Abstract
We observed the biphasic production of ethylene and reactive oxygen species (ROS) in susceptible tobacco (Nicotiana tabacum ‘Wisconsin 38’) plants after shoot inoculation with Phytophthora parasitica var nicotianae. The initial transient increase in ROS and ethylene at 1 and 3 h (phase I), respectively, was followed by a second massive increase at 48 and 72 h (phase II), respectively, after pathogen inoculation. This biphasic pattern of ROS production significantly differed from the hypersensitive response exhibited by cryptogein-treated wild-type tobacco plants. The biphasic increase in ROS production was mediated by both NADPH oxidase isoforms, respiratory burst oxidase homolog (Rboh) D and RbohF. Conversely, different 1-aminocyclopropane-1-carboxylic acid synthase members were involved in specific phases of ethylene production: NtACS4 in the first phase and NtACS1 in the second phase. Biphasic production of ROS was inhibited in transgenic antisense plant lines expressing 1-aminocyclopropane-1-carboxylic acid synthase/oxidase or ethylene-insensitive3 as well as in transgenic plants impaired in ROS production. All tested transgenic plants were more tolerant against P. parasitica var nicotianae infection as determined based on trypan blue staining and pathogen proliferation. Further, silencing of NtACS4 blocked the second massive increase in ROS production as well as pathogen progression. Pathogen tolerance was due to the inhibition of ROS and ethylene production, which further resulted in lower activation of ROS-detoxifying enzymes. Accordingly, the synergistic inhibition of the second phase of ROS and ethylene production had protective effects against pathogen-induced cell damage. We conclude that the levels of ethylene and ROS correlate with compatible P. parasitica proliferation in susceptible plants.
In aerobic organisms, reactive oxygen species (ROS) are produced during normal cellular metabolism as byproducts of metabolic pathways and electron flows in the mitochondria and chloroplasts. Respiratory burst oxidase homolog (Rboh), named NADPH oxidase, is a transmembrane protein that generates superoxide radicals in plant cells. The superoxide species produced by Rbohs are unable to permeate cell membranes under ambient pH conditions due to the presence of a negative charge (Sagi and Fluhr, 2006). An enzymatic dismutation reaction converts superoxide into a more stable, membrane-permeable hydrogen peroxide (H2O2) derivative, which is required for cell-to-cell signaling (Allan and Fluhr, 1997). Moreover, the tissue-specific expression of NADPH oxidase transcript occurs in three ways: expression throughout the entire plant (Atrboh D and F), in the roots (Atrboh A–G, I), and in a pollen-specific manner (Atrboh H and I; Sagi and Fluhr, 2006).
The generation of ROS in response to incompatible pathogens and elicitors is biphasic in many plant systems (Yoshioka et al., 2001). Previously, it was reported that treatment of potato (Solanum tuberosum) tubers with hyphal wall components from Phytophthora infestans causes a rapid and transient accumulation of H2O2 at 1 h (phase I), followed by a massive oxidative burst at 6 to 9 h (phase II; Kobayashi et al., 2007). Similarly, biphasic ROS production was recently observed in plants subjected to other stresses such as ozone and H2O2 (Xie et al., 2011). Despite these results, detailed studies on biphasic ROS production have not been performed. Along the same line, several studies have suggested that ethylene contributes to preimmunity, possibly through the regulation of pathogen-associated molecular pattern triggered ROS production. Ethylene is an important regulator of plant development and growth and is known to be associated with plant defense, although its role is diverse and remains largely uncharacterized in plant-microbe interactions (van Loon et al., 2006). It was demonstrated in an analysis of ethylene-insensitive2 (ein2) mutant in Arabidopsis (Arabidopsis thaliana) that ethylene plays, at best, a minor role in hypersensitive response (HR)-mediated cell death. Conversely, ethylene is a virulence factor of many biotropic or semibiotrophic pathogens (Mur et al., 2008). Further, a previous study reported that ethylene production in tobacco (Nicotiana tabacum) leaves after inoculation with HR-inducing Pseudomonas syringae follows a biphasic pattern reminiscent of H2O2 production (Mur et al., 2008). However, P. syringae pv tabaci, which causes disease symptoms in tobacco, elicits only a single peak in ethylene production.
There are two steps involved in ethylene biosynthesis, conversion of S-adenosyl-Met to 1-aminocyclopropane-1-carboxylic acid (ACC) followed by oxidation to ethylene, which are regulated by ACC synthase (ACS) and ACC oxidase, respectively (Bleecker et al., 1988). In a previous study, we reported that the biphasic accumulation of ROS in response to abiotic stress is significantly reduced in transgenic antisense plants expressing ACS gene (CAS-AS-2, 3, 4), thereby resulting in decreased necrosis and cell death (Wi et al., 2010). Prior studies have demonstrated the potential interplay of biphasic ROS production and ethylene biosynthesis during abiotic responses based upon the biphasic generation of ethylene in response to pathogen (Mur et al., 2008) and the response to oxidative stresses such as H2O2 treatment (Wi et al., 2010). Here, we extend our previous investigations into biphasic ROS and ethylene production during the defense response against compatible hemibiotroph Phytophthora parasitica var nicotianae (Ppn) in susceptible tobacco. We further provide evidence that a pathogen-induced oxidative burst is required as an upstream regulator of ethylene production in both phases. Further, we investigated each phase of biphasic ROS and ethylene production after infection with this compatible oomycete in transgenic tobacco plants down-regulated in the expression of NADPH oxidase genes as well as ethylene biosynthetic and signaling genes.
RESULTS
Biphasic ROS and Ethylene Biosynthesis in Tobacco Plants Responding to Infection with the Compatible Oomycete Ppn
To investigate ROS generation in susceptible tobacco (cv Wisconsin 38) leaves after shoot inoculation with the soilborne oomycete Ppn, which is a compatible pathogen that causes black shank disease (Véronési et al., 1996), we monitored ROS levels in tobacco leaf discs during and up to 120 h using dichlorofluorescein diacetate (DCFH-DA). As a result, we observed prominent biphasic ROS accumulation accompanied by gradual necrotic symptoms after challenge with compatible Ppn pathogen. The first smaller increase in ROS accumulation transiently peaked at about 1 h and then declined (phase I) in pathogen-inoculated wild-type plants (Fig. 1A). The second massive ROS accumulation started at 30 h after inoculation and peaked at 48 h, followed by a gradual decline until 96 h (phase II). This second burst did not return back to basal levels by 120 h and was about 40% higher compared with that in control plants.
Figure 1.
Kinetics of ROS production and detoxification in response to Ppn attack. A and B, ROS accumulation in wild-type plants after treatment with pathogen (A) or cryptogein (B). Wild-type plants were shoot inoculated with Ppn. Leaf discs of wild-type plants were treated with cryptogein (20 nm) for 24 h. ROS accumulation in initial 3 h after Ppn infection was shown in inserted figure. RFU, Relative fluorescence units compared with the untreated control. C, Relative mRNA levels of RbohD and RbohF genes in wild-type plants infected by Ppn. Shoot-inoculated wild-type plants with Ppn were subjected to real-time qRT-PCR analysis. Relative mRNA expression levels were expressed as means ± sd. An asterisk indicates a significant difference between pathogen- or cryptogein-treated and untreated wild-type plants (one asterisk [P < 0.05] or two asterisks [P < 0.01]).
Cryptogein, which is secreted from Phytophthora cryptogea (Ricci et al., 1989), triggers the activation of a number of tobacco defense responses, including HR. Upon cryptogein treatment, biphasic ROS accumulation was observed at 0.5 and 6 h for phases I and II, respectively, and a return to basal level occurred after 12 h (Fig. 1B). Therefore, there was a clear biphasic oxidative burst; the first burst at 30-min postinoculation included a sharp increase in ROS generation that was about 70% higher than that in Ppn-treated plants, followed by a rapid decrease to almost basal level (Fig. 1B). Three hours later, we observed a more massive increase in ROS generation, which reached maximal level at 6 h. As shown, the compatible pathogen was unable to produce the same response as the elicitor cryptogein. Although these differences in peak times could be attributed to the type of plant tissue and treatment method, it is possible that peak time was also dependent on the virulence of the pathogen. Therefore, the magnitude and timing of biphasic ROS production are important determinants of HR in incompatible pathogen-plant interactions.
