Synaptic neurotransmitter release is driven by Ca2+ influx through active zone voltage-gated calcium channels (VGCCs)1,2. Control of active zone VGCC abundance and function remains poorly understood. We show that a trafficking step likely sets synaptic VGCC levels as overexpression of the pore-forming α1A fails to change synaptic VGCC abundance or function. α2δs are a family of GPI-anchored VGCC-associated subunits3, which in addition to being the target of the potent neuropathic analgesics gabapentin and pregabalin (α2δ-1, α2δ-2)4,5, were also identified in a forward genetic screen for pain genes (α2δ-3)6. We show that these proteins confer powerful modulation of presynaptic function through two distinct molecular mechanisms. α2δ subunits set synaptic VGCC abundance, as predicted from their chaperone-like function when expressed in non-neuronal cells3,7. Secondarily, α2δs configure synaptic VGCCs to drive exocytosis through an extracellular metal ion-dependent adhesion site (MIDAS), a conserved set of amino acids within α2δ’s predicted von Willebrand A (VWA) domain. Expression of α2δ with an intact MIDAS motif leads to an 80% increase in release probability, while simultaneously protecting exocytosis from blockade by an intracellular Ca2+ chelator. α2δs harboring MIDAS site mutations still drive synaptic accumulation of VGCCs however, they no longer change release probability or sensitivity to intracellular Ca2+ chelators. Our data reveal dual functionality of these clinically important VGCC subunits, allowing synapses to make more efficient use of Ca2+ entry to drive neurotransmitter release.
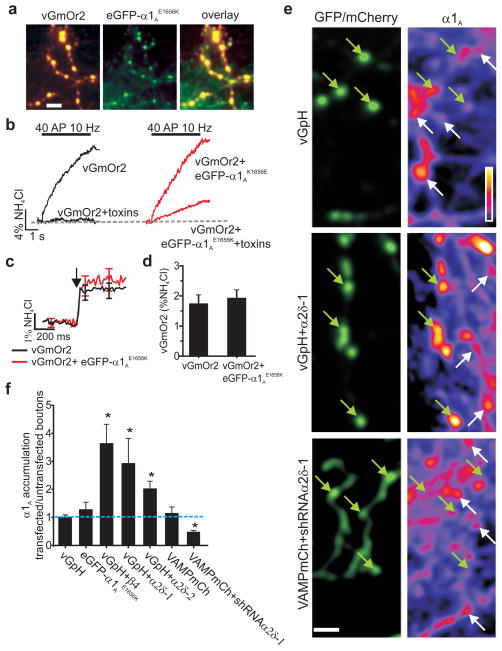
VGCCs are composed of pore-forming α1 and auxiliary β and α2δ subunits8,9. In central synapses neurotransmitter release is generally driven by P/Q-type (α1A) and/or N-type (α1B)10 VGCCs. Based on the failure of α1A overexpression to increase synaptic strength, it had been suggested that VGCCs functionally coupled to presynaptic release machinery is limited by a fixed number of available “slots” where channels can insert into the synaptic membrane11. We examined the existence of such a bottleneck by expressing eGFP-α1A12 together with a reporter of presynaptic exocytosis (vGlut1 with a luminal tag mOrange2, vGmOr2) and carried out retrospective immunocytochemistry to probe the abundance of α1A in transfected compared to control neurons. eGFP-α1A correctly trafficked to nerve terminals as it co-localized well with the vesicle-targeted reporter (Fig. 1a). In order to ensure eGFP-α1A functionally integrated with endogenous channels to drive neurotransmitter release we introduced a point mutation (E1656K), rendering this channel insensitive to the antagonist ω-agatoxin IVA13. Under control conditions a combination of ω-agatoxin IVA and the α1B inhibitor ω-conotoxin GVIA completely blocked vGmOr2 responses to action potential (AP) firing, however in the presence of eGFP-α1AE1656K a significant fraction of the response remains (Fig 1b). Measurements of single AP responses showed that expression of this exogenous α1A did not alter exocytosis efficiency compared to controls (Fig. 1c–d), consistent with the “slot” hypothesis11. However, retrospective immunocytochemistry using an anti-α1A antibody whose specificity was verified using shRNA-mediated α1A knockdown (Fig. S1) showed that transfected and control nerve terminals had similar immunoreactivity (Fig. 1e–f) while at the cell soma it had doubled (Fig S2). These results demonstrate that synaptic VGCC abundance is likely limited by trafficking from the cell soma and failure to increase synaptic performance does not result from a fixed number of active zone insertion sites. α2δ and β auxiliary VGCC subunits are both strong candidates for modulating such trafficking as they control functional expression of α1 subunits when co-expressed in non-neuronal cells14,15. We coexpressed individual auxiliary subunits with the reporter vGlut1-pHluorin (vGpH) in neurons and carried out measurements of exocytosis and immunocytochemistry as described above. These experiments demonstrated that expression of either α2δ-1 or β4 subunits led to a significant increase (~3-fold, p>0.05) in synaptic abundance of α1A (Fig. 1e–f). Similar results were obtained with overexpression of α2δ-2 (Fig. 1f). Furthermore, introduction of shRNA targeting α2δ-1 caused depletion of α1A at nerve terminals (Fig. 1e–f, Figure S3), while leaving the somatic concentration unaltered (data not shown). These results demonstrate that synaptic α1A levels are titrated by expression of auxiliary VGCC subunits.
