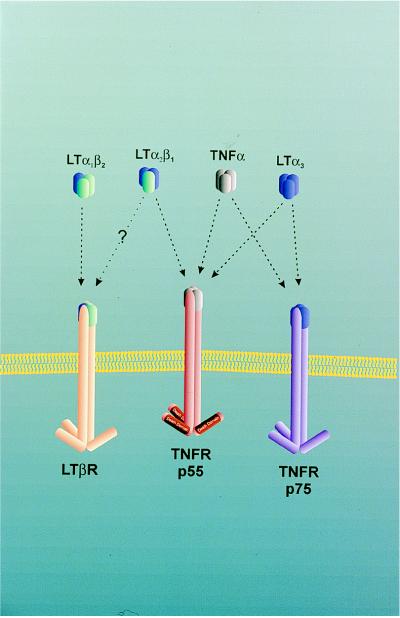
Tumor necrosis factor (TNF) and lymphotoxins (LT) belong to a family of structurally related cytokines (1) that were originally recognized for their cytotoxic effects on normal or transformed cells (2–4). The genes for the lymphotoxins α (LTα) and β (LTβ) as well as for TNF are clustered within the major histocompatibility complex (5–7). Whereas TNF can be expressed and secreted by a variety of cells including lymphocytes, NK cells, and monocytes (1), the lymphotoxins are mostly produced by activated lymphocytes and NK cells (8). TNF exists as a homotrimer that interacts with two TNF receptors, p55 TNFR and p75 TNFR. The lymphotoxins can be found either as homotrimers or heterotrimers. The LTα homotrimer lacks a transmembrane domain. The LTα,β2 heterotrimer can be retained at the cell surface because LTβ is a type II transmembrane protein (6). The LTα homotrimer binds like TNF to the p55 TNF and p75 TNF receptors (6), whereas the LTα,β2 heterotrimer binds to the LTβ receptor (3) (Fig. 1). The p55 and p75 TNF receptors are expressed in a variety of tissues including hemopoetic and epithelial cells (9). LTβ expression is found in primary and secondary lymphoid tissues but is absent in cells of peripheral blood (9).
Figure 1.
TNF, LT, and their receptors.
A number of studies in mice deficient in either TNF or LT as well as their receptors have now provided persuasive evidence that these cytokines do not only mediate cytotoxic effector functions as their names suggest but are, perhaps more importantly, involved in lymphoid organogenesis (10). The paper by Alimzhanov et al. (11) in this issue of the Proceedings makes an important contribution to this field by examining the role of LTβ in the development of secondary lymphoid tissue.
Early studies in TNF-deficient mice showed that TNF was the key mediator of septic shock induced by lipopolysaccharide and superantigens but also a key mediator of resistance to Listeria monocytogenes because p55 TNFR−/− mice were resistant to endotoxic shock but susceptible to Listeria infection (12). Apart from their deficiency in effector functions, TNF−/− mice exhibited also a deficiency in the organization of lymphoid tissue in that no primary lymphoid follicles were found in the spleen, and mature follicular dendritic cells were absent. Significantly, such mice could not form germinal centers after antigenic stimulation (13, 14).
Normally lymphoid organs are organized into compartments of T cell zones and B cell follicles. T cell zones are found in the paracortex of lymphnodes and periarteriolar sheets of the spleen whereas B cells form either resting primary follicles or activated secondary follicles—i.e., germinal centers. A third compartment of B cells exists in the marginal zone of the spleen (10). Until recently, little was known about the molecular mechanisms responsible for these structural organizations of lymphocytes around follicular dendritic cells in the case of primary or secondary follicles and around macrophages and metallophils in the marginal zone of the spleen. In TNF−/− mice the marginal zones in the spleen were expanded whereas follicles and follicular dendritic cells were absent (13). This may be due to a block of migration of marginal zone B cells, perhaps caused by the absence of adhesion receptors on the marginal zone endothelial cells because TNF can induce the expression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), MadCAM-1, and peanut agglutinin d (PNA-d) (15–18). Indeed in TNF−/− mice MadCAM-1 could not be detected in spleenic marginal zones (13). Thus, while T cells appear to migrate normally from marginal zones in TNF−/− mice, B cell migration appears to be blocked. The absence of follicular dendritic cells (FDC) may in fact be caused by the absence of follicular B cells because generally B cell-deficient mice lack FDC (19, 20). This is well in line with the observation that transfer of normal bone marrow cells into TNF−/− mice can restore normal lymphoid organ structure (21).
