Abstract
Coexistence of competitors may result if resources are sufficiently abundant to render competition unimportant, or if species differ in resource requirements. Detritus type has been shown to affect interspecific competitive outcomes between Aedes albopictus (Skuse) and Aedes aegypti (L.) larvae under controlled conditions. We assessed the relationships among spatial distributions of detritus types, nutrients, and aquatic larvae of these species in nature. We collected mosquitoes, water, and detritus from artificial containers across 24 Florida cemeteries that varied in relative abundances of Ae. aegypti and Ae. albopictus.We measured nutrient content of fine particulate organic matter in water samples as total N, P, and C and ratios of these nutrients. We quantified food availability via a bioassay, raising individual Aedes larvae in the laboratory in standard volumes of field-collected, particulate-containing water from each cemetery. Quantities of detritus types collected in standard containers were significant predictors of nutrients and nutrient ratios. Nutrient abundances were significant predictors of relative abundance of Ae. aegypti, and of larval survival and development by both species in the bioassay. Survival and development of larvae reared in particulate-containing water from sites decreased with decreasing relative abundance of Ae. aegypti. These data suggest that N, P, and C availabilities are determined by detritus inputs to containers and that these nutrients in turn determine the feeding environment encountered by larvae, the intensity of interspecific competition among larvae, and subsequent relative abundances of species at sites. Detritus inputs, nutrients, and food availability thus seem to contribute to distributions of Ae. aegypti and Ae. albopictus in cemetery containers throughout Florida.
Keywords: Culicidae, Aedes, interspecific competition, ecological stoichiometry, detritus
Distributions and abundances of species occupying similar niches can be determined by spatial variation in the effects of interspecific resource competition (Tilman 1982, Chase and Leibold 2003). Two competing speciesmaycoexist if the effects of interspecific competition are less severe than intraspecific effects (Chesson 2000), a situation that can arise when competitors make differential use of more than one resource (Tilman 1982, Grover 1997). In contrast, if effects of interspecific competition are more severe than intraspecific effects, or if one species is more strongly affected by interspecific competition than the other, competitive exclusion of one species is may occur (Grover 1997, Vandermeer and Goldberg 2003). Although controlled studies of effects of resources on the outcomes of interspecific competition (reviewed by Grover 1997) and field studies of effects of habitat heterogeneity on species’ distributions (Schumacher and Parrish 2005, Ayala et al. 2007, Menzel and Nebeker 2008) have both been done, few studies have attempted to link the results of laboratory experiments on resource effects on competition directly to patterns of species distributions and coexistence in the field. Establishing such a link would be particularly important when the competitors in question are medically important mosquitoes.
In many ecosystems, including container habitats dominated by medically important mosquitoes, the majority of energy and essential nutrients pass through detritus pathways (O’Neill and Reichle 1980; Walker et al. 1991; Moore et al. 2004a,b; Yee and Juliano 2006). Despite this, there has been relatively little investigation of how the types of detritus entering a system, and detritus nutrient composition (e.g., C, N, and P) may affect competition and community structure in detritus-based communities (Yee et al. 2007). In terrestrial systems, the effects of essential nutrient composition of the soil on the outcome of competition among plants is well understood theoretically (Grover 1997) and empirically (Tilman et al. 1982; Tilman and Wedin 1991a,b). The resulting community composition of plants may in turn determine the community assemblages of higher trophic levels (Hunter and Price 1992, Haddad et al. 2001). In contrast, in aquatic systems, where detritus forms the base of a food web, essential nutrients are transferred from nonliving matter entering the system to microbial communities (Moore et al. 2004a). Microorganisms in turn are the food of animal consumers such as mosquito larvae (Merritt et al. 1992, Walker et al. 1996). Differences in essential nutrient composition of the detritus may affect the nutrient composition of the microbial community (Cross et al. 2003, Moore et al. 2004b), which ultimately may have effects on interspecific competition at higher trophic levels in detritus food chains (Yee et al. 2007) and biodiversity of assemblages of detritivores (Moore et al. 2004a,b).
If detritus composition can affect competitive outcomes among higher trophic levels in detritus food webs, then spatial or temporal heterogeneity in detritus inputs—containing different combinations of essential nutrients—may produce spatial or temporal variation in species’ distributions (Moore et al. 2004b, Greenwood et al. 2007, Greico et al. 2007). This effect creates the possibility of coexistence of competitors under some conditions and competitive exclusion under others (Yee et al. 2007). To demonstrate that differences in detritus compositions can affect nutrient environments in ways that affect distribution and abundance of detritivorous species, it must be shown that 1) interspecific competition of detritivorous species can be altered by changing the detrital environment, 2) nutritional value of microbial communities is altered by changing the detrital environment, 3) both detritus inputs and microorganism nutrient composition vary in nature in ways that can affect detritivores, and 4) nutrient composition can be used to predict spatial or temporal distribution of detritivores in nature.
The role of detritus composition in interspecific resource competition among detritivores may be important for understanding the distributions of competing larval mosquitoes Aedes aegypti (L.) and Aedes albopictus (Skuse). Both species are important vectors of human disease and are problem invasive species in much of the world (Lounibos 2002; Braks et al. 2003, 2004; Juliano 2009). Ae. aegypti was introduced into the Americas in the 17th century (Christophers 1960; Lounibos 2002). Ae. albopictus was introduced into North America in the 1980s (Hawley 1988) and has since spread widely in the southeastern United States (Lounibos 2002). In Florida, invasion by Ae. albopictus has coincided with local declines and some local extinctions of Ae. aegypti (O’Meara et al. 1995), but the two species coexist in other areas of the state, and still other locations have not been successfully invaded by Ae. albopictus (O’Meara et al. 1995; Juliano et al. 2004). This pattern of exclusion or coexistence suggests environmentally induced variation in the outcome of interspecific competition. Patterns of coexistence or exclusion are correlated with climate (Juliano et al. 2002) and land use (Braks et al. 2003, Rey et al. 2006, Leisnham and Juliano 2009).
Interspecific competition between Ae. aegypti and Ae. albopictus larvae is well studied and is likely to be an important influence on their distribution and abundance. These species share similar life histories, cooccur in water-filled artificial containers such as tires or cemetery vases (O’Meara et al. 1995; Juliano et al. 2002, 2004), and feed primarily on fine particulate organic matter (FPOM) by filter feeding and browsing surfaces (Merritt et al. 1992, Walker et al. 1996). Microorganisms that are dependent on nutrients in detritus are a major food source (Merritt et al. 1992, Walker et al. 1996). Effects of competition among larvae on survival, growth, and development are readily detectable at typical densities in nature (Juliano 1998, Braks et al. 2004, Juliano et al. 2004). In laboratory experiments using natural detritus resources (e.g., plants, invertebrate carcasses), larval Ae. albopictus are usually superior competitors (Barrera 1996, Juliano 1998, Daugherty et al. 2000, Costanzo et al. 2005, Murrell and Juliano 2008, Leisnham et al. 2009, Leisnham and Juliano 2010), but this superiority can be altered by detritus type (Barrera 1996, Daugherty et al. 2000, Murrell and Juliano 2008, Juliano 2009).
