Abstract
We tested whether a gene regulating nodule number in Medicago truncatula, Super Numeric Nodules (SUNN ), is involved in root architecture responses to carbon (C) and nitrogen (N) and whether this is mediated by changes in shoot-to-root auxin transport. Nodules and lateral roots are root organs that are under the control of nutrient supply, but how their architecture is regulated in response to nutrients is unclear. We treated wild-type and sunn-1 seedlings with four combinations of low or increased N (as nitrate) and C (as CO2) and determined responses in C/N partitioning, plant growth, root and nodule density, and changes in auxin transport. In both genotypes, nodule density was negatively correlated with tissue N concentration, while only the wild type showed significant correlations between N concentration and lateral root density. Shoot-to-root auxin transport was negatively correlated with shoot N concentration in the wild type but not in the sunn-1 mutant. In addition, the ability of rhizobia to alter auxin transport depended on N and C treatment as well as the SUNN gene. Nodule and lateral root densities were negatively correlated with auxin transport in the wild type but not in the sunn-1 mutant. Our results suggest that SUNN is required for the modulation of shoot-to-root auxin transport in response to altered N tissue concentrations in the absence of rhizobia and that this controls lateral root density in response to N. The control of nodule density in response to N is more likely to occur locally in the root.
Nitrogen (N) is one of the limiting nutrients for plant growth, and plants show large phenotypic plasticity in the response to N availability in the environment. N is available as nitrate or ammonium from the soil, and many legumes additionally gain N from a symbiosis with N-fixing bacteria called rhizobia. Rhizobia initiate the development of root nodules in specific legume hosts. They invade the nodules and convert atmospheric N into ammonia, which is exported to the plant as amino acids. In return, the plant provides rhizobia with a carbon (C) source because N fixation has high energy demands (White et al., 2007).
Little is known about the genetic regulation of the developmental plasticity responses to N. Some of the first identified genes regulating N phenotypes are a class of genes regulating nodule number by a systemic mechanism called autoregulation (Reid et al., 2011b). Mutation of these autoregulation genes causes supernodulation, mostly even in the presence of high N concentrations, and were thus first termed nitrate tolerant symbiotic (nts) mutants in soybean (Glycine max; Carroll et al., 1985a, 1985b).
In legumes, the number and density of nodules on a root system are strictly regulated to balance the need for N with the supply of C as an energy source for N fixation. A Leu-rich repeat receptor-like kinase is responsible for the systemic regulation of nodule number and density. This Leu-rich repeat receptor-like kinase, also named Nodulation Autoregulation Receptor Kinase (NARK), has been cloned from different legumes, including the crops soybean (Searle et al., 2003) and pea (Pisum sativum; Krusell et al., 2002) and the model legumes Lotus japonicus (Krusell et al., 2002; Nishimura et al., 2002) and Medicago truncatula (Schnabel et al., 2005). Autoregulation mutants, in which NARK is dysfunctional, are unable to down-regulate nodule number, and their roots are supernodulated with high densities of nodules along the whole root system. Grafting experiments have shown that NARK acts in the shoot to regulate nodule density by long-distance signaling (Delves et al., 1986). Split-root experiments demonstrated that infection of the root by rhizobia induces a signal that moves to the shoot and activates NARK, and subsequently, a signal is sent back from the shoot to the root to inhibit further nodule development, at least temporarily (Kosslak and Bohlool, 1984). Interestingly, autoregulation mutants also show the nts phenotype (Carroll et al., 1985b; Sagan et al., 1995); that is, they still nodulate in the presence of nitrate concentrations that inhibit nodulation in the wild type, although various mutants differ in their degree of nitrate tolerance. Again, grafting studies showed that the nts phenotype is exerted by the shoot in soybean and M. truncatula (Day et al., 1989; Sagan et al., 1995; Jeudy et al., 2010). However, there is also a local effect of N supply on nodulation in M. truncatula that affects nodule size and color (Jeudy et al., 2010). Therefore, the inhibitory action of nitrate on nodulation is expected to interact with the autoregulation signal, perceived through NARK, to down-regulate nodule density. It is likely that NARK directly or indirectly perceives the C/N ratio in the shoot and then sends a long-distance signal to the root to adjust nodule density according to N demand and C supply. Several autoregulation mutants also show nodulation-independent phenotypes, for example, an increased density of lateral roots and a short root system (Wopereis et al., 2000), suggesting that these phenotypes are also under the control of a long-distance signaling system. So far, the long-distance signals that regulate nodule or lateral root density have not been identified, but auxin is one candidate for a shoot-to-root signal regulating nodule and lateral root development.
Auxin is mainly synthesized in the shoot and transported to the root via phloem transport and by active polar cell-to-cell transport (Friml, 2003). Auxin is a crucial regulator of lateral root (Fukaki et al., 2007) and nodule (Mathesius, 2008) development. Lateral root initiation is dependent on auxin transport from the root tip to the lateral root initiation zone (Reed et al., 1998; Casimiro et al., 2001), whereas auxin transport from the shoot to the root is required for lateral root elongation (Bhalerao et al., 2002).
The regulation of lateral root initiation and elongation by nitrate is likely to be mediated by auxin signaling. In general, local patches of high nitrate availability trigger lateral root elongation, whereas high systemic levels of N in the plant cause an inhibition of lateral root emergence (Zhang and Forde, 1998; Zhang at al., 1999; Forde, 2002).
Studies focusing on the local stimulation of lateral root elongation in response to nitrate showed that the auxin-resistant Arabidopsis (Arabidopsis thaliana) mutant arx4 is resistant to nitrate-induced lateral root elongation (Zhang et al., 1999), although this was not confirmed in a different study (Linkohr et al., 2002). The demonstration that the nitrate transporter (NRT1.1) also transports auxin and thus directly links nitrate sensing with lateral root elongation strongly links local auxin transport into an elongating lateral root with nitrate sensing (Krouk et al., 2010). In addition, application of a synthetic auxin transport inhibitor above the site of localized nitrate supply in Arabidopsis prevented the outgrowth of lateral roots, suggesting that auxin transport is involved in the lateral root response to nitrate (Guo et al., 2005).
Studies examining the inhibition of lateral root emergence in response to high (generally more than 10 mm) systemic treatment with nitrate showed that high-nitrate treatment causes changes in auxin concentration and response in the root. For example, microarray and other gene expression experiments provide evidence for the regulation of many auxin response and auxin transport genes in nitrate-treated Arabidopsis plants (Gutiérrez et al., 2007; Gifford et al., 2008). The repression of lateral root initiation in Arabidopsis seedlings grown under high-C and low-N conditions was linked to high auxin response in the hypocotyl and low response in the root and could be rescued by auxin addition to the roots (Malamy and Ryan, 2001). Shifting roots from high- to low-nitrate medium increased root auxin content in pineapple (Ananas comosus; Tamaki and Mercier, 2007) and in Arabidopsis, where this increase was accompanied by increased lateral root emergence (Walch-Liu et al., 2006). In maize (Zea mays), nitrate treatment led to the inhibition of root growth, and this was correlated with reduced auxin concentration in the root, particularly close to the root tip (Tian et al., 2008). Bao et al. (2007) showed reduced expression of the synthetic auxin-responsive promoter:GUS fusion, DR5:GUS, in response to high nitrate, suggesting that the auxin response might be affected by nitrate.
To our knowledge, no genes mediating systemic effects of N or C on lateral root development have been identified. However, autoregulated nodulation genes have been suggested as candidates (Walch-Liu et al., 2005).
