Abstract
Pharmacological treatments for serious mental illness (SMI) can cause weight gain and adverse metabolic effects. Many second generation antipsychotics and mood stabilizers appear to be particularly problematic in this regard. Several studies have investigated interventions for antipsychotic-induced, or less commonly mood stabilizer –induced, weight gain. Both lifestyle and pharmacological interventions have demonstrated effectiveness. We systematically review randomized controlled trials of pharmacological interventions for weight gain related to these medications. We conducted a meta-analysis of clinical trials for the most studied agents to estimate mean weight loss: metformin (2.93 kg, 95% C.I. 0.97–4.89, p=0.003), H2 antagonists (1.78 kg (95% C.I. −0.50–4.06, p=0.13), topiramate (3.95 kg 95% C.I. 1.77–6.12, p=0.0004), and norepinephrine reuptake inhibitors (1.30 kg (95% C.I. −0.06–2.66, p=0.06). Among the studied options for antipsychotic-related weight gain, metformin has the strongest evidence base and may improve vascular risk factors beyond obesity. The use of topiramate is also supported by the literature and may improve psychotic symptoms in those refractory to treatment. A marginal benefit is seen with norepinephrine reuptake inhibitors, and any vascular benefits from such weight loss may be counteracted by increases in blood pressure or heart rate. Pharmacological therapies may offer benefits as a means of supplementing the effects of lifestyle changes for weight loss. However, the existing evidence provides little evidence of specificity for pharmacological therapies to antipsychotic-induced weight gain and has not studied any connection between benefits and reduced incidence of diabetes mellitus or any vascular outcomes.
Keywords: Antipsychotic agents, Schizophrenia, Bipolar Disorder, Dyslipidemias, Major Depression, Meta-analysis, Obesity, Psychotic Disorders, Vascular Diseases, Weight Loss
INTRODUCTION
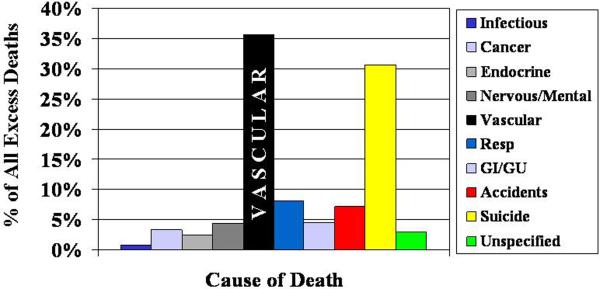
Individuals with serious mental illness (SMI) are at a greater risk of dying early as a result of vascular disease. In a meta-analysis of 37 studies in schizophrenia, Saha et al. reported the mean standardized mortality ratio for cardiovascular diseases in schizophrenia as 2.0, a ratio that has increased since the introduction of second generation antipsychotics [1]. Similar elevations in cardiovascular mortality have been reported for bipolar disorder [2–4]. Cardiovascular diseases represent the leading cause of death in much of the industrialized world [5], and account for much of the excess death seen in people with bipolar disorder and schizophrenia as illustrated in Figure 1 [3, 6]. The excess cardiovascular disease seen in SMI represents a growing disparity of considerable public health relevance.
Figure 1. Causes of Excess Death in Serious Mental Illness.
Vascular disease is the leading cause of excess mortality in bipolar disorder and schizophrenia. Data for figure aggregated from similarly designed studies in Sweden by Osby et al. Archives of General Psychiatry 2001 and Osby et al. Schizophrenia Research 2000.
Pharmacological treatments for SMI may adversely influence the risk factors for vascular disease. For example, lithium has been associated with weight gain [7] and valproic acid derivatives with weight gain and insulin resistance [8]. Valproate results in considerably more weight gain than lithium (1.1 kg vs. 0.2 kg in 12 weeks) [9] and perhaps similar weight gain to the second generation antipsychotics [10, 11]. Several of the second generation antipsychotics have been increasingly associated with significant metabolic complications including weight gain / obesity [12–15], dyslipidemia [13, 16–19], and insulin resistance/diabetes mellitus [13, 20–26]. First generation antipsychotics may also adversely affect cardiovascular risk [27].
Outside of large pharmacoepidemiological studies or meta-analysis of clinical trials in a high risk population, it is hardly feasible to recruit a sample large enough to detect differences in vascular events, due to the low base rate of events and interval of observation required. Because of the ease of measurement, sensitivity to change, and well-established association with atherosclerosis; studies of intervention to mitigate the adverse metabolic effects of antipsychotics have largely focused on body weight. Thus, we reviewed the literature on pharmacological interventions for the management of antipsychotic or mood stabilizer-induced weight gain.
A variety of non-pharmacological interventions have demonstrated positive results including but not limited to those based on behavioral or cognitive-behavioral therapy [28–30] or education [31]. Therefore, non-pharmacological interventions are recommended for all at-risk individuals [29]. Nonetheless, pharmacological therapy should also be considered since many patients with SMI may have difficulty implementing non-pharmacological interventions, and because combining the two may offer additive benefits [32].
WEIGHT GAIN WITH ANTIPSYCHOTICS
Antipsychotics vary with regard to their propensity to induce weight gain [33]. Clozapine and olanzapine have been associated with the greatest weight gain, but significant weight gain has also been reported with quetiapine and risperidone. On the other hand, molindone, ziprasidone, fluphenazine, haloperidol, pimozide, and loxapine appear to result in the least weight gain, at least in adults [34, 35]. Aripiprazole is also considered to have less of an effect on weight [36]. The newest second generation antipsychotics iloperidone, asenapine, and lurasidone are also purported to cause less weight gain [37] but comparative data to other second generation antipsychotics are lacking. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) confirm these findings with the greatest weight gain occurring with olanzapine, followed by quetiapine and risperidone then perphenazine and ziprasidone [38]. Assessments regarding absolute magnitude of expected weight gain are difficult as many clinical trials included participants who had been on various antipsychotics prior to enrollment. For example, in CATIE, 72% of the participants were on antipsychotic medications at baseline [38]. In the Comparison of Atypicals for First Episode (CAFÉ) study, one year of treatment with olanzapine, risperidone, or quetiapine was associated with gains in weight of 11.0, 6.4, and 5.5 kg, respectively, in those with no more than 4 months exposure to antipsychotics [39]. Across groups, this corresponded to an increase in body mass index (BMI) of 2.4 for women and 3.1 for men [39]. In a European trial of agents for first episode schizophrenia or schizophreniform disorder, one year of treatment with olanzapine, quetiapine, amisulpride, haloperidol, or ziprasidone were associated with estimated weight gains at 12 months of 13.9 kg, 10.5 kg, 9.7 kg, 7.3 kg, and 4.8 kg, respectively [40]. In the non-randomized Second-Generation Antipsychotic Treatment Indications, Effectiveness and Tolerability in Youth (SATIETY) cohort study, a median of 10.8 weeks of treatment with olanzapine, quetiapine, risperidone, and aripiprazole was associated with weight gains of 8.5 kg, 6.1 kg, 5.3 kg, and 4.4 kg, respectively, in antipsychotic-naïve children [35]. On the other hand, in the double-blind Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study, where 68% of the participants had taken antipsychotics prior to enrollment, weight increased by 6.1kg on olanzapine, 3.6kg on risperidone, and 0.3 kg on molindone [41].
CLINICAL RELEVANCE OF WEIGHT GAIN ON RISK OF VASCULAR DISEASE
The impact of weight or weight change on vascular mortality is not straightforward. Some studies have reported that higher weight appears to be associated with vascular mortality in the obesity range, defined as a BMI ≥ 30 [42]. However, two recent large prospective studies have found a 31% higher overall mortality [43] and a 23% higher cardiovascular events [44] for subjects with an increase of ~5 kg/m2 greater BMI, starting from a BMI of 25. Most, though not all, of the excess cardiovascular risk from obesity can be accounted for by worsening of conventional risk factors such as cholesterol, blood pressure, and diabetes [44]. With regard to weight change, a 33 to 53% increase in mortality, independent of other risk factors, has been found prospectively following large weight gains (changes 3 to 5 units of BMI over two years) in those with a BMI of greater than or equal to 35 [45]. While several conditions accounted for this elevated risk, cardiovascular disease is the key cause of obesity-related mortality [43].
