Abstract
Intracellular trafficking of auxin transporters has been implicated in diverse developmental processes in plants. Although the dynamic trafficking pathways of PIN-FORMED auxin efflux proteins have been studied intensively, the trafficking of ATP-binding cassette protein subfamily B proteins (ABCBs; another group of auxin efflux carriers) still remains largely uncharacterized. In this study, we address the intracellular trafficking of ABCB4 in Arabidopsis (Arabidopsis thaliana) root epidermal cells. Pharmacological analysis showed that ABCB4 barely recycled between the plasma membrane and endosomes, although it slowly endocytosed via the lytic vacuolar pathway. Fluorescence recovery after photobleaching analysis revealed that ABCB4 is strongly retained in the plasma membrane, further supporting ABCB4’s nonrecycling property. The endocytosis of ABCB4 was not dependent on the GNOM-LIKE1 function, and the sensitivity of ABCB4 to brefeldin A required guanine nucleotide exchange factors for adenosyl ribosylation factor other than GNOM. These characteristics of intracellular trafficking of ABCB4 are well contrasted with those of PIN-FORMED proteins, suggesting that ABCB4 may be a basic and constitutive auxin efflux transporter for cellular auxin homeostasis.
Cell-to-cell auxin transport produces local auxin gradients for diverse plant developmental processes such as cell and tissue differentiation and organogenesis (Petrásek and Friml, 2009). This auxin transport is mediated by several membrane transport proteins such as PIN-FORMED (PIN), AUXIN RESISTANT1 (AUX1)/LIKE-AUXIN RESISTANT1, and ABCB/PGP/MDR (for ATP-binding cassette protein subfamily B/P-glycoprotein/multidrug resistance; Vanneste and Friml, 2009). Directional auxin transport is achieved by asymmetric localization of PINs in the plasma membrane (PM), and the dynamic subcellular trafficking is responsible for the regulation of PIN protein localization (Petrásek and Friml, 2009).
Previous studies have demonstrated that both common and unique trafficking pathways cooperate for the subcellular localization of PIN1, PIN2, and AUX1. PIN1 and PIN2 proteins undergo recycling, and their basal localization is regulated by brefeldin A (BFA)-sensitive GNOM, a guanine nucleotide exchange factor for adenosyl ribosylation factor (ARF-GEF; Geldner et al., 2001, 2003; Kleine-Vehn et al., 2008a). However, trafficking of AUX1 and apical localization of PIN2 are regulated by ARF-GEFs other than GNOM (Geldner et al., 2003; Kleine-Vehn et al., 2006, 2008a). In addition to ARF-GEFs, retromer complexes such as SORTING NEXIN 1 (SNX1) and vacuolar protein-sorting proteins also affect the recycling of PINs from the lytic vacuolar pathway (Kleine-Vehn et al., 2008b). Whereas GNOM is involved in the recycling of PIN1 (Geldner et al., 2003), GNOM-LIKE1 (GNL1; a BFA-resistant ARF-GEF) is required for endocytosis of PIN2 (Teh and Moore, 2007). In the presence of BFA, BEN1 (for BFA-visualized endocytic trafficking defective 1; an ARF-GEF) affects the early step of endocytic trafficking for both PIN1 and V-type ATPase (Tanaka et al., 2009). These previous studies indicate that different ARF-GEFs are involved in the regulation of endocytosis and recycling processes of different PINs and other auxin transporters.
Of the many ABCB family members, only ABCB1, ABCB4, and ABCB19 have been implicated in auxin transport (Noh et al., 2001; Multani et al., 2003; Geisler et al., 2005; Terasaka et al., 2005; Cho et al., 2007). For example, the ABCB4 loss-of-function mutants show several root phenotypes, such as agravitropism, abnormal lateral root formation, and enhanced root hair elongation (Santelia et al., 2005; Terasaka et al., 2005; Cho et al., 2007; Lewis et al., 2007). These ABCBs (AT-ABCBs, where AT stands for auxin transporting) localize to the PM in either a polar or nonpolar manner, depending on the protein and the cell type in which they are expressed (Geisler et al., 2005; Terasaka et al., 2005; Blakeslee et al., 2007; Cho et al., 2007; Wu et al., 2007; Mravec et al., 2008). Although the effect of BFA on the trafficking of AT-ABCBs has been studied, only intracellular trafficking of ABCB19 was examined in relatively more detail (Cho et al., 2007; Titapiwatanakun et al., 2009; Wu et al., 2010). Insensitivity to short-term treatment of BFA and the long recovery time after photobleaching of ABCB19 indicated that ABCB19 is stably anchored in the PM and that the recycling from PM to endosomes is not dynamic, which is different from PINs and ABCB1 (Titapiwatanakun et al., 2009). In addition, trafficking of ABCB19 to the PM is dependent on GNL1 (Titapiwatanakun et al., 2009). It also was shown that endoplasmic reticulum-localized TWISTED DWARF1 is required for the proper targeting of the three AT-ABCBs to the PM, whereas PIN2 localization is not affected by TWISTED DWARF1 (Wu et al., 2010).
To characterize the trafficking of auxin-transporting ABCB proteins in greater detail, we compared the subcellular behavior of ABCB4 with PINs using a variety of cytological, genetic, and pharmacological tools in Arabidopsis (Arabidopsis thaliana). Our study suggests that ABCB4 proteins are strongly retained in the PM and show a distinct regulation mechanism relative to PIN proteins.
RESULTS
ABCB4 Proteins Exhibit Lower Levels of Endosomal Localization Than PINs
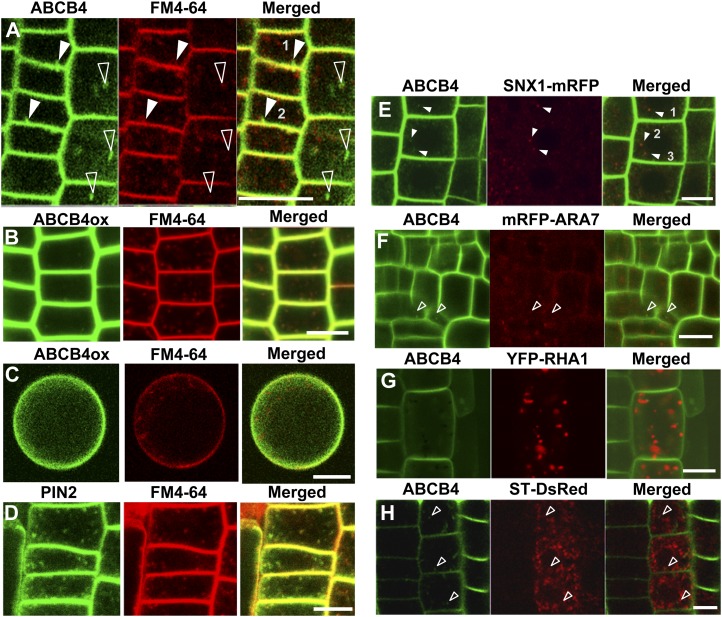
To characterize the intracellular trafficking of ABCB4, its subcellular localization in root epidermal cells was observed. Colocalization analysis of ProABCB4:ABCB4-GFP transformants with the endocytic tracer FM4-64 revealed that only a small number (less than five in a cell; n + 20 seedlings) of ABCB4-containing vesicles overlapped with FM4-64-positive endosomes (Fig. 1A). Because the abundance of ABCB4 in endosomes is low, we tested ABCB4 endosomal localization in the ABCB4 overexpression line and compared this with PIN2 localization. Similar low abundance of ABCB4 in endosomal vesicles was also observed in the root tissues of Pro-35S (cauliflower mosaic virus 35S promoter):ABCB4-YFP transformants and with leaf protoplast cells (Fig. 1, B and C; n + 50 seedlings). However, in root epidermal cells, PIN2 exhibited higher levels of endosomal localization (less than 15 in a cell; n + 12) compared with ABCB4 (Fig. 1D).
Figure 1.
ABCB4 shows weak endosomal localization. A, Colocalization of ABCB4-GFP and the endocytic tracer FM4-64 (2 μm, 15 min). B and C, Colocalization of ABCB4-YFP and the endocytic tracer FM4-64 (2 μm, 15 min) in root epidermal cells (B) and a leaf mesophyll protoplast (C). D, Colocalization of PIN2-GFP and FM4-64 (2 μm, 15 min) in root epidermal cells. E, Colocalization analysis of ABCB4-GFP and a TGN and/or PVC marker, SNX1-mRFP. F, Colocalization analysis of ABCB4-GFP and a PVC marker, mRFP-ARA7. G, Colocalization analysis of ABCB4-GFP and a PVC marker, YFP-RHA1. H, Colocalization analysis of ABCB4-GFP and a Golgi marker, ST-RFP. White arrowheads indicate colocalization, and open arrowheads indicate no colocalization. Bars + 10 μm.
