Abstract
Previous studies have indicated phase-related differences in HPA activity and amygdala responsiveness in women, such that the response to negative emotional images is reduced during high-estrogen phases of the menstrual cycle. Other research has indicated an opposite effect of exogenous progesterone, increasing amygdala activity at some doses. However, no study to date has assessed the response of the brain’s arousal circuitry to negative images during the luteal phase, when both progesterone and estrogen levels are elevated. To address this question, 17 naturally cycling women were each scanned during the early follicular and mid-luteal phases of the cycle, and response to IAPS images was assessed by fMRI. The results indicated significantly increased activity in hippocampus and amygdala during mid-luteal scans when compared to scans in the early follicular phase. These findings suggest that progesterone-mediated effects dominate during the luteal-phase, and further suggest that estrogen and progesterone may play opposing roles in modulating the brain’s arousal circuitry.
Keywords: progesterone emotion fMRI amygdala hippocampus
Introduction
Numerous studies have observed significant sex differences in both the physiological and neural responses to stress. For equivalent stressors, women on average show reduced glucocorticoid release relative to men (Uhart et al., 2006; Kudielka et al., 2005; Kirschbaum et al., 1999), but a greater physiological response as measured by skin conductance (Bradley et. al, 2001). Neuroimaging studies have identified significantly differing activation between the sexes during the viewing of emotionally arousing material in regions including the amygdala and anterior cingulate (Wrase et al., 2003; Hamann 2005). In the amygdala, there are significant sex differences in functional connectivity even at rest (Kilpatrick et al., 2006).
One potential explanation for these sex differences in emotional processing is that they are related to changes in ovarian hormone levels in women over the course of the menstrual cycle. Studies that have considered menstrual phase have observed larger basal glucocorticoid levels during the luteal phase (Bouma et al., 2003) when progesterone levels are at their peak. Similarly, stress-evoked ACTH (Altemus et al., 2001) and glucocorticoid responses are increased in luteal women, relative to levels evoked during the follicular phase (Andreano, Arjomandi & Cahill, 2008; Kirschbaum et al., 1999; Tersman, Collins & Eneroth, 1991). Studies of hormone replacement have further indicated that progesterone, which reaches peak levels during the mid-luteal phase, enhances HPA responding to a stressor (Roca et al., 2003), whereas estrogen, which peaks in the late follicular phase, seems to decrease both the glucocorticoid and noradrenergic response (Lindheim et al., 1994).
Neuroimaging studies of emotionally arousing tasks have similarly shown phase-related changes in activity in brain regions associated with stress and arousal. During the anticipation of an expected reward, activity in the amygdala and orbitofrontal cortex varies significantly by menstrual phase in women, with increased activity in the follicular relative to luteal phase. At reward delivery, increased follicular activity can be observed in midbrain and striatal areas (Dreher et al., 2007). The response of the OFC also varies by phase during the viewing of negatively valenced words, although activity in this case is increased premenstrually, during the luteal phase (Protopopescu et al., 2005).
Studies of the encoding of negatively valenced, high arousal visual stimuli have also shown significant differences in the response of affective circuitry between phases, and provided clues to the specific roles of estrogen and progesterone in modulating these responses. During the late follicular phase, when estrogen levels are at their peak, activity in several limbic, frontal, and hypothalamic regions show a significantly reduced response to negative material compared to the early follicular phase, when estrogen levels are low (Goldstein et al., 2005). These phase-related effects may underlie sex differences in stress responding (Goldstein et al., 2010). In contrast, the effects of progesterone appear to stimulate the activity of arousal circuitry. Van Wingen and colleagues (2008) have demonstrated that the application of exogenous progesterone at levels similar to those seen during the mid-luteal phase significantly increases the amygdala response to emotional material relative to neutral images.
However, these findings do not fully describe the entirety of the natural menstrual cycle. Goldstein’s findings are limited in their interpretation to the first half of the cycle. While Van Wingen’s results provide information on the effects of progesterone, the use of exogenous progesterone during the early follicular phase differs from progesterone release during the luteal phase in at least two ways. First, in a natural cycle, progesterone release is always accompanied by release of estrogen, which may alter progesterone’s effects. Additionally, progesterone release naturally occurs after ovulation, preceded by surges in estradiol and luteinizing hormone. It is possible that the effects of these hormones may alter the responsiveness of the brain to progesterone.
To address these possibilities, the following study will compared activity evoked by negatively arousing stimuli between the early follicular and mid-luteal phase, thus assessing the neural effects of endogenous progesterone release. It was hypothesized that amygdala activity during the encoding of negative material would be increased during the mid-luteal phase, despite the potentially inhibitory influence of estrogen, and that emotion-induced amygdala activity would be correlated with ovarian hormone levels.
Methods
Participants
17 healthy, naturally cycling women were recruited on the UCI campus. Participants ranged in age from 18 to 27, and the mean age was 20.875, +/−3.125. All participants were right handed and had at least a high school education. 53% of participants (9) were Caucasian, 29% (5) were Asian, 12% (2) were Hispanic, and 6% (1) was African-American. Exclusion criteria included use of hormonal contraception, history of substance abuse, psychiatric diagnosis, treatment with psychiatric drugs, or head injury resulting in loss of consciousness. All participants were contacted in the month prior to testing and asked to track their cycle’s length. All confirmed that their cycles were regular and stable in length, and those reporting skipped cycles within the past 6 months were excluded.