We further investigated the gene expression profile of tobacco plants in the context of ROS production during Ppn infection. Even though the Arabidopsis genome contains 10 Rboh genes, AtrbohD and AtrbohF are required for ROS accumulation in the plant defense response against pathogen infection (Torres et al., 2002). To examine the function of the Rboh genes in biphasic ROS generation, we analyzed the transcript levels of two Rboh genes, RbohD and RbohF, in tobacco leaf discs using relative real-time quantitative reverse transcription (qRT)-PCR analysis. The RbohD transcript showed biphasic kinetics upon pathogen inoculation, displaying an early transient peak in phase I at 1 h and a massive peak in phase II at 48 h (Fig. 1C, left section). Regarding RbohF, a small increase in transcript level was observed at 1 h, followed by a much larger increase at 30 h that peaked at 48 h. This increase in transcription was observed for up to 96 h (Fig. 1C, right section). These results indicate that RbohD and RbohF share functions in biphasic H2O2 generation in response to biotic stress. More specifically, the relative expression level of RbohD normalized to the expression level of β-actin was about 10 times higher than that of RbohF, which suggests one possibility that RbohD might be much more actively involved in the biotic stress response. However, the other possibilities of posttranscriptional and/or translational regulation cannot be ruled out during ROS production. These findings suggest that the biphasic expression profiles of RbohD and RbohF genes in response to Ppn infection (Fig. 1C) significantly differ from those induced by incompatible pathogen interactions and elicitors, even though both are biphasic.
A biphasic pattern of ethylene production was observed in tobacco leaf discs after shoot inoculation with Ppn (Fig. 2A). The initial increase in ethylene production appeared to transiently peak at about 3 h, followed by a decline to basal level at 9 h. The second increase in ethylene production began at 12 h after inoculation, after which it steadily increased and peaked at 72 h. Even though similar biphasic kinetics were observed between ROS and ethylene production in response to Ppn inoculation, the biphasic peaks of ethylene production were significantly delayed relative to those of ROS accumulation. Therefore, ROS generation occurred more rapidly as well as functioned upstream of ethylene production. The biphasic pattern of cryptogein-induced ethylene production differed greatly (Fig. 2B). Specifically, the initial peak in ethylene production in cryptogein-treated plants occurred at 6 h, the same time as the second massive ROS burst, and was 6.7 times larger than that observed in Ppn-treated wild-type plants. However, the second peak, which occurred between 24 and 36 h, was much smaller than that observed in Ppn-treated wild-type plants. These results suggest that the first massive transient peak in cryptogein-induced ethylene production resulted in pathogen resistance through HR. Therefore, in this elicitation process, transient and massive induction of ROS production within 1 h serves as a determinant of the defense response. However, the second massive peaks of ROS and ethylene production after 36 h in response to compatible pathogen infection might be related to pathogen proliferation and cell death.
Figure 2.
Kinetics of ethylene production and expression profiles of ACS isoforms in response to Ppn or cryptogein treatment. A and B, Ethylene production in wild-type plants after treatment with Ppn (A) or cryptogein (B). Wild-type plants were shoot inoculated with Ppn. Leaf discs of wild-type plants were treated with cryptogein (20 nm) for 72 h. C, Transcript levels of NtACS gene family members, NtACS1 and NtACS4, in wild-type plants after treatment with pathogen. Transcript amounts were expressed relative to the reference gene of β-actin after qRT-PCR. Relative mRNA expression levels were expressed as means ± sd. An asterisk indicates a significant difference between pathogen- or cryptogein-treated and untreated wild-type plants (one asterisk [P < 0.05] or two asterisks [P < 0.01]).
Our previous results have shown that abiotic stresses induce gene-specific expression of ACS members in a time-dependent manner (Wi et al., 2010). In response to H2O2-induced oxidative stress, the transcription level of NtACS4 in wild-type tobacco leaf discs peaked at 1 h, whereas induction of NtACS1 transcription occurred between 6 and 24 h and peaked at 24 h. In this study, we measured the levels of NtACS1 and NtACS4 transcription after Ppn infection using real-time qRT-PCR. Neither NtACS1 nor NtACS4 transcription was induced in wild-type plants in the absence of Ppn inoculation. On the other hand, NtACS4 expression was transiently up-regulated starting at 1 h after pathogen treatment and reached an undetectable level after 6 h (Fig. 2C, left section). In contrast, NtACS1 expression was not induced at all until 24 h after Ppn inoculation, after which it increased starting at 30 h and peaked at 72 h (Fig. 2C, right section). The relative expression of NtACS1 normalized to the expression level of β-actin was about 102 higher than that of NtACS4. Therefore, NtACS4 contributes to ethylene production in the first phase by functioning as a trigger of the wider pathogen response. On the other hand, NtACS1 is active during the second phase and plays a different role from that of NtACS4. Based on these results, the physiological effects of ethylene might be regulated by the activation of specific ACS isoforms. Further, we cannot rule out the possibility that isoforms other than NtACS1 and NtACS4 are involved and posttranscriptional regulation of two ACS gene members is involved in ethylene production during plant-pathogen interactions.
Impaired ROS Production Decreases Ethylene Biosynthesis and Weakens Pathogenicity in Susceptible Plants
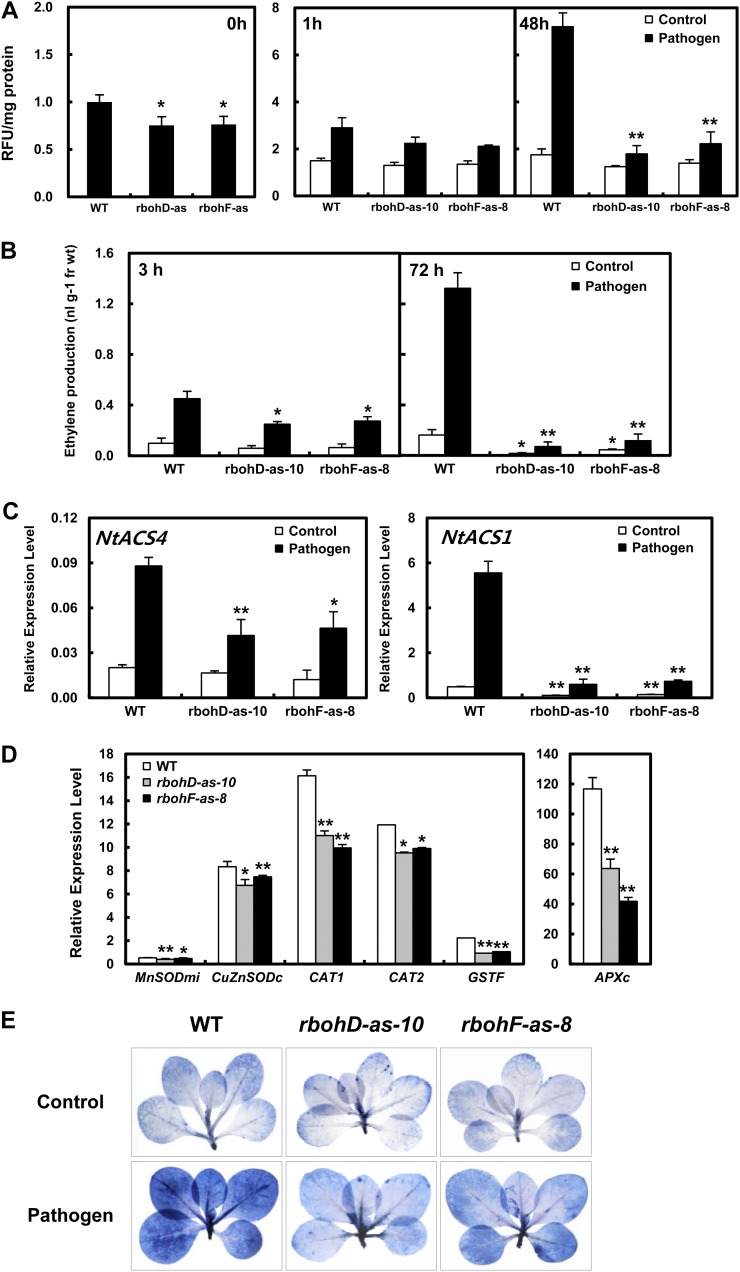
According to our results, environmental stresses (Wi et al., 2010) and pathogen infection (Fig. 1 and 2) both stimulated massive accumulation of ROS accompanied by ethylene biosynthesis. Therefore, transgenic tobacco plants were produced in which ROS production was impaired by the antisense expression of RbohD and RbohF (Supplemental Fig. S1). Compared to wild-type plants, lower ROS levels were detected in rbohD-as and rbohF-as transgenic plants not infected with pathogen (Fig. 3A, left section). At 1 and 48 h after Ppn infection, much weaker accumulation of ROS was observed in rbohD-as and rbohF-as plants relative to wild-type plants (Fig. 3A, middle and right sections). Notably, in phase II, ROS accumulation was 4-fold higher in Ppn-infected wild-type plants compared with control plants; however, it was only 50% to 57% higher in Ppn-treated transgenic plants in comparison with untreated transgenic plants. The relative transcript level of RbohD normalized to the expression level of β-actin was much higher than that of RbohF (Fig. 1C), indicating that RbohD plays a greater role in the response to pathogen infection. Further, a lower level of pathogen-induced ROS accumulation in phase II was observed in rbohD-as plants compared to rbohF-as plants, indicating that ROS production during phase II is highly associated with RbohD.