In order to identify the endosomes in which ABCB4 localizes, we performed colocalization studies with certain known endosomal markers. ABCB4-GFP partially overlapped with the scattered cytoplasmic particles of SNX1-mRFP (approximately 54%; n + 21 seedlings; Fig. 1E). SNX1 is known as a prevacuolar compartment (PVC; a synonym of multivesicular bodies) marker (Jaillais et al., 2008; Kleine-Vehn et al., 2008b) that acts in the degradation pathway of PIN2 and BOR1 (a PM boron transporter; Jaillais et al., 2008; Kleine-Vehn et al., 2008b) and is also responsible for redirecting the proteins to the trans-Golgi network (TGN) and the PM (Jaillais et al., 2007, 2008). However, SNX1 seems to localize not only to the PVC but also predominantly in the TGN, as the recycling point for vacuolar sorting receptors from the PVC to the TGN (Niemes et al., 2010). Next, we examined whether ABCB4 colocalizes with Rab5 orthologs ARABIDOPSIS RAB GTPASE HOMOLOG F2B (ARA7/RabF2b) and ARABIDOPSIS RAB HOMOLOG F2A (RHA1/RabF2a), other PVC markers (Lee et al., 2004; Haas et al., 2007). The F1 double transgenic seedlings of ProABCB4:ABCB4-GFP and Pro-35S:mRFP-ARA7/RabF2b failed to show any distinct ABCB4 localization in the ARA7/RabF2b-positive endosomes (Fig. 1F). Like ARA7/RabF2b, ABCB4 was also not observed in the RHA1/RabF2a-positive endosomes (Fig. 1G). Additionally, internal ABCB4-GFP signals (ProABCB4:ABCB4-GFP) did not overlap with the Golgi marker sialyl transferase (ST)-DsRed (Saint-Jore et al., 2002; Fig. 1H).
To test whether ABCB4 localized in the endosomes that are involved in PIN2 recycling, the trafficking inhibitor ENDOSIDIN1 (ES1) was employed. ES1 blocks early endocytosis of PIN2 as well as AUX1 and BRI1, but not PIN1 or PIN7, resulting in the internal accumulation of characteristic endosidin bodies defined by two TGN/early endosome markers, syntaxin SYP61 and the V-ATPase subunit VHA-a1 (Robert et al., 2008). Although ES1 caused internal PIN2 aggregations, it failed to form similar endosidin bodies with ABCB4 (Supplemental Fig. S1), which indicates that ABCB4 trafficking is predominantly ES1 independent.
BFA blocks the retargeting of endocytosed vesicles from recycling to the PM as well as the degradation pathway to the vacuole and results in aggregates (BFA compartments) inside the cell (Geldner et al., 2001; Kleine-Vehn et al., 2008b). Because ABCB4 generated BFA compartments like PIN (Cho et al., 2007), BFA treatment analysis together with the use of subcellular compartment markers could provide information about the trafficking pathways of ABCB4. Thus, we tried to identify the subcellular organelles that are present in the ABCB4-containing BFA compartments.
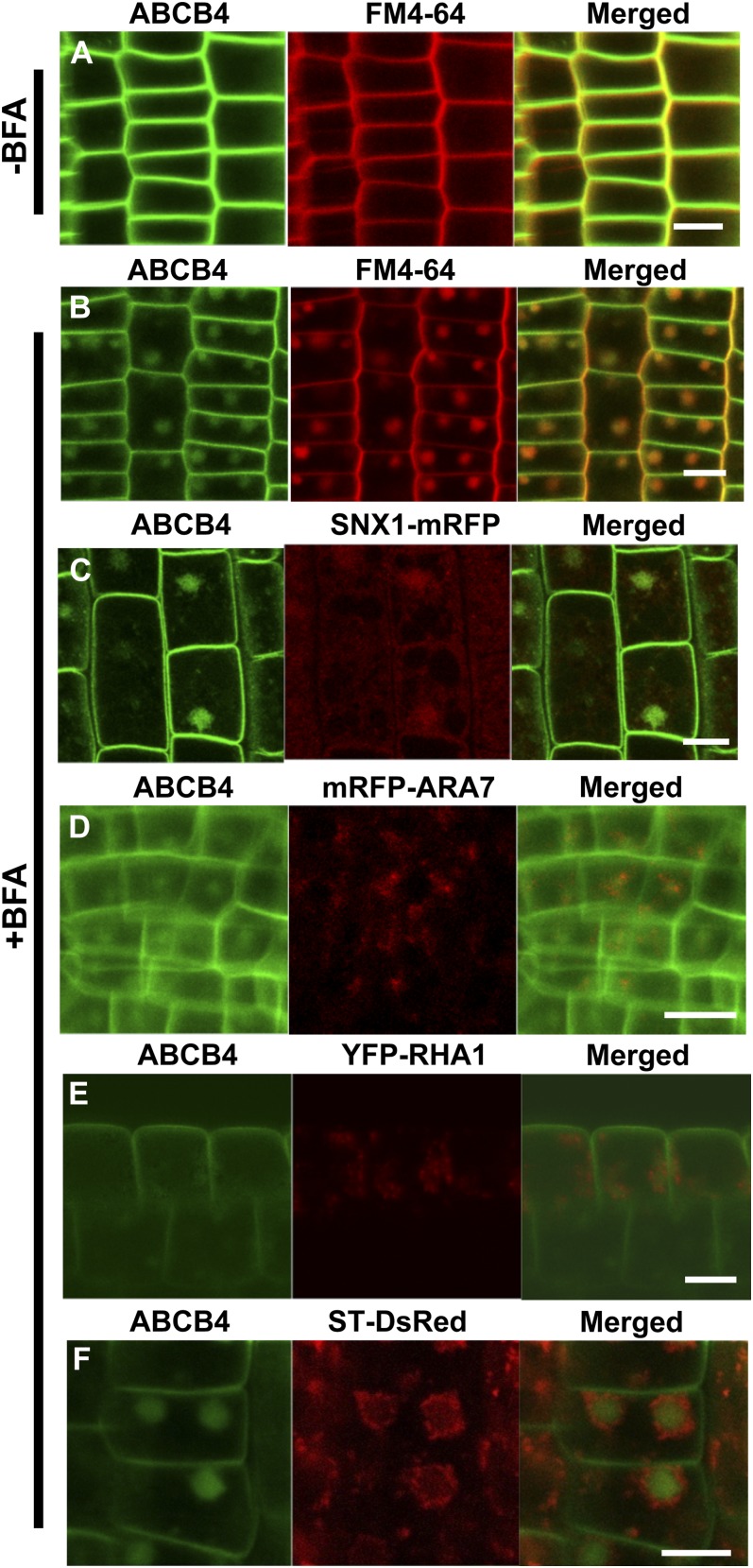
Although ABCB4 did not show strong endosomal localization, BFA induced its aggregation into BFA compartments that merged closely with FM4-64 positive endosomes (Fig. 2, A and B). BFA treatment caused both ABCB4 and SNX1 to form ball-shaped BFA compartments, and these two signals appeared to be merged (Fig. 2C), which is consistent with the results shown in Figure 1E. However, ARA7/RabF2b was partially merged with the BFA compartments of ABCB4 in response to BFA, and some of them were located at the edge of the ABCB4 aggregates (Fig. 2D). Another PVC marker, RHA1/RabF2a, did not merge with BFA compartments of ABCB4 (Fig. 2E). The RHA1 endosomes did not aggregate together upon BFA treatment like ABCB4 but surrounded the ABCB aggregates.
Figure 2.
BFA compartments of ABCB4 colocalized with SNX1. A and B, Colocalization of ABCB4-GFP and FM4-64 in the BFA compartment. Transgenic seedlings were treated with 50 μm BFA for 2 h and then cotreated with 2 μm FM4-64 for 15 min (B). The same volume of DMSO was administered as a control (A). C, Colocalization analysis of ABCB4-GFP and SNX1-mRFP with BFA (50 μm, 2 h). D, Colocalization analysis of ABCB4-GFP and mRFP-ARA7 with BFA (50 μm, 2 h). E, Colocalization analysis of ABCB4-GFP and YFP-RHA1 with BFA (50 μm, 2 h). F, Colocalization analysis of ABCB4-GFP and ST-RFP with BFA (50 μm, 2 h). Bars + 10 μm.
Also, ABCB4-containng BFA compartments did not colocalize with that of ST-DsRed (Fig. 2F). Upon BFA treatment of this line, ABCB4-GFP and ST-DsRed signals formed large BFA compartments in a similar way: a round ball-like shape for ABCB-GFP and a ring-like organization for ST-DsRed. However, the ABCB4-GFP signals resided within the circular Golgi structure, and the two signals barely overlapped (Fig. 2F).
In summary, part of ABCB4 endocytoses through SNX1-positive endosomes, and the number of ABCB4-containing endosomes is less abundant compared with that of PIN2.
ABCB4 Is Unlikely to Recycle between the PM and Endosomes
Whereas PIN2 recycling between the PM and endosomes was demonstrated using a green-to-red photoconvertible version of PIN2 (PIN2-EosFP; Dhonukshe et al., 2007), the recycling status of ABCB4 has not been demonstrated. From the results in Figure 1, we considered that the recycling pathways of ABCB4 from the PM and endosomes are not active; instead, an endocytic pathway to the vacuole could be the relevant trafficking pathway for ABCB4, which is also responsive to BFA (Kleine-Vehn et al., 2008b). In order to address this hypothesis, we examined from which pathway ABCB4-positive BFA compartments are produced. For this purpose, we also used a UV-induced green-to-red photoconvertible protein (Kaede) for tracing the trafficking of PM proteins (Ando et al., 2002). The abcb4 mutant root grows slightly longer hairs, most likely due to the partial lack of ABCB4-mediated auxin efflux in the root hair cell (Cho et al., 2007). The N-terminal Kaede fusion of ABCB4 (ProABCB4:Kaede-ABCB4) partially rescued the abcb4 mutant phenotype as the C-terminal GFP version did (Cho et al., 2007; Supplemental Fig. S2), indicating that the fusion construct is functional.
