Abstract
Phospholipase D (PLD) is involved in responses to abiotic stress and abscisic acid (ABA) signaling. To investigate the roles of two Arabidopsis (Arabidopsis thaliana) PLDs, PLDα1 and PLDδ, in ABA signaling in guard cells, we analyzed ABA responses in guard cells using Arabidopsis wild type, pldα1 and pldδ single mutants, and a pldα1 pldδ double mutant. ABA-induced stomatal closure was suppressed in the pldα1 pldδ double mutant but not in the pld single mutants. The pldα1 and pldδ mutations reduced ABA-induced phosphatidic acid production in epidermal tissues. Expression of either PLDα1 or PLDδ complemented the double mutant stomatal phenotype. ABA-induced stomatal closure in both pldα1 and pldδ single mutants was inhibited by a PLD inhibitor (1-butanol ), suggesting that both PLDα1 and PLDδ function in ABA-induced stomatal closure. During ABA-induced stomatal closure, wild-type guard cells accumulate reactive oxygen species and nitric oxide and undergo cytosolic alkalization, but these changes are reduced in guard cells of the pldα1 pldδ double mutant. Inward-rectifying K+ channel currents of guard cells were inhibited by ABA in the wild type but not in the pldα1 pldδ double mutant. ABA inhibited stomatal opening in the wild type and the pldδ mutant but not in the pldα1 mutant. In wild-type rosette leaves, ABA significantly increased PLDδ transcript levels but did not change PLDα1 transcript levels. Furthermore, the pldα1 and pldδ mutations mitigated ABA inhibition of seed germination. These results suggest that PLDα1 and PLDδ cooperate in ABA signaling in guard cells but that their functions do not completely overlap.
Stomatal pores are formed by pairs of guard cells and mediate transpiration and carbon dioxide uptake. Abscisic acid (ABA) synthesized in plants subjected to drought stress induces stomatal closure in order to suppress water loss in plants (Assmann and Shimazaki, 1999; Schroeder et al., 2001).
Phospholipase D (PLD) activity increases in response to hyperosmotic stress and dehydration in Craterostigma plantagineum (Frank et al., 2000; Munnik et al., 2000) and Arabidopsis (Arabidopsis thaliana; Katagiri et al., 2001). PLDs hydrolyze phospholipids, phosphatidylcholine (PC), and phosphatidylethanolamine (PE), releasing phosphatidic acid (PA) and their head group in the plasma membrane. The released PA is thought to function as a signal molecule in cellular signaling.
The Arabidopsis genome has 12 PLD genes that are classified into six subfamilies, α, β, γ, δ, ε, and ζ (Wang, 2005). Pharmacological studies examining the effect of a PLD inhibitor (1-butanol [1-BuOH]) and exogenous application of PA in Vicia faba have suggested that ABA signaling is mediated by PLDs (Jacob et al., 1999). Previous studies have reported that PLDα1 positively regulates ABA-induced stomatal closure (Zhang et al., 2004) and the inhibition of stomatal opening by ABA (Mishra et al., 2006). Furthermore, PLDα1 activity is reported to be modulated by a G protein, GPA1, in the process of inhibition of stomatal opening by ABA (Zhao and Wang, 2004; Mishra et al., 2006). However, a pldα1 loss-of-function mutation alone did not inhibit ABA-induced stomata closure (Siegel et al., 2009), which suggests that other PLDs are involved in ABA signaling in guard cells.
PLDδ is involved in responses to drought and salinity stress (Katagiri et al., 2001) and cold stress (Li et al., 2004). PLDα1 and PLDδ are required for tolerance to high salinity and hyperosmotic stress (Bargmann et al., 2009). Therefore, we hypothesized that PLDδ functions cooperatively with PLDα1 in the ABA signal pathway in guard cells.
ABA signal transduction in guard cells is mediated by various signaling molecules, including reactive oxygen species (ROS), nitric oxide (NO), Ca2+, sphingosine-1-phosphate, and inositol 1,4,5-triphosphate (Zhang et al., 2001; Desikan et al., 2002; Coursol et al., 2003; Sokolovski et al., 2005; Lee et al., 2007; Roelfsema and Hedrich, 2010). In addition, ABA-induced stomatal closure is accompanied by various events, including cytosolic free calcium concentration ([Ca2+]cyt) oscillation/elevation and cytosolic alkalization (Pei et al., 2000; Murata et al., 2001; García-Mata et al., 2003; Sokolovski and Blatt, 2004; Suhita et al., 2004; Islam et al., 2010a).
In this study, we investigated the roles of PLDα1 and PLDδ in (1) ABA-induced stomatal closure, (2) ABA-induced production of ROS and NO, cytosolic pH alkalization, and cytosolic free Ca2+ elevation, and (3) ABA inhibition of inward-rectifying K+ (K+in) channel currents and stomatal opening. We report that PLDα1 and PLDδ have overlapping functions in ABA-induced stomatal closure and different functions in the ABA inhibition of light-induced stomatal opening.
RESULTS
Expression of PLDα1 and PLDδ in Guard Cells
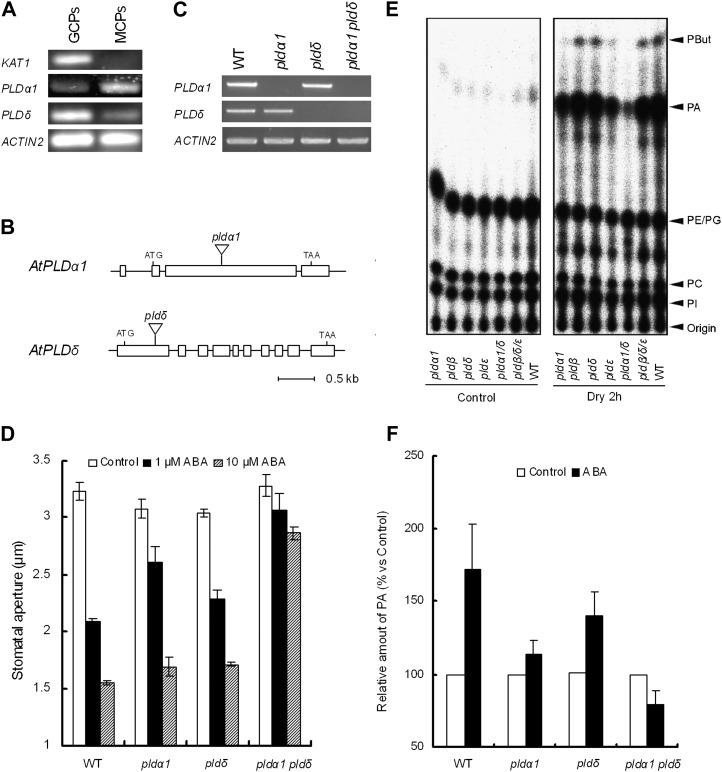
We examined the accumulation of transcripts of PLDα1 (At3g15370) and PLDδ (At4g35790) in isolated guard cell protoplasts (GCPs) using reverse transcription (RT)-PCR. POTASSIUM CHANNEL IN ARABIDOPSIS THALIANA1 (KAT1) was used as a specific GCP marker (Leonhardt et al., 2004). As shown in Figure 1A, the transcripts of PLDα1 and PLDδ were detected in both GCPs and mesophyll cell protoplasts (MCPs). The PLDα1 transcription level in GCPs appeared to be lower than that in MCPs, whereas the PLDδ transcription level in GCPs was slightly higher than that in MCPs (Fig. 1A).
Figure 1.
PLDα1 and PLDδ expression and the effect of mutation of PLDs in ABA-induced stomatal closure and PA production. A, RT-PCR analysis of PLDα1 and PLDδ gene expression in wild-type GCPs and MCPs. ACTIN2 was used as a positive control for the RT-PCR. KAT1 was used as a control of GCP gene expression. B, The positions of T-DNA insertions in pldα1 and pldδ mutants. Boxes represent exons. C, RT-PCR analysis of PLDα1 and PLDδ gene expression in the wild type (WT) and pldα1, pldδ, and pldα1 pldδ mutants. ACTIN2 was used as a positive control for the RT-PCR. D, ABA-induced stomatal closure in the wild type and pldα1, pldδ, and pldα1 pldδ mutants. Averages from three independent experiments (60 stomata per bar) are shown. E, Drought-induced PA accumulation in rosette leaves of the wild type and pld mutants. F, ABA-induced PA accumulation in epidermal tissues of the wild type and pld mutants. Radioactivity was normalized to the control values. Averages from six independent experiments are shown. Error bars represent se.
