Abstract
There is increasing research interest in the risk stratification of emergency department (ED) syncope patients. A major barrier to comparing and synthesizing existing research is wide variation in the conduct and reporting of studies. The authors wished to create standardized reporting guidelines for ED syncope risk stratification research using an expert consensus process. In that pursuit, a panel of syncope researchers was convened and a literature review was performed to identify candidate reporting guideline elements. Candidate elements were grouped into four sections: eligibility criteria, outcomes, electrocardiogram findings, and predictors.
A two-round, modified Delphi consensus process was conducted using an internet-based survey application. In the first round, candidate elements were rated on a five-point Likert scale. In the second round, panelists re-rated items after receiving information about group ratings from the first round. Items that were rated by >80% of the panelists at the two highest levels of the Likert scale were included in the final guidelines.
There were 24 panelists from eight countries who represented five clinical specialties. The panel identified an initial set of 183 candidate elements. After two survey rounds, the final reporting guidelines included 92 items that achieved >80% consensus. These included 10 items for study eligibility, 23 items for outcomes, 9 items for electrocardiogram abnormalities, and 50 items for candidate predictors. Adherence to these guidelines should facilitate comparison of future research in this area.
INTRODUCTION
The emergency department (ED) evaluation of syncope is characterized by high practice variation and costs. Admission rates for adults with syncope range among 12% in Canada,1 55% in a national U.S. ED sample,2 and >80% at U.S. academic medical centers3; the underlying reasons for practice variance are unclear. In the United States alone, annual health care costs associated with hospitalizations for syncope exceed $2.4 billion,4 although there is limited evidence demonstrating benefit from inpatient evaluation and management.5
Improved risk stratification is a fundamental first step to narrowing practice variation and safely reducing hospital admissions for syncope.6 There is increasing worldwide interest in improving the ED evaluation and management of syncope, and at least nine ED-based risk-stratification instruments have been published in the past 15 years.7–15 Because a minority of well-appearing patients will experience a short-term, serious event after syncope,14 large sample sizes are likely required to derive and validate a clinically relevant risk tool. Important logistical challenges to performing rigorous validation studies may include the need for multi-site enrollment and significant external funding.
Alternatively, literature review, data pooling, and meta-analysis can potentially combine information across multiple studies. A major barrier to this approach is the large variation in the existing literature for reported eligibility criteria, outcome measures, electrocardiogram (ECG) findings, and candidate predictors.16,17 The creation of standardized research reporting guidelines may improve the ability to compare and combine data produced by different research groups.18 To address the lack of consistent research reporting, we developed standardized reporting guidelines for ED syncope risk stratification research using an expert panel modified Delphi process.
OVERVIEW OF DELPHI METHODOLOGY
We performed a two-round modified Delphi consensus study using internet-based surveys. The modified Delphi method is a systematic approach to achieve consensus among a panel of experts on a topic where existing knowledge is incomplete.19,20 The approach is characterized by iteration, controlled feedback, and statistical group response. After an initial round of anonymous ratings, panelists are given feedback on group responses and discuss items that did not achieve consensus. This allows panelists to share knowledge in a structured format and potentially improve consensus. A second round of anonymous ratings is then performed. The modified Delphi approach is suited for generating guidelines in the absence of definitive information and has been widely used in health care applications.20,21
PARTICIPANTS
Thirty-four first or senior authors on selected ED-based risk-stratification studies7–15 and panelists on recent professional society syncope guidelines22–27 were invited to participate. Additional potential panelists were identified through recommendations of interested participants. The final set of 24 participating panelists provided informed consent to participate (Table 1). The panelists represent a diversity of professional backgrounds, including cardiology (n = 9), emergency medicine (n = 6), internal medicine (n = 5), neurology (n = 2), and geriatrics (n = 2). The respondents practice in eight countries, and 54% have previously been involved in preparing professional society guidelines for the evaluation of syncope.16,22,23,26 To minimize possible investigator bias, the study chair, co-chair, and research assistant (BCS, VT, and JDC) were process moderators and did not complete the surveys themselves.
THE CONCEPTUAL MODEL
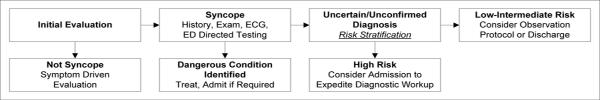
To focus the wide expertise of the group to risk stratification in the ED, we used an adapted conceptual framework from the European Society of Cardiology Guidelines for the Diagnosis and Management of Syncope.22 This conceptual framework explicitly describes the role of risk stratification in the ED diagnostic evaluation of syncope (Figure 1).
Figure 1.
Conceptual Model of ED Risk Stratification for Syncope
This conceptual model has three critical branch points. First, the clinician must distinguish `syncope' from other conditions that may have distinct diagnostic pathways from syncope. Second, a directed history, exam, ECG, and selective testing will identify a subset of patients who require hospital treatment for a dangerous condition recognized in the ED.
