Abstract
Immunolabeling, combined with chemical analyses and transcript profiling, have provided a comprehensive temporal and spatial picture of the deposition and modification of cell wall polysaccharides during barley (Hordeum vulgare) grain development, from endosperm cellularization at 3 d after pollination (DAP) through differentiation to the mature grain at 38 DAP. (1→3)-β-d-Glucan appears transiently during cellularization but reappears in patches in the subaleurone cell walls around 20 DAP. (1→3, 1→4)-β-Glucan, the most abundant polysaccharide of the mature barley grain, accumulates throughout development. Arabino-(1-4)-β-d-xylan is deposited significantly earlier than we previously reported. This was attributable to the initial deposition of the polysaccharide in a highly substituted form that was not recognized by antibodies commonly used to detect arabino-(1-4)-β-d-xylans in sections of plant material. The epitopes needed for antibody recognition were exposed by pretreatment of sections with α-l-arabinofuranosidase; this procedure showed that arabino-(1-4)-β-d-xylans were deposited as early as 5 DAP and highlighted their changing structures during endosperm development. By 28 DAP labeling of hetero-(1→4)-β-d-mannan is observed in the walls of the starchy endosperm but not in the aleurone walls. Although absent in mature endosperm cell walls we now show that xyloglucan is present transiently from 3 until about 6 DAP and disappears by 8 DAP. Quantitative reverse transcription-polymerase chain reaction of transcripts for GLUCAN SYNTHASE-LIKE, Cellulose Synthase, and CELLULOSE SYNTHASE-LIKE genes were consistent with the patterns of polysaccharide deposition. Transcript profiling of some members from the Carbohydrate-Active Enzymes database glycosyl transferase families GT61, GT47, and GT43, previously implicated in arabino-(1-4)-β-d-xylan biosynthesis, confirms their presence during grain development.
In cereal species the endosperm, the nutritive part of the grain, begins development as a syncytium, where the triploid endosperm nucleus undergoes numerous rounds of division without cytokinesis (Olsen, 2004). Thus, the syncytium consists of a highly vacuolated central cell with nuclei positioned around its perimeter. Approximately 3 d after pollination (DAP) and in the absence of mitosis and phragmoplasts, anticlinal cell walls grow out centripetally from the periphery of the central cell and form partitions between individual nuclei. Each nucleus then undergoes mitotic division, followed immediately by cytokinesis and the laying down of a cell plate and periclinal cell wall. This alternating cycle of free-growing anticlinal cell walls followed by mitosis and the laying down of a periclinal cell wall continues in a centripetal fashion until the entire endosperm is compartmentalized into cells (Brown et al., 1994, 1997). This sequence of events makes grass (cereal) endosperm ideal for studying mechanisms of cell wall growth and development. Cereals are also the world’s major source of nutrition with much of their caloric content deposited as complex carbohydrates in developing and maturing endosperm cells. Given its unique biology and economic importance, it is not surprising that the cereal endosperm has attracted much attention from scientists with both pure and applied research interests.
The polysaccharide composition of the starchy endosperm cell walls in barley (Hordeum vulgare) has been known for some time and chemical analyses have shown mature grain to contain approximately 70% (w/w) (1→3, 1→4)-β-d-glucan, 20% (w/w) arabino-(1-4)-β-d-xylan, 3% to 4% (w/w) (1→4)-β-d-glucan (cellulose), 3% to 4% (w/w) hetero-(1→4)-β-d-mannan, and some protein (Fincher, 1975). In contrast, walls of the aleurone consist of approximately 70% (w/w) arabino-(1-4)-β-d-xylan, 25% (w/w) (1→3, 1→4)-β-d-glucan, and minor amounts of (1→4)-β-d-glucan (cellulose) and hetero-(1→4)-β-d-mannan (Bacic and Stone, 1981a, 1981b). In a recent study we used immuno-light and -electron microscopy (EM) to describe the temporal and spatial deposition of key wall polysaccharides during the cellularization phase of barley grain development, 3 to 8 DAP (Wilson et al., 2006). (1→3)-β-d-Glucan (callose) is the first polysaccharide to be laid down in the cellularizing grain, at approximately 3 DAP. However, its presence is only transient and by 6 DAP it can only be found in those cell wall regions surrounding plasmodesmata. (1→3, 1→4)-β-d-Glucan appears next between 4 and 5 DAP and continues to accumulate in the grain throughout development. The hetero-(1→4)-β-d-mannan antibody detects hetero-mannans at 6 DAP whereas arabino-(1-4)-β-d-xylan was first detected at 8 DAP. During cellularization each polysaccharide is uniformly deposited in the endosperm cell walls except for arabino-(1-4)-β-d-xylan where labeling is restricted to those cell walls immediately adjacent to the grain crease (Wilson et al., 2006). Similar studies have been performed on developing rice (Oryza sativa; Brown et al., 1997) and wheat (Triticum aestivum) grains (Philippe et al., 2006).
Polysaccharides [in particular (1→3, 1→4)-β-d-glucan and arabino-(1→4)-β-d-xylan] of the cell walls in cereal grains are not digested by enzymes secreted into the small intestine of monogastric animals and are hence components of dietary fiber in human nutrition and therefore have positive effects on human health by reducing both the risk of cardiovascular disease by lowering plasma cholesterol levels (Topping, 2007) and on non-insulin-dependent diabetes by lowering postprandial blood glucose and insulin levels (Wood, 2007). Furthermore, recent pig feeding trials indicate that consumption of arabino-(1→4)-β-d-xylan attenuates the somewhat damaging effect of the western diet on gut health (Belobrajdic et al., 2011). Conversely, (1→3, 1→4)-β-d-glucan also reduces digestibility and hence available metabolizable energy in monogastric animals such as pigs and poultry and lowers extraction potential and reduces filterability during malting and brewing (Brennan and Cleary, 2005).
Considerable effort has been made toward identifying the genes and biochemical mechanisms that contribute to the biosynthesis of cell wall polysaccharides although progress in this area has been slow (Fincher, 2009; Doblin et al., 2010). Only relatively recently have specific genes and gene families been implicated in (1→3, 1→4)-β-d-glucan synthesis. After introducing the rice Cellulose Synthase-Like F (CslF) genes into Arabidopsis (Arabidopsis thaliana), a species known not to ordinarily contain (1→3, 1→4)-β-d-glucan in its walls, (1→3, 1→4)-β-d-glucan was detected in transgenic lines using immuno-EM (Burton et al., 2006). Similar gain-of-function experiments were performed by transforming Arabidopsis with the barley CslH gene. (1→3, 1→4)-β-d-Glucan was immunologically detected in the walls of transgenic plants and confirmed with biochemical analysis of wall extracts (Doblin et al., 2009). The genes encoding the xylan synthases and key side chain glycosyl transferases are largely unconfirmed biochemically but studies of mutant lines and transcript profiles of cereal species accumulating arabino-(1-4)-β-d-xylan implicate the GT43, GT47, and GT61 gene families (Mitchell et al., 2007; Scheller and Ulvskov, 2010). Experimental evidence confirming the role of these genes, particularly the xylan synthases, is an area of intense interest given the importance of plant materials as feedstocks for biofuels and the potential human health benefits from diets inclusive of arabino-(1-4)-β-d-xylan.
Some gene families have also been implicated in the synthesis of the other, less-abundant, polysaccharides of the developing barley grain. For example, there is ample evidence associating the GLUCAN SYNTHASE-LIKE (GSL) gene family with callose [(1→3)-β-d-glucan] biosynthesis (Brownfield et al., 2009). Heterologous expression experiments coupled with gene expression profiles have implicated the CslC gene family in the synthesis of the glucan backbone of xyloglucan (Cocuron et al., 2007) and cellulose (Dwivany et al., 2009) whereas members of the CslA gene family have been shown to have mannan or (gluco)mannan synthase activity (Dhugga et al., 2004).
In this study, we focus on the second phase, the differentiation phase, of barley endosperm development and apply antibodies to key wall polysaccharides from 10 to 28 DAP to describe their distribution, using both light and EM. We also have quantified the levels of (1→3, 1→4)-β-d-β-glucan and the monosaccharides arabinose, Xyl, and Man from cellularization (3 DAP) through to the mature grain (28 DAP). In addition, RNA has been isolated from developing grains between 6 and 38 DAP and quantitative real-time reverse transcription-PCR (QPCR) analysis performed in an attempt to determine whether transcript patterns of cell wall synthesis genes can be correlated with polysaccharide deposition and accumulation in the grain.
RESULTS
Endosperm Differentiation in Barley from 10 to 28 DAP
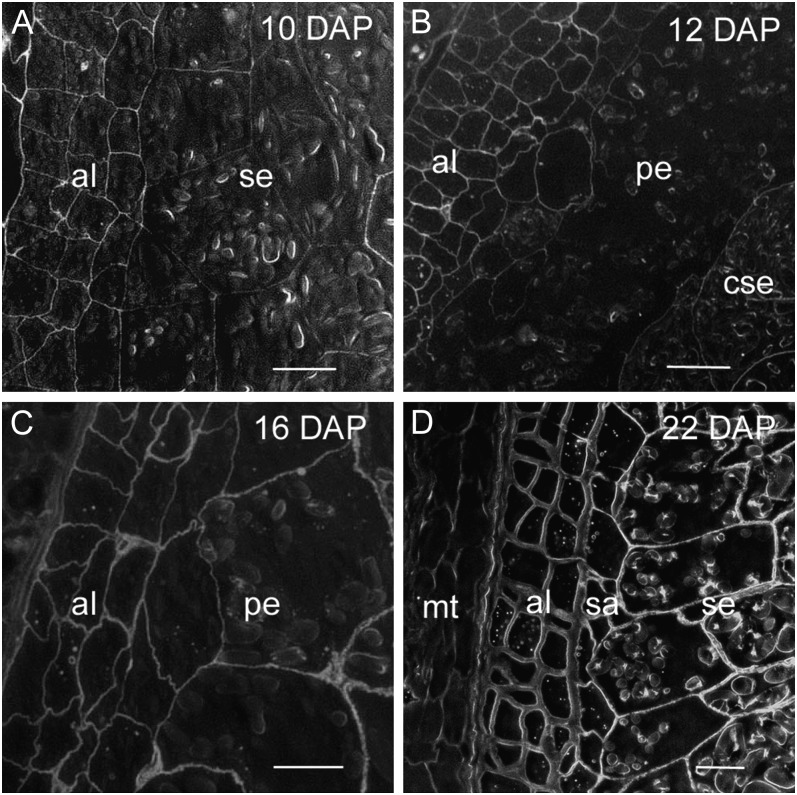
During the differentiation phase, a number of changes to the endosperm were observed using light microscopy and toluidine blue staining of sectioned grain. The beginning of the differentiation phase in barley endosperm is marked by the appearance of three to four distinct layers of aleurone cells surrounding the starchy endosperm. At 10 DAP aleurone cells are easily distinguished from the rest of the endosperm by their small size, isodiametric shape, regular, brick-like arrangement, and by their complete lack of starch granules (Fig. 1A). By 14 DAP a histologically distinct subaleurone layer separates the differentiating aleurone from the starchy endosperm. Subaleurone cells are larger than those of the aleurone but smaller than the starchy endosperm and contain small starch granules and protein bodies (Fig. 1B). Differentiation continues with the thickening of the endosperm cell walls, particularly those of the aleurone, and with the accumulation of starch granules and protein bodies. It is difficult to determine when differentiation ends and maturation of the grain begins but aleurone cell walls appear to reach their maximum thickness by approximately 22 DAP (Fig. 1C). Beyond this stage, the barley grain continues to accumulate starch, progressively loses water, and subsequently becomes difficult to section for either light or EM. Therefore, observations on grain development and polysaccharide distribution are only described up to 28 DAP.
Figure 1.