Beyond vascular mortality, weight gain may have broad reaching effects on quality of life. Increases in body mass index negatively impact physical and mental domains of health-related quality of life [46]. In new onset bipolar disorder, clinical significant weight gain has also been associated with functional impairments [47]. Despite these negative effects on function and quality of life, the clinician must consider vascular risk with any interventions for weight gain, particularly since sympathomimetic weight loss agents have a dubious history in that their effectiveness for weight loss has been counterbalanced with greater vascular mortality [48].
LITERATURE SEARCH
We conducted a computerized literature search of PubMed for pharmacological interventions for antipsychotic or mood stabilizer-related weight gain using the following keywords on our primary search: (randomized controlled trial) AND (antipsychotics) AND (weight gain). Follow-up searches replaced the search term (randomized controlled trial) with a variety of weight reduction medications, (antipsychotics) with the names of second generation antipsychotics and mood stabilizers, and (weight gain) with (weight) and (obesity). No language restrictions were applied. Reference lists from identified and related studies were used to identify additional studies. We restricted our review to pharmacological interventions investigated in randomized, double-blind placebo-controlled trials of any duration published between 1/1/1990 and 6/30/2011. Results of unblinded, uncontrolled, or non-randomized studies, such as open label trials, are not discussed. A total of 32 trials of pharmacological interventions for treatment-related weight gain were identified, 30 of which focused on antipsychotic-related weight gain while the other two involved divalproex or lithium. Two trials in healthy individuals, without serious mental illness, are also discussed. Lamotrigine, which has been associated with stable weight or weight loss relative to placebo [7, 49], and carbamazepine, which has been associated with weight loss [50], were not considered for this review. Our review and meta-analysis is distinct from that of Maayan et al. 2010 [51] in our exclusion of open label trials, interest in weight gain with antipsychotics or mood stabilizers, inclusion of 6 additional trials [52–57], and focus on clinical relevance of findings in the context of risk of vascular disease.
ANALYTIC METHODS
For any treatment or class of treatment, in which three or more studies for antipsychotic-related weight gain meeting the aforementioned criteria were present, we conducted a meta-analysis using Review Manager 5.1 [58]. Trials were heterogeneous with regard to methodology, and given the paucity of studies with similar methods, we included studies with incident or prevalent users of psychotropic agents as well as studies with or without a concomitant lifestyle intervention. For this analysis, weight change in kilograms was assessed as the outcome of interest. This weight change could represent weight loss in prevalent users or reduced weight gain in incident users of a medication. Data were abstracted for mean and standard deviation of change in weight in kilograms. If the requisite data was not available or could not be calculated from data presented in published trials, corresponding authors were contacted for additional information. Individual studies were modeled as a random effect and weighted by inverse variance. Heterogeneity was assessed using I2 and sensitivity analyses pursued potential methodological reasons for excess heterogeneity where applicable and able to be addressed. Random effects were selected given the heterogeneity in observed results and because samples differed from each other in a manner that could impact outcome. When multiple doses of a single agent were used in a trial, these were modeled as separate studies, again utilizing data from the placebo comparison group. Given the limited number of studies for some agents, medications from similar classes were aggregated in the meta-analysis assuming similar mechanisms of action.
PHARMACOLOGICAL INTERVENTIONS FOR WEIGHT GAIN WITH ANTIPSYCHOTICS AND MOOD STABILIZERS
Metformin
Metformin is the most studied agent to combat antipsychotic-associated weight gain. Weight loss was demonstrated in most studies in addition to lifestyle intervention. Table 1 contrasts the selected metformin studies, including data on whether studies included a lifestyle intervention for all groups or studied metformin with incident (to prevent weight gain) or prevalent (to reverse weight gain) users of antipsychotics. In a 2×2 factorial study by Wu and colleagues including 128 individuals with schizophrenia who had gained more than 10% of their body weight on an antipsychotic, metformin yielded significantly greater weight loss (−3.2 kg) relative placebo (+3.1 kg) or a lifestyle intervention (−1.4 kg), consisting of eduction with a prescribed diet and exercise [32]. The combination of metformin with the lifestyle intervention (−4.7 kg) was superior to either intervention alone [32]. A RCT by Wu et al. demonstrated that metformin significantly attenuated the weight gain associated with incident use of olanzapine (1.9 vs. 6.9 kg) in those with new-onset psychosis [59]. However, another study involving incident users of olanzapine failed to show any significant differences in weight gain between metformin and placebo (5.5 kg versus 6.2 kg gained) [60]. In a study of prevalent (as opposed to incident or new) users of olanzapine that did not set a requirement for prior weight gain, metformin resulted in weight loss (−1.4 kg) although this did not significantly differ from placebo (−0.2 kg) [61]. A similarly designed 14 week study of prevalent users of clozapine [62], which also did not set an inclusion threshold for prior weight gain, found a significant weight loss advantage to metformin over placebo on the order of 2 kg.
Table 1. Summary of studies of metformin for antipsychotic-related weight gain.
Key differences in sample characteristics, interventions, and outcomes are highlighted. If lifestyle interventions were recommended in addition to the pharmacological intervention, the nature of the intervention is highlighted. The final column notes the percentage of participants randomized who were analyzed in the primary analysis.
| Study | Diagnosis | Inclusion | Lifestyle | Metformin Dose | Durati on | Mean Age | Delta kg (SD) | % Analyzed |
|---|---|---|---|---|---|---|---|---|
| Arman et al. 2008 | Schizophrenia or schizoaffective disorder | Incident use of risperidone 2–6 mg/day in antipsychotic naive | Not reported | 500 mg/day × 1 wk then 1000 mg/day Placebo |
12 weeks | 11 9 |
0.81 (0.33) 2.2 (2.54) |
32/49 = 65% |
| Baptista et al. 2006 | Schizophrenia or schizoaffective disorder | Olanzapine 10 mg/day added to prior antipsychotics | Balanced diet of 2500–3000 kcal | 850–1700 mg/day Placebo |
14 weeks | 48 | 5.5(3.3) 6.3 (2.3) |
37/40 = 93% |
| Baptista et al. 2007 | Schizophrenia (76) or bipolar disorder (4) | Olanzapine for ≥ 4 months | Education regarding diet and activity | 850–2250 mg/day as tolerated Placebo |
12 weeks | 44 45 |
−1.40(3.2) −0.18(2.8) |
72/80 = 90% |
| Carrizo et al. 2009 | Mainly schizophrenia | Clozapine for ≥ 3 months | Education regarding diet and activity | 500 mg/day × 2 wks then 1000 mg/day Placebo |
14 weeks | 40 38 |
−1.87(2.9)† 0.16(2.9) |
54/61 = 89% |
| Klein et al. 2006 | Various | >10% weight gain in less than 12 months on olanzapine, risperidone, or quetiapine | Nutritional counseling | 500 × 1 wk, 1000 × 1 wk, then 1700 mg/day Placebo |
16 weeks | 13 13 |
−0.13 (2.88)† 4.01 (6.23) |
38/39 = 97% |
| Wu et al. 2008a (JAMA) | First psychotic episode of schizophrenia | >10% weight gain in less than 12 months on clozapine, olanzapine, risperidone, or sulpride | Education, monitored diet, directed exercise embedded in 2×2 factorial design | 750 mg/day after 8 days with lifestyle 750 mg/day after 8 days alone Lifestyle alone Placebo |
12 weeks | 26 27 26 26 |
−4.7 (3.3)*† −3.2 (2.0)*† −1.4 (1.9)*† 3.1 (2.0)* |
127/128 = 99% (for intention to treat) |
| Wu et al. 2008b (Am J Psychiatry) | Hospitalized first psychotic episode of schizophrenia | Incident use of olanzapine 15 mg/day | Hospital diet, 30 minutes exercise per day | 750 mg/day Placebo |
12 weeks | 25 25 |
1.90 (2.72)† 6.87 (4.23) |
37/40 = 93% |
Indicates significant difference from placebo intervention.
Standard deviation calculated from confidence interval reported.
Less consistent results with metformin have been reported from child and adolescent samples. Over 16 weeks, children and adolescents ages 10–17 years who had gained more than 10% of their body weight during 12 months of treatment with selected second generation antipsychotics (risperidone, olanzapine, or quetiapine) lost on average 4.1 kg relative to placebo [63]. On the other hand, a younger sample of children started on risperidone did not reveal significant differences between metformin at 1000 mg/day and placebo at endpoint of 12 weeks though differences were significant at 4 weeks [64]. This latter study is notable for involving the youngest sample and incident users of risperidone.