Scan Scheduling
Each woman reported the date of most recent menses, and these dates were used to schedule two fMRI scans, one occurring during the early follicular phase (defined as 0-7 days since the onset of menses), and one occurring during the mid-luteal phase (defined as 18-24 days since the onset of menses). As no participants were scanned on day 0, the actual early follicular range was 1-7 days. Participants were scheduled in a counterbalanced fashion, such that half were scanned first during the early follicular phase, and half were scanned first at mid-luteal, to control for any effects of novelty or unfamiliarity with the scanning situation. Scans were scheduled in the afternoon to control for the morning cortisol surge, although in 2 cases, due to scheduling conflicts, scans took place in the late morning.
Stimuli
All images were selected from the International Affective Picture System (IAPS; Lang et al., 2008), a standardized set commonly used in emotion research. Images were selected according to standardized valence and arousal ratings. Emotional images were selected for low (negative) valence, and high arousal, while neutral images were selected for moderate (neutral) valence and low arousal. Mean valence and arousal ratings for emotional slides were 3.46 and 6.87, respectively.
Experimental Design
Each participant was scanned once during their early follicular, and once during their mid-luteal phase. Each visit began with a high resolution T1 weighted anatomical scan, upon which functional activations would be overlaid. This was followed by three functional scans, each six minutes in length. During each scan, each participant was presented with four blocks each of negative IAPS images, neutral IAPS images, and fixation crosses. Each block was 30 seconds long, and included 6 images presented for 5 seconds each. Thus in a single visit, each participant was exposed to 12 blocks of negatively emotional and neutral images, for a total of 72 images in each category.
Participants were instructed to attend to the images, and to attempt to retain as much information from them as possible.
Procedures during the second visit were identical to those in the first, with only the hormonal status of the participants differing.
Salivary Analysis
Each participant provided a saliva sample at the beginning of each session, from which sex hormone levels were determined. Samples were taken by direct expectoration into a sterile tube, as cotton salivette based methods may distort estrogen readings (Shirtcliff et al., 2001). Saliva samples were centrifuged at 2080 × g for 15 minutes to pellet out any potential contaminants. Cleared saliva was decanted into a separate tube and frozen to separate mucous material. Upon thawing, samples were decanted again and centrifuged for an additional 10 minutes.
Salivary estradiol and progesterone levels were assessed using Salimetrics (State College, PA) ELISA kits. For estradiol, these kits have a calibrated detection range from 1-32 pg/mL, and a sensitivity of .1pg/mL. For progesterone, the range is 10-2430 pg/mL, and sensitivity is 5 pg/mL. Inter-assay variance for estradiol and progesterone is 8.1% and 9.6%, respectively. Optical densities, inversely proportional to the amount of hormone in the sample, were measured using a ThermoLabSystems Multiskan (Waltham, MA) ascent spectrophotometer. Measurements showing intra-assay variance greater than 10% were retested.
Imaging
Scanning was performed at UCI’s 3T Phillips scanner. During anatomical scanning, 160 T1 weighted 1mm slices covering the whole brain were acquired in descending order, with a repetition time of 8 ms. FOV was 240 × 240 × 160, with a voxel size of 1mm3
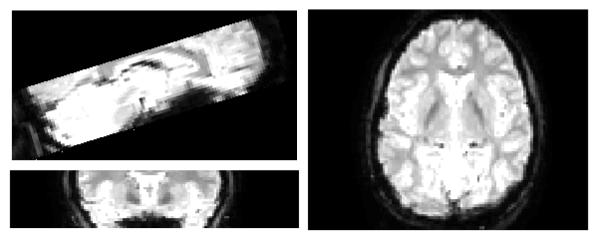
Functional scanning was performed in three separate scan sessions per visit. In each session, 24 T2* weighted slices were acquired with a repetition time of 2s. Slices were angled to include both medial temporal and frontal lobe, while more dorsal regions were not acquired. (See figure 1). Slice thickness was 2mm, and functional voxel size was 2mm3. In each 6 minute scanning session, 180 time points were acquired.
Fig. 1.
Data Analysis
fMRI data was processed in SPM5 (Wellcome Department of Imaging Neuroscience, London) running in Matlab 2007b (Mathworks, Natick, MA).
Preprocessing began with spatial realignment of functional and anatomical images to correct for head motion. All participants included in this study showed no head motion greater than 2mm. Anatomicals were segmented using SPM’s segmentation algorithm. This segmentation includes normalization to MNI space. Functional images were normalized to the segmented anatomicals, such that all images were in a common space. Following normalization, functional voxel size was 2 mm3 smoothed using a 4mm FWHM Gaussian smoothing kernel.