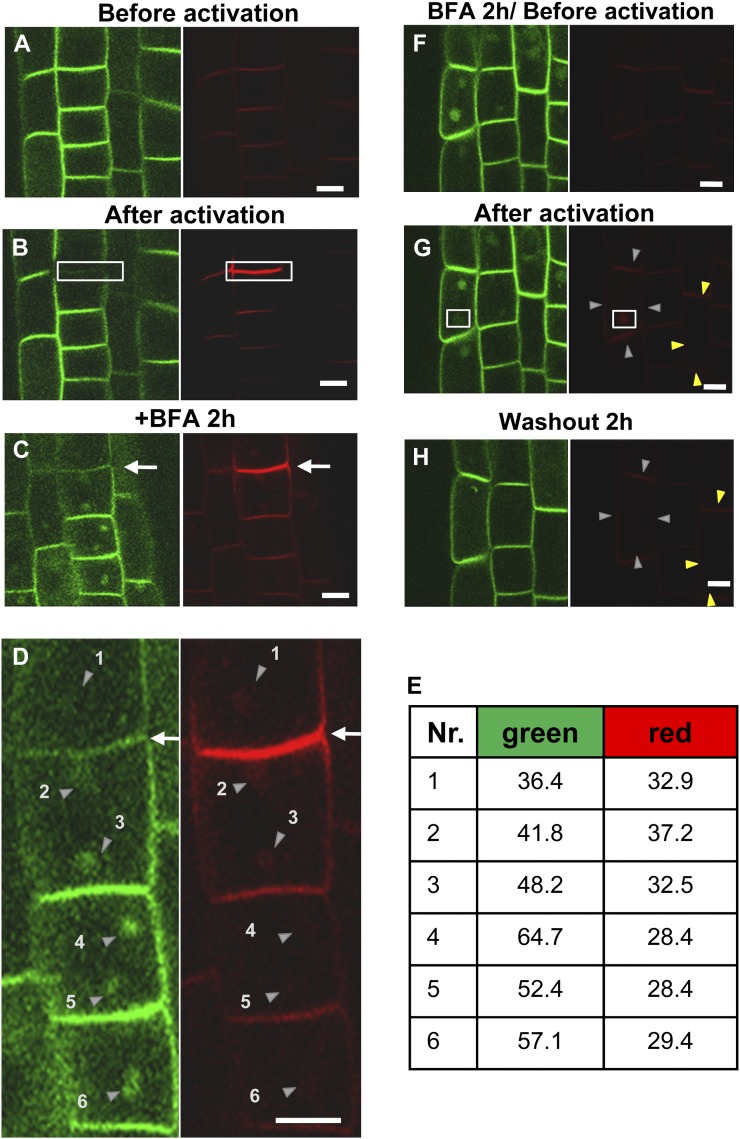
UV (364 nm) radiation under a 40× object lens effectively converted the fluorescence of Kaede-ABCB4 fusion proteins from green to red in root epidermal cells (Fig. 3, A and B). Only a small part of the green-Kaede-ABCB4-containing PM was activated, and then the formation of ABCB4-containing BFA compartments was observed (Fig. 3, A–E). Although derived from limited endocytic resources (i.e. a small photoactivated portion of the PM), BFA compartments containing the weak red-Kaede signals were formed near the activated (red signal) PM regions (Fig. 3, C and D). BFA compartments that were formed distal to the photoactivated PM section (Fig. 3, D, right panel, nos. 4–6, and E) exhibited weaker red-Kaede signals than proximal compartments (Fig. 3, D, right panel, nos. 1–3, and E), whereas the opposite pattern was observed for green-Kaede-ABCB4 (Fig. 3D, left panel). The same tendency was observed in nine out of 12 independent seedlings, but the other three contained only one BFA compartment in the cells adjacent to photoactivated PM; thus, we excluded them from the comparison. These findings suggest that endocytosis is responsible for the PM-derived vesicles that reside in the ABCB4-containing BFA compartments.
Figure 3.
ABCB4 does not seem to recycle between the PM and endosomes. A, Fluorescence of Kaede-ABCB4 in root epidermal cells prior to green-to-red photoconversion (left, green channel; right, red channel, for A–H). B, Fluorescence of Kaede-ABCB4 after selective green-to-red photoconversion. Only the ROI (boxed), which contained green-Kaede-ABCB4, was photoconverted to red. C, Tracking of the photoconverted red-Kaede-ABCB4 signal after BFA treatment (25 μm, 2 h). Arrows in C and D indicate the photoactivated PM region. D, Magnified images of C. Arrowheads indicate the positions of BFA compartments. E, Relative fluorescence intensity (in arbitrary units) of the numbered BFA compartments in D. F, Fluorescence of Kaede-ABCB4 after BFA treatment (25 μm, 2 h) prior to photoconversion. G, Fluorescence of Kaede-ABCB4 after selective green-to-red photoconversion. Only the ROI (boxed), which contained a BFA compartment of green-Kaede-ABCB4, was photoconverted to red. H, Tracking of the photoconverted red-Kaede-ABCB4 signal after washout with 1/2 MS for 2 h. Gray arrowheads indicate the PM region containing a photoconverted BFA compartment, and yellow arrowheads indicate neighbor PMs (background). Bars + 10 μm.
To address whether ABCB4-containing BFA compartments are derived by blocking ABCB4 recycling to the PM after endocytosis and/or trafficking to the vacuole, we tracked the ABCB4 signal from BFA compartments after washing out the BFA. Kaede-ABCB4-containing cells were treated with BFA for 2 h before photoconversion (Fig. 3F). One large BFA compartment of green-Kaede-ABCB4 was marked as a region of interest (ROI) and converted to red-Kaede-ABCB4 upon 364-nm radiation (Fig. 3G). After a 2-h washout, the BFA compartment disappeared (Fig. 3H) and the red-Kaede-ABCB4 membrane signals (gray arrowheads) including neighbor membranes (yellow arrowheads, background) were compared before and after washing out the BFA. The sum of red-Kaede signal intensity for the gray and yellow arrowheads marking the PM are 36.3 and 20.1 before the washout (Fig. 3G) and 34.0 and 17.7 after washout of BFA (Fig. 3H), respectively. The red-Kaede signal level was not significantly changed after the washout of BFA and was seen repeatedly in nine independent cells from nine trials. Our data suggest that ABCB4 recycling between the PM and endosomes is unlikely, and the BFA compartments of ABCB4 seem to be generated mostly by blocking endocytosis to the vacuole. However, the signal of green-Kaede-ABCB4 in the BFA compartment was much weaker than the ABCB4 signal at the PM (Figs. 2B and 3, C and D), because only approximately 21.5% of the ABCB signal at the PM is BFA sensitive (see Fig. 6A below). This affected the intensity of the photoconverted red-Kaede-ABCB4 BFA compartment and sequentially the red-Kaede-ABCB4 signal at the PM after washout. Thus, the possibility that some undetectable amounts of red-Kaede-ABCB4 recycles to the PM cannot be completely ruled out.
Figure 6.
BFA sensitivity of ABCB4 is different from PINs. A to C, Relative intensities of ABCB4-GFP (A), PIN2-GFP (B), and PIN1-GFP (C) in the PM of root epidermal cells after BFA treatment. Transgenic seedlings were treated with 0.1% (v/v) DMSO and 50 μm CHX for 2 h. For BFA treatment, seedlings were pretreated with 50 μm CHX for 30 min and then cotreated with 50 μm BFA for 2 h before observation. Data represent means ± se (n + 95–110 cells from 19–22 roots for ABCB4-GFP; n + 85–235 cells from 17–47 roots for PIN2-GFP; n + 90–160 cells from 18–32 roots for PIN1-GFP, from three to five independent experiments). BFA sensitivity between ABCB4-GFP, PIN2-GFP, and PIN1-GFP was significantly different as determined by one-way ANOVA (P < 0.001). D to L, Representative confocal microscopy images of root epidermal cells expressing ABCB4-GFP (D–F), PIN2-GFP (G–I), and PIN1-GFP (J–L), as described in A to C. Bars + 10 μm. [See online article for color version of this figure.]