Finally, the remaining patients will have either an unknown or unconfirmed diagnosis. This group includes patients with presumptive diagnoses such as vasovagal or orthostatic syncope, as `criterion standard' confirmatory tests do not exist.28–30 The clinician must then risk-stratify for a serious outcome and decide whether to admit or discharge the patient. Patients at low to intermediate risk (including patients with presumptive benign causes of syncope) may be discharged or evaluated in an observation unit setting. Patients at high risk may benefit from an inpatient diagnostic evaluation. Explicit risk models1,7–14 can enhance decision-making at this final branch point.
To be clinically useful, a risk stratification model must be feasible to implement in an ED setting. Important constraints unique to the ED that panelists were asked to consider included: 1) availability and accuracy of information about the syncopal episode, 2) availability and accuracy of information about patient co-morbidities, 3) time to evaluate patients and determine disposition, and 4) availability of specialized testing. These criteria were developed by the study co-authors (BCS and VT) to maximize the face validity and feasibility of the final set of guidelines elements.
GUIDELINE ELEMENTS
Initial Identification
We performed a comprehensive literature review of primary syncope research to identify a preliminary set of guideline elements (Data Supplement S1).1,7–15,22,23,25,26,31–42
Based on our literature review and our perceived areas of reporting variation, we organized the candidate elements into four major sections: study eligibility, outcomes, ECG findings and reporting, and candidate predictors. Study eligibility items focused on constructing an operational definition of syncope and identifying `universal' exclusion criteria. The outcomes section focused both on outcome time frames relevant to ED management as well as on clinically significant conditions, procedures, and health service use. Because all prior studies have collected ECG data, we sought to define a core set of `abnormal' ECG findings. Finally, candidate predictors included demographic characteristics, symptoms, physical exam findings, co-morbidities, medications, and laboratory tests. Symptom items were loosely grouped by presumptive cause (e.g. cardiac, neurologic, vasovagal, orthostatic hypotension). All panelists reviewed the initial set of candidate guideline elements for completeness and clarity.
Criteria for Inclusion
The instructions for all items were: `Please rate all survey items on the following Likert scale.' The response scale for all items was: 1) Strongly Agree; 2) Agree; 3) Don't Know/ Depends; 4) Disagree; 5) Strongly Disagree.43 The specific question stems varied by item and are described in Data Supplement S1.
We a priori defined guideline elements as those items which were rated as `strongly agree' or `agree' by at least 80% of the panelists after the first or second survey round.44 All panelists were aware of the 80% consensus threshold for all parts of the study. All other candidate elements were excluded from the final reporting guidelines.
DELPHI PROCESS
Text describing the conceptual model and ED-specific constraints was e-mailed to all participants prior to survey administration and preceded question items on the on-line surveys. All panelists were asked to review the conceptual model and ED-specific constraints prior to completing the survey.
Round One
In the first round of the Delphi process, we administered a structured, internet-based questionnaire using a commercially available survey application (SurveyMonkey, Palo Alto, CA) managed by a research assistant (JDC). Panelists received individualized e-mails with a web-link to complete the survey. The survey questions were preceded by an overview of the study goal and a description of the conceptual model. The survey included questions about each panelist's specialty, country of practice, and prior participation in writing syncope guidelines sponsored by a professional society.16,22–26
Panelists then rated each of the candidate guideline elements on the five-point Likert scale.44 At the end of each block of items, a free text response box allowed panelists to make suggestions about the wording of items or to recommend additional items. Free text suggestions were then discussed during the group feedback and structured panelist interaction. There were no interactions among panelists prior to completion of the first survey round.
Group Feedback and Structured Panelist Interaction
We analyzed the results of the first round and provided individualized feedback to each of the panelists. For all candidate elements, we provided a summary of the group responses including the median and interquartile range (IQR). For each individualized report, we also provided that panelist's ratings for all items in the first round. The reports did not provide identifiable responses for other participants. An electronic file containing individual and group response data was e-mailed to all participants. An example of an individualized summary file is provided in Data Supplement S2.
To encourage panelist interaction and to potentially resolve areas of poor consensus, we created structured opportunities for discussion. A one-hour, moderated conference call was scheduled to discuss the results of the first round survey. As we could not schedule a single conference call that could be attended by all participants, we created a moderated e-mail forum to allow panelists to discuss the first round survey results. The process moderators (BCS, VT, and JDC) summarized all phone conference and e-mail forum comments, and these summaries were forwarded to all participants prior to the second round survey (Data Supplement S3).
Round Two
In the second round, participants received an adapted version of the first round questionnaire. In general, items that were rated by >80% of the panelists as `strongly agree' or `agree' were not included in the second round questionnaire. However, such items could be re-rated if substantive problems were identified during the structured panel interactions. All items that did not achieve >80% consensus on the first round were re-rated. All elements that were rated as `strongly agree' or `agree' by >80% of the panelists after the second round were included in the final guidelines.