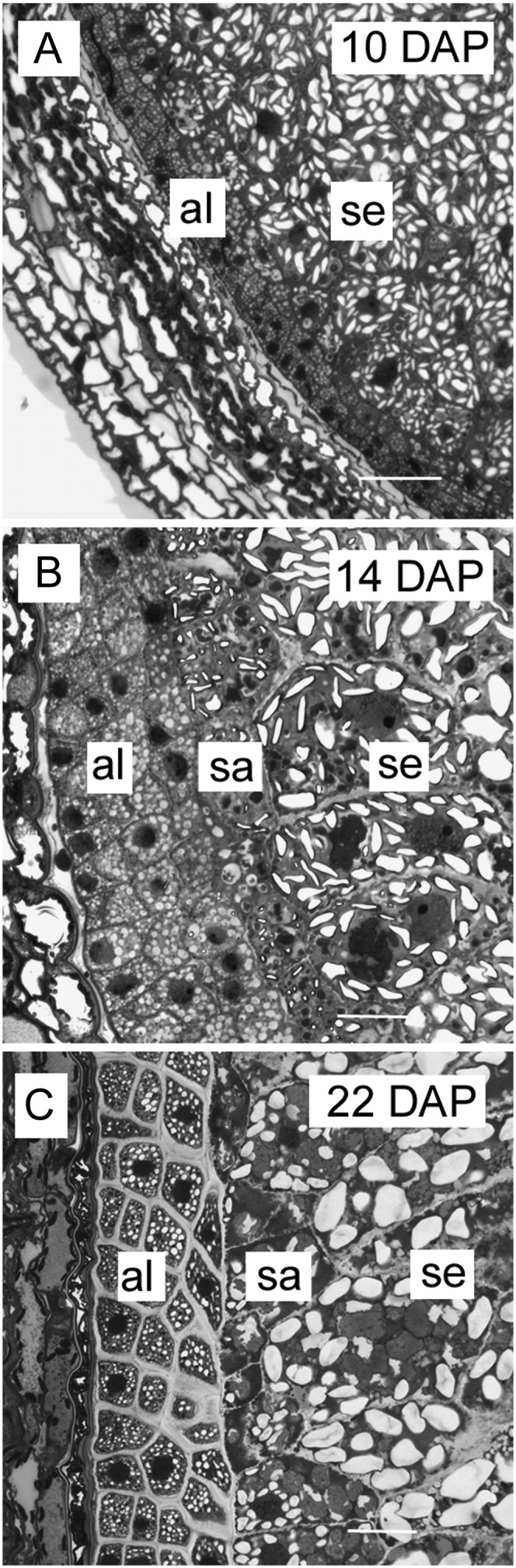
Light micrographs of toluidine-blue-stained sections through barley grains during endosperm differentiation. A, At 10 DAP several cell layers of maternal tissue surround the brick-like arrangement of cells distinguishing the differentiating aleurone layers from the starch-rich central endosperm. Bar = 200 μm. B, At 14 DAP a subaleurone cell layer marked by small starch granules and protein bodies lies between the aleurone cell layers and starchy endosperm. Bar = 100 μm. C, By 22 DAP the aleurone cells appear to have fully differentiated with their cell walls attaining maximum thickness whereas large endosperm cells continue to accumulate starch. Bar = 100 μm. al, Aleurone; se, starchy endosperm; sa, subaleurone.
(1→3)-β-d-Glucan (Callose) Appearance in Differentiating Endosperm (10–28 DAP)
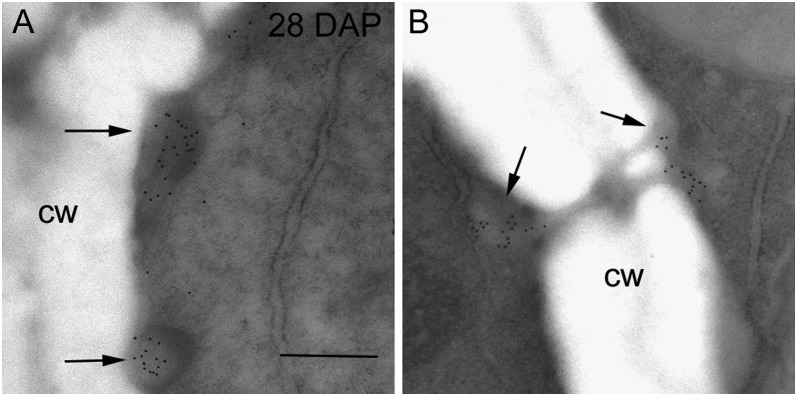
We (Wilson et al., 2006) and others (Brown et al., 1997; Philippe et al., 2006) have reported the transient nature of (1→3)-β-d-glucan during early endosperm development. Callose is the first polysaccharide to appear in the walls of the cellularizing endosperm at 3 DAP, persisting until around 6 DAP (Wilson et al., 2006). From 6 DAP onwards callose becomes restricted to those cell wall regions surrounding plasmodesmata throughout all endosperm cells. At 28 DAP, immunogold labeling with the (1→3)-β-d-glucan antibody reveals newly deposited callose in electron-dense, nonuniform patches at the edges of those subaleurone cell walls directly abutting the aleurone cells (Fig. 2A). Elsewhere in the 28 DAP endosperm callose is associated only with plasmodesmata (Fig. 2B).
Figure 2.
Transmission electron micrographs of endosperm cells at 28 DAP labeled with (1→3)-β-d-glucan antibody. A, Deposits of callose were seen in electron-dense regions at the edges of subaleurone cells (arrows). B, In the starchy endosperm gold labeling was restricted to the callosic-rich plugs surrounding plasmodesmata (arrows). Bar = 0.5 μm. cw, Cell wall.
Xyloglucan Deposition during Cellularization (3–8 DAP)
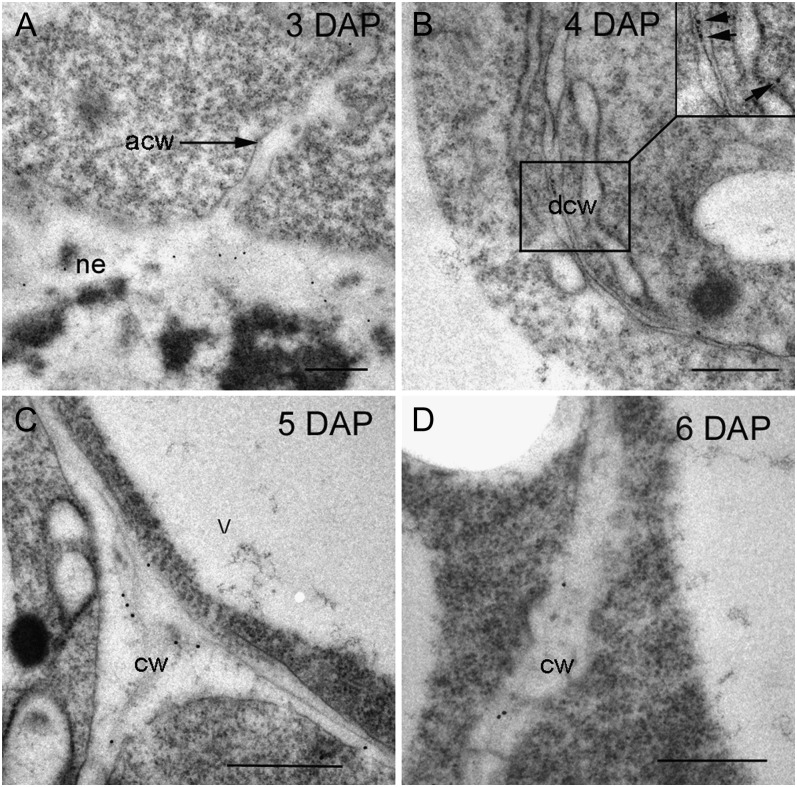
Because an antibody (LM15) has only recently been generated (Marcus et al., 2008) that recognizes an epitope unique to xyloglucans and made commercially available, we were unable to confirm the presence of xyloglucan during cellularization in our previous article (Wilson et al., 2006). The cellulose-degrading enzyme, cellobiohydrolase II (CBHII or Cel6A) from the fungus Trichoderma reesei, coupled to gold was used to detect cellulose in the central cell wall at 3 DAP and the first-formed anticlinal walls at 4 DAP (see fig. 5 in Wilson et al., 2006). However, CBHII also binds to the (1→4)-β-oligoglucoside structures in (1→3, 1→4)-β-d-glucan and xyloglucan (Henriksson et al., 1995; Amano et al., 1996). We assumed at 3 and 4 DAP, CBHII was binding to cellulose because (1→3, 1→4)-β-d-glucan is not deposited until after 4 DAP and xyloglucan is absent in the mature grain (Fincher, 1975; Bacic and Stone, 1981a, 1981b). This assumption was incorrect and we now show that xyloglucan is present transiently in the endosperm from 3 until about 6 DAP. It appears first in the central cell wall at 3 DAP just as the first anticlinal walls are forming (Fig. 3A). Labeling of the LM15 antibody is present, albeit at low levels, at 4 and 5 DAP (Fig. 3, B and C) but is barely detectable at 6 DAP (Fig. 3D). By 8 DAP no gold labeling can be found anywhere in the endosperm (images not shown). As yet, there is no specific probe for cellulose so we cannot comment when this polysaccharide is first deposited but chemical analysis shows cellulose to comprise approximately 3% to 4% (w/w) of the mature barley endosperm walls (Fincher, 1975).
Figure 5.
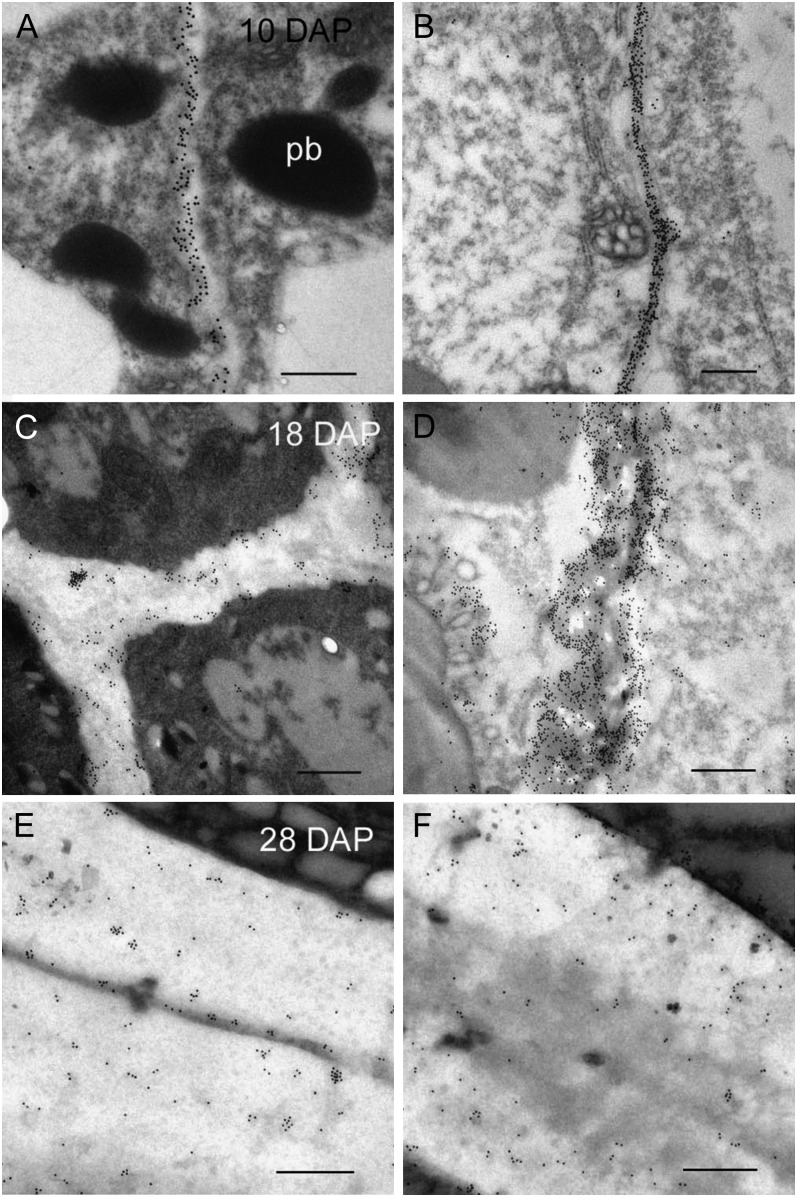
Transmission electron micrographs of (1→3, 1→4)-β-d-glucan labeling of differentiating aleurone and starchy endosperm cells 10 to 28 DAP. A, At 10 DAP there appears to be comparable levels of labeling in the cell walls of the aleurone and starchy endosperm (B). C, By 18 DAP there is less labeling in the thickened cell walls of the aleurone than the starchy endosperm (D). E and F, At 28 DAP the density of gold labeling in the aleurone cell walls (E) is similar to that observed at 18 DAP, whereas labeling in cell walls of the starchy endosperm (F) appears less dense. Bars = 0.5 μm. pb, Protein body.
Figure 3.
Transmission electron micrographs of developing endosperm cell walls labeled with xyloglucan antibody, LM15, at 3 to 8 DAP. A, At 3 DAP xyloglucan is present in the central cell wall but absent in the first forming anticlinal cell walls. B, At 4 DAP light labeling can be detected in the early endosperm cell walls. Inset shows enlarged image of cell wall with gold particles (arrows). C, At 5 DAP that xyloglucan labeling is at its most abundant in the endosperm. D, By 6 DAP gold labeling is barely detectable in the starchy endosperm but still present in the central cell wall. By 8 DAP xyloglucan labeling in the starchy endosperm is almost negligible (data not shown). Bar = 0.5 μm. acw, Anticlinal cell wall; cw, cell wall; dcw, developing cell wall; ne, nucellar epidermis; V, vacuole.