Different types of detritus often differ in nutrient composition (Palik et al. 2006) so that different detritus types are expected to support different quantities of microorganisms (Yee and Juliano 2006) and to favor different microbial taxa with different nutrient composition (Moore et al. 2004a). Detritus types that decay more rapidly contain lower C:N and C:P ratios than slower decaying detritus (Enriquez et al. 1993), suggesting that detrital stoichiometry contributes to heterogeneity of microbial communities. Such differences probably affect individual and population growth, and species composition of FPOM consumers such as these container Aedes.
Container Aedes larvae raised with animal detritus or with rapidly decaying plant detritus develop faster and grow larger than do larvae raised with slowly decaying plant detritus (Fish and Carpenter 1982, Barrera 1996, Daugherty et al. 2000, Dieng et al. 2002, Yee and Juliano 2006, Yee et al. 2007). The presence of high-quality detritus types alters the intensity of interspecific competition, shifting the outcome from competitive asymmetry and competitive exclusion to low interspecific competition and possible stable coexistence (reviewed by Juliano 2009). For Ae. albopictus competing with Ae. aegypti, grass clippings as a detritus source yielded no significant effect of interspecific densities on the two competitors (Murrell and Juliano 2008). Similarly, additions of relatively small amounts of insect carcasses resulted in reductions of the intensity of interspecific competition between Ae. albopictus and Ae. aegypti (Daugherty et al. 2000). Ratios of leaf and animal detritus also alter the outcome of competition between Ae. albopictus and a native mosquito Aedes triseriatus Say (Yee et al. 2007). What remains unknown is whether natural spatial variation in abundances of detritus types affects container community composition in nature, and if so, whether variation in availability of essential nutrients in detritus and associated microorganisms is the basis for detritus-dependent outcomes of competition.
We propose three hypotheses that link the relative abundances of Aedes species across sites to resource availability. First, we hypothesize that this nutrient heterogeneity is driven primarily by differences in detritus input among sites. Second, we hypothesize that heterogeneity in nutrient ratios across sites determines relative abundances of Aedes species at those sites. Third, we hypothesize that sites with greater relative abundance of Ae. aegypti will have greater abundance or higher quality microbial food. These hypotheses yield several predictions that we test by using field and laboratory data. 1) Sites with more rapidly decaying detritus will have FPOM with lower C:N and C:P ratios than sites with slow-decaying detritus. 2) Sites with FPOM with lower C:N and C:P will be associated with greater relative abundance of the poorer competitor Ae. aegypti. 3) Sites with greater relative abundance of Ae. aegypti will have greater survivorship and faster development of both Aedes species when they are reared on a standard volume of water and FPOM from such sites.
Materials and Methods
Sampling Mosquitoes
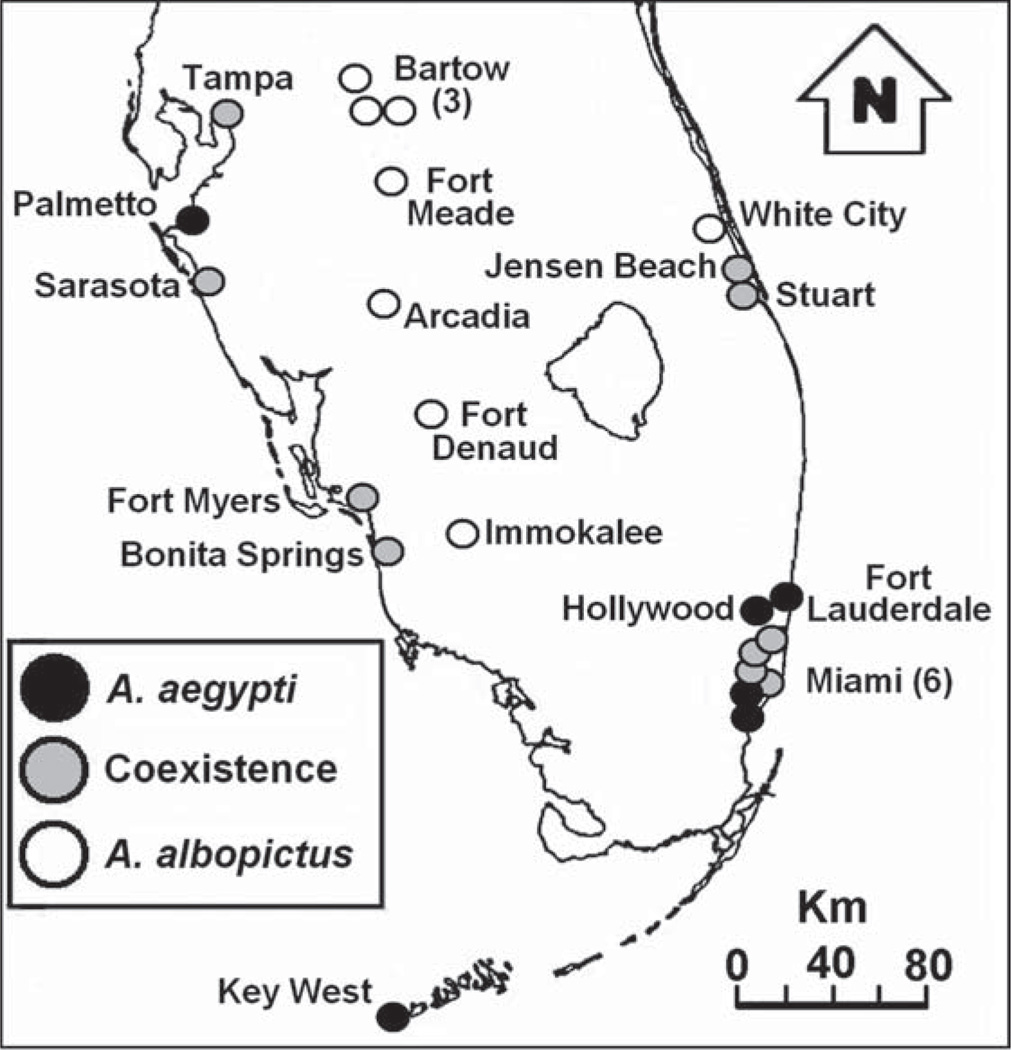
Between 3 June and 10 August 2006, we sampled 24 cemeteries in southern Florida (Fig. 1) that each had at least eight vases containing live Ae. aegypti or Ae. albopictus larvae. Among the 24 sites, six yielded only Ae. aegypti larvae, eight yielded only Ae. albopictus larvae, and 10 yielded both species (Fig. 1), with Ae. aegypti relative abundance at coexistence sites ranging from 0.22 to 0.89 (see Results). Six of these sites (three with both species, three with Ae. albopictus only) were the used in past field experiments that showed significant effects of resource competition among larvae at natural densities (Juliano et al. 2004). Our estimates of Aedes species compositions at these cemeteries are consistent with published data (O’Meara et al. 1995, Juliano et al. 2002, 2004). Aedes aegypti-only sites are less common than are Ae. albopictus-only, or coexistence sites; hence, our sample sizes for site types probably do not reflect the frequencies of site types.
Fig. 1.
Map depicting the locations of the 24 Florida cemeteries and species present at each site. Sites designated as “aegypti” or “albopictus” contained 100% of the corresponding Aedes species; sites designated as “coexistence” contained varying proportions of both species.
Mosquito larvae and water were collected from 8 to 12 preexisting (“extant”) vases per cemetery. Larvae from each vase were counted and identified to species. Water within the vase was stirred and then passed through a 106-µm sieve to remove larvae and large detritus. A portion of this water was used for the in-laboratory bioassay of food availability (see Bioassay); the remaining water samples were bottled by individual vase, stored at −60°C and assayed for N, C, and P in associated FPOM (see Nutrients). All collections and experiments for this paper complied with the current laws of the United States of America.