SURVEY RESULTS
We initially developed a set of 183 candidate guideline elements that were rated in the first survey round. (Data Supplement 1) These included 12 items for study eligibility, 65 items for outcomes, 18 items for ECG abnormalities and reporting, and 88 items for candidate predictors. All 24 panelists completed the first round survey. There were 73 items that achieved >80% consensus after the first round (Data Supplement 2).
During the structured interaction phase, the panelists requested re-wording of three items. In addition, two items which achieved >80% consensus were re-ranked in the second survey round due to conceptual concerns that were raised during the panel interactions (Data Supplement 3). Both of these items still achieved >80% consensus after the second survey round. No other items that received >80% consensus in the first round were discussed by panelists during the structured interaction phase.
The second survey round was completed by 23 (96%) of the panelists. (Data Supplement 4) After two survey rounds, there were 92 items that achieved >80% consensus and were included in the final reporting guidelines (Tables 2 through 5). These included 10 items for study eligibility, 23 items for outcomes, nine items for ECG abnormalities and reporting, and 50 items for candidate predictors.
Table 1.
Demographics of the Expert Panel (n=24)
| Characteristic | n (%) |
|---|---|
| Primary Specialty | |
| Cardiology | 9 (37) |
| Emergency medicine | 6 (25) |
| Internal medicine | 5 (21) |
| Neurology | 2 (9) |
| Geriatrics | 2 (8) |
| Country of practice | |
| Italy | 7 (29) |
| United States | 7 (29) |
| Canada | 3 (13) |
| Netherlands | 3 (13) |
| France | 1 (4) |
| Japan | 1 (4) |
| Switzerland | 1 (4) |
| United Kingdom | 1 (4) |
| Previous participation in developing professional society syncope guidelines* | |
| None | 11 (46) |
| European Society of Cardiology | 9 (38) |
| American College of Emergency Physicians | 2 (8) |
| American Heart Association | 1 (4) |
| Other^ | 4 (16) |
some panelists participated in multiple guidelines
Canadian Cardiovascular Society; Japanese Society of Cardiology; United Kingdom National Institute for Health and Clinical Excellence
DISCUSSION
Using an iterative expert panel process, we developed reporting guidelines for ED-based syncope risk stratification studies. This effort identified a core set of reporting elements for eligibility criteria, outcomes, ECG findings, and candidate predictors. We did not attempt to propose methodological standards for performing risk stratification studies, as these have been previously described by others.45,46 A recent systematic review and meta-analysis of the existing ED syncope risk stratification literature identified wide variation in research methodology and reporting.17 Meaningful comparison of studies is seriously limited by these inconsistencies. Our reporting guidelines directly address this problem and provide a common reporting template for future syncope risk stratification research.
Professional society groups offer varying definitions of syncope22–26 (Table 6), and even more variants exist in the research literature.17,47 Our panel constructed an operational definition of syncope (Table 2, items 1–5) that closely matches the criteria suggested by the American College of Emergency Physicians.24 We also identified exclusion criteria for loss of consciousness caused by substance abuse, seizure, stroke, head trauma, and hypoglycemia.
Table 5b.
Guideline Elements- Candidate Predictors- (>80% Panel Consensus)
| Data on the following elements should be collected and reported: |
| Medications |
| 81 Diuretics |
| 82 Beta-blockers |
| 83 Nitrates |
| 84 Other antiarrhythmics not listed above (e.g. amiodarone, sotalol) |
| Physical Exam Findings |
| 85 Triage systolic blood pressure |
| 86 Lowest systolic blood pressure measured in ED |
| 87 Triage pulse |
| 88 Lowest pulse measured in ED |
| 89 Orthostatic vital signs (blood pressure and pulse measured lying and standing) |
| 90 Heart murmur |
| 91 New neurologic deficits |
| Laboratory Tests |
| 92 Hematocrit or Hemoglobin |
Although most professional societies23–26 and all risk stratification studies17 have used a symptoms-based definition of syncope, the European Society of Cardiology (ESC) advocates a definition based on a pathophysiological mechanism of global cerebral hypoperfusion.22 The intent of the ESC definition is to minimize conceptual and diagnostic confusion by excluding conditions caused by other mechanisms, such as seizures and concussion. Our expert panel was divided between those who advocated for the inclusion of `global hypoperfusion' in the eligibility criteria and those who believed that a mechanism-based definition would be impractical in ED settings. Although the `global hypoperfusion' item did not achieve >80% consensus, the panel felt that the exclusion criteria were consistent with the intent of the ESC guidelines by excluding conditions that were clearly not due to global cerebral hypoperfusion.
Existing studies have reported a wide range of outcomes time frames and outcomes.17 Studies variably include events that were identified while the patient was still in the ED, and outcome periods have varied from seven days to one year after the initial ED evaluation.7–15 Outcomes have included various combinations of death, arrhythmias, myocardial infarction, pulmonary embolism, hemorrhage, stroke, subarachnoid hemorrhage, acute procedures, abnormal electrophysiology study findings, and ED return visits and hospitalizations. Clinically significant arrhythmias have also been variably defined; for example, some investigators consider non-sustained ventricular tachycardia and symptomatic atrial tachyarrhythmias as dangerous outcomes, whereas others have not.
