Abstract
The effects of low root temperature on growth and root cell water transport were compared between wild-type Arabidopsis (Arabidopsis thaliana) and plants overexpressing plasma membrane intrinsic protein 1;4 (PIP1;4) and PIP2;5. Descending root temperature from 25°C to 10°C quickly reduced cell hydraulic conductivity (Lp) in wild-type plants but did not affect Lp in plants overexpressing PIP1;4 and PIP2;5. Similarly, when the roots of wild-type plants were exposed to 10°C for 1 d, Lp was lower compared with 25°C. However, there was no effect of low root temperature on Lp in PIP1;4- and PIP2;5-overexpressing plants after 1 d of treatment. When the roots were exposed to 10°C for 5 d, Lp was reduced in wild-type plants and in plants overexpressing PIP1;4, whereas there was still no effect in PIP2;5-overexpressing plants. These results suggest that the gating mechanism in PIP1;4 may be more sensitive to prolonged low temperature compared with PIP2;5. The reduction of Lp at 10°C in roots of wild-type plants was partly restored to the preexposure level by 5 mm Ca(NO3)2 and protein phosphatase inhibitors (75 nm okadaic acid or 1 μm Na3VO4), suggesting that aquaporin phosphorylation/dephosphorylation processes were involved in this response. The temperature sensitivity of cell water transport in roots was reflected by a reduction in shoot and root growth rates in the wild-type and PIP1;4-overexpressing plants exposed to 10°C root temperature for 5 d. However, low root temperature had no effect on growth in plants overexpressing PIP2;5. These results provide strong evidence for a link between growth at low root temperature and aquaporin-mediated root water transport in Arabidopsis.
Low soil temperature is often a major factor restricting the growth and yield of plants even if the soil is not frozen (Bonan, 1992; Wan and Zwiazek, 1999; Wan et al., 2001). In plants that are sensitive to cold soils, growth reduction is accompanied by the reduction of water uptake, which usually starts within a few minutes after the temperature decrease (Bigot and Boucaud, 1996; Lee et al., 2005a). Low soil temperature that is accompanied by relatively high air temperature, with high transpirational demand, poses especially serious challenges to plants. Therefore, it is not surprising that low temperature tolerance has been often correlated with drought resistance (Janowiak et al., 2002; Aroca et al., 2003; Costa e Silva et al., 2007) and can be increased by treatments that facilitate root water uptake and/or reduce plant water loss (Pérez de Juan et al., 1997; Aroca et al., 2005; Lee et al., 2008). Reduced water flux at low temperatures is thought to occur due to higher water viscosity (Muhsin and Zwiazek, 2002) and the inhibition of transmembrane water transport (Wan et al., 2001; Lee et al., 2005a, 2005b). Contrary to chilling-sensitive cucumber (Cucumis sativus) plants, low root temperature had little or no effect on cell hydraulic conductivity (Lp) in roots of low-temperature-tolerant figleaf gourd (Cucurbita ficifolia; Lee et al., 2005a, 2005b, 2008), suggesting that the ability of plants to maintain the transmembrane water flow may be among the key factors linked to chilling tolerance.
Transmembrane water flow is regulated by aquaporins, which function as channels for water and other small neutral molecules (Echevarria et al., 1994; Ishibashi et al., 1994; Murata et al., 2000). Aquaporins have been classified into four different subfamilies based on subcellular localization and sequence similarity: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins, nodulin-like intrinsic proteins, and small basic intrinsic proteins, as well as the recently identified uncharacterized intrinsic proteins (Chaumont et al., 2001; Johanson et al., 2001; Quigley et al., 2002; Danielson and Johanson, 2008; Gupta and Sankararamakrishnan, 2009). Although low temperature was found to increase the transcript levels of some Arabidopsis (Arabidopsis thaliana) aquaporins (Jang et al., 2004), the effects of low temperature on aquaporin expression are not always clear, with down-regulation (Lian et al., 2004; Yu et al., 2005) of some aquaporins also reported. Even less is known about the effects of low temperature on aquaporin gating, which regulates water flux through protein conformational changes (Lee et al., 2005a). Gating factors known to be involved in aquaporin regulation include phosphorylation and dephosphorylation (Johansson et al., 1996, 1998), cytoplasmic pH (Tournaire-Roux et al., 2003; Sutka et al., 2005; Alleva et al., 2006), and divalent cations (Gerbeau et al., 2002).
Protein phosphorylation and dephosphorylation are common regulatory mechanisms through which many enzymes and receptor molecules are altered by environmental stimuli (Johansson et al., 2000; Kerk et al., 2002). Phosphorylation of the plasma membrane aquaporins was demonstrated to be responsible for the regulation of temperature-dependent opening of tulip (Tulipa spp.) petals (Azad et al., 2004). The phosphorylation of the Ser residue is catalyzed by the plasma membrane-associated Ca-dependent protein kinase (Johansson et al., 2000; Karlsson et al., 2003), which has been also implicated in temperature responses (Azad et al., 2004).
In this study, we used genetically transformed Arabidopsis plants overexpressing PIP1;4 and PIP2;5 (Jang et al., 2007) to examine the role of these aquaporins in the responses of plants to low root temperature. The PIP1;4 and PIP2;5 aquaporins were chosen because of the reported increase in their expression levels in roots of plants exposed to low air temperature (Jang et al., 2004). In this study, we subjected Arabidopsis roots to low temperature (10°C) whereas the shoots of plants were exposed to high transpirational demand conditions (23°C/21°C day/night temperatures) to study the effects of low root temperature on Lp and plant growth rates. We also used several inhibitors of protein phosphorylation and dephosphorylation to determine whether these processes may be involved in the responses of Lp to low temperature. We hypothesized that (1) the impact of low temperature on root water transport involves aquaporin gating through the phosphorylation/dephosphorylation processes, and (2) overexpression of the low-temperature-responsive aquaporins PIP1;4 and PIP2;5 would help the plants maintain high Lp values and, in consequence, high growth rates when their roots are exposed to low temperature.