As for lateral root formation, there is evidence that auxin transport control is required for nodule development. Local auxin transport regulation is required for nodule initiation, and young nodule primordia show cell-specific auxin responses (Mathesius et al., 1998; Pacios-Bras et al., 2003; van Noorden et al., 2007). In M. truncatula roots in which the cell cycle regulator CDC16 was silenced, reduced auxin response was linked to lower numbers of lateral roots but increased numbers of nodules, supporting the hypothesis that these two processes require different auxin concentrations (Kuppusamy et al., 2009). In soybean, the auxin content of the roots was increased by inoculation with rhizobia but reduced in the presence of nitrate in the wild type, whereas auxin concentrations in an autoregulation mutant did not respond to nitrate or inoculation (Caba et al., 2000). There is also evidence that auxin acts as a long-distance signal from the shoot to the root. The M. truncatula autoregulation mutant super numeric nodules-1 (sunn-1) shows increased shoot-to-root auxin transport compared with the wild type. After inoculation of M. truncatula wild-type roots, shoot-to-root auxin transport is inhibited at the same time as the autoregulation starts. This down-regulation of auxin transport was not found in the sunn-1 mutant, suggesting that shoot-to-root auxin transport inhibition could be part of the long-distance autoregulation control (van Noorden et al., 2006).
These studies suggest that auxin could be a long-distance signal that regulates both lateral root and nodule development in response to N and possibly C supply. To examine this question, we utilized the sunn-1 mutant because of its known phenotype of altered shoot-to-root auxin transport and defects in regulating root organ numbers. We grew M. truncatula wild-type and sunn-1 mutant plants under high and low nitrate and CO2 regimes to test (1) whether the sunn-1 mutant shows altered N-response phenotypes, (2) whether the C or N supply alters shoot-to-root auxin transport, (3) whether this altered auxin transport is correlated with altered root growth and lateral root and nodule formation, and (4) whether SUNN mediates the changes in root architecture to altered C or N supply via the regulation of shoot-to-root auxin transport.
RESULTS
SUNN Affects Growth Responses to C and N
Our first aim was to determine whether the SUNN gene affects the plant responses to externally applied C and N. Therefore, we grew wild-type and sunn-1 mutant seedlings under four different C and N conditions: approximately ambient C (ambient C; 370 μL L−1 CO2 in the atmosphere); high C (800 μL L−1 CO2 in the atmosphere); minus N (no external N source); and plus N (2.5 mm nitrate in the growth medium). This nitrate concentration was chosen because it was sufficient to inhibit nodulation (data not shown) and N fixation (Moreau et al., 2008) in the wild type to the same extent as higher (5 or 10 mm) N concentrations and at the same time increased internal N concentrations significantly (see below). As increasing nitrate concentrations delay nodulation in M. truncatula (Moreau et al., 2008), we kept nitrate concentrations to a minimum to avoid the asynchronous behavior of plants with different nitrate treatments. Half of the plants were inoculated with rhizobia and the other half were mock inoculated 5 d after germination. Root and shoot material was collected at day 20 after germination, at which time pink nodules had formed in both genotypes in the absence of N. We determined the fresh weight of shoots and roots as well as N concentrations (Fig. 1), C concentrations, and C and N contents (Supplemental Fig. S1).
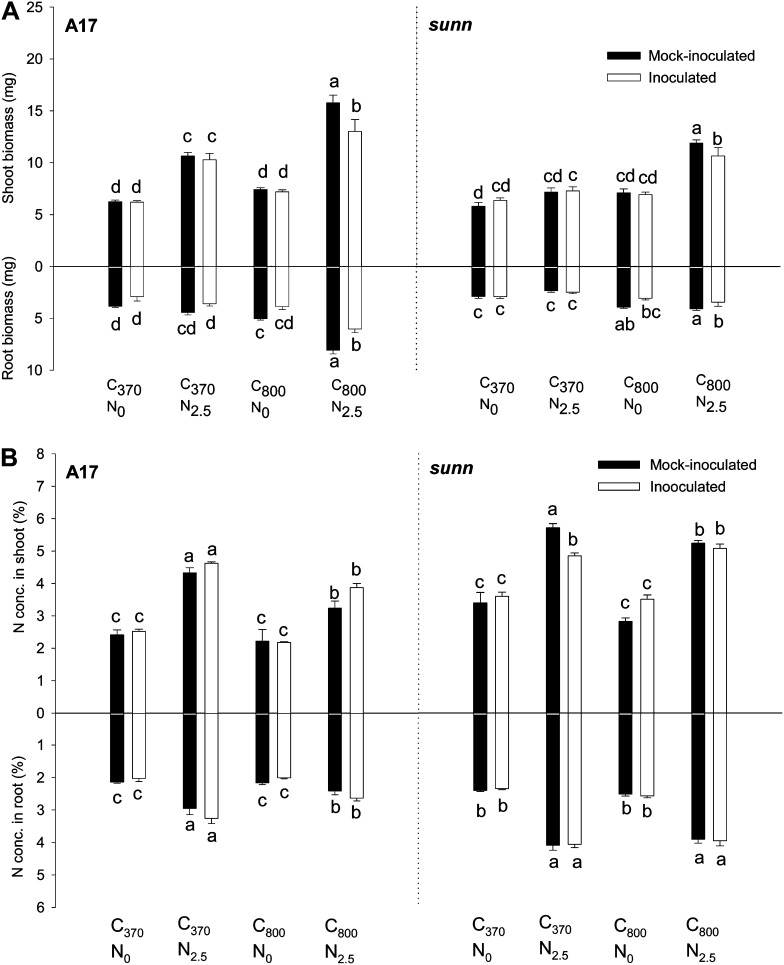
Figure 1.
Biomass and N concentrations of cv Jemalong A17 and sunn-1 seedlings. A, Shoot and root biomass (in mg dry weight). B, N concentrations of A17 (wild type) and sunn-1 shoot and roots determined by mass spectrometry. Data are expressed as percentage dry weight. Data were obtained from 20-d-old A17 and sunn-1 seedlings grown in ambient C (370 μL L−1 CO2 [C370]), high C (800 μL L−1 CO2 [C800]), minus N (0 mm nitrate [N0]), and/or plus N (2.5 mm nitrate [N2.5]). Inoculation with S. meliloti was performed on 5-d-old seedlings. Data are results from four samples of six to eight seedlings each ± se. Treatments labeled with different letters differ significantly within one genotype (P < 0.05; two-way ANOVA).
In general, sunn-1 seedlings were smaller than wild-type seedlings, especially the root systems (Fig. 1A). Whereas the wild type showed significantly increased shoot weight in response to N and high C plus N, sunn-1 mutants showed only moderate increases in shoot weight in response to N and only significantly responded to high C plus N. Root weight increased significantly in response to high C and high C plus N in both genotypes. In both genotypes, inoculation of roots with rhizobia caused a significant reduction in root and shoot weight in high-C-plus-N-treated plants but not in the other treatments.
While application of C and N did not significantly affect C concentrations in any of the treatments or genotypes (Supplemental Fig. S1A), it changed N concentrations significantly (Fig. 1B). In both genotypes, 2.5 mm nitrate significantly increased N concentrations in shoots and roots. Treatment with high C plus N reduced N concentrations significantly compared with ambient C plus N in wild-type shoots and roots but only in sunn-1 mutant shoots. The sunn-1 seedlings generally showed higher N concentrations in shoots and roots compared with the wild type (Fig. 1B).
In summary, these results suggest that, even though sunn-1 seedlings had higher N tissue concentrations compared with the wild type and N concentrations increased in response to nitrate treatment, sunn-1 seedlings generally responded less strongly to N addition with changes in biomass.
SUNN Affects Root Architecture Responses to C and N
Our next question was whether the SUNN gene mediates changes in root architecture in response to C and N. We determined nodule density, lateral root density, and taproot and lateral root length in wild-type and sunn-1 seedlings grown under the C and N treatments described above (Fig. 2). Under our growth conditions, sunn-1 roots developed significantly more nodules and higher nodule density than wild-type roots. In both genotypes, nodule densities were significantly reduced in response to N addition and significantly increased in response to high C. High C did not rescue the low-nodulation phenotype of the plus-N treatment (Fig. 2A).