Our guidelines recommend reporting of outcomes identified during the ED evaluation and up to 30 days after the ED evaluation. Although a minority of panelists felt that prediction of `obvious' conditions identified during the ED evaluation was of questionable clinical value, the majority believed that it was difficult to retrospectively ascertain whether a diagnosis of a serious outcome was made during or after the ED evaluation.
We also identified a core set of serious conditions to be reported in future studies, with an emphasis on cardiac arrhythmias and structural/ischemic heart disease (Table 3). Consensus was not achieved for all-cause mortality, as several panelists felt that it was often difficult to attribute death to a prior episode of syncope. Conversely, the panel thought that it was important to report mortality that could reasonably be related to syncope such as cardiac death.
Table 2.
Guideline Elements: Study Eligibility (>80% Panel Consensus)
| The following components should be included in the definition of syncope for ED-based studies: |
| 1 Transient loss of consciousness (LOC) |
| 2 Inability to maintain postural tone |
| 3 Immediate recovery |
| 4 Spontaneous recovery without medical intervention |
| 5 Complete recovery (to pre-existing mental status and neurological function) |
| The following patients should be excluded from syncope risk stratification studies: |
| 6 Alcohol or illicit drugs as presumptive cause of LOC |
| 7 Seizure as presumptive cause of LOC |
| 8 Stroke/ transient ischemic attack as presumptive cause of LOC |
| 9 Head trauma followed by LOC |
| 10 Hypoglycemia as presumptive cause of LOC |
Although abnormal ECG findings have universally been found to be predictive of poor outcomes, research investigators have used a wide range of definitions for ECG `abnormalities.' Examples of individual criteria that have been variably used include non-sinus rhythms, frequent premature ventricular contractions, bundle branch blocks, ventricular hypertrophy, left or right axis deviation, abnormal conduction intervals, ischemic changes, and any new changes from a prior ECG. Our panel identified a core set of eight ECG findings that should be considered abnormal for the purposes of developing risk stratification instruments (Table 4). Our panelists also recommended that studies clearly report the source of ECG interpretation (e.g. treating physician, cardiology overread, or research personnel).
Table 3.
Guideline Elements: Outcomes (>80% Panel Consensus)
| An ED-based risk stratification tool should: |
| 11 Identify serious outcomes that are recognized during the ED evaluation |
| 12 Identify serious outcomes occurring within 7 days after the ED visit |
| 13 Identify serious outcomes occurring 7–30 days after the ED visit |
| Clinically important serious outcomes that should be predicted by a risk stratification tool include: |
| Mortality: |
| 14 Cardiac death |
| 15 Syncope-related death |
| Arrhythmias |
| 16 Ventricular fibrillation |
| 17 Ventricular tachycardia > 30 seconds |
| 18 Symptomatic ventricular tachycardia < 30 seconds |
| 19 Sick sinus syndrome with alternating sinus bradycardia and tachycardia |
| 20 Sinus pause > 3 seconds |
| 21 Mobitz type II atrioventricular heart block |
| 22 Complete heart block |
| 23 Pacemaker or implantable cardioverter-defibrillator malfunction with cardiac pauses. |
| Structural/ Ischemic Heart Disease |
| 24 Aortic stenosis with valve area ≤ 1 cm2 |
| 25 Hypertrophic cardiomyopathy with outflow tract obstruction |
| 26 Left atrial myxoma or thrombus with outflow tract obstruction |
| 27 Myocardial infarction |
| Other Outcomes |
| 28 Pulmonary embolus |
| 29 Aortic dissection |
| 30 Internal hemorrhage or anemia requiring transfusion |
| 31 Recurrent syncope or fall resulting in major traumatic injury (trauma that requires admission or procedural/surgical intervention) |
| 32 Permanent pacemaker or defibrillator placement |
| 33 Cardiopulmonary resuscitation |
Finally, published studies have considered a wide range of candidate predictors including demographic characteristics, symptoms, exam findings, co-morbidities, medications, and laboratory tests.7–15 Risk stratification instruments may not be easily compared if they are derived from non-overlapping sets of candidate predictors.45 From a starting set of 88 potential candidate predictors, our panel identified 50 as core reporting elements (Table 5).
Our consensus panel effort represents the first step in creating a common template for syncope research reporting. Although all of these elements can feasibly be collected in the context of prospective research protocols,7–15 these guidelines represent a significant measurement burden. Our results create the foundation for future work to streamline reporting guidelines. Elements that are difficult to collect or that have poor inter-rater reliability could be removed in future iterations. Many of the current elements could potentially be grouped into higher level categories; for example, guideline elements such as nausea, lightheadedness, and the presence of a triggering event may be suggestive of vasovagal syncope. Finally, elements that have poor prognostic association with outcomes could potentially be dropped.