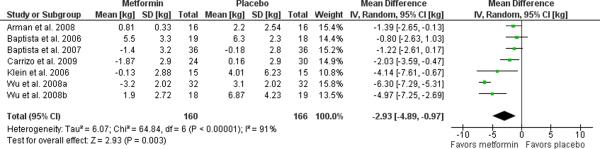
For our meta-analysis, completers data was obtained for all but one study, which presented intention-to-treat data for a study with high retention (92% over 12 weeks) [32]. Randomized clinical trials, 12–16 weeks in duration, have used doses ranging from 750 to 2250 mg/day of metformin and yielded a weight loss of 2.93 kg (95% C.I. 0.97 to 4.89, p=0.003, I2=91%) relative to placebo as illustrated in the Forest plot in Figure 2. Exclusion of the two child and adolescent studies [63, 64] or the two studies assessing incident users [59, 64] did not reduce the heterogeneity.
Figure 2. Meta-analysis of metformin for antipsychotic-related weight gain.
The forest plot summarizes the results of 7 randomized, double-blind, placebo controlled trials of metformin. The overall effect supports the use of metformin for this indication with a mean difference of 2.93 kg (95% C.I. 0.97–4.89 I2=91%) difference in weight loss with metformin over placebo.
Metformin is an antihyperglycemic that inhibits hepatic gluconeogenesis and improves skeletal muscle insulin sensitivity though increases in adenosine monophosphate kinase [66]. Metformin produces substantial improvement in glycemia (−15%), triglycerides (−6%), and LDL-cholesterol (−6%) [67]. Effects on body weight appear to be due to reduction in appetite [68] rather than increases in calorie expenditure [69]. Reduction in appetite may be secondary to taste disturbance and nausea or increases in anorexic glucagon-like peptide-1 [70].
H2 Receptor Antagonists
H2 receptor antagonists have been observed to cause weight loss in some studies. H2 receptor antagonists are hydrophilic but thought to exert their effects peripherally, rather than centrally, perhaps though appetite suppression secondary to increased cholecystokinin [71]. As such, famotidine and nizatidine have been studied for their potential to mitigate antipsychotic-related weight gain. In one small (N=14) study of incident users of olanzapine with new-onset psychosis, famotidine demonstrated no measurable benefit to placebo in preventing weight gain (mean weight gain of 4.8 vs. 4.9 kg over 6 weeks over placebo, p=0.91) [72].
Four RCTS have investigated the potential benefit of nizatidine for antipsychotic-related weight gain. Two smaller 8 week trials yielded promising results [73, 74] while the other two, larger trials failed to separate nizatidine from placebo following 12–16 weeks of treatment [24, 71]. A prior meta-analysis of these four trials found that nizatidine failed to significantly differ from placebo (mean weight loss of 2.07 kg, 95% C.I. of −0.45 to 4.58 kg, I2=95%) [51]. The two positive studies by Atmaca et al. (2003, 2004) investigated nizatidine 300 mg/day (divided bid) versus placebo in individuals with schizophrenia who completed the trials [73, 74]. Assuncao et al. studied 600 mg/day of nizatidine in prevalent users of 5–20 mg/day of olanzapine and did show any significant differences between groups (weight loss of 1.1 kg with nizatidine compared to 0.7 mg on placebo) [71]. In the much larger trial of nizatidine and the only of the nizatidine trials to include only incident users (olanzapine), Cavazzoni et al. randomized 175 (169 included in completers analysis) individuals to placebo, nizatidine 300 mg/day, or nizatidine 600 mg/day and found no significant differences in weight loss between groups at 16 weeks though the high dose group did demonstrate a significant benefit at 3–4 weeks that was not sustained [75].
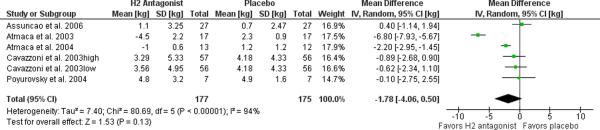
Mixed models estimates were provided for the one study [71] for which variability data also had to be obtained from a secondary source [51]. Completers data was obtained for the remainder. No studies specified any concomitant use of a lifestyle intervention. In our meta-analysis of the five trials of H2 receptor antagonists (famotidine or nizatidine), the experimental interventions yielded a non-significant weight loss of 1.78 kg (95% C.I. −0.50 to 4.06, p=0.13, I2=94%) relative to placebo as illustrated in the Forest plot in Figure 3. Heterogeneity is not improved with removal of the famotidine study. Heterogeneity improves dramatically with removal of the two studies by Atmaca et al. though no compelling methodological difference could be identified to explain this (non-significant weight loss of 0.27 kg (95% C.I. −0.63 to 1.18, p=0.55, I2=0%).
Figure 3. Meta-analysis of H2 antagonists for antipsychotic-related weight gain.
The forest plot summarizes the results of 5 randomized, double-blind, placebo controlled trials of nizatidine (4) or famotidine (1). The overall effect fails to support this indication with a non-significant mean difference of 1.78 kg (95% C.I. −0.50–4.06, I2=94%) between active treatment and placebo. Exclusion of the famotidine study does not substantially change these results.
Topiramate
Topiramate holds some promise as an adjunctive therapy for both weight gain and treatment of psychotic symptoms. Three of the four RCTs have demonstrated positive results on weight. A 10-week study of women on olanzapine demonstrated significantly greater weight loss for those on topiramate 250 mg/day compared with placebo (change in weight of −4.4 kg vs. 1.2 kg) [76]. Those who gained the most weight during their first two months of treatment with olanzapine lost the most weight on topiramate [76]. Ko et al. recruited overweight or obese individuals receiving maintenance treatment with second generation antipsychotics to a 12-week trial of placebo, topiramate 100 mg/day, or topiramate 200 mg/day, demonstrating a dose response with weight loss of −0.3, −1.7, and −5.4 kg, respectively [77]. A study of incident users of olanzapine with new-onset psychosis suggested that topiramate 100 mg/day can avert weight gain (loss of 1.3 kg versus gain of 6.0 kg on placebo) [54]. One smaller trial (total N=16) failed to demonstrate a significant benefit of topiramate, flexibly dosed between 50 and 300 mg/day (mean dose not reported), on BMI though those assigned to topiramate had quantitatively greater reductions in BMI (−0.91 versus +0.21) [55].
One trial assessed topiramate for weight gain with mood stabilizers though not as a primary outcome. A 12-week trial of topiramate (flexible titration, mean dose of 255 mg/day) for those with bipolar I on a stable dose of lithium or valproate (level between 0.5–1.2 mEq/L or 45–100 mg/L respectively) showed greater weight loss with topiramate (−2.5 kg) than placebo (+0.2 kg) in intention-to-treat analysis [56]. Mood ratings did not differ between groups though those on topiramate experienced significantly greater paresthesias (Number needed to harm (NNH) = 5.1) and diarrhea (NNH=11.9) [56].
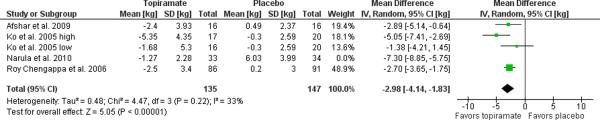
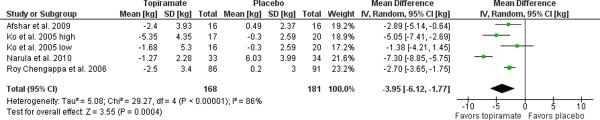
Data for one study [77] was obtained from a secondary published source [51] while another two through personal communication with the corresponding author [54, 55]. Requisite data for one study were not reported and not able to be obtained on request [76]. Completers data was obtained for all four studies. The remaining four trials, yielded a weight loss of 3.95 kg (95% C.I. 1.77 to 6.12, p=0.0004, I2=86%) relative to placebo as illustrated in the Forest plot in Figure 4. Exclusion of the only study to recruit incident, as opposed to prevalent, users [54] substantively improved heterogeneity, resulting in an estimated weight loss of 2.98 kg (95% C.I. 1.83 to 4.14, p=0.00001, I2=33%) relative to placebo.
Figure 4. Meta-analysis of topiramate for antipsychotic-related weight gain.
The forest plot summarizes the results of 4 randomized, double-blind, placebo controlled trials of topiramate. The overall effect supports the use of topiramate for this indication with a mean difference of 3.95 kg (95% C.I. 1.77–6.12, I2=86%) in weight loss with topiramate over placebo. Exclusion of the Narula et al. 2010 study of incident users reduced the heterogeneity with an estimate of the mean difference in weight loss of 2.98 kg (95% C.I. 1.83–4.14, I2=33%).