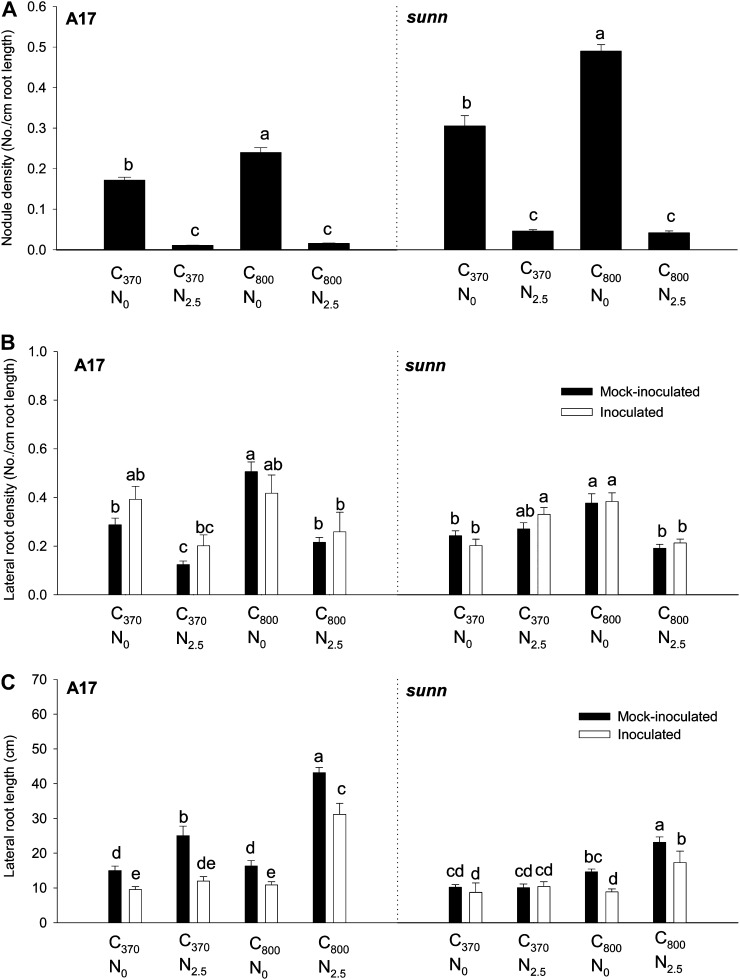
Figure 2.
Root architecture phenotypes of A17 and sunn-1 seedlings. Nodule density (A), first-order lateral root density (B), and lateral root length (total of all lateral roots per plant; C) of 20-d-old cv Jemalong A17 and sunn-1 seedlings grown in ambient C (370 μL L−1 CO2 [C370]), high C (800 μL L−1 CO2 [C800]), minus N (0 mm nitrate [N0]), and/or plus N (2.5 mm nitrate [N2.5]) are shown. Inoculation with S. meliloti was performed on 5-d-old seedlings. Data are results from four samples of six to eight seedlings each ± se. Treatments labeled with different letters differ significantly within one genotype (P < 0.05; two-way ANOVA).
Lateral root density at ambient-C and minus-N conditions was similar between genotypes. Nitrate addition significantly reduced the lateral root density in the wild type, but this was not the case in sunn-1 mutants (Fig. 2B). High C significantly increased lateral root density in uninoculated wild-type plants, while this effect was only significant in minus-N treatments in sunn-1 mutants.
Lateral root length was generally shorter in sunn-1 mutants than in the wild type (Fig. 2C). While N addition significantly increased lateral root length in the wild type, this response only occurred with a combination of high C plus N in sunn-1 mutants and was much less pronounced. Inoculation significantly reduced lateral root length in all treatments in the wild type but only in the high-C treatments in sunn-1 mutants.
Taproot length was not significantly affected by C or N in the wild type but was reduced by ambient C plus N in sunn-1 mutants (Supplemental Fig. S2).
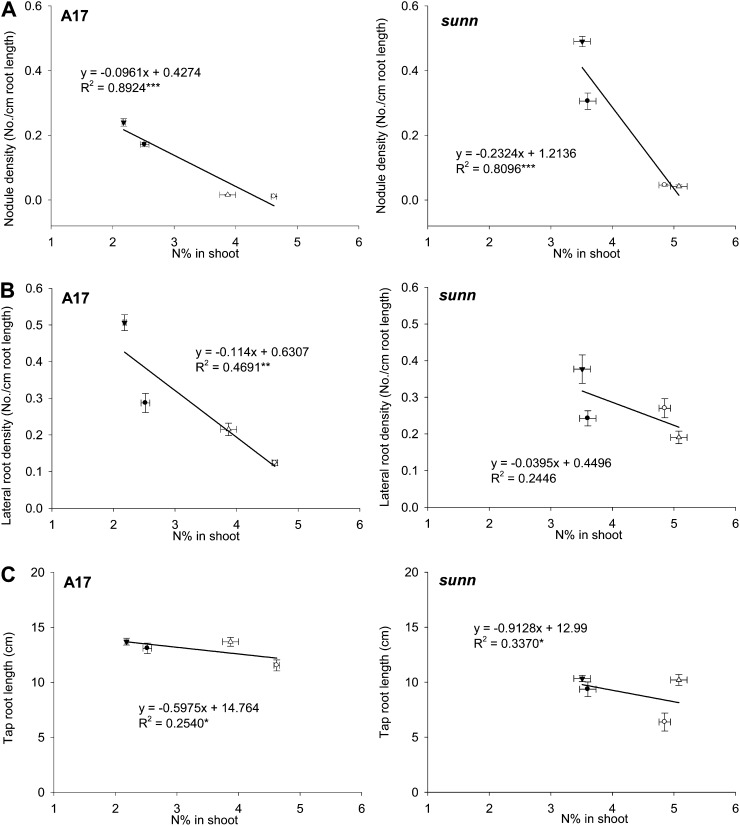
To test whether root organ density and root length are related to the availability of C or N in the shoot or root, we correlated these root architecture phenotypes to C and N tissue concentrations. We show here a correlation with shoot [N], but in all cases very similar results were found for root [N], and in both genotypes shoot [N] and root [N] were strongly positively correlated (Supplemental Fig. S3A). In both wild-type and sunn-1 seedlings, nodule density was strongly negatively correlated with shoot [N] (P < 0.001; Fig. 3A). There was no significant correlation of nodule density with shoot (or root) [C] (Supplemental Fig. S3B). Lateral root density was strongly negatively correlated with shoot [N] in the wild type (P < 0.01) but not in sunn-1 mutants (Fig. 3B). There was no significant correlation between lateral root density and shoot or root [C] (Supplemental Fig. S3C). In both genotypes, lateral root length showed no significant correlation with either shoot or root [N] or [C] (data not shown). Taproot length showed a slight but significant (P < 0.05) negative correlation with shoot [N] in both genotypes (Fig. 3C) but no response to shoot [C] (data not shown).
Figure 3.
Correlation of shoot N concentrations with root architecture phenotypes. A, Correlation between nodule density and shoot N concentration. B, Correlation between lateral root density (of mock-inoculated roots) and shoot N concentration. C, Correlation between taproot length (of mock-inoculated roots) and shoot N concentration. N concentrations are expressed as percentage N of dry weight. All data are from 20-d-old cv Jemalong A17 and sunn-1 seedlings grown in ambient C (370 μL L−1 CO2 [C370]), high C (800 μL L−1 CO2 [C800]), minus N (0 mm nitrate [N0]), and/or plus N (2.5 mm nitrate [N2.5]). Inoculation with S. meliloti was performed on 5-d-old seedlings. Data are results from four samples of six to eight seedlings each ± se. Significant correlations are marked with asterisks (* P < 0.05, ** P < 0.01, *** P < 0.001 [calculated with linear regression]).