Figure 1. Increased expression of α2δ and β subunits leads to increased P/Q Ca2+ channel accumulation at synapses.
a, Co-expression of vGmOr2 (left), eGFP–α1AE1656K (middle), overlay (right). b, Exocytic response for vGmOr2 alone (left; n=9) and vGmOr2 coexpressed with eGFP-α1AE1656K (right; n=6) reveals a significant toxin-resistant response (20±7.1% of pre-toxin). c, Average traces of single AP vGmOr2 responses ±eGFP-α1AE1656K (n>8). Arrow indicates stimulation with 1 AP. d, Average ΔF response for data in (c) (control 1.76±0.28; n=14; + eGFP–α1AE1656K 1.91±0.35; n=9; (p>0.1). e, Presynaptic α1A abundance. Green arrows indicate transfected boutons, white arrows indicate non-transfected immunopositive α1A channel puncta. f, ratio of α1A immunofluorescence intensity in transfected puncta compared to untransfected puncta (n≥8 cells for all conditions). All stated values are mean±SEM. Inset linear pseudo color LUT scale. Scale bar for all images = 4 μm.
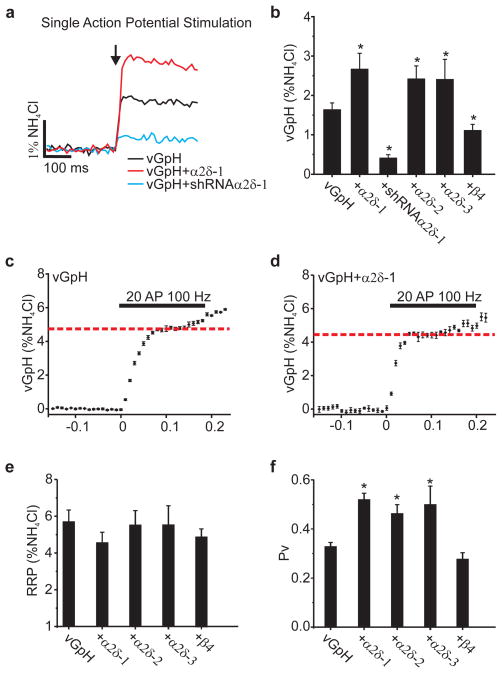
To examine whether changes in VGCC accumulation alters synaptic release properties, we measured single AP-stimulated exocytosis in neurons with altered VGCC levels. Overexpression and depletion of α2δ–1 led to much larger and much smaller single AP responses respectively compared to control (Fig. 2a). Similar increases in exocytosis were observed following expression of all three isoforms of α2δ tested (Fig. 2b). In contrast, expression of β4 did not change exocytosis, despite the synaptic accumulation of α1A (Fig. 2b). Quantitative estimates of α2δ-1 synaptic expression levels suggest a stoichiometric relationship between α2δ and α1A (Fig. S4). These results demonstrate that increasing VGCC abundance does not necessarily lead to increased function, and identify α2δ expression as a key rate-limiting parameter in determining presynaptic function.