ABA-Induced Stomatal Closure in pld Mutants
We isolated mutants with T-DNA inserted in PLDα1 (SALK_063785) and PLDδ (KAZUSA T-DNA tag line) loci (Fig. 1B). A double mutant, pldα1 pldδ, was generated by crossing the single mutants. Disruption of the genes was assessed by RT-PCR with total RNA isolated from whole leaves. The transcripts of PLDα1 were not detected in the pldα1 and pldα1 pldδ mutants, and the transcripts of PLDδ were not detected in the pldδ and pldα1 pldδ mutants (Fig. 1C).
Utilizing these loss-of-function mutants, we examined the involvement of PLDα1 and PLDδ in ABA-induced stomatal closure. Application of 1 μm ABA induced stomatal closure in the wild type (P < 10−4) and both pld single mutants (P < 10−3 for pldα1, P < 10−3 for pldδ) compared with the solvent control (Fig. 1D) but not in the double mutant (P = 0.096). On the other hand, the application of 10 μm ABA slightly induced stomatal closure in the double mutant (Fig. 1D), indicating greatly reduced ABA sensitivity of double mutant stomata.
Drought induced an accumulation of PA in wild-type rosette leaves (Fig. 1E). The drought-induced PA accumulation in rosette leaves was suppressed in the pldα1 pldδ double mutant but not in either single mutant (Fig. 1E). ABA-induced PA accumulation in wild-type rosette leaves was not detected (data not shown), in agreement with previous results (Katagiri et al., 2001). On the other hand, in epidermal tissues, 50 μm ABA induced an accumulation of PA in the wild type (P < 0.05; Fig. 1F). ABA also induced a similar accumulation of PA in the pldδ single mutant (P < 0.03), which was not significantly different from the accumulation induced in the wild type (P = 0.593; Fig. 1F). ABA did not induce PA accumulation in either the pldα1 single mutant (P = 0.222) or the pldα1 pldδ double mutant (P = 0.163; Fig. 1F). Moreover, suppression of PA production by the double mutation was stronger than that of the pldα1 single mutation (P < 0.04). These results suggest that PLDα1 and PLDδ are involved in PA production induced by ABA signaling in guard cells.
Complementation of the Stomatal Phenotype of the pldα1 pldδ Double Mutant with PLDα1 or PLDδ
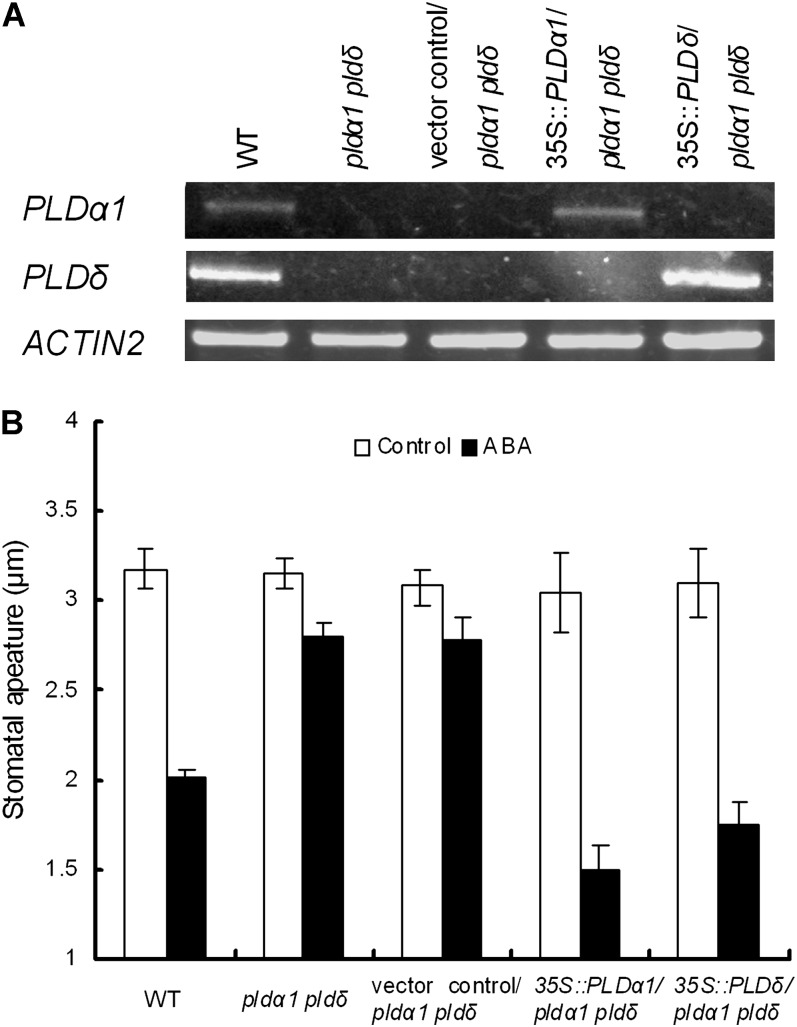
To test whether the expression of PLDα1 or PLDδ complements the stomatal phenotype of the double mutant, we generated the pldα1 pldδ mutants transformed with PLDα1 or PLDδ. PLDα1 and PLDδ transcripts were detected in the respective complement lines (Fig. 2A). The transformed plants showed a restored stomatal response to ABA (Fig. 2B), suggesting that mutations of PLDα1 and PLDδ are responsible for the ABA-insensitive phenotype observed in the pldα1 pldδ mutants.
Figure 2.
Complementation analysis of PLDα1 and PLDδ in pldα1 pldδ. A, RT-PCR analysis of PLDα1 and PLDδ gene expression in the wild type (WT), the pldα1 pldδ double mutant, pldα1 pldδ harboring the pGWB2 vector (vector control/pldα1 pldδ), a complement plant that has the PLDα1 gene cloned into pGWB2 in pldα1 pldδ (35S::PLDα1/pldα1 pldδ), and a complement plant that has the PLDδ gene cloned into pGWB2 in pldα1 pldδ (35S::PLDδ/pldα1 pldδ). ACTIN2 was used as a positive control for the RT-PCR. B, ABA-induced stomatal closure in the wild type, pldα1 pldδ, vector control/pldα1 pldδ, 35S::PLDα1/pldα1 pldδ, and 35S::PLDδ/pldα1 pldδ. Rosette leaves were treated with 10 μm ABA. Averages from three independent experiments (60 stomata per bar) are shown. Error bars represent se.
PA-Induced Stomata Closure in pld Mutants
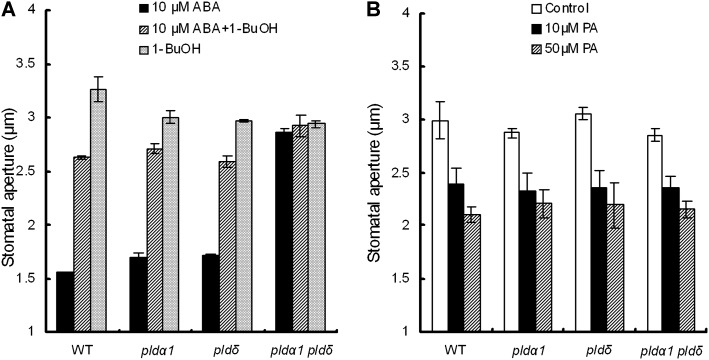
To confirm the function of PLDs in ABA signaling in guard cells, we examined the effects of a PLD inhibitor, 1-BuOH, on ABA-induced stomatal closure. ABA at 10 μm closed stomata of pldα1 and pldδ at a level comparable to the level in the wild type in the absence of 1-BuOH, whereas 1-BuOH at 50 μm suppressed the ABA-induced stomatal closure (Fig. 3A), which suggests that activation of PLDs is involved in ABA-induced stomatal closure.
