Synopsis
This review describes the current literature on the interaction between insulin-like growth factors, endocrine hormones, and branched chain amino acids on muscle physiology in both healthy young individuals and during select pathological conditions. An emphasis is placed on the mechanism by which physical and hormonal signals are transduced at the cellular level to either grow or atrophy skeletal muscle. The key role of the mammalian target of rapamycin and its ability to respond to both hypertrophic and atrophic signals lays the ground work for understanding how a combination of physical, nutritional, and pharmacological therapies may be used in tandem to prevent and/or ameliorate reductions in muscle mass.
IGF and mTOR in skeletal muscle health: the whys and the wherefores
Insulin and insulin-like growth factors (IGFs)-I and –II are evolutionarily conserved peptides that are expressed in organisms as evolutionarily diverse as nematodes and humans (1). In the adult, these peptides orchestrate metabolic responses to nutrients, hormones, and stress (2).
IGFs and their receptors are also critical for the proliferation of muscle stem cells and recovery from muscle injury (3,4). Skeletal muscle is especially dependent upon IGF signaling during development as mice null for the IGF-I receptor (IGF-IR) exhibit severe muscle cell hypoplasia (5,6). Specific deletion of the IGF-IR in skeletal muscle also reduces the number of myonuclei per myofiber and myofiber cross-sectional area suggesting that IGF-IR signaling mediates both the addition of nuclei to fibers and the maintenance of myofiber size (7). In contrast, mice null for IGF-I expression in peripheral tissues (including skeletal muscle) exhibit body weights indistinguishable from control littermates when endocrine IGF-I is supplied from a hepatic transgene, signifying that a combination of autocrine, paracrine, and endocrine derived IGFs contribute to the maintenance of muscle mass (7–10).
A variety of pathophysiological conditions adversely impact muscle mass and function including: glucocorticoid excess, sepsis, muscular injury and dystrophies, inactivity, alcohol, and aging. Almost all of these states have on occasion been linked to a reduction in either circulating IGF-I levels or IGF signaling, although a definitive cause and effect relationship is often lacking (11–20). It is therefore of interest from a human health perspective to understand how IGFs affect muscle at the cellular, molecular and organismal level to determine how and when the clinical use of IGFs or therapies that simulate their signaling pathways may be beneficial 21–25.
Numerous laboratories have determined that nutrients, growth factors, hormones, and muscle activity all generate cellular signals that have the capacity to maintain and/or grow muscle mass. Although signaling through insulin receptor substrate (IRS)-1 and -2 and Akt are important, a key focal point at which all of these signals meet and integrate in skeletal muscle is the protein kinase known as the mammalian target of rapamycin (mTOR) 13,26,27. Because mTOR is critical for muscle function and its tissue-specific deletion results in muscular dystrophy it seems fortuitous at this point in time to review the role played by IGFs and mTOR in muscle during health and disease 28,29. This review will broadly evaluate the role of IGFs and mTOR in the maintenance of muscle mass and function and how this relationship is altered in pathophysiological conditions affecting skeletal muscle.
Muscle IGFs and nutrition: substrates and signaling
It has long been appreciated that optimal nutrition, including sufficient caloric and protein intake, is necessary for muscle growth during development, postnatal growth, and puberty 30–32. The growth hormone (GH)- IGF-I axis and muscle per se are influenced by amino acids, fatty acids, and micronutrients which provide a milieu in which optimal muscle growth may occur 33–37. Essential amino acids (EAAs) account for nearly all of the ability of a well-balanced meal to stimulate muscle protein synthesis 38,39. Furthermore, the branched-chain amino acids, (BCAA, e.g. leucine, isoleucine, and valine) a subset of the EAAs, mediate this response 40. Combined with the ability of amino acids to stimulate both insulin and GH release these nutrients provide a strong and potentially persistent anabolic signal for skeletal muscle 41
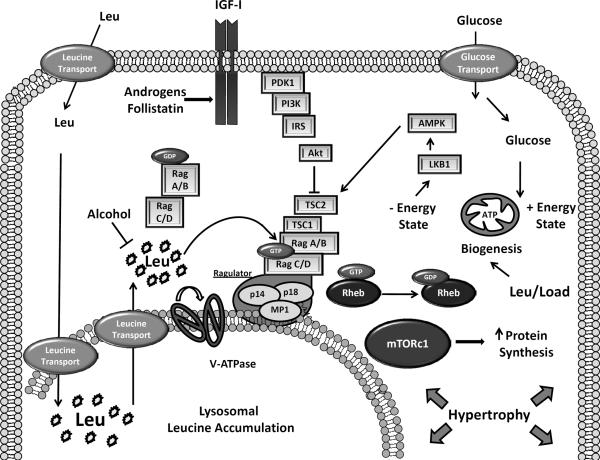
BCAA not only serve as substrates for protein synthesis but also as signalling molecules that act independently of growth factor receptors, phosphoinositide 3-kinase (PI3K), and Akt to activate mTOR by facilitating its direct binding to the Ras-homolog enriched in brain (Rheb) 42–45. EAA uptake is required for this effect because AA uptake inhibitors such as D-phenylalanine block mTOR activation by EAA. The siRNA-mediated knockdown of the solute carrier SLC7A5 / SLC3A2 (an EAA and glutamine co-transporter) also curtails mTOR signaling to its substrates ribosomal protein S6 kinase-1 (S6K1) and eukaryotic initiation factor 4E binding protein (4E-BP1) 46. Optimal mTOR signaling therefore occurs when the intracellular level of glutamine is sufficient for the co-transport of glutamine out of the cell followed by the ensuing movement of leucine into the cell and the activation of mTOR 47. Leucine transport and the stimulation of mTOR are also enhanced by IGF-I providing additive effects on the activation of mTOR in muscle cells (See Figure 1) 48.