(1→3, 1→4)-β-d-Glucan Deposition during Endosperm Differentiation (10–28 DAP)
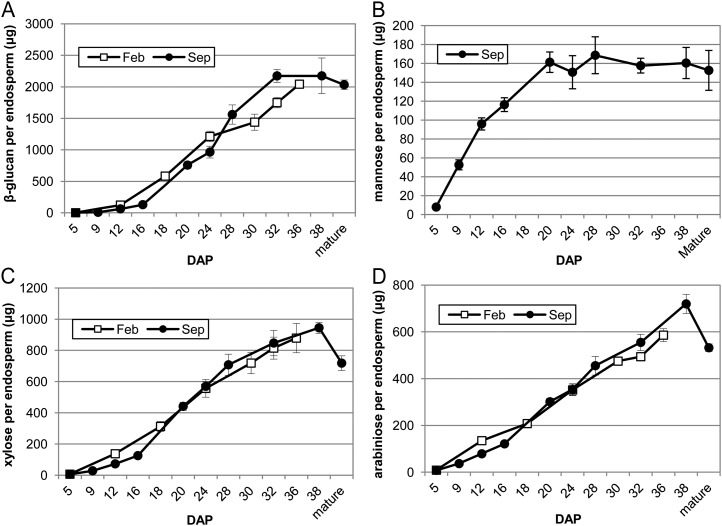
Chemical analysis has shown (1→3, 1→4)-β-d-glucan to be the most abundant polysaccharide in the barley grain (Fincher, 1975). Unlike rice (Brown et al., 1997) but similar to wheat (Philippe et al., 2006), (1→3, 1→4)-β-d-glucan appears in the early walls of the cellularizing endosperm between 4 and 5 DAP and accumulates in the cell walls of the endosperm throughout development. At 10 DAP, immunolabeling for light and EM using the (1→3, 1→4)-β-d-glucan antibody shows (1→3, 1→4)-β-d-glucan to be distributed uniformly throughout the endosperm tissues (Figs. 4A and 5, A and B). However, at 12 DAP a few rows of starchy endosperm cells closest to the differentiating aleurone layers appear to have little or no (1→3, 1→4)-β-d-glucan in their walls (Fig. 4B). This specific lack of labeling in the peripheral endosperm cells has also been reported in wheat (Philippe et al., 2006). In wheat, this observed heterogeneity in labeling intensity within the starchy endosperm persists throughout development whereas in barley, by 16 DAP the cell walls of the peripheral endosperm are labeled (Fig. 4C). Between 18 and 22 DAP, (1→3, 1→4)-β-d-glucan labeling is less intense in the thickened aleurone cell walls than the starchy endosperm (Figs. 4D and 5, C and D). The cell walls of the subaleurone show similar levels of (1→3, 1→4)-β-d-glucan to the starchy endosperm. Indeed, the distribution of all polysaccharides in the subaleurone cell walls seems to resemble the starchy endosperm walls and not the aleurone walls. At 28 DAP there was substantial labeling in both the aleurone and starchy endosperm cell walls (Fig. 5, E and F). This observation was expected because the level of (1→3, 1→4)-β-d-glucan in the developing endosperm shows greatest accumulation between 16 and 32 to 36 DAP (Fig. 8A). Similarly, Coles (1979) reported that the greatest accumulation of nonstarchy polysaccharides in the barley grain occurs after 23 DAP in phytotron-grown plants.
Figure 4.
Light micrographs of silver-enhanced (1→3, 1→4)-β-d-glucan labeling of differentiating endosperm from 10 to 22 DAP. A, At 10 DAP (1→3, 1→4)-β-d-glucan labeling was present in all cell walls of the differentiating endosperm. B, At 12 DAP the (1→3, 1→4)-β-d-glucan monoclonal antibody labeled the cell walls of the differentiating aleurone and central starchy endosperm. Labeling was absent from the cell walls of the peripheral endosperm. C, At 16 DAP (1→3, 1→4)-β-d-glucan was present in the cell walls of the peripheral endosperm. D, Increased labeling intensity implies (1→3, 1→4)-β-d-glucan is in greater abundance in the starchy endosperm cell walls than either the aleurone or maternal tissues. Bars = 100 μm. al, Aleurone; cse, central starchy endosperm; mt, maternal tissues; pe, peripheral endosperm; se, starchy endosperm; sa, subaleurone.
Figure 8.
The amounts of (1→3, 1→4)-β-d-glucan and the monosaccharides Man, Xyl, and arabinose in the developing barley endosperm (cv Sloop). The endosperms were collected from 5 to 38 DAP in February 2008 (Feb) and September 2008 (Sep) as described in the “Materials and Methods.” Mean of three replicates, error bars are sd. A, (1→3, 1→4)-β-d-glucan. B, Man (Sep only). C, Xyl. D, Arabinose expressed as micrograms per endosperm.
Hetero-(1→4)-β-d-Mannan Deposition during Endosperm Differentiation (10–28 DAP)
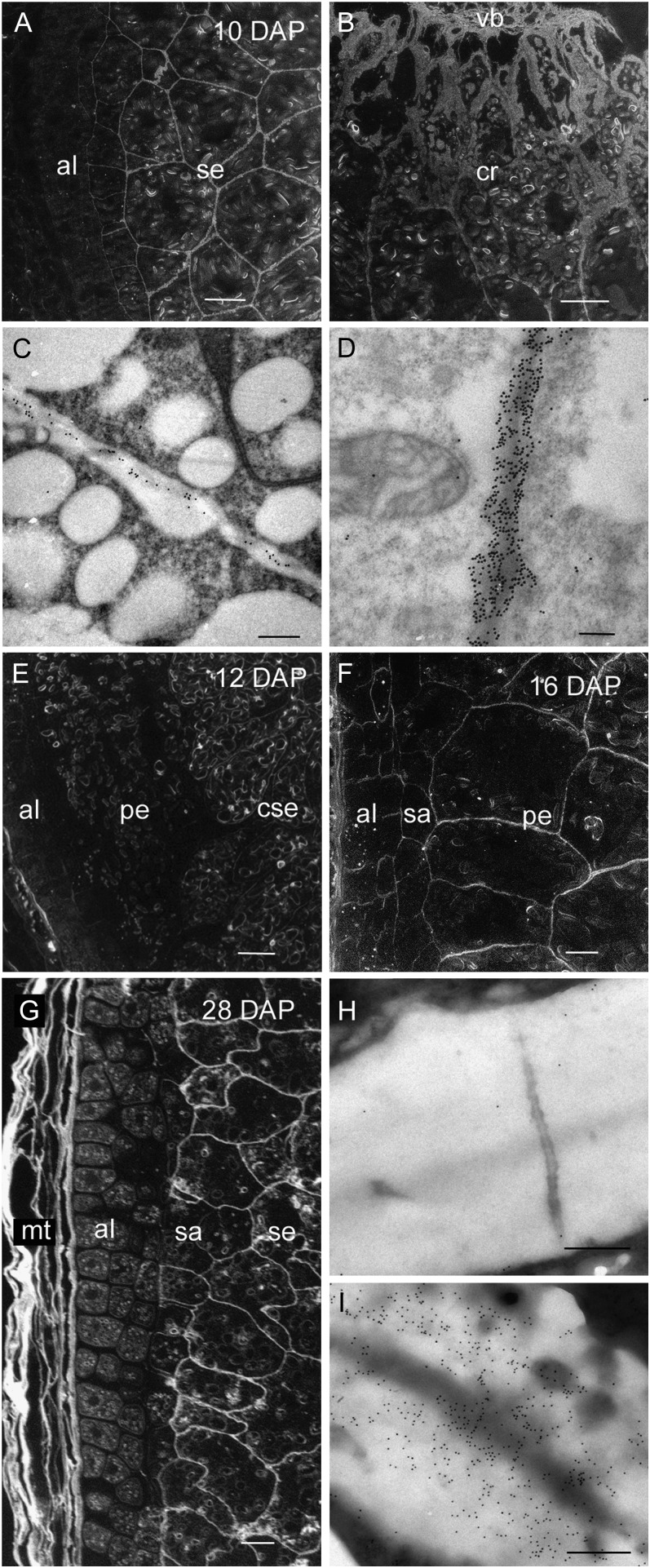
Heteromannan is laid down after cellularization between 5 and 6 DAP and all endosperm cell walls are uniformly labeled with the hetero-(1→4)-β-d-mannan monoclonal antibody at this stage of development (Wilson et al., 2006). At 10 DAP heteromannans could not be detected in the aleurone cell walls using silver enhancement for light microscopy. However, intense labeling was present in the starchy endosperm, in particular, in those walls of the cells adjacent to the grain crease (Fig. 6, A and B). Immunogold labeling of thin sections using transmission EM detects heteromannan in the aleurone cell walls at 10 DAP, albeit at low levels (Fig. 6C). Gold labeling in the starchy endosperm cell walls reaches its peak at this time, although this strong labeling is not indicative of the abundance of heteromannans in barley endosperm (Fig. 6D). It should be noted that differences in antibody avidity for their epitopes prevent us from making conclusions on polysaccharide (heteromannan) abundance based on antibody binding alone. Thus, the strong labeling in this case results from the antibody avidity, rather than from the presence of large amounts of the heteromannans because the levels determined by linkage analyses in starchy endosperm walls of mature barley grain is 3% to 4% (w/w; Fincher, 1975). Sugar composition analysis of the endosperm (Fig. 8B) also shows that the amount of Man in the endosperm is low and increases from 5 DAP and peaks at around 20 DAP. At 12 DAP heteromannan antibody labeling is absent in the walls of the aleurone and peripheral starchy endosperm whereas the central starchy endosperm remains well labeled (Fig. 6E). As was the case for (1→3, 1→4)-β-d-glucan, this absence of labeling in the cell walls of the peripheral endosperm is observed until 16 DAP. At this time, the walls of the peripheral endosperm show a similar labeling intensity to other regions of the starchy endosperm (Fig. 6F). At 28 DAP the cell walls of the remnant maternal tissues, subaleurone, and starchy endosperm, but not those of the aleurone, contain heteromannan (Fig. 6, G–I).
Figure 6.
Light and transmission electron micrographs of hetero-(1→4)-β-d-mannan labeling during endosperm differentiation from 10 to 28 DAP. A, Silver-enhanced antibody labeling at 10 DAP revealing little or no hetero-(1→4)-β-d-mannan in the aleurone cell walls whereas the starchy endosperm is well labeled. Bar = 100 μm. B, At 10 DAP the thicker starchy endosperm cell walls of the crease region are heavily labeled. Bar = 200 μm. C, Antibody labeling at 10 DAP revealing low levels of hetero-(1→4)-β-d-mannan in the aleurone cell walls. Bar = 0.5 μm. D, The cell walls of the starchy endosperm are heavily labeled at 10 DAP. Bar = 0.5 μm. E, Silver-enhanced antibody labeling at 12 DAP showing little labeling in the aleurone cell walls, no labeling in the walls of the peripheral starchy endosperm whereas hetero-(1→4)-β-d-mannan is present in the walls of the central starchy endosperm. Bar = 100 μm. F, By 16 DAP the walls of the peripheral starchy endosperm are labeled. Bar = 100 μm. G, At 28 DAP, silver-enhanced antibody labeling shows hetero-(1→4)-β-d-mannan is present in the walls of the maternal tissues, subaleurone, and starchy endosperm but absent in the thickened aleurone cell walls. Bar = 100 μm. H, Gold-labeled sections confirm hetero-(1→4)-β-d-mannan is absent in the aleurone cell walls but present in the starchy endosperm cell walls (I). Bars = 0.5 μm. al, Aleurone; cse, central starchy endosperm; cr crease region; mt, maternal tissues; pe, peripheral endosperm; sa, subaleurone; se, starchy endosperm; vb; vascular bundle.