Figure 2. Exocytosis is increased in neurons expressing α2δ.
a, Representative single AP vGpH responses (10 trial average, ≈25 boutons). Arrow indicates stimulation with 1 AP. b, Summary of single AP response (%NH4Cl): vGpH= 1.65±0.17; vGpH+α2δ-1= 2.71±0.40; vGpH+α2δ-1shRNA= 0.4±0.08; vGpH+α2δ-2= 2.44±0.36; vGpH+α2δ-3, 2.42±0.51; vGpH+β4= 1.12±0.15, *p<0.01, n≥7. c–d, vGpH-based RRP measurements, dotted line identifies RRP. e, Summary of RRP size (%NH4Cl): vGpH= 5.67±0.64, vGpH+α2δ-1= 4.6±0.58, vGpH+α2δ-2= 5.57±0.78, vGpH+α2δ-3= 5.58±1.05, vGpH+β4= 4.92±0.45; (p>0.1; n≥7). f, Pv measurements (*p <0.01): vGpH= 0.33±0.02, vGpH+α2δ-1 0.52±0.03, vGpH+α2δ-2= 0.46±0.04, vGpH+α2δ-3= 0.50±0.08, vGpH+β4= 0.28±0.03; n≥7. All stated values are mean±SEM.
Measurements of presynaptic strength can be parsed into two biophysical parameters: the number of vesicles available for rapid release upon stimulation, known as the readily-releasable pool (RRP) and the probability that a vesicle in the RRP will undergo fusion with a single AP stimulus (Pv)16. We recently developed a rapid depletion protocol to measure RRP sizes using optical methods17. High frequency stimulation leads to rapid exhaustion of exocytosis in the first 8–15 APs. The fraction of the total pool corresponding to this rapid depletion phase is taken as the RRP17 (Fig. 2c–d). The RRP size in neurons overexpressing α2δ was no different than controls (Fig. 2e). Thus α2δ overexpression changes Pv (Fig. 2f).
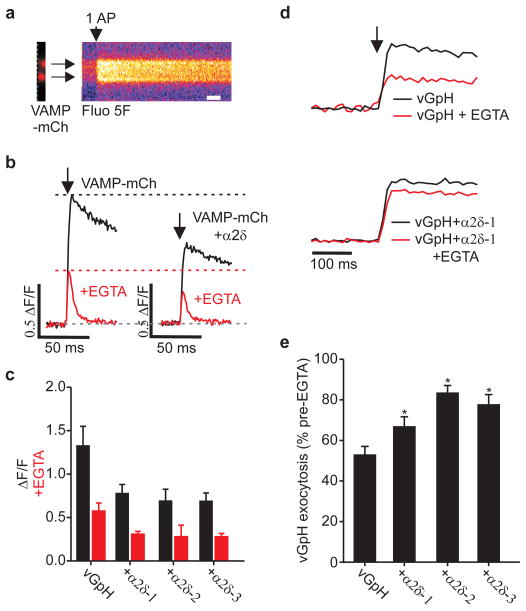
α2δ-driven increases in Pv might arise from increasing total Ca2+ influx (from changes in VGCC gating and/or surface abundance) and/or changing VGCC proximity to release sites 18. To examine this question, we measured intracellular [Ca2+] at synaptic boutons in response to single APs using the fast fluorescent indicator of Ca2+, Fluo5F-AM, visualized by expression of VAMP-mCherry (VAMPmCh; Fig. 3a left panel) with or without α2δ. Single APs resulted in robust Ca2+ signals (Fig. 3a right panel) that peaked within 1 ms. but were reduced by ~40% in synapses overexpressing α2δ isoforms compared to controls (Fig. 3b–c). We verified that the peak signal was not dominated by Ca2+ clearance mechanisms (e.g. endogenous buffers or extrusion) in experiments where the signal decay was set by high concentrations of intracellular EGTA. This treatment led to a ~50% decrease in peak signal and a decay time of ~10 ms in controls as well as α2δ overexpressing synapses. Measurements of Ca2+ signals using a genetically encoded Ca2+ indicator GCaMP319, co-expressed with or without α2δ-1 gave very similar results (Fig. S5). This reduction in Ca2+ was surprising given that α2δ overexpression increases the total number of synaptic VGCCs (surface and intracellular) suggesting that α2δ might additionally control Ca2+ influx. Measurements of somatic AP waveforms revealed that α2δ expression led to a ~30% decrease in AP duration (Fig. S6), providing a possible explanation for the drop in synaptic Ca2+ entry. Given that exocytosis at nerve terminals is steeply dependent on Ca2+ influx20, the proximity of sites of Ca2+ influx to sites of exocytosis can, in principle, have a powerful influence over neurotransmitter release2. The increase in Pv with a commensurate decrease in Ca2+ influx strongly suggests that overexpression of α2δ subunits results in a tighter spatial relationship between sites of Ca2+ entry and exocytosis. We tested this hypothesis by measuring the sensitivity of exocytosis to the presence of a Ca2+ chelator (EGTA-AM). The chelator’s efficiency in reducing exocytosis depends on its ability to buffer Ca2+ before it binds the calcium-sensor for exocytosis following VGCC opening, a process determined by chelator concentration and Ca2+ binding kinetics21. We chose incubation conditions for EGTA-AM that led to a ~50% reduction in single AP exocytosis responses, compared to the pre-EGTA condition in control neurons (Fig. 3d). In neurons transfected with α2δ however, EGTA application led to much smaller decreases in exocytosis (Fig. 3e) indicating that in conditions of α2δ overexpression Ca2+ must bind the calcium sensor more rapidly than in control conditions. Therefore the Ca2+ sensor controlling exocytosis is experiencing higher levels of Ca2+ influx even though overall synaptic Ca2+ transients are reduced. Single AP-driven Ca2+ influx remained equally sensitive to ω-conotoxin GVIA following α2δ overexpression indicating this condition did not lead to a significant shift in VGCC type at nerve terminals (Fig. S7).