Functional data was analyzed as a block design, and each epoch of trials was modeled using a boxcar function. Comparisons were performed using t-contrasts in SPM. Both visits for each subject were included in a single model, considering both task condition Functional images were then and menstrual position. On the first level, these contrasts compared emotional to neutral blocks (E-N) across both visits, as well as comparing the interaction between menstrual phase and emotion condition by subtracting the E-N difference in the follicular phase from luteal, and vice versa ((E-N follicular) – (E-N luteal) and (E-N luteal)-(E-N follicular)).
Results from the individual subject level were submitted to a second level analysis in which subjects were treated as a random effect. For the E-N contrast testing the main effect of picture category, this test was performed as a t test using a threshold of p < .05, FWE corrected. A test using the same threshold was performed for the phase/picture category interaction contrasts. Because this interaction contrast is meaningless in voxels where there is no E-N difference to be modulated by menstrual phase, an additional analysis was performed using the results of the E-N contrast as an inclusive mask on the interaction contrast. In this way, the test for voxels showing significant differences in E-N signal change between menstrual phases was limited to those voxels that exhibited a significant signal difference between emotional and neutral conditions. As a result of this much more anatomically restricted search, substantially fewer comparisons were made. The masked statistical threshold was p < .05.
Region of interest analyses were also performed on the amygdala and hippocampus. Using Automated Anatomical Labeling (AAL) (Tzourio-Mazoyer et al., 2002) in the PickAtlas toolbox for SPM (Maldjian et al., 2003), masks covering the volume of bilateral hippocampi and bilateral amygdalae were generated. The left and right amygdala masks covered 204 and 248 voxels, respectively, while left and right hippocampi covered 696 and 670, respectively. Within these anatomical volumes, the same ((E-N follicular) – (E-N luteal)) and ((E-N luteal)-(E-N follicular)) contrasts were applied, at the statistical threshold of p < .05, small volume corrected.
Regression analyses were performed in several anatomically defined regions of interest. These regions were selected based on those found to be differentially sensitive to negatively arousing material depending upon menstrual phase in Goldstein and colleagues’ (2005) study, and included hippocampus, amygdala, and hypothalamus. The hypothalamic ROI was drawn to include the ventromedial and paraventricular nuclei. Activity in these regions was regressed against salivary estrogen and progesterone levels.
Salivary sex hormone levels were compared between menstrual phase groups by paired t-test to confirm that sex hormone levels aligned with self-reported menstrual position.
Results
Salivary Sex Hormones
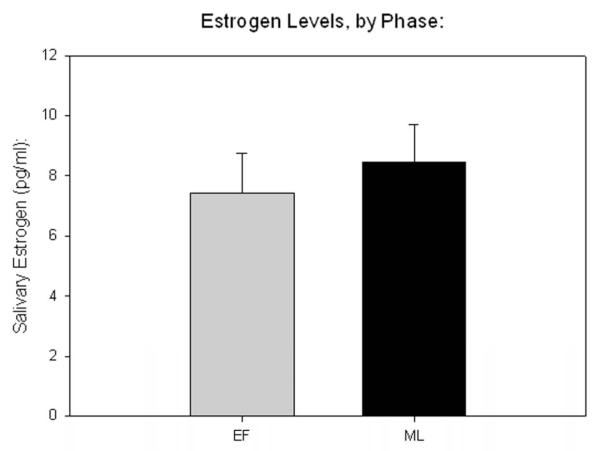
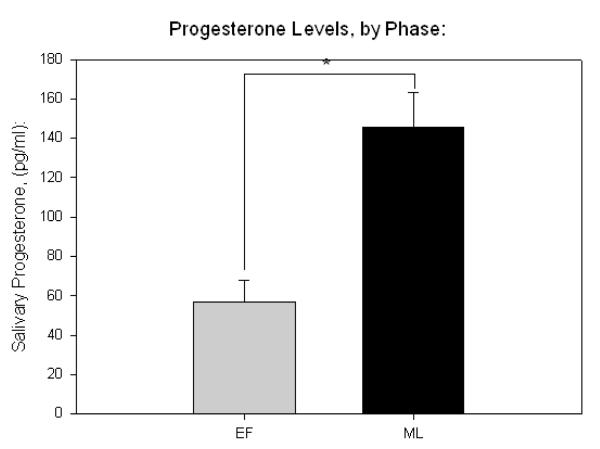
All sex hormone measurements showed and intra-assay variance of less than 10%. Salivary ELISA indicated that mean estrogen and progesterone in the early follicular phase was 7.42 pg/mL, and 56.74 pg/mL, respectively. In the mid-luteal phase, mean estrogen was 8.46 pg/mL, and mean progesterone was 145 pg/mL. Paired t tests indicated greater progesterone in the luteal phase (p < .001, Fig 1b), while no significant difference in estrogen levels was detected (Fig 2a).