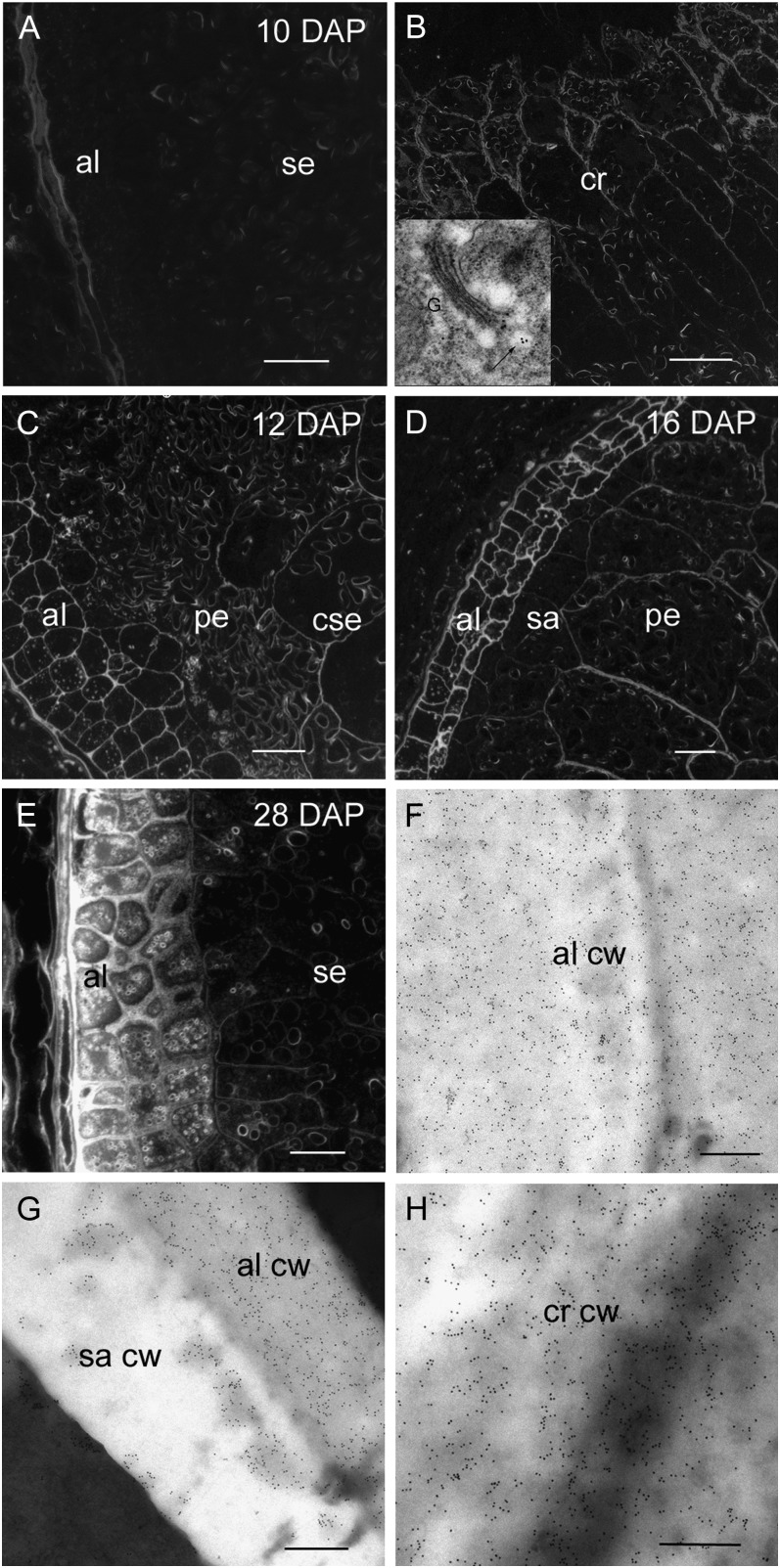
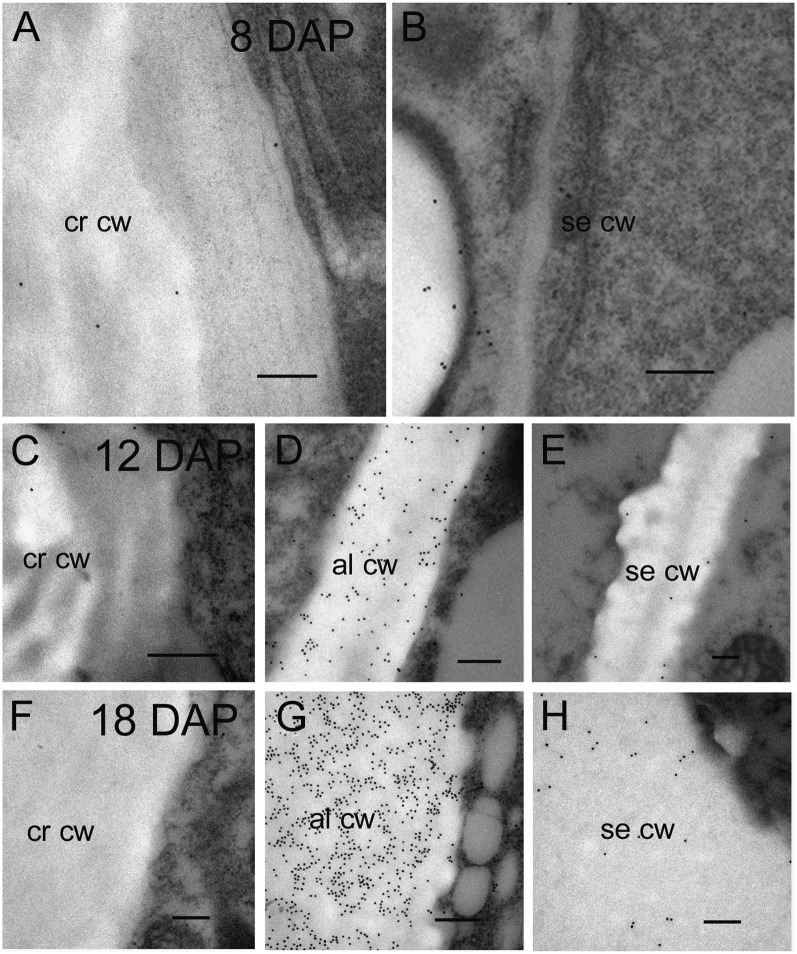
Arabino-(1→4)-β-d-Xylan Deposition during Endosperm Differentiation 10 to 28 DAP
Arabino-(1→4)-β-d-xylan deposition, monitored with the LM11 antibody, is the last polysaccharide to be laid down in the endosperm and first appears at 8 DAP but only in those endosperm cell walls nearest to the vascular strand in the crease region (Wilson et al., 2006). At 10 DAP arabino-(1→4)-β-d-xylan is still restricted to the crease region (Fig. 7, A and B) but by 12 DAP, strong labeling can also be detected in the aleurone and central starchy endosperm cell walls (Fig. 7C). As was the case for (1→3, 1→4)-β-d-glucan and hetero-(1→4)-β-d-mannan, arabino-(1→4)-β-d-xylan appears to be absent from the cell walls of the peripheral starchy endosperm (Fig. 7C). At 16 DAP, all endosperm cell walls contain arabino-(1→4)-β-d-xylan including the subaleurone and peripheral starchy endosperm but the most intense labeling occurs in the aleurone (Fig. 7D). Silver enhancement for light microscopy shows very intense labeling in the thickened aleurone cell walls at 28 DAP and very light labeling in the subaleurone and starchy endosperm (Fig. 7E). Immunogold labeling for transmission EM shows the aleurone cell walls are intensely labeled whereas those of the subaleurone appear to have less arabino-(1→4)-β-d-xylan with a patchy distribution (Fig. 7, F and G). The starchy endosperm cell walls adjacent to the crease continue to display intense labeling equal to that of the aleurone (Fig. 7H).
Figure 7.
Light and transmission electron micrographs of arabino-(1-4)-β-d-xylan labeling using the LM11 antibody during endosperm differentiation from 10 to 28 DAP. A, Silver-enhanced antibody labeling at 10 DAP reveals no arabino-(1-4)-β-d-xylan in aleurone or starchy endosperm cell walls. Bar = 200 μm. B, Arabino-(1-4)-β-d-xylan is present in the starchy endosperm cells walls of the crease region at 10 DAP. Bar = 200 μm. Inset, An electron micrograph of a gold-labeled Golgi apparatus (arrow) located within a cell at the crease. C, At 12 DAP silver-enhanced antibody labeling reveals arabino-(1-4)-β-d-xylan is now present in the aleurone and central starchy endosperm cell walls but absent in the peripheral starchy endosperm. Bar = 100 μm. D, At 16 DAP the cell walls of the peripheral starchy endosperm are labeled with the arabino-(1-4)-β-d-xylan antibody. Bar = 100 μm. E, At 28 DAP the aleurone cell walls are intensely labeled with the arabino-(1-4)-β-d-xylan antibody whereas labeling in the starchy endosperm cell walls is barely detectable. Bar = 100 μm. F, At 28 DAP electron micrographs of antibody-labeled sections show intense gold labeling in the aleurone cell wall. Bar = 0.5 μm. G, A heavily labeled aleurone cell wall abutting a subaleurone cell wall that shows patchy gold labeling with the arabino-(1-4)-β-d-xylan Ab. Bar = 0.5 μm. H, At 28 DAP the cell walls in the crease region continue to be strongly gold labeled. Bar = 0.5 μm. al, Aleurone; cw, cell wall; cse, central starchy endosperm; cr, crease; pe, peripheral endosperm; sa, subaleurone; se, starchy endosperm.
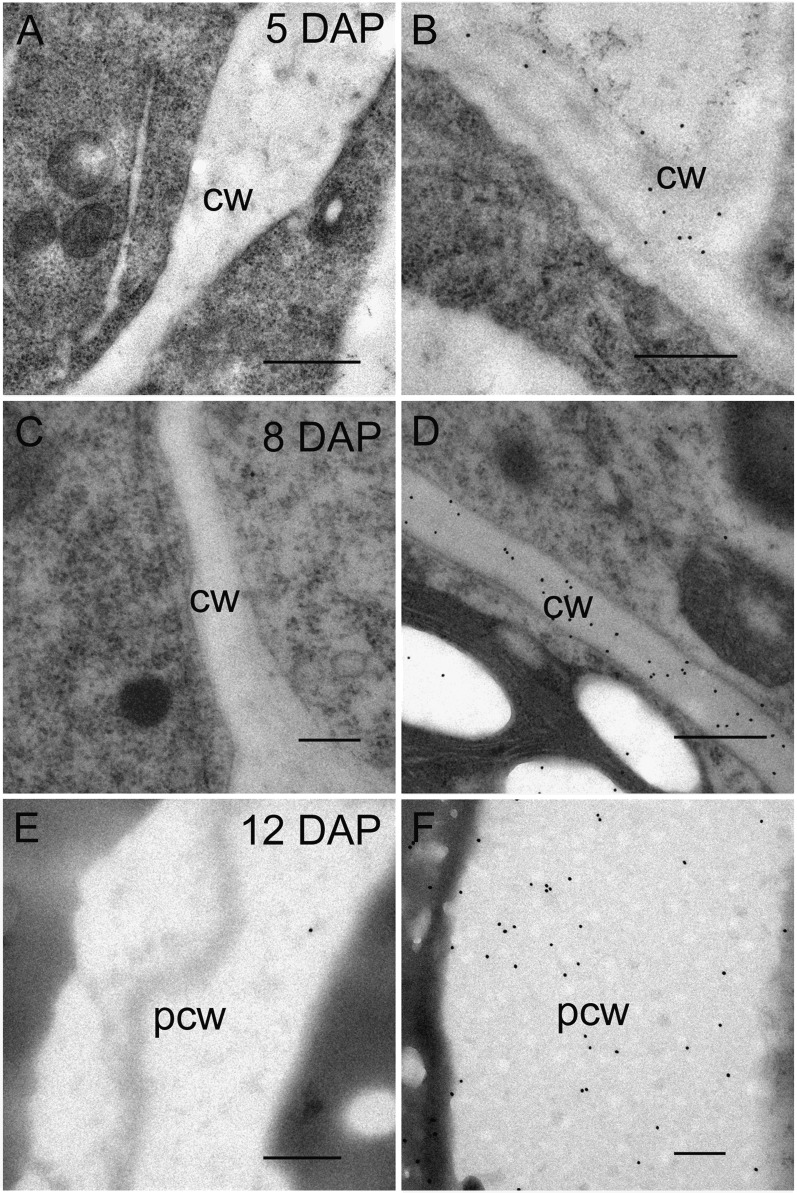
These immunolabeling results were inconsistent with the presence of arabinose and Xyl in alcohol insoluble polymeric material, presumably as arabino-(1→4)-β-d-xylan, in the endosperm from 5 DAP (Fig. 8, C and D). Thus, monosaccharide analysis of the developing endosperm shows a continual increase in arabinose and Xyl from 5 to 38 DAP but the arabino-(1→4)-β-d-xylan is not detected at high levels with the antibodies until 12 DAP. A decrease in both arabinose and Xyl is observed in the mature grain due to the removal of some of the aleurone layer during the pearling process. In an attempt to reconcile these data, we considered whether the xylan backbone may be so heavily substituted with either arabinose or O-acetyl residues during early endosperm development that it is rendered inaccessible to the antibody. To test this hypothesis, we either pretreated sections with alkali (to remove O-acetyl residues) or applied an α-l-arabinofuranosidase (to remove arabinose residues) to sections prior to LM11 labeling at 5, 8, and 12 DAP. Labeling was now observed in all cell walls of the endosperm that had been pretreated with the enzyme (Fig. 9, A–F), confirming that heavily substituted arabino-(1→4)-β-d-xylan is present in walls of the early differentiating endosperm but was not detected in previous LM11 labeling experiments due to arabinose side chains. Pretreating the sections with alkali did not change the pattern of LM11 labeling.
