Abstract
The olfactory system presents a practical model for investigating basic mechanisms involved in patterning connections between peripheral sensory neurons and central targets. Our understanding of olfactory map formation was advanced greatly by the discovery of cAMP signaling as an important determinant of glomerular positioning in the olfactory bulb. Additionally, several cell adhesion molecules have been identified recently that are proposed to regulate homotypic interactions among projecting axons. From these studies a model has emerged to partially explain the wiring of axons from widely dispersed neuron populations in the nasal cavity to relatively stereotyped glomerular positions. These advances have revitalized interest in axon guidance molecules in establishing olfactory topography, but also open new questions regarding how these patterns of guidance cues are established and function, and what other pathways, such as glycosylation, might be involved. This review summarizes the current state of this field and the important molecules that impact on cAMP-dependent mechanism in olfactory axon guidance.
Keywords: Olfactory, Axon Guidance, Glycosyltransferase, Glycosylation
The ability to detect odorant molecules in the external environment is an important sensory modality for survival of many animals. Odor discrimination is essential to identifying food and predators, while pheromone cues influence mate selection, aggression and other aspects of social and sexual behavior. The olfactory system has devised unique neural mechanisms for encoding odor quality that are distinct from the topographic maps utilized to represent visual and somatosensory information in the brain (see Luo and Flanagan, 2007 for a detailed comparison between these systems).
In this review we will focus on the factors that influence axon pathfinding for the class of olfactory sensory neurons (OSNs) that comprise the vast majority of neurons in the olfactory epithelium (OE); those that express odorant receptors (ORs) and signal through cAMP generated by adenylyl cyclase-3 (AC3). Our perceptions of how glomerular targets are selected for these neurons has significantly changed with the recent finding that olfactory signal transduction plays a much larger role in this process than had previously been appreciated. This discovery has also revealed other cellular pathways and proteins that influence signaling directly and indirectly, including glycosylation. Despite these recent advances, there are still significant questions that remain to be addressed that will also be highlighted.
Organization of the First Circuit
The first relay of the olfactory system has been the most intensively studied, and consequently the best understood (see de Castro, 2009). This initial connection is comprised of the OSNs within the OE lining the nasal cavity and their axon projections to the olfactory bulb (OB). The major feature that defines OSN identity is the monoallelic expression of only one OR per neuron from a repertoire of nearly 1,000 potential receptor genes encoded by the mouse genome (Chess et al., 1993; Zhang and Firestein, 2002). The mechanisms involved in selecting an OR during neuronal maturation and maintaining monogenic expression are still poorly understood. The choice of OR is critical, however, for both determining the odorant response profile of the OSN and establishing the targets its axon will project to in the OB.
The population of OSNs expressing a given OR is confined to one of several overlapping zones spanning the dorsal medial to ventral lateral axis of the OE (Ressler et al., 1993; Vassar et al., 1993). Despite this zonality, the OSN subsets are distributed over a relatively large portion of the sensory epithelium in the nose. The ability to genetically tag individual OSN populations expressing a specific OR through targeted insertion of coding sequences for axonally-transported reporter proteins, such as tau-LacZ or GFP, has been a powerful tool for following trajectories of OSN populations, and genetically tracing these projections in the background of gene knockouts for candidate axon guidance molecules (Mombaerts et al., 1996; Wang et al., 1998).
From these broad regions of the OE, the axons of OSNs expressing a given OR mix with other heterogeneous axons in large bundles that project through the underlying lamina propria, cross the cribriform plate and innervate the OB. Within the nerve layer surrounding the OB axons defasciculate from other OR-expressing populations, and converge into glomeruli where they synapse with the dendrites of mitral and tufted cells, the projection neurons that relay sensory stimuli to higher centers in the brain. Each OR-expressing population typically forms a single medial and lateral glomerulus in each OB hemisphere at a position that is largely stereotyped. Thus, each glomerulus receives afferent input from OSNs expressing only a single OR (Vassar et al., 1994; Ressler et al., 1994). In this way, the activity of each dispersed OSN subset is rendered convergent in the OB.
Most ORs do not recognize specific odorant molecules, but are instead more broadly tuned to a range of overlapping structures sharing related molecular features (Mori et al., 1999). Each odorant activates a spectrum of glomeruli at differing levels. There may be a degree of chemical topography in the OB in that some molecularly related odorants activate glomeruli in specific OB subregions. However, it is debatable whether or not these chemotopic maps might be a useful mechanism to facilitate odor processing in the cortex (Soucy et al., 2009). In any case, it is the combinatorial pattern of glomerular activation elicited by odorants that is decoded by higher processing centers in the brain to identify odorant stimuli (Reviewed in Ache and Young, 2005).
The convergence of odorant information from dispersed OR-expressing neuronal populations into glomeruli forms an “olfactory map” of odor quality. This makes the olfactory system fundamentally different from the topographic maps utilized in other sensory areas, such as the visual system, where retinal axons maintain their positional relationship in route to the cortex. The key differences between these methods of encoding sensory information have been expertly reviewed elsewhere (Luo and Flanagan, 2007). This review will focus on the mechanisms by which olfactory axons are guided to glomeruli, and will summarize recent advances in the role of signaling in this process and the factors that modulate signaling pathways.