When LTα−/− mice were analyzed, an even more severe impairment of lymphoid organ structure was noticed: primary and secondary follicules were absent and FDC networks were lacking as in TNF−/− mice, but in addition lymphnodes and Peyer’s patches (PP) were absent (22, 23). Theoretically the more severe phenotype of LTα−/− mice could be due to either the absence of the LTα homotrimer or the LTα,β2 heterotrimer that bind to the p55 or p75 TNFR and the LTβ receptor, respectively (Fig. 1). However, because p55 TNFR−/− (24) and p75 TNFR−/− (25) mice develop lymphnodes and PP, it was more likely that the LTα−/− phenotype was caused by the absence of the LTα,β2 heterotrimer binding to the LTβ receptor. Indeed, it was shown in experiments employing a soluble LTβ receptor–Fc fusion protein in pregnant mice that the genesis of lymphnodes and PPs but not that of mesenteric lymphnodes was inhibited in the embryos (26). These results were confirmed and extended in the papers by Alimzhanov et al. (11) as well as by Koni et al. (27) in LTβ−/− mice.
LTβ−/− mice, like LTα−/− mice, lacked peripheral lymphnodes, PPs, primary and secondary B cell follicles, as well as FDC. Unlike LTα−/− mice, however, these mice contained mesenteric and cervical lymphnodes, suggesting that the LTα homotrimer may have a special role in lymphoid organogenesis of the latter structures. A detailed analysis of the spleen in LTβ−/− mice revealed the absence of MOMA+ metallophilic macrophages in the spleen and the absence of MadCAM-I. In lymphnodes T and B cell zones appeared separated yet B cell follicles and FDC were absent. Here MadCAM-1 expression was found on what appeared to be flattened high endothelial venules. There were no abnormalities detected in primary lymphoid organs even though LTβ was shown to be expressed in the thymus. In lung and liver a marked accumulation of lymphocytes was detected in perivascular areas consisting mostly of B cells and CD4+ T cells (Table 1). Finally, after immunization, germinal centers did not form but aggregates of PNA-binding cells could be detected.
Table 1.
Phenotypes of mice deficient in TNF, LTs, or their receptors
| Splenic marginal zone | Primary lymphoid follicles | Secondary lymphoid follicles (germinal centers) | FDC networks | Lymphnode | PP | Thymus | Organ infiltrates | |
|---|---|---|---|---|---|---|---|---|
| TNF = TNFα | Enlarged | Absent | Absent | Absent | Normal | Present | Normal | Absent |
| p55 TNFR | Absent | Absent | Absent | Normal | Present | Normal | Absent | |
| p75 TNFR | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
| LTα = TNFβ | Disturbed | Absent | Absent | Absent | Absent | Absent | Normal | |
| LTβ | Disturbed | Absent | Absent | Absent | Partially absent* | Absent | Normal | Massive† |
*Cervical and mesenterical lymphnodes present.
CD4+ cells and B cells.
The underlying molecular mechanisms responsible for the defects in lymphoid organogenesis and formation of germinal centers are not yet elucidated, but it appears likely that they involve the regulation of expression of various adhesion molecules as well as of inflammatory molecules that regulate migration of B cells as well as organization of FDC networks.
In summary, the TNF-like cytokine family of lymphotoxins has come a long way from initially being recognized as mediators of immune effector functions to the realization that these molecules play essential roles in the normal genesis of lymphoid organ structures.