Figure 9.
Transmission electron micrographs of 5, 8, and 12 DAP endosperm cell walls labeled with the LM11 (left-hand column) antibody for arabino-(1→4)-β-d-xylan and those pretreated with α-l-arabinofuranosidase before labeling with LM11 (right-hand column). A, Immunogold-labeled section of cellularizing endosperm at 5 DAP showing no gold particles in the cell walls. B, Treatment with α-l-arabinofuranosidase prior to LM11 labeling reveals arabino-(1→4)-β-d-xylan to be present in the early endosperm cell walls. C, No labeling using the LM11 antibody can be seen in the cell walls of the starchy endosperm at 8 DAP. D, LM11 labeling after pretreating 8 DAP sections with α-l-arabinofuranosidase reveals significant gold labeling along starchy endosperm cell walls. E, Gold particles are absent when the peripheral starchy endosperm at 12 DAP has been labeled with the LM11 antibody. F, The presence of arabino-(1→4)-β-d-xylan is revealed in the peripheral starchy endosperm of 12 DAP sections when treated with α-l-arabinofuranosidase prior to LM11 labeling. Bars = 0.2 μm. cw, Central starchy endosperm wall; pcw, peripheral starchy endosperm wall.
5-O-(Transferuloyl)-l-Arabinose Polyclonal Antibody Labeling of Differentiating Endosperm Cell Walls from 10 to 18 DAP
Barley endosperm arabino-(1→4)-β-d-xylan has been shown to vary in its degree of α-arabinofuranosyl substitution at the C(O)2 and/or C(O)3 position of the Xyl and esterification on C(O)5 of the arabinofuranosyl residues by hydroxycinnamic acids, such as ferulic acid or p-coumaric acid (Fincher and Stone, 2004; York and O’Neill, 2008). To examine the temporal pattern of feruloylation throughout endosperm differentiation, we applied a polyclonal antibody specific for ferulic acid ester linked at the C(O)5 position to α-l-arabinosyl residues of arabino-(1→4)-β-d-xylans (Philippe et al., 2007). At 8 DAP there appears to be very little, if any, ferulic acid substitution anywhere in the starchy endosperm that contrasts with the heavy labeling we observed with arabino-(1→4)-β-d-xylan labeling at the grain crease at this time point (Fig. 10, A and B). Again at 12 DAP there are barely detectable levels of labeling at the grain crease whereas the aleurone cell walls, which by now are morphologically distinct from the endosperm, show considerable levels of labeling (Fig. 10, C and D). A low level of feruloylated epitope was detected in the central starchy endosperm (Fig. 10E). This pattern of labeling continues to 18 DAP and beyond where the walls of the grain crease are unlabeled, the aleurone cell walls heavily labeled, whereas the starchy endosperm cell walls show barely detectable levels of the feruloylated epitope (Fig. 10, F and G). The Golgi apparatus in aleurone cells is also well labeled with this antibody (data not shown), indicating the site of synthesis.
Figure 10.
Transmission electron micrographs revealing the labeling of the 5-O-(transferuloyl)-l-arabinose (5-O-Fer-Ara) epitope on arabino-(1→4)-β-d-xylan during barley endosperm differentiation from 10 to 18 DAP. A, Immunogold-labeled section at the grain crease showing barely detectable levels of binding at 10 DAP. B, The central starchy endosperm at 10 DAP also shows little labeling on cell walls although gold particles bind to starch granules nonspecifically. C, Again at 12 DAP there is almost no detectable levels of the 5-O-Fer-Ara epitope at the grain crease. D, The aleurone cell walls that are beginning to differentiate (thicken) at 12 DAP are well labeled with the 5-O-Fer-Ara antibody. E, There is light labeling in the starchy endosperm at 12 DAP. F, No labeling was detected at the grain crease at 18 DAP. G, The aleurone cells are heavily and uniformly labeled throughout their thickened walls at 18 DAP. H, The starchy endosperm walls at 18 DAP are lightly and unevenly labeled with the 5-O-Fer-Ara antibody. Bar = 0.2 μm. al, Aleurone; cr, crease; cw, cell wall; se, starchy endosperm.
Transcript Profiles of GSL Genes
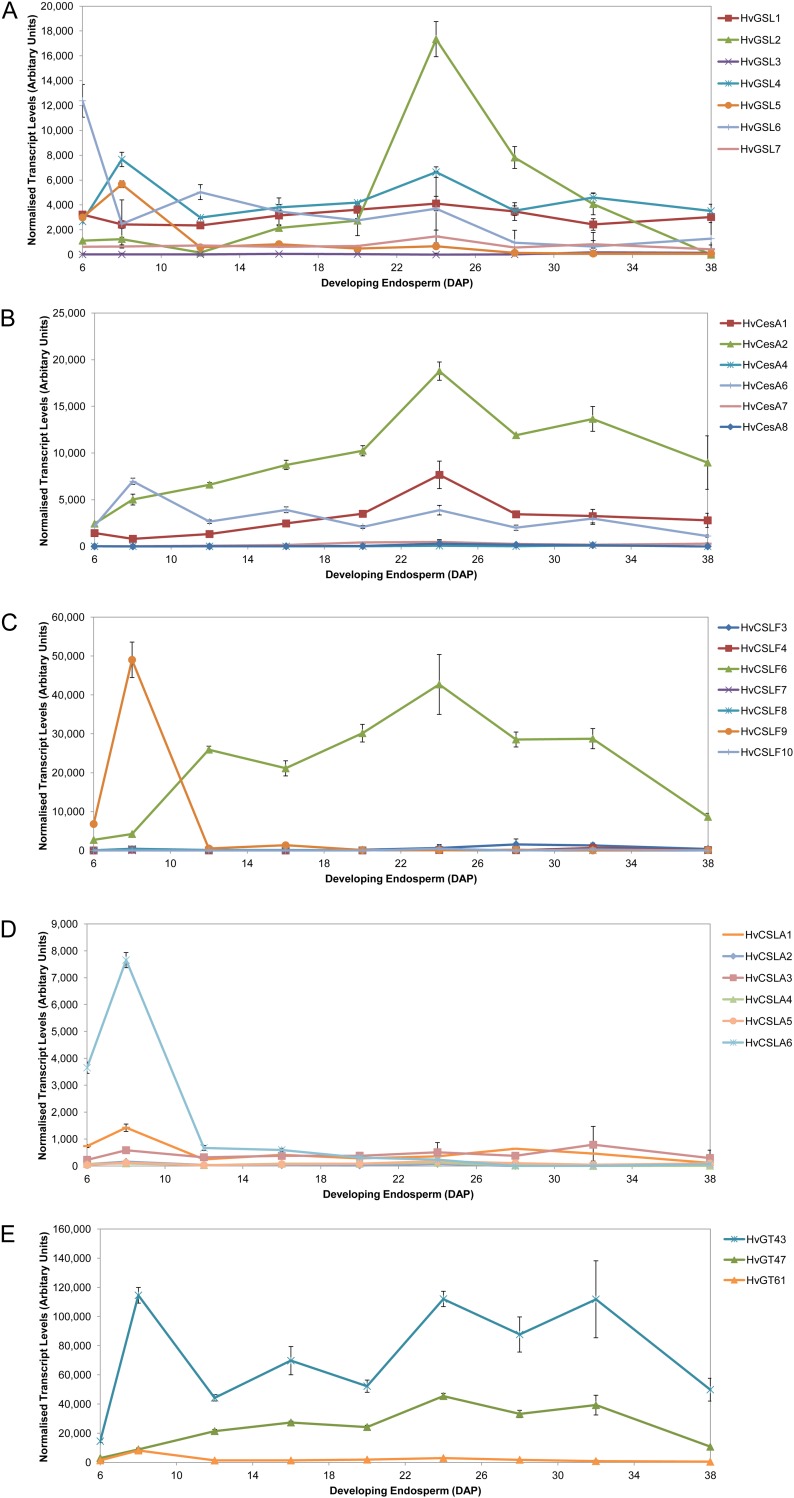
There is now considerable biochemical and molecular evidence suggesting the GSL genes are responsible for the synthesis of (1→3)-β-d-glucan in either a developmentally regulated or wound-activated manner in plants (Brownfield et al., 2009). Seven GSL genes have so far been identified in barley (Schober et al., 2009). QPCR experiments using gene-specific primer pairs on cDNAs prepared from endosperm between 6 and 38 DAP show a number of patterns of steady-state transcript levels. HvGSL6 has the highest transcript level of all the GSLs early in endosperm development at 6 DAP being 3-fold higher than any other GSL gene whereas HvGSL2 transcript dominates the GSL expression profile within the late differentiation stage, peaking at 24 DAP (Fig. 11A). The other GSL genes show lower and relatively even transcript levels throughout endosperm development. These data are similar to those presented by Schober et al. (2009), although in the earlier work GSL gene transcripts were only monitored up to 11 DAP.
Figure 11.
Normalized transcript levels of glycosyl transferase genes in developing barley endosperm from 6 to 38 DAP. A, GSL gene family. B, CesA family genes. C, CSLF family genes. D, CSLA genes. E, Representative Carbohydrate Active Enzymes database GT 43, 47, and 61 gene family members.
Transcript Profiles of Cellulose Synthase Genes
The appearance and distribution of cellulose in the walls of developing barley grain could not be defined because currently there is no probe that specifically detects (1→4)-β-d-oligoglucosides within cellulose and not within (1→3, 1→4)-β-d-glucan and xyloglucan. Nevertheless, transcript abundance of Cellulose Synthase (CesA) genes involved in cellulose synthesis in primary cell walls was monitored throughout endosperm development, because the presence of small amounts of cellulose in the walls of mature endosperm (Fincher, 1975) indicates that these genes should be active. Burton et al. (2008) reported that HvCesA1, HvCesA2, and HvCesA6 are cotranscribed in tissues where primary wall synthesis is occurring. In the developing barley endosperm, relatively low levels of these transcripts were detected throughout starchy endosperm development (Fig. 11B). HvCesA4, HvCesA7, and HvCesA8, which tend to be coexpressed where secondary wall synthesis is occurring, showed extremely low levels of expression throughout development as expected (Burton et al., 2004; Fig. 11B).
Transcript Profiles of Csl Genes
The profiling was restricted to those gene families proposed to have roles in the assembly of the wall polysaccharides present in endosperm walls. Transcript profiles of members of the CslF family, that encode (1→3, 1→4)-β-d-glucan synthases (Burton et al., 2006; Doblin et al., 2009), are shown in Figure 11C. Two genes dominate the expression profile: Transcripts of CslF9 peak early in grain development at 8 DAP and decrease to negligible levels by 12 DAP whereas the transcript levels of CslF6 shows a concurrent increase to become the dominant transcript present throughout the majority of grain development (12–38 DAP). Significant levels of CslF6 transcript persist until late in grain development and despite a decline at 32 DAP (Fig. 11C) mRNA levels of this gene are still far higher than any other CslF or indeed CslH gene, whose transcript levels are extremely low (<200 normalized transcript units; data not shown). Thus, CslF6 is a likely candidate necessary for the synthesis of much of the (1→3;1→4)-β-glucan found in the mature grain (Burton et al., 2008).
The CslA genes from a broad range of species including Arabidopsis, guar (Cyamopsis tetragonoloba), poplar (Populus trichocarpa), loblolly pine (Pinus taeda), moss (Physcomitrella patens), and rice have been shown to have either mannan or glucomannan synthase activity when heterologously expressed (Dhugga et al., 2004; Liepman et al., 2005, 2007; Suzuki et al., 2006). The expression levels of six known barley CslA genes were followed over the differentiation phase of endosperm development. Only one of the genes, CslA6, shows significant transcript abundance, albeit at a level well below the dominant transcripts of the other gene families described above. CslA6 expression levels began to rise around 5 DAP, peaking at 8 DAP, and declining sharply to the low levels of the other CslA genes at around 12 DAP (Fig. 11D).
Low levels of HvCslC gene transcripts, proposed to encode the polysaccharide synthase for both the (1→4)-β-d-glucan backbone of xyloglucans (Cocuron et al., 2007) and cellulose (Dwivany et al., 2009), were also detected throughout endosperm development (data not shown; see Dwivany et al., 2009 for profiles from whole grain), but the functional significance of these gene transcripts is currently unknown.