RESULTS
Effects of Low Root Temperature on Relative Growth Rates
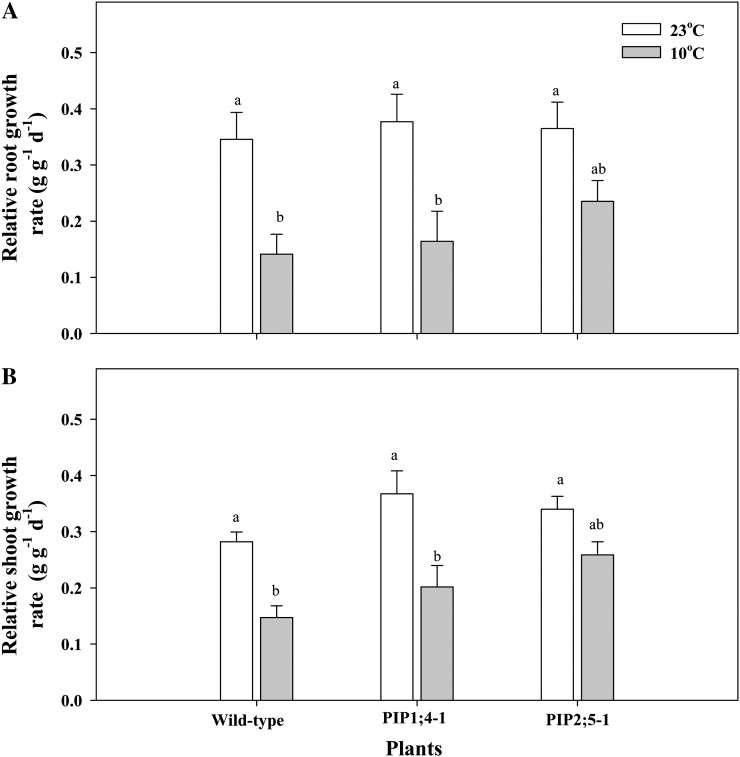
There were no significant differences in root and shoot relative growth rates between the different plant groups when exposed to 23°C root temperature (Fig. 1). However, when root zone temperature was decreased from 23°C to 10°C for 5 d, there was a sharp and statistically significant reduction of the root and shoot relative growth rates in plants of the wild type and those overexpressing PIP1;4 (Fig. 1). However, plants overexpressing PIP2:5 showed no significant differences in relative shoot and root growth rates at both root zone temperatures (Fig. 1).
Figure 1.
Shoot (A) and root (B) relative growth rates in wild-type Arabidopsis plants and in plants overexpressing PIP1;4 and PIP2;5. The plants were subjected to root temperature of 23°C or 10°C for 5 d. Data are means ± se (n = 40). The results were analyzed by ANOVA followed by Tukey’s multiple comparison.
Hydraulic Properties of Root Cells
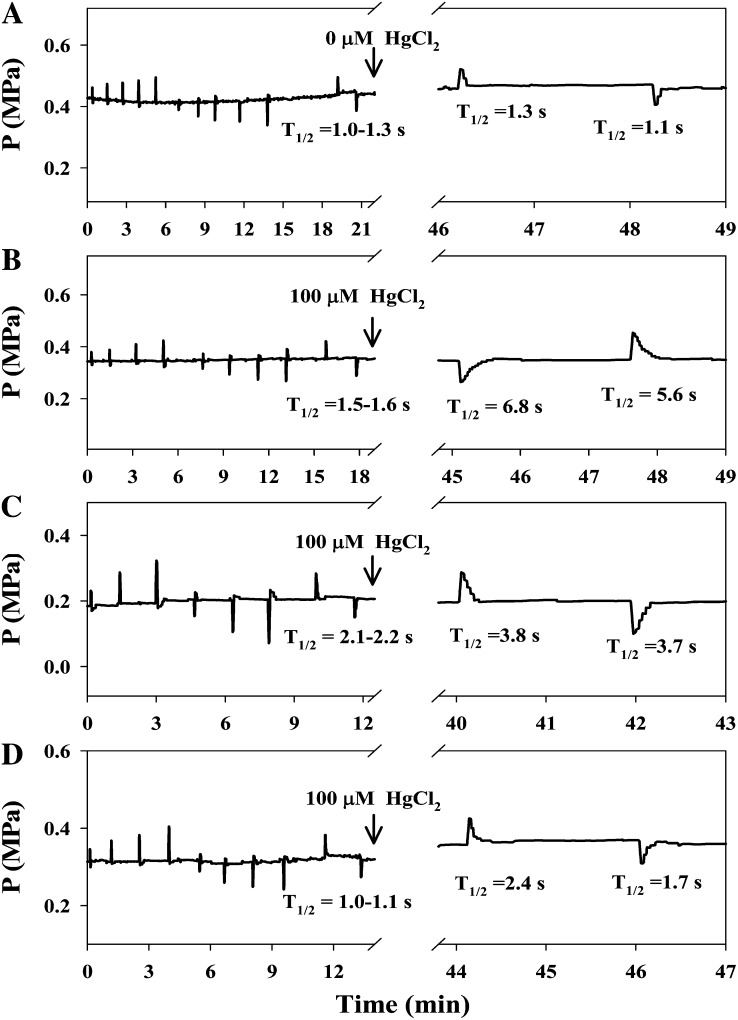
Cell dimensions and the water relations parameters turgor pressure (P), half-time of water exchange (T1/2), and cell elasticity (ε) of the root cortex cells were similar in the wild-type and PIP-overexpressing plants (Table I). The Lp values were in the range of 6.2 to 9.0 × 10−7 m s−1 MPa−1 (Tables I and II). T1/2 values in wild-type plants (Table I; Fig. 2, A and B) and overexpression plants (Table I; Fig. 2, C and D) were similar, ranging between 1 and 2 s. The addition of 100 µm HgCl2 significantly increased T1/2 by 4-fold (Fig. 2B) and decreased Lp values (Table II) in the wild-type plants but did not affect the stability of P (Fig. 2), demonstrating that mercury did not affect cell integrity in our experimental system. Similar changes, but of lower magnitude (2-fold or less), were recorded in PIP1;4- and PIP2;5-overexpressing plants (Table II; Fig. 2, C and D).
Table I. Effect of HgCl2 on the Lp of root cortical cells in Arabidopsis.
HgCl2 (100 μm) was added to the solution for 20 to 30 min, and the cell water permeability was measured before and after HgCl2 treatment. Different letters in each row and column for the wild-type and transgenic plants indicate significant differences (paired t test; P = 0.05). Values are means ± se (n = 6 cells from six plants).
| Plants |
Lp × 107 |
|
|---|---|---|
| Control | HgCl2 (100 μm) | |
| m s-1 MPa-1 | ||
| Wild type | 7.4 ± 0.2 a | 1.5 ± 0.2 b |
| PIP1;4-1 | 9.0 ± 0.2 a | 4.7 ± 0.2 b |
| PIP1;4-3 | 6.9 ± 1.8 a | 3.4 ± 0.4 b |
| PIP2;5-1 | 7.1 ± 0.2 a | 4.0 ± 0.4 b |
| PIP2;5-3 | 6.8 ± 1.1 a | 3.3 ± 0.5 b |
Table II. Effect of HgCl2 on the Lp of root cortical cells in Arabidopsis.
