Summary
Background
As the demand for minimally invasive rejuvenation is increasing, micro-peel resurfacing using Erbium:Yttrium Aluminium Garnet (Er:YAG ) laser 2940 nm has been reported for the treatment of photoaged skin without ablation of the epidermis. However, little is known about the efficacy and underlying histologic changes associated with this type of treatment.
Aims
The purpose of this study is to evaluate the clinical effect and objectively quantify the histological changes in response to multiple sessions of Er:YAG laser 2940 nm mini-peels.
Patients and methods
Six female volunteers of Fitzpatrick skin type III-IV and Glogau’s class I-III wrinkles were subjected to six microresurfacing peels at 2-week intervals using Er:YAG 2940 nm laser at sub-ablative fluences of 2 - 3 J/cm2 to treat periorbital rhytides. Quantitative evaluation of collagen types I, III and VII, newly synthesized collagen, total elastin and tropoelastin was performed by histochemistry and immunohistochemistry coupled with computerized morphometric analysis at base line, end of treatment, and three months post treatment.
Results
Compared to the base line, evaluation of volunteers revealed obvious clinical improvement in response to Er:YAG mini-peels. Collagen types I, III, and VII, as well as newly synthesized collagen, together with tropoelastin showed a statistically significant increase in response to treatment, while the mean level of total elastin was significantly decreased in response to treatment. However, this was followed by regression of improvement at 3 months post treatment, but was still better than baseline.
Conclusions
The present study revealed that multiple Er:YAG mini-peels is a promising treatment option for photoaging as it reverses the signs of photoaged skin with little downtime and side effects. However, to maintain the short term improvement achieved after treatment, continued Er:YAG 2940 nm laser mini-peels is required.
Keywords: Erbium YAG laser, collagen, elastin, noninvasive, skin aging, rejuvenation
Introduction
Skin aging is a complex multifactorial biological phenomenon; wherein ongoing intrinsic changes combine synergistically with cumulative effects of chronic exposure to the elements, primarily UV radiation, causing the skin to lose its thickness and elasticity and develop wrinkles (1-3). Previous clinical studies have shown that changes in the mechanical properties of the skin are accompanied by histological changes in the different skin layers, with increased tendency for wrinkle formation (4-8).
Although Erbium:Yttrium Aluminum Garnet (Er:YAG ) laser 2940 nm is used primarily for ablative rejuvenation, it has also been reported as a minimally invasive method for skin resurfacing (9). Micro-resurfacing, also known as mini-peels, is a technique that employs the use of Er:YAG laser at pulse fluences below the ablation threshold, “sub-ablative or thermal mode”, to increase the temperature in the upper dermal layers without epidermal ablation (10). The use of multiple passes of Er:YAG laser would build up residual heat in tissues resulting in a zone of thermal injury to the dermal layer with subsequent denaturation of collagen fibrils (11, 12). Over time, as a thermally mediated healing response, fibroblasts are stimulated leading to deposition of new collagen and an overall increase in collagen content (13).
The aim of the present study was to evaluate the clinical effect of, and objectively quantify the histological facial skin response to multiple Er:YAG 2940 nm laser mini-peel treatments as a minimally invasive approach to photoaging, and to assess whether multiple passes at fluences below the ablation threshold would improve clinical outcome. This was accomplished with histochemical and immunostaining techniques, as well as histometric evaluation of skin at the base line, end of treatment, and 3 months post treatment.
Patients and methods
Study population and treatment protocol
This study was conducted on a cohort of six female volunteers from the attendants of the dermatology outpatient clinic of Al-Minya University Hospital, Al-Minya, Egypt, ranging in age from 38 to 72 years, with an average of 52.8 ± 14.0, who desired an improvement in the appearance of skin laxity and treatment of periorbital wrinkles. The details of treatment and study protocol were fully explained to the subjects. All volunteers gave an informed consent for study participation and having photographs as well as skin biopsies before treatment, after 3 months (end of treatment) and 6 months after the start of treatment (post treatment). The volunteers of Fitzpatrick skin type III-IV and Glogau class I-III wrinkles (14) were subjected during a three month period at 2-week intervals for 6 sessions of mini-peel (microresurfacing) with Er:YAG 2940 nm laser (SkinPlus Erbium:YAG device, Fotona Medical Lasers, Ljubljana, Slovenia). The laser was used in a thermal mode and set to deliver a sequence of short pulses, each of a sub-ablation threshold fluence with a total energy of 2-3 J/cm2 and pulse duration of 200–250 milliseconds.