Figure 3.
Kinetics of ROS and ethylene production and transcripts for ROS detoxification in response to Ppn attack. A, ROS accumulation in wild-type plants and transgenic plants with impaired ROS production (rbohD-as and rbohF-as) after treatment with Ppn. Left section: uninoculated plants; middle section: 1-h-inoculated plants; right section: 48-h-inoculated plants. B, Ethylene production in plants after treatment with pathogen. Three hours (left, phase I) or 72 h (right, phase II) after shoot inoculation with Ppn, ethylene measurements were performed. C, Transcript levels of NtACS gene family members in wild-type and transgenic plants after shoot inoculation with Ppn for 1 h (NtACS4) or 72 h (NtACS1). D, Transcript accumulation of endogenous ROS-detoxification enzymes, MnSODmi, CuZnSODc, CAT1, CAT2, GSTF, and APXc, in response to Ppn infection. Transcript amounts were expressed relative to the reference gene of β-actin after qRT-PCR. Relative mRNA expression levels were expressed as means ± sd. An asterisk indicates a significant difference between wild-type and transgenic plants with Ppn-treated or untreated cases (one asterisk [P < 0.05] or two asterisks [P < 0.01]). E, Necrotic areas in Ppn-inoculated wild-type and transgenic plants (rbohD-as and rbohF-as) were determined using trypan blue staining, and then imaged with a digital camera.
To determine whether the biphasic pattern of ethylene biosynthesis is influenced by the impairment of NADPH oxidase genes in response to pathogen infection, ethylene production as well as the gene expression of ACS members was assessed after infecting the stem bases of 10-week-old transgenic plants grown on agar plugs with Ppn mycelia (Fig. 3, B and C). Pathogen-induced ethylene production was significantly perturbed in both rbohD-as and rbohF-as plants, and the level of ethylene was dramatically lower at 72 h (phase II) than at 3 h (Fig. 3B). Ppn-induced ethylene production at 72 h decreased by 95% in rbohD-as mutants and 91% in rbohF-as mutants. These results suggest that RbohD and RbohF are more significantly involved in ethylene production in phase II than phase I.
We also investigated changes in the expression profiles of ACS gene members in rbohD-as and rbohF-as mutants during ethylene production in response to Ppn infection. NtACS1 and NtACS4 expression after Ppn challenge in wild-type and rboh-as tobacco mutants was measured by real-time qRT-PCR (Fig. 3C). The NtACS4 transcript level in phase I significantly decreased by about 50% in both rbohD-as and rbohF-as mutants after pathogen infection compared to Ppn-inoculated wild-type tobacco plants. Meanwhile, the NtACS1 transcript level in phase II dramatically decreased by 89% in rbohD-as plants and 87% in rbohF-as plants. NtACS1 expression was also dramatically lower in both types of transgenic plants without Ppn treatment, in comparison with uninfected wild-type plants. These results indicate that both RbohD and RbohF serve as upstream regulators of pathogen-induced ethylene biosynthesis.
The level of ROS is tightly regulated by enzymes involved in ROS-detoxifying pathways: mitochondrial manganese-superoxide dismutase (MnSODmi), cytosolic copper/zinc superoxide dismutase (CuZnSODc), cytosolic ascorbate peroxidase (APXc), catalase (CAT1 and CAT2), and ϕ glutathione-S-transferase (GSTF; Fukao et al., 2011). As shown in Supplemental Figure S2, Ppn more effectively induced the expression of all tested genes in phase II (48 h) than at 1 h. However, the mRNA levels of almost all the ROS detoxification enzymes decreased at 72 h after infection, corresponding to the gradual decrease in ROS accumulation. We further examined the transcript levels of ROS detoxification enzymes in wild-type and rbohD-as and rbohF-as mutants in response to Ppn inoculation to determine the effectiveness of the ROS detoxification machinery in mutants impaired in ROS production (Fig. 3D). Interestingly, impairment of the RbohD and RbohF genes via antisense expression effectively decreased the mRNA levels of the ROS detoxification enzymes at phase II (48 h) in response to Ppn infection compared to Ppn-treated wild-type plants. Although APXc transcription was induced by only 55% in rbohD-as plants and 36% in rbohF-as plants, the transcript levels of the other tested enzymes such as MnSODmi, CuZnSODc, and CAT2 were induced by 75% to 90% relative to Ppn-treated wild-type plants. Further, CAT1 and GSTF transcript levels were reduced by 32% to 38% and 53% to 59%, respectively, in both mutants. This decrease in the expression of antioxidative enzymes in phase II could be attributed to impaired ROS production in both mutants in response to pathogen. Therefore, ROS-detoxifying capacity is dependent on the level of compatible pathogen-induced ROS production.
Inoculation of tobacco plant with the compatible pathogen Ppn significantly increased the rate of cell death. In compatible interactions with tobacco cell cultures, encystment of zoospores occurs within 2 h, followed by the penetration of cells and eventually prolific pathogen growth (Able et al., 1998). To monitor pathogen growth and the rate of cell death, we examined Ppn-inoculated plant leaves from wild-type and transgenic rbohD-as and rbohF-as mutants by photography after staining with lactophenol trypan blue (Takemoto et al., 2005). At 48 h after Ppn inoculation, cell death had significantly decreased in both transgenic plants, especially rbohD-as, in comparison with Ppn-inoculated wild-type plants (Fig. 3E). Further, almost all of the cells from Ppn-inoculated wild-type plants were positively stained with trypan blue at 48 h after inoculation. Of note, although some of the cells from the uninfected wild-type plants stained positive as well, we believe that these cells died (blue-stained cells) due to the weakness of the in vitro-cultured plantlets, which did not form complete cuticles.
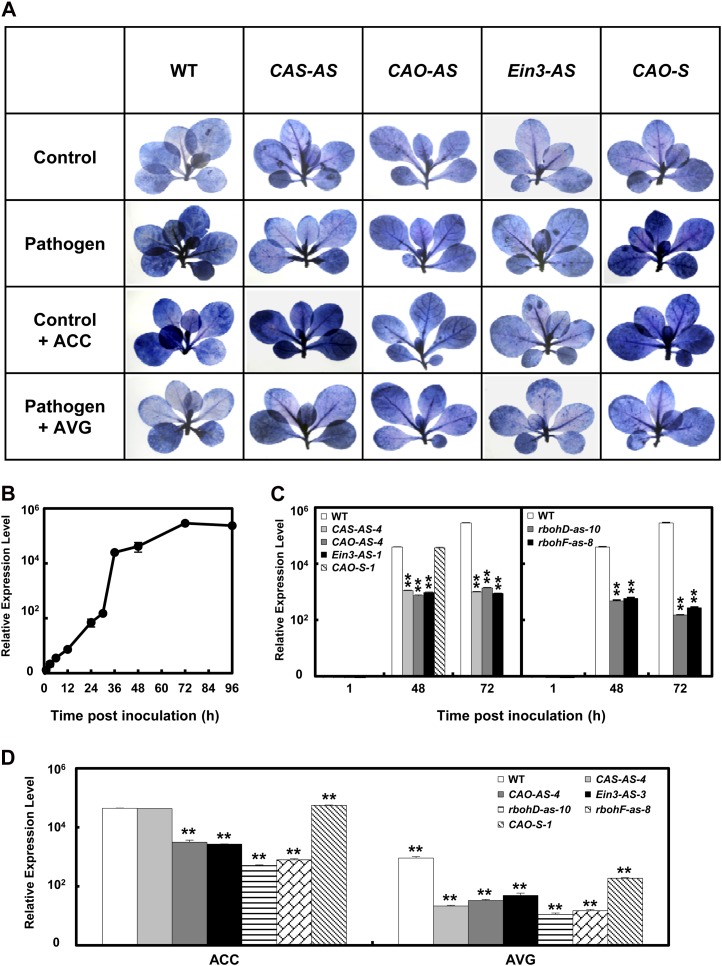
There were no differences in staining level between the tested mutants (CAS-AS, CAO-AS, and Ein3-AS) in the absence of pathogen infection (Fig. 4A, first lane). After inoculation with Ppn, similar amounts of blue-stained cells were observed among the transgenic mutants in comparison with uninfected mutant controls. However, the levels of staining in the transgenic mutants were much lower than those observed in the wild-type and CAO-S plants (Fig. 4A, second lane). These results indicate that impaired ethylene production or signaling during plant-pathogen infection can alleviate cell damage and disease progression.
Figure 4.