Fig. 2.
fMRI
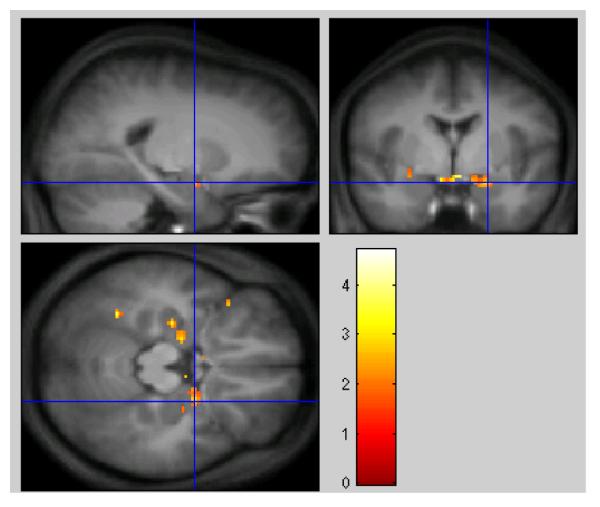
Table 1 shows clusters found to exhibit a significantly greater BOLD response to negatively arousing images, compared to neutral images (coordinates represent peak activation in this and subsequent tables). The 237 voxels total that reached threshold in this analysis defined the search volume for the inclusive mask. Areas showing significant differences between the negative and neutral conditions, including the amygdala and hippocampus are shown in figure 3
Table 1.
E-N contrast, clusters significant at p < .05, FWE corrected
| Region: | x | y | z | Z | Voxels | p | |
|---|---|---|---|---|---|---|---|
| E-N: | |||||||
| Fusiform Gyrus | R | 40 | −48 | −22 | 6.11 | 48 | <.05 |
| Inf. Temporal Gyrus |
R | 46 | −38 | −18 | 5.31 | <.05 | |
| Fusiform Gyrus | L | −42 | −46 | −22 | 5.92 | 57 | <.05 |
| Hippocampus | L | −24 | −10 | −18 | 5.8 | 59 | <.05 |
| Amygdala | L | −28 | −4 | −14 | 4.93 | <.05 | |
| Hippocampus | R | 16 | −4 | −14 | 5.75 | 31 | <.05 |
| Amygdala | R | 22 | −2 | −22 | 5.35 | 5 | <.05 |
| Cerebellum | R | 8 | −78 | −42 | 4.78 | 5 | <.05 |
Fig. 3.
No significant clusters were found using the E-N in L-F or E-N in F-L contrasts on the whole brain level.
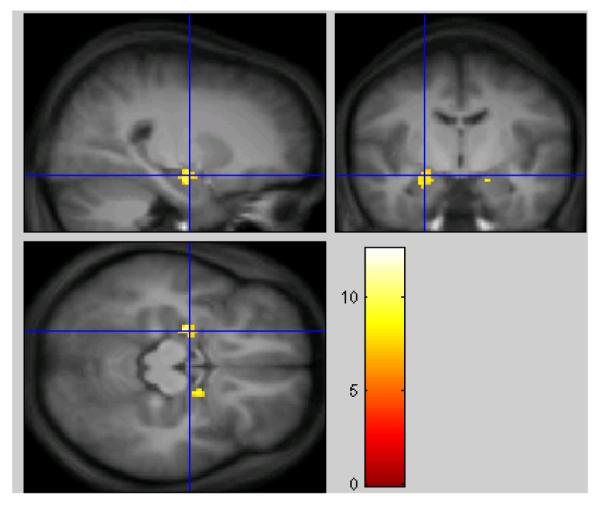
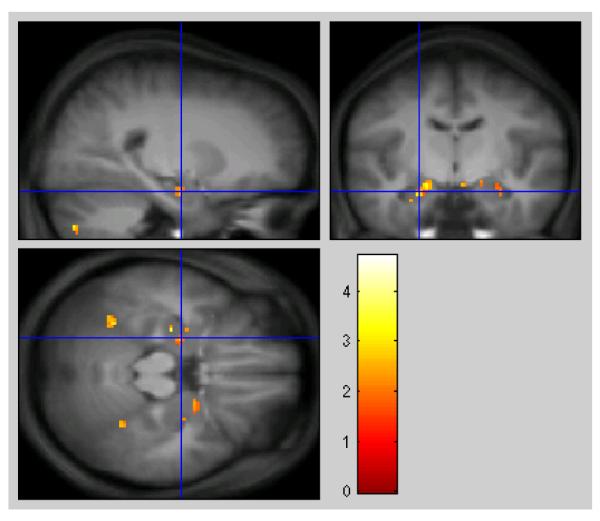
Table 2 shows the results of the inclusive mask. Clusters included show significantly greater increases in activity to negative material in the mid-luteal phase compared to early follicular, when the search is restricted to those regions showing significant differences in the E-N contrast. Areas of activation in the hippocampus and amygdala can be seen in anatomical context in figure 4.
Table 2.