A topical anesthetic cream (Lidocaine 2.5% + Prilocaine 2.5%) was applied to the treatment area as a thick coating and left for 90 minutes under occlusion. Prior to starting the treatment, the cream was gently removed and the volunteer’s eyes were covered with protective gauze and special eye goggles. For each volunteer, the laser parameters used were similar across treatment sessions. At each session, the entire treatment area received 2-3 consecutive passes with 10% overlap producing a clinical endpoint of skin whitening; no wiping was performed between the passes. The applied parameters and technique allowed us to increase the skin temperature but preserve the epidermis from being ablated; care was taken each session to keep the skin cool during the laser irradiation with cryogen spray cooling. After each session, volunteers were instructed to use topical antibiotic (Fusidic acid) for 3-4 days and sun screens during day light. Potential side effects, including erythema, edema, hypo- or hyperpigmentation were monitored during each session and throughout the treatment period.
Punch biopsies (3 mm) were obtained from the facial skin (treatment site) at base line, end of treatment and three months post treatment. All histological and immunostaining evaluation were carried out in the Department of Dermatology and Cutaneous Biology, Thomas Jefferson University, Philadelphia, PA, USA.
Histometric measurements
Using a computer-based software (Image-Pro Plus 6.1; Media Cybernetics, Silver Spring, MD), epidermal thickness was determined using hematoxylin and eosin (H&E) stained sections. Five measurements for each section were calculated between the top of the granular cell layer to the dermo-epidermal junction.
Histological staining
Specimens were stained for standard H&E, Verhoeff-van Gieson (elastic fibers) (HT25A; Sigma, St Louis, MO, USA), and picrosirius red staining (Direct Red 80, Sigma) for newly synthesized collagen (15, 16).
Immunohistochemical staining
The immunoperoxidase technique was used for evaluation of collagen types I and III, as well as total elastin as previously described (3, 13, 17). The antibodies used were against type I collagen (1:400; sc-59772; Santa Cruz Biotechnology, Santa Cruz, CA, USA), type III collagen (1:600; ab6310; Abcam, Cambridge, MA, USA) and total elastin (1:300; E4013; Sigma, St Louis, MO, USA).
Collagen type VII was detected by using indirect immunofluorescence (IF) staining technique. Tissues were incubated with antibodies to type VII collagen (1:600; sc-33710; Santa Cruz Biotechnology), secondary antibody Alexa Fluor goat anti-mouse IgG 594 (1:400; Molecular Probes, Eugene, OR, USA) and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (1:1000; D8417; Sigma) for nuclear staining.
To detect tropoelastin, the microprobe system (Fisher Thermo Scientific #FD-188-10A) was used with reagents from Open Biosystems (Huntsville, AL, USA) (13, 18). Antibodies used were tropoelastin GA317 (1:400; Elastin Products, Owensville, MO, USA) and Alexa Fluor goat anti-rabbit IgG 594 (1:400; Molecular Probes).
Quantitative evaluation and statistical analysis
A Nikon microscope equipped with filters to provide circularly polarized illumination was used to evaluate picrosirius red stained tissues. Quantitative evaluation of picrosirius red staining and immunostained tissues was carried out using Image-Pro Plus 6.1 software; a representative square area of 1×1 cm was used to measure luminosity for fluorescent stained sections, while another 2.5×2.5 cm square was used to measure the color density for immunoperoxidase staining. All values were normalized to the base line. Histological measurements and quantitative evaluation were tabulated and analyzed using the Software Package for Statistical Science (SPSS) (Version 16, SPSS Inc.; Chicago, IL, USA). Statistical analysis was performed using One-way ANOVA, Wilcoxon-matched pairs signed ranks, and Chi-square tests. Data were expressed as the mean value ± standard deviation (SD). Statistical significance was defined as p ≤ 0.05.