Comparison of cell death and pathogen growth in wild-type and antisense transgenic plants with ACS, ACC oxidase, and ein3 (CAS-AS, CAO-AS, and Ein3-AS) after Ppn infection. A, Necrotic areas in Ppn-inoculated wild-type and transgenic plants were stained with trypan blue, and then imaged with a digital camera. B, Detection of Ppn in wild-type plants after inoculation by real-time qRT-PCR of the 5.8S rRNA of Ppn. C, Comparison of pathogen growth in wild-type and transgenic plants after inoculation by real-time qRT-PCR of the 5.8S rRNA of Ppn. D, Comparison of pathogen growth in Ppn-inoculated wild-type and transgenic plants after treatment with ACC or AVG by real-time qRT-PCR of the 5.8S rRNA of Ppn. Transcript amounts were expressed relative to the reference gene of plant β-actin. Relative mRNA expression levels were expressed as means ± sd. These data showed a significant difference between wild-type and transgenic plants with Ppn treatment (one asterisk [P < 0.05] or two asterisks [P < 0.01]).
Ethylene has been implicated as a virulence factor of pathogens as well as a signaling molecule in disease resistance (van Loon et al., 2006). To further determine whether ethylene functions as a physiological factor in cell death, we incubated plantlets with ACC, a precursor of ethylene. Application of ACC without pathogen inoculation significantly induced the formation of blue spots in wild-type, CAS-AS, and CAO-S plants, but it had no effect in CAO-AS and Ein3-AS plants in comparison with corresponding untreated controls (Fig. 4A, third lane). This result suggests that ethylene biosynthesis and signaling act as propagating factors of cell death. In contrast, treatment with aminoethoxyvinyl-Gly (AVG), an inhibitor of ACS, significantly reduced cell death in Ppn-infected wild-type and CAO-S plants, as measured by trypan blue staining, but did not affect cell death in Ppn-infected CAS-AS, CAO-AS, and Ein3-AS plants (Fig. 4A, fourth lane). Therefore, Ppn-induced pathogen growth and cell death were considerably alleviated in wild-type and CAO-S plants by the inhibition of ethylene production. Further, cell death induced by ACC treatment or Ppn inoculation was effectively inhibited in Ein3-AS plants, suggesting that ethylene perception is required for cell death. These data raise the possibility that inhibition of ethylene production could alleviate cell death after pathogen attack. Therefore, we can conclude that ethylene promotes cell death as a virulence factor in tobacco plants infected with Ppn.
Real-time qRT-PCR has been proven to be a reliable technique for the detection and quantification of plant pathogens, and it is increasingly being used in plant pathology. To correlate the various symptoms observed in wild-type and mutant plants after inoculation with pathogen, PCR-based detection of Ppn was performed (Rancé et al., 1998). It was recently reported that qRT-PCR is a highly sensitive method for the quantification of Ppn in plants (Kox et al., 2007). Accordingly, we used real-time qRT-PCR along with oligonucleotide primers designed to target an rRNA sequence specific to the genome of Ppn pathogen. Previously, Yan and Liou (2006) characterized 18 housekeeping genes as potential suitable internal controls to study the gene expression profile in response to Ppn infection. They reported that the expression of ribosomal protein S3A (WS21) shows minimal changes in mRNA expression at all stages, making this gene a suitable internal control to study the gene expression profile of Ppn pathogenesis. Further, in European beech (Fagus spp.), the only gene unaffected by abiotic and biotic stresses was determined to be actin (Olbrich et al., 2008). Therefore, we designed primers specific to 5.8S rRNA (GenBank AY769953) as an internal control for monitoring Ppn growth. Using real-time qRT-PCR, expression of the 5.8S rRNA gene was compared to that of tobacco β-actin, which was used as an internal plant reference gene. The relative expression profile of pathogen 5.8S rRNA showed an S-shaped growth curve, increasing progressively until 36 h after inoculation and then increasing exponentially until 72 h, at which point maximal expression of pathogen rRNA was observed (Fig. 4B). Since tobacco plants are susceptible to Ppn, expression of pathogen rRNA was maintained until 72 h after inoculation, implying that the pathogen population continually increased. Symptoms did not actually appear in inoculated wild-type plants until 36 h, after which significant accumulation of the pathogen was observed, as determined by trypan blue staining (Fig. 4A) and real-time qRT-PCR (Fig. 4, B and C). Pathogen rRNA expression was detected in plants as early as 3 h after inoculation by real-time qRT-PCR, indicating that colonization of this pathogen occurred immediately after inoculation. Further, extensive growth of the pathogen based on relative expression of Ppn 5.8 rRNA was observed in tobacco between 36 and 48 h after infection with Ppn (Fig. 4B). The pathogen population was much higher in wild-type plants compared with other tested transgenic lines at both 48 and 72 h (Fig. 4C). Growth of the pathogen also increased by 14% by 48 h after ACC treatment in Ppn-inoculated wild-type plants and by 53% in Ppn-inoculated CAO-S transgenic plants, in comparison with untreated Ppn-inoculated wild-type controls and CAO-AS controls, respectively (Fig. 4D). Pathogen population after ACC treatment was still much lower in CAO-AS and Ein3-AS plants relative to wild-type plants, implying that ethylene production and signaling might be a crucial component of pathogen growth. Pathogen population after ACC treatment was declined to lowest level in rbohD-as and rbohF-as mutants. These results suggest that ROS production might be an essential component of ethylene-induced pathogen growth. However, AVG treatment dramatically decreased pathogen proliferation not only in wild-type plants but also in all tested transgenic lines in comparison with untreated Ppn-inoculated wild-type plants, which implies again that pathogen growth in tobacco plants is dependent on ethylene and ROS production. According to our results, the growth of compatible pathogens might be activated through ethylene and ROS signaling pathways. Ppn infection mediates its virulence through induction of ethylene production. Ethylene-induced cell death therefore results in the enhanced growth of the pathogen in plants.
We previously reported that the modulation of ethylene production and signaling is a key determinant in enhancing tolerance against abiotic stresses (Wi et al., 2010). Here, we found that enhanced ethylene production altered susceptibility of tobacco plants to Ppn infection. Ethylene production decreased considerably in phases I and II in pathogen-inoculated transgenic plants (CAS-AS-4, CAO-AS-4, and Ein3-AS-3) in comparison with wild-type plants. Inhibition of ethylene production was more effective in phase II (72 h), when large quantities of ethylene were produced, than in phase I (3 h; Supplemental Fig. S3A). In addition, NtACS1 expression in phase II was reduced more significantly than expression of NtACS4 in phase I (Supplemental Fig. S3B). Further, the severity of pathogen-induced cell damage was dependent on the magnitude of ethylene production (Fig. 4A), which suggests that ethylene biosynthesis and cell death are positively correlated after pathogen infection. Therefore, these results further confirm that ethylene production is a virulence factor in response to Ppn infection in tobacco. These phenomena were also consistent with previously published studies that reported an increase in ethylene production or ethylene-sensitivity-stimulated lesion expansion and pathogen growth after infection (Knoester et al., 1999). However, these results do not correspond with other articles that reported that ethylene insensitivity could also enhance disease severity (Ton et al., 2002). These conflicting results indicate that the effects of changes in ethylene production and signaling depend on the types of host-pathogen interactions.
Impaired Ethylene Synthesis and Signaling Reduces ROS Accumulation, Thereby Improving Pathogen Tolerance in Plants
To investigate the effects of ethylene on the biphasic production of ROS in tobacco plants inoculated with Ppn in the context of ethylene as a virulence factor, the levels of ROS was first determined using a spectrofluorophotometer with DCFH-DA in shoot-inoculated tobacco leaves. Pathogen-induced ROS accumulation was inhibited by about 30% in phase I (1 h) and about 50% in phase II (48 h) in all transgenic plants displaying impaired ethylene biosynthesis or signaling (Fig. 5A). The accumulation of pathogen-induced RbohD transcription was notably inhibited at 48 h in all transgenic lines compared to wild-type plants (Fig. 5B). Further, inhibition of pathogen-induced RbohD transcription was less prominent in all transgenic plants at 1 h compared with those at 48 h. However, after pathogen inoculation, changes of RbohF transcript levels in all transgenic plants, except CAS-AS at 1 h, were not statistically significant in both phases at 1 and 48 h compared to wild-type plants (Fig. 5C). We observed that the relative expression level of RbohD mRNA was on the order of 1, whereas the relative expression of RbohF mRNA was on the order of 1/10. According to our findings, ROS accumulation was primarily derived from RbohD activity. Therefore, ethylene might modulate pathogen-induced ROS production by manipulating RbohD expression, even though there were the possibilities of posttranscriptional and translational regulation.
Figure 5.