LIMITATIONS
The modified Delphi technique combines both anonymous voting and a structured format to elicit expert feedback. This approach allows panelists to synthesize their collective expertise while limiting the potential bias introduced by group interpersonal dynamics (e.g. domination of the process by a few members).20 However, there are possible limitations inherent to any expert panel process.
First, our panelists may not be representative of all experts in this field. However, our group includes clinical researchers in syncope research who span multiple clinical specialties and countries, and many have previously participated in professional society syncope consensus guidelines.
Second, our approach applies equal weighting of all panelists' opinions, regardless of level of experience. We believe that this potential limitation was mitigated by the structured conference call and e-mail forum between the two survey rounds, which allowed the participants to articulate and justify their ratings. In addition, there is no evidence that differential weighting by status results in more reliable findings.20
Third, there are no universally accepted standards for scaling responses and defining `consensus' in a Delphi consensus process.20 Although the widely cited RAND/UCLA Appropriateness Method (RAM) uses a nine-point Likert scale,19 the RAM was developed to evaluate the appropriateness of clinical interventions and may be less applicable for creating research reporting guidelines. Other groups have used similar scaling and consensus definitions as those used in our study,43,44 and we felt that this approach had high face validity.
Fourth, data on some elements may not be routinely collected for clinical care. However, all of the guideline elements have been collectively reported in published research studies,7–15 and we believe that data measurement is feasible in the context of a prospective research study. Investigators should consider item missingness and the potential for bias when applying these reporting guidelines to chart or administrative data.
Fifth, we did not stratify candidate items into `mandatory,' `optional,' and `not-important' categories. Although others have used an iterative consensus conference approach to create tiered recommendations,18 we found that the use of multiple thresholds was confusing in the context of a Delphi panel process. Our use of a binary threshold for element inclusion or exclusion is consistent with most Delphi consensus efforts.19,43,44,48
Finally, it is possible that items that did not achieve consensus or were not rated by the panel may be important for syncope risk stratification. Our panel identified a `core' set of reporting elements felt to be important for all studies. Our results are not meant to preclude the study of populations, outcomes, or data elements that are not explicitly described in the final guidelines.
CONCLUSIONS
We developed reporting guidelines for ED-based risk stratification studies. Our expert panel effort addresses the wide variation in study reporting in the existing literature.16,17 Adherence to our reporting guidelines should facilitate future literature review, data pooling, and meta-analysis of ED syncope risk stratification studies.
Supplementary Material
Table 4.
Guideline Elements: Electrocardiogram (>80% Panel Consensus)
| ECG Findings |
| The following ECG findings should be considered abnormal: |
| 34 Non-sinus rhythms (includes paced rhythm) |
| 35 Sinus bradycardia ≤ 40 per minute |
| 36 Complete left bundle branch block |
| 37 Delta waves (e.g. Wolff-Parkinson-White) |
| 38 Prolonged QRS (>120 ms) |
| 39 Prolonged QTc (> 450 ms) |
| 40 Brugada pattern |
| 41 Q/ST/T changes consistent with acute or chronic ischemia |
| ECG Interpretation: |
| 42 Report who is interpreting the ECG (e.g. emergency physician, cardiologist, research team, etc.) |
Table 5a.
Guideline Elements: Candidate Predictors (>80% Panel Consensus)
| Data on the following elements should be collected and reported: |
| Demographic characteristics |
| 43 Age |
| 44 Sex |
| Historical features |
| 45 Exertion |
| 46 While driving |
| 47 Time of syncope event |
| 48 Supine position |
| 49 Sitting position |
| 50 Lack of warning symptoms |
| 51 Chest discomfort |
| 52 Shortness of breath |
| 53 Palpitations |
| 54 Traumatic injury (laceration, fracture, intracranial bleed, thoraco-abdominal injury) |
| 55 Lightheadedness |
| 56 Standing from supine/ sitting position |
| 57 Post-prandial (within 1 hour of meal) |
| 58 Nausea/ vomiting |
| 59 Feeling of warmth |
| 60 Diaphoresis |
| 61 Blurred vision |
| 62 Any prodromes lasting greater than 5 seconds |
| 63 Triggered by painful/ emotionally distressing stimulus |
| 64 Triggered by turning head/ cough/ micturation/ defecation |
| Co-morbidities |
| 65 Premature (<50 years) sudden death in sibling or parents |
| 66 Congestive heart failure |
| 67 Coronary artery disease (past MI/ PTCA/ CABG) |
| 68 Congenital heart disease |
| 69 Structural heart disease- aortic stenosis |
| 70 Structural heart disease- outflow tract disease, excluding aortic stenosis (e.g. idiopathic hypertrophic subaortic stenosis) |
| 71 Structural heart disease- ejection fraction <40% by objective testing (e.g. echocardiogram, cardiac catheterization) within one year |
| 72 Structural heart disease- pulmonary hypertension |
| 73 Structural heart disease- valve disease, excluding aortic stenosis and mitral prolapse |
| 74 Arrhythmia- ventricular tachycardia/ ventricular fibrillation/ sudden death |
| 75 Arrhythmia- SVTs, including PSVT, atrial fibrillation, atrial flutter |
| 76 Arrhythmia- sick sinus syndrome, Mobitz II or complete heart block, junctional rhythm |
| 77 Implanted permanent pacemaker |
| 78 Implanted defibrillator |
| 79 Hypertension requiring medication |
| 80 Syncope in the prior year |
MI = myocardial infarction; PTCA = percutaneous transluminal coronary angioplasty; CABG = coronary artery bypass grafting; SVT = supraventricular tachycardia; PSVT = paroxysmal supraventricular tachycardia
Table 6.