References
- 1.Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 2.Li C B, Gray P W, Lin P F, McGrath K M, Ruddle F H, Ruddle N H. J Immunol. 1987;138:4496–4501. [PubMed] [Google Scholar]
- 3.Conta B S, Powell M B, Ruddle N H. J Immunol. 1985;134:2185–2190. [PubMed] [Google Scholar]
- 4.Powell M B, Conta B S, Horowitz M, Ruddle N H. Lymphokine Res. 1985;4:13–26. [PubMed] [Google Scholar]
- 5.Müller U, Jongeneel C V, Nedospasov S A, Fisher Lindahl K, Steinmetz M. Nature (London) 1987;325:265–267. doi: 10.1038/325265a0. [DOI] [PubMed] [Google Scholar]
- 6.Browning J L, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow E P, Hession C, O’Brine-Greco B, Foley S F, Ware C F. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 7.Pokholok D K, Maroulakou I G, Kuprash D V, Alimzhanov M B, Kozlov S V, Novobrantseva T I, Turetskaya R L, Green J E, Nedospasov S A. Proc Natl Acad Sci USA. 1995;92:674–678. doi: 10.1073/pnas.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware C F, Crowe P D, Grayson M H, Androlewicz M J, Browning J L. J Immunol. 1992;149:3881–3888. [PubMed] [Google Scholar]
- 9.Ware C F, VanArsdale T L, Crowe P D, Browning J L. Curr Top Microbiol Immunol. 1995;198:175–218. doi: 10.1007/978-3-642-79414-8_11. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y J, Banchereau J. J Exp Med. 1996;184:1207–1211. doi: 10.1084/jem.184.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alimzhanov M B, Kuprash D V, Kosco-Vilbois M H, Luz A, Turetskaya R L, Tarakhovsky A, Rajewsky K, Nedospasov S A, Pfeffer K. Proc Natl Acad Sci USA. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeffer K, Matsuyama T, Kündig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Krönke M, Mak T W. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 13.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasparakis M, Alexopoulou L, Grell M, Pfizenmaier K, Bluethmann H, Kollias G. Proc Natl Acad Sci USA. 1997;94:6319–6323. doi: 10.1073/pnas.94.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pober J S, Lapierre L A, Stolpen A H, Brock T A, Springer T A, Fiers W, Bevilacqua M P, Mendrik D L, Gimbrone M A. J Immunol. 1987;138:3319–3324. [PubMed] [Google Scholar]
- 16.Cavender D E, Edelbaum D, Ziff M. Am J Pathol. 1989;134:551–560. [PMC free article] [PubMed] [Google Scholar]
- 17.Sikorski E E, Hallmann R, Berg E L, Butcher E C. J Immunol. 1993;151:5239–5250. [PubMed] [Google Scholar]
- 18.Broudy V C, Harlan J M, Adamson J W. J Immunol. 1987;138:4298–4302. [PubMed] [Google Scholar]
- 19.Cernay A R, Zinkernagel R M, Groscurth P. Cell Tissue Res. 1988;254:449–454. doi: 10.1007/BF00225818. [DOI] [PubMed] [Google Scholar]
- 20.Kapasi Z F, Burton G F, Schultz L D, Tew J, Szakal A K. J Immunol. 1993;150:2648–2658. [PubMed] [Google Scholar]
- 21.Müller M, Eugster H P, Le Hir M, Shakhov A, DiPadova F, Maurer C, Quesniaux V F J, Ryffel Mol Med. 1996;2:247–255. [PMC free article] [PubMed] [Google Scholar]
- 22.De Tongi P, Goellner J, Ruddle N H, Streeter P R, Fick A, Mariathasan S, Smith S C, Carlson R, Shornick L P, Strauss-Schoenberger J, Russel J H, Karr R, Chaplin D D. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 23.Banks T A, Rouse B T, Kerly M K, Blair P J, Godfrey V L, Kuklin N A, Bouley D M, Thomas J, Kanangat S, Mucenski M L. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 24.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Nature (London) 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 25.Erickson S L, de Sauvage F J, Kikly K, Carver-Moore K, Pitts-Meek S, Gillet N, Sheehan K C, Schreiber R D, Goeddel D V, Moore M W. Nature (London) 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 26.Rennert P D, Browning J L, Mebius R, Mackay F, Hochman P S. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koni P A, Sacca R, Lawton P, Browning J L, Ruddle N H, Flavell R A. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]