Because extant cemetery vases varied in age, size, and shape, we sampled detritus inputs by setting out new (“standard”) green plastic vases (mouth and base 10 and 5.5 cm in diameter, respectively; depth, 22.5 cm) placed inside or adjacent to each extant vase from which we collected larvae and water. Each standard vase was filled with 0.5 liter of water and allowed to collect detritus for 10–12 d, a sufficient time for both animal and plant detritus to accumulate, but not sufficient for complete decay of soft plant or animal detritus. We then collected detritus from the standard vases and sorted it into six types: coniferous, deciduous, herbaceous, animal, unidentified particulate matter (>106 µm in size), and other (primarily flower buds of undetermined origin). We dried (at 60°C for ≥48 h) and weighed all detritus samples. No water samples or mosquito larvae were collected from the standard vases.
Bioassay
We quantified food availability and quality for detritivores via bioassays measuring growth and development of a single Aedes larva when given a standard allotment of water and FPOM under standard conditions. A similar approach was used as a bioassay for food availability and quantity associated with solid detritus (Palik et al. 2006). Our bioassay was designed to quantify average food availability of the FPOM for each of the 24 cemeteries we sampled. We conducted this bioassay at the Florida Medical Entomology Laboratory in Vero Beach, FL, by using the newly collected water samples from each cemetery. Because we did not have enough water to conduct replicated bioassays for each individual vase, we instead pooled 50–70-ml samples of water from 7 to 10 randomly chosen sieved water samples per cemetery, creating a single aggregate sample for each cemetery. The aggregate sample was used to create three food treatments determined by the volume of water from the aggregate used to rear one larva in a 25-ml vial. Treatments were 20, 10, and 5 ml of aggregate water, with distilled water added to the 10- and 5-ml treatments to bring total volume to 20 ml. Thus, for each cemetery, availability of edible FPOM for Aedes larvae is reduced to 50 and 25% for the 10- and 5-ml treatments, respectively.
Bioassay larvae of Ae. aegypti or Ae. albopictus were from laboratory colonies at Florida Medical Entomology Laboratory, Vero Beach, FL. These colonies originated from field-collected individuals from multiple sites in Florida and have been maintained in the laboratory for multiple generations, with supplemental additions of field-collected mosquitoes. One first instar larva was added to each vial within 48 h of collecting the water. There were five replicates per species-volume treatment for each cemetery for a total of 30 individuals per cemetery. Larvae were hatched by submerging eggs in nutrient broth 24 h before the experiment. Larvae were reared in these containers at room temperature (25–28°C), and ambient summer photoperiod, for 14 d. Developmental stage (first–fourth-instar larva, pupa, or adult) and survival of each individual were recorded daily.
Nutrients
Frozen water samples from the cemeteries were thawed, mixed thoroughly, and divided in half. Each half was separately vacuum-filtered through a 0.7-µm glass fiber filter to collect FPOM between 0.7 and 106 µm. One set of glass fiber filters was processed by acid-persulfate digestion (APHA 1998) and analyzed using a Lambda 35 UV/VIS spectrometer to determine total nitrate nitrogen (N-NO3) and total phosphorus (P-PO4). These values, along with the volume filtered per sample, were used to calculate N and P concentrations for each water sample.
The second set of glass fiber filters was dried at 100°C for 24 h, weighed, and ashed at 540°C for 6h. Ash residue was weighed and organic content of each sample determined by subtraction from dry weight. These values, along with the volume filtered per sample, were used to calculate organic matter concentrations for each water sample. N concentration was then subtracted from the organic concentration to estimate C concentration for each sample.
Statistical Analyses for Hypothesis 1: Nutrient Heterogeneity Is Driven Primarily by Differences in Detritus Input
To assess the effect of detritus types on concentrations of nutrients, we used transformed (log abundance + 1) of detritus types per vase as independent variables to predict log-transformed concentrations of C, N, and P in FPOM per vase by using stepwise linear regression. Because of the log-log scaling, this regression is a suitable test if ratios of certain detritus types are predictors of nutrient concentrations. We also used stepwise linear regression to test the log-transformed detritus abundances as predictors of log-transformed ratios of C:N, N:P, and C:P per individual vase.
Statistical Analyses for Hypothesis 2: Heterogeneity in Nutrient Ratios Across Sites Determines Relative Abundances of Aedes Species
We analyzed median amounts of C, N, and P as predictors of proportion Ae. aegypti by using stepwise multiple logistic regression to determine whether total abundance of these nutrients is related to community composition. Because these two species were the only mosquito larvae we collected, the relative abundance of Ae. albopictus would simply be 1 − (proportion Ae. aegypti); hence, analysis of relative abundance of Ae. albopictus would be redundant. We also tested multiple logistic regression of proportion Ae. aegypti versus log10 of C, N, and P. By using log-log–scaled nutrient abundances we can test whether ratios of C, N, and P are related to proportion Ae. aegypti. All logistic regressions were conducted at two spatial scales: individual vase and cemetery. For the cemetery analysis, we used log10 of median C, N, and P. Although larval competition occurs at the vase level, it is also important to address distributions of these species at the cemetery level, because individual adult females may oviposit in multiple containers, and because local populations are not confined to individual vases but rather are maintained in an area by the set of vases at a suitable site. The analyses at the cemetery level also enable us to compare these data directly to those from the bioassay, which was conducted at the cemetery level due to limited water volume per vase.
Effects of cemetery and vase type (plastic, metal, stone, glass) on median C, N, P, and Aedes density also were analyzed by factorial analysis of variance (ANOVA; PROC GLM, SAS 9.1).
Statistical Analyses for Hypothesis 3: Sites With Greater Relative Abundance of Ae. aegypti Will Have Greater Abundance or Higher Quality Microbial Food
For the bioassay, we tested effects of species, FPOM availability, and proportion Ae. aegypti found at each cemetery on developmental time to third instar and time to larval death by using failure-time analysis (PROCLIFEREG,SAS9.1; Fox 2001). We chose larval death and third instar because these two states were best represented across all treatments. Our hypothesis 3 predicts that time to death should increase and time to third instar should decrease with volume of FPOM-bearing water and with proportion of Ae. aegypti. As a test of whether Aedes larvae originally present in the extant vases could deplete measurable food availability, we used failure-time analysis to test the effects of mean Aedes density at each cemetery on times to death and the third instar. For this analysis, if food availability in the water sample had been depleted by high abundances of foraging Aedes in the original samples, we expect that time to death and time to the third instar for individual larvae in the bioassay should be negatively and positively related, respectively, to mean Aedes density in the original sample. Finally, we tested the effects of median C, N, P, and amounts of detritus types at each cemetery on time to third instar and to death, again using failure-time analyses.
Results
Hypothesis 1: Nutrient Heterogeneity is Driven Primarily by Differences in Detritus Input Among Sites
Of all detritus types, only the relative abundance of “other” detritus was significantly and negatively correlated with the C:P and N:P ratios, and significantly and positively correlated with P abundance (Table 1). Coniferous and animal detritus showed negative trends as predictors of C:P, deciduous detritus showed a positive trend as a predictor of C abundance, and coniferous detritus showed a positive trend as a predictor of P abundance. Vase type was strongly associated with cemetery site, and after taking into account effects of site on nutrient concentrations, there was no significant effect of vase type on nutrient concentrations (P = 0.8692).