Canonical Olfactory Transduction
Odor detection initiates in the nasal epithelium, where the major components of canonical olfactory signaling are highly concentrated in specialized sensory cilia that protrude into the nasal cavity from the single apical dendrite of OSNs. The regulation of each of these proteins has been extensively studied and is known in detail, although other pathways that directly or indirectly influence cilia targeting and signaling continue to emerge (Von Dannecker et al., 2005; McEwen et al., 2007). All ORs are seven transmembrane receptors that couple to downstream signaling through heterotrimeric G-proteins. During normal olfaction this complex includes the Gαolf subunit, a Gs homologue that is highly expressed in OSNs. Odorant binding to ORs stimulates Gαolf, which activates the olfactory-type adenylyl cyclase isoform AC3. Stimulation of AC3 generates an increase in intracellular cAMP concentration, which opens a heterotetrameric cyclic nucleotide gated channel complex, composed of a dimer of the principal CNGA2 subunit and the modulatory subunits CNGA4 and CNGB1b. Gating of this calcium-permeable channel complex results in a depolarizing membrane current and efflux of chloride through ion channels that further depolarizes the membrane to generate an action potential.
The action of cAMP generated by AC3, either through odor-evoked or spontaneous activity, is not limited to gating of the CNG channel. A number of intracellular pathways are activated by cAMP, including protein kinase-A (Boekhoff and Breer, 1992) and ERK/MAP kinase (Watt and Storm, 2001). Stimulation of these pathways also leads to CREB phosphorylation and CRE-mediated transcriptional activation in OSNs. These downstream effectors generate opportunities for cross talk between cAMP-dependent odor-evoked activity and other intracellular pathways, and might also limit the duration of AC3 activation by feedback inhibition (Wei et al, 1996).
Axon Guidance Mechanisms
There are several significant characteristics that distinguish olfactory axon guidance from mechanisms described in other systems: 1) The absence of target-derived cues, 2) mosaic expression of guidance and adhesion molecules, 3) the restricted role played by neuronal activity in axon targeting. These distinct mechanisms, summarized below, may have arisen in the olfactory system by necessity to enable homotypic projections from the broadly dispersed OSN populations to distinguish self from non-self, fasciculate, and form glomeruli at reproducible positions in the OB. Each of these unique characteristics will be considered in more detail.
1. Target derived cues play relatively minor roles in the olfactory system. Since the initial identification of repulsive and attractive axon guidance molecules, such as Collapsin in chick and Netrin in mice (Luo et al., 1993; Serafini et al., 1994), it has been shown in vertebrates and invertebrates that neurons in the prospective targets express factors that directly influence growth cone dynamics. In some systems gradients of attractive and repulsive guidance cues in the target field are used to establish topography (Flanagan, 2006; Sanchez-Camacho and Bovalenta, 2009). Such multiple overlapping gradients present an effective mechanism for achieving concentration-dependent positive and negative effects on guidance. For example, during formation of retinotopic maps in visual development, graded expression of ephrins and Eph receptors in the axon target field are known to be critical for establishing the topography of retinal projections to the tectum (reviewed in Luo and Flanagan, 2007). Although ephrin gradients function in map formation in other sensory and non-sensory systems as well, in the olfactory system ephrins are not expressed in a gradient, nor do ephrin positive axons respond to ligand gradients in the OB (Cutforth et al., 2003; Flanagan, 2006).
In fact, none of the major neuronal populations of the developing OB have been shown to express functionally relevant attractive or repulsive influences on growth cones of OSNs. Furthermore, deletion of OB projection neurons by gene targeting of the T-box transcription factor Tbr-1 and OB interneurons by ablation of the homeobox genes Dlx-1 and Dlx-2 resulted in normal targeting of P2 axons to appropriate positions in the ventral OB (Bulfone et al., 1998). Even removal of the OB altogether does not prevent axonal convergence into homotypic populations (St John et al., 2003). With a few possible exceptions, target derived guidance cues appear to have little influence on olfactory targeting, at least in mice.
2. Most of the axonally expressed molecules that play a role in axon guidance are expressed mosaically in glomeruli. That is, glomeruli are composed of axons that express discrete levels (high, medium or low) of these proteins, including neuropilin 1 (Nrp1), kirrel2 and 3, ephrinA5, Big-2 and lactosamine. Furthermore, glomeruli that express different amounts of these proteins are not positioned in a descending or ascending linear gradient along any particular axis but appear to be distributed in a mosaic pattern in which glomeruli expressing widely differing levels of these molecules are randomly spaced across the two dimensional glomerular layer in the OB. For example, glomeruli that express high levels of Nrp1 or BIG-2 are surrounded by glomeruli that express medium or very low levels of Nrp1 and BIG-2. This is a feature that would be best suited for specifying local compartments (perhaps at the individual glomeruli level) by influencing axon fasciculation or defasciculation through differential adhesive and repulsive mechanisms, respectively.
3. Odor-evoked activity plays a more limited role in OSN axon guidance relative to other sensory systems. In the visual system, activity-independent mechanisms are used by axons to find an approximate target followed by activity-dependent mechanisms that refine the map to the needed precision. In attempts to demonstrate the role of activity in olfactory axon guidance, all of the major proteins involved in this odor-evoked activity have been targeted by gene deletion. Gene targeting of Gαolf generated anosmic mice that retained apparently normal glomerular convergence (Belluscio et al., 1998). Deletion of the CNGA2 subunit of the cyclic nucleotide gated channel complex also resulted in anosmia with a surprisingly mild targeting phenotype (Brunet et al., 1996; Zheng et al., 2000), although mosaic analysis revealed that the axons of electrically silenced neurons were progressively lost from the OB, apparently by competition from wild-type neurons (Zhao and Reed, 2001). When naries are unilaterally occluded at birth, thereby limiting neuronal activity on one side, the number of heterogeneously innervated glomeruli remained only marginally increased on that side in adults (Zou et al., 2004), implying a more significant role for molecular rather that activity-dependent determinants in olfactory axon guidance. Likewise, experiments that inhibited action potentials in OSNs in either a competitive or non-competitive context suggested a permissive rather than instructive requirement for neural firing for axon targeting (Yu et al., 2004). When firing was suppressed in a subset of OR-defined neurons, those neurons were no longer detected in the OE, suggesting either that they did not survive or that they switched to the transcription of a different OR gene.