All four qualifying clinical trials demonstrated significant improvement on relevant clinical measures of psychopathology for those on added topiramate compared with placebo in samples with SMI [54, 55, 76, 77]. Two of these studies utilized the PANSS, and demonstrated varied results in difference in total PANSS change between groups ranging from 1 to 18 points [54, 55].
Topiramate has diverse mechanisms that may facilitate weight loss. Peripherally, these may involve stimulation of lipoprotein lipase as well as inhibition of carbonic anhydrase and subsequently lipogenesis [54]. Topiramate clearly decreases appetite and increases satiety but does not alter energy expenditure [78], perhaps through inhibition of carbonic anhydrase. Beneficial effects for schizophrenia are proposed to be mediated by antagonism of glutamate-induced excitotoxicity at AMPA and kainate receptors [55]. In trials for antipsychotic-related weight gain, topiramate has been associated with paresthesia, psychomotor retardation, drooling, dizziness, headache, and mild cognitive impairment [55, 79].
Norepinephrine reuptake inhibitors
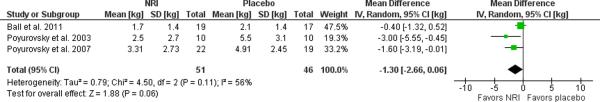
Studies of the norepinephrine reuptake inhibitor reboxetine have been encouraging though one study of atomoxetine was negative. Poyurovsky et al. (2003) studied reboxetine (2 mg bid) in a sample of incident users of olanzapine (10 mg/day) and found that weight gain after six weeks was significantly attenuated by 3 kg compared to placebo [80]. A larger, similarly designed follow-up study by the same group found a significant though lesser (1.6 kg) benefit [81]. Both studies additionally found significantly lower scores on the Hamilton Depression Rating Scale for those treated with reboxetine. A 24 week trial of atomoxetine, however, failed to demonstrate any weight reduction in a sample of prevalent users of olanzapine or clozapine [53]. Mixed models estimates were provided for the atomoxetine study and completers data for the reboxetine studies. A meta-analysis of these three trials of norepinephrine reuptake inhibitors (reboxetine or atomoxetine) yielded a small, marginally significant weight loss 1.30 kg (95% C.I. −0.06 to 2.66, p=0.06, I2=56%) relative to placebo as illustrated in the Forest plot in Figure 5. Sensitivity analyses were deferred given the presence of only three studies.
Figure 5. Meta-analysis of norepinephrine reuptake inhibitors for antipsychotic-related weight gain.
The forest plot summarizes the results of 3 randomized, double-blind, placebo controlled trials of reboxetine (2) or atomoxetine (1). A non-significant effect is observed for this class of agents with a mean difference of 1.30 kg (95% C.I. −0.06–2.66, p=0.06, I2=56%) in weight loss between norepinephrine reuptake inhibitors and placebo.
Norepinephrine reuptake inhibitors are thought to result in weight loss by reducing appetite through action on the hypothalamus [53] and perhaps by increasing energy expenditure [80, 81]. Norepinephrine reuptake inhibitors may increase heart rate and blood pressure, primarily through sympathomimetic effects on the heart [82]. The aforementioned reboxetine studies did not report changes in blood pressure or heart rate [80, 81]. Although actual changes were not reported, no significant differences in blood pressure were reported for the atomoxetine study, which was underpowered to detect clinically significant differences [53]. Increases in heart rate or blood pressure could cancel out any cardiovascular benefits of weight loss with these agents.
Sibutramine
Sibutramine is a norepinephrine and serotonin reuptake inhibitor with a potential for weight loss. Sibutramine stimulates the exocytotic release of dopamine resulting in increased extracellular concentrations of dopamine [83]. Two RCTs have investigated sibutramine for antipsychotic-associated weight gain with contrasting results. In an RCT of prevalent users of olanzapine, sibutramine was associated with greater weight loss at week 12 in a completers analysis (3.8 vs. 0.8 kg, total N completed = 31) [84] though a similarly designed study of prevalent users of clozapine found no significant difference in weight loss at week 12 (1.9 vs. 0.5 kg, total N completed = 18) [85]. In the first study, the total Positive and Negative Syndrome Scale (PANSS) score dropped 5.5 points for the placebo group compared to 1.9 points for the sibutramine group, which was statistically significant only for the negative symptoms sub-scale [84] though a significant difference was not observed in the subsequent study [85]. Adverse effects on blood pressure have also been noted with, a mean increase of 3.5 mm Hg compared to a decrease of 7.4 mm Hg for placebo at 12 weeks [84].
In a small sample of prevalent olanzapine users with schizophrenia, the combination of sibutramine and metformin for 12 weeks was no different than placebo (2.8 vs. 1.4 kg) while increases in the Brief Psychiatric Rating Scores (BPRS) were quantitatively but not significantly higher among those randomized to metformin + sibutramine (increase of 20.7 versus 14.7 points) [86]. Although this difference was not statistically significant, it remains of some clinical concern given that this study may have been underpowered to detect clinically significant differences and that sibutramine may induce psychosis in a biologically plausible manner.
Sibutramine has been withdrawn from the U.S., Canadian, European, and Australian markets [87] after a clinical trial demonstrated an increased risk of cardiovascular outcomes, presumed secondary to sympathomimetic effects on blood pressure and heart rate [48]. Phentermine and diethylpropion, similar agents that share sympathomimetic and cardiovascular properties with sibutramine, are still available. Given the increased cardiovascular risk of sibutramine and concern for potential to worsen psychosis, these agents cannot be recommended for the treatment of antipsychotic-related weight gain.
Amantadine
Two trials of amantadine for weight gain related to olanzapine have demonstrated positive results. In a small 12 week trial (total N=21), Graham et al. found a large benefit (loss of 0.4 kg versus gain of 4.0 kg) to amantadine 300 mg/day over placebo in those who had gained at least 5 pounds on 5–30 mg of olanzapine [88]. A larger 16 week trial (total N analyzed = 123) of lower dose amantadine (100–300 mg/day, mean of 236 mg/day) yielded a smaller effect (loss of 0.2 kg versus gain of 1.3 kg) in those who had gained at least 5% of body weight on 5–20 mg of olanzapine [89]. While both of these studies were significant, a random effects meta-analysis of these two studies did not cross the threshold of significance with an estimated mean difference in weight of −2.27 (95% C.I. −4.77–0.23) between amantadine and placebo [51].
Amantadine exerts its effect through inhibition of the reuptake of dopamine, norepinephrine, and serotonin [89]. It antagonizes N-methyl-D-aspartate (NMDA) receptors [90]. Both actions may increase levels of dopamine. Theoretically, any such increase in extracellular dopamine has the potential to worsen psychosis. The aforementioned trial of Deberdt et al. did not reveal any worsening on the Brief Psychiatric Rating Scale (BPRS) [89]. Similarly, the trial by Graham et al. did not detect differences between amantadine and placebo on the PANSS although it was underpowered to detect clinically meaningful differences and scores were not reported [88].
Aripiprazole
A relatively large trial of aripiprazole (flexible dosing 5–15 mg/day) added on to a stable dose of clozapine lost more weight than those on placebo (loss of 2.5 kg versus loss of 0.4 kg) over 16 weeks [52]. Clozapine doses were similar between groups and held constant during the study. In addition to weight loss, those randomized to aripiprazole had significant reductions in total and low density lipoprotein cholesterol. There were no significant differences in the PANSS score between aripiprazole and placebo [52].
Other
A cadre of other medications has been investigated for their potential to mitigate the effects of antipsychotics on weight gain without convincing benefit. Orlistat failed to show significant results in a study of overweight or obese individuals with serious mental illness taking olanzapine or clozapine although a significant benefit for men was seen on a sub-group analysis [91]. In an open-label follow-up of this same study, this potential sex difference was again seen [92], however, neither analysis specifically tested a sex by treatment interaction. In two studies of olanzapine-associated weight gain, fluoxetine failed to demonstrate any significant benefit over placebo [93, 94]. Phenylpropanolamine and rosiglitazone did not demonstrate any significant benefit over placebo in individuals treated with clozapine and olanzapine, respectively [95, 96]. A study of L-carnitine in 60 individuals with bipolar I or II who had experienced clinically significant weight gain after at least six months of treatment with valproate also failed to show any significant benefit over placebo [57].