Table 1.
Standardized coefficients from stepwise multiple regressions
| Variable | Standardized estimate |
P | R2 |
|---|---|---|---|
| Log10 C:P ratio | 0.0092 | 0.0639 | |
| Other | −7.0426 | 0.0302 | 0.0226 |
| Coniferous | −1.5764 | 0.0744 | 0.0151 |
| Animal | −9.5218 | 0.0683 | 0.0156 |
| Herbaceous | −0.5103 | 0.1314 | 0.0106 |
| Log10 N:P ratio | 0.0252 | 0.0353 | |
| Other | −6.9172 | 0.0307 | 0.0225 |
| Coniferous | −1.4306 | 0.1005 | 0.0128 |
| Log10 C | 0.0467 | 0.0382 | |
| Animal | −13.8693 | 0.1050 | 0.0127 |
| Deciduous | 1.3946 | 0.0765 | 0.0150 |
| Herbaceous | −0.7151 | 0.1375 | 0.0105 |
| Log10 P | 0.0251 | 0.0353 | |
| Other | 9.5490 | 0.0435 | 0.0196 |
| Coniferous | 2.3471 | 0.0695 | 0.0157 |
Table shows the log10 detritus abundances as predictors of log10 ratios of nutrients, and log10 abundances of nutrients within each individual vase (n = 205). Only nutrients and nutrient ratios with significant P values are shown. Values of significant contributors to the models are in bold.
Hypothesis 2: Heterogeneity in Nutrient Ratios Across Sites Determines Relative Abundances of Aedes Species
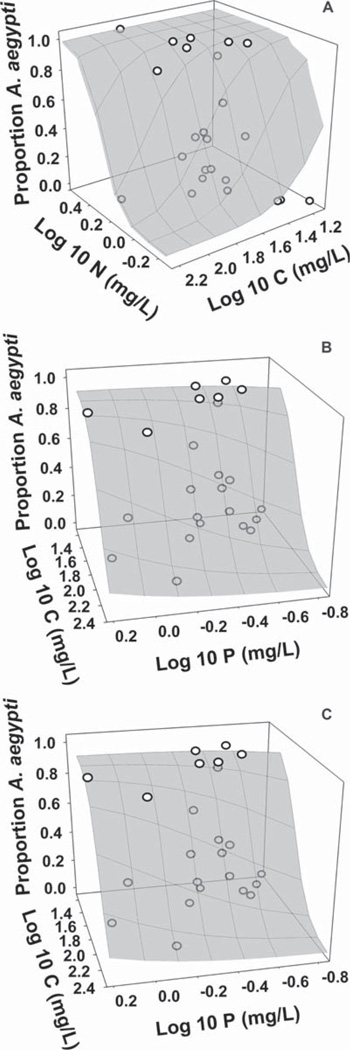
Both median concentrations and log-transformed median concentrations of nutrients were significant predictors of proportion Ae. aegypti across sites, and yielded similar R2 and concordance (a measure of the predictive accuracy of the model; SAS Institute 1990) (Table 2). Proportions of Ae. aegypti increased with concentrations of both N and P (Fig. 2A–C) but decreased with concentration of C (Fig. 2A and C). For the log-scaled analysis, this pattern suggests maximal Ae. aegypti relative abundance at sites with a low C:N ratio, and to a lesser degree, a low C:P ratio. Concordance and R2 values for regressions versus nutrients were higher than for regressions versus detritus types (Tables 1 and 2). Concordance and R2 values for regression versus nutrients were also much higher at the cemetery level than at the vase level (Table 2), although the direct of the relationships between nutrients and proportion Ae. aegypti were consistent at both levels. Nutrient amounts were not significant predictors of mean Aedes density per site (P = 0.0627), and only N abundance was a significant, and positive, predictor of Aedes abundance per vase (P = 0.0220).
Table 2.
Standardized coefficients from the stepwise multiple logistic regressions, testing log-transformed nutrient abundances as predictors for proportion Ae. aegypti
| Variable | Standardized estimate |
P | R2 | % concordant |
|---|---|---|---|---|
| Vase analyses (n = 205) | ||||
| Median nutrient concn | 0.0924 | 67.9 | ||
| C | −0.2506 | <0.0001 | ||
| N | 0.6171 | <0.0001 | ||
| P | 0.1425 | 0.0001 | ||
| Log (median nutrient concn) | 0.0967 | 67.6 | ||
| C | −0.2089 | <0.0001 | ||
| N | 0.4140 | <0.0001 | ||
| P | 0.1618 | <0.0001 | ||
| Cemetery analyses (n = 24) | ||||
| Median nutrient concn | 0.5337 | 87.6 | ||
| C | −0.9247 | <0.0001 | ||
| N | 2.2760 | <0.0001 | ||
| P | 0.5105 | <0.0001 | ||
| Log (median nutrient concn) | 0.4914 | 84.0 | ||
| C | −0.8211 | <0.0001 | ||
| N | 1.5917 | <0.0001 | ||
| P | 0.3470 | <0.0001 |
Analyses at both the individual vase and cemetery level are shown.
Fig. 2.
Comparisons of log10-transformed medians of C and N (A), N and P (B), and C and P (C) to proportion Ae. aegypti found at each cemetery (n = 24). Gray surface plots indicate proportions predicted by the logistic regression, and circles represent actual values per cemetery.