In contrast, altering neuronal activity, using hemizygous mice in which OSNs randomly inactivate one CNGA2 allele, can modulate expression of adhesion molecules that play important roles in axon fasciculation (Serizawa et al., 2006; Kaneko-Goto et al., 2008). Furthermore, regulation of axon-axon interactions, though not a guidance mechanism per se, could play a role in axon segregation and convergence into distinct glomeruli.
Guidance and Adhesion Molecules
Because of the limited role for targets, gradients and activity, olfactory axons must utilize other means for pathfinding to glomeruli. One important mechanism involves the interaction of cues derived from olfactory ensheathing glial cells with OSN axons. During the initial stages of OE development Nrp1 is expressed by subsets of axons that innervate lateral and medial aspects of the OB (Schwarting et al., 2000). Nrp1 expressing axons are deflected from the anterior-ventral OB towards medial and lateral targets by the repulsive cue semaphorin3A (Sema3A), which is produced by olfactory ensheathing cells that are present in the lamina propria, along axon pathways between the OE and OB, and in the developing OB nerve layer (Schwarting et al., 2000; Imai et al., 2009). A mosaic pattern of Nrp1 expression is visible in early postnatal development shortly after distinct glomeruli are observed, suggesting that Nrp1 levels are regulated prior to innervating the OB. In Sema3A null mice, Nrp1 axons are misrouted to anterior and ventral OB targets from which they are normally excluded, and in the case of P2, innervate supernumerary glomeruli within these aberrant projection sites (Schwarting at al., 2004). Repulsive axon-axon interactions may also play a role in guidance. In addition to its expression in glial cells, Sema3A is weakly expressed by some OSNs (Imai et al., 2009; Henion et al., 2011). Recently, Sema3F has also been identified as a neuronally expressed guidance cue that restricts later-arriving Nrp2+ axons from entering the dorsal OB through repulsive interactions (Takeuchi et al., 2010).
Different glomeruli are also mosaic for ephrin expression. Combined ablation of ephrinA3 and ephrinA5 throughout the OE causes a posterior shift in the position at which reporter-labeled glomeruli form in the OB. Overexpression of ephrinA5 in P2 axons, however, induces relatively minor segregation from wild-type P2 glomeruli (Cutforth et al., 2003). Similarly, members of the Robo family of guidance receptors that play critical roles in a number of axon guidance contexts are also expressed in the developing OE. Robo2 is expressed in axons targeting the dorsal OB, and loss of Robo2 expression results in a small number of aberrant Robo2+ glomeruli in the ventral OB, whereas most axons target the dorsal OB correctly (Cho et al., 2007). Although Eph receptors and Slit-1 receptors are expressed in subsets of mitral cells, it remains to be seen whether ephrinA5+ or Robo-2+ axons are exceptions that respond directly to guidance influences from target neurons in the OB.
Several adhesion molecules have been identified recently that are thought to participate in glomerular convergence (Serizawa et al., 2006; Kaneko-Goto et al., 2008). Kirrel2 and Kirrel3 are Ig-superfamily proteins that are mosaically expressed by glomeruli in a reciprocal fashion. Their expression correlates with specific OSN populations and both exhibit homotypic adhesion properties in vitro, suggesting they may regulate axonal association for incoming populations expressing the same OR (Serizawa et al., 2006). In addition, ephrinA5 and EphA5 are also expressed in a complementary manner in glomeruli (Serizawa et al., 2006). Another Ig-superfamily member, BIG-2 (contactin-4) also displays similar mosaic expression and glomerular segregation (Kaneko-Goto et al., 2008). In BIG-2 knockout mice, glomeruli are found in the correct position but often split into two or more smaller glomeruli. Together, these studies provide a framework for understanding how individual OR-defined axon populations segregate from heterotypic axons in the OB nerve layer. The existence of sets of adhesion molecules that are either reciprocally or independently regulated have the potential to impart unique identities to each of the many axon populations present, even within relatively restricted subregions of the OB.
Initial studies in mice on the role of ORs in targeting suggested that OR proteins might directly provide axon guidance information. Replacement of the coding sequence for the P2 OR with that of an M12-ires-tau-lacZ cassette resulted in targeting to a novel position, intermediate between that of the endogenous P2 and M12 glomerulus (Mombaerts et al., 1996). Deletion of the P2 coding region altogether resulted in a complete failure of axons to converge into a glomerulus (Wang et al., 1998). These findings were further corroborated with other receptor swap experiments suggesting this was a conserved feature of all ORs. Precisely how these alterations induced targeting abnormalities was unknown, although one conclusion was that OR proteins themselves could be involved in the process of sorting axons by mediating selective axon fasciculation (Feinstein et al., 2004). However, more recent evidence suggests that their role in targeting may not be so direct.
cAMP Signaling in Axon Guidance
It now appears that the axon guidance defects associated with receptor swap experiments are derived from the changes in cAMP signaling rather than a specific function for ORs in homotypic adhesion or axon fasciculation. Each OR is hypothesized to generate intrinsically different levels of cAMP that influences targeting to specific positions in the OB (Imai and Sakano, 2008). Swapping one OR for another is predicted to alter these signals, changing the location at which the glomeruli form. The apparent failure of axons to converge in OR-deleted mice results from stochastic activation of other ORs in each targeted neuron, leading to a myriad of projection sites. In support of this, the replacement of the M71 OR with the coding sequence for the β-adrenergic receptor, which also couples to G-proteins to generate cAMP signals, results in axon convergence (Feinstein et al., 2004; Chesler et al., 2007). Thus the OR protein is dispensable for targeting while the downstream signals they generate are not.