In short-term studies of lean, healthy men housed on an inpatient study site with controlled food intake, the glucocorticoid and progesterone receptor antagonist mifepristone reduced weight gain from risperidone or olanzapine (by ~ 2 kg) [97, 98]. These findings suggest dysregulation of the hypothalamic-pituitary-adrenal axis as a potential target, but will need replication in clinical samples.
Specificity
Each of the medications used to treat antipsychotic-related weight gain are similar in that each has demonstrated weight loss in other populations, with the exception of mifepristone. Studies have not demonstrated that any observed weight loss is specific to users of antipsychotic medications. Therefore, although not directly studied, it is possible that agents such as metformin, found to be helpful in antipsychotic-treated individuals, will similarly be useful at attenuating weight gain from mood stabilizers or those not taking any of these agents. Further studies are needed to explore this hypothesis.
Other Metabolic Effects
In the selected trials, potentially beneficial effects beyond weight were noted for several agents. We highlight those from studies with a significant effect of treatment on weight. Metformin was associated with decreased glucose/hemoglobin A1C [32, 60, 62], insulin resistance [32, 59], and triglyceride/high density lipoprotein-cholesterol ratio [62]. Topiramate demonstrated reduced glucose [54], total cholesterol [54], low density-lipoprotein cholesterol [54], and blood pressure [54]. Aripiprazole resulted in significant decreases in total and low density lipoprotein cholesterol [52].
DISCUSSION
Summary of Evidence Base
A variety of agents have been studied for their potential to attenuate the potential of antipsychotics or mood stabilizers to increase weight. Only one of these medications, orlistat, is approved by the United States Food and Drug Administration for the indication of weight loss though did not produce significant results in the one study for antipsychotic-related weight gain [91]. Of all the medication reviewed, metformin has been the most extensively studied. In our meta-analysis of seven studies of metformin for antipsychotic-related weight gain, metformin differed from placebo by a mean of approximately 3 kg (6.6 pounds) in trials ranging from 12–16 weeks. For an individual of average height (170 cm), this would correspond to a reduction in BMI by 1 kg/m2. The clinical significance of such a difference is not clear, though it may be greater for those who are already obese or receive prolonged treatment. Importantly, metformin has substantial benefits on risk factors that are worsened by weight gain, including glycemia, triglycerides and LDL-cholesterol [67] though further evidence in this particular population is needed. Thus, the cardiovascular benefit of metformin could be greater than expected by from changes in weight alone. However, few studies have examined the long-term effects of metformin on weight and no studies have assessed the potential benefits of treatment on vascular outcomes. Although there has been more limited study, available evidence also supports the use of topiramate, which differed from placebo by a mean of 4 kg (8.8 pounds) and may have additional benefits on psychotic symptoms. The impact of norepinephrine reuptake inhibitors on weight gain was small (1.3 kg), only marginally significant, and with potential for adverse cardiovascular effects on heart rate and blood pressure.
Any potential benefits of medications for antipsychotic- or mood stabilizer-related weight gain, of course, must be balanced against potential adverse effects, drug interactions, and other monitoring parameters which may influence the clinician's decision to utilize a specific agent. Metformin requires periodic monitoring of renal function, glucose, and other hematological measures [99]. Gastrointestinal side effects (e.g. diarrhea, flatulence, indigestion) may occur in approximately 10−50% of patients taking metformin. There is also a rare risk of life threatening lactic acidosis. Even though they are approved for weight loss, sympathomimetic agents such as phentermine and diethylpropion cannot be recommended for the treatment of antipsychotic-associated weight gain due to the risk for life threatening cardiac events. Monitoring of serum bicarbonate is recommended during treatment with topiramate [100]. Importantly, neurological side effects such as confusion, dizziness, memory impairment, and concentration difficulties may occur in up to ~1/3 of patients taking this medication and are important considerations in the context of the cognitive impairment often observed in patients with SMI. As previously mentioned, cardiovascular effects of norepinephrine reuptake inhibitors are important considerations, particularly when used at higher doses and in patients with pre-existing hypertension. It is important to note that while many of these medications are safe to use in psychiatric patient populations, it remains essential to consider risk-benefit ratios when considering them in the context of multiple medications or comorbid medical conditions. It is also important not to forget that non-pharmacological strategies are effective and should be instituted early during antipsychotic treatment [101].
Limitations of Research
The existing trials are generally of short duration and typically only analyze data from completers. The short duration of available trials renders it difficult to assess the long-term risk-benefit ratio for use of these agents. The use of completers analysis may yield overly optimistic benefits for these agents on weight loss compared to that expected in a real world practice though it does provide an estimate of the effect in those adherent to and able to tolerate the medications. Studies have focused on weight to a greater degree than other cardiovascular risk factors. Studies have used variable inclusion/exclusion criteria. Some studies were tailored only to those with obesity or who have gained substantial weight (even > 10%) on antipsychotics. The included studies are quite heterogeneous in other methods and this reflects in the heterogeneity seen in the meta-analyses. The trials also disproportionately target individuals taking olanzapine and clozapine. The findings to date may therefore not be broadly generalizable.
Prior research has largely focused on weight gain as an outcome. The relevance of these changes in weight to vascular disease risk is uncertain. Previous epidemiological studies have found a change in BMI of 3–5 kg/m2 in those with high BMIs to be associated with excess mortality [45]. Only three studies reported changes in BMI relative to placebo (2.4 kg/m2 – 2.9 kg/m2) that approached this magnitude [32, 54, 73]. Whether longer trials would yield more significant weight loss remains to be seen. Moreover, no studies in antipsychotic- or mood stabilizer-treated participants have linked metformin use to a vascular outcome or assessments of vascular function, though a randomized trial of metformin in patients with type 2 diabetes mellitus showed fewer cardiovascular events and 27% lower overall mortality, that persisted for 10 years after study completion [102]. It is nonetheless critical to verify whether an improvement in weight and associated cardiovascular risk factors translate into improved vascular function and, ultimately, long-term reduction in cardiovascular events as has been done with patients with diabetes. With prior metformin trials also noting improvements in visceral obesity, insulin resistance, and lipid profile; it is tempting to speculate that this will result in vascular benefits although well-designed longitudinal studies must confirm it.
It is difficult to determine whether the effects of metformin or other pharmacological interventions are specific to antipsychotic treatment or represent more general effects. Metformin has also been more generally prescribed for obesity although interestingly many of these studies involved samples with polycystic ovarian syndrome [103]. It seems plausible that metformin is only effective for weight loss in samples with insulin resistance, whether due to obesity, polycystic ovarian syndrome, or use of antipsychotics. Given the heterogeneity in results of clinical trials previously noted and a plausible mechanistic explanation for specificity, a study designed to determine whether any benefit of metformin treatment is specific to antipsychotics or mood stabilizers, or confined to a particular subgroup, is welcomed.
Conclusions
Available evidence is sufficient to support the select use of metformin or topiramate in at-risk patients, for whom lifestyle interventions alone are not adequate. Broad recommendations for the use of adjunctive pharmacological agents for antipsychotic-related weight gain are premature without data linking use of these agents for this indication to vascular outcomes. Given the limited benefit and potential risk due to sympathomimetic side-effects, we caution against the use of norepinephrine reuptake inhibitors to specifically target psychotropic-induced weight gain, unless another indication for their use is present.
Future Directions
Better understanding of the mechanisms linking antipsychotics to weight gain or other metabolic complications may be useful in the design of novel therapies to target antipsychotic-induced weight gain. Although antipsychotics have been linked with weight gain, the degree to which they contribute to the elevated risk of vascular mortality that has been consistently documented among those with serious mental illness is unclear. Obese individuals with SMI are certainly at risk of vascular disease and untoward vascular outcomes and this risk may be compounded by the metabolic effects of psychotropic medications. Inclusion of broader samples of at risk individuals with SMI could help determine whether effects of any intervention specifically target the adverse effects of antipsychotics and could benefit a broader at-risk group. The clinical relevance of antipsychotic-induced weight gain is primarily, albeit not exclusively, related to the impact of weight and other metabolic sequelae on mortality. The impact of therapeutic interventions on hard vascular outcomes (e.g. myocardial infarction, stroke, or cardiovascular mortality) in more general populations with SMI may not be feasible for experimental study, outside of very select high-risk groups. Alternatively, use of surrogate vascular outcomes such as those obtained through vascular phenotyping methods (e.g. flow-mediated dilation) could facilitate quantitative assessment of the impact of any intervention on the development of vasculopathy or occurrence of vascular events.