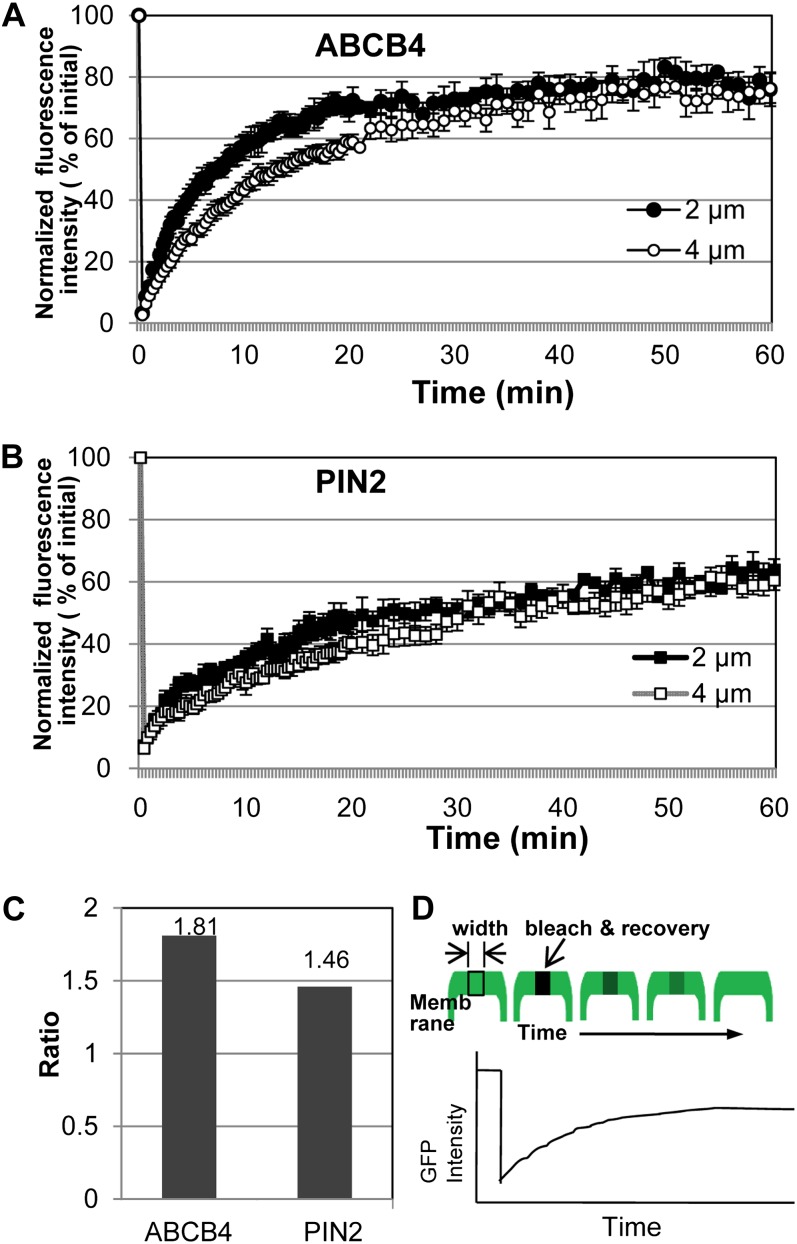
Fluorescence Recovery after Photobleaching Analysis Reveals a Stable Association of ABCB4 in the PM
BFA resistance and the low endocytosis rate of ABCB4, as indicated by its stable residence in the PM, raise the question of whether ABCB4 associates strongly with the PM. Fluorescence recovery after photobleaching (FRAP) analysis was performed to assess the strength of association between ABCB4 and the PM. This technique determines protein mobility in living cells by measuring the rate of fluorescence recovery at a bleached site (Sprague and McNally, 2005). The mobility of ABCB4-GFP and PIN2-GFP was compared using FRAP analysis. The GFP signals of ABCB4-GFP and PIN2-GFP were bleached from 2- or 4-µm-wide PM regions of the root epidermal cells, and fluorescence recovery was recorded over time (Fig. 4D). This experiment was designed to test the following three possibilities. First, if the PM fluorescence recovers only by lateral diffusion of unbleached proteins, then the recovery time will be proportional to the width of the bleached area. This parameter can indicate whether the protein has strong residence in the PM or low recycling between the PM and endosomes. Second, if recycling processes are the only contributors to recovery, then the fluorescence recovery rate will be similar regardless of the size of the bleached area. Third, if both lateral diffusion and recycling contribute to recovery, then the recovery rate will vary according to their relative contribution levels (Rotblat et al., 2004; Goodwin and Kenworthy, 2005).
Figure 4.
FRAP analyses demonstrate the stable association of ABCB4 in the PM. A, Fluorescence recovery curves after photobleaching of ABCB4-GFP in the PM of the root epidermal cell (n + 8–9). B, Fluorescence recovery curves after photobleaching of PIN2-GFP in the PM of the root epidermal cell (n + 8). Data represent means ± se. C, Ratio comparison of the t1/2 (4 µm versus 2 µm) of the extrapolated maximum recovery for ABCB4-GFP and PIN2-GFP. D, Schematic illustration of the FRAP analysis. [See online article for color version of this figure.]
Fluorescence was observed for approximately 60 min after bleaching. ABCB4-GFP fluorescence was observed to recover faster with a 2-µm bleach compared with a 4-µm bleach (Fig. 4A). To estimate the process of recovery, the maximum recovery value (%) from the recovery curves of ABCB4 and PIN2 after photobleaching was extrapolated using SPSS software, and then the ratio of the time required to attain 50% recovery (t1/2; 4 µm versus 2 µm) of the extrapolated maximum recovery was calculated (Supplemental Table S1). Theoretically, a t1/2 ratio of 2 should indicate that fluorescence has recovered by lateral diffusion within the PM, whereas a ratio of 1 would indicate recovery by the recycling process (Niv et al., 2002; Goodwin and Kenworthy, 2005). Values between 1 and 2 can be considered to arise from a mix of the two processes, where both diffusion and recycling contribute to recovery. However, because cycloheximide (CHX) was not employed for these experiments, the contribution of recycling and de novo secretion of the ABCB4-GFP and PIN2-GFP recovery could not be distinguished. Both ABCB4 and PIN2 showed faster recovery with the 2-µm bleach than with the 4-µm bleach. However, the difference between the two recovery curves of ABCB4 was distinctively larger than that of PIN2 within 20 min (Fig. 4, A and B), and they exhibited different 4 µm:2 µm ratios of t1/2, namely 1.81 for ABCB4 and 1.48 for PIN2 (Fig. 4C; Supplemental Table S1). ABCB4 recovery by lateral diffusion is more predominant than recovery by recycling or de novo ABCB4 secretion, suggesting that the ABCB4 protein is associated with the PM and that its intracellular trafficking is less compared with PIN2.
ABCB4 Is Slowly Trafficked to the Vacuole
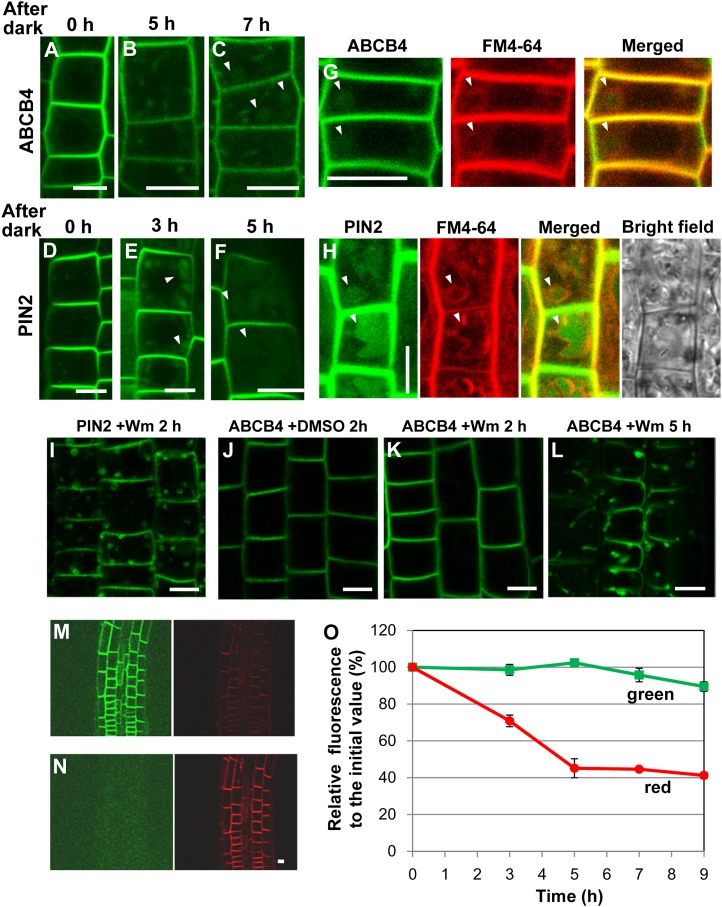
In general, stable PM-resident proteins degrade more slowly than actively recycling PM proteins, because recycling PM proteins create more opportunities to contact other compartments and proteolytic enzymes located in the cytoplasm (Hare and Taylor, 1991). The rate of ABCB4 degradation was examined to confirm that most ABCB4 proteins reside in the PM. Because light causes the degradation of GFP due to conformational changes and also affects GFP signal by inducing a pH change in the vacuole (Tamura et al., 2003), we transferred ABCB4-GFP and PIN2-GFP transgenic lines to the dark, the respective transgenic lines were observed for ABCB4-GFP and PIN2-GFP signals, and the time required for these proteins to be detected in the vacuole was measured. This time could reflect the degradation rate of ABCB4 and PIN2.
Sizable ABCB4-GFP-containing vesicles were detected in the intracellular space of root epidermal cells 5 h after transfer to the dark, and the earliest vacuolar accumulation was observed after 7 h of darkness (Fig. 5, A–C). In contrast, PIN2-GFP appeared in the vacuole within 3 h of darkness (Fig. 5, D–F). The PM and endocytic tracer FM4-64 have been shown to reach the vacuolar tonoplast within 2 h of dark treatment (Dettmer et al., 2006). When the roots of transgenic seedlings were treated with FM4-64 for the final 3 h in the dark, ABCB4- and PIN2-GFP showed colocalization with FM4-64 in the epidermal cell vacuoles (Fig. 5, G and H).
Figure 5.