Comparison of ROS production and detoxification in wild-type and transgenic plants with impaired ethylene synthesis or signaling (CAS-AS, CAO-AS, and Ein3-AS). A, ROS in plants after treatment with pathogen. One hour (left, phase I) or 48 h (right, phase II) after plants with five leaves were shoot inoculated with Ppn, ROS content was measured using DCFH-DA. B and C, Relative mRNA levels of RbohD and RbohF genes in wild-type and transgenic plants infected by Ppn. Transcript abundances of RbohD 1 h after inoculation (phase I; B) or RbohF 48 h after inoculation (phase II; C) were expressed as means ± sd. Transcript amounts were expressed relative to the reference gene of β-actin after qRT-PCR. Relative mRNA expression levels were expressed as means ± sd. An asterisk indicates a significant difference between wild-type and transgenic plants with Ppn-treated or untreated cases (one asterisk [P < 0.05] or two asterisks [P < 0.01]).
Transcripts of genes associated with ROS detoxification were also analyzed in wild-type and antisense-transgenic tobacco plants using real-time qRT-PCR (Supplemental Fig. S4). Among ROS detoxification enzymes, all tested genes were less expressed in transgenic lines after pathogen infection. This indicates that, in general, transcription of these genes in transgenic plants would be less effectively induced in response to pathogen infection. Based on our previous result that the level of the second ROS burst was significantly reduced at 48 h after pathogen inoculation in transgenic plants (Fig. 5A), we can assert that ethylene down-regulates ROS production, thereby decreasing the induction capability of ROS-detoxifying enzymes. APX activity gradually increased in wild-type and transgenic plants until 48 h after Ppn inoculation, but this increase was much slower in the transgenic plants (Supplemental Fig. S5).
These observations led us to consider whether the expression levels of pathogenesis-related (PR) genes are altered in CAS-AS, CAO-AS, Ein3-AS, rbohD-as, and rbohF-as, after Ppn infection. Pathogen infection up-regulated the expression of PR proteins induced by compatible plant-pathogen interactions (van Loon et al., 2006). Although the accumulation of PR proteins is a hallmark of pathogen-induced resistance by salicylic acid-dependent or jasmonic acid/ethylene-dependent signaling, little information on the interrelationship between PR proteins and ROS is available. In our experiments, the expression levels of all PR proteins and defense-related proteins such as proteinase inhibitor II and SAR 8.2 were up-regulated in both wild-type and transgenic plants after pathogen infection in comparison with uninfected controls. However, the magnitude of pathogen-induced expression was much lower in transgenic plants relative to wild-type plants (Supplemental Fig. S6). Expression of the PR and SAR 8.2 genes increased significantly at 48 h (as evidenced by massive ROS accumulation) and then declined at 72 h (as evidenced by massive ethylene production), indicating that ROS is a crucial factor for the induction of PR transcription. Less effective induction of each PR and defense-related protein was also observed in the transgenic plants at 48 h after Ppn infection, in which the levels of ROS or ethylene were lower in comparison with wild-type plants. Therefore, the expression levels of PR proteins are dependent on the second burst of ROS accumulation.
NtACS4-Silenced Plants Are Impaired in RbohD-Induced ROS Production and Display Increased Tolerance Toward Ppn Infection
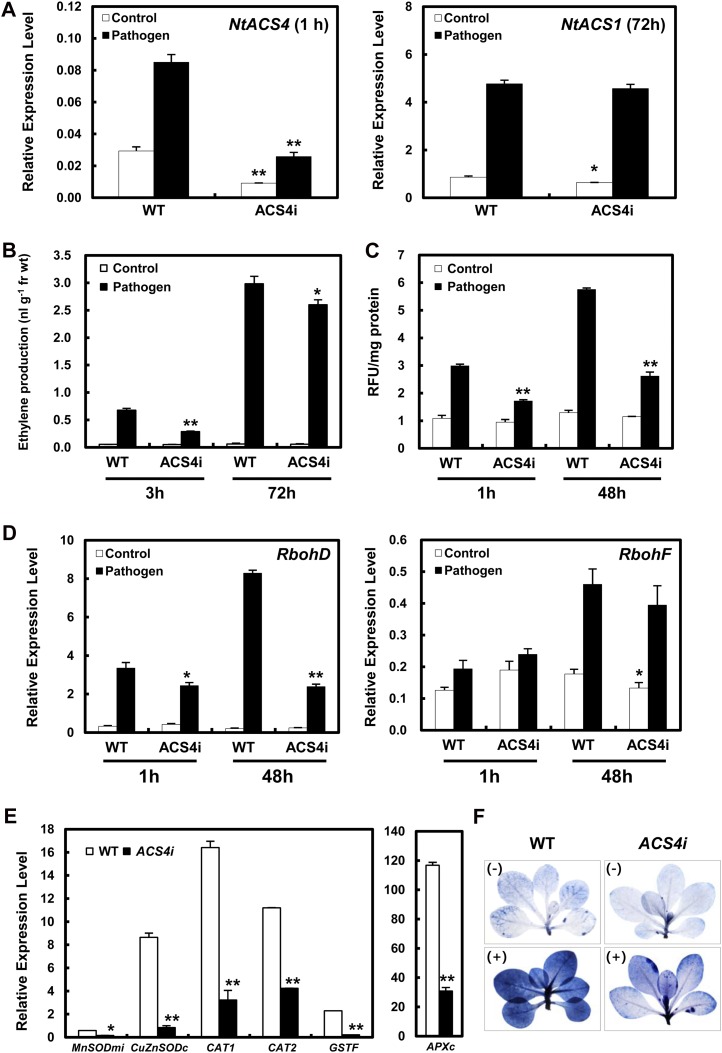
Since we observed that biphasic ROS accumulation and ethylene biosynthesis function differently and are induced by various pathways in response to pathogen infection, we chose to focus on NtACS4, which is a specific ACS isoform active in phase I of ethylene production (Fig. 2C). To determine whether NtACS4 influences ROS accumulation and the defense response, we constructed NtACS4-silenced transgenic plants (ACS4i) via RNA interference-mediated repression. Untreated transgenic lines expressing NtACS4i exhibited no alterations in phenotype. The expression of NtACS4 was significantly reduced in both untreated and Ppn-treated ACS4i lines at 1 h (phase I); however, no differences in NtACS1 expression were observed in the plants at 72 h after pathogen infection (phase II; Fig. 6A). These results suggest that pathogen-induced NtACS1 expression is specifically induced in phase II and independently regulated by NtACS4. Although NtACS4 was specifically suppressed in ACS4i plants at 1 h, lower levels of pathogen-induced ethylene production were observed, compared with Ppn-infected wild-type plants (Fig. 6B). The silencing of NtACS4 more significantly inhibited Ppn-induced ethylene production by around 60% at 3 h, compared with around 10% at 72 h. We hypothesized that changes in ethylene-responsive gene expression could be modulated with endogenous ethylene. Based on this hypothesis, it was assumed that EIN3 and ETHYLENE RESPONSE FACTOR1 (Solano et al., 1998) act as a node, mediating cross talk between the first and second phases of ethylene production. To test this hypothesis, we examined the expression of EIN3 and ERF1 using real-time qRT-PCR at a time point between phase I and phase II (48 h). We previously demonstrated that transcription of EIN3 and ERF1 reaches a maximum level between phases I and II after treatment with abiotic stresses (Wi et al., 2010). Here, expression of EIN3 and ERF1 was not significantly affected in the ACS4i transgenic line after pathogen inoculation (Supplemental Fig. S7). These results indicate that expression of EIN3 and ERF1 is independent of NtACS4, which is consistent with the unresponsiveness of NtACS1.
Figure 6.
Comparison of relative mRNA expression levels of ACS isoforms, ethylene biosynthesis, and ROS production, and then cell death in response to Ppn infection in ACS4i plants. Relative mRNA expression levels were expressed as means ± sd. An asterisk indicates a significant difference between wild-type and ACS4i plants with pathogen or control treatment (one asterisk [P < 0.05] or two asterisks [P < 0.01]). A, Transcript levels of NtACS gene family members in wild-type and ACS4i plants 1 h (NtACS4, phase I) or 72 h (NtACS1, phase II) after shoot inoculation with Ppn. B, Ethylene production levels in wild-type and ACS4i plants 3 or 72 h after shoot inoculation with Ppn. C, ROS accumulation in wild-type and ACS4i plants 1 or 48 h after shoot inoculation with Ppn. RFU, Relative fluorescence units compared with the untreated control. D, Relative mRNA levels of RbohD and RbohF genes in wild-type and transgenic plants infected by Ppn. Transcript abundances of RbohD (Left) or RbohF (Right) 1 or 48 h after inoculation. E, Relative transcript accumulation of endogenous ROS-detoxifycation enzymes, MnSODmi, CuZnSODc, CAT1, CAT2, GSTF, and APXc were determined in response to Ppn infection after 48 h. F, Necrotic areas in wild-type and ACS4i transgenic plants were determined using trypan blue staining after Ppn infection, and then imaged with a digital camera.