Figure 3.
Effects of the PLD inhibitor 1-BuOH on ABA-induced stomata closure and PA in the wild type (WT) and pld mutants. A, Rosette leaves of the wild type and pld mutants were treated with 10 μm ABA and with or without 50 μm 1-BuOH. 1-BuOH was applied to the assay solution before ABA treatment. B, PA induced stomatal closure in pld mutants. Rosette leaves of the wild type and pldα1, pldδ, and pldα1 pldδ mutants were treated with 10 μm ABA, 10 μm PA, and 50 μm PA. Averages from three independent experiments (60 stomata per bar) are shown. Error bars represent se.
PLDs hydrolyze phospholipids, releasing PA. The released PA mediates ABA signaling, leading to stomatal closure (Jacob et al., 1999; Mishra et al., 2006; Zhang et al., 2009). We examined exogenous PA-induced stomatal closure in the pldα1 and pldδ mutants. PA at 10 and 50 μm induced stomatal closure in the wild type and the pld mutants (Fig. 3B), suggesting that downstream of PA production in the ABA signal cascade is intact in the pld mutants.
Abolishment of ABA-Induced Production of ROS and NO and Cytosolic Alkalization in pldα1 pldδ Guard Cells
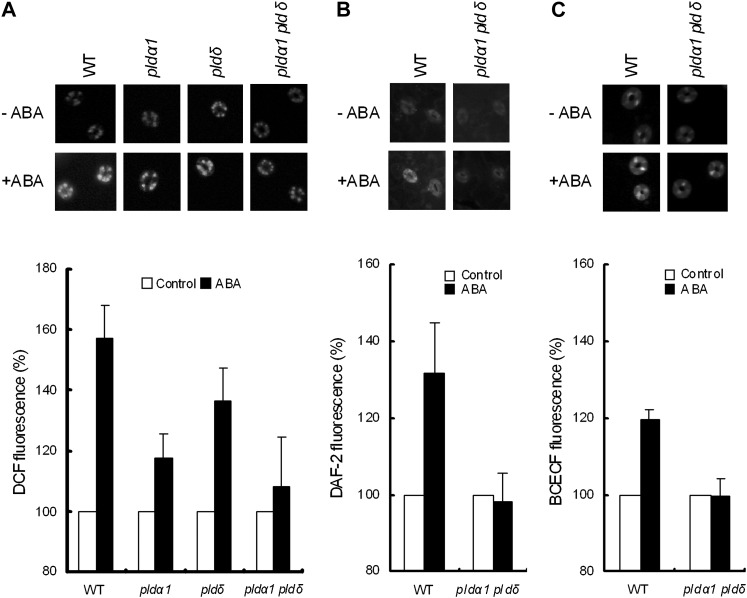
We measured ABA-induced ROS accumulation in guard cells using a hydrogen peroxide (H2O2)-sensitive fluorescent dye, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA). ABA induced ROS accumulation in guard cells in the wild type (P < 0.003; Fig. 4A), in agreement with previous results (Pei et al., 2000; Murata et al., 2001; Munemasa et al., 2007). ABA significantly induced ROS production in guard cells of both single mutants (P < 0.05 for pldα1, P < 0.02 for pldδ), while the amounts of accumulated ROS were decreased. However, ROS production was nearly abolished in the double mutant (P = 0.619; Fig. 4A).
Figure 4.
Effects of ABA (50 μm) on ROS production, NO production, and cytosolic alkalization in the wild type (WT) and pld mutants. A, Representative gray-scale DCF fluorescence images (top panel) and ROS production as shown by DCF fluorescence in the wild type, pldα1, pldδ, and pldα1 pldδ (bottom panel). B, Representative gray-scale DAF-2 images (top panel) and NO production as shown by DAF-2 fluorescence (bottom panel). C, Representative BCECF images (top panel) and cytosolic alkalization as shown by BCECF fluorescence (bottom panel). In each graph, fluorescence intensity was normalized to the control values. Bars indicate averages of three independent experiments (60 guard cells per bar). Error bars represent se.
Stomatal closure in response to H2O2 in the pld mutants was examined. Application of 100 μm H2O2 induced stomatal closure in the wild type (P < 10−3; Supplemental Fig. S1), as reported previously (Pei et al., 2000; Murata et al., 2001; Kwak et al., 2003; Zhang et al., 2009, 2011), and also in the pldα1 (P < 0.03), pldδ (P < 10−2), and pldα1 pldδ (P < 0.03) mutants (Supplemental Fig. S1). These results indicate that PLDα1 and PLDδ function upstream of ROS production in ABA signaling.
Production of NO in guard cells was examined using 4,5-diaminofluorescein-2 diacetate (DAF-2DA). ABA induced NO accumulation in wild-type guard cells (P < 0.05) but not in the pldα1 pldδ double mutant (P = 0.79; Fig. 4B). Cytosolic alkalization was investigated using 2′,7′-bis-(2-carboxyethyl)-5,(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM). ABA elicited cytosolic alkalization in the wild-type guard cells (P < 0.002) but not in the pldα1 pldδ double mutant (P = 0.95; Fig. 4C).
Exogenous PA induced ROS accumulation and cytosolic alkalization in guard cells in the wild type and the pldα1 pldδ mutant (Supplemental Fig. S2, A and C) but did not affect NO accumulation in guard cells in either the wild type or the double mutant (Supplemental Fig. S2B).
ABA-Induced Cytosolic Ca2+ Oscillations in pldα1 pldδ Guard Cells
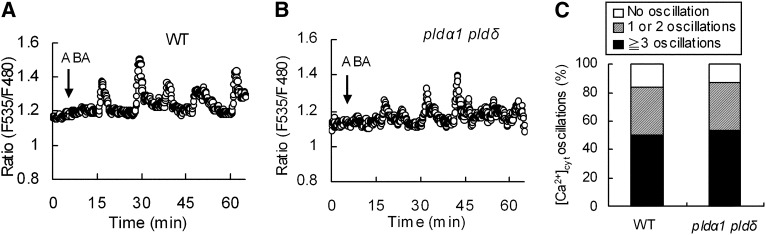
We monitored [Ca2+]cyt in guard cells using a Ca2+-sensing fluorescent protein, Yellow Cameleon 3.6 (YC3.6). When the wild-type guard cells were treated with 10 μm ABA, 83% of the guard cells showed [Ca2+]cyt transient elevation(s), hereafter [Ca2+]cyt oscillation (n = 30; Fig. 5, A and C). When the double mutant guard cells were treated with 10 μm ABA, 87% of the guard cells showed [Ca2+]cyt oscillations (n = 32; Fig. 5, B and C). The frequency of [Ca2+]cyt oscillations was not significantly different between the wild type and the double mutant, suggesting that neither PLDα1 nor PLDδ is involved in [Ca2+]cyt oscillation in guard cells in response to ABA.
Figure 5.
ABA-elicited [Ca2+]cyt oscillations in wild-type (WT) and pldα1 pldδ guard cells. A and B, Representative fluorescence emission ratios (F535/F480 nm) showing [Ca2+]cyt oscillations in 10 μm ABA-treated wild-type guard cells (n = 25 of 30 cells; 83% [A]) and pldα1 pldδ guard cells (n = 28 of 32 cells; 87% [B]). C, Stack column representation of ABA-induced [Ca2+]cyt oscillations (%) in wild-type guard cells (n = 30) and pldα1 pldδ guard cells (n = 32).