In short, these results suggest that the SUNN gene is required for changes in lateral root density in response to [N], while nodule density and taproot length are affected by shoot [N] similarly in both genotypes.
SUNN Is Required for Changes in Shoot-to-Root Auxin Transport in Response to [N]
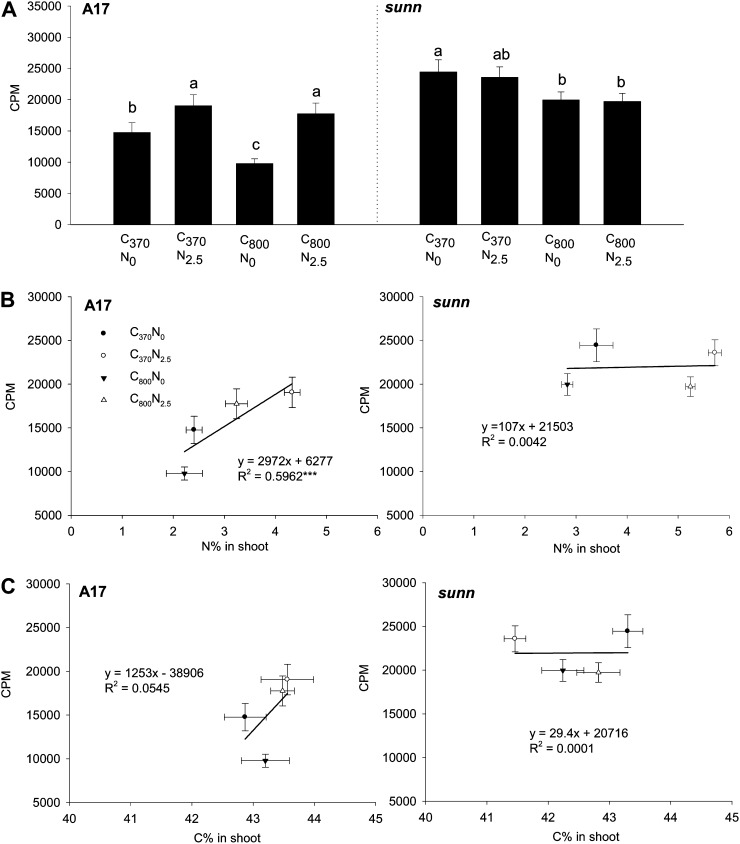
One of our hypotheses was that root organ density was determined by the amount of shoot-to-root auxin transport, which we previously found to be regulated by the SUNN-1 gene under conditions of N limitation and ambient CO2 (van Noorden et al., 2006). We now wanted to determine whether this shoot-to-root auxin transport mediated changes in root architecture in response to C and N. Measurements of shoot-to-root auxin transport in 10-d-old mock-inoculated seedlings showed (1) that sunn-1 mutants had significantly higher auxin transport than the wild type, as reported previously (van Noorden et al., 2006), (2) that treatment with 2.5 mm nitrate increased auxin transport in the wild type but not in sunn-1 mutants, and (3) that supply of high C reduced auxin transport, although this could be rescued by N addition in the wild type (Fig. 4A). To further examine the relationship between C and N supply and shoot-to-root auxin transport, we correlated total auxin transport with shoot and root [N] and [C]. We found positive and statistically significant (P < 0.001) correlation between shoot (and root; data not shown) [N] with total auxin transport in the wild type but not in sunn-1 mutants (Fig. 4B). There was no significant correlation of auxin transport with shoot (or root) [C] (Fig. 4C). This result suggests that the SUNN gene is required for the modulation of shoot-to-root auxin transport in response to N tissue concentrations.
Figure 4.
Shoot-to-root auxin transport phenotypes in response to C and N treatments. A, Total transported [3H]IAA (in cpm) in 10-d-old, mock-inoculated cv Jemalong A17 and sunn-1 seedlings grown in ambient C (370 μL L−1 CO2 [C370]), high C (800 μL L−1 CO2 [C800]), minus N (0 mm nitrate [N0]), and/or plus N (2.5 mm nitrate [N2.5]). Radiolabeled auxin was applied between the cotyledons at the shoot apex and allowed to transport into the root. After 3 h, eight 5-mm segments, starting just below the cotyledons, were excised into scintillation fluid. Segment 1 was not taken into account for the analysis because of diffusion from the site of application. The results show the sum of the total amount of radiolabeled auxin transported into segments 2 to 8 as means ± se (n = 16–20). Treatments labeled with different letters differ significantly within one genotype (P < 0.05; one-way ANOVA). B, Correlation of total shoot-to-root auxin transport with N concentration in the shoot. C, Correlation of total shoot-to-root auxin transport with C concentration in the shoot. Significant correlations are marked with asterisks (*** P < 0.001 [calculated with unbalanced linear regression]).
SUNN Couples Shoot-to-Root Auxin Transport Regulation to Root Organ Density
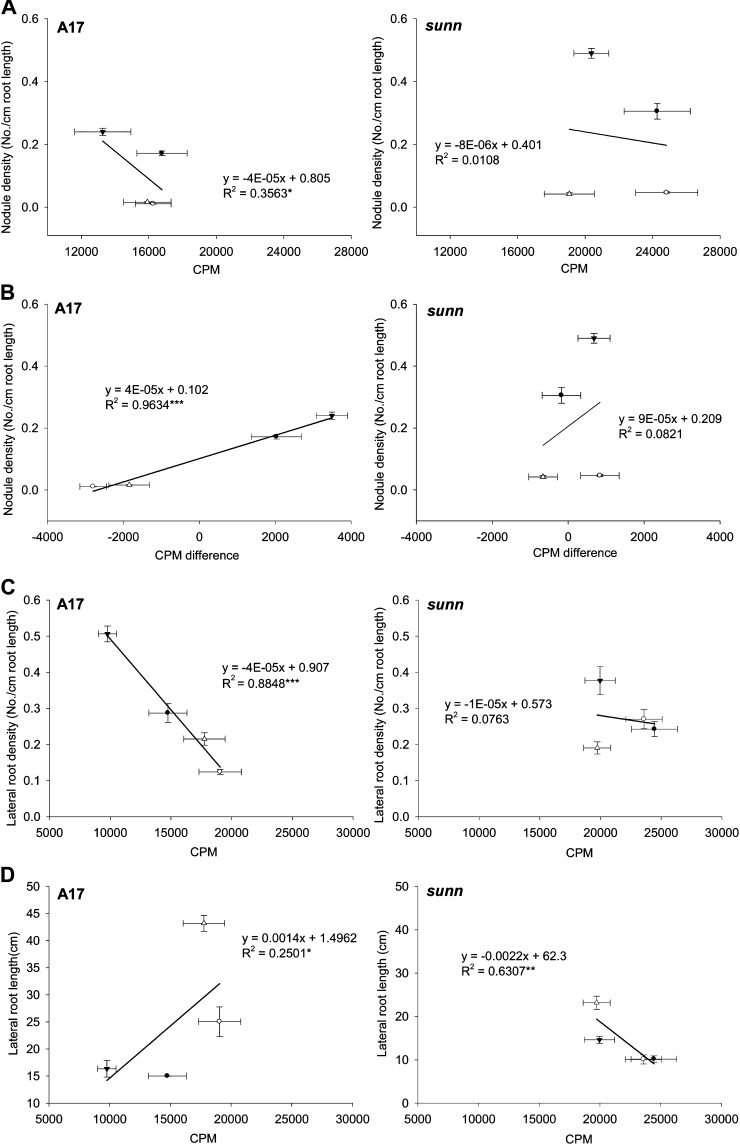
To find out whether long-distance auxin transport could determine the density of root organs formed, we correlated auxin transport with the density of emerged lateral roots and nodules. For this comparison, we examined 6- and 10-d-old seedlings, which had been inoculated 5 d after germination (i.e. measurements were made 24 and 120 h after [mock] inoculation). The 24-h time point was chosen because at this time, autoregulation (i.e. temporary inhibition of nodule formation) was determined to start in M. truncatula, and this was associated with a drop in shoot-to-root auxin transport in the wild type but not in sunn-1 mutants (van Noorden et al., 2006). After 120 h, nodule formation resumed in the wild-type and lateral roots started to emerge.