ABCB4 degrades more slowly than PIN2. A to C, Changes in the subcellular localization of ABCB4-GFP after dark incubation of transformant seedlings for 5 h (B) and 7 h (C). A control image was taken before dark treatment (A). D to F, Changes in the subcellular localization of PIN2-GFP after dark incubation of the transformant seedlings for 3 h (E) and 5 h (F). A control image was taken before dark treatment (D). G, Colocalization of ABCB4-GFP and FM4-64 after dark treatment. The transgenic seedlings were incubated in the dark for 7 h and treated with 2 μm FM4-64 for 3 h before observation. H, Colocalization of PIN2-GFP and FM4-64 after dark treatment. The transgenic seedlings were incubated in the dark for 4 h and treated with 2 μm FM4-64 for the final 3 h. I to L, ProPIN2:PIN2-GFP transgenic seedlings were treated with wortmannin (Wm; 33 μm) for 2 h (I), and ProABCB4:ABCB4-GFP transformants were treated without wortmannin (J) or with wortmannin for 2 h (K) and 5 h (L). M and N, Fluorescence images of root epidermal cells expressing Kaede-ABCB4 (ProABCB4:Kaede-ABCB4) before (M) and after (N) UV-induced green-to-red photoconversion. Bars + 10 μm. O, Lifetime of ABCB4 in the PM (n + 7–14 for each time point of red-Kaede-ABCB4 from four independent experiments; n + 7–14 for each time point of green-Kaede-ABCB4 from three independent experiments). Data represent means ± se.
Wortmannin is an inhibitor of phosphatidylinositiol-3-kinase and interferes with the endocytic process from the PM to the vacuole (Matsuoka et al., 1995; Kleine-Vehn et al., 2008b). Wortmannin was used to acquire additional evidence for the vacuolar targeting of ABCB4 and also to compare the vacuolar targeting of ABCB4 and PIN2. In all of the seedlings observed, treatment with 33 μm wortmannin for 2 h caused a relocalization of PIN2-GFP to wortmannin-induced compartments (previously described as PVC/multivesicular bodies by Kleine-Vehn et al., 2008b; Fig. 5I). However, the wortmannin sensitivity of ABCB4 was different. Only four out of 21 seedlings responded to wortmannin within 2 h (Fig. 5, J and K), whereas 10 out of 21 seedlings responded within 5 h (Fig. 5L). In addition, fewer wortmannin compartments were observed for ABCB4 than for PIN2. These results indicate that, as with PIN2, the lytic vacuolar pathway of ABCB4 is dependent upon the phosphatidylinositol-dependent pathway and the different sensitivities to wortmannin are likely due to differences in rates (or amounts) of endocytosis to the vacuole. Kaede-ABCB4 was used to quantify ABCB4 signals in the PM of root epidermal cells over time (Fig. 5, M and N). Green-Kaede-ABCB4 in the PM signals remained at 89.5% of their initial value after 9 h. However, red-Kaede-ABCB4 signals in the PM were reduced to 45.1% at 5 h after UV photoactivation, and this value continued until 9 h with slight reduction (Fig. 5O). Because we measured ABCB4 signals in the PM without considering the ABCB4 signal in the vacuole, these data could restrictively provide the t1/2 of ABCB4 in the PM, which is approximately 2.5 h.
In summary, the ABCB4 endocytic event to the vacuoles is a slow process that is related to the stable anchoring of ABCB4 in the PM.
BFA Sensitivity of ABCB4 Trafficking Requires ARF-GEFs Other Than GNOM, and Its Endocytosis Is Independent of GNL1
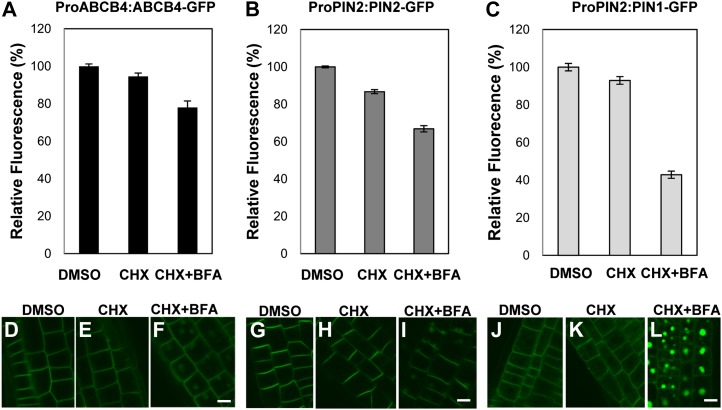
Although ABCB4 trafficking is responsive to BFA, which induces the formation of internal ABCB4-containing compartments, a substantial proportion of the ABCB4 signal remains in the PM (Figs. 1 and 3; Cho et al., 2007). To determine how much of the ABCB4 residing in the PM is resistant to BFA, a quantitative confocal microscopy program was used to compare the intensities of ABCB4 (ProABCB4:ABCB4-GFP), PIN1 (ProPIN2:PIN1-GFP), and PIN2 (ProPIN2:PIN2-GFP) signals in the respective areas of localization in the PM of BFA-treated root epidermal cells. Compared with the control (i.e. only dimethyl sulfoxide [DMSO] treatment), 94.6% of the ABCB4-GFP signal was retained in the PM after treatment with CHX only and 78.0% with cotreatments of CHX and BFA (Fig. 6, A, D, E, and F). The PM PIN2-GFP signal was decreased to 86.7% of the control value after CHX treatment (Fig. 6, B, G, and H), and cotreatment of CHX and BFA reduced the PIN2-GFP signal in the PM to 66.9% (Fig. 6, B, G, and I). In contrast, cotreatment of CHX and BFA reduced the PIN1-GFP signal in the PM to 42.8% of the control level (Fig. 6, C, J, and L), whereas CHX alone slightly decreased the PIN1-GFP signal to 92.9% (Fig. 6, C, J, and K). The differential sensitivities to CHX and/or BFA of ABCB4, PIN2, and PIN1 indicate that their traffickings are differently regulated.
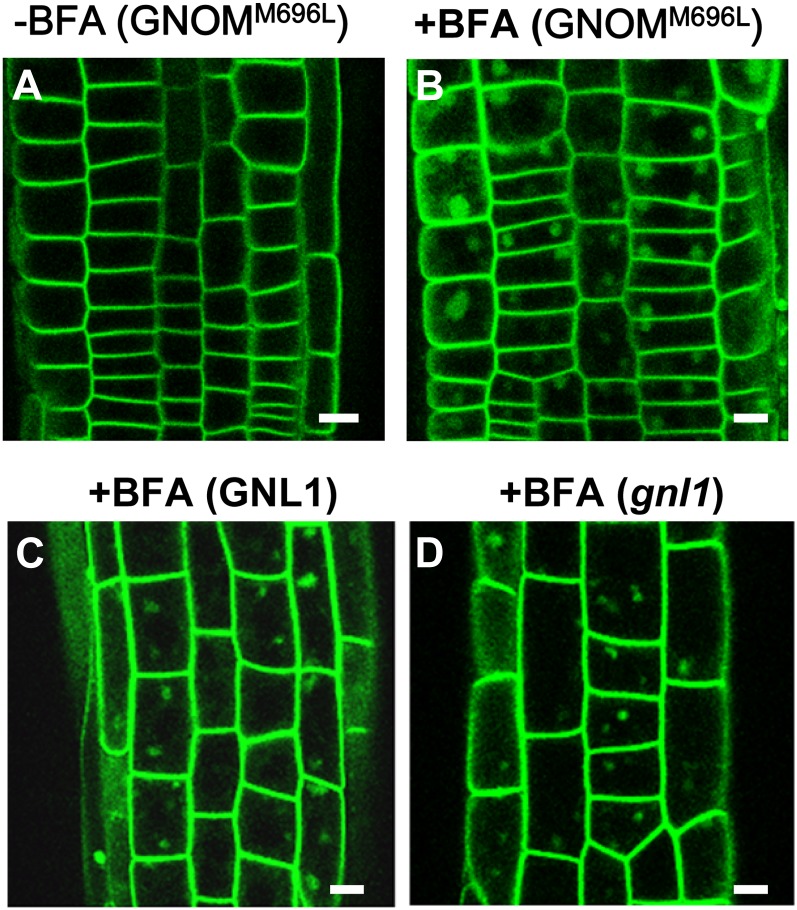
Because ARF-GEFs such as GNOM and GNL1 have been implicated in PIN trafficking, their potential roles in ABCB4 trafficking were investigated using the same BFA concentration as was used for individual experiments. ProABCB4:ABCB4-GFP was introduced into the GNOMM696L-myc line, which is resistant to BFA (Geldner et al., 2003). Similar to observations in the wild-type background, application of 50 μm BFA for 1 h caused the formation of ABCB4-containing BFA compartments in the BFA-resistant GNOMM696L-myc line (Fig. 7, A and B). In the GNOMM696L-myc line, PIN1 did not aggregate in response to 50 μm BFA because BFA sensitivity of PIN1 trafficking is regulated only by GNOM (Geldner et al., 2003). Thus, these data suggest that other BFA-sensitive ARF-GEFs are required for the BFA sensitivity of ABCB4 trafficking, although the involvement of GNOM could not be completely ruled out. Endocytosis of PIN2 is more affected by GNL1, a BFA-insensitive ARF-GEF, than by PIN1, and PIN2 is not properly internalized to the BFA compartments in the gnl1 mutant (Teh and Moore, 2007). If GNL1 mediates the endocytosis of ABCB4, BFA would not be expected to cause ABCB4 internalization. The response of ABCB4 to 25 μm BFA for 1 h did not differ significantly in the GNL1 and gnl1-2 backgrounds (Fig. 7, C and D). These data indicate that, unlike the PIN proteins, ABCB4 trafficking operates independently of GNL1. It will be the next challenge to identify and characterize the responsible ARF-GEFs for ABCB4 trafficking.