Results
Clinical evaluation
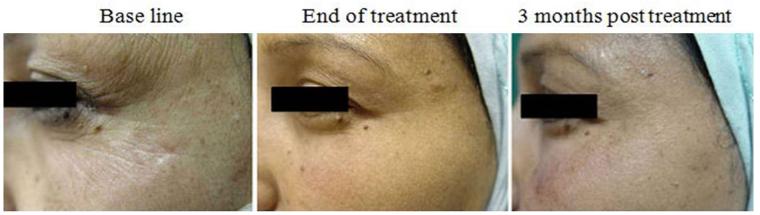
The clinical changes and improvement in periorbital wrinkles, skin tightening and texture as well as overall satisfaction were rated and evaluated by the volunteers, two dermatologists and two independent observers before treatment and at 3 and 6 months after the start of the treatment, based on a five-point scale (none = 0%, mild = 1 - 25%, moderate = 26 - 50%, good = 51 - 75% and very good = 76 - 100%). All volunteers completed the study and showed short term clinical improvement of skin tightening and rhytides in the periorbital area. A noticeable enhancement in skin appearance, wrinkles and volunteers’ satisfaction was observed at the end of the treatment when compared to the base line, however this was followed by regression of the clinical improvement at 3 months post treatment compared to the end of treatment, but was still better than base line (Figure 1). No potential side effects were observed.
Figure 1.
Clinical evaluation in response to Er:YAG laser mini-peels. Representative photographs of periorbital area at base line, end of treatment, and 3 months post treatment.
Data obtained from the structured questionnaire was tabulated and compared to base line for statistical significance with the Pearson Chi-square test. At the end of treatment, subjects showed good improvement (50-55%) in skin tightening (p = 0.01), good improvement (50-55%) in skin texture (p = 0.01), good improvement (55-60%) in wrinkles (p = 0.01), and very good overall satisfaction (85-90%) (p = 0.001). On comparing the clinical changes at 3 months post treatment to that of end of treatment, there was mild insignificant decrease in the improvement of skin texture, tightening, and wrinkles by 5%, as well as of volunteers’ satisfaction by 20% (good satisfaction), yet the volunteers were still satisfied (Table I).
Table I.
Clinical improvement relative to base line in response to Erbium:YAG 2940 nm mini-peels
| Percent improvement (%) | ||||
|---|---|---|---|---|
| Skin tightening | Skin texture | Wrinkles | Overall satisfaction |
|
| End of treatment | 50-55* good |
50-55* good |
55-60* good |
85-90* very good |
| Three months post treatment |
45-50* moderate |
45-50* moderate |
50-55* good |
65-70* good |
p≤0.05; Chi-square test
Epidermal changes
Compared to the base line, microscopic evaluation of H&E stained sections revealed a significant increase from 54.3 ± 6.3 μm to 63 ± 6.5 μm at the end of treatment and to 66.4 ± 5.1 μm 3 months post treatment (p = 0.042 and 0.005, respectively) (Table II).
Table II.
Quantitative analysis of collagen (newly synthesized and types I, III and VII), elastin and tropoelastin before and after Erbium: YAG 2940 nm mini-peels.
| (n = 6) | Percent dermis positive (Mean ± SD) |
P-value | ||||
|---|---|---|---|---|---|---|
| Base line | End of treatment |
3 Months post treatment |
Base line vs End of treatment |
End of treatment vs 3 Months post treatment |