HgCl2 (100 μm) was added to the solution for 20 to 30 min, and the cell water permeability was measured before and after HgCl2 treatment. Different letters in each row and column for the wild-type and transgenic plants indicate significant differences (paired t test; P = 0.05). Values are means ± se (n = 6 cells from six plants).
| Plants |
Lp × 107 |
|
|---|---|---|
| Control | HgCl2 (100 μm) | |
| m s-1 MPa-1 | ||
| Wild type | 7.4 ± 0.2 a | 1.5 ± 0.2 b |
| PIP1;4-1 | 9.0 ± 0.2 a | 4.7 ± 0.2 b |
| PIP1;4-3 | 6.9 ± 1.8 a | 3.4 ± 0.4 b |
| PIP2;5-1 | 7.1 ± 0.2 a | 4.0 ± 0.4 b |
| PIP2;5-3 | 6.8 ± 1.1 a | 3.3 ± 0.5 b |
Figure 2.
Effect of HgCl2 on T1/2 in individual cortical cells of Arabidopsis roots. On the left side of the trace (8–12 min), quick changes in pressure were imposed for the determination of cell volumetric ε, and then T1/2 was continually measured in the absence and presence of HgCl2. A and B, Wild type. C, PIP1;4-1. D, PIP2;5-1.
Effects of Low Temperature on T1/2 and Lp
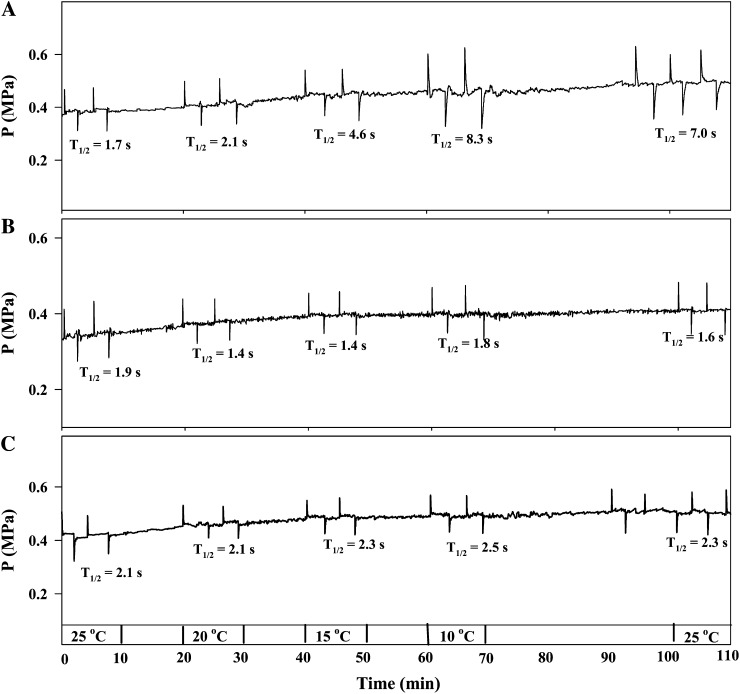
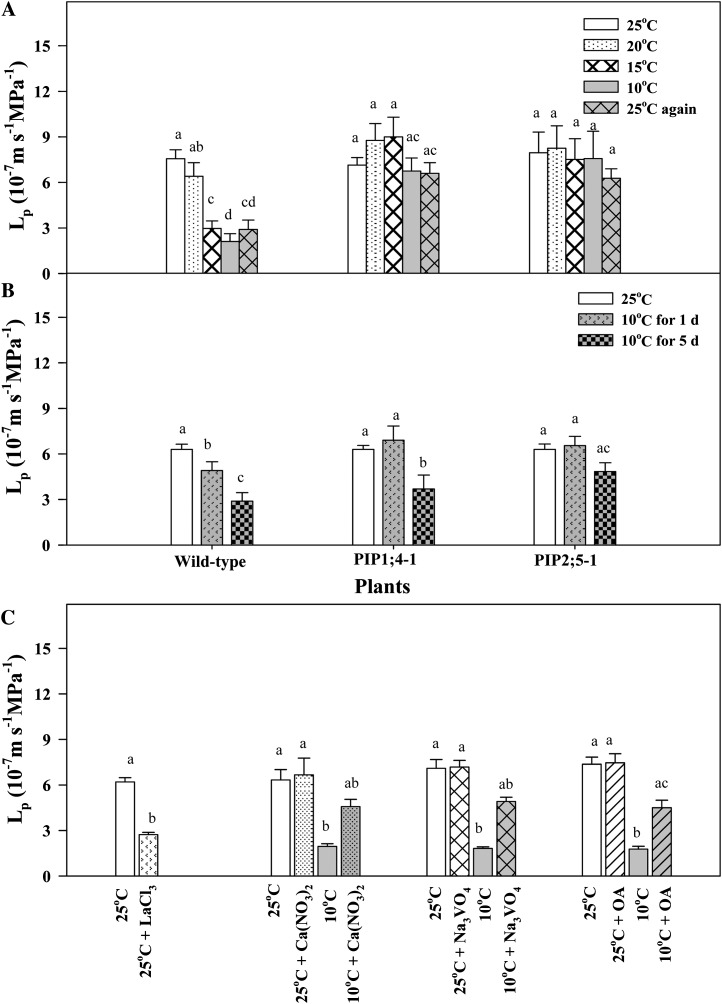
As the root temperature gradually decreased from 25°C to 10°C, T1/2 values increased in root cortex cells of the wild type (Fig. 3A) but remained unchanged in PIP-overexpressing plants (Fig. 3, B and C). Furthermore, T1/2 values in the wild-type plants did not recover after the root temperature was raised back from 10°C to 25°C (Fig. 3A). Similar results were also observed for Lp values (Fig. 4A). Short-term treatment (for 1 d) of root at 10°C did not affect Lp in PIP1;4- and PIP2;5-overexpressing plants (Fig. 4B). However, when plants were exposed to 10°C for 5 d, Lp was significantly reduced in PIP1;4-overexpressing plants but not in plants overexpressing PIP2:5 (Fig. 4B).