Several recent studies from the Sakano group examining the effects of modulating cAMP levels in OSNs have convincingly demonstrated the direct role of signaling in olfactory axon targeting (Imai et al., 2006; 2009; Serizawa et al., 2007). When OR protein expression was effectively decoupled from AC3, via a transgenic approach in which the G-protein binding site on the OR I7 was mutagenized, olfactory axons failed to innervate glomeruli (Imai et al., 2006). Overexpressing a constitutively active form of Gαs restored convergence, crucially in an OR-independent manner. Furthermore, overexpression of a dominant negative form of protein kinase-A or CREB shifted glomeruli towards anterior or posterior positions, respectively. These results suggested that increasing cAMP levels in OSN axons induced convergence of I7 at progressively more posterior glomeruli in the OB. Although cAMP can directly influence growth cone turning, much of the affect on targeting may be mediated through differential regulation of genes for axon guidance molecules.
The level of cAMP generated within an OSN subset determines Nrp1 transcription. Nrp1 mRNA expression is higher in OSNs genetically manipulated to express elevated cAMP levels versus those where downstream AC3 signaling is decoupled from ORs (Imai et al., 2006). The ablation of AC3 altogether leads to the loss of Nrp1 expression on OB axons (Col. et al., 2007). Sema3A in OSNs is also regulated in part by cAMP. Normally, Sema3A is weakly expressed in the OE postnatally but is upregulated when OR signaling is inhibited (Imai et al., 2009). Thus, the reciprocal expression of Nrp1 and Sema3A levels could mediate repulsive guidance cues for subsets of axons.
In I7 neurons that overexpress either Nrp1 or constitutively active G-protein or protein kinase-A signaling, axons project to more posterior Nrp1+ OB positions. Conversely, I7 neurons with suppressed signaling project to more anterior Nrp1-negative glomeruli (Imai et al., 2006, 2009). These results have led to the hypothesis that olfactory axon populations form glomeruli along the anterior - posterior axis in accordance with lower to higher levels of Nrp1, respectively. Nrp1+ axons are already excluded from the anterior nerve layer by glial-derived Sema3A (Schwarting et al., 2000), and reduced expression of Nrp1 is likely to allow axons to innervate this domain, which may influence this glomerular shift. Moreover, more posterior glomeruli are also highly variable for levels of Nrp1 expression, suggesting that other mechanisms must be involved beyond a linear Nrp1 gradient on axons. It is likely that additional guidance or adhesion molecules cooperate in this process.
Although cAMP determines Nrp1 levels, it is important to note that this is apparently independent of odor-evoked activity. In the mouse, most olfactory axon populations have already targeted the approximate site in the OB and formed protoglomeruli before birth and exposure to the external environment. As noted before, only the targeted disruption of ORs and AC3 disturbs the initial formation of glomeruli while downstream signaling components such as Gαolf and CNGA2 are dispensable for grossly normal map formation developmentally. This in part is explained by redundancy in G-protein expression in the OE (Imai et al., 2006; Chesler et al., 2007). Whether such signals emanate from intrinsic stimuli or spontaneous activity, it will be important to show that each OR-expressing population is defined by specific level of cAMP-signaling that directs Nrp1 expression and glomerular targeting.
β3GnT2 Glycosylation and Olfactory System Development
The importance of cAMP signaling for olfactory map formation is also apparent from studies on another mouse model with defective OB innervation. The glycosyltransferase β3GnT2 is highly expressed in the developing OE where it synthesizes lactosamine-based glycans (Henion et al., 2005). β3GnT2 null mice exhibit multiple defects in OE development and axon guidance. In mutants, olfactory axons grow normally to the OB, but some subsets are misguided to ectopic targets. Other axon populations fail to form glomeruli altogether, and are subsequently significantly reduced in expression. Unexpectedly, these defects appear to result mainly from hypoglycosylated AC3, which is severely deficient in its ability to generate cAMP. Thus, glycosylation is also an important component for maintaining olfactory signaling and proper glomerular targeting.
The β3GnT2 glycosyltransferase, originally termed β3GnT1 in earlier reports under a prior nomenclature, is a member of a large family of β1,3-N-acetylglucosaminyltransferases that are expressed tissue-specifically, and have differing acceptor specificities (Shiraishi et al., 2001; Zhou et al., 2001). β3GnT2 exhibits the strongest enzyme activity in vitro towards a range of non-reducing, β1-linked galactose oligosaccharide acceptors typical of N-glycan branches. In vivo, the free N-acetylglucosamine structures generated by β3GnT2 are subsequently modified by ubiquitously expressed galactosyltransferase enzymes to generate the terminal disaccharide lactosamine (Galactose-β1,4N-acetylglucosamine-β1,3-R). These lactosamine units are substrates for repeated β3GnT2 and galactosyltransferase modification, leading to the synthesis of extended polylactosamine (PLN) chains of varying length. β3GnT2 is the only member of this family that is widely expressed at significant levels in mouse OSNs. Transcription initiates by E10.5 as the olfactory placode invaginates to form the OE, and the earliest olfactory axons target the presumptive OB. Expression is maintained in immature and differentiated OSNs throughout the remainder of the lifespan of the mouse. The analysis of β3GnT2 null mice has firmly established that this enzyme regulates PLN expression (Henion et al., 2011).
The effects of β3GnT2 loss on olfactory development are apparent in late embryogenesis (Henion et al., 2005). At birth, most glomeruli of wild-type mice have already condensed into loosely rounded clusters of intermingled axons and mitral cell dendrites. In contrast, the OB of β3GnT2 null mice is largely composed of disorganized axon aggregates and individual fibers that rarely form morphologically normal glomeruli. There is also a large reduction in the number of axons within the OB nerve layer, which is noticeably thinner relative to wild-types. In parallel with the loss of OB innervation, subsets of olfactory neurons are differentially eliminated from the OE. In adults, the P2 and I7 OSNs populations that fail to form glomeruli are decreased greater than 75%.