ABA Inhibition of K+in Channel Currents in Guard Cells
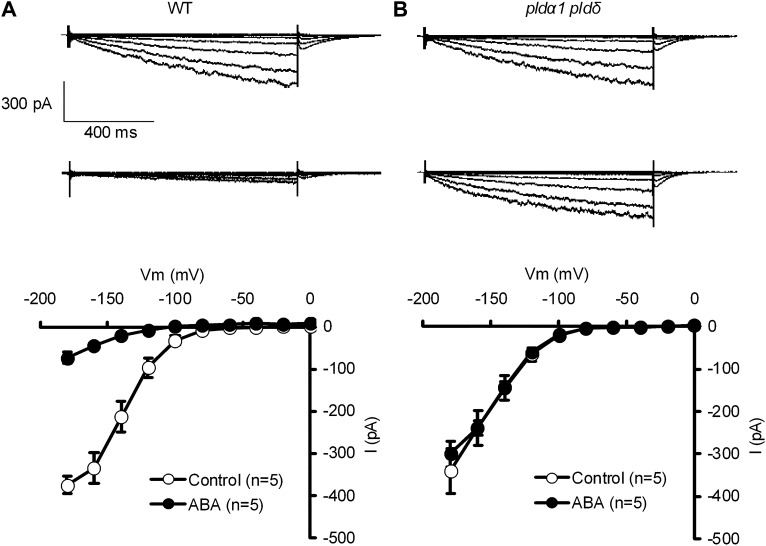
In the absence of ABA, K+in channel currents in GCPs were not significantly different between the wild type and the double mutant (P = 0.70). However, in the presence of ABA, K+in channel currents were reduced in the wild type (P < 10−3; Fig. 6, A and B) but not in the double mutant (P = 0.24; Fig. 6, C and D). These results indicate that PLDα1 and PLDδ are involved in the inhibition of the K+in channel by ABA signaling.
Figure 6.
Effects of ABA on K+in channel currents in wild-type (WT) GCPs and pldα1 pldδ double mutant GCPs. A, K+in channel currents in wild-type GCPs treated without ABA (top trace) or with 10 μm ABA (bottom trace). B, K+in channel currents in pldα1 pldδ double mutant GCPs treated without ABA (top trace) or with 10 μm ABA (bottom trace). C, Current-voltage relationships for effects of ABA on K+in channel currents in wild-type GCPs as recorded in A. D, Current-voltage relationships for effects of ABA on K+in channel currents in pldα1 pldδ double mutant GCPs as recorded in B. The voltage protocol was stepped up from 0 to −180 mV in 20-mV decrements (holding potential, −40 mV). Error bars represent se.
Inhibition of Stomatal Opening by ABA
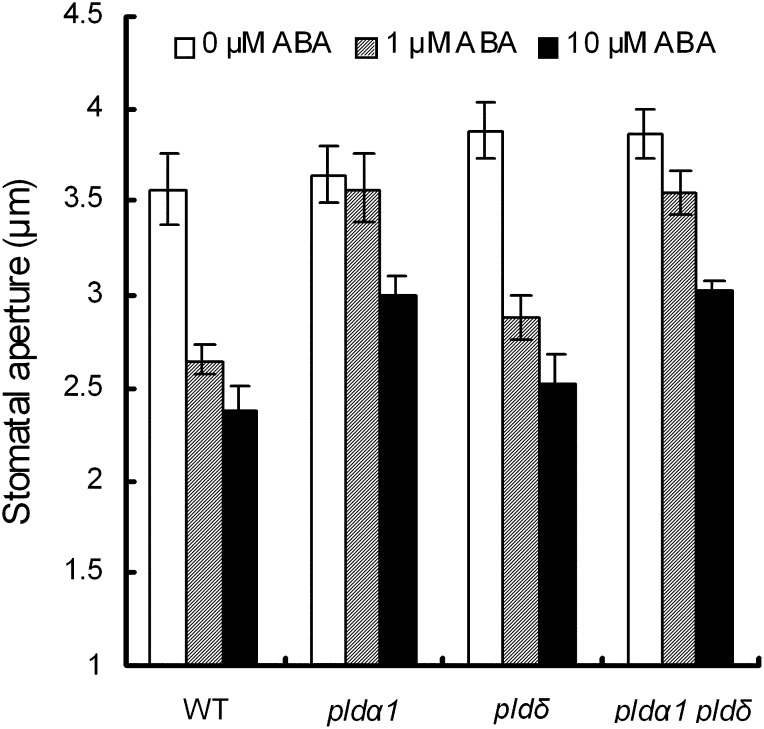
One of ABA’s many roles is to inhibit light-induced stomatal opening (Shimazaki et al., 2007), and PLDα1 is reported to be involved in the ABA inhibition of light-induced stomata opening (Mishra et al., 2006). ABA at 1 μm inhibited stomatal opening in the wild type (P < 0.02) and the pldδ single mutant (P < 0.01) but did not inhibit it in the pldα1 single mutant (P = 0.76) or the pldα1 pldδ double mutant (P = 0.15; Fig. 7). On the other hand, 10 μm ABA slightly inhibited stomatal opening in the pldα1 single mutant and the pldα1 pldδ double mutant. These results suggest that PLDδ functions differently from PLDα1 in the inhibition of light-induced stomatal opening.
Figure 7.
Inhibition of light-induced stomatal opening by ABA in pld mutants. Rosette leaves of wild type (WT), pldα1, pldδ, and pldα1 pldδ plants were placed in the dark for 2 h. These leaves were replaced in the light in the presence and absence of 1 or 10 μm ABA. Averages from three independent experiments (60 total stomata per bar) are shown. Error bars represent se.
Inhibition of Seed Germination by ABA
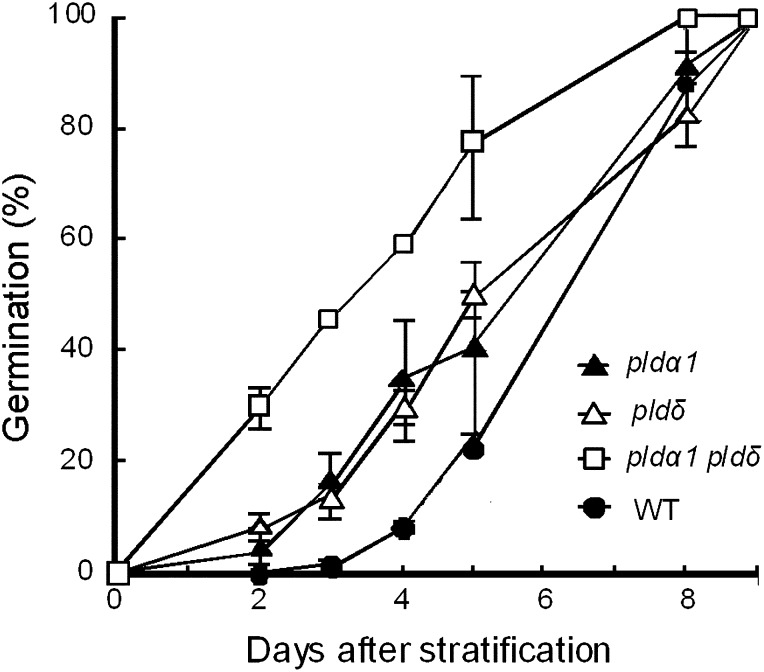
The inhibitory effect of ABA on seed germination was slightly reduced in both of the single mutants and strongly reduced in the double mutant (Fig. 8). These results suggest that PLDα1 and PLDδ cooperatively function not only in stomatal closure but also in seed germination. In contrast, root growth was inhibited by ABA in a dose-dependent manner in the wild type and the pld mutants (data not shown). Hence, PLDα1 and PLDδ do not appear to be involved in all ABA signaling in Arabidopsis.
Figure 8.
Effects of 3 μm ABA on the germination of wild-type (WT) and pld mutant seeds. Averages from three independent experiments (100 seeds per replication) are shown. Error bars represent se.