Transcript Profiles of Genes Implicated in Arabino-(1→4)-β-d-Xylan Synthesis
Although the genes involved in arabino-(1→4)-β-d-xylan biosynthesis have not been biochemically defined unequivocally, there are a number of studies that have implicated glycosyl transferases from the GT43, GT47, and GT61 families in the synthesis of this polysaccharide (Mitchell et al., 2007). The transcript abundance of selected genes from these families were therefore monitored and shown to be present throughout grain development, with relatively high levels of GT43 mRNA (Fig. 11F).
DISCUSSION
This study extends our earlier work on endosperm cellularization in barley, insofar as it is focused on the temporal and spatial appearance of wall polysaccharides during the latter differentiation stage of endosperm development. Furthermore, observations made after application of newly available polysaccharide-specific probes have unveiled important new information about the deposition of polysaccharides within the developing barley grain. This has also led to a revision of some of our original observations on polysaccharide deposition during cellularization. We have examined the monosaccharide composition of the endosperm over the course of development, applied the recently generated xyloglucan antibody to early endosperm development, and modified our approach to studying arabino-(1→4)-β-d-xylan using the LM11 antibody. Previous studies identifying genes involved in the synthesis of cell wall polysaccharides have shown that transcript accumulation correlates with the time of polysaccharide synthesis and deposition (Pear et al., 1996; Doblin et al., 2001; Dhugga et al., 2004; Brown et al., 2005; Persson et al., 2005; Cocuron et al., 2007; Mitchell et al., 2007). By coupling our immuno-EM observations with transcript profiles of candidate cell wall synthesis genes, we can now infer which subfamily members of these gene families is functional during key stages of endosperm development.
(1→3)-β-d-Glucan (Callose)
(1→3)-β-d-Glucan is the first polysaccharide to be deposited in the developing endosperm, appearing in the central cell wall at 3 DAP and within the newly formed anticlinal and periclinal cell walls that encapsulate the nuclei of the syncytium until cellularization is complete at 5 to 6 DAP (Wilson et al., 2006). Callose has a transient appearance in the cellularizing endosperm except in those regions surrounding plasmodesmata (Wilson et al., 2006). This pattern of callose deposition in the early endosperm is similar to what has been described for rice and wheat endosperm (Brown et al., 1997; Philippe et al., 2006).
Early studies on wheat and barley grains report callose in mature endosperm cell walls as evidenced by deposits of the aniline blue fluorochrome at the aleurone/starchy endosperm interface (Fulcher et al., 1977; Bacic and Stone, 1981a). Fulcher et al. (1977) described different labeling patterns in two barley varieties: One, cv Vanier, shows a continuous layer of the aniline blue fluorochrome whereas the other, cv Himalaya, has discrete patches of fluorescence along the thick walls directly adjacent to the aleurone. Similarly, Bacic and Stone (1981a) observed a continuous layer of aniline blue fluorochrome staining at the subaleurone/aleurone interface in wheat (cv Insignia) while describing droplets of fluorescent material at the same location for barley (cv Himalaya). These aniline blue fluorescent deposits match the noncontinuous patches of (1→3)-β-d-glucan antibody labeling observed here in cv Sloop within the walls of subaleurone cells directly adjacent to the aleurone (Fig. 2A). Fulcher et al. (1977) suggests that these deposits may be the primary substrate for high levels of β-(1,3)-glucanase activity present at the onset of germination. Both Fulcher et al. (1977) and Bacic and Stone (1981a) recognized that variation in the pattern and size of callose deposits between barley varieties does not support a specific physiological function, although Fincher (1989) suggested that the variable patterns of callose distribution might have reflected a variable number of transient water stress events that caused plasmolysis and callose deposition during grain development. The deposits may either influence the movement of substances during germination or they could simply be a remnant of developing walls (Fulcher et al., 1977). However, another possible explanation is that callose is being synthesized as a wound response. The subaleurone cell would be expected to be under enormous mechanical pressure as they are pushed up against the thick aleurone cell during grain filling. It may also explain the variability in the pattern of callose deposition as it is likely the degree of pressure exerted and extent of wounding is nonuniform across grain varieties and individual grains.
Considerable molecular, biochemical, and functional evidence has now accumulated, supporting a role for GSL proteins in the synthesis of (1→3)-β-d-glucan in plants (for review, see Brownfield et al., 2009). Two HvGSL genes show particularly interesting patterns during endosperm development. HvGSL6 has by far the highest transcript levels during cellularization, indicating that the encoded gene products are likely responsible for callose deposition in the early stages of endosperm development when the first cell walls are laid down (Wilson et al., 2006). In contrast, HvGSL2 transcript levels peak at 24 DAP within the late differentiation stage just as callose appears as deposits at the perimeter of subaleurone/aleurone interface. Hence, HvGSL2 may be involved in the synthesis of callose in this later stage of endosperm development.
Xyloglucan
We were not expecting to detect xyloglucan in the developing endosperm because previous chemical analyses were negative in both the starchy endosperm and aleurone cell walls of mature grain (Fincher, 1975; Bacic and Stone, 1981b) and the amount of xyloglucan in the primary cell walls of the commelinoid monocots is the lowest of any of the flowering plants. However, despite these assumptions, the (1→4)-β-oligoglucoside structures detected by the CBHII-AU probe at 3 and 4 DAP attributed to cellulose binding (Wilson et al., 2006), were at least partly belonging to the xyloglucan backbone. We can now draw this conclusion because the recently generated xyloglucan-specific antibody, LM15 (Marcus et al., 2008), binds to the first formed endosperm cell walls at 3 DAP and persists till 6 DAP (Fig. 3). After this time, xyloglucan labeling diminishes in endosperm walls but persists in the surrounding maternal tissues, indicating that xyloglucan is either hydrolyzed from the developing endosperm walls or undergoes modification such that the LM15 antibody does not recognize it (Dwivany et al., 2009). The former possibility seems the more likely given that xyloglucan-specific linkages are not detected in mature endosperm walls by either chemical analysis (Fincher, 1975; Bacic and Stone, 1981b) or by the more sensitive immuno-EM method used in this study (data not shown). Xyloglucan is therefore the second polysaccharide to be shown to undergo turnover in endosperm walls. The reason why xyloglucan is only temporarily deposited during endosperm cellularization is unknown but, like callose, it is a polysaccharide that is laid down early in phragmoplast formation and is subsequently removed (Samuels et al., 1995; Otegui and Staehelin, 2000a, 2000b; Otegui et al., 2001), presumably because other polysaccharide(s) supersede its role later in development. Although a definitive role for xyloglucan during early development cannot be deduced from this study, it is of interest that the low levels of xyloglucan often seen in the cell walls of the commelinoid monocots appears to be associated with young and/or expanding cells, although xyloglucan is present in the mature endosperm cell walls of rice (Kato et al., 1982; Kato and Nevins, 1984, 1991; Gibeaut et al., 2005) and has recently been reported to be present in mature tissues of specific cell types in some members of the Poales (Brennan and Harris, 2011).
Cocuron et al. (2007) proposed that the CSLC proteins function is to synthesize the xyloglucan backbone although Dwivany et al. (2009) have also proposed a role for some CSLC family members in the synthesis of cellulose in certain specialized cell types. Quantitative reverse transcription-PCR analysis undertaken here showed that the abundance of barley CSLC gene transcripts was extremely low (data not shown), consistent with the low levels of xyloglucan (Fincher, 1975; Bacic and Stone, 1981b).
Cellulose
The temporal or spatial deposition of cellulose in the developing endosperm is difficult to assess because the CBHII-Au probe used to detect cellulose in our previous study also binds the (1→4)-β-glucan backbone of xyloglucan. Over the years, a number of molecular probes have been used to detect cellulose including histochemical stains such as Congo Red, the fluorophore/optical brightener Calcofluor white (FB28; Wood and Fulcher, 1978; Wood, 1980), and various carbohydrate-binding modules (CBMs; McCartney et al., 2006). However, to our knowledge, there is no probe for cellulose that does not also detect the (1→4)-β-oligoglucoside structures of (1→3, 1→4)-β-d-glucan and/or xyloglucan, including CBM3a (S. Wilson, unpublished data) and the CBM from Clostridium thermocellum directed toward crystalline cellulose (Blake et al., 2006; Hervé et al., 2010).
Cellulose comprises 3% to 4% (w/w) of barley endosperm walls at maturity (Fincher, 1975) and must therefore be deposited sometime during endosperm development. It seems reasonable to assume that cellulose would also be deposited during the cellularization phase, concomitant with the other polysaccharides we have shown to be deposited during this time (Wilson et al., 2006). To our knowledge, there is no reported case of xyloglucan existing within a plant cell wall in the absence of cellulose in either eudicots or monocots. One of the postulated roles of xyloglucan in the primary wall is to noncovalently cross-link cellulose microfibrils so that the xyloglucan-cellulose network becomes the major load-bearing structure of the wall (Cavalier et al., 2008). Whether the low levels of xyloglucan permit or are necessary for such a role early in endosperm development is debatable; in any event it cannot fulfill this role in the endosperm beyond 8 DAP when LM15 labeling disappears in endosperm cells. Xyloglucan may serve a different purpose, possibly to coat nascent cellulose microfibrils as they are extruded into the early walls because endosperm walls are neither exposed to the load nor turgor that is experienced by the cell walls of vegetative tissues, thereby obviating the need for an extensive cellulose/xyloglucan network. Cellulose is considered a carbon sink and is not turned over to any great degree after microfibril deposition hence it would be expected to persist in endosperm walls in the absence of xyloglucan during development.
QPCR analysis of barley CesA genes during endosperm development suggests that cellulose synthesis does occur during endosperm cellularization. The primary wall CesA genes, HvCesAs1, -2, and -6 (Burton et al., 2004), were anticipated to show the highest steady-state transcript levels in developing endosperm and transcripts of these genes were indeed detected here. However, HvCesAs4, -7, and -8 showed equally low levels of expression barely detectable on the transcript profile. This result was unexpected because these genes tend to be coordinately expressed in tissues where secondary wall synthesis is occurring (Burton et al., 2004).
(1→3, 1→4)-β-d-Glucan
(1→3, 1→4)-β-d-Glucan accumulates from about 5 DAP and is by far the most abundant polysaccharide of the mature barley grain (Fincher, 1975; Wilson et al., 2006). Its abundance is fairly uniform across the grain during early development then changes to being concentrated to the starchy endosperm and to a much lesser extent the thickened aleurone cell walls by the end of differentiation.
Two gene families are known to be involved in (1→3, 1→4)-β-d-glucan synthesis, the CSLF (Burton et al., 2006) and CSLH (Doblin et al., 2009) gene families. Only one CSLH gene has been identified in the barley genome whereas there are seven CSLF genes. Previously published transcript profiles show that CSLH1 peaks at 4 DAP, one day before (1→3, 1→4)-β-d-glucan appears in endosperm cell walls (Doblin et al., 2009) and then declines to low levels by 8 DAP, implicating its involvement in early endosperm development. However, CSLH1 transcript levels are generally much lower than CSLF transcript levels in endosperm and in vegetative tissues. At their peak, CSLH1 transcript levels are considerably lower than both CSLF6 (<200 normalized transcripts levels; data not shown) that is steady in the early stages of endosperm development and CSLF9 that is highest at 8 DAP, declining to baseline levels by 12 DAP (Burton et al., 2008; Doblin et al., 2009). After this time, only CSLF6 has significant levels of expression throughout the developing endosperm, suggesting that this gene is responsible for the accumulation of (1→3, 1→4)-β-d-glucan during differentiation. Indeed, the barley blg [(1,3;1,4)-β-d-glucan less] mutant that has a mutation within the HvCSLF6 gene lacks (1→3, 1→4)-β-d-glucan in both endosperm and aleurone walls (Tonooka et al., 2009), confirming that this is the major gene contributing to (1→3, 1→4)-β-d-glucan content within the grain. Reduction of CSLF6 gene expression in wheat using RNA interference constructs has shown that this gene is also a major contributor to wheat endosperm cell wall (1→3, 1→4)-β-d-glucan (Nemeth et al., 2010).