As expected from these developmental defects, β3GnT2 null mice have an early functional deficit in smell perception, displaying decreased performance in food-finding tasks (Henion et al., 2005). Despite the initial disruptions in OB innervation, a significant degree of functional connectivity is established during postnatal development. A large increase in basal progenitor cell proliferation occurs by P10 and morphologically distinguishable glomeruli appear by P15, although these remain irregularly shaped and do not occupy the most dorsoposterior aspect of the OB. Despite this, P2 and I7 glomeruli never reform, although null mice continue to produce a reduced number of these neurons in adults. M72 axons are also heterotypically maintained in numerous glomeruli innervated by other OR subsets at ectopic positions in the anterior OB (Schwarting and Henion, 2007).
From this continued level of disorganization it is clear that the olfactory map remains dramatically altered in adult β3GnT2 null mice. In fact, gene array analysis reveals that upwards of one third of all ORs are expressed at very low levels, and perhaps in compensation, many other ORs are expressed at significantly higher levels in β3GnT2 null mice than in wild-types. It is not known if the loss of OR subsets is due to a failure to survive or a shift in those cells to the transcription of a new OR gene. In addition, analysis of axon trajectories suggests that the guidance of remaining OSN populations is aberrant in null mice. In most cases examined to date, mutant OR-specific axons innervate glomeruli that are more anterior and ventral compared to controls.
In spite of the significant structural defects in β3GnT2 null mice, they have a surprising ability to discriminate odorants in a food based olfactory task. This result is reminiscent of earlier studies in which significant portions of the OB were surgically removed but resulted in only small deficits in olfactory perception (Lu and Slotnick, 1998). Similarly, mice in which OR71 was overexpressed in 95% of OSNs retained a considerable ability to discriminate odors, regardless of the fact that their endogenous receptors were expressed at very low levels (Fleischmann et al., 2008). These studies suggest that even though an OR population may be severely diminished in number and incapable of forming a mature glomerulus, if enough of these neurons make a connection to mitral cells in the correct region of the OB, the animal is able to maintain a diminished sense of smell. Likewise in β3GnT2 null mice, the significant decrease in the numbers of many neuron populations results in relatively mild impairment in olfactory discrimination. Since only a very small number of OR binding events are needed to signal the brain, mice with many fewer receptor-specific OSNs may still retain modest discriminatory ability (Ben-Chaim et al., 2011).
cAMP Signaling in β3GnT2 Null Mice
The olfactory defects in β3GnT2 null mice closely parallel those reported for gene-targeted AC3 mice. Along with a loss of electrophysiological responses to odor challenge, AC3 nulls exhibit a disorganized pattern of glomerular formation (Trinh and Storm, 2003). More detailed analyses of these mice revealed regional differences in OB innervation. The dorsocaudal OB in particular was sparsely innervated in AC3 mutants similar to β3GnT2 nulls (Col et al., 2007). Furthermore, the loss of projections was OR-dependent, displaying differential effects on OSN subsets that paralleled those we observed for β3GnT2 null mice (Henion et al., 2005). P2 OSNs were largely eliminated from OE and failed to form glomeruli, whereas M72 axons were abnormally displaced to the anterior OB (Chesler et al., 2007; Col et al., 2007). Importantly, in the absence of either enzyme heterotypic M72 glomeruli are maintained well past their ‘critical period’ for elimination, consistent with a reduction in activity-dependent refinement for some subsets. The similarities strongly suggest that the effects of β3GnT2 loss are manifested through AC3.
AC3 protein is heavily concentrated in cilia where olfactory signal transduction initiates, but is also present on OSN cell bodies and axons projecting to the OB (Col et al., 2007; Zou et al., 2007). Reactivity for the plant lectin Lycopersicum esculantum (LEA), which specifically recognizes PLN, colocalizes with AC3 in all OSNs and glomeruli. In β3GnT2 nulls, LEA reactivity for PLN is ablated, and there is a 50 - 60% reduction in the number of AC3+ OSNs that closely parallels the decrease in mature OSNs. Despite this, AC3 appears to be expressed at wild-type levels in remaining neurons, and traffics normally to cilia (Henion et al., 2011).
AC3 is a heavily glycosylated glycoprotein that migrates diffusely on protein gels at a mass far greater than its native 129 Kda size. Although the glycan structures that modify AC3 in OSNs have not been resolved, most of this protein is retained on LEA affinity columns, indicating a substantial amount of PLN modification. This glycosylation is β3GnT2-dependent, as AC3 from null OSNs is devoid of PLN and migrates very near its predicted molecular mass. β3GnT2 also modifies the guidance receptor plexinA4 and kirrel2. Although these glycoproteins are expressed at wild-type levels in null OSN cell bodies, AC3 and kirrel2 are absent from glomeruli, suggesting that PLN may stabilize their expression on axons. Alternatively, AC3 may be actively trafficked to dendritic cilia, at the expense of axonal expression.