Comparison of auxin transport in mock-inoculated compared with inoculated seedlings showed that in the wild type, rhizobia caused a significant decrease in auxin transport after 24 h in ambient-C and no-N conditions, as reported previously (data not shown; van Noorden et al., 2006). After 120 h, auxin transport increased after inoculation in the no-N treatments (which formed nodules) but significantly decreased in the plus-N treatments (which formed very few or no nodules; compare with Fig. 2; Supplemental Fig. S4). In sunn-1 seedlings, inoculation with rhizobia did not significantly alter auxin transport in any of the treatments (Supplemental Fig. S4). Correlation of total auxin transported in 10-d-old inoculated seedlings with nodule density showed a slight but significant (P < 0.05) negative correlation in wild-type seedlings but not in sunn-1 seedlings (Fig. 5A). When correlating the difference in auxin transport between inoculated and mock-inoculated seedlings with the density of nodules formed, we found a significant (P < 0.001) and positive correlation (Fig. 5B) in the wild type (i.e. a significant increase in auxin transport 120 h after inoculation was correlated with higher nodule density). Again, this correlation was absent in sunn-1 seedlings. These data indicate that SUNN is required for the alteration of auxin transport by rhizobia, as found by van Noorden et al. (2006), and that this change in auxin transport in inoculated roots could be responsible for controlling the density of nodules formed.
Figure 5.
Correlation of auxin transport with root architecture phenotypes. A, Correlation of total auxin transport (as cpm) with nodule density in cv Jemalong A17 and sunn-1. B, Correlation of the cpm difference between inoculated and mock-inoculated roots with nodule density in A17 and sunn-1 (i.e. positive values indicate more auxin transport in inoculated compared with mock-inoculated roots and vice versa for negative values). C, Correlation of total auxin transport with lateral root density in mock-inoculated seedlings of A17 and sunn-1. D, Correlation of total auxin transport with lateral root length in mock-inoculated seedlings of A17 and sunn-1. Seedlings were grown in ambient C (370 μL L−1 CO2 [C370]), high C (800 μL L−1 CO2 [C800]), minus N (0 mm nitrate [N0]), and/or plus N (2.5 mm nitrate [N2.5]). Inoculation with S. meliloti was performed on 5-d-old seedlings, and auxin transport measurements were made in 10-d-old seedlings. Significant correlations are marked with asterisks (* P < 0.05, ** P < 0.01, *** P < 0.001 [calculated with unbalanced linear regression]). Data points indicate means ± se with n = 16 to 20 for auxin transport measurements and n = 24 to 32 for nodule and lateral root measurements.
Correlation of lateral root density with auxin transport in mock-inoculated seedlings showed a significant (P < 0.01) negative correlation in the wild type but not in sunn-1 mutants (Fig. 5C). Lateral root length was significantly positively correlated with auxin transport in the wild type but negatively correlated in sunn-1 mutants (Fig. 5D). These results suggest that total shoot-to-root auxin transport influences lateral root density and length and that this depends on the function of SUNN.
DISCUSSION
In this study, we wanted to test whether a nodule autoregulation gene, SUNN, plays a role in other aspects of root plasticity to C and N availability and whether these responses might be mediated by changes in shoot-to-root auxin transport. These questions were based on two main aspects of autoregulation. First, many autoregulation mutants show nodulation under high N availability, suggesting that the autoregulation signal interacts with an N signal to regulate nodule number or density (Day et al., 1989). Furthermore, many autoregulation mutants show lateral root and other growth phenotypes even in mock-inoculated conditions, although it has never been tested systematically whether these phenotypes respond to N or C availability. Second, the autoregulation mutant sunn-1 is deficient in the regulation of shoot-to-root auxin transport in response to rhizobia at the onset of autoregulation in M. truncatula, and sunn-1 nodule number can be reduced to wild-type levels by applying an auxin transport inhibitor at the shoot-root junction (van Noorden et al., 2006).
sunn-1 Affects Root Phenotypes in Response to N
Our results showed that the sunn-1 mutant was characterized by lower root and shoot biomass, shorter lateral root length and taproot length, but similar lateral root density compared with the wild type at ambient-C and minus-N conditions. This was the case in inoculated as well as mock-inoculated conditions. As expected, sunn-1 seedlings had an increased nodule number compared with the wild type, although the nodule number was not as much increased as in the soybean (nts), pea (sym29), and L. japonicus (har1) autoregulation mutants (Carroll et al., 1985a, 1985b; Sagan et al., 1995; Wopereis et al., 2000) or in sunn-1 mutants grown under hydroponic conditions (Schnabel et al., 2010). Taproot length was previously found to be reduced in sunn-1 (Penmetsa et al., 2003; Schnabel et al., 2010), har1 (Wopereis et al., 2000), and nts382 (Carroll et al., 1985a, 1985b) mutants. Lower plant dry weight was reported for har1 (Wopereis et al., 2000) and soybean nts (Hansen et al., 1989) mutants, although the difference in plant mass between mock-inoculated nts and wild-type soybean plants was not significant (Day et al., 1986), in contrast to sunn-1 plants. Lateral root density varies in different autoregulation mutants and was reported to be increased in har1 (Wopereis et al., 2000) and nts382 (Day et al., 1986) mutants but was unchanged in sunn mutants (Schnabel et al., 2005) compared with the wild type.
When supplied with altered [N] and [C], sunn-1 mutants showed significantly different responses to the wild type, again under both inoculated and mock-inoculated conditions. The root phenotypes of the wild type to added N were mostly as expected from other species: increased tissue [N] led to increased biomass (Carroll et al., 1985a; Hansen et al., 1992), a significant inhibition of nodule density (Streeter 1988), but no changes to taproot length (Drew et al., 1973; Zhang and Forde, 1998). Lateral root density in the wild type was reduced in response to increased shoot [N], which is thought to be a systemic effect (Zhang et al., 1999; Walch-Liu et al., 2006). However, high shoot N was also expected to inhibit lateral root emergence, resulting in less total root length (Zhang and Forde, 1998; Zhang et al., 1999; Walch-Liu et al., 2006), which was not observed in the wild type. Rather, increased lateral root elongation in the wild type in response to higher N resembled the responses of roots to a high localized supply of nitrate (Zhang and Forde, 1998). It is possible that these differences are due to the different nitrate concentrations used in the different studies, as inhibition of lateral root emergence in Arabidopsis was shown to require 10 mm or greater nitrate, while we only used 2.5 mm.
Inoculation only led to minor changes in biomass, lateral root density, and taproot length. Only lateral root length was significantly reduced in inoculated wild-type plants and in sunn-1 plants under high C. The reason for this reduction is unknown but is unlikely to be due to resource reallocation to nodules, as it also occurred in plants forming almost no nodules. Interestingly, inoculation did not significantly increase tissue [N] in the plants growing in the absence of nitrate. Even though nodules were pink at the time of harvest at 20 d post germination, it is possible that N supply from nodulation was not sufficient to cover the plant’s N needs, as reported previously for M. truncatula (Moreau et al., 2008).
