso CC. (2001) The acyl-CoA synthetases encoded within FAA1 and FAA4 in Saccharomyces cerevisiae function as components of the fatty acid transport system linking import, activation, and intracellular utilization. J Biol Chem 276: 37051–37059 [DOI] [PubMed] [Google Scholar]
- Faergeman NJ, DiRusso CC, Elberger A, Knudsen J, Black PN. (1997) Disruption of the Saccharomyces cerevisiae homologue to the murine fatty acid transport protein impairs uptake and growth on long-chain fatty acids. J Biol Chem 272: 8531–8538 [DOI] [PubMed] [Google Scholar]
- Gardiner SE, Roughan PG, Slack CR. (1982) Manipulating the incorporation of [1-14C]acetate into different leaf glycerolipids in several plant species. Plant Physiol 70: 1316–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbeck JH, Martin IF, Fowler CF. (1980) Mechanism of linolenic acid-induced inhibition of photosynthetic electron transport. Plant Physiol 65: 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SC, Cahoon EB. (2007) Enhancing vitamin E in oilseeds: unraveling tocopherol and tocotrienol biosynthesis. Lipids 42: 97–108 [DOI] [PubMed] [Google Scholar]
- Inoue S, Ejima K, Iwai E, Hayashi H, Appel J, Tyystjärvi E, Murata N, Nishiyama Y. (2011) Protection by α-tocopherol of the repair of photosystem II during photoinhibition in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1807: 236–241 [DOI] [PubMed] [Google Scholar]
- Jones A, Davies HM, Voelker TA. (1995) Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell 7: 359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarzyk D, Fulda M. (2010) Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol 152: 1598–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F, Hamilton JA. (1992) pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc Natl Acad Sci USA 89: 11367–11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll LJ, Johnson DR, Gordon JI. (1995) Complementation of Saccharomyces cerevisiae strains containing fatty acid activation gene (FAA) deletions with a mammalian acyl-CoA synthetase. J Biol Chem 270: 10861–10867 [DOI] [PubMed] [Google Scholar]
- Koo AJ, Ohlrogge JB, Pollard M. (2004) On the export of fatty acids from the chloroplast. J Biol Chem 279: 16101–16110 [DOI] [PubMed] [Google Scholar]
- Koo AJK, Fulda M, Browse J, Ohlrogge JB. (2005) Identification of a plastid acyl-acyl carrier protein synthetase in Arabidopsis and its role in the activation and elongation of exogenous fatty acids. Plant J 44: 620–632 [DOI] [PubMed] [Google Scholar]
- Kunz HH, Scharnewski M, Feussner K, Feussner I, Flügge UI, Fulda M, Gierth M. (2009) The ABC transporter PXA1 and peroxisomal β-oxidation are vital for metabolism in mature leaves of Arabidopsis during extended darkness. Plant Cell 21: 2733–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sheng J, Curtiss R., III (2011) Fatty acid production in genetically modified cyanobacteria. Proc Natl Acad Sci USA 108: 6899–6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loqué D, Lalonde S, Looger LL, von Wirén N, Frommer WB. (2007) A cytosolic trans-activation domain essential for ammonium uptake. Nature 446: 195–198 [DOI] [PubMed] [Google Scholar]
- Maeda H, DellaPenna D. (2007) Tocopherol functions in photosynthetic organisms. Curr Opin Plant Biol 10: 260–265 [DOI] [PubMed] [Google Scholar]
- Maeda H, Sakuragi Y, Bryant DA, Dellapenna D. (2005) Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol 138: 1422–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre TM, Zimmerman GA, Prescott SM. (1999) Biologically active oxidized phospholipids. J Biol Chem 274: 25189–25192 [DOI] [PubMed] [Google Scholar]
- Neiss WF. (1983) Extraction of osmium-containing lipids by section staining for TEM. Histochemistry 79: 245–250 [DOI] [PubMed] [Google Scholar]