Figure 3.
Typical results of descending and ascending temperature effects on T1/2 in individual cortical cells of Arabidopsis roots. T1/2 was continually measured in descending temperatures (25°C, 20°C, 15°C, and 10°C) for 10 min followed by ascending temperatures to 25°C. A, Wild type. B, PIP1;4-1. C, PIP2;5-1.
Figure 4.
Effect of temperature and exogenous treatments on Lp. A, Effects of descending root temperatures (25°C, 20°C, 15°C, and 10°C) followed by the ascending root temperature of 25°C on Lp. B, Lp values in roots of plants subjected to 10°C for 1 and 5 d and in roots exposed to 25°C. The measurements were carried out in the respective treatment temperatures. C, Effects of 5 mm Ca(NO3)2, 1 mm LaCl3, 1 µm Na3VO4, and 75 nm okadaic acid (OA) examined in the wild-type plants at either 25°C or 10°C after 20- to 30-min exposure. Means ± se (n = 6) are shown. The data were analyzed for statistically significant differences using ANOVA with Tukey’s multiple comparison.
Effects of Ca(NO3)2, LaCl3, and Protein Phosphatase Inhibitors on Lp
Application of 1 mm LaCl3 (calcium channel blocker) in the wild-type plants at 25°C resulted in an over 2-fold decrease in Lp (Fig. 4C). The addition of 5 mm Ca(NO3)2 at 25°C showed no effect on Lp (Fig. 4C). However, when 5 mm Ca(NO3)2 was added at 10°C, the value of Lp was increased almost to the same level as the one measured at 25°C (Fig. 4C). Similarly, 1 mm Na3VO4 and 75 mm okadaic acid increased Lp when added to roots at 10°C (Fig. 4C).
Activation Energy for Root Water Transport
Activation energy (Ea) for Lp was 63 kJ mol−1 in the wild-type plants (Table III). In both PIP overexpression plants, Ea values for Lp were below 10 kJ mol−1 (Table III).
Table III. Ea values for water flow in root cortical cells.
Ea was measured at the temperature range of 283 to 298 K. Different letters for the wild-type and transgenic Arabidopsis plants indicate significant differences (unpaired t test; P = 0.05). Values are means ± se (n = 7).
| Plants | Ea |
|---|---|
| kJ mol−1 | |
| Wild type | 63.0 ± 11.6 a |
| PIP1;4-1 | 9.3 ± 1.6 b |
| PIP2;5-1 | 7.3 ± 2.5 b |
Relative Expression Profiles of the 13 PIP Genes in Arabidopsis Exposed to Low-Temperature Stresses
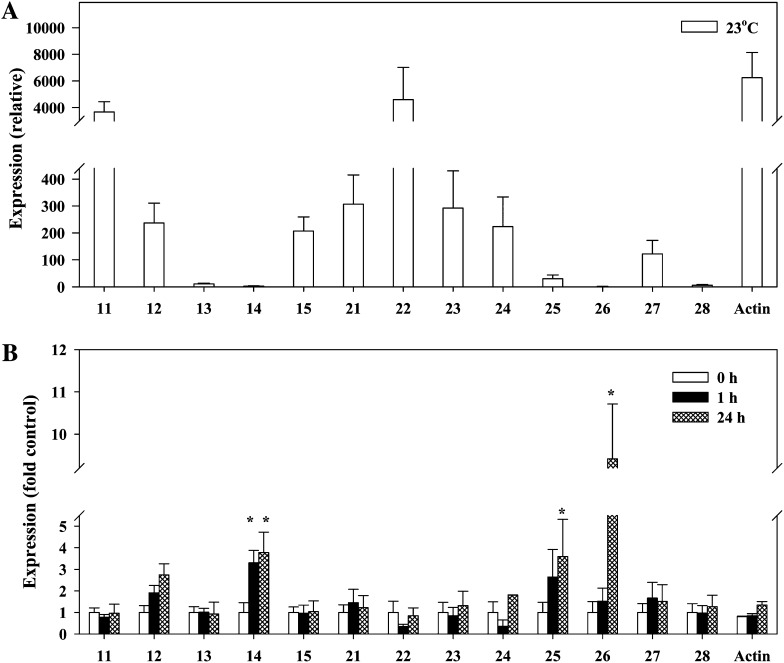
Based on the expression levels at 23°C, the 13 PIP genes in 3-week-old wild-type Arabidopsis seedlings could be classified into the high expression group (PIP1;1 and PIP2;2), the intermediate expression group (PIP1;2, PIP1;5, PIP2;1, PIP2;3, PIP2;4, and PIP2;7), and the low expression group (PIP1;3, PIP1;4, PIP2;5, PIP2;6, and PIP2;8; Fig. 5A). After subjecting the roots to 10°C for 1 and 24 h, the expression of PIP1;2, PIP1;4, PIP2;5, and PIP2;6 increased (Fig. 5B). The greatest increase (8-fold) was that of PIP2;6 in plants treated for 24 h (Fig. 5B). The expression of the low-temperature response marker RD29A increased 40-fold at 10°C after 1 h, and no significant change in the transcript level of actin was observed (data not shown).
Figure 5.
Analyses of PIP expression. A, Relative expression profile of the 13 PIP genes in roots of 3-week-old wild-type Arabidopsis seedlings at a root temperature of 23°C. The transcript levels of each PIP are plotted as the relative expression (fold) of the lowest gene expression level (PIP2;6). B, Transcript levels of the 13 PIP genes in Arabidopsis seedlings subjected to 10°C root temperature treatment for 1 and 24 h and plotted as the relative expression (fold) of the preexposure (0-h) level (plant roots at 23°C). Means ± se (n = 3) from three independent experiments are shown. The data were analyzed by an unpaired t test, and asterisks above the bars indicate statistically significant differences.
DISCUSSION
Direct and continuous measurements of water relation parameters with the cell pressure probe demonstrated that aquaporin-mediated water transport is a major factor that is responsible for growth inhibition of plants growing in cold soils. These results also emphasize once again the pivotal role of aquaporins in stress responses in plants, because many environmental stresses directly or indirectly upset water balance in plants (Fennell and Markhart, 1998; Holmberg and Bülow, 1998; Sutka et al., 2011).