Base line vs 3 Months post treatment |
|
| Epidermal Thickness (μm) |
54.3 ± 6.3 | 63 ± 6.5 | 66.4 ± 5.1 | 0.042* | 0.340 | 0.005* |
| Collagen I | 64 ± 4.5 | 71.1 ± 3.9 | 69.7 ± 4.4 | 0.044* | 0.685 | 0.158 |
| Collagen III | 59.7 ± 2.1 | 62.9 ± 1.1 | 60.7 ± 2.6 | 0.037* | 0.178 | 0.619 |
| Collagen VII | 8.4 ± 1.4 | 11.7 ± 1.5 | 10.8 ± 2.1 | 0.004* | 0.264 | 0.010* |
| Newly synthesized collagen |
11.3 ± 2.1 | 16.1 ± 3.8 | 13.7 ± 1.5 | 0.001* | 0.197 | 0.117 |
| Total elastin | 68.2 ± 7.8 | 56.5 ± 4.5 | 59.8 ± 6.1 | 0.025* | 0.316 | 0.107 |
| Tropoelastin | 9.7 ± 2.4 | 13.5 ± 2.1 | 13.1 ± 1.1 | 0.017* | 0.779 | 0.041* |
p ≤ 0.05
Dermal changes
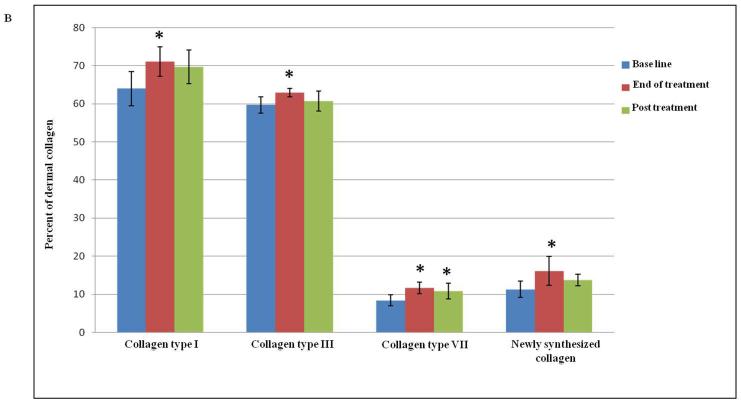
Dermal collagen is primarily comprised of type I (80- 85%) and type III (10-15%) collagen (6, 7). In photodamaged skin, collagen, which comprises more than 80% of the total dry weight of the dermis, becomes disorganized with enhanced breakdown and reduced network formation (5, 19). These alterations contribute to the skin sagging and wrinkling (20). The effect of Er:YAG 2940 nm mini-peels on collagen types I and III was quantitatively evaluated and the values were compared to the base line for statistical significance. Assessment of collagen type I expression revealed an increased level from 64.0 ± 4.5 % before treatment to 71.1 ± 3.9 % at the end of treatment (p = 0.04); type I collagen level decreased to 69.7 ± 4.4 % 3 months post treatment when compared to the end of treatment level (p=0.685). Collagen type III showed a slight, but significant increase from 59.7 ± 2.1 % at the base line to 62.9 ± 1.1 % at the end of treatment (p = 0.037), but the level of collagen type III expression did not differ significantly (p = 0.178) 3 months post treatment (60.7 ± 2.6 %) when compared to the end of treatment (Table II and Figures 2A and B).
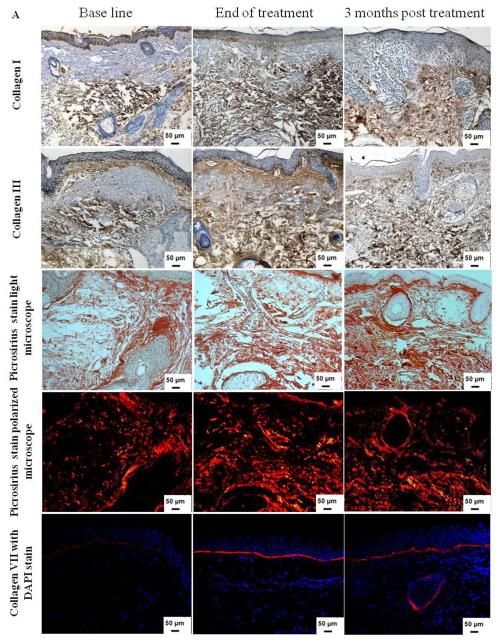
Figure 2.
(A) Increase in dermal collagen content after Er:YAG 2940 nm laser mini-peels. Immunohistochemical staining of skin tissues for collagen types I and III (1st and 2nd rows, respectively) showing an increase in collagen content. Shown in the 3rd and 4th rows are representative examples of skin tissues stained with picrosirius red viewed under bright field (3rd row) and polarized field (4th row). Bright field captures total collagen content while polarized light showed yellow to orange birefringence reflecting newly synthesized collagen in yellow to orange and total collagen in red. An increase in collagen type VII expression (red; 5th row) was observed after Er:YAG mini-peels compared to base line, and then decreased 3 months post treatment. Nuclei stained in blue with DAPI (Immunohistochemical and Picrosirius red; X 200); (B) Percent of dermis occupied by collagen levels. Data showed a statistically significant increase in both collagen types I, III and VII, as well as newly synthesized collagen at the end of Er:YAG laser mini-peels (*,p≤ 0.05).