Figure 7.
BFA sensitivity of ABCB4 requires other ARF-GEFs besides GNOM, but endocytosis is independent from GNL1. A and B, Fluorescence images of ABCB4-GFP in the BFA-resistant GNOM background (GNOMM696L) after treatments without BFA (A) or with BFA (50 μm, 1 h; B). C and D, Fluorescence images of ABCB4-GFP after treatment with BFA (25 μm, 1 h) in GNL1(C) or in gnl1 (D). Bars + 10 μm. [See online article for color version of this figure.]
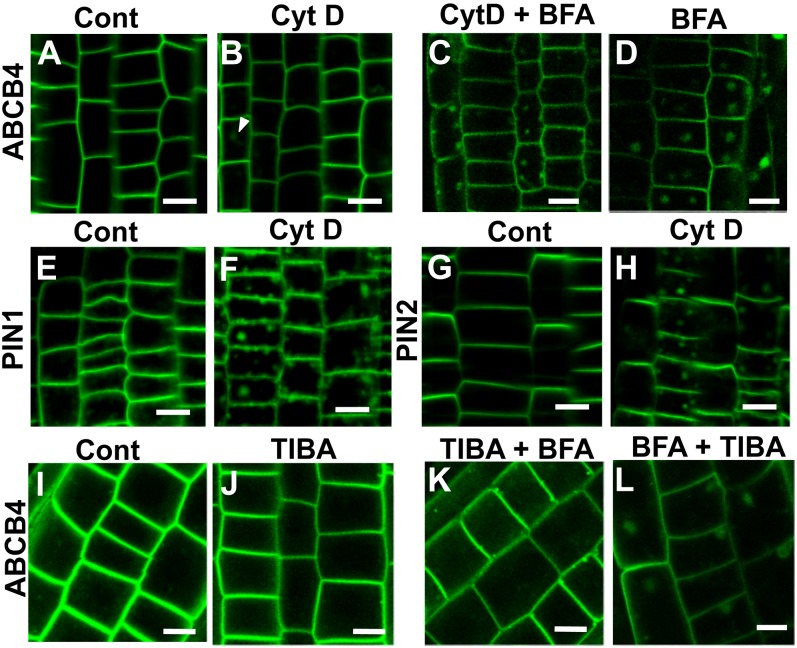
Cytochalasin D Treatment Shows That ABCB4 Trafficking Is Actin Dependent But Slower and Less Active Than PIN
To determine whether the cytoskeleton plays an important role in ABCB4 trafficking, we examined the effects of actin and microtubule inhibitors on the localization and the intracellular trafficking of ABCB4. Cytochalasin D (CytD) is an actin-depolymerizing drug that affects the polar localization and trafficking of PIN1 (Geldner et al., 2001). ABCB4 signal barely responded to 30 μm CytD for 2 h (Fig. 8, A and B), whereas PIN1 and PIN2 signals showed intracellular aggregation under the same conditions (Fig. 8, E–H). However, an extended treatment of up to 3 h induced the intracellular aggregation of ABCB4 (Supplemental Fig. S3). In addition, the effect of CytD on ABCB4 trafficking was observed when CytD was cotreated with BFA; the formation of ABCB4-containing BFA compartments was considerably reduced in the presence of CytD (Fig. 8, C and D).
Figure 8.
ABCB4 trafficking is less sensitive to actin inhibitors. A to D, Fluorescence images of ABCB4-GFP. The transgenic seedlings were treated with 30 μm CytD for 2 h (B), pretreated with CytD for 30 min and then cotreated with 50 μm BFA for 45 min (C), or treated solely with BFA for 45 min (D). Controls (Cont; A) were treated with DMSO. E and F, Fluorescence images of PIN1-GFP treated with 30 μm CytD for 2 h (F) and the same volume of DMSO (Cont; E). G and H, Fluorescence images of PIN2-GFP treated with 30 μm CytD for 2 h (H) and the same volume of DMSO (Cont; G). I to L, ProABCB4:ABCB4-GFP transgenic seedlings were treated with 50 μm TIBA for 2 h (J), pretreated with TIBA for 30 min and then cotreated with BFA for 90 min (K), or treated with BFA for 2 h and then washed with TIBA for 90 min (L). Controls (Cont; I) were treated with DMSO. Bars + 10 μm. [See online article for color version of this figure.]
2,3,5-Triiodobenzoic acid (TIBA), a weak auxin as well as an auxin transport inhibitor, also inhibits endocytosis and vesicle motility by interfering with actin stabilization (Dhonukshe et al., 2008a), although ABCB4 localization seemed to be unaffected by prolonged treatment (2 h) with TIBA alone (Fig. 8, I and J). However, pretreatment with TIBA (50 μm for 30 min) followed by cotreatment with BFA inhibited the formation of ABCB4-containing BFA compartments in the root epidermis (Fig. 8K). Similarly, the BFA compartments of ABCB4 were unable to disappear after washing out with TIBA (Fig. 8L).
In contrast to actin, microtubules do not play a key role in ABCB4 trafficking. The microtubule-disrupting agent oryzalin (20 μm for 3 h) did not significantly affect ABCB4 trafficking. Only higher concentrations of oryzalin (40 μm for 3 h) could interfere with the ABCB4 signal in the PM, presumably by causing morphological changes to the cell (Supplemental Fig. S4).
These data demonstrate that actin is involved in the endocytosis of ABCB4 as well as in ABCB4’s targeting to the PM, but the reaction time and degree of intracellular aggregation of ABCB4 after treatment with CytD are different from those of PIN1 and PIN2.
DISCUSSION
Although the intracellular trafficking of PIN proteins has been actively studied, the trafficking of ABCB-type auxin efflux proteins remains poorly understood. This study revealed both similarities and differences in the trafficking of ABCB4 and PINs. PIN proteins show dynamic intracellular trafficking and polar localization in the PM. By contrast, ABCB4 localizes in a nonpolar manner and is stably retained in the PM. The primary intracellular trafficking of ABCB4, which is the degradation pathway to the vacuole, is slow. This endocytic pathway of ABCB4 is routed through SNX1-positive endosomes like PIN2 (Kleine-Vehn et al., 2008b). However, the subcellular localization of SNX1 still remains controversial. Two groups reported that SNX1 localized to the PVC (Jaillais et al., 2008; Kleine-Vehn et al., 2008b), whereas another group showed partial colocalization of SNX1 with both PVC and TGN (Phan et al., 2008). Moreover, the specific localization of SNX1 to TGN has been reported recently (Niemes et al., 2010). Our result showed that SNX1 likely localizes to TGN, because the core BFA compartment of ABCB4, which is derived by TGN, is SNX1 positive (Robinson et al., 2008). However, FRAP analysis and the results using Kaede-ABCB4 suggest that ABCB4 is stably localized in the PM and that only undetectable levels of ABCB4 might recycle between the PM and endosomes. So, trafficking of ABCB4 to the vacuole is via SNX1-positive endosomes, and ABCB4-containing BFA compartments might be generated by the BFA blocking the degradation pathway. Like PIN2, the BFA sensitivity of ABCB4 is dependent on GNOM and also other ARF-GEFs, whereas the BFA sensitivity of PIN1 trafficking predominantly depends on GNOM (Geldner et al., 2003; Kleine-Vehn et al., 2008b). On the other hand, the endocytosis of ABCB4 was not affected by GNL1, a protein important for the endocytosis of PIN2 (Teh and Moore, 2007). In addition, the endocytic process of ABCB4 is a slow process compared with PIN2. This likely resulted in the slow response of ABCB4 trafficking to CytD and wortmannin.
Although some features of ABCB4 trafficking are characterized in this study, two new questions are raised. First, if ABCB4 trafficking is very slow, how does ABCB4 quickly respond to BFA to make aggregates? We traced the ABCB4 signals right after BFA treatment. Interestingly, BFA initially increased the number of intracellular vesicles from the PM (Supplemental Fig. S5, A and B; Supplemental Movie S1). The BFA action was very fast. The internalizing effect of BFA on ABCB4 vesicles was already observed within 2 min after BFA treatment and lasted up to 20 min, after which they aggregated with each other to form BFA compartments (Supplemental Fig. S5B). These BFA-induced internal ABCB4 vesicles localized to the endosomes, which were FM4-64 positive (Supplemental Fig. S5, C and D). Even considering the life time of ABCB4 in the PM (Fig. 5O), the BFA-mediated blocking of ABCB4 trafficking to the degradation pathway alone hardly can explain this prompt ABCB4 response to BFA. We speculate that BFA initially promotes the endocytosis of ABCB4 and then blocks the endocytosis of ABCB4 to the vacuole, resulting in endosomal aggregates. The increased endocytosis of PM proteins or tracers into endosomes by BFA treatment has been demonstrated with BRI1 (the brassinosteroid receptor) in the Arabidopsis root, FM1-43 in tobacco (Nicotiana tabacum) BY-2 cells, and FM4-64 in the pollen tube of Picea meyeri (Emans et al., 2002; Wang et al., 2005; Geldner et al., 2007).