Effects of ABA on Transcription of PLDδ
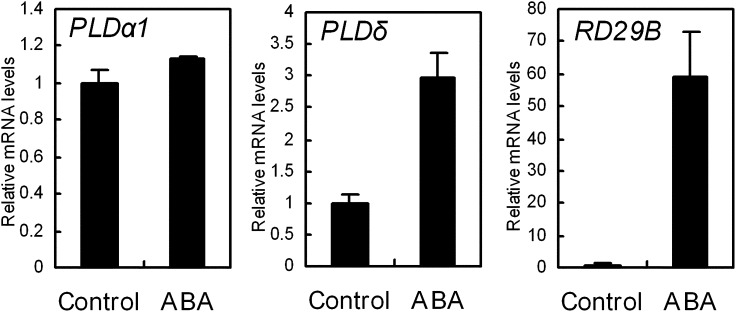
Rosette leaves were placed in stomatal assay solution with and without 50 μm ABA under the light condition for 2 h. Total RNA was isolated from the leaves, and transcript levels of PLDα1, PLDδ, and RESPONSIVE TO DEHYDRATION B (RD29B), a positive control for the ABA response (Yamaguchi-Shinozaki and Shinozaki, 1993), were measured by quantitative real-time PCR. The amounts of PLDα1 transcripts in the untreated and treated leaves were not significant different (P = 0.13; Fig. 9, left panel), but the amount of PLDδ transcript in the ABA-treated leaves was three times higher than that in the untreated leaves (P < 0.002; Fig. 9, middle panel). RD29B transcripts were remarkably increased by ABA (Fig. 9, right panel), as expected. These results indicate that transcription of PLDα1 is constitutive and that transcription of PLDδ is ABA inducible. They also suggest that PLDδ functions in ABA signaling by regulating gene transcription.
Figure 9.
Real-time PCR analysis of PLDα1, PLDδ, and RD29B gene expression in the wild type. Total RNA was isolated from leaves treated with 50 μm ABA and from untreated leaves, as was done in the stomatal assay procedure. Transcript levels were normalized to the expression of ACTIN2 in the control. Three independent experiments were done. Error bars represent se.
DISCUSSION
Cooperative Function of PLDα1 and PLDδ in ABA Signaling in Arabidopsis Guard Cells
In the pldα1 single mutant, most studies have reported that ABA-induced stomatal closure is impaired (Zhang et al., 2004, 2009; Mishra et al., 2006), although one study found no strong ABA-insensitive phenotype (Siegel et al., 2009), in agreement with our research here.
Our results show that the pldα1 pldδ double mutation disrupted ABA-induced PA production and ABA-induced stomatal closure but that the single mutations did not, suggesting that not only PLDα1 but also PLDδ positively regulate ABA-induced stomatal closure. The disruption by the double mutation was complemented by the expression of PLDα1 or PLDδ by the 35S promoter, suggesting that PLDα1 and PLDδ cooperatively function in ABA signaling in guard cells.
A PLD inhibitor, 1-BuOH, inhibited ABA-induced stomatal closure in the pldα1 and pldδ single mutants as it did in the wild type (Fig. 3A), suggesting that other PLDs are involved in ABA-induced stomatal closure in each pld mutant. Moreover, ABA-induced stomatal closure in the pldα1 pldδ double mutant was not completely inhibited (Fig. 1D), but it was completely inhibited by the application of 1-BuOH (Fig. 3A). This implies that other PLDs are involved in ABA signaling in guard cells.
PLDα1 and PLDδ Are Involved in ROS and NO Production and Cytosolic Alkalization But Not in Calcium Oscillation in ABA-Induced Stomatal Closure
ABA induces ROS production in guard cells, resulting in stomatal closure (Pei et al., 2000; Murata et al., 2001). The ROS production is mediated by NADPH oxidases, encoded by RESPIRATORY BURST OXIDASE HOMOLOG D (AtrbohD) and AtrbohF genes (Kwak et al., 2003). OPEN STOMATA1 kinase has been reported to activate AtrbohF via phosphorylation in ABA signaling (Sirichandra et al., 2009), and PA has also been reported to activate AtrbohF via binding (Zhang et al., 2009), suggesting that ABA-induced ROS production occurs downstream of PA production in ABA signaling in guard cells.
In this study, ABA-induced ROS production in guard cells was partially suppressed in the single mutants and completely suppressed in the double mutant (Fig. 4A), suggesting that both PLDα1 and PLDδ are involved in ABA-induced ROS production and that PLDα1 and PLDδ cooperatively function upstream of ABA-induced ROS production in guard cells. Like ROS production, NO production and cytosolic alkalization are also accompanied by ABA-induced stomatal closure (Irving et al., 1992; Desikan et al., 2002; Suhita et al., 2004; García-Mata and Lamattina, 2007; Gonugunta et al., 2008; Islam et al., 2010a). ABA-induced NO production and cytosolic alkalization were also impaired in the pldα1 pldδ double mutant (Fig. 4, B and C), suggesting that PLDα1 and PLDδ positively regulate NO production and cytosolic alkalization in ABA signaling.
PLDs hydrolyze phospholipids, releasing PA as a second messenger. Our findings that PA induces ROS production (Supplemental Fig. S2A) and cytosolic alkalization (Supplemental Fig. S2C) confirm that PLDα1 and PLDδ function upstream of ROS production and cytosolic alkalization in ABA signaling.
In our study, PA treatment did not evoke NO production in guard cells (Supplemental Fig. S2B). Previous reports have shown that NO production in guard cells is dependent on ABA-induced H2O2 (Bright et al., 2006), suggesting that NO production is downstream of ROS production. However, another report has shown that NO induces PA production (Distéfano et al., 2008). The protein phosphatase 2C abi1 mutation impaired ABA-induced ROS production (Murata et al., 2001) but not ABA-induced NO production (Desikan et al., 2002). Moreover, Lozano-Juste and León (2010) have proposed a NO-independent regulatory mechanism of ABA-induced stomatal closure. Taken together, these results indicate that PA is closely involved in ROS production and cytosolic alkalization in ABA signaling and that the roles of NO production in ABA signaling remain to be investigated.
[Ca2+]cyt oscillation/elevation is known to occur during ABA-induced stomatal closure (Allen et al., 2000, 2001) and is closely related with ROS production in guard cells (Pei et al., 2000; Islam et al., 2010a, 2010b). Activation of PLDα1 and PLDδ requires Ca2+, since these PLDs contain a conserved C2 domain that participates in Ca2+/phospholipid binding (Li et al., 2009), suggesting that PLD activities are affected by [Ca2+]cyt elevation in guard cells. PA production and [Ca2+]cyt oscillation/elevation may occur not only in tandem but also in parallel in ABA signaling in guard cells.
In this study, ABA induced [Ca2+]cyt oscillation but not ROS production in the pldα1 pldδ double mutant (Fig. 5). These results contradict the current ABA-signaling model, in which Ca2+-permeable cation channels in the plasma membrane are activated by H2O2 (Pei et al., 2000; Kwak et al., 2003). However, long-term Ca2+ programmed stomatal closure requires stimulus-specific calcium oscillations; that is, certain specific Ca2+ signatures inhibit stomatal reopening after Ca2+ (reactive) stomatal closure (Allen et al., 2000, 2001). Therefore, the H2O2-independent [Ca2+]cyt oscillations in the pldα1 pldδ double mutant may have failed to induce stomatal closure and may be attributed to a malfunction of [Ca2+]cyt homeostasis due to the double mutation. Furthermore, spatiotemporal modulation of ROS production and/or differences of pattern of [Ca2+]cyt elevation are important in guard cell ABA signaling (Allen et al., 2001; Jannat et al., 2011a, 2011b). Spatiotemporal analysis of ROS production and [Ca2+]cyt elevation should resolve this discrepancy.
Inhibition of K+in Channel Currents by ABA in GCPs
Three second messengers, H2O2, NO, and sphingoshine-1-phosphate, have been reported to inhibit K+in channel currents of GCPs in V. faba (Zhang et al., 2001; Sokolovski et al., 2005) and of Arabidopsis GCPs (Coursol et al., 2003). Exogenous PA was also reported to inhibit K+in channel currents of V. faba GCPs (Jacob et al., 1999). Our findings that exogenous PA inhibited K+in channel currents in Arabidopsis GCPs (Supplemental Fig. S3) and that ABA strongly inhibited K+in channel currents of wild-type GCPs but not of the pldα1 pldδ GCPs (Fig. 6) suggest that, in ABA signaling, PLDα1 and PLDδ are also involved in the inhibition of K+in channel currents.