To determine whether NtACS4 affects pathogen-induced ROS production, we measured the cellular levels of ROS after Ppn inoculation. Pathogen-induced ROS accumulations were significantly suppressed by 42% at 1 h and by 55% at 48 h in ACS4i plants compared with wild-type plants (Fig. 6C). We also analyzed the expression of RbohD and RbohF in phases I and II after pathogen inoculation using real-time qRT-PCR (Fig. 6D). The expression level of RbohD decreased slightly by 27% at 1 h and then dramatically by 71% at 48 h upon silencing of NtACS4 compared with Ppn-infected wild-type plants. However, the expression level of RbohF decreased by only 15% in ACS4i transgenic lines at 48 h after inoculation compared with wild-type plants. According to this finding, it appears that pathogen-induced RbohD expression, which increased specifically at 48 h, is involved in ethylene-dependent ROS production through the expression of NtACS4. Interestingly, transcription of ROS-detoxifying enzymes was much lower in response to pathogen infection in ACS4i transgenic lines compared with wild-type plants (Fig. 6E), which indicates that the expression of these enzymes is dependent on the cellular ROS level. Therefore, silencing of ethylene production by NtACS4 does not effectively induce the expression of ROS-detoxifying enzymes in response to Ppn infection, which is in agreement with our observation that induction of these enzymes was low in CAS-AS, CAO-AS, rbohD-as, and rbohF-as mutants (Figs. 3D and 6E). Further, pathogen-induced cell damage was significantly suppressed in ACS4i transgenic plants (Fig. 6F). Therefore, we can postulate that expression of NtACS4 and NtACS1 is independently induced in response to pathogen infection and that NtACS4-induced ethylene production early on is responsible for RbohD-induced ROS production later, which ultimately contributes to cell death after infection with compatible pathogens.
DISCUSSION
One of the prominent phenomena of plants in the response to both compatible and incompatible pathogens is the production pattern of ROS. Biphasic ROS production with first minor burst and second major burst has been reported in several incompatible plant-pathogen interactions (Mandal et al., 2011). With regards to incompatible interactions only, hydroxyl/superoxide radicals are produced in a minor burst between 0 and 2 h and then followed by a major burst between 8 and 10 h postinoculation, immediately causing HR in host plants (Able et al., 1998). The first burst is common to both compatible and incompatible interactions as well as elicitor-mediated defense in plant systems (Galletti et al., 2008). However, it has been reported that no second burst is observed in compatible interactions, in which HR does not occur. In some cases, a much smaller single-phase ROS burst or no burst at all occurs in plants infected with susceptible pathogens. Therefore, the biphasic production of ROS is more important in the context of resistant cultivars than susceptible cultivars.
The HR elicited in plant cells by incompatible pathogens has been widely studied in a variety of plant/pathogen systems. Treatment with elicitin induces typical defense responses, which is a form of programmed cell death, rapid generation of ROS, production of phytoalexins, expression of various defense-marker genes such as PR protein genes, and systemic acquired resistance to various pathogens in responsive plants (Takemoto et al., 2005). Consistent with this, a large first ROS burst at 0.5 h (Fig. 1B) as well as a significant ethylene production peak at 6 h (Fig. 2B) were observed in cryptogein-treated tobacco plants. On the other hand, a large second ROS burst as well as ethylene production occurred at 48 and 72 h, respectively, in Ppn-inoculated plants (Figs. 1B and 2B). Cryptogein is a 10-kD proteinaceous elicitor purified from culture filtrates of the oomycete P. cryptogea, an avirulent pathogen of tobacco (Ricci et al., 1989), and triggers an HR-like response. Lherminier et al. (2009) reported that initial production of H2O2 is mediated by RbohD and occurs within a few minutes after challenge with cryptogein. This event is not sufficient to induce HR development or establish acquired resistance; rather, it is most likely involved in signaling pathways associated with cell protection. The second H2O2 production peak, which occurs within 1 d, may induce HR and contribute to disease resistance strategies. However, in the compatible interaction with Ppn, in which pathogen growth was extensive after 30 h, a second massive ROS peak occurred between 36 and 72 h (Figs. 1A and 4B). Our findings differ from those of other articles on plant-pathogen interactions, in which a monophasic ROS burst was reported for compatible pathogens with susceptible cultivars. Further, in transgenic plants with low ROS levels, pathogen growth was much less abundant at 48 h (Fig. 4, C and D). Therefore, these results suggest that the second massive ROS burst is positively related to pathogen proliferation.
The results presented here demonstrate that biphasic production of ROS and ethylene properly coordinates the physiological and molecular responses to compatible pathogen infection in a phase-dependent manner. Here, we studied the different physiological functions of ROS and ethylene in each phase by analyzing transgenic plants displaying antisense expression of ACS, ACO, and Ein3 related to ethylene biosynthesis and signaling as well as RbohD and RbohF related to ROS generation in response to Ppn. Although we observed much lower levels of ROS production in the first phase in Ppn-infected tobacco plants relative to cryptogein-treated plants (Fig. 1, A and B), biphasic ROS production was still observed in tobacco plants infected with compatible pathogen. Further, each ROS burst was followed by biphasic ethylene production (Fig. 2, A and B). These results also suggest that the biphasic kinetics of ROS and ethylene production are obvious in compatible plant-pathogen interactions. Specifically, suppression of the first ethylene peak by silencing of NtACS4, which was specifically induced in phase I, significantly reduced the level of RbohD transcription in plants infected with Ppn (Fig. 6D). In addition, when RbohD or RbohF expression was inhibited in transgenic plants infected with Ppn, both peaks of ethylene production were significantly inhibited, suggesting a positive correlation between ROS and ethylene production (Fig. 3, A and B). The observation that the ROS-detoxifying enzymes and PR proteins were effectively induced by compatible pathogen only at 48 h suggests a different physiological function for each phase of ROS accumulation. These results imply that the expression of those proteins may have the potential to serve as an independent marker of oxidative stress, which is induced by second massive ROS burst during compatible interaction.
Ethylene has diverse functions in plant-microbe interactions. Ethylene production during the first phase after pathogen infection occurs not only in HR-eliciting and nonpathogenic strains, but also in disease-forming ones. However, the second phase of ethylene production within 1 d occurs only after infection with HR-eliciting strains (Mur et al., 2008). The fact that the early response of biphasic ROS and ethylene production was determined to be an important factor for disease resistance is in agreement with a study published by Ding et al. (2011). They found that delayed activation of the first phase is associated with increased susceptibility. Since the low level of ROS and ethylene production in the early phase in Ppn-inoculated plants did not induce HR, the result is relevant to pathogen expansion. The highest growth rate of pathogen was observed between 30 and 48 h after compatible pathogen inoculation, when the second ROS burst occurred, which were accompanied by an extremely large amount of ethylene production (Figs. 1A and 2A). In this case, the degrees of plant damage and pathogen expansion in the compatible interaction were dependent on the cellular levels of ROS and ethylene in both phases, which was confirmed in the CAS-AS, CAO-AS, Ein3-AS, rbohD-as, and rbohF-as mutants. Further, silencing of NtACS4, which specifically induced ethylene production at phase I after Ppn infection, significantly reduced RbohD-mediated ROS production at 48 h (Fig. 6D). This result indicates that the first peak in ethylene production might act as a signal for the second massive burst of ROS. Further, ethylene production might also be the central component of a self-amplifying loop in which transient biphasic ROS bursts play a critical regulatory role. Higher transcript levels of ROS-detoxifying enzymes (Supplemental Fig. S2) and PR proteins (Supplemental Fig. S6) were obtained at 48 h, at which point pathogen growth rate and ROS accumulation were at their highest levels. Therefore, this result suggests that pathogen proliferation and cell death induced by compatible interactions are related to this massive increase in ROS. However, it is too difficult to determine whether this ROS burst is the cause or the result of pathogen proliferation.
ERF1 transcription was highly induced after Ppn infection in wild-type plants (Supplemental Fig. S7). This result is consistent with a report that found that six of 17 compatible genes in downy mildew infection belong to the ERF family, suggesting that ERF plays a role in compatibility (Huibers et al., 2009). The transcript levels of Ein3 and ERF1 did not significantly change at 48 h with or without pathogen infection (Supplemental Fig. S7), and the expression level of NtACS1 in the later phase (Fig. 6A) was not altered in ACS4i plants. These results lead us to hypothesize that ethylene production in each phase might be independently coordinated by unknown signaling components, in addition to each phase of ROS signaling.