Second, what is the physiological relevance of auxin transporters such as ABCBs that are stably associated with the PM? The polarity of PINs is generated and maintained by constant polar recycling of nonpolar PM-localized PINs and can be altered in response to internal and external stimuli (Dhonukshe et al., 2008b; Kleine-Vehn and Friml, 2008; Petrásek and Friml, 2009). These dynamic changes in subcellular PIN localization linked with the dynamic intracellular trafficking of PIN proteins contribute to the modulation of local auxin gradients and thus to the alteration of developmental programs in response to internal and environmental cues (Petrásek and Friml, 2009; Zazímalová et al., 2010). On the contrary, AT-ABCBs are retained in the PM in a nonpolar and persistent manner (Blakeslee et al., 2007; Titapiwatanakun et al., 2009; this study). ABCB4, being nonpolar, may not need similar mechanisms for polarization and hence may not need rapid endocytosis and recycling, which may be suitable for functioning as basal auxin efflux transporters to maintain homeostatic cellular auxin levels. For instance, whereas PIN2 is expressed from the root tip to the elongation zone of root epidermal cells, where it is involved in the basipetal auxin stream and root gravitropism, ABCB4 is expressed in the entire root epidermis (Blilou et al., 2005; Cho et al., 2007). ABCB4 might be implicated in supplying auxin throughout the epidermal cells so that the cells maintain a certain auxin concentration necessary for their growth and other auxin-mediated fundamental events. Stable association of ABCB proteins with the PM might also provide a platform for PIN proteins to reside in the PM. Regardless of relocation, the proximity between PINs and AT-ABCBs may enable effective physical interactions between them. The contribution by ABCB19 to PIN1 function is associated with their colocalization in the membrane microdomains (Titapiwatanakun et al., 2009). ABCB4 was also detectable in the detergent-resistant membrane (Borner et al., 2005). However, whether ABCB4 actually stabilizes PIN2 in the detergent-resistant membrane fraction has yet to be demonstrated.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) Columbia ecotype was used as the wild type. The following Arabidopsis mutants and transgenic lines were also employed: abcb4/pgp4 (Cho et al., 2007), gnl1 (Teh and Moore, 2007), ProABCB4:ABCB4-GFP (Cho et al., 2007), Pro-35S:ABCB4-YFP (Cho et al., 2007), ProPIN2:PIN1-GFP (Lee et al., 2010), ProPIN2:PIN2-GFP (Xu and Scheres, 2005), Pro-35S:mRFP-ARA7/RabF2b (Haas et al., 2007), Pro-35S:YFP-RHA1/RabF2a (Haas et al., 2007), ST-DsRed (Saint-Jore et al., 2002), SNX-mRFP (Jaillais et al., 2006), and GNOMM696L (Geldner et al., 2003). Seedlings were grown on plates containing one-half-strength (1/2) Murashige and Skoog (MS) nutrient mix (Duchefa Biochemie), 1% Suc, 0.5 g L−1 MES (pH 5.7) with KOH, and 0.8% agarose (SeaKem; Cambrex BioScience Rockland). Seeds were cold treated for 3 d at 4°C and germinated at 23°C under a 16-h-light/8-h-dark photoperiod.
Transgene Constructs
The ProABCB4:Kaede-ABCB4 construct was generated by sequential cloning. The first ABCB4 genomic fragment, containing the region from =1 to =1,583 (relative to the transcription initiation site), was obtained by PCR using the primers 5′-TGAACCCGGGATGGCTTCAGAGAGCGGC-3′ and 5′-TCTTTGAGCTCAATATCTCCCTTAATGTC-3′. This fragment was used to replace GFP in the pGPTV-GFP vector (Cho and Cosgrove, 2002). The second ABCB4 fragment, containing the region from =1,584 to =4,743, was generated by PCR using the primers 5′-AGGGAGATATTGAGCTCAAAGATGTTTAC-3′ and 5′-ATAAGAGCTCTCAAGAAGCCGCGGTTAG-3′. This fragment was inserted into the SacI site of the vector carrying the first ABCB4 fragment. The Kaede-coding region was obtained by PCR amplification of the pKaede-N1 vector using the primers 5′-ACCGGTCGACACCATGGTGAGTCTGATTA-3′ and 5′-CGGCCCGGGCTTGACGTTGTCCGGCAAT-3′. This region was inserted upstream of the first ABCB4 fragment. The ABCB4 promoter region (ProABCB4), from −12 to −2,174, was obtained by PCR using the primers 5′-ACATGTCGACTAAAGGATTTGGGTCTA-3′ and 5′-AGAGGTCGACAGATACCTCACGATCC-3′. The promoter region was inserted upstream of the Kaede region in the ProABCB4:Kaede-ABCB4 construct.
The constructs confirmed by DNA sequencing were transformed into Arabidopsis (ecotype Columbia) via Agrobacterium tumefaciens (strain C58C1)-mediated infiltration (Bechtold and Pelletier, 1998). The insertion of transgenes was confirmed by PCR analysis of genomic DNA from transformants. Homozygous plants exhibiting a 3:1 segregation ratio on antibiotic plates were selected for further analyses.
Observation of Reporter Markers and Chemical Treatments
Subcellular localization and expression of transporter-reporter fusion proteins was observed in 3- or 4-d-old seedlings. For chemical treatments, 4-d-old seedlings were transferred into 1/2 MS liquid medium containing different chemicals and incubated for the time periods indicated. The control liquid medium and treatments included the same amounts of solvent. FM4-64, BFA, TIBA, CytD, naphthylacetic acid, wortmannin, and ES1 were dissolved in 100% DMSO. Oryzalin and cycloheximde were dissolved with 100% ethanol and water, respectively. Fluorescence from reporter proteins (GFP, yellow fluorescent protein [YFP], and red fluorescent protein [RFP]) was observed using a confocal laser scanning microscope (LSM 510; Carl Zeiss). GFP, YFP, and RFP signals were detected using 488/505- to 530-nm, 514/greater than 53-nm, and 543/greater than 560-nm excitation/emission filter sets, respectively. To isolate GFP and YFP for double transgenic lines of ProABCB4:ABCB4-GFP and Pro-35S:YFP-RHA1/RabF2a, a 488-nm laser line was employed, images were taken in 11-nm bandwidth from 511 to 618 nm, and then GFP and YFP images were separated through linear unmixing. Fluorescence images were digitalized with LSM Image Browser. Overlapping between ABCB4-FP (or PIN-FP) with the fluorescent molecular markers was qualitatively analyzed where overlapping of the intracellular particles was mostly partial but obvious. The relationships of the various trafficking components and chemicals and the molecular markers are illustrated in Supplemental Figure S6.
LSM 510 quantification software was used to analyze green fluorescence intensity at the apical and/or basal sides at the PM in the epidermis cells of ProABCB4:ABCB4-GFP, ProPIN2:PIN2-GFP, and ProPIN2:PIN1-GFP transgenic lines (Fig. 6). Background signals were subtracted.
Photoconversion of Kaede Protein
For green-to-red photoconversion of ROI as in Figure 3, one side of the PM was marked using a rectangle and illuminated with the 364-nm laser at 12.3% excitation power for 1 min. After the photoconversion, green and red signals were detected using the 488/505- to 530-nm and 543/greater than 560-nm excitation/emission filter sets (LSM 510; Carl Zeiss).
Images of green-Kaede:ABCB4 and red-Kaede:ABCB4 were taken using fluorescence microscopy (Olympus BX60) for the degradation rate analysis in the PM (Fig. 5, M–O). For the green-to-red photoconversion, the root tip region of ProABCB4:green-Kaede:ABCB4 transformants was subjected to 1 min of UV illumination under a 20× objective lens. Images of green- and red-Kaede ABCB4 were taken after 0, 3, 5, 7, and 9 h of incubation in 1/2 MS liquid medium from a different set of seedlings to rule out photobleaching damage. The green and red signals were detected by the 480/510- to 520-nm and 520- to 550/greater than 580-nm filters, respectively. The mean values of fluorescence intensity in the PM were measured using ImageJ.
FRAP Analysis
The transgenic lines ProABCB4:ABCB4-GFP and ProPIN2:PIN2-GFP were used for analyses. Seedlings were mounted on a glass slide containing 1.5% agarose and 1/2 MS nutrient mix. For fixing, roots were covered with a coverslip and the edges of the coverslip were attached to the slide with tape. FRAP analysis was performed with an LSM 510 confocal laser scanning microscope equipped with a water-corrected 40× objective, numerical aperture + 1.2 (Carl Zeiss). For bleaching, 2- or 4-µm-wide ROIs were selected and then bleached with 100 iterations of a 488-nm argon laser at full power. GFP recoveries were detected by 488-nm excitation and greater than 505-nm emission filters and measured every 20 s for 20 min after photobleaching and then every 1 min for the next 40 min. After photobleaching, the intensity of green fluorescence was determined using the LSM 510 quantification software. To correct for the variation in expression levels of ABCB4-GFP and PIN2-GFP, values were normalized with the following equation (Goodwin and Kenworthy, 2005):
where F(t)ROI + fluorescence of ROI for each time point (t), Fbkgd + background fluorescence, F(t)ngb + fluorescence of a comparable neighbor PM for each time point, Fi_ROI + initial fluorescence of ROI, and Fi_ngb + initial fluorescence of a comparable neighbor PM.