PLDα1 and PLDδ Differentially Function in ABA Signaling in Guard Cells
In this study, we observed several differences of phenotype between the pldα1 and pldδ single mutants. In the case of ABA-induced stomatal closure, the pldα1 single mutation partially impaired stomatal closure induced by 1 μm ABA but the pldδ single mutation did not (Fig. 1D). ROS production by ABA in the pldα1 single mutant was smaller than that in the pldδ single mutant, even though the difference was not significant (Fig. 4A). Similarly, ABA-induced PA production in the pldα1 single mutant was less than that in the pldδ single mutant (Fig. 1F). Moreover, the pldα1 single mutation weakened the ABA inhibition of light-induced stomatal opening but the pldδ single mutation did not (Fig. 7). Together, these results suggest that PLDα1 and PLDδ have somewhat different roles in ABA signaling.
PLDα1 is located in the cytosol and plasma membrane and prefers PC to PE as a substrate (Fan et al., 1999; Li et al., 2009), whereas PLDδ is mainly located at the plasma membrane and prefers PE to PC (Gardiner et al., 2001; Qin et al., 2002; Li et al., 2009). These differences in phenotype between the pldα1 and pldδ mutants thus may be due to differences in the location of PA production and the molecular species of the produced PA between PLDα1 and PLDδ.
Moreover, PLDα1 activity is regulated by the binding of PLDα1 with the G protein α-subunit GPA1 at a DRY motif (Zhao and Wang, 2004), while PLDδ potentially interacts with G protein because it contains a DRY motif and a hydrophobic motif, which are highly conserved in G protein-binding proteins. Hence, the difference in affinity for G protein may also be responsible for the phenotype differences between the pldα1 and pldδ mutants.
The PLDα1 gene is constitutively expressed even under drought and saline conditions, whereas the PLDδ gene is inductively expressed by dehydration and salinity (Katagiri et al., 2001). Antisense suppression of PLDα1 increases PLDδ gene expression (Mane et al., 2007). Our study shows that expression of the PLDα1 gene was constitutive regardless of ABA treatment and that expression of the PLDδ gene was ABA inducible (Fig. 9). This suggests that PLDδ activity is regulated at the transcriptional level in the stomatal response to ABA. Thus, the pldα1 single mutant phenotype is susceptible to a change in PLDδ expression that is influenced by growth conditions. As a result, we could see both ABA-sensitive and ABA-hyposensitive phenotypes in the pldα1 single mutant. By contrast, PLDα1 activity may be posttranslationally regulated by other factors, such as GPA1. In other words, PLDα1 may mainly function under moderate environmental stress conditions and PLDδ may cooperatively work with PLDα1 under severe environmental stress conditions.
Other Physiological Functions of PLDα1 and PLDδ in Response to ABA
In this study, the inhibitory effect of ABA on seed germination was slightly reduced in both of the single mutants and strongly reduced in the double mutant (Fig. 8), in agreement with the result of Katagiri et al. (2005) that an accumulation of PA facilitates the inhibition of seed germination by ABA in Arabidopsis. However, in Oryza sativa, PLDβ1 mutation mitigates the inhibition of germination by ABA (Li et al., 2007). It is unknown whether the mutation also reduces PA production.
In this study, root growth was inhibited by ABA in a dose-dependent manner in the wild type and the pld mutants (data not shown), while a reduction of PA production due to the pldα1 pldδ double mutation increased the sensitivity to hyperosmotic and salt stress in Arabidopsis roots (Bargmann et al., 2009). In roots, PLDα1 and PLDδ appear to be involved in the responses to salinity and hyperosmolarity but not in the response to ABA.
CONCLUSION
Our results show that PLDα1 and PLDδ cooperatively function upstream of the production of ROS and NO and the cytosolic alkalization in ABA signaling of Arabidopsis guard cells.
MATERIALS AND METHODS
Plant Materials, Growth, and Transformation
Arabidopsis (Arabidopsis thaliana) wild type (Columbia-0) as well as pldα1, pldδ, and pldα1 pldδ mutants were grown in a growth chamber at 22°C and 60% humidity with a 16-h light period with 80 μmol m−2 s−1 photon flux density and 8 h of dark. Water containing 0.1% Hyponex was applied two to three times in 1 week on the plant growth tray. [Ca2+]cyt in guard cells was measured using a Ca2+-sensing fluorescent protein, YC3.6 (Nagai et al., 2004; Mori et al., 2006). To obtain YC3.6-expressing plants, wild-type and pldα1 pldδ double mutant plants were crossed with a Columbia-0 plant that had previously been transformed with YC3.6.
For a germination test, 100 seeds of the same age were sown on germination medium agar plates (Katagiri et al., 2001) supplemented with 1% (w/v) Suc. Germination was defined as the emergence of the radicle.
Measurement of Stomatal Aperture
Stomatal apertures were measured as described previously (Hossain et al., 2011). Briefly, excised rosette leaves were floated on an assay solution containing 5 mm KCl, 50 μm CaCl2, and 10 mm MES-Tris, pH 6.15, for 2 h in the light to induce stomatal opening followed by the addition of ABA or PA or H2O2. After a 2-h incubation, the leaves were shredded in a commercial blender for 30 s, and the remaining epidermal tissues were collected using nylon mesh. For stomatal opening, excised rosette leaves were floated on the assay solution for 2 h in the dark to induce stomatal closure. These leaves were transferred in the light for 3 h with ABA. The leaves were shredded for 30 s, and the remaining epidermis was collected. For each sample, 20 stomatal apertures were measured.
Isolation of MCPs and GCPs
MCPs and GCPs were enzymatically isolated from 4-week-old Arabidopsis plants as described previously (Leonhardt et al., 2004).
RNA Extraction and Real-Time PCR
Total RNA was isolated from whole leaves, isolated GCPs, and MCPs using Trizol reagent (Invitrogen). cDNA was prepared from 1 μg of RNA using Moloney murine leukemia virus reverse transcriptase (Takara) and oligo(dT) primers according to the manufacturer’s instructions. PCR was performed with 1 μL of RT reaction mixture using BIOTAQ DNA polymerase (Bioline) and primer sets as follows: PLDα1 (At3g15370), 5′-GCACGCCGTTTCATGATTTACGTC-3′ and 5′-TTGCACCGTCCATTGACCTCT-3′; PLDδ (At4g35790), 5′-CTGGCCCTGTGCAAGAAA-3′ and 5′-TTGCTATAACATCATACATCATCTGC-3′; and RD29B (At5g52300), 5′-AGAAGGAATGGTGGGGAAAG-3′ and 5′-CAACTCACTTCCACCGGAAT-3′. Arabidopsis ACTIN2 (At3g18780) was amplified as an internal positive control using primer set 5′-GCTGAGAGATTCAGATGCCCA-3′ and 5′-GTGGATTCCAGCAGCTTCCAT-3′. KAT1 (At5g46240) was amplified as a guard cell-expressed control using primer set 5′-AAGCATGGGATGGGAAGAGTGG-3′ and 5′-CCATTAGAGCAGTGTCGGAAGT-3′. Primers were constructed on exons of the gene that interfere with an intron. Amplified DNA products were separated using an agarose gel followed by image analysis with LAS-3000 (GE Healthcare).
Quantitative real-time PCR was performed with an Mx3000P QPCR System (Agilent Technologies) using SYBR Green (Brilliant II QPCR Master Mix; Stratagene) to monitor double-stranded DNA synthesis. A standard curve was constructed for each gene using gene fragments that had been previously amplified and quantified. The levels of gene transcript were normalized to that of ACTIN2 and expressed relative to the amounts observed under control conditions.