Professional Society Definitions of Syncope
| Organization | Definition |
|---|---|
| American College of Emergency Physicians23 | Brief loss of consciousness with an inability to maintain postural tone that spontaneously and completely resolves without medical intervention. |
| American College of Physicians25 | Transient loss of consciousness accompanied by loss of postural tone. |
| American Heart Association26 | Transient loss of consciousness. |
| European Society of Cardiology22 | Transient loss of consciousness due to transient global cerebral hypoperfusion characterized by rapid onset, short duration, and spontaneous recovery. |
Acknowledgments
Funding Sources: Dr. Sun was supported by NIH/ NIA grants (K12AG001004, RC1AG035664, and P30-AG028748) at the time of this work. The content does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. Dr. Thiruganasambandamoorthy is supported by grants from Physicians' Services Incorporated Foundation (09q4017), the Canadian Institute of Health Research (245124), and the Heart and Stroke Foundation Canada.
Appendix: Consortium to Standardize ED Syncope Risk Stratification Reporting Expert Panel
| Name | Degree (s) | Institution | Institution City, State, Country |
|---|---|---|---|
| Haruhiko Abe | MD, PhD | University of Occupational and Environmental Health | Kitakyushu, Japan |
| Franca Barbic | MD | Neuroscience Research Association | Milan, Italy |
| Jean-Jacques Blanc | MD | Brest University | Brest, France |
| Furio Colivicchi | MD | San Filippo Neri Hospital | Rome, Italy |
| Franca Dipaola | MD | Istituti Clinici di Perfezionamento | Sesto S.G., Milan, Italy |
| Raffaello Furlan | MD | Bolognini Hospital | Seriate (BG), Italy |
| Georgi Costantino | MD | Medicina II, Ospedale L. Sacco | Milan, Italy |
| Shamai Grossman | MD, MS | Harvard University | Boston, MA, USA |
| Erik Hess | MD, MSc | Mayo Clinic College of Mediine | Rochester, MN, USA |
| Andrew Krahn | MD | University of Western Ontario | London, Ontario, Canada |
| Lew Lipsitz | MD | Beth Israel Deaconess Medical Center | Boston, MA, USA |
| Carlos Morillo | MD | McMaster University | Hamilton, ON, Canada |
| Brian Olshansky | MD | University of Iowa | Iowa City, IA, USA |
| James Quinn | MD, MS | Stanford University | Stanford, CA, USA |
| Antonio Raviele | MD | dell'Angelo Hospital | Venice-Mestre, Italy |
| Matthew Reed | MD, MA, MB | Edinburgh University | Edinburgh, UK |
| Francois Sarasin | MD, MSc | Hôpitaux Universitaires de Gèneve | Geneva, Switzerland |
| Satish Raj | MD, MSCI | Vanderbilt University School of Medicine | Nashville, TN, USA |
| Luis Serrano | MD, MS | Mayo Clinic College of Medicine | Rochester, MN, USA |
| Robert Sheldon | MD, PhD | University of Calgary | Calgary, Alberta, Canada |
| Roland Thijs | MD, PhD | Dutch Epilepsy Clinics Foundation | Hoofddorp, The Netherlands |
| Andrea Ungar | MD, PhD | AOU Careggi and Univeristy of Florence | Florence, Italy |
| Gert van Dijk | MD, PhD | Leiden University Medical Centre | Leiden, The Netherlands |
| Nynke van Dijk | MD, PhD | Academic Medical Center, University of Amsterdam | Amsterdam, The Netherlands |
Footnotes
Prior Presentations: none
Disclosures: The authors have no additional financial disclosures or conflicts of interest to report.