Figure 3. α2δ leads to reduced Ca2+ influx and tighter coupling of calcium channels to exocytosis.
a, Ca2+ influx stimulated by 1 AP (vertical arrow) from boutons identified by VAMP-mCh (left), and visualized by Fluo5F (kymograph, right). Scale bar = 20 ms. b, Single traces of Ca2+ influx. c, Peak Fluo5F signal vs control (*p<0.05): VAMP-mCh 1.3±0.2; +α2δ-1 0.78±0.09; +α2δ-2 0.69±0.13; +α2δ-3 0.69±0.13 (n>8). ΔF/F of Ca2+ transient post EGTA (red): VAMP-mCh 0.58±0.09; +α2δ-1 0.32±0.03; +α2δ-2 0.28±0.13; +α2δ-3 0.29±0.03. d, Representative traces vGpH±α2δ-2 stimulated by 1 AP (vertical arrow). Traces normalized to response pre-EGTA treatment. e, Resistance to EGTA treatment (% exocytosis pretreatment): vGpH 53±4; +α2δ-1 68±6; +α2δ-2 83±4; +α2δ-3 77±5 *p<0.05, n≥7 for all conditions. All stated values are mean±SEM.
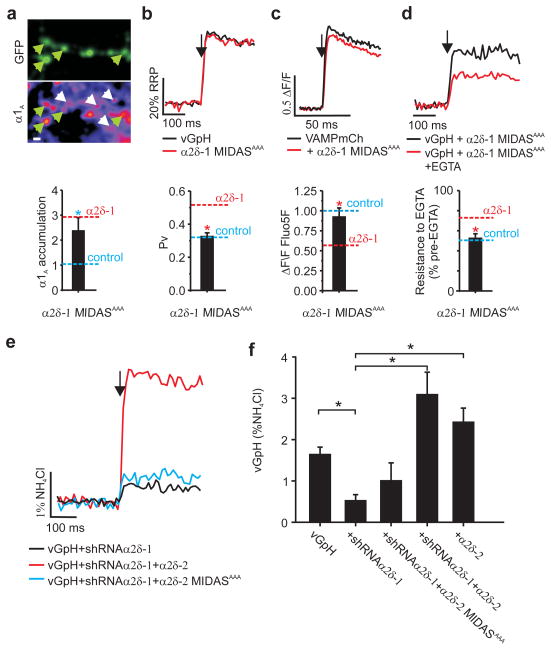
The finding that α2δ subunits form GPI-anchored proteins3 implies that their ability to change VGCC-exocytosis coupling is likely conveyed through an extracellular interaction. One possible candidate for exerting such influence lies in the highly-conserved VWA domain within α2δ22. A characteristic feature of this domain is its ability to interact with adhesion proteins via the MIDAS motif by sharing coordination of a divalent cation23–25. To examine the role of α2δ’s MIDAS motif we mutated three of five conserved key metal coordinating residues within the MIDAS motif to alanine22 and expressed the mutant protein (α2δ-1 MIDASAAA) in neurons together with functional reporters. α2δ-1 MIDASAAA was similar to wild type α2δ-1 in its ability to drive α1A accumulation at synapses (Fig. 4a). However, measurements of exocytosis from α2δ-1 MIDAS-mutants showed no enhancement of Pv (Fig. 4b), normal Ca2+ influx (Fig. 4c) and normal sensitivity to EGTA block of exocytosis (Fig. 4d). Furthermore α2δ-2 MIDASAAA, unlike intact α2δ-2, was unable to rescue the decrease in exocytosis resulting from shRNA-mediated α2δ-1 depletion (Fig. 4e,f). These data are consistent with the ability of this mutation to block enhancement of Ca2+ currents when expressed in heterologous systems22 (Fig. S8), but show that they do not prevent endogenous α2δ from functioning. Taken together, these results demonstrate that α2δ exerts its powerful control of synaptic VGCC function through at least two separate molecular mechanisms: a forward trafficking-step from cell body to presynaptic terminal that is independent of MIDAS motif integrity; and a local MIDAS-dependent interaction critical for proper VGCC function and coupling to exocytosis.