E-N in L-F, masked with E-N. The masked threshold is p < .05
| Region: | x | y | z | Z | Voxels | p | |
|---|---|---|---|---|---|---|---|
| Amygdala | R | 22 | −2 | −18 | 2.55 | 57 | <.005 |
| Inf. Frontal Gyrus |
L | −38 | 20 | −16 | 3.1 | 8 | <.001 |
| Hippocampus | L | −28 | −16 | −20 | 2.95 | 9 | <.005 |
| Fusiform Gyrus | L | −34 | −52 | −18 | 2.94 | 18 | <.005 |
| Amygdala | L | −18 | −10 | −16 | 2.89 | 35 | <.005 |
| Hippocampus | L | −24 | −10 | −22 | 2.53 | <.005 | |
| Cerebellum | L | −16 | −50 | −46 | 2.89 | 6 | <.005 |
| Cerebellum | R | 18 | −72 | −40 | 2.37 | 8 | <.01 |
| Fusiform Gyrus | R | 32 | −48 | −20 | 2.3 | 10 | <.05 |
| Hippocampus | L | −28 | −6 | −22 | 2.24 | 8 | <.05 |
| Caudate Nucleus | R | 18 | 16 | 4 | 2.2 | 7 | <.05 |
| Amygdala | R | 28 | −8 | −22 | 2.15 | 8 | <.05 |
Fig. 4.
ROI Analysis
The results of the ROI analyses in hippocampus and amygdala are shown below in Table 3. Analysis of the hippocampal ROI revealed several significant clusters where activity in response to negative material was significantly larger in the mid-luteal phase, bilaterally. Analysis of the amygdalar ROI revealed only one such cluster, in the right amygdala. No clusters were detected where the response to negatively arousing images was greater in the early follicular phase compared to mid-luteal.
Table 3.
Clusters detected by ROI analysis in hippocampus and amygdala, thresholded at p < .05, small volume corrected
| Region: | x | y | z | Z | Voxels | p | |
|---|---|---|---|---|---|---|---|
| Hippocampus | R | 32 | −30 | −10 | 3.75 | 29 | <.0001 |
| Hippocampus | L | −12 | −8 | −18 | 3.26 | 77 | <.005 |
| Hippocampus | R | 18 | −12 | −14 | 3.21 | 32 | <.005 |
| Hippocampus | L | −28 | −16 | −20 | 2.95 | 28 | <.005 |
| Hippocampus | L | −28 | −34 | −8 | 2.89 | 15 | <.005 |
| Hippocampus | L | −34 | −24 | −16 | 2.88 | 5 | <.01 |
| Hippocampus | L | −32 | −22 | −10 | 2.72 | 8 | <.005 |
| Amygdala | R | 22 | −2 | −18 | 2.66 | 22 | <.005 |
| Hippocampus | R | 30 | −10 | −22 | 2.52 | 54 | <.01 |
| Amygdala | L | −20 | −8 | −16 | 2.28 | 5 | <.05 |
Regression Analysis
Regression analysis failed to find any significant relationship between amygdala signal and sex hormone levels, using either univariate or multivariate models. Similarly, a multiple regression model treating the interaction between estrogen and progesterone as a factor also failed to significantly predict amygdala activity, as did an analysis comparing amygdala signal to estrogen/progesterone ratio. However, a univariate model comparing signal change in the amygdala to salivary estrogen levels did approach significance (R =−.43, p = .107), indicating a possible negative relationship between estrogen and amygdala activity.
In the luteal phase, estradiol correlated negatively with hypothalamic activity, whether considering activity during negative encoding (R = −.58, p = .05) or during neutral encoding (R = −.61, p < .01).
Discussion
The results of this study indicate that the difference in neural response between negatively arousing and neutral stimuli is significantly larger during the mid-luteal phase than during the early follicular phase in a network including the amygdala and hippocampus. This suggests that affective processing in response to negative stimuli differs depending upon circulating levels of ovarian hormones. These findings provide further support to a growing number of neuroimaging studies showing phase-related changes in the activity of limbic regions. Additionally, because the amygdala and hippocampus are well known to be involved in both learning and memory and HPA regulation, the implications of the present data for these fields should also be considered.
Sex Hormones and Emotional Arousal in the Brain
Of the two ovarian hormones studied here, a significant difference between the early follicular and mid-luteal phases was found for salivary progesterone, but not for estradiol. These findings may suggest, therefore, that the mid-luteal increase in the reactivity of the amygdala and hippocampus to negative emotional material is likely to be related to increased progesterone levels. This outcome is in agreement with those of Van Wingen and colleagues (2008) who found a similar result using exogenous progesterone during the early follicular phase. This interpretation is limited, however, by the fact that estradiol levels were not significantly elevated in the luteal phase.
Similar studies comparing affective responses across the cycle that have targeted contrasts in estrogen have indicated that increased estrogen levels reduce the reactivity of arousal circuitry. Goldstein and colleagues (2010; 2005) report that responses to IAPS stimuli in regions related to stress and emotion are significantly greater in the early follicular phase relative to the late-follicular phase, when estrogen is at its peak. Similarly, a recent study has shown greater activity in amygdala and orbitofrontal cortices following stress induction during the late luteal phase when compared to late follicular (Ossewarde et al., 2010). Taken together, these findings seem to indicate that estrogen’s influence on the activity of arousal circuitry in the brain is generally inhibitory, whereas increased levels of progesterone seem to increase the responsiveness of these regions to negative emotion. The results of the current study are consistent with this view.