Hypothesis 3: Sites With Greater Relative Abundance of Ae. aegypti Will Have Greater Abundance or Higher Quality Microbial Food
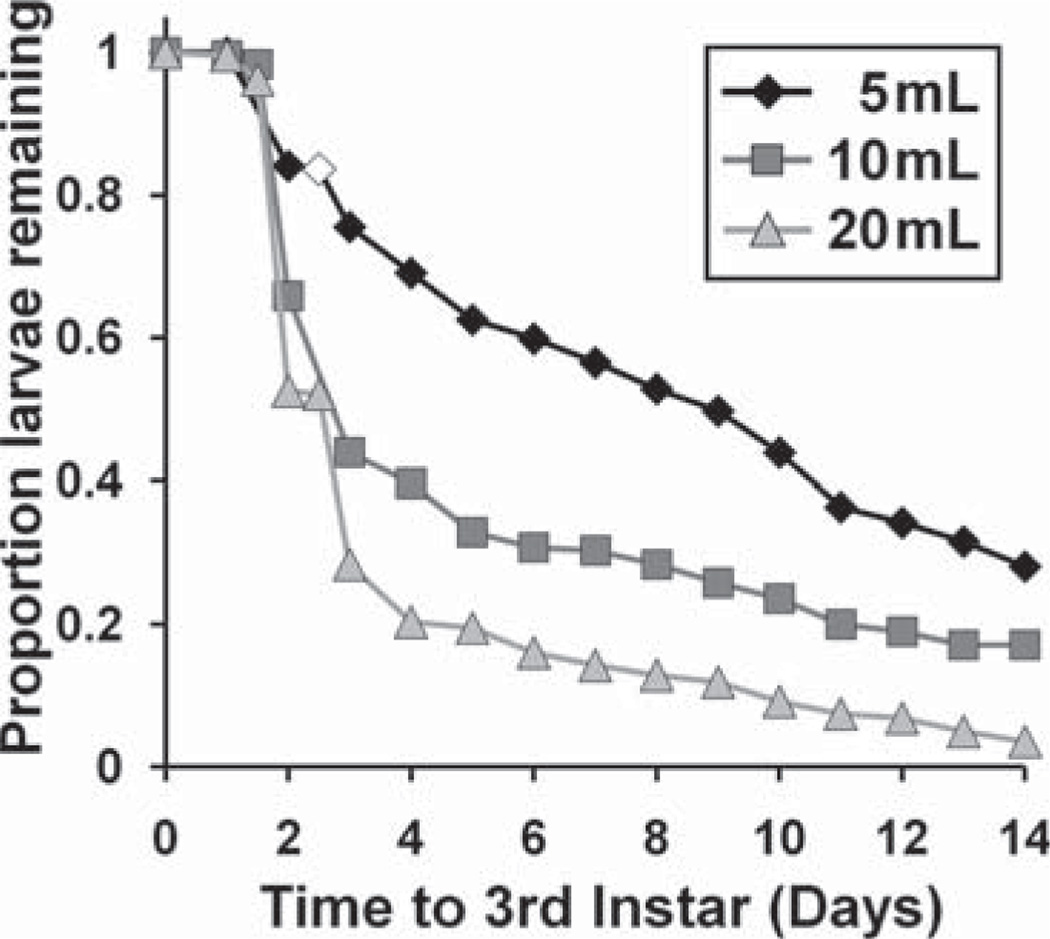
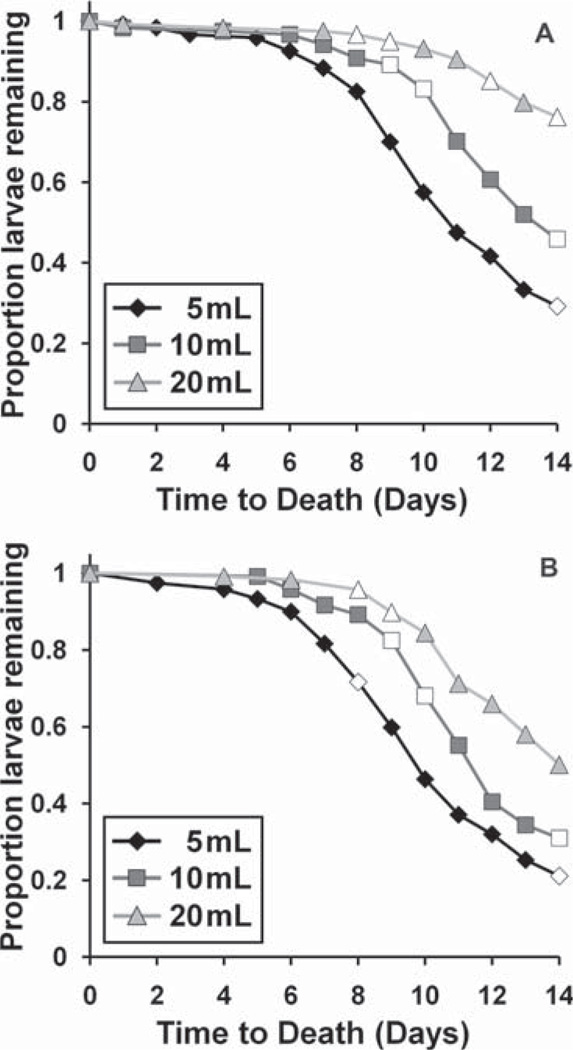
For the bioassay, volume of FPOM-bearing water was negatively related with time to third instar (P < 0.0001, Fig. 3) and positively related with time to death (P < 0.0001) for both species (Fig. 4A and B). There was a significant species effect on time to death, with Ae. albopictus living significantly longer (Fig. 4A) than Ae. aegypti (Fig. 4B), but no significant species effect for time to third instar. Species × volume interaction effect on time to death approached significance (P = 0.0531), with Ae. albopictus showing somewhat longer survival times than Ae. aegypti at high-volume treatments. Cemeteries with greater proportion Ae. aegypti and greater total Aedes density yielded FPOM-bearing water more favorable for mosquito growth (i.e., positively related to time to death and negatively related to time to third instar; P < 0.0001 in all cases). Some detritus types and nutrient amounts were also significant predictors of bioassay performance (Tables 3 and 4). Greater N and greater herbaceous detritus were consistently favorable for mosquito growth, shortening time to third instar and prolonging time to death. Greater animal detritus at a site was consistently unfavorable for mosquito growth, prolonging time to third instar and shortening time to death.
Fig. 3.
Comparison of developmental time to third instar of all bioassay larvae among different volumes of FPOM-bearing cemetery water. White data points denote days with censored values (e.g., larvae that died before attaining third instar).
Fig. 4.
Comparison of time to death of Ae. albopictus (A) and Ae. aegypti (B) bioassay larvae among different volumes of FPOM-bearing cemetery water. White data points denote days with censored values (e.g., larvae that eclosed or had not yet died at 14 d).
Table 3.
Failure-time analysis of detritus types as predictors of bioassay performance
| Parameter | Time to death | Time to third instar | ||||||
|---|---|---|---|---|---|---|---|---|
| df | Estimate | χ2 | P | df | Estimate | χ2 | P | |
| Intercept | 1 | 3.0687 | 4830.14 | <0.0001 | 1 | 0.8718 | 159.74 | <0.0001 |
| Vol | 2 | 101.18 | <0.0001 | 2 | <0.0001 | |||
| Species | 1 | −0.1356 | 25.59 | <0.0001 | 1 | −0.0706 | 1.77 | 0.1831 |
| Coniferous | 1 | −19.4687 | 1.66 | 0.1982 | 1 | −192.682 | 22.90 | <0.0001 |
| Deciduous | 1 | −0.1851 | 0.15 | 0.7002 | 1 | −1.4460 | 1.26 | 0.2610 |
| Herbaceous | 1 | 13.9856 | 21.47 | <0.0001 | 1 | −55.6310 | 125.25 | <0.0001 |
| Animal | 1 | −125.047 | 24.97 | <0.0001 | 1 | 227.3967 | 18.75 | <0.0001 |
| Fine particulate | 1 | −2.5702 | 34.63 | <0.0001 | 1 | 15.0325 | 211.35 | <0.0001 |
Significant effects are in bold. Estimates for each detritus types summarize the directional effect of that detritus type on death and developmental time.
Table 4.
Failure-time analysis of nutrients as predictors of bioassay performance
| Parameter | Time to death | Time to third instar | ||||||
|---|---|---|---|---|---|---|---|---|
| df | Estimate | χ2 | P | df | Estimate | χ2 | P | |
| Intercept | 1 | 3.0114 | 2147.650 | <0.0001 | 1 | 1.6784 | 231.140 | <0.0001 |
| Vol | 2 | 116.584 | <0.0001 | 2 | 137.111 | <0.0001 | ||
| Species | 1 | −0.1507 | 33.590 | <0.0001 | 1 | −0.0250 | 0.179 | 0.6721 |
| Log10 median N | 1 | 0.1558 | 15.241 | <0.0001 | 1 | −0.4322 | 28.998 | <0.0001 |
| Log10 median P | 1 | −0.0981 | 4.277 | 0.0386 | 1 | −0.1812 | 1.741 | 0.1870 |
| Log10 median C | 1 | −0.0004 | 0.763 | 0.0866 | 1 | 0.0026 | 10.140 | 0.0015 |
| Proportion Ae. aegypti at cemetery | 1 | 0.3356 | 76.563 | <0.0001 | 1 | −0.5192 | 39.391 | <0.0001 |
Significant effects are in bold. Estimates for each nutrient type and proportion Ae. aegypti summarize the directional effect of that variable on death and developmental time.