ACKNOWLEDGEMENTS
Dr. Fiedorowicz has no potential conflicts of interest. He is supported by the National Institutes of Health (1K23MH083695-01A210), the Nellie Ball Trust Research Fund, and the Institute for Clinical and Translational Science at the University of Iowa (3 UL1 RR024979-03S4).
Dr. Miller serves as a consultant on Data and Safety Monitoring Boards for Schering-Plough, Otsuka Pharmaceuticals, and Glaxo-Smith Kline.
Dr. Bishop is supported by the National Institutes of Health (K08MH083888) and has received an honorarium from Eli Lilly and research support from Ortho-McNeil Jannssen.
Dr. Calarge has no potential conflicts of interest to report. He is currently supported by the National Institutes of Health (K23MH085005-01, R21MH080968-S2, R01MH090072-01A1).
Dr. Ellingrod has no potential conflicts of interest to report. She is supported by the National Institutes of Health (R01MH090072 (Calarge) and UL1 RR024986 (Clauw/Pienta)).
Dr. Haynes has no potential conflicts of interest and is supported by the National Institutes of Health (P01 HL014388).
REFERENCES
- 1.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–31. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 2.Murray DP, Weiner M, Prabhakar M, Fiedorowicz JG. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11(6):475–80. doi: 10.1007/s11920-009-0072-3. [DOI] [PubMed] [Google Scholar]
- 3.Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844–50. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 4.Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23(1):40–47. [PMC free article] [PubMed] [Google Scholar]
- 5.Meetoo D. Chronic diseases: the silent global epidemic. Br J Nurs. 2008;17(21):1320–5. doi: 10.12968/bjon.2008.17.21.31731. [DOI] [PubMed] [Google Scholar]
- 6.Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. Bmj. 2000;321(7259):483–4. doi: 10.1136/bmj.321.7259.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs G, Bowden C, Calabrese JR, Ketter T, Thompson T, White R, et al. Effects of lamotrigine and lithium on body weight during maintenance treatment of bipolar I disorder. Bipolar Disord. 2006;8(2):175–81. doi: 10.1111/j.1399-5618.2006.00308.x. [DOI] [PubMed] [Google Scholar]
- 8.Pylvanen V, Knip M, Pakarinen A, Kotila M, Turkka J, Isojarvi JI. Serum insulin and leptin levels in valproate-associated obesity. Epilepsia. 2002;43(5):514–7. doi: 10.1046/j.1528-1157.2002.31501.x. [DOI] [PubMed] [Google Scholar]
- 9.Bowden CL, Mosolov S, Hranov L, Chen E, Habil H, Kongsakon R, et al. Efficacy of valproate versus lithium in mania or mixed mania: a randomized, open 12-week trial. Int Clin Psychopharmacol. 2010;25(2):60–7. doi: 10.1097/YIC.0b013e328333ac1b. [DOI] [PubMed] [Google Scholar]
- 10.Maina G, Albert U, Salvi V, Mancini M, Bogetto F. Valproate or olanzapine add-on to lithium: an 8-week, randomized, open-label study in Italian patients with a manic relapse. J Affect Disord. 2007;99(1–3):247–51. doi: 10.1016/j.jad.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Pavuluri MN, Henry DB, Findling RL, Parnes S, Carbray JA, Mohammed T, et al. Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder. Bipolar Disord. 2010;12(6):593–605. doi: 10.1111/j.1399-5618.2010.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson GM. Atypical antipsychotics and the burden of disease. Am J Manag Care. 2005;11(8 Suppl):S235–41. [PubMed] [Google Scholar]
- 13.Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: A five-year naturalistic study. Am J Psychiatry. 2000;157(6):975–81. doi: 10.1176/appi.ajp.157.6.975. [DOI] [PubMed] [Google Scholar]
- 14.Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159(2):255–62. doi: 10.1176/appi.ajp.159.2.255. [DOI] [PubMed] [Google Scholar]
- 15.Zipursky RB, Gu H, Green AI, Perkins DO, Tohen MF, McEvoy JP, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537–43. doi: 10.1192/bjp.187.6.537. [DOI] [PubMed] [Google Scholar]
- 16.Huang TL, Chen JF. Serum lipid profiles and schizophrenia: effects of conventional or atypical antipsychotic drugs in Taiwan. Schizophr Res. 2005;80(1):55–9. doi: 10.1016/j.schres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Spivak B, Lamschtein C, Talmon Y, Guy N, Mester R, Feinberg I, et al. The impact of clozapine treatment on serum lipids in chronic schizophrenic patients. Clin Neuropharmacol. 1999;22(2):98–101. doi: 10.1097/00002826-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gaulin BD, Markowitz JS, Caley CF, Nesbitt LA, Dufresne RL. Clozapine-associated elevation in serum triglycerides. Am J Psychiatry. 1999;156(8):1270–2. doi: 10.1176/ajp.156.8.1270. [DOI] [PubMed] [Google Scholar]
- 19.Osser DN, Najarian DM, Dufresne RL. Olanzapine increases weight and serum triglyceride levels. J Clin Psychiatry. 1999;60(11):767–70. doi: 10.4088/jcp.v60n1109. [DOI] [PubMed] [Google Scholar]
- 20.Guo JJ, Keck PE, Jr, Corey-Lisle PK, Li H, Jiang D, Jang R, et al. Risk of diabetes mellitus associated with atypical antipsychotic use among patients with bipolar disorder: A retrospective, population-based, case-control study. J Clin Psychiatry. 2006;67(7):1055–61. doi: 10.4088/jcp.v67n0707. [DOI] [PubMed] [Google Scholar]
- 21.Lambert BL, Chou CH, Chang KY, Tafesse E, Carson W. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf. 2005;14(6):417–25. doi: 10.1002/pds.1092. [DOI] [PubMed] [Google Scholar]
- 22.Ollendorf DA, Joyce AT, Rucker M. Rate of new-onset diabetes among patients treated with atypical or conventional antipsychotic medications for schizophrenia. MedGenMed. 2004;6(1):5. [PMC free article] [PubMed] [Google Scholar]
- 23.Sernyak MJ, Gulanski B, Rosenheck R. Undiagnosed hyperglycemia in patients treated with atypical antipsychotics. J Clin Psychiatry. 2005;66(11):1463–7. doi: 10.4088/jcp.v66n1117. [DOI] [PubMed] [Google Scholar]
- 24.Carlson C, Hornbuckle K, Delisle F, Kryzhanovskaya L, Breier A, Cavazzoni P. Diabetes mellitus and antipsychotic treatment in the United Kingdom. Eur Neuropsychopharmacol. 2005 doi: 10.1016/j.euroneuro.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Henderson DC, Cagliero E, Copeland PM, Borba CP, Evins E, Hayden D, et al. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62(1):19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- 26.Gianfrancesco F, White R, Wang RH, Nasrallah HA. Antipsychotic-induced type 2 diabetes: evidence from a large health plan database. J Clin Psychopharmacol. 2003;23(4):328–35. doi: 10.1097/01.jcp.0000085404.08426.3a. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Iglesias R, Crespo-Facorro B, Amado JA, Garcia-Unzueta MT, Ramirez-Bonilla ML, Gonzalez-Blanch C, et al. A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J Clin Psychiatry. 2007;68(11):1733–40. doi: 10.4088/jcp.v68n1113. [DOI] [PubMed] [Google Scholar]
- 28.Kwon JS, Choi JS, Bahk WM, Yoon Kim C, Hyung Kim C, Chul Shin Y, et al. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: A 12-week randomized controlled clinical trial. J Clin Psychiatry. 2006;67(4):547–53. doi: 10.4088/jcp.v67n0405. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Jimenez M, Gonzalez-Blanch C, Vazquez-Barquero JL, Perez-Iglesias R, Martinez-Garcia O, Perez-Pardal T, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: A randomized controlled trial. J Clin Psychiatry. 2006;67(8):1253–60. doi: 10.4088/jcp.v67n0812. [DOI] [PubMed] [Google Scholar]
- 30.Khazaal Y, Fresard E, Rabia S, Chatton A, Rothen S, Pomini V, et al. Cognitive behavioural therapy for weight gain associated with antipsychotic drugs. Schizophr Res. 2007;91(1–3):169–77. doi: 10.1016/j.schres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Mauri M, Simoncini M, Castrogiovanni S, Iovieno N, Cecconi D, Dell'Agnello G, et al. A psychoeducational program for weight loss in patients who have experienced weight gain during antipsychotic treatment with olanzapine. Pharmacopsychiatry. 2008;41(1):17–23. doi: 10.1055/s-2007-992148. [DOI] [PubMed] [Google Scholar]