To assess whether the increase in collagen level observed by immunohistochemistry was due to increase in newly synthesized collagen formation, picrosirius red staining was used to detect newly synthesized collagen as collagen fibers display characteristic optical properties under polarized microscopy; large fibers stain red, while the thinner ones, which represent the newly synthesized collagen fibers, show a yellow to orange staining (15, 16). Er:YAG 2940 nm mini-peels showed a noticeable effect on enhancing collagen formation, as picrosirius red staining revealed a statistically significant increase from 11.3 ± 2.1% before treatment to 16.1 ± 3.8% at the end of treatment (p = 0.001); this was followed by insignificant decrease to 13.7 ± 1.5 % 3 months post treatment (p = 0.16) (Table II and Figures 2A and B).
In the human skin, the stability of the dermal-epidermal junction is maintained in part by type VII collagen, the main component of anchoring fibrils, which is synthesized by both fibroblasts and keratinocytes (21) and the effects of aging on its biosynthesis and degradation has been previously noted (22, 23). Quantitative evaluation of type VII collagen expression showed a statistically significant increase from 8.4 ± 1.4 % before treatment to 11.7 ± 1.5 % at the end of treatment (p = 0.004). Collagen type VII was slightly decreased at 3 months post treatment to 10.8 ± 2.1 % when compared to the end of treatment (p = 0.25), but the level was still statistically significantly higher when compared to the base line (p = 0.01) (Table II and Figures 2A and B).
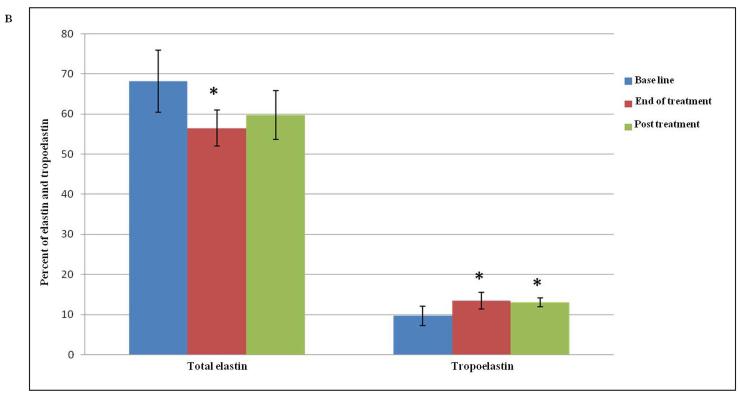
Normal elastic fibers, which are responsible for the resilient properties of the skin, constitute less than 2-4% of the extracellular matrix in sun protected areas (6, 7, 18). The hallmark feature of photoaging is dermal/solar elastosis whereby the elastic fibers appear disorganized, thickened and tangled. The effect of Er:YAG microresurfacing on total dermal elastin was examined by measuring the percent area of dermis occupied by immunohistochemically detectable elastin, and then values were normalized to the base line. The total elastin level was decreased significantly from 68.2 ± 7.8 % at base line to 56.5 ± 4.5 % at the end of treatment (p = 0.03). This was followed by an insignificant increase in the elastin level to 59.8 ± 6.1 % 3 months post treatment. Yet, the level of elastin was still lower than the pretreatment baseline level (Table II and Figures 3A and B). All tissues were stained with the Verhoeff-van Gieson special stain to differentiate the elastic fibers (blue-black to black) from collagen fibers (red), which showed restoration of normal appearing elastic fibers within the papillary and reticular dermis in response to treatment (Figure 3A).
Figure 3.