Our results demonstrated that the shoot and root growth in Arabidopsis plants overexpressing PIP2;5 aquaporin was less sensitive to low root temperature compared with the wild-type plants. Low soil temperature is a common factor that inhibits plant growth (Wan et al., 2001; Lee et al., 2005a) and upsets plant water balance by reducing root water flux to the transpiring leaves (Wan and Zwiazek, 1999; Wan et al., 2001). Synchronization of leaf and root water transport in a plant is required to maintain stomatal conductance that is essential for CO2 uptake (Fischer et al., 1998; Siefritz et al., 2002; Kaldenhoff et al., 2008). In this study, higher root and shoot growth rates at 10°C root temperature in plants overexpressing PIP2;5 clearly indicate that aquaporins are involved in the processes that limit the growth of Arabidopsis plants under low-root-temperature conditions.
Our results indicate that the functional significance of the aquaporin isoforms that are weakly expressed at favorable growth temperatures, such as PIP1;4 and PIP2;5, increases when the roots of Arabidopsis are exposed to low temperature. Similar to an earlier report (Jang et al., 2004), in this study, expression levels of PIP1;4 and PIP2;5 in the wild-type plants increased after 1- and 24-h exposures of roots to 10°C. We also found a severalfold increase in another weakly expressed aquaporin, PIP2;6, but only after 24 h of low-root-temperature treatment.
It is interesting that the overexpression of PIP1;4 was effective in maintaining the high water permeability of root cortical cells at 10°C for 1 d, but not when low root temperature was maintained for 5 d. This may explain why, in contrast to Arabidopsis plants overexpressing PIP2;5, the PIP1;4-overexpressing plants showed growth reductions when exposed to 10°C root temperature and provides additional evidence for a direct link between growth responses and aquaporin function at low root temperature. The differences in low-root-temperature responses between the PIP1;4- and PIP2;5-overexpressing plants may reflect functional differences between the two aquaporins (Jang et al., 2004, 2007; Alexandersson et al., 2005; Wudick et al., 2009; Almeida-Rodriguez et al., 2010) or the differences in tissue distribution (Javot et al., 2003; Suga et al., 2003; Da Ines, 2008; Alexandersson et al., 2010). However, they may also reflect possible differences in regulation mechanisms between PIP1;4 and PIP2;5 or in their water-transporting effectiveness, assuming no major differences in PIP1;4 and PIP2;5 membrane density in plants overexpressing the respective aquaporins.
Interestingly, the reduction of Lp in the wild-type plants at 10°C was reversed to as much as 70% of the preexposure level by the addition of 5 mm Ca(NO3)2 and protein phosphatase inhibitors (75 nm okadaic acid and 1 μm Na3VO4), pointing to protein phosphorylation and/or dephosphorylation as likely gating mechanisms involved in the low-root-temperature response of aquaporins. Okadaic acid and vanadate are commonly used as inhibitors of protein phosphorylation (Cohen and Cohen, 1989; Gordon, 1991). We have also demonstrated that the calcium channel blocker, 1 mm LaCl3, significantly inhibited Lp at 25°C, further supporting the notion that aquaporin gating is linked to the calcium signal (Johansson et al., 1996; Carvajal et al., 2000; Németh-Cahalan and Hall, 2000; Azad et al., 2004).
Although several PIPs and tonoplast intrinsic proteins are insensitive to mercury (Daniels et al., 1994; Biela et al., 1999), many aquaporins are blocked by mercury reagents (Maggio and Joly, 1995; Wan and Zwiazek, 1999) that are thought to bind to sulfhydryl groups, resulting in a reversible alteration of protein conformation and channel closure (Zhang and Tyerman, 1999). We have previously demonstrated that the overexpression of aquaporins in plants alleviates the effect of mercury on Lp, likely because the inhibition of water transport by mercury is only partial and there are more water-transporting channels present in the PIP-overexpressing plants (Jang et al., 2007; Lee et al., 2009). PIP1;4 and PIP2;5 do not have Cys-189, which is known to be present in many mercury-sensitive aquaporins. If PIP1;4 and PIP2;5 are indeed mercury insensitive, than the reduced mercury sensitivity of Lp in both overexpression lines may also be due to their increased overall contribution to water transport. However, there are other Cys residues that these PIPs have (83, 105, 141, and 146 in PIP1;4 and 74, 96, 132, and 137 in PIP2;5), which could potentially affect their mercury sensitivity. In higher concentrations, mercury can also inhibit water transport by acting as a nonspecific metabolic inhibitor (Wan and Zwiazek, 1999; Zhang and Tyerman, 1999). In this study, turgor pressure remained stable during the measurements, suggesting that metabolic inhibition was not a major factor in the reduction of Lp in plants treated with 100 µm HgCl2. Similar to the earlier studies (Jang et al., 2007; Lee et al., 2009), the inhibition of Lp was less effective in Arabidopsis plants overexpressing PIP1;4 and PIP2;5 compared with the wild-type plants, reflecting the increased aquaporin abundance and the water-transporting functionality of both PIPs.
In this study, high Ea (63 kJ mol−1) was measured in the wild-type plants, demonstrating a high dependency of cell water transport on temperature in Arabidopsis roots. Therefore, the 7- to 9-fold lower Ea values measured in PIP1;4 and PIP2;5 overexpression lines provide strong evidence for the importance of these proteins in cell water transport at low temperature. Ea depends on the nature of the rate-limiting barrier for water movement and on the energetics of the water-pore interactions (Verkman et al., 1996). The low Ea (less than 25 kJ mol−1) for water transport is among the typical features of membranes with water-transporting pores (Finkelstein, 1987; Niemietz and Tyerman, 1997), because water moving across a channel does not have to overcome a large energy barrier (Tyerman et al., 1999). On the other hand, water movement through the lipid bilayer would need to surmount the high energy barrier of water partitioning into the hydrophobic lipid phase (46–63 kJ mol−1; Tyerman et al., 1999). Most of the studies in higher plants and algae showed Ea values ranging from 18 to 48 kJ mol−1 at the cell and membrane levels (Hertel and Steudle, 1997; Niemietz and Tyerman, 1997; Gerbeau et al., 2002; Lee et al., 2005a). However, Ea values as high as 186 kJ mol−1 were reported for Tradescantia virginiana leaf epidermal tissues (Tomos et al., 1981), and Ea close to 100 kJ mol−1 was measured in cucumber root cortical cells (Lee et al., 2005a). These large differences in reported values may reflect the differences in measurement methods and/or in lipid bilayer properties of different plants and tissues, the effects of which have not been extensively studied. High Ea values, exceeding 57 kJ mol−1, in plasma membrane vesicles from tobacco (Nicotiana tabacum) were used as evidence for diffusional water transport across the lipid matrix (Maurel et al., 1997). However, in this study, Lp of the wild-type plants not only showed high temperature sensitivity but was also sharply inhibited by HgCl2, pointing to the significant involvement of aquaporins in water transport. We suggest that the concept of Ea for cell water transport may need to be revisited to consider likely differences in the temperature sensitivities of different aquaporins that are involved in water transport processes of different plant species and plant tissues.