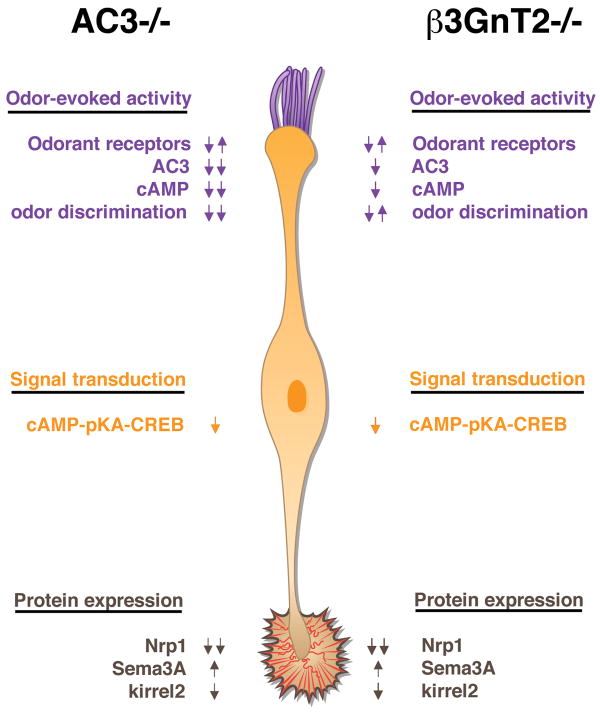
Despite continued AC3 expression in the β3GnT2-/- OE, measurements of adenylyl cyclase activity revealed that cAMP production is decreased by 80 to 90% in newborns and adults (Henion et al., 2011). This result indicates a role for PLN in the maintenance of cAMP-dependent signaling that is independent of OSN survival or AC3 localization and expression. One intriguing possibility is that PLN is required to maintain AC3 in an active complex with other signaling proteins that facilitates cAMP-mediated signal transduction. The severely reduced levels of cAMP result in decreased Nrp1 levels in OSNs and axon projections, and increased Sema3A expression in the OE. These changes in important axon guidance cues, which closely parallel those reported in AC3-/- mice, may in part explain the severe targeting abnormalities shared by both knockout models. The developmental defects in β3GnT2 nulls are also independent of odor-evoked activity, as changes in OSN survival and OB targeting are evident by E17, prior to exposure to the external odorant environment. These results are consistent with a dual role for cAMP in odor-evoked activity and transcriptional regulation of axon guidance and adhesion molecules (Figure 1).
Figure 1.
In AC3-/- cilia, AC3 is absent, cAMP is greatly decreased, and the mice are anosmic. In β3GnT2-/- mice, AC3 and cAMP are partially reduced but olfactory perception is only slightly diminished. In AC3-/- and β3GnT2-/- axons, cAMP signaling is greatly reduced. Expression of the axon guidance and adhesion molecules neuropilin-1 (Nrp1) and and kirrel2 is decreased in both mouse models, while Semaphorin 3A (Sema3A) expression is increased.
Surprisingly, despite these abnormalities it is clear that β3GnT2 null mice are not completely anosmic. Unlike other knockout mouse models for signaling components (Brunet et al., 1996; Belluscio et al., 1998, Wong et al., 2000), most β3GnT2 pups survive the neonatal period, indicating that they can follow olfactory cues required for suckling. Moreover, the performance of β3GnT2-/- mice in buried food tests and behavioral assays for AC3-dependent odors, while impaired relative to wild-types, suggests a considerable degree of olfactory function. These results reveal apparent differences in the levels of cAMP needed to regulate Nrp1 and Sema3A transcription, versus maintenance of odor-evoked signaling. In this regard, it is interesting to note that a number of genes reported to be regulated by odor-evoked activity independent of cAMP, including S100A5, calretinin, and Lrrc3b (Bennett et al., 2010) are largely unaffected in β3GnT2-/- OSNs (Henion et al., unpublished results).
Future Directions
Our understanding of the mechanisms that regulate olfactory map formation has progressed greatly in the last several years. The identification of cAMP as a primary regulator of Nrp1 and Sema3A expression and glomerular positioning has tentatively linked OR-mediated signal transduction to the broader field of established axon guidance cues. Once axons target an approximate OB subdomain, it appears that mosaically expressed molecules, including glycans, ephrins, BIG-2 and Kirrels 2/3, facilitate adhesive and repulsive interactions during glomerular formation. This model explains how the axons for approximately 1,000 distinct OR-defined OSNs subsets can converge at great distance upon largely stereotyped OB targets from widely dispersed locations in the nasal cavity (Imai et al., 2008).
Despite this progress, our understanding of the role of signaling in olfactory axon targeting is still preliminary, and there are many questions that remain unanswered. Most importantly, how is the level of AC3 activity and cAMP signaling regulated prenatally as olfactory axons target the OB? Not only is AC3 activity influenced by OR activation and β3GnT2 expression, other G protein-coupled receptors and their ligands such as CXCR4/SDF-1 are likely to modulate cAMP levels in subsets of embryonic OSNs. It was recently shown that SDF-1 could activate cAMP signaling in zebrafish retinal axons to antagonize their responses to midline chemorepellents (Xu et al., 2010). CXCR4 is highly expressed in OSNs developmentally (Schwarting et al., 2006). It will be interesting to see how these and other GPCR pathways cooperate to regulate cAMP responses during targeting. It is also intriguing that the components of canonical AC3 olfactory signal transduction pathway are expressed and function in both OSN cilia and axons (Maritan et al., 2009). How are these signals correlated with each other and does axonal signaling predominate during axon extension to the OB as predicted (Imai and Sakano, 2008)?
Although Nrp1 appears to play an important role in glomerular positioning in the OB, it is likely from the nonlinear pattern of Nrp1 expression in axons that other guidance cues are involved. How does this mosaicism fit in with the current model and what guidance mechanisms account for the targeting of axons in the anterior-medial and ventral OB compartments where Nrp1 axons are excluded through semaphorin3A repulsion (Nagao et al., 2003; Schwarting et al., 2004; Taniguchi et al., 2003). In the visual system, multiple sets of guidance cues are utilized to pattern each axis (Flanagan, 2006). Are there other molecules that act cooperatively with Nrp1 to ensure proper positioning?