REFERENCES
- 1.Thiruganasambandamoorthy V, Hess EP, Alreesi A, Perry JJ, Wells GA, Stiell IG. External validation of the San Francisco Syncope Rule in the Canadian setting. Ann Emerg Med. 2010;55:464–72. doi: 10.1016/j.annemergmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Sun BC, Emond JA, Camargo CA., Jr Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992–2000. Acad Emerg Med. 2004;11:1029–34. doi: 10.1197/j.aem.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum A, Esses D, Bijur P, Wollowitz A, Gallagher EJ. Failure to validate the San Francisco Syncope Rule in an independent emergency department population. Ann Emerg Med. 2008;52(2):151–9. doi: 10.1016/j.annemergmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Sun BC, Emond JA, Camargo CA., Jr Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005;95:668–71. doi: 10.1016/j.amjcard.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Crane SD. Risk stratification of patients with syncope in an accident and emergency department. Emerg Med J. 2002;19:23–7. doi: 10.1136/emj.19.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benditt DG, Can I. Initial evaluation of “syncope and collapse” the need for a risk stratification consensus. J Am Coll Cardiol. 2010;55:722–4. doi: 10.1016/j.jacc.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 7.Martin TP, Hanusa BH, Kapoor WN. Risk stratification of patients with syncope. Ann Emerg Med. 1997;29:459–66. doi: 10.1016/s0196-0644(97)70217-8. [DOI] [PubMed] [Google Scholar]
- 8.Sarasin FP, Hanusa BH, Perneger T, Louis-Simonet M, Rajeswaran A, Kapoor WN. A risk score to predict arrhythmias in patients with unexplained syncope. Acad Emerg Med. 2003;10:1312–7. doi: 10.1111/j.1553-2712.2003.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 9.Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J. 2003;24:811–9. doi: 10.1016/s0195-668x(02)00827-8. [DOI] [PubMed] [Google Scholar]
- 10.Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43:224–32. doi: 10.1016/s0196-0644(03)00823-0. [DOI] [PubMed] [Google Scholar]
- 11.Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to general hospital: the EGSYS score. Heart. 2008;94(12):1620–6. doi: 10.1136/hrt.2008.143123. [DOI] [PubMed] [Google Scholar]
- 12.Costantino G, Perego F, Dipaola F, et al. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol. 2008;51:276–83. doi: 10.1016/j.jacc.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 13.Reed MJ, Newby DE, Coull AJ, Prescott RJ, Jacques KG, Gray AJ. The ROSE (risk stratification of syncope in the emergency department) study. J Am Coll Cardiol. 2010;55:713–21. doi: 10.1016/j.jacc.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 14.Sun BC, Derose SF, Liang LJ, et al. Predictors of 30-day serious events in older patients with syncope. Ann Emerg Med. 2009;54:769–78. doi: 10.1016/j.annemergmed.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman SA, Fischer C, Lipsitz LA, et al. Predicting adverse outcomes in syncope. J Emerg Med. 2007;33:233–9. doi: 10.1016/j.jemermed.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheldon RS, Morillo CA, Krahn AD, et al. Standardized approaches to the investigation of syncope: Canadian Cardiovascular Society position paper. Can J Cardiol. 2011;27:246–53. doi: 10.1016/j.cjca.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Serrano LA, Hess EP, Bellolio MF, et al. Accuracy and quality of clinical decision rules for syncope in the emergency department: a systematic review and meta-analysis. Ann Emerg Med. 2010;56:362–73. doi: 10.1016/j.annemergmed.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollander JE, Blomkalns AL, Brogan GX, et al. Standardized reporting guidelines for studies evaluating risk stratification of emergency department patients with potential acute coronary syndromes. Ann Emerg Med. 2004;44:589–98. doi: 10.1016/S0196064404012806. [DOI] [PubMed] [Google Scholar]
- 19.Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care. 1986;2:53–63. doi: 10.1017/s0266462300002774. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2:i–iv. 1–88. [PubMed] [Google Scholar]
- 21.Sanmartin C, Murphy K, Choptain N, et al. Appropriateness of healthcare interventions: concepts and scoping of the published literature. Int J Technol Assess Health Care. 2008;24:342–9. doi: 10.1017/S0266462308080458. [DOI] [PubMed] [Google Scholar]
- 22.Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC) Eur Heart J. 2009;30(21):2631–71. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huff JS, Decker WW, Quinn JV, et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with syncope. Ann Emerg Med. 2007;49:431–44. doi: 10.1016/j.annemergmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;127:76–86. doi: 10.7326/0003-4819-127-1-199707010-00014. [DOI] [PubMed] [Google Scholar]
- 25.Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 1: value of history, physical examination, and electrocardiography. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;126:989–96. doi: 10.7326/0003-4819-126-12-199706150-00012. [DOI] [PubMed] [Google Scholar]