Responses to increased C were generally not as pronounced as those to increased N and mainly resulted in enhanced plant growth, as observed in other studies (Stitt and Krapp, 1999; Fischinger et al., 2010). As expected, increased C also reduced tissue [N], which could be due to a dilution effect by increased C assimilation and by a decrease in N uptake under high CO2 concentrations (Taub and Wang, 2008).
sunn-1 plants showed a smaller increase in biomass in response to increased C and N supply than the wild type, a smaller reduction in lateral root density, a smaller increase in lateral root length, but a decrease in taproot length in response to high N. However, in contrast to other autoregulation mutants, including sunn-4, sunn-1 mutants still showed significant reductions in nodule number and density in response to increased N supply, and this is consistent with a previous study (Schnabel et al., 2010).
When taking into account the tissue [C] and [N], we showed that sunn-1 seedlings had consistently higher shoot and root [N] than the wild type, whereas [C] was fairly constant in both genotypes. Tissue [N] has not previously been reported for sunn mutants. In soybean nts mutants, N content and concentration were found to be elevated, although this was only measured under inoculated conditions (Day et al., 1986; Hansen et al., 1989). Overall, the sunn-1 mutant does not seem defective in uptake of N (or C) but in “translating” tissue [N] into the appropriate phenotypes.
Correlations of tissue [C] and [N] with root phenotypes showed that it was specifically the [N], not the [C], that drove changes in root architecture in the wild type. Increased shoot (or root) [N] showed significant negative correlations with nodule density, lateral root density, and taproot length in cv Jemalong A17. sunn-1 plants still showed significant negative correlations of tissue [N] with nodule density and taproot length, but not with lateral root density. Therefore, it appears that the sunn-1 mutant has lost some of its plasticity in regulating lateral root density in response to [N].
Our next question was whether changes in root architecture in response to [N] in sunn-1 mutants are controlled by shoot-to-root auxin transport.
Does Shoot-to-Root Auxin Transport Mediate Root Developmental Changes in Response to C or N Supply?
We found previously that shoot-to-root auxin transport was inhibited by inoculation of the root with rhizobia within 24 h, corresponding to the time of onset of autoregulation under those conditions in wild-type M. truncatula. The sunn-1 mutant showed elevated auxin transport from the shoot to the root compared with the wild type, and auxin transport inhibition did not occur after inoculation (van Noorden et al., 2006). In addition, inhibition of shoot-to-root auxin transport with the synthetic auxin transport inhibitor naphthylphthalamic acid reduced nodule number in sunn-1 plants compared with the wild type (van Noorden et al., 2006). This led us to hypothesize that shoot-to-root auxin transport could be one of the autoregulation signals that control nodule number.
Here, we tested whether shoot-to-root auxin transport responds to N and C supply and whether this correlates with nodule density as well as lateral root density and elongation. It had been hypothesized that a systemic N signal could control lateral root emergence through shoot-to-root auxin transport, with the prediction that nitrate would reduce auxin transport from shoot to root, thus preventing lateral root emergence (Forde, 2002; Walch-Liu et al., 2006). In the wild type, shoot-to-root auxin transport was significantly affected by [N] but not by [C]. However, we found a significant increase in auxin transport with increasing shoot [N]. This led to a significant negative correlation between auxin transport and nodule and lateral root density but a positive correlation with lateral root length. Auxin transport measurements from shoot to root in maize showed that a 0.5 mm nitrate treatment reduced auxin transport, and this was correlated with increased lateral root length and no change to lateral root density (Liu et al., 2010). However, that study measured auxin after 1 h of nitrate treatment, while we measured auxin transport after 10 d, at a time when lateral roots started to emerge. Another study in maize demonstrated that root indoleacetic acid (IAA) content and IAA concentration in phloem sap were reduced by the application of at least 5 mm nitrate, but this was correlated with decreased lateral root length, while lateral root density was not affected (Tian et al., 2008). In Arabidopsis, application of a synthetic auxin transport inhibitor above the site of nitrate treatment prevented the enhancement of lateral root length in response to nitrate, strengthening the idea that shoot-to-root auxin transport in response to N can influence lateral root length (Guo et al., 2005). The detailed relationship between nitrate treatment, auxin transport, and lateral root density and elongation might depend on plant species, time of measurement, nitrate concentration, and method of nitrate application. Our finding that auxin transport was strongly negatively correlated with lateral root density suggests that auxin concentration in the root might be superoptimal for lateral root initiation or early emergence in M. truncatula. This seems counterintuitive, as external auxin application usually increases lateral root number (Wightman et al., 1980), but it agrees with detailed measurements of auxin gradients in the root, which show that lateral root founder cells are established at sites of auxin minima (Dubrovsky et al., 2011).
We also found a negative correlation between nodule density and auxin transport. Again, this was unexpected, because higher auxin transport in sunn-1 mutants is correlated with higher nodule density. However, as for lateral root initiation, it might be the establishment of local auxin gradients, rather than total auxin transport or content in roots, that determines the optimum conditions for nodule initiation. The local inhibition of auxin transport by rhizobia, which transiently establishes local auxin minima in the root before nodule initiation (Mathesius et al., 1998) and which also occurs in sunn-1 mutants (van Noorden et al., 2006), might be a critical local control point for nodule initiation. It would be interesting in the future to find out in detail how shoot-to-root auxin transport interacts with local auxin gradients in the root.
In sunn-1 plants, auxin transport was constitutively higher than in wild-type plants, and the amount of auxin transported did not respond to inoculation, confirming previous results (van Noorden et al., 2006). The interesting new finding was that in sunn-1 mutants, auxin transport did not respond to shoot [N] as in the wild type. This suggests that SUNN is one of the regulators “translating” a systemic N signal into changes in long-distance auxin transport. Thus, the correlation between lateral root and nodule density with auxin transport was abolished in sunn-1 mutants. This finding suggests two new hypotheses. First, nodule density seems not to be regulated primarily by the total amount of shoot-to-root auxin transport in response to [N], because while sunn-1 mutants showed a strong correlation of nodule density with tissue [N], like the wild type, auxin transport in sunn-1 was not correlated with nodule density in response to [N]. It is likely that in sunn-1 mutants, local inhibition of nodulation by nitrate in the root overrides a defective autoregulation control. In this respect, sunn-1 mutants might be an exception to the rule that most autoregulation mutants, including other sunn mutant alleles, still nodulate in the presence of nitrate. The local regulation of nodulation by nitrate is supported by the finding that nitrate induces a CLE-like peptide in soybean, GmNIC1, that probably interacts with NARK in the root to inhibit nodulation (Reid et al., 2011a). Another local regulator of nodulation could be NIP/LATD, a putative nitrate transporter expressed in roots and nodules that affects meristem activity in nodules and lateral roots (Bright et al., 2005; Yendrek et al., 2010).
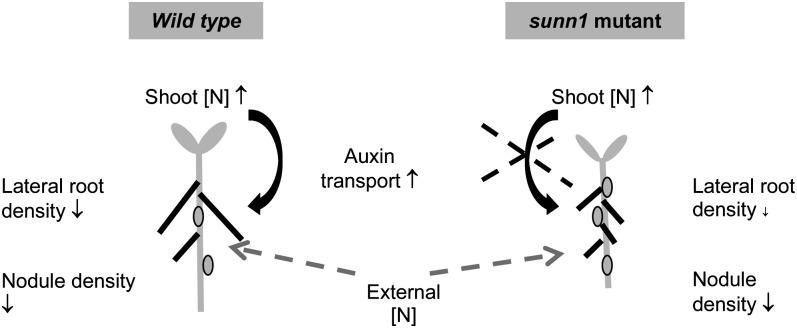
Second, lateral root density seems likely to be under the control of shoot-to-root auxin transport in response to [N], because in the wild type, lateral root density was strongly dependent on shoot [N] and strongly correlated with auxin transport. This relationship appears to be under the control of SUNN, because sunn-1 mutants failed to show a significant correlation between shoot [N] and lateral root density and between lateral root density and auxin transport. Therefore, we hypothesize that SUNN regulates lateral root development in response to tissue [N] systemically by the modulation of shoot-to-root auxin transport (Fig. 6).