Unaltered, reduced, or enhanced bacterial numbers have all been reported in ethylene-impaired or insensitive mutants, depending on infection type (Pieterse et al., 1998). It was recently reported that ein2-1 mutants are more susceptible to biotrophic P. syringae pv tomato DC3000 (Rosebrock et al., 2007), suggesting the importance of pathogen-triggered ethylene production in the plant immune response. Ethylene contributes to preinvasive immunity, possibly through regulation of elicitor-triggered ROS production, which suggests that its differential effects are mediated by the specific stage of infection. Further, ethylene synthesis occurs more rapidly in response to avirulent strains than virulent ones (Ciardi et al., 2000). Based on this, the result that the massive peak in ethylene synthesis occurred relatively late, when pathogen growth was extensive, indicates that ethylene might play a role in pathogen compatibility and not defense. Ethylene production seems likely to reflect pathogen penetration into host plants and then symptom development. Therefore, it is plausible that the rapid transient increases in ROS generation followed by ROS-induced ethylene production act as a determinant of HR, whereas the later massive ROS burst and subsequent massive ethylene production act as a positive determinant of pathogen expansion and cell death in compatible interactions. Although the second massive ROS burst in infected plants induced the expression of defense-related genes, growth of the pathogen population was not disrupted. In addition, a very large ethylene production peak occurred in the later phase. These phenomena may induce significant damage to plant tissue. Compatible-specific genes differ from defense-related ones, which are triggered by pathogen-associated molecular patterns, in that they are differentially regulated depending on whether the interaction is compatible or incompatible (Huibers et al., 2009). Therefore, the increased tolerance of our transgenic plants was the result of their lower capability to produce ROS and ethylene, and not due to higher activation of the ROS-detoxification machinery. These phenomena indicate that the levels of ethylene and ROS production determine the severity of Ppn virulence.
Pathogens can be divided into two or three classes: necrotrophs, biotrophs, and more rarely, hemibiotrophs (Oliver and Ipcho, 2004). Biotrophs, which are dependent on live tissues, do not trigger necrosis and cause little damage to the host plant. In addition, they are controlled by salicyclic acid-dependent defense pathways. Meanwhile, necrotrophs, which produce toxins, are controlled by jasmonic acid- and ethylene-dependent defense pathways. Hemibiotrophs have been defined as an initial asymptomatic period of biotrophs, feeding initially on living cells, followed by necrotrophic extracellular hyphae (Perfect and Green, 2001). When challenged with the black shank hemibiotroph pathogen Ppn, plants were extensively colonized within 2 to 3 d, developing brown necrotic lesions that subsequently continued to expand until they covered the entire plant (Narusaka et al., 2004). Mechanisms of resistance to necrotrophic and hemibiotrophic pathogens are complicated. In our study, enhanced disease resistance to hemibiotrophic Ppn was observed in transgenic plants impaired in ethylene synthesis and signaling, indicating that ethylene-dependent signaling increases susceptibility to this type of pathogen.
Although H2O2 is better known for its cytotoxic effects, it has been shown recently to be an important regulator of eukaryotic signal transduction. Further, there is increasing evidence that antioxidant enzymes play multiple key roles as sensors and regulators of signal transduction in response to H2O2-mediated oxidative stress in eukaryotic cells (Veal et al., 2007). Recent studies on mammalian cells have revealed concentration-specific responses to H2O2. These studies demonstrated that antioxidant enzyme expression is induced in response to low levels of H2O2, resulting in decreased ROS levels and protection of cells from DNA damage. On the other hand, higher levels were found to stimulate the expression of prooxidants involved in apoptosis (Sablina et al., 2005). Accordingly, we hypothesize that the late massive ROS burst stimulates higher biosynthesis of ethylene, which could promote disease susceptibility. In this process, the biphasic production of ethylene and ROS might be regulated by a self-amplifying loop. The issue of ROS signal specificity has recently received considerable attention. One possibility is that the specific features of ROS signaling (amplitude, frequency, and/or duration) could be perceived and decoded into specific responses, which determine gene expression patterns (Mittler et al., 2011). Our findings shed light on the mechanisms behind the resistance to hemibiotrophic pathogen infection in plants, and they will assist the design of strategies to curb pathogen-mediated diseases.
MATERIALS AND METHODS
Plant Materials, Fungal Culture, and Inoculation
Transgenic tobacco (Nicotiana tabacum ‘Wisconsin 38’) plants impaired in genes for ethylene synthesis or signaling and ROS generation (CAS-AS, CAO-AS, Ein3-AS, rbohD-as, and rbohF-as) were used in this study (Wi et al., 2010; Ji and Park, 2011). Surface-sterilized seeds were cultured on solid Murashige and Skoog medium (pH 5.8) under light (16L/8D, 50 μmol photons m−2 s−1) at room temperature (25°C ± 5°C). Ppn was maintained on oatmeal agar at 25°C in the dark. Tobacco shoots with four to five leaves were inoculated directly with a pathogen plug (1 cm in diameter) in a culture bottle containing solid half-strength Murashige and Skoog medium. Inoculated plants were then placed in a growth chamber at 25°C and maintained at 100% relative humidity.
RNA Isolation and Real-Time qRT-PCR
Total RNA isolation was performed as described previously (Wi and Park, 2002). To analyze the relative abundance of transcripts by real-time qRT-PCR, 1 μg of total RNA from the leaf discs was reverse transcribed for 30 min at 42°C in a 20 μL reaction volume using a High Fidelity PrimeScript RT-PCR kit (Takara), according to the manufacturer’s instructions. Gene-specific PCR primers, sequence information of which was obtained from the GenBank database, were designed using a stringent set of criteria (Supplemental Table S1), including a predicted melting temperature of 60°C ± 5°C, primer lengths of 20 to 24 nucleotides, a guanine-cytosine content of 50% to 60%, and PCR amplicon lengths of 100 to 250 bp. Real-time qRT-PCR was performed in optical 96-well plates using a Chromo 4 continuous fluorescence detector (Bio-Rad). Reactions (20 μL) contained 10 μL of 2× SYBR Green master mix, 0.5 μm of each primer, and 10 ng of cDNA. PCR conditions were as follows: 95°C for 15 min; followed by 45 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 30 s; and then 72°C for 10 min. Fluorescence threshold data were analyzed using MJ Opticon monitor software version 3.1 (Bio-Rad) and then exported to Microsoft Excel for further analysis. Relative expression levels in each cDNA sample were normalized to the reference gene β-actin. PCR efficiencies (90%–95%) for all primers were determined by serial dilution of cDNA from RNA samples.
Quantification of ROS
Quantification of ROS levels was based on the oxidation of DCFH-DA (Sigma). Mechanistically, DCFH-DA can be transported across the cell membrane and deacetylated by esterase to form nonfluorescent DCFH. This compound is then trapped inside of the cells, after which it is converted into the highly fluorescent compound DCF through the action of H2O2 and peroxidase, which can be detected and quantified based on fluorescence intensity. In brief, plant tissues were homogenized with 10 mm Tris buffer (pH 7.2) and then centrifuged at 2,000g for 5 min. The supernatant was then incubated with DCFH-DA at room temperature for 10 min in the dark. DCF fluorescence was detected by spectrofluorophotometer at an excitation wavelength of 485 nm and an emission wavelength of 525 nm (RF-1501; Shimadzu). Data were expressed as relative fluorescence units per milligram of protein.
Ethylene Measurement
Ethylene production by pathogen-treated plants was measured by enclosing the sample in 20-mL vials for 1 h. The ethylene content was determined from 1 mL of headspace gas by gas chromatography (Hewlett Packard 5890 series II) using an activated alumina column at 250°C and a flame ionization detector.
Trypan Blue Staining
To monitor plant cell death and fungal growth, plant leaves were stained as described previously (Wi et al., 2010). Shoots with several leaves inoculated with Ppn were immersed for 1 min in a boiling solution of 10 mL of lactic acid, 10 mL of glycerol, 10 g of phenol, and 0.4% (w/v) trypan blue. After the plants had cooled to room temperature for 1 h, the solution was replaced with 70% (w/v) chloral hydrate. Stained plants were decolorized overnight and then photographed using a digital camera.
Measurements of APX Activity
APX activity was determined as described by Nakano and Asada (1987). APX was assayed based on the rate of ascorbic acid oxidation at 290 nm (absorption coefficient 2.8 mm−1 cm−1) upon addition of 0.1 mm H2O2 to the reaction mixture containing 0.5 mm ascorbate, 50 mm potassium phosphate (pH 7.0), and enzyme. One unit of APX activity was defined as the amount of enzyme required for oxidation of 1 μmol ascorbate per 1 min.
Construction of RNA Interference Construct and ACS4i Transgenic Tobacco Plants
To clone sequences encoding the inverted-repeated RNA into pSK-int intermediate vector, sense and antisense PCR fragments of NtACS4 were separately cloned into both the 5′ and 3′ arms of the intron, resulting in pSK-NtACS4i. The SpeI-XhoI fragment from pSK-NtACS4i was then cloned into a modified binary vector pTA7001 containing a 35S promoter and nopaline synthase terminator, followed by induction with dexamethazone. The resulting construct was introduced into tobacco by Agrobacterium-mediated transformation. After hygromycin selection, the transgenic plants with dexamethazone treatment were used in the pathogen infection experiments.