- 26.Strickberger SA, Benson DW, Biaggioni I, et al. AHA/ACCF Scientific Statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation: in collaboration with the Heart Rhythm Society: endorsed by the American Autonomic Society. Circulation. 2006;113:316–27. doi: 10.1161/CIRCULATIONAHA.105.170274. [DOI] [PubMed] [Google Scholar]
- 27.Benditt DG. The ACCF/AHA Scientific statement on syncope: a document in need of thoughtful revision. Europace. 2006;8:1017–21. doi: 10.1093/europace/eul134. [DOI] [PubMed] [Google Scholar]
- 28.Benditt DG, Brignole M. Syncope: is a diagnosis a diagnosis? J Am Coll Cardiol. 2003;41:791–4. doi: 10.1016/s0735-1097(02)02928-5. [DOI] [PubMed] [Google Scholar]
- 29.Kapoor WN, Brant N. Evaluation of syncope by upright tilt testing with isoproterenol. A nonspecific test. Ann Intern Med. 1992;116:358–63. doi: 10.7326/0003-4819-116-5-358. [DOI] [PubMed] [Google Scholar]
- 30.Brignole M, Sutton R, Menozzi C, et al. Lack of correlation between the responses to tilt testing and adenosine triphosphate test and the mechanism of spontaneous neurally mediated syncope. Eur Heart J. 2006;27:2232–9. doi: 10.1093/eurheartj/ehl164. [DOI] [PubMed] [Google Scholar]
- 31.Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med. 2006;47:448–54. doi: 10.1016/j.annemergmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. 2001;37:1921–8. doi: 10.1016/s0735-1097(01)01241-4. [DOI] [PubMed] [Google Scholar]
- 33.Calkins H, Shyr Y, Frumin H, Schork A, Morady F. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med. 1995;98:365–73. doi: 10.1016/S0002-9343(99)80315-5. [DOI] [PubMed] [Google Scholar]
- 34.Da Costa A, Gulian JL, Romeyer-Bouchard C, et al. Clinical predictors of cardiac events in patients with isolated syncope and negative electrophysiologic study. Int J Cardiol. 2006;109:28–33. doi: 10.1016/j.ijcard.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Denes P, Uretz E, Ezri MD, Borbola J. Clinical predictors of electrophysiologic findings in patients with syncope of unknown origin. Arch Intern Med. 1988;148:1922–8. [PubMed] [Google Scholar]
- 36.Gabayan GZ, Derose SF, Asch SM, et al. Predictors of short-term (seven-day) cardiac outcomes after emergency department visit for syncope. Am J Cardiol. 2010;105:82–6. doi: 10.1016/j.amjcard.2009.08.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hing R, Harris R. Relative utility of serum troponin and the OESIL score in syncope. Emerg Med Australas. 2005;17:31–8. doi: 10.1111/j.1742-6731.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 38.Oh JH, Hanusa BH, Kapoor WN. Do symptoms predict cardiac arrhythmias and mortality in patients with syncope? Arch Intern Med. 1999;159:375–80. doi: 10.1001/archinte.159.4.375. [DOI] [PubMed] [Google Scholar]
- 39.Pfister R, Diedrichs H, Larbig R, Erdmann E, Schneider CA. NT-pro-BNP for differential diagnosis in patients with syncope. Int J Cardiol. 2009;133(1):51–4. doi: 10.1016/j.ijcard.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 40.Reed MJ, Newby DE, Coull AJ, Prescott RJ, Gray AJ. Diagnostic and prognostic utility of troponin estimation in patients presenting with syncope: a prospective cohort study. Emerg Med J. 2010;27:272–6. doi: 10.1136/emj.2008.068635. [DOI] [PubMed] [Google Scholar]
- 41.Sheldon R, Rose S, Connolly S, Ritchie D, Koshman ML, Frenneaux M. Diagnostic criteria for vasovagal syncope based on a quantitative history. Eur Heart J. 2006;27:344–50. doi: 10.1093/eurheartj/ehi584. [DOI] [PubMed] [Google Scholar]
- 42.Galizia G, Abete P, Mussi C, et al. Role of early symptoms in assessment of syncope in elderly people: results from the Italian group for the study of syncope in the elderly. J Am Geriatr Soc. 2009;57:18–23. doi: 10.1111/j.1532-5415.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 43.Vandelanotte C, Dwyer T, Van Itallie A, Hanley C, Mummery WK. The development of an internet-based outpatient cardiac rehabilitation intervention: a Delphi study. BMC Cardiovasc Disord. 2010;10:27. doi: 10.1186/1471-2261-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colucci E, Kelly CM, Minas H, Jorm AF, Chatterjee S. Mental health first aid guidelines for helping a suicidal person: a Delphi consensus study in India. Int J Ment Health Syst. 2011;5:e12. doi: 10.1186/1752-4458-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 1997;277:488–94. [PubMed] [Google Scholar]
- 46.Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313:793–9. doi: 10.1056/NEJM198509263131306. [DOI] [PubMed] [Google Scholar]
- 47.Thijs RD, Benditt DG, Mathias CJ, et al. Unconscious confusion--a literature search for definitions of syncope and related disorders. Clin Auton Res. 2005;15:35–9. doi: 10.1007/s10286-005-0226-2. [DOI] [PubMed] [Google Scholar]
- 48.Beattie E, Mackway-Jones K. A Delphi study to identify performance indicators for emergency medicine. Emerg Med J. 2004;21:47–50. doi: 10.1136/emj.2003.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.