CONCLUSION
Overexpression of PIP1;4 and PIP2;5 in Arabidopsis was effective in alleviating the short-term effects of low root temperature on Lp in root cells. However, contrary to the overexpression of PIP2;5, this alleviation was no longer present after 5 d of root exposure to 10°C in plants overexpressing PIP1;4. The temperature sensitivity of cell water transport in Arabidopsis roots was reflected by the reductions in shoot and root growth rates in the wild-type and PIP1;4-overexpressing plants exposed to 10°C root temperature for 5 d. However, low root temperature had no effect on root and shoot growth in plants overexpressing PIP2;5. The inhibition of Lp by low temperature could be partially prevented by the application of Ca(NO3)2 and by protein phosphatase inhibitors, suggesting that the effect of low temperature on root water transport may involve the calcium-dependent protein phosphorylation/dephosphorylation processes. These results provide strong evidence for a link between growth responses to low root temperature and aquaporin-mediated root water transport in Arabidopsis and suggest that the low temperature sensitivity of root water transport may be connected to the aquaporin phosphorylation/dephosphorylation processes. Our data point to the functional and biological significance of a low-expression single PIP isoform in growth processes of plants exposed to stress conditions.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) wild-type (ecotype Columbia) or overexpressing (PIP1;4 and PIP2;5) plants were used for the study. Overexpressing PIP1;4 or PIP2;5 seeds (Jang et al., 2007) were kindly provided by Dr. Hunseung Kang. The seeds were sown in Jiffy pots (Jiffy Co.) for 2 d at 4°C before being transferred to the controlled-environment growth room with a 16-h photoperiod, approximately 350 μmol m−2 s−1 photosynthetic photon flux density, and 23°C/21°C (day/night) temperatures. After 12 to 14 d, the roots were gently washed and the plants were transplanted to the solution culture. The plants were grown in 15-L containers with aerated nutrient solution containing 1.25 mm KNO3, 1.5 mm Ca(NO3)2, 0.75 mm MgSO4, 0.5 mm KH2PO4, 50 μm H3BO3, 10 μm MnCl2, 2 μm ZnSO4, 1.5 μm CuSO4, 0.075 μm NH4Mo7O24, and 74 μm Fe-EDTA. The plants were 3 weeks old at the beginning of the low-temperature treatment.
Relative Growth Rate in Low-Temperature Treatment
At the commencement of the experiment, 40 plants from each experimental group (wild type, overexpressing PIP1;4, and overexpressing PIP2;5) were harvested as a reference (time 0), and the remaining 80 plants from each experimental group were divided into two groups and exposed to 10°C or 23°C root temperature for 5 d using a 15-l circulating water bath (Thermomix BU coupled to Frigomix U, B. Braun Biotech International, Melsungen, Germany). For dry weight determinations, shoots and roots were harvested and dried in an oven at 80°C for 72 h. The relative growth rates of roots and shoots were calculated from the initial and final dry weights (Hoffmann and Poorter, 2002; n = 40).
Cell Pressure Probe Measurements
Cell pressure probe measurements were conducted on the cortical cells of roots at 25- to 30-mm distance from the root apex after excising about 50-mm-long distal root segments. Nutrient solution of the same composition as for solution culture was circulated along the roots during experiments. A cell pressure probe was used to measure T1/2, ε, and P (Steudle, 1993). An oil-filled microcapillary was attached to the probe with a tip diameter ranging from 5 to 6 μm and then punctured the cortical cells with the microcapillary. Once a meniscus was formed between cell sap and oil, it was gently pushed to a position close to the surface of the root. P became steady within a few minutes, and then hydraulic parameters of the cell under hydrostatic water flow were determined (Steudle, 1993). The nutrient solution was circulated along the roots during the experiments, and treatment solution was added into the nutrient solution to determine the responses of the same cells. To calculate Lp of root cortical cells, T1/2, cell dimensions (cell volume and cell surface area), ε, and osmotic pressure of the cell were required (Steudle, 1993). Cell dimensions were examined microscopically in thin root sections embedded in Spurr’s resin (Spurr, 1969).
Root Temperature Control for Cell Pressure Probe Measurements and Application of Chemicals
To control the temperature, nutrient solution was pumped through a tube using a circulating water bath (F3; Haake). The first cell pressure probe measurements of T1/2 were taken at 25°C, and then the temperature was decreased to 20°C for 10 min and the measurements were taken again for 10 min. This was repeated at 15°C and 10°C when the last sets of measurements were taken. Root temperature during the measurements was monitored with the thermocouple that was placed near the root surface. The measurements lasted for about 110 min with the same cells measured for all temperatures. For some of the roots, the measurements were conducted with ascending temperatures from 10°C to 25°C.
When cells had attained a steady P and T1/2 at either 10°C or 25°C for 30 min, roots were treated with 5 mm Ca(NO3)2, 1 mm LaCl3, 1 µm Na3VO4, or 75 nm okadaic acid, which were added to the circulating solution to examine whether protein phosphorylation and calcium signals play a role in cell water transport responses to low root temperature. Also, 100 µm HgCl2 was added to the circulating root medium to determine the contribution of mercury-sensitive water transport. Water relations parameters were continuously measured and compared before and after 20- to 30-min exposure to chemicals on the same individual cells.
Measurements of Ea
Ea for Lp was calculated for all plants from the slopes of Arrhenius plots where the natural log of cortical Lp was plotted against the inverse of absolute temperature (T = 283–298 K), because:
RNA Extraction, Reverse Transcription-PCR, and Real-Time PCR
To investigate the effect of low temperature on the transcript level of each PIP gene, we monitored the expression of all 13 PIP genes in roots of wild-type plants that were grown in solution culture at 23°C root temperature and then exposed to 10°C for 1 and 24 h. We used 3′ untranslated region gene-specific primers (Jang et al., 2004) except PIP1;3, PIP1;5, PIP2;1, PIP2;3, and PIP2;7 (Supplemental Table S1) and quantified the abundance of PIP transcripts using real-time PCR analysis.