Hetero-(1→4)-β-d-Mannan
Heteromannan first appears in the developing endosperm walls at 5 DAP and immunogold labeling reaches its maximum intensity in the starchy endosperm cell walls at around 10 DAP. Later in development heteromannan is present in the starchy endosperm but completely absent from aleurone cell walls. Experiments in which a number of CSLA genes from various plant species have been individually expressed heterologously in either plant or insect systems have shown that these genes encode (gluco)mannan synthases (Dhugga et al., 2004; Liepman et al., 2005, 2007; Suzuki et al., 2006; Doblin et al., 2010). However, this activity has not yet been demonstrated for any members of the cereal-specific clade of the CSLA family (van Erp and Walton, 2009). To date, six CSLA genes have been identified in barley (Schreiber et al., 2008). Of these, phylogenetic analyses show that two genes, HvCSLA2 and HvCSLA6, are located within the same clade as the known (gluco)mannan synthases are positioned and hence are predicted to encode enzymes with the same activities (M. Doblin, unpublished data). The four other barley CSLA genes lie within the cereal-specific CSLA clade (van Erp and Walton, 2009) and, given their lower sequence similarity to the experimentally verified (gluco)mannan synthases, may encode enzymes with other polysaccharide synthase activities. Only one CSLA gene, CSLA6, shows significant levels of expression during endosperm development. The appearance and accumulation of heteromannan in the endosperm at 10 DAP coincides with an increase in CSLA6 expression. Given the transcript profiles of the HvCSLA genes as well as the phylogenetic evidence, it appears CSLA6 may be responsible for heteromannan deposition during endosperm development.
Arabino-(1→4)-β-d-Xylan
In our first study we reported arabino-(1→4)-β-d-xylan, based upon LM11 binding, as the last polysaccharide to be deposited in the endosperm at 8 DAP once cellularization was complete (Wilson et al., 2006). The distribution pattern of arabino-(1→4)-β-d-xylan deposition was different to the other polysaccharides in that it was restricted to the crease region until 12 DAP. All other polysaccharides were laid down uniformly across the developing endosperm (Wilson et al., 2006). The observation that arabino-(1→4)-β-d-xylan appears later in development than the other polysaccharides is not supported by monosaccharide analyses that show Xyl and arabinose to be present from 5 DAP, albeit in low amounts (Fig. 8, C and D). In light of the chemical analyses, it seemed most likely that arabino-(1→4)-β-d-xylan was present earlier in endosperm development but in a highly substituted form that was not recognized by the LM11 antibody (McCartney et al., 2005). To test this hypothesis, we applied the enzyme α-l-arabinofuranosidase to thin sections in attempts to remove arabinosyl substituents from the polysaccharide and hence to expose the xylan backbone to the LM11 antibody (Fig. 9). Only those endosperm sections pretreated with the enzyme had labeling along early endosperm cell walls, confirming arabino-(1→4)-β-d-xylan does appear earlier in development but in a highly substituted form.
At 12 DAP, strong labeling was seen in the aleurone and central starchy endosperm but absent in the peripheral starchy endosperm cell walls. Similarly in wheat, arabino-(1→4)-β-d-xylan is reported to appear during early differentiation at 12 DAP and labeling is strongest in the differentiating aleurone layers and central starchy endosperm but scant in the cell walls of the peripheral endosperm (Philippe et al., 2007). The same pattern of labeling was seen using the (1→3, 1→4)-β-d-glucan antibody in wheat. In our study, none of the antibodies we applied to thin sections of endosperm between 12 and 16 DAP labeled the cell walls at the peripheral starchy endosperm. Given this puzzling result in both barley and wheat, we decided to treat endosperm sections at 12 and 14 DAP with the α-l-arabinofuranosidase enzyme prior to labeling with the LM11 antibody. As was the case for early cell walls at 5 DAP, those sections pretreated with the enzyme had significant gold labeling in the cell walls of the peripheral endosperm whereas untreated sections did not (Fig. 9). This result suggests arabino-(1→4)-β-d-xylan in this region is in a highly substituted form that the LM11 antibody does not recognize. Consistent with this, members of the GT43 and GT47 gene families, which have been implicated in arabino-(1→4)-β-d-xylan synthesis in other plant systems (Mitchell et al., 2007; York and O’Neill, 2008; Zeng et al., 2008) were transcribed throughout the developmental window used in this study (Fig. 11E). Transcript levels of the GT43 gene were particularly high in comparison to levels for other genes but levels of mRNA encoding the GT61 gene examined here were low (Fig. 11E). This was somewhat surprising given that GT61 genes are proposed to encode arabinosyl transferases (Mitchell et al., 2007).
CONCLUSION
The availability of increasingly sophisticated antibodies that discriminate between the fine structural variations on polysaccharide backbones enables us to describe the structural variation during assembly or after deposition in a spatial and temporal manner by microscopy that is not possible by bulk chemical analysis techniques. Here we demonstrate the strength of these complementary approaches particularly for the heteroxylans, the most complex class of wall polysaccharides in the grass endosperm. These data are invaluable in informing the interpretation of transcriptomic data thereby identifying putative candidate synthase/glycosyl transferase genes involved in polysaccharide assembly and/or modification.
MATERIALS AND METHODS
Plant Material
Barley (Hordeum vulgare) grain (cv Sloop) from 5 to 38 DAP was collected from glasshouse-grown plants grown in February and September, 2008, as described in Wilson et al. (2006). Samples of developing grain collected for QPCR were prepared as described in Burton et al. (2008).
Immunolabeling for Light and EM
A complete description of the methods and the probes used is provided in Wilson et al. (2006). Grain samples from 10 to 28 DAP were prepared for microscopy and changes to the endosperm were monitored by taking transverse sections of the developing grains. The distribution and relative abundance of polysaccharides in the differentiating grain were detected on sections using monoclonal antibodies raised against specific polysaccharide epitopes including (1→3)-β-d-glucan (Meikle et al., 1991), xyloglucan LM15 (Marcus et al., 2008), (1→3,1→4)-β-d-glucan (Meikle et al., 1994), hetero-(1→4)-β-d-mannan (Pettolino et al., 2001), and arabino-(1-4)-β-d-xylan LM11 (McCartney et al., 2005). Sections for light microscopy (1 μm) were either stained in toluidine blue to highlight tissue architecture or processed for immunogold labeling. The unstained sections were dried onto glass microscope slides and incubated in diluted primary antibodies followed by either anti-mouse or anti-rat secondary antibodies conjugated to ultrasmall gold (average particle size 0.8 nm). These gold particles are made visible under the light microscope using a silver enhancement kit (Aurion) that amplifies the labeling by coating the gold particles in silver to produce an increasingly intense black stain. Images of labeled samples were captured on a digital camera mounted to a DM2500 Leica compound light microscope. Images were inverted in Adobe Photoshop so antibody labeling appears white against a black background. No control sections have been included as all were black images.
Sections for EM (80 nm) were collected on 200 mesh formvar-coated gold grids and incubated in diluted primary antibodies followed by either anti-mouse or -rat secondary antibodies conjugated to 18-nm gold particles. Some grids were preincubated for 30 min in a 1 mg/mL solution of α-l-arabinofuranosidase (Megazyme), washed, and antibodies applied. Grids were poststained in an aqueous solution of 2% (w/v) uranyl acetate, washed, dried, and viewed on a Philips BioTwin transmission electron microscope and images captured on a Gatan multiscan digital camera (Wilson et al., 2006).
RNA Isolation, cDNA Synthesis, and QPCR
Total RNA was extracted from endosperm tissue homogenates using a phenol-guanidine isothiocyanate reagent (Trizol, Invitrogen) following the recommended protocol with the following exception. After the initial resuspension of the tissue in Trizol, 200 μL of plant RNA isolation aid (Ambion) was added. The sample was centrifuged at 13,200g for 10 min to pellet insoluble material and starch. The supernatant was removed to a clean tube and the standard extraction protocol continued. cDNA synthesis was carried out and used for QPCR as described in Burton et al. (2008), using identical primers for control genes as described therein. QPCR primers for the various cellulose synthase-like genes examined are supplied in Table I. Primers for the following genes are not included in the table because they have been published previously: HvGSL (Schober et al., 2009), HvCESAs (Burton et al., 2004), HvCSLFs (Burton et al., 2008), and HvCSLCs (Dwivany et al., 2009).
Table I. Gene-specific QPCR primers, with PCR product sizes in bp and optimal acquisition temperatures for the genes analyzed.
| Name | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | PCR Product | Acquisition Temperature | Accession No. |
|---|---|---|---|---|---|
| bp | °C | ||||
| HvCSLA1 | CGTTTCTTTTCTTCTGTGCGTCAT | GAAACTGTATAACAGCACTTGGTA | 175 | 76 | AK359952.1 |
| HvCSLA2 | GCGTTCGGGAAGGACAAT | GAGAAGACTGCGTGATCTTTACT | 215 | 80 | AK371878.1 |
| HvCSLA3 | GTATGATTACGTGCTTGGAA | TATGGATTGCCGTTGTCAGAT | 199 | 79 | AK375227.1 |
| HvCSLA4 | TTCCACTCCCAGTGCCGAATAA | GCAGCAAAGAATGGTTGGATCA | 171 | 78 | AK367304.1 |
| HvCSLA5 | GCTCGTGCAGATCCCCATGT | AAGGAAGCTAGATTCAGGTTGGAAAT | 319 | 83 | AK372922.1 |
| HvCSLA6 | CTGCCGCTGCTTCTGCTACC | GCCGAATCCGATGACGAATG | 236 | 84 | AK376872.1 |
| HvGT61 | CCAACCAGCCCAGGTAAAC | CTTTCCAGCAGCAGTAGTAGTGA | 126 | 78 | AK253054.1 |
| HvGT43 | GCTGGTCTGATCTGTGTGAG | GATGTATCGTCTGTGACTTTCT | 124 | 79 | AJ969053.1 |
| HvGT47 | TAGCATCTCTCGCCTCGC | TTGACTTATGGTCAGCACTGTG | 125 | 77 | AK366860.1 |
Polysaccharide Quantitation
Mature grain samples were pearled to remove external maternal tissue until 30% of the grain was removed. For quantification of levels of (1→3,1→4)-β-d-glucan and monosaccharides, endosperm material was harvested, freeze-dried, and ground to a powder. (1→3, 1→4)-β-d-β-Glucan was measured using a small-scale version of the Megazyme mixed-linkage β-glucan assay (McCleary and Codd, 1991). Initially, 15 mg of sample was washed twice with ethanol (75% v/v, 1 mL) for 10 min at 75°C, centrifuged at 10,000g for 10 min, and the supernatant discarded. This was followed by a third wash at room temperature. Samples were dried at 37°C before analysis was undertaken according to the manufacturer’s instruction using appropriately decreased volumes.
The quantitation of the monosaccharides Xyl, arabinose, and Man was performed after acid hydrolysis by separating the monosaccharides by reversed phase HPLC using a Hypersil C18 5 μm, 2.1 × 250 mm column as described in Burton et al. (2011). Twenty-five milligrams of ground, freeze-dried endosperm was hydrolyzed with sulfuric acid (1 m, 1 mL) for 3 h at 100°C, cooled, and centrifuged at 20,000g for 5 min. The samples were diluted 20-fold before derivatization with 1-phenyl-3-methyl-5-pyrazolone (Burton et al., 2011). Talose was included with each sample as an internal standard.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers HvCSLA1, AK359952.1; HvCSLA2, AK371878.1; HvCSLA3, AK375227.1; HvCSLA4, AK367304.1; HvCSLA5, AK372922.1; HvCSLA6, AK376872.1; HvGT61, AK253054.1; HvGT43, AJ969053.1; and HvGT47, AK366860.1.
Acknowledgments
We thank Jelle Lahnstein for general analytical help and Ms. Cherie Walsh for her careful preparation of EM samples.
Glossary
- DAP
d after pollination
- EM
electron microscopy
- QPCR
quantitative real-time PCR
- CBHII
cellobiohydrolase II
- CBMs
carbohydrate-binding modules
References
- Amano Y, Shiroishi M, Nisizawa K, Hoshino E, Kanda T. (1996) Fine substrate specificities of four exo-type cellulases produced by Aspergillus niger, Trichoderma reesei, and Irpex lacteus on (1→3), (1→4)-β-D-glucans and xyloglucan. J Biochem 120: 1123–1129 [DOI] [PubMed] [Google Scholar]
- Bacic A, Stone BA. (1981a) Isolation and ultrastructure of aleurone cell walls from wheat Triticum aestivum cultivar Insignia and barley Hordeum vulgare cultivar Clipper. Aust J Plant Physiol 8: 453–474 [Google Scholar]
- Bacic A, Stone BA. (1981b) Chemistry and organization of aleurone cell wall components from wheat Triticum aestivum cultivar Insignia and barley Hordeum vulgare cultivar Clipper. Aust J Plant Physiol 8: 475–495 [Google Scholar]
- Belobrajdic DP, Lam YY, Mano M, Wittert GA, Bird AR. (2011) Cereal based diets modulate some markers of oxidative stress and inflammation in lean and obese Zucker rats. Nutr Metab (Lond) 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake AW, McCartney L, Flint JE, Bolam DN, Boraston AB, Gilbert HJ, Knox JP. (2006) Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. J Biol Chem 281: 29321–29329 [DOI] [PubMed] [Google Scholar]
- Brennan CS, Cleary LJ. (2005) The potential use of cereal (1,3;1,4)-b-D-glucans as functional food ingredients. J Cereal Sci 42: 1–13 [Google Scholar]
- Brennan M, Harris PJ. (2011) Distribution of fucosylated xyloglucans among the walls of different cell types in monocotyledons determined by immunofluorescence microscopy. Molecular Plant 4: 144–156 [DOI] [PubMed] [Google Scholar]
- Brown DM, Zeef LAH, Ellis J, Goodacre R, Turner SR. (2005) Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17: 2281–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Olsen O-A. (1994) Endosperm development in barley: microtubule involvement in the morphogenetic pathway. Plant Cell 6: 1241–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Stone BA, Olsen OA. (1997) Cell wall (1→3)- and (1→3, 1→4)-beta-glucans during early grain development in rice (Oryza sativa L.). Planta 202: 414–426 [DOI] [PubMed] [Google Scholar]
- Brownfield L, Doblin M, Fincher G, Bacic A. (2009) Biochemical and molecular properties of biosynthetic enzymes for (1,3)-β-glucans in embryophytes, chlorophytes and rhodophytes. In A Bacic, G Fincher, B Stone, eds, Chemistry, Biochemistry and Biology of (1,3)-Beta Glucans and Related Polysaccharides, Ed 2. Academic Press, Elsevier Inc., San Diego, pp 283–326.