Statistical Analyses
All experiments were repeated at least three times with three replicates, and the data from one representative experiment were presented. Statistically significant differences according to the t test between transgenic lines and the respective controls at each time point are indicated with one asterisk (*; P < 0.05) or two asterisks (**; P < 0.01). Two-way ANOVA was also performed to investigate statistical differences between the responses of wild-type and transgenic lines.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Transcript levels of RbohD and RbohF in wild-type and transgenic plants (rbohD-as and rbohF-as) after Ppn infection.
Supplemental Figure S2. Relative transcript accumulation of endogenous ROS-detoxification enzymes, MnSODmi, CuZnSODc, CAT2, GSTF, APXc, and CAT1, after Ppn infection in wild-type plants.
Supplemental Figure S3. Comparison of ethylene biosynthesis in wild-type and transgenic plants with impaired ethylene synthesis or signaling (CAS-AS, CAO-AS, and Ein3-AS) after Ppn infection.
Supplemental Figure S4. Comparison of ROS detoxification in wild-type and transgenic plants with impaired ethylene synthesis or signaling (CAS-AS, CAO-AS, and Ein3-AS).
Supplemental Figure S5. Comparison of APX activity in transgenic plants.
Supplemental Figure S6. Profiles of gene expression for the PR proteins were determined by real-time qRT-PCR during the responses against Ppn infection in wild-type and transgenic plants.
Supplemental Figure S7. Comparison of relative mRNA expression levels of genes responsible for ethylene signaling in wild-type and ACS4i plants 48 h after shoot inoculation with Ppn.
Supplemental Table S1. Sequences of PCR primers used in real-time qRT-PCR.
Supplementary Material
Acknowledgments
We are grateful to Dr. M.Y. Nam (Korea Basic Science Institute) for her helpful discussion and review in preparing this manuscript and Dr. Harald Keller (INRA, France) for generously providing cryptogein.
Glossary
- ROS
reactive oxygen species
- Rboh
respiratory burst oxidase homolog
- H2O2
hydrogen peroxide
- HR
hypersensitive response
- qRT
quantitative reverse transcription
- DCFH-DA
dichlorofluorescein diacetate
- ACC
1-aminocyclopropane-1-carboxylic acid
- AVG
aminoethoxyvinyl-Gly
- APX
ascorbate peroxidase
- Ppn
Phytophthora parasitica var nicotianae
References
- Able AJ, Guest DI, Sutherland MW. (1998) Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of phytophthora parasitica var nicotianae. Plant Physiol 117: 491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AC, Fluhr R. (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9: 1559–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241: 1086–1089 [DOI] [PubMed] [Google Scholar]
- Ciardi JA, Tieman DM, Lund ST, Jones JB, Stall RE, Klee HJ. (2000) Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol 123: 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Xu H, Yi H, Yang L, Kong Z, Zhang L, Xue S, Jia H, Ma Z. (2011) Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 6: e19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. (2011) The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 23: 412–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti R, Denoux C, Gambetta S, Dewdney J, Ausubel FM, De Lorenzo G, Ferrari S. (2008) The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol 148: 1695–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibers RP, de Jong M, Dekter RW, Van den Ackerveken G. (2009) Disease-specific expression of host genes during downy mildew infection of Arabidopsis. Mol Plant Microbe Interact 22: 1104–1115 [DOI] [PubMed] [Google Scholar]
- Ji NR, Park KY. (2011) Stress-induced biphasic ethylene and ROS biosynthesis are synergistically interacted in cell damage. J Plant Biotechnol 38: 22–29 [Google Scholar]
- Knoester M, Pieterse CM, Bol JF, Van Loon LC. (1999) Systemic resistance in Arabidopsis induced by rhizobacteria requires ethylene-dependent signaling at the site of application. Mol Plant Microbe Interact 12: 720–727 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19: 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox LF, Brouwershaven IR, Vossenberg BT, Beld HE, Bonants PJ, Gruyter J. (2007) Diagnostic values and utility of immunological, morphological, and molecular methods for in planta detection of Phytophthora ramorum. Phytopathology 97: 1119–1129 [DOI] [PubMed] [Google Scholar]
- Lherminier J, Elmayan T, Fromentin J, Elaraqui KT, Vesa S, Morel J, Verrier JL, Cailleteau B, Blein JP, Simon-Plas F. (2009) NADPH oxidase-mediated reactive oxygen species production: subcellular localization and reassessment of its role in plant defense. Mol Plant Microbe Interact 22: 868–881 [DOI] [PubMed] [Google Scholar]
- Mandal S, Das RK, Mishra S. (2011) Differential occurrence of oxidative burst and antioxidative mechanism in compatible and incompatible interactions of Solanum lycopersicum and Ralstonia solanacearum. Plant Physiol Biochem 49: 117–123 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. (2011) ROS signaling: the new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Mur LA, Laarhoven LJ, Harren FJ, Hall MA, Smith AR. (2008) Nitric oxide interacts with salicylate to regulate biphasic ethylene production during the hypersensitive response. Plant Physiol 148: 1537–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. (1987) Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate depleted medium. Plant Cell Physiol 28: 131–140 [Google Scholar]
- Narusaka Y, Narusaka M, Seki M, Umezawa T, Ishida J, Nakajima M, Enju A, Shinozaki K. (2004) Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Mol Biol 55: 327–342 [DOI] [PubMed] [Google Scholar]
- Olbrich M, Gerstner E, Welzl G, Fleischmann F, Osswald W, Bahnweg G, Ernst D. (2008) Quantification of mRNAs and housekeeping gene selection for quantitative real-time RT-PCR normalization in European beech (Fagus sylvatica L.) during abiotic and biotic stress. Z Naturforsch C 63: 574–582 [DOI] [PubMed] [Google Scholar]
- Oliver RP, Ipcho SV. (2004) Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens. Mol Plant Pathol 5: 347–352 [DOI] [PubMed] [Google Scholar]
- Perfect SE, Green JR. (2001) Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol Plant Pathol 2: 101–108 [DOI] [PubMed] [Google Scholar]
- Pieterse CM, van Wees SC, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon LC. (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancé I, Fournier J, Esquerré-Tugayé MT. (1998) The incompatible interaction between Phytophthora parasitica var. nicotianae race 0 and tobacco is suppressed in transgenic plants expressing antisense lipoxygenase sequences. Proc Natl Acad Sci USA 95: 6554–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci P, Bonnet P, Huet JC, Sallantin M, Beauvais-Cante F, Bruneteau M, Billard V, Michel G, Pernollet JC. (1989) Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur J Biochem 183: 555–563 [DOI] [PubMed] [Google Scholar]
- Rosebrock TR, Zeng L, Brady JJ, Abramovitch RB, Xiao F, Martin GB. (2007) A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448: 370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. (2005) The antioxidant function of the p53 tumor suppressor. Nat Med 11: 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D, Hardham AR, Jones DA. (2005) Differences in cell death induction by Phytophthora elicitins are determined by signal components downstream of MAP kinase kinase in different species of Nicotiana and cultivars of Brassica rapa and Raphanus sativus. Plant Physiol 138: 1491–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Van Pelt JA, Van Loon LC, Pieterse CM. (2002) Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis. Mol Plant Microbe Interact 15: 27–34 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Geraats BP, Linthorst HJ. (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11: 184–191 [DOI] [PubMed] [Google Scholar]
- Veal EA, Day AM, Morgan BA. (2007) Hydrogen peroxide sensing and signaling. Mol Cell 26: 1–14 [DOI] [PubMed] [Google Scholar]
- Véronési C, Rickauer M, Fournier J, Pouénat ML, Esquerré-Tugayé MT. (1996) Lipoxygenase gene expression in the tobacco-Phytophthora parasitica nicotianae interaction. Plant Physiol 112: 997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wi SJ, Jang SJ, Park KY. (2010) Inhibition of biphasic ethylene production enhances tolerance to abiotic stress by reducing the accumulation of reactive oxygen species in Nicotiana tabacum. Mol Cells 30: 37–49 [DOI] [PubMed] [Google Scholar]
- Wi SJ, Park KY. (2002) Antisense expression of carnation cDNA encoding ACC synthase or ACC oxidase enhances polyamine content and abiotic stress tolerance in transgenic tobacco plants. Mol Cells 13: 209–220 [PubMed] [Google Scholar]
- Xie YJ, Xu S, Han B, Wu MZ, Yuan XX, Han Y, Gu Q, Xu DK, Yang Q, Shen WB. (2011) Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant J 66: 280–292 [DOI] [PubMed] [Google Scholar]
- Yan HZ, Liou RF. (2006) Selection of internal control genes for real-time quantitative RT-PCR assays in the oomycete plant pathogen Phytophthora parasitica. Fungal Genet Biol 43: 430–438 [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Sugie K, Park HJ, Maeda H, Tsuda N, Kawakita K, Doke N. (2001) Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol Plant Microbe Interact 14: 725–736 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.