Total RNA was extracted from the frozen samples using the Plant RNeasy extraction kit (Qiagen). The concentration of RNA was accurately quantified by spectrophotometer measurements using a NanoDrop 1000 apparatus, and cDNAs were obtained using a reverse transcriptase kit (Qiagen). Real-time PCR was performed in a Biosystems 7500 real-time thermal cycling system (Applied Biosystems) using the QuantiTect SYBR Green PCR kit (Qiagen). After normalization of the DNA content using the actin gene expression pattern in each sample, the expression level of each gene in plants under low-temperature conditions was calculated by comparing the expression level with plants exposed to 23°C root temperature. These experiments were repeated at least three times, and the histograms represent mean values and se of different experiments conducted with different RNA preparations.
Statistical Analyses
Statistical analyses were performed using SigmaPlot version 11.0 (Systat Software) at α = 0.05 confidence level. The data were analyzed using unpaired t tests to compare the water relations parameters, cell dimension, activation energy, and aquaporin gene expression, and a paired t test was used for the HgCl2 effects. ANOVA with Tukey’s multiple comparison was used for growth rate and temperature effects on cell hydraulic conductivity.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Gene-specific primer pairs used in PCR.
Supplementary Material
Acknowledgments
We thank Prof. Hunseung Kang (Chonnam National University) for supplying the Arabidopsis seeds overexpressing PIP1;4 and PIP2;5.
Glossary
- Lp
cell hydraulic conductivity
- P
turgor pressure
- T1/2
half-time of water exchange
- ε
elastic modulus
- Ea
activation energy
References
- Alexandersson E, Danielson JÅH, Råde J, Moparthi VK, Fontes M, Kjellbom P, Johanson U. (2010) Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant J 61: 650–660 [DOI] [PubMed] [Google Scholar]
- Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P. (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59: 469–484 [DOI] [PubMed] [Google Scholar]
- Alleva K, Niemietz CM, Sutka M, Maurel C, Parisi M, Tyerman SD, Amodeo G. (2006) Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J Exp Bot 57: 609–621 [DOI] [PubMed] [Google Scholar]
- Almeida-Rodriguez AM, Cooke JE, Yeh F, Zwiazek JJ. (2010) Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii×balsamifera clones with different drought resistance strategies. Physiol Plant 140: 321–333 [DOI] [PubMed] [Google Scholar]
- Aroca R, Amodeo G, Fernández-Illescas S, Herman EM, Chaumont F, Chrispeels MJ. (2005) The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 137: 341–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R, Irigoyen JJ, Sánchez-Díaz M. (2003) Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol Plant 117: 540–549 [DOI] [PubMed] [Google Scholar]
- Azad AK, Sawa Y, Ishikawa T, Shibata H. (2004) Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant Cell Physiol 45: 608–617 [DOI] [PubMed] [Google Scholar]
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R. (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18: 565–570 [DOI] [PubMed] [Google Scholar]
- Bigot J, Boucaud J. (1996) Short-term responses of Brassica rapa plants to low root temperature: effects on nitrate uptake and its translocation to the shoot. Physiol Plant 96: 646–654 [Google Scholar]
- Bonan GB (1992) Soil temperature as an ecological factor in boreal forest. In HH Shugart, R Leemans, GB Bonan, eds, System Analysis of the Global Boreal Forest. Cambridge University Press, Cambridge, UK, pp 126–143
- Carvajal M, Cerda A, Martínez V. (2000) Does calcium ameliorate the negative effect of NaCl on melon root water transport by regulating aquaporin activity? New Phytol 145: 439–447 [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125: 1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Cohen PTW. (1989) Protein phosphatases come of age. J Biol Chem 264: 21435–21438 [PubMed] [Google Scholar]
- Costa e Silva F, Shvaleva A, Almeida MH, Chaves MM, Pereira JS. (2007) Responses to chilling of two Eucalyptus globulus clones with contrasting drought resistance. Funct Plant Biol 34: 793–802 [DOI] [PubMed] [Google Scholar]
- Da Ines O (2008) Functional analysis of PIP2 aquaporins in Arabidopsis thaliana. PhD thesis. Ludwig Maximilians Universität, Munich, Germany
- Daniels MJ, Mirkov TE, Chrispeels MJ. (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol 106: 1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson JÅH, Johanson U. (2008) Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria M, Windhager EE, Tate SS, Frindt G. (1994) Cloning and expression of AQP3, a water channel from the medullary collecting duct of rat kidney. Proc Natl Acad Sci USA 91: 10997–11001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell A, Markhart AH. (1998) Rapid acclimation of root hydraulic conductivity to low temperature. J Exp Bot 49: 879–884 [Google Scholar]
- Finkelstein A (1987) Water movement through lipid bilayers, pores, and plasma membranes: theory and reality. Distinguished Lecture Series of the Society of General Physiologists, Vol 4. Wiley-Interscience, New York
- Fischer RA, Rees D, Sayre KD, Lu Z-M, Condon AG, Larque Saavedra A. (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38: 1467–1475 [Google Scholar]
- Gerbeau P, Amodeo G, Henzler T, Santoni V, Ripoche P, Maurel C. (2002) The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J 30: 71–81 [DOI] [PubMed] [Google Scholar]
- Gordon JA. (1991) Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol 201: 477–482 [DOI] [PubMed] [Google Scholar]
- Gupta AB, Sankararamakrishnan R. (2009) Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol 9: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel A, Steudle E. (1997) The function of water channels in Chara: the temperature dependence of water and solute flow provides evidence for composite membrane transport and for a slippage of small organic solutes across water channels. Planta 202: 324–335 [Google Scholar]
- Hoffmann WA, Poorter H. (2002) Avoiding bias in calculations of relative growth rate. Ann Bot (Lond) 90: 37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg N, Bülow L. (1998) Improving stress tolerance in plant by gene transfer. Trends Plant Sci 3: 61–66 [Google Scholar]
- Ishibashi K, Sasaki S, Fushimi K, Uchida S, Kuwahara M, Saito H, Furukawa T, Nakajima K, Yamaguchi Y, Gojobori T, et al. (1994) Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci USA 91: 6269–6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JY, Kim DG, Kim YO, Kim JS, Kang H. (2004) An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol Biol 54: 713–725 [DOI] [PubMed] [Google Scholar]