It is also likely that other differentially expressed molecules exist in addition to those already identified that facilitate sorting of axons into homotypic glomeruli. Considering that kirrels, ephrins and BIG-2 have relatively localized effects on glomerular refinement that might not be evident to casual observation, there are likely to be other known molecules that need to be reexamined for functions in this late stage of glomerular formation. In this regard, carbohydrates are good candidates, given their mosaic expression both developmentally and in adult animals (Schwarting et al., 1992; Key and Akeson, 1993; Lipscomb et al., 2002). Perhaps a more detailed analysis relating the expression of these and other glycan moieties to odor-evoked activity and glomerular formation in OR-reporter mice will reveal previously overlooked phenotypes.
Acknowledgments
Grant Sponsor: NIH Grant Numbers: RO1-DC00953 and RO1-DC09034
Grant Sponsor: Mizutani Foundation for Glycoscience
Literature cited
- Ache BW, Young J. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Ben-Chaim Y, Cheng MM, Yau KY. Unitary response of mouse olfactory receptor neurons. Proc Natl Acad Sci USA. 2011;108:822–827. doi: 10.1073/pnas.1017983108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Kulaga HM, Reed RR. Odor-evoked gene regulation and visualization in olfactory receptor neurons. Mol Cell Neurosci. 2010;43:353–362. doi: 10.1016/j.mcn.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoff I, Breer H. Termination of second messenger signaling in olfaction. Proc Natl Acad Sci U S A. 1992;89:471–474. doi: 10.1073/pnas.89.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;4:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, Meneses J, Pedersen R, Axel R, Rubenstein JL. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998;21:1273–1282. doi: 10.1016/s0896-6273(00)80647-9. [DOI] [PubMed] [Google Scholar]
- Chesler AT, Zou DJ, Le Pichon CE, Peterlin ZA, Matthews GA, Pei X, Miller MC, Firestein S. A G protein/cAMP signal cascade is required for axonal convergence into olfactory glomeruli. Proc Natl Acad Sci USA. 2007;104:1039–1044. doi: 10.1073/pnas.0609215104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Cho JH, Lepine M, Andrews W, Parnavelas J, Cloutier JF. Requirement for Slit-1 and Robo-2 in zonal segregation of olfactory sensory neuron axons in the main olfactory bulb. J Neurosci. 2007;27:9094–9104. doi: 10.1523/JNEUROSCI.2217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Col JA, Matsuo T, Storm DR, Rodriguez I. Adenylyl cyclase-dependent axonal targeting in the olfactory system. Development. 2007;134:2481–2489. doi: 10.1242/dev.006346. [DOI] [PubMed] [Google Scholar]
- Cutforth T, Moring L, Mendelsohn M, Nemes, Shah NM, Kim MM, Frisén J, Axel R. Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell. 2003;114:311–322. doi: 10.1016/s0092-8674(03)00568-3. [DOI] [PubMed] [Google Scholar]
- de Castro F. Wiring olfaction: the cellular and molecular mechanisms that guide the development of synaptic connections from the nose to the cortex. Front Neurosci. 2009;3:1–17. doi: 10.3389/neuro.22.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein P, Mombaerts P. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell. 2004;117:817–831. doi: 10.1016/j.cell.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Flanagan JG. Neural map specification by gradients. Curr Opin Neurobiol. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Shykind BM, Sosulski DL, Franks KM, Glinka ME, Mei DF, Sun Y, Kirkland J, Mendelsohn M, Albers MW, Axel R. Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron. 2008;60:1068–1081. doi: 10.1016/j.neuron.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henion TR, Zhou D, Wolfer DP, Jungalwala FB, Hennet T. Cloning of a mouse beta 1,3 N-acetylglucosaminyltransferase GlcNAc(beta 1,3)Gal(beta 1,4)Glc-ceramide synthase gene encoding the key regulator of lacto-series glycolipid biosynthesis. J Biol Chem. 2001;276:30261–30269. doi: 10.1074/jbc.M102979200. [DOI] [PubMed] [Google Scholar]
- Henion TR, Raitcheva D, Grosholz R, Biellmann F, Skarnes WC, Hennet T, Schwarting GA. Beta1-3-N-Acetylglucosaminytransferase 1 glycosylation is required for axon pathfinding by olfactory sensory neurons. J Neurosci. 2005;25:1894–1903. doi: 10.1523/JNEUROSCI.4654-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Suzuki M, Sakano H. Odorant receptor-derived cAMP signals direct axonal targeting. Science. 2006;314:657–661. doi: 10.1126/science.1131794. [DOI] [PubMed] [Google Scholar]
- Imai T, Sakano H. Odorant receptor-mediated signaling in the mouse. Curr Opin Neurobiol. 2008;18:251–260. doi: 10.1016/j.conb.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Imai T, Yamazaki T, Kobayakawa R, Kobayakawa K, Abe T, Suzuki M, Sakano H. Pre-Target Axon Sorting Establishes the Neural Map Topography. Science. 2009;325:585–590. doi: 10.1126/science.1173596. [DOI] [PubMed] [Google Scholar]
- Kaneko-Goto T, Yoshihara S, Miyazaki H, Yoshihara Y. BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron. 2008;57:834–846. doi: 10.1016/j.neuron.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010;11:188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- Key B, Akeson RA. Distinct subsets of sensory olfactory neurons in mouse: possible role in the formation of the mosaic olfactory projection. J Comp Neurol. 1993;335:355–368. doi: 10.1002/cne.903350306. [DOI] [PubMed] [Google Scholar]
- Lipscomb BW, Treloar HB, Greer CA. Cell surface carbohydrates reveal heterogeneity in olfactory receptor cell axons in the mouse. Cell Tissue Res. 2002;308:7–17. doi: 10.1007/s00441-002-0532-0. [DOI] [PubMed] [Google Scholar]