The maximum recoverable fluorescence intensity (F∞) and t1/2 required to reach the recoverable fluorescence for a Gaussian spot were calculated using nonlinear regression analysis and fitted to the following equation with SPSS (Statistical Package for the Social Sciences; IBM) software (r2 > 95%):
where F0 + initial fluorescence and t + time. The value derived from 2 µm was used for the F∞ value of the 4-µm width. Statistical analyses were performed using Student’s t test.
The Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: GNL1 (At5g39500), GNOM (At1g13980), ABCB4/PGP4 (At2g47000), PIN1 (At1g73590), PIN2 (At5g57090), SMT1 (At5g13710), SNX1 (At5g06140), RHA1/RabF2a (At5g45130), ARA7/RabF2b (At4g19640), and ST (At2g03760).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. ES1 does not alter ABCB4 trafficking.
Supplemental Figure S2. ProABCB4:Kaede-ABCB4 transgenic plants partially complements the abcb4 root-hair phenotype.
Supplemental Figure S3. ABCB4 trafficking is less affected by CytD.
Supplemental Figure S4. ABCB4 trafficking does not depend on microtubules.
Supplemental Figure S5. BFA increases the endosomal abundance of ABCB4.
Supplemental Figure S6. Endomembrane trafficking pathways with the trafficking components and their inhibitors.
Supplemental Table S1. Summary of FRAP analysis.
Supplemental Movie S1. Endocytic trafficking of ABCB4 after BFA treatment.
Supplementary Material
Acknowledgments
We thank Jiří Friml, Thierry Gaude, Chris Hawes, Glenn Hicks, Gerd Jürgens, Ian Moore, Marisa S. Otegui, and Ben Scheres for sharing published plant materials and chemicals. We also thank Edgar P. Spalding for his kind cooperation in revising the manuscript in his laboratory and Anindya Ganguly for his help in the preparation of the manuscript.
Glossary
- PM
plasma membrane
- BFA
brefeldin A
- PVC
prevacuolar compartment
- TGN
trans-Golgi network
- ST
sialyl transferase
- ROI
region of interest
- FRAP
fluorescence recovery after photobleaching
- t1/2
time required to attain 50% recovery
- DMSO
dimethyl sulfoxide
- CytD
cytochalasin D
- TIBA
2,3,5-triiodobenzoic acid
- 1/2
half-strength
- MS
Murashige and Skoog
- RFP
red fluorescent protein
- YFP
yellow fluorescent protein
- CHX
cycloheximide
References
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. (2002) An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci USA 99: 12651–12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G. (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. In JM Martinez-Zapater, J Salinas, eds, Arabidopsis Protocols. Humana, Totowa, NJ, pp 259–266. [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J, et al. (2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Borner GH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, Macaskill A, Napier JA, Beale MH, Lilley KS, Dupree P. (2005) Analysis of detergent-resistant membranes in Arabidopsis: evidence for plasma membrane lipid rafts. Plant Physiol 137: 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ. (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 14: 3237–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, Lee SH, Cho HT. (2007) P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell 19: 3930–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, Paciorek T, Petrásek J, Seifertová D, Tejos R, et al. (2008a) Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA 105: 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Tanaka H, Goh T, Ebine K, Mähönen AP, Prasad K, Blilou I, Geldner N, Xu J, Uemura T, et al. (2008b) Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456: 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Emans N, Zimmermann S, Fischer R. (2002) Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, et al. (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44: 179–194 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G. (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. (2007) Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev 21: 1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Kenworthy AK. (2005) Photobleaching approaches to investigate diffusional mobility and trafficking of Ras in living cells. Methods 37: 154–164 [DOI] [PubMed] [Google Scholar]
- Haas TJ, Sliwinski MK, Martínez DE, Preuss M, Ebine K, Ueda T, Nielsen E, Odorizzi G, Otegui MS. (2007) The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. Plant Cell 19: 1295–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JF, Taylor K. (1991) Mechanisms of plasma membrane protein degradation: recycling proteins are degraded more rapidly than those confined to the cell surface. Proc Natl Acad Sci USA 88: 5902–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miège C, Gaude T. (2008) Evidence for a sorting endosome in Arabidopsis root cells. Plant J 53: 237–247 [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miège C, Rollin C, Gaude T. (2006) AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443: 106–109 [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miège C, Gaude T. (2007) The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer PB, Wiśniewska J, Paciorek T, Benková E, Friml J. (2008a) ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr Biol 18: 526–531 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J. (2006) Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18: 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Friml J. (2008) Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol 24: 447–473 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, Friml J. (2008b) Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci USA 105: 17812–17817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Sohn EJ, Lee MH, Hwang I. (2004) The Arabidopsis rab5 homologs rha1 and ara7 localize to the prevacuolar compartment. Plant Cell Physiol 45: 1211–1220 [DOI] [PubMed] [Google Scholar]
- Lee OR, Kim SJ, Kim HJ, Hong JK, Ryu SB, Lee SH, Ganguly A, Cho HT. (2010) Phospholipase A(2) is required for PIN-FORMED protein trafficking to the plasma membrane in the Arabidopsis root. Plant Cell 22: 1812–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Miller ND, Splitt BL, Wu G, Spalding EP. (2007) Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-like ABC transporter genes. Plant Cell 19: 1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K. (1995) Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol 130: 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J, Kubes M, Bielach A, Gaykova V, Petrásek J, Skůpa P, Chand S, Benková E, Zazímalová E, Friml J. (2008) Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135: 3345–3354 [DOI] [PubMed] [Google Scholar]
- Multani DS, Briggs SP, Chamberlin MA, Blakeslee JJ, Murphy AS, Johal GS. (2003) Loss of an MDR transporter in compact stalks of maize br2 and sorghum dw3 mutants. Science 302: 81–84 [DOI] [PubMed] [Google Scholar]
- Niemes S, Langhans M, Viotti C, Scheuring D, San Wan Yan M, Jiang L, Hillmer S, Robinson DG, Pimpl P. (2010) Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J 61: 107–121 [DOI] [PubMed] [Google Scholar]
- Niv H, Gutman O, Kloog Y, Henis YI. (2002) Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J Cell Biol 157: 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J, Friml J. (2009) Auxin transport routes in plant development. Development 136: 2675–2688 [DOI] [PubMed] [Google Scholar]
- Phan NQ, Kim SJ, Bassham DC. (2008) Overexpression of Arabidopsis sorting nexin AtSNX2b inhibits endocytic trafficking to the vacuole. Mol Plant 1: 961–976 [DOI] [PubMed] [Google Scholar]
- Robert S, Chary SN, Drakakaki G, Li S, Yang Z, Raikhel NV, Hicks GR. (2008) Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc Natl Acad Sci USA 105: 8464–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Langhans M, Saint-Jore-Dupas C, Hawes C. (2008) BFA effects are tissue and not just plant specific. Trends Plant Sci 13: 405–408 [DOI] [PubMed] [Google Scholar]
- Rotblat B, Prior IA, Muncke C, Parton RG, Kloog Y, Henis YI, Hancock JF. (2004) Three separable domains regulate GTP-dependent association of H-ras with the plasma membrane. Mol Cell Biol 24: 6799–6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jore CM, Evins J, Batoko H, Brandizzi F, Moore I, Hawes C. (2002) Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. Plant J 29: 661–678 [DOI] [PubMed] [Google Scholar]
- Santelia D, Vincenzetti V, Azzarello E, Bovet L, Fukao Y, Düchtig P, Mancuso S, Martinoia E, Geisler M. (2005) MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett 579: 5399–5406 [DOI] [PubMed] [Google Scholar]
- Sprague BL, McNally JG. (2005) FRAP analysis of binding: proper and fitting. Trends Cell Biol 15: 84–91 [DOI] [PubMed] [Google Scholar]
- Tamura K, Shimada T, Ono E, Tanaka Y, Nagatani A, Higashi SI, Watanabe M, Nishimura M, Hara-Nishimura I. (2003) Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J 35: 545–555 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kitakura S, De Rycke R, De Groodt R, Friml J. (2009) Fluorescence imaging-based screen identifies ARF GEF component of early endosomal trafficking. Curr Biol 19: 391–397 [DOI] [PubMed] [Google Scholar]
- Teh OK, Moore I. (2007) An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature 448: 493–496 [DOI] [PubMed] [Google Scholar]
- Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, Lee OR, Richards EL, Murphy AS, Sato F, et al (2005) PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17: 2922–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titapiwatanakun B, Blakeslee JJ, Bandyopadhyay A, Yang H, Mravec J, Sauer M, Cheng Y, Adamec J, Nagashima A, Geisler M, et al. (2009) ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J 57: 27–44 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J. (2009) Auxin: a trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Wang Q, Kong L, Hao H, Wang X, Lin J, Samaj J, Baluska F. (2005) Effects of brefeldin A on pollen germination and tube growth: antagonistic effects on endocytosis and secretion. Plant Physiol 139: 1692–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lewis DR, Spalding EP. (2007) Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. Plant Cell 19: 1826–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Otegui MS, Spalding EP. (2010) The ER-localized TWD1 immunophilin is necessary for localization of multidrug resistance-like proteins required for polar auxin transport in Arabidopsis roots. Plant Cell 22: 3295–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Scheres B. (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazímalová E, Murphy AS, Yang H, Hoyerová K, Hosek P. (2010) Auxin transporters: why so many? Cold Spring Harb Perspect Biol 2: a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.