These results do appear to conflict, however, with the recent evidence that activity in the amygdala during the anticipation of reward is greater 4-8 days post-menses than during the mid-luteal phase (Dreher et al., 2007). While the present data indicate that the amygdala’s sensitivity to negatively arousing stimuli increases from the early follicular to mid-luteal, findings from reward anticipation show the opposite pattern. These disparate findings may be related to differences in the tasks studied. While the present study measured amygdala and hippocampal responses to passive viewing of negatively arousing stimuli, Dreher and colleagues specifically probed activity in response to a signal which probabilistically predicted monetary gain. (It should be noted, however, that follicular women showed a greater left amygdala response at the time of reward as well). Additionally, while Dreher and colleagues used signals for appetitive stimuli, the arousing material used in this study was clearly aversive.
Another region of note which was revealed to be differentially sensitive to arousal during the mid-luteal phase was the inferior frontal gyrus. This structure has previously shown enhanced activity in response to emotional vs. neutral words (Kuchinke et al., 2005). The IFG’s response to arousing material seems to be related to the intentional suppression of emotional responses (Wang et al., 2008). If the IFG is acting in a similar way in this study, related to the downregulation of emotional responses to the arousing pictures, these results would suggest it is more active in this capacity during the mid-luteal phase. IFG activity has also been previously related to ovarian hormone levels, as it was shown to be positively correlated with estradiol levels on a verbal task (Craig et al., 2008).
Regression Analysis
Our regression analyses showed a relationship which approached significance between E-N signal change in the amygdala and salivary estrogen during the luteal phase. This relationship was negative, indicating an inhibitory influence of estrogen, as other studies have suggested. However, this relationship was observed only in the mid-luteal phase. The fact that such a relationship was not found in our early follicular participants may be because estrogen levels in that phase were too consistently low for an effect to be detected.
Nonetheless, despite significant progesterone differences between the menstrual phase groups studied here, and significant phase differences in amygdalar and hippocampal response to negative material, regression analyses did not detect any significant correlation between sex hormone levels and activity in these regions during either neutral or emotional encoding. The lack of correlation with estradiol may be explained by the fact that no significant differences in estradiol levels were found between phase groups, thus the range of estradiol levels may have been insufficient to detect a relationship. A possible explanation for this lack of difference may be that our early follicular range (days 1-7 post-menses) was too broad, and thus may have included some women in the mid-follicular phase with somewhat elevated estradiol levels. It has also been reported that alterations in gonadal activity can be induced by stress in mid-follicular women, although these alterations have only been consistently reported with stressors more intense and prolonged than those used here (Tarin, Hamatani, & Cano, 2010). It should also be noted that other studies using the same early follicular range have reported significant differences in estradiol (Amin et al., 2006).
As for progesterone, one possible explanation for the lack of observed association between these variables is that sex hormones influence these regions in a non-linear way, which was not captured by the linear models tested. Alternatively, sex hormone effects on amygdala activity may be mediated through an interaction with cortisol. In a previous study comparing estrogen, progesterone, and cortisol with subsequent memory, the interaction of cortisol and estrogen was found to be a significant predictive factor, while estrogen alone did not predict memory (Andreano et al., 2008). Unfortunately, the slow time course of the cortisol response made independent measurements of cortisol levels in emotional versus neutral blocks impossible in this study.
Ovarian Influences on the HPA
Several of the structures found to show menstrual phase effects on their sensitivity to arousing material in this study, including the amygdala and hippocampus, and several nuclei of the hypothalamus, have been implicated in the regulation of the hypothalamic-pituitary adrenal axis. (Herman et al., 2005). This suggests that HPA activity may also be affected by menstrual phase through cyclic effects on these regulation sites.
Studies of stress hormone responses have consistently shown increased basal (Andreano et al., 2008) and evoked (Kirschbaum et al., 1999) cortisol during the mid-luteal phase compared to follicular phase, or following progesterone replacement (Roca et al., 2003). These phase related changes in HPA responsiveness cannot be directly connected to the phase related changes in neural activity in HPA regulatory sites observed here. However, animal models have indicated that stimulation of the hippocampus and amygdala influences HPA activity. Direct amygdala stimulation has been shown to increase glucocorticoid release, (Dunn & Whitener, 1986; Redgate & Fahringer, 1973 ; Matheson et al., 1971), while hippocampal stimulation inhibits the HPA (Herman & Cullinan, 1997; Dunn & Orr, 1984). Thus it seems more likely mid-luteal increases in cortisol release are mediated by progesterone effects on the amygdala.
Consistent with the view that estrogen’s role in modulating the arousal circuitry is inhibitory, a significant negative correlation between estrogen and hypothalamic activity was found. Negative relationships were present during the encoding of both negative and neutral images, and these relationships did not significantly differ. Thus, under our conditions, while hypothalamic activity was influenced by estrogen levels, this influence was not dependent upon the level of arousal. This outcome seems to conflict with the findings of Goldstein and colleages (2005), who report a significant effect of a difference in estrogen levels on the response to negative stimuli in this region. However, as the correlation found in the present study was found only in the luteal phase, which was not included in Goldstein’s study, it is possible that estradiol’s effects in this region differ in the presence of luteal levels of progesterone.