- Nunn WD, Simons RW. (1978) Transport of long-chain fatty acids by Escherichia coli: mapping and characterization of mutants in the fadL gene. Proc Natl Acad Sci USA 75: 3377–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeyer T, Fraisl P, DiRusso CC, Black PN. (2007) Topology of the yeast fatty acid transport protein Fat1p: mechanistic implications for functional domains on the cytosolic surface of the plasma membrane. J Lipid Res 48: 2354–2364 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Katoh S, Murakami S. (1977) Effects of linolenic acid on spinach chloroplast structure. Plant Cell Physiol 18: 551–560 [Google Scholar]
- Okazaki K, Sato N, Tsuji N, Tsuzuki M, Nishida I. (2006) The significance of C16 fatty acids in the sn-2 positions of glycerolipids in the photosynthetic growth of Synechocystis sp. PCC6803. Plant Physiol 141: 546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisareva T, Shumskaya M, Maddalo G, Ilag L, Norling B. (2007) Proteomics of Synechocystis sp. PCC 6803: identification of novel integral plasma membrane proteins. FEBS J 274: 791–804 [DOI] [PubMed] [Google Scholar]
- Pollard M, Ohlrogge J. (1999) Testing models of fatty acid transfer and lipid synthesis in spinach leaf using in vivo oxygen-18 labeling. Plant Physiol 121: 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111: 1–61 [Google Scholar]
- Savidge B, Weiss JD, Wong YH, Lassner MW, Mitsky TA, Shewmaker CK, Post-Beittenmiller D, Valentin HE. (2002) Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 129: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. (2005) Chemistry and biology of vitamin E. Mol Nutr Food Res 49: 7–30 [DOI] [PubMed] [Google Scholar]
- Schnurr JA, Shockey JM, de Boer GJ, Browse JA. (2002) Fatty acid export from the chloroplast: molecular characterization of a major plastidial acyl-coenzyme A synthetase from Arabidopsis. Plant Physiol 129: 1700–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine WE, Mancha M, Stumpf PK. (1976) Fat metabolism in higher plants: differential incorporation of acyl-coenzymes A and acyl-acyl carrier proteins into plant microsomal lipids. Arch Biochem Biophys 173: 472–479 [DOI] [PubMed] [Google Scholar]
- Shockey JM, Fulda MS, Browse J. (2003) Arabidopsis contains a large superfamily of acyl-activating enzymes: phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme A synthetases. Plant Physiol 132: 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler PA. (1972) Aging of the photosynthetic apparatus. IV. Similarity between the effects of aging and unsaturated fatty acids on isolated spinach chloroplasts as expressed by volume changes. Biochim Biophys Acta 275: 182–191 [DOI] [PubMed] [Google Scholar]
- Singh SC, Sinha RP, Hader DP. (2002) Role of lipids and fatty acids in stress tolerance in cyanobacteria. Acta Protozool 41: 297–308 [Google Scholar]
- Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. (1998) Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94: 131–141 [DOI] [PubMed] [Google Scholar]
- Thompson GA, Roughan PG, Browse JA, Slack CR, Gardiner SE. (1986) Spinach leaves desaturate exogenous [14C]palmitate to hexadecatrienoate: evidence that de novo glycerolipid synthesis in chloroplasts can utilize free fatty acids imported from other cellular compartments. Plant Physiol 82: 357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjellström H, Yang Z, Allen DK, Ohlrogge JB. (2012) Rapid kinetic labeling of Arabidopsis cell suspension cultures: implications for models of lipid export from plastids. Plant Physiol 158: 601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Elert E, Stampfl P. (2000) Food quality for Eudiaptomus gracilis: the importance of particular highly unsaturated fatty acids. Freshw Biol 45: 189–200 [Google Scholar]
- Wada H, Murata N. (1990) Temperature-induced changes in the fatty acid composition of the cyanobacterium, Synechocystis PCC6803. Plant Physiol 92: 1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Murata N. (1998) Membrane lipids in cyanobacteria. PA Siegenthaler, N Murata, eds, Lipids in Photosynthesis: Structure, Function and Genetics. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 65–81 [Google Scholar]
- Yao JK, Rastetter GM. (1985) Microanalysis of complex tissue lipids by high-performance thin-layer chromatography. Anal Biochem 150: 111–116 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.