- *32.Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. Jama. 2008;299(2):185–93. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 33.Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: A systematic review and meta-analysis. Schizophr Res. 2010;123(2–3):225–33. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 35.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA : the journal of the American Medical Association. 2009;302(16):1765–73. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komossa K, Rummel-Kluge C, Schmid F, Hunger H, Schwarz S, El-Sayeh HG, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009;(4):CD006569. doi: 10.1002/14651858.CD006569.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Citrome L. Iloperidone, asenapine, and lurasidone: a brief overview of 3 new second-generation antipsychotics. Postgrad Med. 2011;123(2):153–62. doi: 10.3810/pgm.2011.03.2273. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 39.Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, et al. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophrenia Res. 2009;111(1–3):9–16. doi: 10.1016/j.schres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085–97. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 41.Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165(11):1420–31. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama. 2007;298(17):2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 43.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myrskyla M, Chang VW. Weight change, initial BMI, and mortality among middle- and older-aged adults. Epidemiology. 2009;20(6):840–8. doi: 10.1097/EDE.0b013e3181b5f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cameron AJ, Magliano DJ, Dunstan DW, Zimmet PZ, Hesketh K, Peeters A, et al. A bidirectional relationship between obesity and health-related quality of life: evidence from the longitudinal AusDiab study. Int J Obes (Lond) doi: 10.1038/ijo.2011.103. In Press. [DOI] [PubMed] [Google Scholar]
- 47.Bond DJ, Kunz M, Torres IJ, Lam RW, Yatham LN. The association of weight gain with mood symptoms and functional outcomes following a first manic episode: prospective 12-month data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM) Bipolar Disord. 2010;12(6):616–26. doi: 10.1111/j.1399-5618.2010.00855.x. [DOI] [PubMed] [Google Scholar]
- 48.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–17. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 49.Bowden CL, Calabrese JR, Ketter TA, Sachs GS, White RL, Thompson TR. Impact of lamotrigine and lithium on weight in obese and nonobese patients with bipolar I disorder. Am J Psychiatry. 2006;163(7):1199–201. doi: 10.1176/ajp.2006.163.7.1199. [DOI] [PubMed] [Google Scholar]
- 50.Coxhead N, Silverstone T, Cookson J. Carbamazepine versus lithium in the prophylaxis of bipolar affective disorder. Acta Psychiatr Scand. 1992;85(2):114–8. doi: 10.1111/j.1600-0447.1992.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 51.Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520–30. doi: 10.1038/npp.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleischhacker WW, Heikkinen ME, Olie JP, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J of Neuropsychopharmacol. 2010;13(8):1115–25. doi: 10.1017/S1461145710000490. [DOI] [PubMed] [Google Scholar]
- *53.Ball MP, Warren KR, Feldman S, McMahon RP, Kelly DL, Buchanan RW. Placebo-controlled trial of atomoxetine for weight reduction in people with schizophrenia treated with clozapine or olanzapine. Clin Schizophr Relat Psychoses. 2011;5(1):17–25. [PubMed] [Google Scholar]
- *54.Narula PK, Rehan HS, Unni KE, Gupta N. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trial. Schizophrenia Res. 2010;118(1–3):218–23. doi: 10.1016/j.schres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- *55.Afshar H, Roohafza H, Mousavi G, Golchin S, Toghianifar N, Sadeghi M, et al. Topiramate add-on treatment in schizophrenia: a randomised, double-blind, placebo-controlled clinical trial. J Psychopharmacol. 2009;23(2):157–62. doi: 10.1177/0269881108089816. [DOI] [PubMed] [Google Scholar]
- *56.Roy Chengappa KN, Schwarzman LK, Hulihan JF, Xiang J, Rosenthal NR. Adjunctive topiramate therapy in patients receiving a mood stabilizer for bipolar I disorder: a randomized, placebo-controlled trial. J Clin Psychiatry. 2006;67(11):1698–706. doi: 10.4088/jcp.v67n1105. [DOI] [PubMed] [Google Scholar]
- 57.Elmslie JL, Porter RJ, Joyce PR, Hunt PJ, Mann JI. Carnitine does not improve weight loss outcomes in valproate-treated bipolar patients consuming an energy-restricted, low-fat diet. Bipolar disord. 2006;8(5 Pt 1):503–7. doi: 10.1111/j.1399-5618.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 58.Review Manager (Rev Man) [Computer program] Version 5.1 ed The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- *59.Wu RR, Zhao JP, Guo XF, He YQ, Fang MS, Guo WB, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165(3):352–8. doi: 10.1176/appi.ajp.2007.07010079. [DOI] [PubMed] [Google Scholar]
- *60.Baptista T, Martinez J, Lacruz A, Rangel N, Beaulieu S, Serrano A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51(3):192–6. doi: 10.1177/070674370605100310. [DOI] [PubMed] [Google Scholar]
- *61.Baptista T, Rangel N, Fernandez V, Carrizo E, El Fakih Y, Uzcategui E, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophr Res. 2007;93(1–3):99–108. doi: 10.1016/j.schres.2007.03.029. [DOI] [PubMed] [Google Scholar]
- *62.Carrizo E, Fernandez V, Connell L, Sandia I, Prieto D, Mogollon J, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19–26. doi: 10.1016/j.schres.2009.05.007. [DOI] [PubMed] [Google Scholar]
- *63.Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072–9. doi: 10.1176/ajp.2006.163.12.2072. [DOI] [PubMed] [Google Scholar]
- *64.Arman S, Sadramely MR, Nadi M, Koleini N. A randomized, double-blind, placebo-controlled trial of metformin treatment for weight gain associated with initiation of risperidone in children and adolescents. Saudi Med J. 2008;29(8):1130–4. [PubMed] [Google Scholar]
- 65.Ehret M, Goethe J, Lanosa M, Coleman CI. The effect of metformin on anthropometrics and insulin resistance in patients receiving atypical antipsychotic agents: a meta-analysis. J Clin Psychiatry. 2010;71(10):1286–92. doi: 10.4088/JCP.09m05274yel. [DOI] [PubMed] [Google Scholar]
- 66.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wulffele MG, Kooy A, de Zeeuw D, Stehouwer CD, Gansevoort RT. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med. 2004;256(1):1–14. doi: 10.1111/j.1365-2796.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 68.Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6(1):47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 69.Leslie P, Jung RT, Isles TE, Baty J. Energy expenditure in non-insulin dependent diabetic subjects on metformin or sulphonylurea therapy. Clin Sci (Lond) 1987;73(1):41–5. doi: 10.1042/cs0730041. [DOI] [PubMed] [Google Scholar]
- 70.Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, et al. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24(3):489–94. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]
- *71.Assuncao SS, Ruschel SI, Rosa Lde C, Campos JA, Alves MJ, Bracco OL, et al. Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine. Rev Bras Psiquiatr. 2006;28(4):270–6. [PubMed] [Google Scholar]
- *72.Poyurovsky M, Tal V, Maayan R, Gil-Ad I, Fuchs C, Weizman A. The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: a double-blind placebo-controlled pilot study. Eur Neuropsychopharmacol. 2004;14(4):332–6. doi: 10.1016/j.euroneuro.2003.10.004. [DOI] [PubMed] [Google Scholar]
- *73.Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Nizatidine treatment and its relationship with leptin levels in patients with olanzapine-induced weight gain. Hum Psychopharmacol. 2003;18(6):457–61. doi: 10.1002/hup.514. [DOI] [PubMed] [Google Scholar]