Potential Limitations
Sex hormone measurements in this study were taken from saliva, as opposed to plasma, so that a measurement could be taken immediately prior to scanning without producing any additional arousal associated with sample collection. However, there is evidence that salivary concentrations of sex hormones will vary over the course of a day (Wood, 2009). While this likely does not affect the interpretation of our regression analyses, unless sex hormone concentrations changed during the scan session, it may limit the ability to confirm self-reported menstrual position by sex hormone levels. While it remains the case that our mid-luteal and early follicular groups differed significantly by progesterone immediately prior to scanning, the present data only provides information with respect to that single timepoint. Future studies would be advised to either use post-fasting plasma measurements where possible, or else use pooled saliva collected over several hours for verification of menstrual position by hormone levels.
Additionally, because we scheduled scans within a given phase window, rather than a particular day, the time elapsed between the first and second session is not constant across women in this design. Those participants who were first scanned during the mid-luteal phase were scanned between 5-17 days later for the early follicular phase, while subjects first scanned during the early follicular phase were scanned 11-24 days later. Future studies might take this into account during scheduling to assure that the time elapsed between the first and second scans is uniform across participants.
Lastly, subjective arousal was not measured in this study, so the changes in activity in regions associated with emotion can only be inferred to represent subjective changes. Arousal judgments would additionally have served as a verification of the participants attention to the stimuli. Future studies would benefit from consideration of subjective arousal ratings, as well as state-level measurements of mood.
Implications for Learning and Memory
The hippocampus and amygdala are well known to be critically involved in the formation and consolidation of memory, particularly the modulation of memory by stress and emotion (McGaugh, 2004). Several studies have shown that activity at encoding in the amygdala (Kensinger & Schacter, 2006; Dolcos et al., 2004; Cahill et al., 1996) and hippocampus (Spaniol et al., 2009; Davachi & Wagner, 2002) predicts subsequent memory. In emotional memory, successful encoding appears to depend uniquely on the interactions between these two structures (Strange & Dolan, 2006). The evidence that amygdalar and hippocampal activity during encoding predict subsequent memory, together with the evidence shown here of phase related changes in emotional responses in these structures suggests that fluctuations in ovarian hormone levels across the cycle should affect the formation of emotional memories. More specifically, it would predict that emotional memory formation should be stronger during cycle phases when progesterone levels are elevated.
This issue has rarely been studied in humans, but several animal studies have shown that ovarian hormone levels at training profoundly influence the effects of stress on memory (Shansky et al., 2006; Rubinow et al., 2004; Shors et al., 2001). At least one human study, however, has reported significantly different relationships between cortisol levels at encoding and subsequent memory between hormonally distinct menstrual phases (Andreano et al., 2008). The findings of that study are consistent with those reported here, in that a significant positive relationship with cortisol was found in the mid-luteal phase, but no relationship between cortisol and memory was found in either early or late follicular. Thus the increased mid-luteal amygdala response to arousing material reported here may relate to a tighter coupling of cortisol release with successful memory consolidation.
Implications for Future Studies in Affective Neuroscience
It is worth noting that these analyses showed no impact of menstrual phase on encoding activity when picture category was not considered, or when neutral stimuli were considered separately. However, during emotional encoding, the hormonal status of the participants related significantly to the level of activity in the hippocampus and amygdala. This finding is supported by multiple other studies targeting different menstrual phases, all showing that changes in ovarian hormone levels significantly alter processing during emotional encoding. It would seem, therefore, that the influence of ovarian hormones on neural processes occurring during encoding differs for arousing and non-arousing stimuli.
This conclusion is of particular relevance to future research in affective neuroscience, as the tasks used in affective experiments can be expected to be particularly sensitive to ovarian hormone levels. In every study to date which has addressed the question of phase related effects on emotional processing, significant phase differences in neural activity have been found. Menstrual status, however, is rarely controlled for in neuroimaging studies. While consideration of sex as a factor in neuroimaging analysis is increasingly common, this approach may not provide much additional precision if menstrual position and use of hormonal contraception is not controlled for in female groups, as variability within women may rival that between the sexes. With respect to affective processing, the present data suggests that women can be a heterogeneous group, and that attempts to limit sex-related variability by only studying women may not be effective at reducing statistical noise if ovarian hormone levels are not considered. Thus we recommend that future neuroimaging studies of affect in women should include sex hormone measurements.
In conclusion, these data confirm evidence that amygdala activity during the encoding of negatively arousing material is increased by progesterone. During the mid-luteal phase, when endogenous progesterone levels are elevated, signal change is significantly greater compared to the early follicular phase, when ovarian hormone levels are low. These results also represent new evidence of a similar increase activity in the hippocampus during mid-luteal viewing of negative material. While this study did not measure memory or stress responding, it is nonetheless suggestive that activity associated with encoding of negative images increased during the mid-luteal phase, when the cortisol response is both elevated and more tightly correlated with memory, relative to other phases. Future studies should test whether these phase-related changes in activity relate to subsequent memory.
Research Highlights.