Figure 6.
Model for the role of SUNN in lateral root and nodule development in response to tissue N concentrations. Our model hypothesizes that increasing shoot N concentration increases shoot-to-root auxin transport in the wild type but not in sunn-1. This is correlated with a reduction in lateral root density in the wild type but not in sunn-1. Nodule density reduces with increased [N] in both genotypes, suggesting a local inhibition of nodule initiation in the root in response to external N that is not correlated with total shoot-to-root auxin transport.
CONCLUSION
Developmental plasticity toward changes in the C and N supply enables plants to maximize N capture while minimizing the costs associated with increased growth. Increasing N use efficiency in plants has focused mainly on improved N use uptake, transport, and metabolism (Garnett et al., 2009). The identification of genes that act in the root to sense N and subsequently regulate lateral root outgrowth, including auxin response genes, has suggested a local regulation of root plasticity toward N by auxin (Zhang and Forde, 1998; Zhang et al., 1999; Krouk et al., 2010). However, so far, very few genes have been identified that translate shoot N status into appropriate changes in root architecture. Our results suggest that the SUNN gene may mediate changes in shoot N status into altered lateral root development by controlling shoot-to-root auxin transport. Therefore, SUNN could help in our understanding of N use efficiency at the level of developmental responses to N.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of Medicago truncatula ‘Jemalong A17’ (wild type) or sunn-1 mutants were scarified with sandpaper, surface sterilized with 6% (w/v) sodium hypochlorite for 20 min, washed eight times in sterile distilled water, and transferred to 1% (w/v) water agar plates. After a 2-d period in the dark at 4°C, plates were transferred to 28°C overnight to germinate. Seedlings of approximately 1 cm in length were transferred to 15-cm-diameter petri dishes containing Fåhraeus medium (Fåhraeus, 1957). Fåhraeus plates contained either 2.5 mm KCl (with no further N source) or 2.5 mm KNO3 as an N source. Each plate carried six to eight seedlings. Each treatment was replicated four times independently. Plates were kept vertical, the bottom one-third of each plate was sealed with Nescofilm, and the sides were covered for two-thirds with black paper. A sterilized aluminum foil spacer was placed between the lid and the petri dish to allow for air exchange. Plates were incubated in a growth chamber supplied with either 370 μL L−1 ambient CO2 or 800 μL L−1 elevated CO2. All treatments were randomized for their position in the growth chamber.
CO2 concentration inside the petri dishes was measured using an infrared CO2 analyzer (model LI-6251; Li-Cor).
The other conditions were consistent for all seedlings, with a temperature of 21°C, a light intensity of approximately 100 μmol m−2 s−1, and 16 h of light per day. Five days after germination, the roots were inoculated with 5 μL of Sinorhizobium meliloti at the zone of emerging root hairs. S. meliloti strain 1021 was grown in liquid Bergensen’s modified medium (Rolfe et al., 1980) at 28°C overnight and diluted with sterile water to an optical density at 600 nm of 0.1. As a control, the roots were inoculated with an equivalent volume of diluted Bergensen’s modified medium.
Long-Distance Auxin Transport Measurement
Auxin transport was measured at 24 h (6-d-old seedlings) and 120 h (10-d-old seedlings) after inoculation with S. meliloti. Sixteen to 20 seedlings from each treatment were supplied with 12 pmol of [3H]IAA (Amersham Bioscience; specific activity of 850 GBq mmol−1) in 2 μL of ethanol that was applied between the cotyledons of the seedling. Seedlings were incubated vertically for 3 h in the dark and then cut into eight root segments of 5 mm length each, starting at the root-shoot junction. Each was placed in a scintillation tube with 3 mL of scintillation fluid (Perkin-Elmer). Samples were shaken overnight before being analyzed in a Beckman LS6500 scintillation counter (Beckman Instruments).
Root Morphology Measurement
At 20 d after germination, the numbers of first-order lateral roots and nodules were counted with a stereomicroscope. Root lengths were determined with an Epson 1680 modified flatbed scanner and WINRhizo software (Régent Instruments). Roots were placed in the Plexiglas tray in a shallow depth of water and scanned at 400 dots per square inch. Scans were first imported into Adobe Photoshop 7.0, and images were then analyzed with WINRhizo software for root length.
C and N Measurement
At harvest, the dry mass (oven dried at 70°C for 48 h) of shoot and root was recorded. Total C and N concentrations of shoot or root were determined using a Europa 20-20 isotope ratio mass spectrometer (Europa Scientific Instruments).
Statistical Analysis
Sample means and se values were calculated for all parameters for each sampling date. ANOVA, residual maximum likelihood, and linear regressions were calculated using Genstat for Windows (version 11.1). Residual maximum likelihood was calculated when the data were unbalanced. The probability of a significant difference was set at P < 0.05.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. C and N contents of wild-type and sunn-1 seedlings.
Supplemental Figure S2. Taproot length of cv Jemalong A17 and sunn-1 seedlings.
Supplemental Figure S3. Correlations of shoot with root N concentration and of C concentration with nodule and lateral root density.
Supplemental Figure S4. Shoot-to-root auxin transport in response to inoculation and C and N treatments.
Supplementary Material
Acknowledgments
We thank Dr. Julia Frugoli (Clemson University) for seeds of the sunn-1 mutant, Dr. Richard Phillips of the Commonwealth Scientific and Industrial Research Organization Analytical Chemistry Group for mass spectrometric C and N analyses, and Dr. Chin Wong (Australian National University) for measuring CO2 content in the air of the growth chamber and petri dishes.