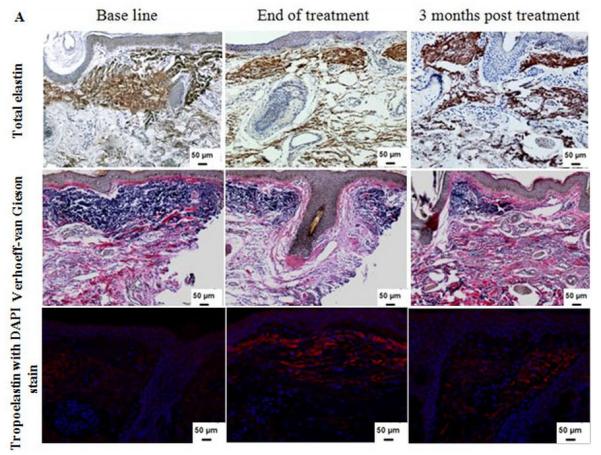
(A) Dermal elastin in response to Er:YAG laser mini-peels. Tissues were immunostained for total elastin (1st row) and Verhoeff-van Gieson stain (2nd row), showing a decrease in elastic fibers content. Immunofluorescence staining for tropoelastin (3rd row) shows increased deposition of newly synthesized tropoelastin in dermis (red), the sections were counterstained blue for nuclei with DAPI (Immunohistochemical and Verhoeff-van Gieson; X 200); (B) Percent of dermis occupied by elastin and tropoelastin showing significant changes after treatment. The values are mean ± SD (*, p ≤ 0.05).
The biosynthesis rate of normal elastin was evaluated by quantifying for newly synthesized tropoelastin, the precursor of elastic fibers (18). Compared to the base line, morphometric analysis of tropoelastin stained sections showed a statistically significant increase in the mean level from 9.7 ± 2.4 % to 13.5 ± 2.1 % (p = 0.02) at the end of Er:YAG mini-peels and to 13.1 ± 1.1 % at 3 months post treatment (p = 0.04) (Table II and Figures 3A and B).
Discussion
The interest in minimally invasive treatments of skin aging with decreased down time and complications is increasing steadily (13, 20). Evaluation of clinical results with subjective observations by physicians and/or volunteers, even combined with photo-documentation, has been thought to be an insufficient way of representing the efficacy of treatment (11, 24, 25). The basic concern with most rejuvenation studies is an issue of methodology; meanwhile there are few standard and objective approaches to quantitate the histological changes after treatment. In the present study, we aimed to strengthen the subjective evaluation by objective means of evaluating the effect of multiple Er:YAG 2940 nm laser mini-peels on reversing the signs of skin aging. Also, as some studies (10, 26) reported good clinical and histological changes in response to 2-3 sessions using single pass of Er:YAG 2940 nm laser mini-peels, we aimed to evaluate the efficacy of multiple sessions using multiple passes of Er:YAG thermal laser pulses at fluences below the ablation threshold.
At the end of multiple Er:YAG 2940 nm mini-peel treatments (6 sessions at 2-week intervals), clinical evaluation of volunteers revealed a significant (p ≤ 0.05) but short term improvement in skin tightening, texture and wrinkles, as well as in volunteers’ overall satisfaction which was statistically significantly decreased 3 months post treatment (p ≤ 0.05). These findings are consistent with the study by Christian (10) which reported that five of six females treated with 8 sessions every 4-6 weeks using Er:YAG 2940 nm laser (7 J/cm2 with single pass per session), showed good clinical improvement at the end of treatment. Additionally, Kunzi-Rapp et al (12) reported that improvement in wrinkles at 1-3 months follow-up was graded as excellent in 19%, good in 19%, fair in 31%, and no improvement in 31% of cases using sub-ablative fluences 2.1-3.1 J/cm2, yet two treatments were applied 2 months apart and the authors used a single pass per treatment.
The clinical changes seen with facial rejuvenation seems to correlate with a number of histological findings. One interesting aspect of our results is that the epidermal thickness was statistically significantly increased (p = 0.04) at the end of Er:YAG 2940 mini-peels. The epidermal thickness continued to increase even after cessation of treatment but this increase was not statistically significant (p = 0.34) when compared to the end of treatment; suggesting that there might be some cross talk between the dermis and the epidermis (17), which lead to increase in the rate of cellular proliferation as well as epidermal thickness, and perhaps may contribute to the improvement of skin appearance, however further studies are required to clarify this point.
This finding agrees with the experimental study of Majaron et al (27) which reported that the thickness of rat dorsal skin, that is comparable to human periorbital facial skin, showed a statistically significant increase 4 weeks after Er:YAG 2940 nm mini-peels.
Compared to the base line, our current study revealed statistically significant decrease in total elastin (p=0.03) which was accompanied by a significant increase in tropoelastin level (p=0.02) at the end of treatment. These changes in elastin level were accompanied by the downward translocation of the elastotic material with re-orientation of the elastic fibers suggesting that Er:YAG mini-peels may activate dermal fibroblasts to produce new elastin and contribute in enhancing the skin texture and appearance.