Discussion
Hypothesis 1: Nutrient Heterogeneity is Driven Primarily by Differences in Detritus Input Among Sites
Of all the detritus categories we tested, only “other” detritus was a significant predictor of measured nutrients ratios in suspended FPOM. This detritus category consisted mostly of fallen flower buds from various plants. However, several other detritus categories, although not individually significant, were contributors to the significant overall stepwise regression model. This weak relationship between nutrients and detritus may arise because they were collected from extant and adjacent standard vases, respectively. Larval densities varied tremendously among vases, and the differential effects of larvae feeding on nutrients in the extant vases may have caused nutrient values in those vases to differ from the nutrients that may be found within the newly collected detritus from standard vases, with limited colonization by Aedes.
The unidentified particulate detritus category was one of the best predictors of species’ relative abundances and food availability in the bioassay, but it was also the most enigmatic variable in that its origin and composition are unknown. Under the microscope, this particulate detritus seems to be a mixture of soil and small particles of animal and plant material. These particles may be products of lawn mowing at the sites, or possibly particles from polystyrene florists’ blocks used in cemetery vases, but the precise origin of this category could not be determined. It also followed a trend opposite to all other categories of detritus, as its increase predicted lower proportions of Ae. aegypti and poorer bioassay performance. This suggests that it is a nutrient-poor detritus type. A nearly significant (P = 0.0533) negative linear regression of P in suspended FPOM versus unidentified particulate detritus is consistent with this interpretation. Thus, greater amounts of this unidentified fine detritus may affect species relative abundances by favoring the species with better ability to survive on poor-quality resources (i.e., Ae. albopictus).
Hypothesis 2: Heterogeneity in Nutrient Ratios Across Sites Determines Different Relative Abundances of Aedes Species
The nutrient composition of suspended FPOM was a significant predictor of Aedes composition for both individual vases and for sites. FPOM suspended in the water column is an important food source for Aedes species and includes bacteria, other microorganisms, and fine particulate detritus (Merritt et al. 1992). Although our study design did not enable us to control or to analyze nutrients at all three trophic levels (i.e., in macroscopic detritus, suspended FPOM, and larvae), our nutrient analyses of suspended FPOM yielded significant prediction of species composition. Nutrient analysis of detritus substrates in future competition experiments could provide a better understanding of how key nutrients, independent of variation in detritus type, may affect the outcome of resource competition.
The difference in R2 values between the cemetery and vase level could be due to at least two factors. First, our sampling period for each vase (10 d) was short in relation to the total length of the study (9 wk). This short sampling period may not account for natural variation at the vase level in detritus deposition and larval densities at the time of collection. Second, the detritus data were collected from our standard vases, whereas larva and nutrient data were collected from extant vases. Although the use of standard vases was necessary to obtain comparable sampling of detritus types across all locations, our method nevertheless introduced another level of variation that may have increased the stochasticity of the data. However, the relationship between nutrient composition and Aedes composition was consistent at both the vase and the cemetery levels, and the predictive power of the cemetery analyses was much greater than the vase analyses (Table 2). Beyond these statistical considerations, species composition within a single vase is not the relevant observation for understanding population level persistence and coexistence of mosquitoes. Populations of mosquitoes, particularly container mosquitoes, exist as cohorts of larvae distributed among many small water bodies, and mobile adults that may originate and oviposit in multiple larval development sites. As such it is more likely that the aggregate sample from a large site (i.e., a cemetery) indicates the relative abundances of multiple species’ populations and that variation among individual containers at a site represents within-population stochasticity.
Hypothesis 3: Sites With Greater Relative Abundance of Ae. aegypti Will Have Greater Abundance or Higher Quality Microbial Food
The performance of individuals in the bioassay clearly indicates that FPOM-bearing water from sites where Ae. aegypti (typically the poorer competitor) is abundant provides better nutrition to mosquito larvae of both species than does FPOM-bearing water from predominantly Ae. albopictus sites. The positive relationship between nutrient concentrations, particularly N, and bioassay performance (Table 4) suggests that nutrition of these Aedes larvae is primarily N limited. Furthermore, greater total Aedes abundance at cemeteries predicts greater performance in the bioassay, and greater N is a significant predictor of greater Aedes abundance within a vase. These results suggest that food availability and nutrient abundance in this system are not determined by depletion of resources by foraging larva. If that were so, greater total Aedes abundance at collection should have been negatively related to N and to performance in the bioassay. These results suggest, instead, that the nutrient abundances and edible FPOM availability and quality are determined primarily by quantity and quality of detritus inputs to these small aquatic systems, rather than by consumption. This pattern of donor control seems to be common in detritus-based systems (Moore et al. 2004a).
Of all the factors we tested, nutrient abundances (particularly N) were the best predictor of Aedes relative abundances across cemeteries. C, the second best predictor of species’ relative abundance, had a negative effect on performance and predicted lower proportions of Ae. aegypti. Because C was 100× more abundant than N or P, its abundance relative to other nutrients may lead to lower food quality in containers with a high C:N ratio, forcing mosquito larvae to feed more vigorously to obtain adequate N, or to be deprived of N when they feed to satiation. Neither nutrients nor detritus amounts provided good prediction of total Aedes abundance for a site, suggesting that the primary effects of nutrients and detritus are to shift species’ relative abundances within the community, rather than to modify total abundance of Aedes supported.
Detritus amounts showed complex significant predictive relationships to bioassay performance. Coniferous and herbaceous detritus were consistently associated with faster development time (Table 3). Although neither of these detritus types were significant predictors of nutrients, coniferous detritus was consistently associated with high phosphorus and low C:P ratios, and herbaceous detritus was associated with low carbon and low C:P ratios (Table 1). Previous studies have demonstrated that mosquito larva development improves with increasing amounts of phosphorus (Peck and Walton 2006, Kaufman et al. 2002) and nitrogen (Kaufman et al. 2002), and decreasing C:P ratios (Peck and Walton 2005). Therefore, the relationship between these detritus types and both nutrients and bioassay performance is consistent with our predictions, and with previous published data.
Contrary to the other detritus types, animal detritus was associated with poorer survival and longer development time. Mean animal detritus per cemetery was 0.0039 ± 0.0014 g; however, of the six cemeteries with the highest amounts of animal detritus, five cemeteries had less than the mean amount of total detritus (0.2454 ± 0.0528 g). Therefore, the performance of larvae in the bioassay may not be determined by amount of animal detritus per se, but rather the association, in some cemeteries, of large amounts of animal detritus with low total amounts of detritus. No other detritus type had a similar negative association with total amounts of detritus.
Alternative Hypotheses
It is evident in our study sites (Fig. 1) and from previous studies (O’Meara et al. 1995) that Ae. aegypti and Ae. albopictus distributions in Florida follow a distinct geographic pattern. Ae. aegypti is now found predominantly in southern urban/coastal regions, whereas Ae. albopictus is predominant in northern inland/rural regions. Thus the question arises: are detritus types and resource competition the primary determinant of Aedes abundances? Or is detritus composition merely correlated with other factors, such as oviposition behavior, climate, or land use, which are the primary determinants of species distributions? Sampling of natural communities, even with analysis of resource abundance and quality, is unlikely to answer this question. The hypotheses that detritus affects Aedes abundances, and that behavior, land use, and climate affect Aedes abundances are not mutually exclusive. We consider several alternative hypotheses here, although our list is not exhaustive.