Glossary
- C
carbon
- N
nitrogen
- IAA
indoleacetic acid
References
- Bao J, Chen F, Gu R, Wang G, Zhang F, Mi G. (2007) Lateral root development of two Arabidopsis auxin transport mutants, auxl-7 and eirl-1, in response to nitrate supplies. Plant Sci 173: 417–425 [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29: 325–332 [DOI] [PubMed] [Google Scholar]
- Bright L, Liang Y, Mitchell DM, Harris JM. (2005) The LATD gene of Medicago truncatula required for both nodule and root development. Mol Plant Microbe Interact 18: 521–532 [DOI] [PubMed] [Google Scholar]
- Caba JM, Centeno ML, Fernández B, Gresshoff PM, Ligero F. (2000) Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulating mutant and the wild type. Planta 211: 98–104 [DOI] [PubMed] [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM. (1985a) Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA 82: 4162–4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM. (1985b) A supernodulation and nitrate-tolerant symbiotic (nts) soybean mutant. Plant Physiol 78: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Caroll BJ, Delves AC, Gresshoff PM. (1989) Relationship between autoregulation and nitrate inhibition in soybeans. Physiol Plant 75: 37–42 [Google Scholar]
- Day DA, Lambers H, Bateman J, Carroll BJ, Gresshoff PM. (1986) Growth comparisons of a supernodulating soybean (Glycine max) mutant and its wild-type parent. Physiol Plant 68: 375–382 [Google Scholar]
- Delves AC, Mathews A, Day DA, Carter AS, Carroll BJ, Gresshoff PM. (1986) Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol 82: 588–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, Saker LR, Ashley TW. (1973) Nutrient supply and growth of seminal root system in barley. 1. Effect of nitrate concentration on growth of axes and laterals. J Exp Bot 24: 1189–1202 [Google Scholar]
- Dubrovsky JG, Napsucialy-Mendivil S, Duclercq J, Cheng Y, Shishkova S, Ivanchenko MG, Friml J, Murphy AS, Benková E. (2011) Auxin minimum defines a developmental window for lateral root initiation. New Phytol 191: 970–983 [DOI] [PubMed] [Google Scholar]
- Fåhraeus G. (1957) The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol 16: 374–381 [DOI] [PubMed] [Google Scholar]
- Fischinger SA, Hristozkova M, Mainassara Z-A, Schulze J. (2010) Elevated CO2 concentration around alfalfa nodules increases N2 fixation. J Exp Bot 61: 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG. (2002) Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Biol 53: 203–224 [DOI] [PubMed] [Google Scholar]
- Friml J. (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Okushima Y, Tasaka M. (2007) Auxin-mediated lateral root formation in higher plants. Int Rev Cytol 256: 111–137 [DOI] [PubMed] [Google Scholar]
- Garnett T, Conn V, Kaiser BN. (2009) Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ 32: 1272–1283 [DOI] [PubMed] [Google Scholar]
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. (2008) Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci USA 105: 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YF, Chen FJ, Zhang FS, Mi GH. (2005) Auxin transport from shoot to root is involved in the response of lateral root growth to localized supply of nitrate in maize. Plant Sci 169: 894–900 [Google Scholar]
- Gutiérrez RA, Lejay LV, Dean A, Chiaromonte F, Shasha DE, Coruzzi GM. (2007) Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis. Genome Biol 8: R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AP, Peoples MB, Gresshoff PM, Atkins CA, Pate JS, Carroll BJ. (1989) Symbiotic performance of supernodulating soybean (Glycine max (L.) Merrill) mutants during development on different nitrogen regimes. J Exp Bot 40: 715–724 [Google Scholar]
- Hansen AP, Yoneyama T, Kouchi H. (1992) Short-term nitrate effects on hydroponically-grown soybean cv Bragg and its supernodulating mutant. 1. Carbon, nitrogen and mineral element distribution, respiration and the effect of nitrate on nitrogenase activity. J Exp Bot 43: 1–7 [Google Scholar]
- Jeudy C, Ruffel S, Freixes S, Tillard P, Santoni AL, Morel S, Journet E-P, Duc G, Gojon A, Lepetit M, et al. (2010) Adaptation of Medicago truncatula to nitrogen limitation is modulated via local and systemic nodule developmental responses. New Phytol 185: 817–828 [DOI] [PubMed] [Google Scholar]
- Kosslak RM, Bohlool BB. (1984) Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol 75: 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al. (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18: 927–937 [DOI] [PubMed] [Google Scholar]
- Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, et al. (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420: 422–426 [DOI] [PubMed] [Google Scholar]
- Kuppusamy KT, Ivashuta S, Bucciarelli B, Vance CP, Gantt JS, Vandenbosch KA. (2009) Knockdown of CELL DIVISION CYCLE16 reveals an inverse relationship between lateral root and nodule numbers and a link to auxin in Medicago truncatula. Plant Physiol 151: 1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO. (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29: 751–760 [DOI] [PubMed] [Google Scholar]
- Liu J, An X, Cheng L, Chen F, Bao J, Yuan L, Zhang F, Mi G. (2010) Auxin transport in maize roots in response to localized nitrate supply. Ann Bot (Lond) 106: 1019–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS. (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127: 899–909 [PMC free article] [PubMed] [Google Scholar]
- Mathesius U. (2008) Auxin: at the root of nodule development? Funct Plant Biol 35: 651–668 [DOI] [PubMed] [Google Scholar]
- Mathesius U, Bayliss C, Weinman JJ, Schlaman HRM, Spaink HP, Rolfe BG, McCully ME, Djordjevic MA. (1998) Flavonoids synthesized in cortical cells during nodule initiation are early developmental markers in white clover. Mol Plant Microbe Interact 11: 1223–1232 [Google Scholar]
- Moreau D, Voisin A-S, Salon C, Munier-Jolain N. (2008) The model symbiotic association between Medicago truncatula cv. Jemalong and Rhizobium meliloti strain 2011 leads to N-stressed plants when symbiotic N2 fixation is the main N source for plant growth. J Exp Bot 59: 3509–3522 [DOI] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, et al. (2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429 [DOI] [PubMed] [Google Scholar]
- Pacios-Bras C, Schlaman HRM, Boot K, Admiraal P, Langerak JM, Stougaard J, Spaink HP. (2003) Auxin distribution in Lotus japonicus during root nodule development. Plant Mol Biol 52: 1169–1180 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Frugoli JA, Smith LS, Long SR, Cook DR. (2003) Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol 131: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118: 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DE, Ferguson BJ, Gresshoff PM. (2011a) Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Mol Plant Microbe Interact 24: 606–618 [DOI] [PubMed] [Google Scholar]
- Reid DE, Ferguson BJ, Hayashi S, Lin Y-H, Gresshoff PM. (2011b) Molecular mechanisms controlling legume autoregulation of nodulation. Ann Bot (Lond) 108: 789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe BG, Gresshoff PM, Shine J. (1980) Rapid screening for symbiotic mutants of Rhizobium and white clover. Plant Sci Lett 19: 277–284 [Google Scholar]
- Sagan M, Morandi D, Tarenghi E, Duc G. (1995) Selection of nodulation and mycorrhizal mutants in the model-plant Medicago truncatula (Gaertn) after gamma-ray mutagenesis. Plant Sci 111: 63–71 [Google Scholar]
- Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J. (2005) The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol 58: 809–822 [DOI] [PubMed] [Google Scholar]
- Schnabel E, Mukherjee A, Smith L, Kassaw T, Long S, Frugoli J. (2010) The lss supernodulation mutant of Medicago truncatula reduces expression of the SUNN gene. Plant Physiol 154: 1390–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM. (2003) Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299: 109–112 [DOI] [PubMed] [Google Scholar]
- Stitt M, Krapp A. (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22: 583–621 [Google Scholar]
- Streeter J. (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci 7: 1–23 [Google Scholar]
- Tamaki V, Mercier H. (2007) Cytokinins and auxin communicate nitrogen availability as long-distance signal molecules in pineapple (Ananas comosus). J Plant Physiol 164: 1543–1547 [DOI] [PubMed] [Google Scholar]
- Taub DR, Wang X. (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50: 1365–1374 [DOI] [PubMed] [Google Scholar]
- Tian Q, Chen F, Liu J, Zhang F, Mi G. (2008) Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J Plant Physiol 165: 942–951 [DOI] [PubMed] [Google Scholar]
- van Noorden GE, Kerim T, Goffard N, Wiblin R, Pellerone FI, Rolfe BG, Mathesius U. (2007) Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti. Plant Physiol 144: 1115–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U. (2006) Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol 140: 1494–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Liu P, Filleur S, Gan YB, Forde BG. (2005) Signaling mechanisms integrating root and shoot responses to changes in the nitrogen supply. Photosynth Res 83: 239–250 [DOI] [PubMed] [Google Scholar]
- Walch-Liu P, Ivanov II, Filleur S, Gan YB, Remans T, Forde BG. (2006) Nitrogen regulation of root branching. Ann Bot (Lond) 97: 875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Prell J, James EK, Poole P. (2007) Nutrient sharing between symbionts. Plant Physiol 144: 604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman F, Schneider EA, Thimann KV. (1980) Hormonal factors controlling the initiation and development of lateral roots. II. Effects of exogenous growth factors on lateral root formation in pea roots. Physiol Plant 49: 304–314 [Google Scholar]
- Wopereis J, Pajuelo E, Dazzo FB, Jiang QY, Gresshoff PM, De Bruijn FJ, Stougaard J, Szczyglowski K. (2000) Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23: 97–114 [DOI] [PubMed] [Google Scholar]
- Yendrek CR, Lee Y-C, Morris V, Liang Y, Pislariu CI, Burkart G, Meckfessel MH, Salehin M, Kessler H, Wessler H, et al. (2010) A putative transporter is essential for integrating nutrient and hormone signaling with lateral root growth and nodule development in Medicago truncatula. Plant J 62: 100–112 [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG. (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279: 407–409 [DOI] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG. (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96: 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.