Figure 4. α2δ MIDAS motif is essential for coupling Ca2+ channels to exocytosis.
a,Top: Presynaptic α1A abundance. Green arrows indicate transfected boutons, white arrows indicate non-transfected immunopositive α1A channel puncta.. Scale bar = 2 μm. Bottom: Ratio of α1A staining in synaptic boutons. Dashed lines represent ratios taken from Fig. 1f as indicated. b, Top: Representative vGpH responses to 1 AP (arrow) as a fraction of the measured RRP Bottom: vGpH and α2δ-1 MIDASAAA (Pv=0.33±0.017) compared to data from Fig. 2f as indicated. c, Top: Representative responses to 1 AP-driven Ca2+ influx (Fluo5F 3F/F). Bottom: Peak 1 AP Fluo 5F ΔF/F values in cells co-transfected with VAMPmCh (n=11) and α2δ-1 MIDASAAA (0.88±0.1; n=6) normalized to VAMPmCh alone (*p<0.05). d,Top: Representative vGpH response to 1 AP in a neuron co-expressing α2δ-1 MIDASAAA as indicated. Bottom: Resistance to EGTA block (% block = 51±5, p=0.63) dashed lines compare data from Fig. 3e as indicated. e, Representative vGpH responses to 1 AP. f, 1 AP response (%NH4Cl): vGpH=1.65±0.17; vGpH+α2δ-1shRNA=0.4±0.08, v G p H +α2δ-1shRNA+α2δ-2=3.10±.53; vGpH+α2δ-1shRNA+α2δ-2 MIDASAAA=1.02±.41; vGpH+α2δ-2=2.44±0.36. Values are mean±SEM, *p<0.01, n≥7, (*p<0.05).
α2δ-1 and α2δ-2 are the targets of the analgesic gabapentin whose binding site lies in close proximity to the MIDAS site26. We found no significant impact of gabapentin application (30 min and >72 h) on Pv in either control or cells overexpressing α2δ-1 or -2 (results not shown) similar to previous findings in hippocampal neurons27. We also examined gabapentin’s impact on VGCC trafficking to nerve terminals by incubating neurons with gabapentin from the time of transfection with eGFP-α1A. Analysis of the presynaptic abundance of this probe after 7 days showed that even though gabapentin appears to impact α2δ-2 trafficking in non-neuronal cells28 it appears unable to impact VGCC trafficking or function in cultured hippocampal neurons (Fig. S9).
Our results reveal that α2δ subunits are potent modulators of synaptic transmission. They function through at least two distinct molecular mechanisms: a trafficking step from the cell soma and a local step at the presynaptic terminal allowing synapses to exhibit increased exocytosis with decreased Ca2+ influx. We speculate that increased presynaptic abundance of VGCCs results in increased abundance of active zone-VGCCs, and hence a higher density in the vicinity of release sites. This active zone accumulation depends on α2δ’s VWA domain, presumably through interactions with extracellular active zone-specific proteins. The identity of the interaction partner(s) at present remains unknown however it is tempting to speculate that α2δs might recognize cues established in the correct juxtaposition of pre and postsynaptic membranes, consistent with synaptic defects observed in Drosophila α2δ-3 mutants29. Additionally this model requires that α2δ interact with a partner resulting in AP shortening to limit total calcium entry. As the MIDAS motif is well conserved throughout the α2δ family, identification of α2δ interaction partners in specific neuronal circuits could provide novel targets in the development of future therapeutics, given the potency that these subunits show in controlling synapse function.