- In this study, the neural response to negatively arousing images was compared between women in the early follicular phase and mid-luteal phase of the menstrual cycle using fMRI
- Negatively arousing pictures produced significantly increased activity in the amygdala and hippocampus.
-This effect was modulated by menstrual phase, such that the difference in signal in both amygdala and hippocampus between negative and neutral images was larger in the mid-luteal phase, when progesterone is elevated, than it was during the early follicular phase, when both estrogen and progesterone are at low levels.
- These findings suggest that the responsiveness of the brain’s arousal circuitry varies significantly over the course of the menstrual cycle, and further suggest that the coupling of activity in these regions to arousal is increased with higher circulating progesterone levels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage. 2006;32(1):457–464. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long term memory. Psychoneuroendocrinology. 2008;33(6):874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Journal of Clinical Endocrinology and Metabolism. 2001;86(6):2525–2530. doi: 10.1210/jcem.86.6.7596. [DOI] [PubMed] [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents cortisol responses to awakening and social stress; effects of gender, menstrual phase, and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34(6):884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh Amygdala activity at encoding correlated with long-term free recall of emotional information. Proceedings of the National Academy of Sciences, USA. 1996;93(15):8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learning and Memory. 2004;11(3):261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in neural basis of emotional memories. Proceedings of the National Academy of Sciences. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampetro V, Murphy DG. Physiological variation in estradiol and brain function: a functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Hormones and Behavior. 2008;53(4):503–508. doi: 10.1016/j.yhbeh.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. Journal of Neurophysiology. 2002;88(2):982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42(5):855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Orr SE. Differential responses to hippocampal stimulation. Experimental Brain Research. 1984;54(1):1–6. doi: 10.1007/BF00235813. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Whitener J. Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitechtural specificity. Neuroendocrinology. 1986;42(3):211–217. doi: 10.1159/000124442. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weis S, Stoeffel-Wagner B, Tendolkar I, Reuber M, Beyenburg S, Klaver P, Fell J, de Grieff A, Ruhlmann J, Reul J, Elger CE. Menstrual cycle dependent neural plasticity in the adult human brain is hormone, task, and region specific. Journal of Neuroscience. 2003;23(9):3790–3795. doi: 10.1523/JNEUROSCI.23-09-03790.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. Journal of Neuroscience. 2010;30(2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic systems mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuropsychopharmacology & Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source memory for positive and negative stimuli. Journal of Neuroscience. 2006;26(9):2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–62. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo ML, Conrad M, Herrmann M. Neuroimage. 2005;28(4):1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. 2005. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA responses to stress: a review. Biological Psychology. 2005;69(1):113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lindheim SR, Legro RS, Morris RS, Wong IL, Tran DQ, Vijod MA, Stanczyk FZ, Lobo RA. The effect of progestins on behavioral stress responses in postmenopausal women. Journal of the Society for Gynecologic Investigation. 1994;1(1):79–83. doi: 10.1177/107155769400100116. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matheson GK, Branch BJ, Taylor AN. Effects of amygdaloid stimulation on pituitary-adrenal activity in conscious cats. Brain Research. 1971;32(1):151–167. doi: 10.1016/0006-8993(71)90160-0. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Ossewarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, Fernandez G. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35(1):47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Redgate ES, Fahringer EE. A comparison of the pituitary adrenal activity elicited by electrical stimulation of preoptic, amygdaloid and hypothalamic sites in the rat brain. Neuroendocrinology. 1973;12(6):334–343. doi: 10.1159/000122182. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. Journal of Clinical Endocrinology and Metabolism. 2003;88(7):3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Arseneau LM, Beverly JL, Juraska JM. Effect of the estrous cycle on water maze acquisition depends on the temperature of the water. Behavioral Neuroscience. 2004;118(4):863–8. doi: 10.1037/0735-7044.118.4.863. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress induced working memory impairment. Behavioral and Brain Functions. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26(2):165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiology of Learning and Memory. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood. Neuropsychologia. 2009;47(8-9):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Anterior medial temporal lobe in human cognition: Memory for fear and the unexpected. Cognitive Neuropsychiatry. 2006;11(3):198–218. doi: 10.1080/13546800500305096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin J, Hamatani T, Cano A. Acute stress may induce ovulation in women. Reproductive Biology and Endocrinology. 2010;8:53. doi: 10.1186/1477-7827-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersman Z, Collins A, Eneroth P. Cardiovascular consequences to psychological and physiological stressors during the menstrual cycle. Psychosomatic Medicine. 1991;53(2):185–197. doi: 10.1097/00006842-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal axis reactivity. Psychoneuroendocrinology. 2006;31(5):642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- van Wingen, van Broekhoven, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Molecular Psychiatry. 2008;13(3):325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G. Prefontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research. 2008;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. Salivary steroid assays: research or routine? Annals of Clinical Biochemistry. 2009;46(3):183–196. doi: 10.1258/acb.2008.008208. [DOI] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruessner SM, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Gender differences in the processing of standardized emotional visual stimuli in humans: A functional magnetic resonance imaging study. Neuroscience Letters. 2003;348(1):41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]