Reversing the signs of photoaging and clinical improvement from laser skin resurfacing, by means of thermal heat delivered to the dermis, is based on the stimulation of new collagen formation (28, 29), and in this study, quantitative evaluation of dermal collagen revealed significant increase in types I, III and VII levels as well as newly synthesized collagen (p<0.05). Three months post treatment, apart from elastin which was increased, tropoelastin, collagen types I, III and VII newly formed collagen levels were decreased as compared to the end of treatment (p>0.05). These data could explain the lack of long-term effects of Er:YAG laser mini-peels.
These findings are in agreement with the study by Christian (10) which investigated the changes in elastic fibers and collagen expression in response to Er:YAG 2940 nm using Verhoeff-van Gieson and Mason trichrome stains. The author reported increased and organized elastic fibers 1 to 2 months post treatment, while on the other hand, collagen expression showed an accentuation of the horizontal bands in the upper papillary dermis at the end of Er:YAG mini-peels treatment.
One obvious limitation of our study is the relatively small number of volunteers. Nevertheless, the results showed evidence of clinical improvement that seems to correlate to the histological changes observed after treatment. Although previous publications have suggested improvement following Er:YAG 2940 nm mini-peels with skin changes, including face tightening, few have objectively analyzed skin after treatment at the histology level (10, 12, 30).
Conclusions
Multiple Erbium:YAG 2940 nm laser mini-peels is an effective, well tolerated and minimally invasive treatment option for photoaging as it stimulates collagen formation and remodeling of extracellular matrix proteins without ablation of the epidermis. This is accompanied by a noticeable clinical improvement of wrinkles and photoaged skin with the advantage of minimal downtime and side effects. The findings of the present study showed short term clinical and histological effects in response to the treatment, suggesting that continued Erbium:YAG 2940 nm laser mini-peels is required to maintain the clinical and the histological improvement. However, further in-depth, long-term studies with a larger number of patients are needed to investigate the maximum duration of treatment required to maintain such effect.
Acknowledgments
The authors would like to thank the Cultural and Educational Bureau of the Republic of Egypt for the financial support of this work.
Funding Sources: This work was supported by the Cultural and Educational Bureau of the Republic of Egypt to WM, and by NIH grant R01 AR28450 to JU.
References
- 1.Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157:874–87. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 2.Farage MA, Miller KW, Berardesca E, et al. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10:73–86. doi: 10.2165/00128071-200910020-00001. [DOI] [PubMed] [Google Scholar]
- 3.El-Domyati M, El-Ammawi TS, Medhat W, et al. Electro-optical synergy technique: a new and effective nonablative approach to skin aging. J Clin Aesthet Dermatol. 2010;3:22–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Uitto J. Understanding premature skin aging. N Engl J Med. 1997;337:1463–65. doi: 10.1056/NEJM199711133372011. [DOI] [PubMed] [Google Scholar]
- 5.El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Domyati M, Attia S, Saleh F, et al. Trichloroacetic acid peeling versus dermabrasion: a histometric, immunohistochemical, and ultrastructural comparison. Dermatol Surg. 2004;30:179–88. doi: 10.1111/j.1524-4725.2004.30061.x. [DOI] [PubMed] [Google Scholar]
- 7.Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7:s12–6. [PubMed] [Google Scholar]
- 8.Flynn C, McCormack BA. Simulating the wrinkling and aging of skin with a multi-layer finite element model. J Biomech. 2009;43:442–48. doi: 10.1016/j.jbiomech.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Sadick NS. Update on non-ablative light therapy for rejuvenation: a review. Lasers Surg Med. 2003;32:120–28. doi: 10.1002/lsm.10127. [DOI] [PubMed] [Google Scholar]