Methods
Hippocampal CA3–CA1 regions were dissected from 1- to 3-day-old Sprague Dawley rats, dissociated, plated and transfected as previously described30. Live-cell images were acquired with an Andor iXon+ (model #DU-897E-BV) camera. A solid-state diode pumped 488 nm (vGpH and MgG imaging) or 532 nm (vGmOr2 imaging) laser was shuttered using acousto-optic modulation. For vGpH and GCaMP3 imaging data were acquired at 100 Hz by integrating for 9.74 ms in frame transfer mode and restricting imaging to a sub-area of the CCD chip. Fluo5F imaging data was acquired at 1 kHz (0.974 ms integration time). To estimate 1 AP ΔFs of vGpH, we took the difference between the average 20 frames before and after the stimulus. The rise in vGpH fluorescence in response to a single AP always took two frames when acquiring at 100 Hz time resolution. For display purposes the images in Fig. 1E were given a 2 pixel gaussian average filter. Pv and single AP calcium signals were measured with 4 mM extracellular CaCl2. All stated values are mean±SEM, statistical significance for groups of 3 or more were determined by one-way ANOVA with Tukey’s HSD for Post-Hoc analysis. Otherwise Student’s t-test was used for determining statistics.
Supplementary Material
Acknowledgments
We thank Wendy Pratt for performing mutagenesis on α2δ-1 and Marianna D’Arco for advice on immunocytochemistry. We thank Sunghyun Kim for designing the vGmOr2 and VAMPmCh plasmids, Pablo Ariel for helpful discussion and analysis of exocytosis data, Ricky Kwan and Yogesh Gera for technical assistance in cell culture, Gerald Obermair (Division of Physiology, Innsbruck Medical University) for providing the wild type eGFP-α1A plasmid and Loren Looger (Janelia Farms, Howard Hughes Medical Institute) for providing the GCaMP3 plasmid and helpful communication.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature
Author Contributions: MHB performed opto-physiological experiments, BL and WM performed electrophysiological experiments, AD, BL & WM designed electrophysiological experiments, MHB, AD & TAR designed all other experiments, MHB, AD & TAR wrote the manuscript.
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare that they have no competing financial interests.
References
- 1.Llinas R, Steinberg IZ, Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976;73:2918–22. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–72. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Davies A, et al. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci U S A. 2007;107:1654–9. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field MJ, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103:17537–42. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Offord J, Oxender DL, Su TZ. Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. Biochem J. 1999;342 (Pt 2):313–20. [PMC free article] [PubMed] [Google Scholar]
- 6.Neely GG, et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–38. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao B, et al. Functional properties of a new voltage-dependent calcium channel alpha(2)delta auxiliary subunit gene (CACNA2D2) J Biol Chem. 2000;275:12237–42. doi: 10.1074/jbc.275.16.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 9.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 11.Cao YQ, et al. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron. 2004;43:387–400. doi: 10.1016/j.neuron.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Watschinger K, et al. Functional properties and modulation of extracellular epitope-tagged Ca(V)2.1 voltage-gated calcium channels. Channels (Austin) 2008;2 doi: 10.4161/chan.2.6.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winterfield JR, Swartz KJ. A hot spot for the interaction of gating modifier toxins with voltage-dependent ion channels. J Gen Physiol. 2000;116:637–44. doi: 10.1085/jgp.116.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–44. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Pragnell M, et al. Calcium channel beta-subunit binds to a conserved motif in the I–II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 16.Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25:206–12. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- 17.Ariel P, Ryan TA. Optical mapping of release properties in synapses. Front Neural Circuits. 2010;4 doi: 10.3389/fncir.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodge FA, Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–32. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol. 2008;586:3043–54. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canti C, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11230–5. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer TA. Complement and the multifaceted functions of VWA and integrin I domains. Structure. 2006;14:1611–6. doi: 10.1016/j.str.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacy DB, Wigelsworth DJ, Scobie HM, Young JA, Collier RJ. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2004;101:6367–72. doi: 10.1073/pnas.0401506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 2002;13:3369–87. doi: 10.1091/mbc.E02-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies A, et al. The calcium channel alpha2delta-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: implications for localization and function. J Neurosci. 2006;26:8748–57. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JT, Randall A. Gabapentin fails to alter P/Q-type Ca2+ channel-mediated synaptic transmission in the hippocampus in vitro. Synapse. 2005;55:262–9. doi: 10.1002/syn.20115. [DOI] [PubMed] [Google Scholar]
- 28.Tran-Van-Minh A, Dolphin AC. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J Neurosci. 2010;30:12856–67. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12:1415–23. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SH, Ryan TA. CDK5 serves as a major control point in neurotransmitter release. Neuron. 2010;67:797–809. doi: 10.1016/j.neuron.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.