- Burton RA, Collins HM, Kibble NAJ, Smith JA, Shirley NJ, Jobling SA, Henderson M, Singh RR, Pettolino F, Wilson SM, et al. (2011) Over-expression of specific HvCslF cellulose synthase-like genes in transgenic barley increases the levels of cell wall (1,3;1,4)-β-d-glucans and alters their fine structure. Plant Biotechnol J 9: 117–135 [DOI] [PubMed] [Google Scholar]
- Burton RA, Jobling SA, Harvey AJ, Shirley NJ, Mather DE, Bacic A, Fincher GB. (2008) The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley. Plant Physiol 146: 1821–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Shirley NJ, King BJ, Harvey AJ, Fincher GB. (2004) The CesA gene family of barley: quantitative analysis of transcripts reveals two groups of co-expressed genes. Plant Physiol 134: 224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, Stone BA, Newbigin EJ, Bacic A, Fincher GB. (2006) Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-D-glucans. Science 311: 1940–1942 [DOI] [PubMed] [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M, et al. (2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20: 1519–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocuron J-C, Lerouxel O, Drakakaki G, Alonso AP, Liepman AH, Keegstra K, Raikhel N, Wilkerson CG. (2007) A gene from the cellulose synthase-like C family encodes a β-1,4 glucan synthase. Proc Natl Acad Sci USA 104: 8550–8555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles G. (1979) Relationship of mixed link beta-glucan accumulation to accumulation of free sugars and other glucans in the developing barley endosperm. Carlsberg Res Commun 44: 439–453 [Google Scholar]
- Dhugga KS, Barreiro R, Whitten B, Stecca K, Hazebroek J, Randhawa GS, Dolan M, Kinney AJ, Tomes D, Nichols S, et al. (2004) Guar seed beta-mannan synthase is a member of the cellulose synthase super gene family. Science 303: 363–366 [DOI] [PubMed] [Google Scholar]
- Doblin MS, De Melis L, Newbigin E, Bacic A, Read SM. (2001) Pollen tubes of Nicotiana alata express two genes from different β-glucan synthase families. Plant Physiol 125: 2040–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblin MS, Pettolino F, Bacic A. (2010) Plant cell walls: the skeleton of the plant world. Funct Plant Biol 37: 357–381 [Google Scholar]
- Doblin MS, Pettolino FA, Wilson SM, Campbell R, Burton RA, Fincher GB, Newbigin E, Bacic A. (2009) A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-D-glucan synthesis in transgenic Arabidopsis. Proc Natl Acad Sci USA 106: 5996–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivany FM, Yulia D, Burton RA, Shirley NJ, Wilson SM, Fincher GB, Bacic A, Newbigin E, Doblin MS. (2009) The CELLULOSE-SYNTHASE LIKE C (CSLC) family of barley includes members that are integral membrane proteins targeted to the plasma membrane. Mol Plant 2: 1025–1039 [DOI] [PubMed] [Google Scholar]
- Fincher GB. (1975) Morphology and chemical composition of barley endosperm cell walls. J Inst Brew 81: 116–122 [Google Scholar]
- Fincher GB. (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol 40: 305–346 [Google Scholar]
- Fincher GB. (2009) Revolutionary times in our understanding of cell wall biosynthesis and remodeling in the grasses. Plant Physiol 149: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GB, Stone BA. (2004) Chemistry of nonstarch polysaccharides. In C Wrigley, H Corke, CE Walker, eds, Encyclopedia of Grain Science. Elsevier, Oxford, pp 206-223.
- Fulcher RG, Setterfield G, McCully ME, Wood PJ. (1977) Observations on the aleurone layer. II. Fluorescence microscopy of the aleurone-sub-aleurone junction with emphasis on possible β-1,3-glucan deposits in barley. Aust J Plant Physiol 4: 917–928 [Google Scholar]
- Gibeaut DM, Pauly M, Bacic A, Fincher GB. (2005) Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta 221: 729–738 [DOI] [PubMed] [Google Scholar]
- Henriksson K, Teleman A, Suortti T, Reinikainen T, Jaskari J, Teleman O, Poutanen K. (1995) Hydrolysis of barley (1→3), (1→4)-[beta]–glucan by a cellobiohydrolase II preparation from Trichoderma reesei. Carbohydr Polym 26: 109–119 [Google Scholar]
- Hervé C, Rogowski A, Blake AW, Marcus SE, Gilbert HJ, Knox JP. (2010) Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc Natl Acad Sci USA 107: 15293–15298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Ito S, Iki K, Matsuda K. (1982) Xyloglucan and β-D-glucan in cell walls of rice seedlings. Plant Cell Physiol 23: 351–364 [Google Scholar]
- Kato Y, Nevins DJ. (1984) Enzymic dissociation of zea shoot cell wall polysaccharides: I. Preliminary characterization of the water-insoluble fraction of zea shoot cell walls. Plant Physiol 75: 740–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Nevins DJ. (1991) Enzymic dissociation of zea shoot cell-wall polysaccharides. V. Dissociation of xyloglucan by urea. Plant Cell Physiol 32: 713–720 [Google Scholar]
- Liepman AH, Nairn CJ, Willats WGT, Sørensen I, Roberts AW, Keegstra K. (2007) Functional genomic analysis supports conservation of function among cellulose synthase-like a gene family members and suggests diverse roles of mannans in plants. Plant Physiol 143: 1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Wilkerson CG, Keegstra K. (2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA 102: 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SE, Verhertbruggen Y, Hervé C, Ordaz-Ortiz JJ, Farkas V, Pedersen HL, Willats WG, Knox JP. (2008) Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney L, Blake AW, Flint J, Bolam DN, Boraston AB, Gilbert HJ, Knox JP. (2006) Differential recognition of plant cell walls by microbial xylan-specific carbohydrate-binding modules. Proc Natl Acad Sci USA 103: 4765–4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney L, Marcus SE, Knox JP. (2005) Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J Histochem Cytochem 53: 543–546 [DOI] [PubMed] [Google Scholar]
- McCleary BV, Codd R. (1991) Measurement of (1→3),(1→4)-β-D-glucan in barley and oats: a streamlined enzymic procedure. J Sci Food Agric 55: 303–312 [Google Scholar]
- Meikle PJ, Bonig I, Hoogenraad NJ, Clarke AE, Stone BA. (1991) The location of 1-3-b-glucans in the walls of pollen tubes of Nicotiana alata using a (13)-b-glucan-specific monoclonal antibody. Planta 185: 1–8 [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hoogenraad NJ, Bonig I, Clarke AE, Stone BA. (1994) A (1—>3,1—>4)-β-glucan-specific monoclonal antibody and its use in the quantitation and immunocytochemical location of (1—>3,1—>4)-β-glucans. Plant J 5: 1–9 [DOI] [PubMed] [Google Scholar]
- Mitchell RAC, Dupree P, Shewry PR. (2007) A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol 144: 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth C, Freeman J, Jones HD, Sparks C, Pellny TK, Wilkinson MD, Dunwell J, Andersson AAM, Åman P, Guillon F, et al. (2010) Down-regulation of the CSLF6 gene results in decreased (1,3;1,4)-β-D-glucan in endosperm of wheat. Plant Physiol 152: 1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OA. (2004) Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell (Suppl) 16: S214–S227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui M, Staehelin LA. (2000a) Syncytial-type cell plates: a novel kind of cell plate involved in endosperm cellularization of Arabidopsis. Plant Cell 12: 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui M, Staehelin LA. (2000b) Cytokinesis in flowering plants: more than one way to divide a cell. Curr Opin Plant Biol 3: 493–502 [DOI] [PubMed] [Google Scholar]
- Otegui MS, Mastronarde DN, Kang BH, Bednarek SY, Staehelin LA. (2001) Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell 13: 2033–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear JR, Kawagoe Y, Schreckengost WE, Delmer DP, Stalker DM. (1996) Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. Proc Natl Acad Sci USA 93: 12637–12642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Wei H, Milne J, Page GP, Somerville CR. (2005) Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proc Natl Acad Sci USA 102: 8633–8638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettolino FA, Hoogenraad NJ, Ferguson C, Bacic A, Johnson E, Stone BA. (2001) A (1—>4)-beta-mannan-specific monoclonal antibody and its use in the immunocytochemical location of galactomannans. Planta 214: 235–242 [DOI] [PubMed] [Google Scholar]
- Philippe S, Saulnier L, Guillon F. (2006) Arabinoxylan and (1—>3),(1—>4)-β-glucan deposition in cell walls during wheat endosperm development. Planta 224: 449–461 [DOI] [PubMed] [Google Scholar]
- Philippe S, Tranquet O, Utille J-P, Saulnier L, Guillon F. (2007) Investigation of ferulate deposition in endosperm cell walls of mature and developing wheat grains by using a polyclonal antibody. Planta 225: 1287–1299 [DOI] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH, Jr, Staehelin LA. (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P. (2010) Hemicelluloses. Annu Rev Plant Biol 61: 263–289 [DOI] [PubMed] [Google Scholar]
- Schober MS, Burton RA, Shirley NJ, Jacobs AK, Fincher GB. (2009) Analysis of the (1,3)-β-D-glucan synthase gene family of barley. Phytochemistry 70: 713–720 [DOI] [PubMed] [Google Scholar]
- Schreiber AW, Shirley NJ, Burton RA, Fincher GB. (2008) Combining transcriptional datasets using the generalized singular value decomposition. BMC Bioinformatics 9: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Li L, Sun Y-H, Chiang VL. (2006) The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiol 142: 1233–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonooka T, Aoki E, Yoshioka T, Taketa S. (2009) A novel mutant gene for (1-3, 1-4)-β-D-glucanless grain on barley (Hordeum vulgare L.) chromosome 7H. Breed Sci 59: 47–54 [Google Scholar]
- Topping D. (2007) Cereal complex carbohydrates and their contribution to human health. J Cereal Sci 46: 220–229 [Google Scholar]
- van Erp H, Walton JD. (2009) Regulation of the cellulose synthase-like gene family by light in the maize mesocotyl. Planta 229: 885–897 [DOI] [PubMed] [Google Scholar]
- Wilson SM, Burton RA, Doblin MS, Stone BA, Newbigin EJ, Fincher GB, Bacic A. (2006) Temporal and spatial appearance of wall polysaccharides during cellularization of barley (Hordeum vulgare) endosperm. Planta 224: 655–667 [DOI] [PubMed] [Google Scholar]
- Wood PJ. (1980) Specificity in the interaction of direct dyes with polysaccharides. Carbohydr Res 85: 271–287 [Google Scholar]
- Wood PJ. (2007) Cereal [beta]-glucans in diet and health. J Cereal Sci 46: 230–238 [Google Scholar]
- Wood PJ, Fulcher RG. (1978) Interaction of some dyes with cereal β-glucans. Cereal Chemistry 55: 952–966 [Google Scholar]
- York WS, O’Neill MA. (2008) Biochemical control of xylan biosynthesis—which end is up? Curr Opin Plant Biol 11: 258–265 [DOI] [PubMed] [Google Scholar]
- Zeng W, Chatterjee M, Faik A. (2008) UDP-xylose-stimulated glucuronyltransferase activity in wheat microsomal membranes: characterization and role in glucurono(arabino)xylan biosynthesis. Plant Physiol 147: 78–91 [DOI] [PMC free article] [PubMed] [Google Scholar]