- 10.Christian MM. Microresurfacing using the variable-pulse erbium:YAG laser: a comparison of the 0.5- and 4-ms pulse durations. Dermatol Surg. 2003;29:605–11. doi: 10.1046/j.1524-4725.2003.29145.x. [DOI] [PubMed] [Google Scholar]
- 11.Grema H, Greve B, Raulin C. Facial rhytides--subsurfacing or resurfacing? A review. Lasers Surg Med. 2003;32:405–12. doi: 10.1002/lsm.10172. [DOI] [PubMed] [Google Scholar]
- 12.Kunzi-Rapp K, Dierickx CC, Cambier B, et al. Minimally invasive skin rejuvenation with Erbium: YAG laser used in thermal mode. Lasers Surg Med. 2006;38:899–907. doi: 10.1002/lsm.20380. [DOI] [PubMed] [Google Scholar]
- 13.El-Domyati M, El-Ammawi TS, Medhat W, et al. Radiofrequency facial rejuvenation: evidence-based effect. J Am Acad Dermatol. 2011;64:524–35. doi: 10.1016/j.jaad.2010.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 1996;15:134–38. doi: 10.1016/s1085-5629(96)80003-4. [DOI] [PubMed] [Google Scholar]
- 15.Whittaker P, Kloner RA, Boughner DR, et al. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 16.Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22:97–104. [Google Scholar]
- 17.El-Domyati M, El-Ammawi TS, Medhat W, et al. Effects of the Nd:YAG 1320-nm laser on skin rejuvenation: Clinical and histological correlations. J Cosmet Laser Ther. 2011;13:98–106. doi: 10.3109/14764172.2011.586423. [DOI] [PubMed] [Google Scholar]
- 18.Mahoney MG, Brennan D, Starcher B, et al. Extracellular matrix in cutaneous ageing: the effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis. Exp Dermatol. 2009;18:205–11. doi: 10.1111/j.1600-0625.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 19.El-Domyati M, Attia S, Saleh F, et al. Effect of topical tretinoin on photoaged facial skin: a histometric, immunohistochemical and ultrastructural study. J Cosmet Dermatol. 2004;3:191–201. doi: 10.1111/j.1473-2130.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Geronemus RG. Nonablative laser and light therapies for skin rejuvenation. Arch Facial Plast Surg. 2004;6:398–409. doi: 10.1001/archfaci.6.6.398. [DOI] [PubMed] [Google Scholar]
- 21.Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28:93–105. doi: 10.1016/j.det.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villone D, Fritsch A, Koch M, et al. Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J Biol Chem. 2008;283:206–13. doi: 10.1074/jbc.M802415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amano S. Possible involvement of basement membrane damage in skin photoaging. J Investig Dermatol Symp Proc. 2009;14:2–7. doi: 10.1038/jidsymp.2009.5. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg DJ, Rogachefsky AS, Silapunt S. Non-ablative laser treatment of facial rhytides. A comparison of 1450 nm diode laser treatment with dynamic cooling device as opposed to treatment with dynamic cooling alone. Lasers Surg Med. 2002;30:79–81. doi: 10.1002/lsm.10011. [DOI] [PubMed] [Google Scholar]
- 25.Fournier N, Dahan S, Barneon G, et al. Nonablative remodeling: clinical, histologic, ultrasound imaging, and profilometric evaluation of a 1540 nm Er:glass laser. Dermatol Surg. 2001;27:799–806. doi: 10.1046/j.1524-4725.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 26.Drnovsek-Olup B, Beltram M, Pizem J. Repetitive Er:YAG laser irradiation of human skin: a histological evaluation. Lasers Surg Med. 2004;35:146–51. doi: 10.1002/lsm.20080. [DOI] [PubMed] [Google Scholar]
- 27.Majaron B, Kelly KM, Park HB, et al. Er:YAG laser skin resurfacing using repetitive long-pulse exposure and cryogen spray cooling: I. Histological study. Lasers Surg Med. 2001;28:121–30. doi: 10.1002/lsm.1026. [DOI] [PubMed] [Google Scholar]
- 28.DeHoratius DM, Dover JS. Nonablative tissue remodeling and photorejuvenation. Clin Dermatol. 2007;25:474–79. doi: 10.1016/j.clindermatol.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Shang Y, Wang Z, Pang Y, et al. The role of mast cells in non-ablative laser resurfacing with 1,320 nm neodymium:yttrium-aluminium-garnet laser. Lasers Med Sci. 2010;25:371–77. doi: 10.1007/s10103-009-0703-2. [DOI] [PubMed] [Google Scholar]
- 30.Kunzi-Rapp K, Cambier B, Drosner M, et al. Non-ablative skin rejuvenation with Erbium:YAG laser pulses-investigation of structural changes in the skin. Lasers Med Sci. 2003;18:1–6. [Google Scholar]