- Lu XCM, Slotnick BM. Olfaction in rats with extensive lesions of the olfactory bulb: Implications for odor coding. Neuroscience. 1998;84:849–866. doi: 10.1016/s0306-4522(97)00520-4. [DOI] [PubMed] [Google Scholar]
- Luo L, Flanagan JG. Development of continuous and discrete neural maps. Neuron. 2007;56:284–300. doi: 10.1016/j.neuron.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Maritan M, Monaco G, Zamparo I, Zaccolo M, Pozzan T, Lodovichi C. Odorant receptors at the growth cone are coupled to localized cAMP and Ca2+ increases. Proc Natl Acad Sci USA. 2009;106:3537–3542. doi: 10.1073/pnas.0813224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DP, Jenkins PM, Martens JR. Olfactory cilia: our direct neuronal connection to the external world. Curr Top Dev Biol. 2008;85:333–370. doi: 10.1016/S0070-2153(08)00812-0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Nagao H, Yoshihara Y, Mitsui S, Fujisawa H, Mori K. Two mirror-image sensory maps with domain organization in the mouse main olfactory bulb. Neuroreport. 2003;11:3023–3027. doi: 10.1097/00001756-200009110-00039. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Camacho C, Bovalenta P. Emerging mechanisms in morphogen-mediated axon guidance. BioEssays. 2009;31:1013–1025. doi: 10.1002/bies.200900063. [DOI] [PubMed] [Google Scholar]
- Schwarting GA, Deutsch G, Gattey DM, Crandall JE. Glycoconjugates are stage- and position-specific cell surface molecules in the developing olfactory system, 2: Unique carbohydrate antigens are topographic markers for selective projection patterns of olfactory axons. J Neurobiol. 1992;23:130–142. doi: 10.1002/neu.480230204. [DOI] [PubMed] [Google Scholar]
- Schwarting GA, Kostek C, Ahmad N, Dibble C, Pays L, Puschel AW. Semaphorin 3A is required for normal guidance of olfactory axons in mice. J Neurosci. 2000;20:7691–7697. doi: 10.1523/JNEUROSCI.20-20-07691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting GA, Raitcheva D, Crandall JE, Burkhardt C, Puschel AW. Semaphorin 3A mediated axon guidance regulates convergence and targeting of P2 odorant receptor axons. Eur J Neurosci. 2004;19:1800–1810. doi: 10.1111/j.1460-9568.2004.03304.x. [DOI] [PubMed] [Google Scholar]
- Schwarting GA, Henion TR. Lactosamine differentially affects olfactory sensory neuron projections to the olfactory bulb. Dev Neurobiol. 2007;67:1627–1640. doi: 10.1002/dneu.20536. [DOI] [PubMed] [Google Scholar]
- Schwarting GA, Henion TR. Olfactory axon guidance: the modified rules. J Neurosci Res. 2008;86:11–17. doi: 10.1002/jnr.21373. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, Sakano H. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127:1057–1069. doi: 10.1016/j.cell.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Shiraishi N, Natsume A, Togayachi A, Endo T, Akashima T, Yamada Y, Imai N, Nakagawa S, Koizumi S, Sekine S, Narimatsu H, Sasaki K. Identification and characterization of three novel beta 1,3-N-acetylglucosaminyltransferases structurally related to the beta 1,3-galactosyltransferase family. J Biol Chem. 2001;276:3498–3507. doi: 10.1074/jbc.M004800200. [DOI] [PubMed] [Google Scholar]
- St John JA, Clarris HJ, McKeown S, Royal S, Key B. Sorting and convergence of primary olfactory axons are independent of the olfactory bulb. J Comp Neurol. 2003;464:131–140. doi: 10.1002/cne.10777. [DOI] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Inokuchi K, Aoki M, Suto F, Tsuboi A, Matsuda I, Suzuki M, Aiba A, Serizawa S, Yoshihara Y, Fujisawa H, Sakano H. Sequential arrival and graded secretion of Sema3F by olfactory neuron axons specify map topography at the bulb. Cell. 2010;6:1056–1067. doi: 10.1016/j.cell.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Nagao H, Takahashi YK, Yamaguchi M, Mitsui S, Yagi, Mori K, Shimizu T. Distorted odor maps in the olfactory bulb of semaphorin 3A-deficient mice. J Neurosci. 2003;23:1390–1397. doi: 10.1523/JNEUROSCI.23-04-01390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Von Dannecker LE, Mercadante AF, Malnic B. Ric-8B promotes functional expression of odorant receptors. Proc Natl Acad Sci U S A. 2006;103:9310–9314. doi: 10.1073/pnas.0600697103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nuñez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Wang F, Nemes A, Mendelsohn M, Axel R. ORs govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Watt WC, Storm DR. Odorants stimulate the ERK/mitogen-activated protein kinase pathway and activate cAMP-response element-mediated transcription in olfactory sensory neurons. J Biol Chem. 2001;276:207–2052. doi: 10.1074/jbc.M006703200. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhao AZ, Chan GC, Baker LP, Impey S, Beavo JA, Storm DR. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in neurons: a mechanism for attenuation of olfactory signals. Neuron. 1998;21:495–504. doi: 10.1016/s0896-6273(00)80561-9. [DOI] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;3:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Zhou D, Dinter A, Gutiérrez Gallego R, Kamerling JP, Vliegenthart JF, Berger EG, Hennet T. A beta-1,3-N-acetylglucosaminyltransferase with poly-N- acetyllactosamine synthase activity is structurally related to beta-1,3-galactosyltransferases. Proc Natl Acad Sci USA. 1999;96:406–411. doi: 10.1073/pnas.96.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou DJ, Chesler AT, Le Pichon CE, Kuznetsov A, Pei X, Hwang EL, Firestein S. Absence of adenylyl cyclase 3 perturbs peripheral olfactory projections in mice. J Neurosci. 2007;27:6675–6683. doi: 10.1523/JNEUROSCI.0699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]