Oviposition behavior of female mosquitoes is sometimes cited as a possible determinant of species’ distributions. Other mosquito genera, such as Culex and Culiseta, will actively avoid ovipositing in containers with predators (Blaustein 1998, Eitam and Blaustein 2004), and ovipositing Aedes are sensitive to bacterial abundances and presence of conspecifics in containers (Edgerly et al. 1998, Bentley and Day 1989). However, most research indicates that mosquito oviposition is not deterred by the presence of interspecific competitors per se (e.g., Stav et al. 1999). Thus, even if oviposition choice is driving species’ distributions, this behavior should directly correlate with nutrient availability across sites (and subsequent larval performance). Furthermore, Ae. aegypti was abundant in rural, inland Florida before the introduction of Ae. albopictus (O’Meara et al. 1995), which is inconsistent with the hypothesis that Ae. aegypti simply avoids ovipositing in rural, inland, or low nutrient areas.
A recent study on Ae. albopictus (Obenauer et al. 2010) showed that this species oviposits preferentially in containers with different detritus types, laying more eggs in oak (Quercus spp.) infusion and oak–pine (Pinus spp.) infusion containers than in grass infusion or control (water only) containers. No similar study has yet been conducted on Ae. aegypti; however, if Aedes species do show oviposition preferences for different detritus types, this could cause the differences in relative abundances that we have observed. This hypothesis is not inconsistent with our results, because such oviposition behavior is probably driven by the mosquitoes seeking suitable nutrient conditions for their larvae, and avoiding harmful volatiles, when choosing a habitat for oviposition (Obenauer et al. 2010).
Climatic differences, due either to urbanization or coastal effects, are a more plausible alternative hypothesis for Aedes distributions (Juliano et al. 2002, Lounibos et al. 2010). Our previous lab study (Murrell and Juliano 2008), yielded evidence that differences in detritus resources can switch competitive outcomes from an Ae. albopictus advantage to minimal competitive effects and probably coexistence; however, we have not identified a resource environment in which Ae. aegypti became the dominant competitor. However, there is at least one study that has shown Ae. aegypti can be superior in competition with Ae. albopictus when climate (specifically, habitat drying and desiccation) affects eggs and adults (Costanzo et al. 2005). Results from Costanzo et al. (2005) and the current study suggests that detritus does affect species’ abundances but is probably only one of several factors affecting species’ distributions in Florida. We hypothesize that, in areas of low larval resource availability or quality, larval competition is the primary factor that determines species’ abundances (thus giving the competitive advantage to Ae. albopictus). However, as quantity or quality of resources for larvae increase, larval competitive asymmetry may be reduced or eliminated, and ecological impacts on adults or eggs then determine species’ distributions and abundances, potentially giving Ae. aegypti the competitive advantage under some circumstances (Costanzo et al. 2005). The data in the current study are consistent with this hypothesis; however, because this investigation focused only on larvae, we cannot test this alternative hypothesis in this paper.
In summary, previous laboratory investigations showed that high-quality, rapidly decaying detritus reduces or eliminates competitive effects between Ae. aegypti and Ae. albopictus (Daugherty et al. 2000, Murrell and Juliano 2008, Juliano 2009). An earlier field study at some of these same cemeteries showed that competition between these species was strong at typical densities (Juliano et al. 2004). In the present paper, we have demonstrated a relationship between detritus and nutrient content of FPOM, and the strong predictive ability of detritus amounts and nutrient concentrations for species’ relative abundances across sites and vases. Thus, we find support for the hypotheses that 1) spatial variation in detritus inputs determines nutrient availability and 2) different nutrient environments alter competitive outcomes in nature and thus may account for spatial patterns distributions of these Aedes in Florida. Investigations of this mosquito system have therefore linked field studies of effects of habitat heterogeneity on species’ distributions (Schumacher and Parrish 2005, Ayala et al. 2007, Menzel and Nebeker 2008) to investigations of the role of nutrient availability in food resources in competitive interactions (Grover 1997). Further research is necessary to show how variation in resource availability interacts with oviposition behavior, land use, and other environmental factors to produce observed distributions of Ae. albopictus and Ae. aegypti in Florida.
Acknowledgments
We thank G. F. O’Meara, N. Nishimura, B. W. Alto, D. Bustamante, K. Kirkham, W. L. Perry, and S. K. Sakaluk for aid in the field or laboratory, comments, and useful discussion; Florida Medical Entomology Laboratory, University of Florida, for providing facilities; the management of all cemeteries for allowing access to their sites; and J. King and two anonymous reviewers for comments on the manuscript. This research was funded by a subaward from National Institute of Allergy and Infectious Diseases grant R01AI44793 for S.A.J. and by an E. L. Mockford fellowship and grants from the Beta Lambda chapter of Phi Sigma and grants from Illinois State University Graduate Student Association to E.G.M.
References Cited
- Ayala JR, Rader RB, Belk MC, Schaalje GB. Ground-truthing the impact of invasive species: spatiotemporal overlap between native least chub and introduced western mosquitofish. Biol. Invasions. 2007;9:857–869. [Google Scholar]
- [APHA] American Public Health Association. Standard methods for the examination of water and waste-water. 19th ed. Baltimore, MD: United Book Press; 1998. [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol. Entomol. 1996;21:112–127. [Google Scholar]
- Bentley MD, Day JF. Chemical ecology and behavioral aspects of mosquito oviposition. Annu. Rev. Entomol. 1989;34:401–421. doi: 10.1146/annurev.en.34.010189.002153. [DOI] [PubMed] [Google Scholar]
- Blaustein L. Influence of the predatory backswimmer, Notonecta maculata, on invertebrate community structure. Ecol. Entomol. 1998;23:246–252. [Google Scholar]
- Braks MAH, Honório NA, Lourenço-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J. Med. Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourençode-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann. Entomol. Soc. Am. 2004;97:130–139. [Google Scholar]
- Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. 1st ed. Chicago, IL: University of Chicago Press; 2003. [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. [Google Scholar]
- Christophers RS. Aedes. aegypti. The yellow fever mosquito: its life history, bionomics, and structure. 1st ed. Cambridge, United Kingdom: Cambridge University Press; 1960. [Google Scholar]
- Costanzo KS, Kesavaraju B, Juliano SA. Condition-specific competition in container mosquitoes: the role of non-competing life-history stages. Ecology. 2005;86:3289–3295. doi: 10.1890/05-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross WF, Benstead JP, Rosemond AD, Wallace JB. Consumer-resource stoichiometry in detritus-based streams. Ecol. Lett. 2003;6:721–732. [Google Scholar]
- Daugherty MP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieng H, Mwandawiro C, Boots M, Morales R, Satho T, Nobuko T, Tsuda Y, Takagi M. Leaf litter decay process and the growth performance of Aedes albopictus larvae (Diptera: Culicidae) J. Vector Ecol. 2002;27:31–38. [PubMed] [Google Scholar]