- Jang JY, Lee SH, Rhee JY, Chung GC, Ahn SJ, Kang H. (2007) Transgenic Arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. Plant Mol Biol 64: 621–632 [DOI] [PubMed] [Google Scholar]
- Janowiak F, Maas B, Dörffling K. (2002) Importance of abscisic acid for chilling tolerance of maize seedlings. J Plant Physiol 159: 635–643 [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, Martin-Laurent F, Güçlü J, Vinh J, Heyes J, Franck KI, Schäffner AR, Bouchez D, et al. (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15: 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P. (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126: 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P. (2000) The role of aquaporins in cellular and whole plant water balance. Biochim Biophys Acta 1465: 324–342 [DOI] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P. (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10: 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Larsson C, Ek B, Kjellbom P. (1996) The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 8: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Ribas-Carbo M, Sans JF, Lovisolo C, Heckwolf M, Uehlein N. (2008) Aquaporins and plant water balance. Plant Cell Environ 31: 658–666 [DOI] [PubMed] [Google Scholar]
- Karlsson M, Fotiadis D, Sjövall S, Johansson I, Hedfalk K, Engel A, Kjellbom P. (2003) Reconstitution of water channel function of an aquaporin overexpressed and purified from Pichia pastoris. FEBS Lett 537: 68–72 [DOI] [PubMed] [Google Scholar]
- Kerk D, Bulgrien J, Smith DW, Barsam B, Veretnik S, Gribskov M. (2002) The complement of protein phosphatase catalytic subunits encoded in the genome of Arabidopsis. Plant Physiol 129: 908–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Chung GC, Steudle E. (2005a) Gating of aquaporins by low temperature in roots of chilling-sensitive cucumber and chilling-tolerant figleaf gourd. J Exp Bot 56: 985–995 [DOI] [PubMed] [Google Scholar]
- Lee SH, Chung GC, Steudle E. (2005b) Low temperature and mechanical stresses differently gate aquaporins of root cortical cells of chilling-sensitive cucumber and -resistant figleaf gourd. Plant Cell Environ 28: 1191–1202 [Google Scholar]
- Lee SH, Chung GC, Zwiazek JJ. (2009) Effects of irradiance on cell water relations in leaf bundle sheath cells of wild-type and transgenic tobacco (Nicotiana tabacum) plants overexpressing aquaporins. Plant Sci 176: 248–255 [Google Scholar]
- Lee SH, Zwiazek JJ, Chung GC. (2008) Light-induced transpiration alters cell water relations in figleaf gourd (Cucurbita ficifolia) seedlings exposed to low root temperatures. Physiol Plant 133: 354–362 [DOI] [PubMed] [Google Scholar]
- Lian HL, Yu X, Ye Q, Ding X, Kitagawa Y, Kwak SS, Su WA, Tang ZC. (2004) The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol 45: 481–489 [DOI] [PubMed] [Google Scholar]
- Maggio A, Joly RJ. (1995) Effects of mercuric chloride on the hydraulic conductivity of tomato root systems: evidence for a channel-mediated water pathway. Plant Physiol 109: 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Tacnet F, Güclü J, Guern J, Ripoche P. (1997) Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity. Proc Natl Acad Sci USA 94: 7103–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhsin T, Zwiazek JJ. (2002) Ectomycorrhizas increase apoplastic water transport and root hydraulic conductivity in Ulmus americana seedlings. New Phytol 153: 153–158 [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. (2000) Structural determinants of water permeation through aquaporin-1. Nature 407: 599–605 [DOI] [PubMed] [Google Scholar]
- Németh-Cahalan KL, Hall JE. (2000) pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem 275: 6777–6782 [DOI] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD. (1997) Characterization of water channels in wheat root membrane vesicles. Plant Physiol 115: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez de Juan J, Irigoyen JJ, Sánchez-Díaz M. (1997) Chilling of drought-hardened and non-hardened plants of different chilling-sensitive maize lines: changes in water relations and ABA contents. Plant Sci 122: 71–79 [Google Scholar]
- Quigley F, Rosenberg JM, Shachar-Hill Y, Bohnert HJ. (2002) From genome to function: the Arabidopsis aquaporins. Genome Biol 3: H0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14: 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr AR. (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43 [DOI] [PubMed] [Google Scholar]
- Steudle E (1993) Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level. In JAC Smith, H Griffith, eds, Water Deficits: Plant Responses from Cell to Community. Bios Scientific Publishers, Oxford, pp 5–36
- Suga S, Murai M, Kuwagata T, Maeshima M. (2003) Differences in aquaporin levels among cell types of radish and measurement of osmotic water permeability of individual protoplasts. Plant Cell Physiol 44: 277–286 [DOI] [PubMed] [Google Scholar]
- Sutka M, Alleva K, Parisi M, Amodeo G. (2005) Tonoplast vesicles of Beta vulgaris storage root show functional aquaporins regulated by protons. Biol Cell 97: 837–846 [DOI] [PubMed] [Google Scholar]
- Sutka M, Li G, Boudet J, Boursiac Y, Doumas P, Maurel C. (2011) Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol 155: 1264–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomos AD, Steudle E, Zimmermann U, Schulze E-D. (1981) Water relations of leaf epidermal cells of Tradescantia virginiana. Plant Physiol 68: 1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C. (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397 [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Niemietz CM. (1999) Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot 50: 1055–1071 [Google Scholar]
- Verkman AS, van Hoek AN, Ma T-H, Frigeri A, Skach WR, Mitra A, Tamarappoo BK, Farinas J. (1996) Water transport across mammalian cell membranes. Am J Physiol 270: C12–C30 [DOI] [PubMed] [Google Scholar]
- Wan X, Zwiazek JJ. (1999) Mercuric chloride effects on root water transport in aspen seedlings. Plant Physiol 121: 939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Zwiazek JJ, Lieffers VJ, Landhäusser SM. (2001) Hydraulic conductance in aspen (Populus tremuloides) seedlings exposed to low root temperatures. Tree Physiol 21: 691–696 [DOI] [PubMed] [Google Scholar]
- Wudick MM, Luu D-T, Maurel C. (2009) A look inside: localization patterns and functions of intracellular plant aquaporins. New Phytol 184: 289–302 [DOI] [PubMed] [Google Scholar]
- Yu A, Hu Y, Li J, Wu Q, Lin Z. (2005) Sense and antisense expression of plasma membrane aquaporin BnPIP1 from Brassica napus in tobacco and its effects on plant drought resistance. Plant Sci 169: 647–656 [Google Scholar]
- Zhang WH, Tyerman SD. (1999) Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiol 120: 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.