- *74.Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Kilic N. Nizatidine for the treatment of patients with quetiapine-induced weight gain. Human psychopharmacology. 2004;19(1):37–40. doi: 10.1002/hup.477. [DOI] [PubMed] [Google Scholar]
- *75.Cavazzoni P, Tanaka Y, Roychowdhury SM, Breier A, Allison DB. Nizatidine for prevention of weight gain with olanzapine: a double-blind placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13(2):81–5. doi: 10.1016/s0924-977x(02)00127-x. [DOI] [PubMed] [Google Scholar]
- 76.Nickel MK, Nickel C, Muehlbacher M, Leiberich PK, Kaplan P, Lahmann C, et al. Influence of topiramate on olanzapine-related adiposity in women: a random, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2005;25(3):211–7. doi: 10.1097/01.jcp.0000162806.46453.38. [DOI] [PubMed] [Google Scholar]
- *77.Ko YH, Joe SH, Jung IK, Kim SH. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169–75. doi: 10.1097/01.wnf.0000172994.56028.c3. [DOI] [PubMed] [Google Scholar]
- 78.Tremblay A, Chaput JP, Berube-Parent S, Prud'homme D, Leblanc C, Almeras N, et al. The effect of topiramate on energy balance in obese men: a 6-month double-blind randomized placebo-controlled study with a 6-month open-label extension. Eur J Clin Pharmacol. 2007;63(2):123–34. doi: 10.1007/s00228-006-0220-1. [DOI] [PubMed] [Google Scholar]
- 79.Egger C, Muehlbacher M, Schatz M, Nickel M. Influence of topiramate on olanzapine-related weight gain in women: an 18-month follow-up observation. J Clin Psychopharmacol. 2007;27(5):475–8. doi: 10.1097/jcp.0b013e31814b98e5. [DOI] [PubMed] [Google Scholar]
- *80.Poyurovsky M, Isaacs I, Fuchs C, Schneidman M, Faragian S, Weizman R, et al. Attenuation of olanzapine-induced weight gain with reboxetine in patients with schizophrenia: a double-blind, placebo-controlled study. Am J Psychiatry. 2003;160(2):297–302. doi: 10.1176/appi.ajp.160.2.297. [DOI] [PubMed] [Google Scholar]
- *81.Poyurovsky M, Fuchs C, Pashinian A, Levi A, Faragian S, Maayan R, et al. Attenuating effect of reboxetine on appetite and weight gain in olanzapine-treated schizophrenia patients: a double-blind placebo-controlled study. Psychopharmacology. 2007;192(3):441–8. doi: 10.1007/s00213-007-0731-1. [DOI] [PubMed] [Google Scholar]
- 82.Mayer AF, Schroeder C, Heusser K, Tank J, Diedrich A, Schmieder RE, et al. Influences of norepinephrine transporter function on the distribution of sympathetic activity in humans. Hypertension. 2006;48(1):120–6. doi: 10.1161/01.HYP.0000225424.13138.5d. [DOI] [PubMed] [Google Scholar]
- 83.Ukai K, Nakagawa T, Ohyama T, Nakanishi H. Sibutramine induces potential-dependent exocytotic release but not carrier-mediated release of dopamine and 5-hydroxytryptamine. Eur J Pharmacol. 2004;484(2–3):209–15. doi: 10.1016/j.ejphar.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 84.Henderson DC, Copeland PM, Daley TB, Borba CP, Cather C, Nguyen DD, et al. A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. Am J Psychiatry. 2005;162(5):954–62. doi: 10.1176/appi.ajp.162.5.954. [DOI] [PubMed] [Google Scholar]
- 85.Henderson DC, Fan X, Copeland PM, Borba CP, Daley TB, Nguyen DD, et al. A double-blind, placebo-controlled trial of sibutramine for clozapine-associated weight gain. Acta psychiatrica Scandinavica. 2007;115(2):101–5. doi: 10.1111/j.1600-0447.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 86.Baptista T, Uzcategui E, Rangel N, El Fakih Y, Galeazzi T, Beaulieu S, et al. Metformin plus sibutramine for olanzapine-associated weight gain and metabolic dysfunction in schizophrenia: a 12-week double-blind, placebo-controlled pilot study. Psychiatry Res. 2008;159(1–2):250–3. doi: 10.1016/j.psychres.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 87.Sibutramine (Meridia) withdrawn. Med Lett Drugs Ther. 2010;52(1350):88. [PubMed] [Google Scholar]
- 88.Graham KA, Gu H, Lieberman JA, Harp JB, Perkins DO. Double-blind, placebo-controlled investigation of amantadine for weight loss in subjects who gained weight with olanzapine. Am J Psychiatry. 2005;162(9):1744–6. doi: 10.1176/appi.ajp.162.9.1744. [DOI] [PubMed] [Google Scholar]
- 89.Deberdt W, Winokur A, Cavazzoni PA, Trzaskoma QN, Carlson CD, Bymaster FP, et al. Amantadine for weight gain associated with olanzapine treatment. Eur Neuropsychopharmacol. 2005;15(1):13–21. doi: 10.1016/j.euroneuro.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci. 2005;25(13):3312–22. doi: 10.1523/JNEUROSCI.4262-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joffe G, Takala P, Tchoukhine E, Hakko H, Raidma M, Putkonen H, et al. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):706–11. doi: 10.4088/jcp.v69n0503. [DOI] [PubMed] [Google Scholar]
- 92.Tchoukhine E, Takala P, Hakko H, Raidma M, Putkonen H, Rasanen P, et al. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week open-label extension phase and both phases of a randomized controlled trial. J Clin Psychiatry. 2011;72(3):326–30. doi: 10.4088/JCP.09m05283yel. [DOI] [PubMed] [Google Scholar]
- 93.Poyurovsky M, Pashinian A, Gil-Ad I, Maayan R, Schneidman M, Fuchs C, et al. Olanzapine-induced weight gain in patients with first-episode schizophrenia: a double-blind, placebo-controlled study of fluoxetine addition. Am J Psychiatry. 2002;159(6):1058–60. doi: 10.1176/appi.ajp.159.6.1058. [DOI] [PubMed] [Google Scholar]
- 94.Bustillo JR, Lauriello J, Parker K, Hammond R, Rowland L, Bogenschutz M, et al. Treatment of weight gain with fluoxetine in olanzapine-treated schizophrenic outpatients. Neuropsychopharmacology. 2003;28(3):527–9. doi: 10.1038/sj.npp.1300089. [DOI] [PubMed] [Google Scholar]
- 95.Borovicka MC, Fuller MA, Konicki PE, White JC, Steele VM, Jaskiw GE. Phenylpropanolamine appears not to promote weight loss in patients with schizophrenia who have gained weight during clozapine treatment. J Clin Psychiatry. 2002;63(4):345–8. doi: 10.4088/jcp.v63n0412. [DOI] [PubMed] [Google Scholar]
- 96.Baptista T, Rangel N, El Fakih Y, Uzcategui E, Galeazzi T, Beaulieu S, et al. Rosiglitazone in the assistance of metabolic control during olanzapine administration in schizophrenia: a pilot double-blind, placebo-controlled, 12-week trial. Pharmacopsychiatry. 2009;42(1):14–9. doi: 10.1055/s-0028-1085438. [DOI] [PubMed] [Google Scholar]
- 97.Gross C, Blasey CM, Roe RL, Belanoff JK. Mifepristone reduces weight gain and improves metabolic abnormalities associated with risperidone treatment in normal men. Obesity. 2010;18(12):2295–300. doi: 10.1038/oby.2010.51. [DOI] [PubMed] [Google Scholar]
- 98.Gross C, Blasey CM, Roe RL, Allen K, Block TS, Belanoff JK. Mifepristone treatment of olanzapine-induced weight gain in healthy men. Adv Ther. 2009;26(10):959–69. doi: 10.1007/s12325-009-0070-1. [DOI] [PubMed] [Google Scholar]
- 99.Product Information: GLUCOPHAGE(R), GLUCOPHAGE(R) XR extended-release oral tablets, oral tablets, metformin hydrochloride extended-release oral tablets, oral tablets. Bristol-Myers Squibb Company; Princeton, NJ: 2009. [Google Scholar]
- 100.Product Information: TOPAMAX(R) oral tablets, oral sprinkle capsules, topiramate oral tablets, oral sprinkle capsules. Ortho-McNeil Neurologics,Inc; Titusville, NJ: 2008. [Google Scholar]
- 101.Alvarez-Jimenez M, Hetrick SE, Gonzalez-Blanch C, Gleeson JF, McGorry PD. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2008;193(2):101–7. doi: 10.1192/bjp.bp.107.042853. [DOI] [PubMed] [Google Scholar]
- 102.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. New Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 103.Nieuwenhuis-Ruifrok AE, Kuchenbecker WK, Hoek A, Middleton P, Norman RJ. Insulin sensitizing drugs for weight loss in women of reproductive age who are overweight or obese: systematic review and meta-analysis. Hum Reprod Update. 2009;15(1):57–68. doi: 10.1093/humupd/dmn043. [DOI] [PubMed] [Google Scholar]