- Edgerly JS, McFarland M, Morgan P, Livdahl T. A seasonal shift in egg-laying behaviour in response to cues of future competition in a treehole mosquito. J. Anim. Ecol. 1998;67:805–818. [Google Scholar]
- Eitam A, Blaustein L. Oviposition habitat selection by mosquitoes in response to predator (Notoecta maculate) density. Physiol. Entomol. 2004;29:188–191. [Google Scholar]
- Enriquez S, Duarte CM, Sand-Jensen K. Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia. 1993;94:457–471. doi: 10.1007/BF00566960. [DOI] [PubMed] [Google Scholar]
- Fish D, Carpenter SR. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology. 1982;63:283–288. [Google Scholar]
- Fox GA. Failure-time analysis: studying times to events and rates at which events occur. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2nd ed. New York: Oxford University Press; 2001. pp. 235–266. [Google Scholar]
- Greenwood JL, Rosemond AD, Wallace JB, Cross WF, Weyers HS. Nutrients stimulate leaf breakdown rates and detritivore biomass: bottom-up effects via heterotrophic pathways. Oecologia. 2007;1511:637–649. doi: 10.1007/s00442-006-0609-7. [DOI] [PubMed] [Google Scholar]
- Greico JP, Rejmánkova E, Achee NL, Klein CN, Andre R, Roberts D. Habitat suitability for three species of Anopheles mosquitoes: larval growth and survival in reciprocal placement experiments. J. Vector Ecol. 2007;32:176–187. doi: 10.3376/1081-1710(2007)32[176:hsftso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Grover JP. Resource competition. 1st ed. London, United Kingdom: Chapman & Hall; 1997. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J. Am. Mosq. Control. 1988;4(Suppl.):1–40. [PubMed] [Google Scholar]
- Haddad NM, Tilman D, Haarstad J, Ritchie M, Knops JMH. Contrasting effects of plant richness and composition on insect communities: a field experiment. Am. Nat. 2001;158:17–35. doi: 10.1086/320866. [DOI] [PubMed] [Google Scholar]
- Hunter MD, Price PW. Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology. 1992;73:724–732. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu. Rev. Entomol. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus and Aedes aegypti in south Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Goodfriend W, Kohler-Garrigan A, Walker ED, Klug MJ. Soluble nutrient effects on microbial communities and mosquito production in Ochlerotatus triseriatus habitats. Aquat. Microbiol. Ecol. 2002;29:73–88. [Google Scholar]
- Leisnham PT, Juliano SA. Spatial and temporal patterns of coexistence between competing Aedes mosquitoes in urban Florida. Oecologia. 2009;160:343–352. doi: 10.1007/s00442-009-1305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Juliano SA. Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion of a superior competitor. Oecologia. 2010;164:221–230. doi: 10.1007/s00442-010-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Lounibos LP, O’Meara GF, Juliano SA. Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology. 2009;90:2405–2413. doi: 10.1890/08-1569.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, O’Meara GF, Juliano SA, Nishimura N, Escher RL, Reiskind MH, Cutwa M, Greene K. Differential survivorship of invasive mosquito species in south Florida cemeteries: do site-specific microclimates explain patterns of coexistence and exclusion. Ann. Entomol. Soc. Am. 2010;103:757–770. doi: 10.1603/AN09142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel TO, Nebeker TE. Distribution of hybrid imported fire ants (Hymenoptera: Formicidae) and some native ant species in relation to local environmental conditions and interspecific competition in Mississippi forests. Ann. Entomol. Soc. Am. 2008;101:119–127. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 1992;37:349–374. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Moore JC, Berlow EL, Coleman DC, de Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ, et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004a;7:584–600. [Google Scholar]
- Moore JW, Ruesink JL, McDonald KA. Impact of supply-side ecology on consumer-mediated coexistence: evidence from a meta-analysis. Am. Nat. 2004b;163:480–487. doi: 10.1086/382091. [DOI] [PubMed] [Google Scholar]
- Murrell EG, Juliano SA. The role of detritus type in interspecific competition and population distributions of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauer PJ, Allan SA, Kaufman PE. Aedes albopictus (Diptera: Culicidae) oviposition response to organic infusions from commonflora of suburban Florida. J. Vector Ecol. 2010;35:301–306. doi: 10.1111/j.1948-7134.2010.00086.x. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- O’Neill RV, Reichle DA. Dimensions of ecosystem theory. In: Waring RH, editor. Forests: fresh perspectives from ecosystem analysis. 1st ed. Corvallis, OR: Oregon State University Press; 1980. pp. 11–26. [Google Scholar]
- Palik B, Batzer DP, Kern C. Upland forest linages to seasonal wetlands: litter flux, processing, and food quality. Ecosystems. 2006;9:142–151. [Google Scholar]
- Peck GW, Walton WE. Effect of different assemblages of larval foods on Culex quinquefasciatus and Culex tarsalis (Diptera: Culicidae) growth and whole body stoichiometry. Environ. Entomol. 2005;34:767–774. doi: 10.1093/jmedent/43.1.25. [DOI] [PubMed] [Google Scholar]
- Peck GW, Walton WE. Effect of bacterial quality and density on growth and whole body stoichiometry of Culex quinquefasciatus and Culex tarsalis (Diptera: Culicidae) J. Med. Entomol. 2006;4:25–33. doi: 10.1093/jmedent/43.1.25. [DOI] [PubMed] [Google Scholar]
- Rey JR, Nishimura N, Wagner B, Braks MAH, O’Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in South Florida. J. Med. Entomol. 2006;43:1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide, vol. 2, version 6, 4th ed. Cary, NC: SAS Institute; 1990. The LOGISTIC procedure; –1126.pp. 1071 [Google Scholar]
- Schumacher BD, Parrish JD. Spatial relationships between an introduced snapper and native goat-fishes on Hawaiian reefs. Biol. Invasions. 2005;7:925–933. [Google Scholar]
- Stav G, Blaustein L, Margalith J. Experimental evidence for predation risk sensitive oviposition by a mosquito, Culiseta longiareolata. Ecol. Entomol. 1999;24:202–207. [Google Scholar]
- Tilman D. Resource competition and community structure. 1st ed. Princeton, NJ: Princeton University Press; 1982. [PubMed] [Google Scholar]
- Tilman D, Wedin DA. Dynamics of nitrogen competition between successional grasses. Ecology. 1991a;72:1038–1049. [Google Scholar]
- Tilman D, Wedin DA. Plant traits and resource reduction for five grasses growing on a nitrogen gradient. Ecology. 1991b;72:685–700. [Google Scholar]
- Tilman D, Kilham S, Kilham P. Phytoplankton community ecology: the role of limiting nutrients. Annu. Rev. Ecol. Syst. 1982;13:349–372. [Google Scholar]
- Vandermeer JH, Goldberg DE. Population ecology: first principles. 1st ed. Princeton, NJ: Princeton University Press; 2003. [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1629–1546. [Google Scholar]
- Walker ED, O’Meara GF, Morgan WT. Bacterial abundance in larval habitats of Aedes albopictus (Diptera: Culicidae) in a Florida cemetery. J. Vector Ecol. 1996;21:173–177. [Google Scholar]
- Yee DA, Juliano SA. Consequences of detritus type in an aquatic microsystem: effects on water quality, micro-organisms and performance of the dominant consumer. Freshw. Biol. 2006;51:448–459. doi: 10.1111/j.1365-2427.2005.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kaufman MG, Juliano SA. The significance of ratios of detritus types and microorganism productivity to competitive interactions between aquatic insect detritivores. J. Anim. Ecol. 2007;76:1105–1115. doi: 10.1111/j.1365-2656.2007.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]