Generation of Complement Transgenic Plants in pldα pldδ
PLDα1 was amplified by PCR from Arabidopsis leaf cDNA using primers 5′-CACCATGGCGCAGCATCTGTTGCACGGG-3′ and 5′-AAAGGTTGTAAGGATTGGAGGCAGGTAG-3′, which were based on GenBank accession At3g15370. Similarly, PLDδ was amplified using primers 5′-CACCATGGCGGAGAAAGTATCGGAGGACG-3′ and 5′-AAACGTGGTTAAAGTGTCAGGAAGAGCC-3′, which were based on At4g35790. These primer sequences were modified to allow insertion in Gateway binary vectors. Amplified DNA fragments were cloned into pENTR/D-TOPO vector (Invitrogen). The final sequence was confirmed by sequencing with an ABI310 sequencer (ABI). The resulting entry clones were introduced into Gateway binary vector pGWB2 (Nakagawa et al., 2007) following the manufacturer’s instructions. The pldα1 pldδ double mutant was transformed using a floral dip procedure (Clough and Bent, 1998), and transformed plants were confirmed to be carrying the transgene by growth on kanamycin- and hygromycin-containing Murashige and Skoog agar medium.
Measurement of ROS and NO Production
ROS production in guard cells was analyzed using H2DCF-DA (Munemasa et al., 2011). The epidermal peels were incubated for 3 h in the assay solution containing 50 mm KCl, 50 μm CaCl2, and 10 mm MES-Tris (pH 6.15), and then 50 μm H2DCF-DA was added to the sample. The epidermal tissues were incubated for 30 min at room temperature, and then the excess dye was washed out with the solution. Collected tissues were again incubated with solution and 50 μm ABA or 50 μm PA for 20 min in the dark condition. The image was captured using a fluorescence microscope (Bio Zero BZ-8000; KEYENCE), and the pixel intensity of the fluorescence in guard cells was measured using ImageJ 1.42q (National Institutes of Health). For ABA- and PA-induced NO detection in guard cells, 10 μm 4,5-diaminofluorescein-2 diacetate was added instead of 50 μm H2DCF-DA (Munemasa et al., 2011).
Measurement of Cytosolic pH
Cytosolic pH elevation in guard cells was analyzed using BCECF-AM (Islam et al., 2010a). The epidermal peels were incubated for 3 h in an assay solution containing 50 mm KCl, 50 μm CaCl2, and 10 mm MES-Tris (pH 6.5), and then 20 μm BCECF-AM was added to the sample. The epidermal tissues were incubated for 30 min at room temperature, and then the excess dye was washed out with the assay solution. Collected tissues were incubated in the solution with 50 μm ABA or 50 μm PA for 20 min in the dark. The images were obtained and analyzed as described above.
Measurement of [Ca2+]cyt Oscillations
Four- to 6-week-old wild-type and pldα1 pldδ plants expressing YC3.6 were used for the measurement of guard cell [Ca2+]cyt oscillations as described previously (Islam et al., 2010a, 2010b; Hossain et al., 2011). The abaxial side of an excised leaf was gently mounted on a glass slide with a medical adhesive (stock no. 7730; Hollister) followed by removal of the adaxial epidermis and the mesophyll tissue with a razor blade in order to keep the lower epidermis intact on the slide. The remaining abaxial epidermis was incubated in a solution containing 5 mm KCl, 50 μm CaCl2, and 10 mm MES-Tris (pH 6.15) under light for 2 h at 22°C to promote stomatal opening. Turgid guard cells were used to measure [Ca2+]cyt oscillations. Guard cells were treated with 10 μm ABA using a peristaltic pump at 5 min after monitoring. For dual-emission ratio imaging of YC3.6, we used a 440AF21 excitation filter, a 445DRLP dichroic mirror, a 480DF30 emission filter for cyan fluorescent protein (CFP), and a 535DF25 emission filter for yellow fluorescent protein (YFP). The CFP and YFP fluorescence intensities of guard cells were imaged and analyzed using the W-View system and AQUA COSMOS software (Hamamatsu Photonics). CFP and YFP fluorescence were simultaneously monitored following simultaneous excitation of CFP and YFP.
Whole-Cell Patch-Clamp Recording of K+in Channel Currents
Arabidopsis GCPs were enzymatically isolated from rosette leaves of 4- to 6-week-old plants as described previously (Munemasa et al., 2007). Whole-cell currents were measured using a patch-clamp amplifier (model CEZ-2200; Nihon Kohden). Data were analyzed with pCLAMP 8.2 software (Molecular Devices). The pipette solution contained 30 mm KCl, 70 mm K-Glu, 2 mm MgCl2, 3.35 mm CaCl2, 6.7 mm EGTA, and 10 mm HEPES adjusted to pH 7.1 with Tris, and the bath solution contained 30 mm KCl, 2 mm MgCl2, 40 mm CaCl2, and 10 mm MES titrated to pH 5.5 with Tris (Saito et al., 2008). Osmolarity of the pipette solution and the bath solution was adjusted with d-sorbitol to 500 and 485 mmol kg−1, respectively. In order to examine the effect of ABA, GCPs were treated with 10 μm ABA for 2 h before recordings.
32P Labeling of Phospholipids of Arabidopsis Leaf Discs and Epidermis
Phospholipids were labeled with 32P as described previously (Katagiri et al., 2001). Leaf discs with a diameter of 3 mm and epidermal tissues were prepared from 3- to 4-week-old Arabidopsis plants. The discs and epidermal peels were incubated in MES-KOH buffer (pH 5.6) supplemented with 3.7 MBq mL−1 [32P]orthophosphoric acid for 12 h. For the control, 32P-labeled leaf discs were transferred into the MES-KOH buffer without [32P]orthophosphoric acid. For dehydration treatment, dry 32P-labeled leaf discs were incubated in an Eppendorf tube for 2 h. For ABA treatment, 32P-labeled leaf discs were transferred into MES-KOH buffer supplemented with 50 μm ABA and then incubated for 2 h. The 32P-labeled epidermal tissues were collected by centrifugation and washed with MES-KOH buffer. The corrected epidermal tissues were incubated in MES-KOH buffer in the absence or presence of 50 μm ABA for 2 h. After each treatment, an equal volume of MES-KOH buffer supplemented with 0.75% (v/v) 1-BuOH was added to each sample. After a 10-min incubation, the reaction was stopped by the addition of 10 μL of 60% (w/v) HClO4. The mixture was incubated in liquid nitrogen for 1 min, and then lipids were extracted from leaf discs and epidermal tissues (Katagiri et al., 2001).
Lipid Extraction and Analysis
Lipids were extracted with the addition of 3.75 volumes of CHCl3:methanol:HCl (50:100:1, v/v) and then with an equal volume of 0.9% (w/v) NaCl and 3.75 volumes of CHCl3 as described previously (Katagiri et al., 2001). The lower organic phase was washed with 3.75 volumes of methanol:water:HCl (50:50:1, v/v). Lipids were dried by vacuum centrifugation, resolved in CHCl3, and separated on a silica-gel 60 thin-layer chromatography plate (Merck) in an ethyl acetate solvent system with an organic upper phase of ethyl acetate:2,2,4-trimethylpentane:formic acid:water (13:2:3:10, v/v).
Statistical Analysis
The significance of differences between mean values of stomatal aperture and root growth were assessed by Student’s t test and two-factor factorial ANOVA. The frequency of [Ca2+]cyt oscillations was assessed by χ2 test. Differences were considered significant at P < 0.05.
Arabidopsis Genome Initiative numbers for the genes discussed in this article are as follows: PLDα1, At3g15730; PLDδ, At4g35790; KAT1, At5g46240; RD29B, At5g52300; ACTIN2, At3g18780.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of exogenous H2O2 (100 μm) on stomatal aperture in the wild type (WT) and pld mutants.
Supplemental Figure S2. Effects of exogenous PA (50 μm) on the production of ROS and NO and cytosolic alkalization in guard cells of the wild type (WT) and pld mutants.
Supplemental Figure S3. Effects of PA (50 μm) on K+in channel currents of guard cells of the wild type.
Supplementary Material
Glossary
- PLD
phospholipase D
- ABA
abscisic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PA
phosphatidic acid
- 1-BuOH
1-butanol
- ROS
reactive oxygen species
- NO
nitric oxide
- [Ca2+]cyt
cytosolic free calcium
- K+in
inward-rectifying K+
- GCP
guard cell protoplast
- RT
reverse transcription
- MCP
mesophyll cell protoplast
- H2O2
hydrogen peroxide
- H2DCF-DA
2′,7′-dichlorodihydrofluorescein diacetate
- BCECF-AM
2′,7′-bis-(2-carboxyethyl)-5,(6)-carboxyfluorescein acetoxymethyl ester
- YC3.6
Yellow Cameleon 3.6
- CFP
cyan fluorescent protein
- YFP
yellow fluorescent protein
References
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Assmann SM, Shimazaki Ki. (1999) The multisensory guard cell: stomatal responses to blue light and abscisic acid. Plant Physiol 119: 809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann BOR, Laxalt AM, ter Riet B, van Schooten B, Merquiol E, Testerink C, Haring MA, Bartels D, Munnik T. (2009) Multiple PLDs required for high salinity and water deficit tolerance in plants. Plant Cell Physiol 50: 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Coursol S, Fan LM, Le Stunff H, Spiegel S, Gilroy S, Assmann SM. (2003) Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 423: 651–654 [DOI] [PubMed] [Google Scholar]
- Desikan R, Griffiths R, Hancock J, Neill S. (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA 99: 16314–16318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distéfano AM, García-Mata C, Lamattina L, Laxalt AM. (2008) Nitric oxide-induced phosphatidic acid accumulation: a role for phospholipases C and D in stomatal closure. Plant Cell Environ 31: 187–194 [DOI] [PubMed] [Google Scholar]
- Fan L, Zheng S, Cui D, Wang X. (1999) Subcellular distribution and tissue expression of phospholipase Dα, Dβ, and Dγ in Arabidopsis. Plant Physiol 119: 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank W, Munnik T, Kerkmann K, Salamini F, Bartels D. (2000) Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell 12: 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. (2007) Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide 17: 143–151 [DOI] [PubMed] [Google Scholar]
- Gardiner JC, Harper JD, Weerakoon ND, Collings DA, Ritchie S, Gilroy S, Cyr RJ, Marc J. (2001) A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell 13: 2143–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonugunta VK, Srivastava N, Puli MR, Raghavendra AS. (2008) Nitric oxide production occurs after cytosolic alkalinization during stomatal closure induced by abscisic acid. Plant Cell Environ 31: 1717–1724 [DOI] [PubMed] [Google Scholar]
- Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y. (2011) Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol 156: 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving HR, Gehring CA, Parish RW. (1992) Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc Natl Acad Sci USA 89: 1790–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Hossain MA, Jannat R, Munemasa S, Nakamura Y, Mori IC, Murata Y. (2010a) Cytosolic alkalization and cytosolic calcium oscillation in Arabidopsis guard cells response to ABA and MeJA. Plant Cell Physiol 51: 1721–1730 [DOI] [PubMed] [Google Scholar]
- Islam MM, Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. (2010b) Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol 51: 302–311 [DOI] [PubMed] [Google Scholar]
- Jacob T, Ritchie S, Assmann SM, Gilroy S. (1999) Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA 96: 12192–12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannat R, Uraji M, Morofuji M, Hossain MA, Islam MM, Nakamura Y, Mori IC, Murata Y. (2011a) The roles of CATALASE2 in abscisic acid signaling in Arabidopsis guard cells. Biosci Biotechnol Biochem 75: 2034–2036 [DOI] [PubMed] [Google Scholar]
- Jannat R, Uraji M, Morofuji M, Islam MM, Bloom RE, Nakamura Y, McClung CR, Schroeder JI, Mori IC, Murata Y. (2011b) Roles of intracellular hydrogen peroxide accumulation in abscisic acid signaling in Arabidopsis guard cells. J Plant Physiol 168: 1919–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Ishiyama K, Kato T, Tabata S, Kobayashi M, Shinozaki K. (2005) An important role of phosphatidic acid in ABA signaling during germination in Arabidopsis thaliana. Plant J 43: 107–117 [DOI] [PubMed] [Google Scholar]
- Katagiri T, Takahashi S, Shinozaki K. (2001) Involvement of a novel Arabidopsis phospholipase D, AtPLDdelta, in dehydration-inducible accumulation of phosphatidic acid in stress signalling. Plant J 26: 595–605 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim YW, Jeon BW, Park KY, Suh SJ, Seo J, Kwak JM, Martinoia E, Hwang I, Lee Y. (2007) Phosphatidylinositol 4,5-bisphosphate is important for stomatal opening. Plant J 52: 803–816 [DOI] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI. (2004) Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Lin F, Xue HW. (2007) Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLD beta 1 in seed germination. Cell Res 17: 881–894 [DOI] [PubMed] [Google Scholar]
- Li M, Hong Y, Wang X. (2009) Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochim Biophys Acta 1791: 927–935 [DOI] [PubMed] [Google Scholar]
- Li W, Li M, Zhang W, Welti R, Wang X. (2004) The plasma membrane-bound phospholipase Ddelta enhances freezing tolerance in Arabidopsis thaliana. Nat Biotechnol 22: 427–433 [DOI] [PubMed] [Google Scholar]
- Lozano-Juste J, León J. (2010) Nitric oxide modulates sensitivity to ABA. Plant Signal Behav 5: 314–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mane SP, Vasquez-Robinet C, Sioson AA, Heath LS, Grene R. (2007) Early PLDalpha-mediated events in response to progressive drought stress in Arabidopsis: a transcriptome analysis. J Exp Bot 58: 241–252 [DOI] [PubMed] [Google Scholar]
- Mishra G, Zhang W, Deng F, Zhao J, Wang X. (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312: 264–266 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al. (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)-permeable channels and stomatal closure. PLoS Biol 4: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. (2011) The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol 155: 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y. (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells: specific impairment of ion channel activation and second messenger production. Plant Physiol 143: 1398–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Meijer HJ, Ter Riet B, Hirt H, Frank W, Bartels D, Musgrave A. (2000) Hyperosmotic stress stimulates phospholipase D activity and elevates the levels of phosphatidic acid and diacylglycerol pyrophosphate. Plant J 22: 147–154 [DOI] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder JI. (2001) Abscisic acid activation of plasma membrane Ca(2+) channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. (2004) Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101: 10554–10559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. (2007) Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Qin C, Wang C, Wang X. (2002) Kinetic analysis of Arabidopsis phospholipase Ddelta: substrate preference and mechanism of activation by Ca2+ and phosphatidylinositol 4,5-biphosphate. J Biol Chem 277: 49685–49690 [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Hedrich R. (2010) Making sense out of Ca(2+) signals: their role in regulating stomatal movements. Plant Cell Environ 33: 305–321 [DOI] [PubMed] [Google Scholar]
- Saito N, Munemasa S, Nakamura Y, Shimoishi Y, Mori IC, Murata Y. (2008) Roles of RCN1, regulatory A subunit of protein phosphatase 2A, in methyl jasmonate signaling and signal crosstalk between methyl jasmonate and abscisic acid. Plant Cell Physiol 49: 1396–1401 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ. (2001) Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410: 327–330 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, Yang Y, Nishimura N, Wang A, Schroeder JI. (2009) Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K channels in Arabidopsis guard cells. Plant J 59: 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, et al. (2009) Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Sokolovski S, Blatt MR. (2004) Nitric oxide block of outward-rectifying K+ channels indicates direct control by protein nitrosylation in guard cells. Plant Physiol 136: 4275–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR. (2005) Protein phosphorylation is a prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J 43: 520–529 [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. (2005) Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol 139: 566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. (1993) Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet 236: 331–340 [DOI] [PubMed] [Google Scholar]
- Zhang W, Jeon BW, Assmann SM. (2011) Heterotrimeric G-protein regulation of ROS signalling and calcium currents in Arabidopsis guard cells. J Exp Bot 62: 2371–2379 [DOI] [PubMed] [Google Scholar]
- Zhang W, Qin C, Zhao J, Wang X. (2004) Phospholipase D α 1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101: 9508–9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP. (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X. (2009) Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21: 2357–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wang X. (2004) Arabidopsis phospholipase Dα1 interacts with the heterotrimeric G-protein α-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J Biol Chem 279: 1794–1800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.