Figure 1.
Skeletal muscle fibers are exposed to a wide range of physical, nutritional, and endocrine signals. When provided on a consistent basis, the amino acid leucine, the peptide hormone IGF-I as well as physical stretch/load stimulate muscle hypertrophy. Amino acid transporters move leucine into the cell from extracellular sources and this process is enhanced by IGF-I. Leucine is transported into the lysosome where it is also generated from the breakdown of cellular proteins by autophagy. The vacuolar ATPase in concert with three “ragulator” proteins allow for release of leucine from the lysosome and recruitment of GTP-loaded Rag heterodimers and mTOR. Tethered to the lysosomal surface by the regulator, mTOR can interact with Rheb and Raptor. The active raptor/mTOR complex phosphorylates mTOR substrates, such as the translation initiation factor 4E-BP1, to stimulate protein synthesis and muscle hypertrophy. IGF-I, by stimulating PDK1, Akt, and TSC2 phosphorylation relieves the negative input of negative energy charge imposed on TSC2 and mTOR by AMPK. Overall energy balance is improved with glucose, leucine and exercise all of which increase mitochondrial biogenesis and the capacity to form ATP.
Although the exact nature of the intracellular BCAA sensor in mammalian cells remains elusive a number of proteins have been identified that mediate signaling between amino acids and Rheb. The key step in this process appears to be the movement of mTOR from a cytosolic location to a late endosomal or lysosomal surface. Small GTPases referred to as the “Rag proteins” (Rag A–D) form heterodimers that when activated by amino acids bring mTOR and Rheb together on the lysosome 49,50. Three targeting proteins — p14, p21, and MP1—known collectively as the “Ragulator” tether the Rags, mTOR, and Rheb to the lysosome 51. Colocalization of Rags and Rheb may allow mTOR to respond to spikes in the intracellular concentration of amino acids as well as amino acids released as a consequence of lysosomal protein breakdown. This positions mTOR to commence signaling and activate translation initiation in response to fresh amino acids after a meal. Amino acids signal using an “inside-out 6 mechanism” by which they must first accumulate within the lysosome followed by a rotation of the vacuolar ATPase which allows the amino acids to be released to the surface and activate the Rag proteins and mTOR (See Figure) 52. The importance of intra-lysosomal accumulation of amino acids in mTOR activation was revealed by the fact that overexpression of a proton-assisted amino acid transporter (PAT-1/Slc36a1) in lysosomes prevented mTOR activation due to the ability of PAT-1to extrude amino acids from the lysosomal lumen 52,53. But, currently the importance of these transporters in mediating catabolism-induced defects in mTOR signaling remains unclear. Regulated translocation of PAT-1 or other transporters between the plasma membrane and the lysosome may be an innate mechanism by which muscle cells regulate mTOR signaling 54,55.
Our current understanding is that mTOR regulates both protein synthesis and autophagic protein degradation in muscle 56. Nowhere is this dual role more apparent than in a premature aging model (the Klotho null mouse) where mice show atrophy of the masseter and tongue muscles which ultimately limits food and protein intake. These mice exhibit normal signaling upstream of mTOR in the masseter but the phosphorylation of mTOR and its downstream substrates is compromised 57. The lack of exogenous amino acids leads to a vicious cycle of continued autophagy and muscle atrophy. Given the known interaction between Klotho and IGF-I it would be interesting to test whether the mTOR defect could be rescued by overexpression or exogenous administration of IGF-I 58. It is likely that even though mTOR is situated on the lysosome it does not always receive a sufficient amino acid signal to prevent autophagic cell death during prolonged periods of nutrient deprivation 59. In this case, loss of mTOR activity on the lysosome surface may result in dephosphorylation of a transcription factor that binds to the E-box (TFEB) and TFEB's translocation to the nucleus where it up regulates 7 the expression of genes involved in vacuolar biogenesis thereby perpetuating the atrophy process 60.
Rag proteins may not be the only small GTPases to regulate the intracellular localization of mTOR. Recently, Saci et al showed that Rac1, a member of the Rho family, is necessary for the activation of mTOR complex (mTORc)-1 and -2 by growth factors. Rac1 binds directly to mTOR via a c-terminal RKR motif and binding is lost when these residues were mutated to alanine 61. Growth factors and serum stimulate the Rac1-dependent translocation of mTOR from a peri-nuclear localization to the plasma membrane 61. Conversely, Rac1 deletion in mouse embryonic fibroblasts decreases cell size and inhibits the phosphorylation of mTOR substrates. Additional studies suggest that BCAA also stimulate mTOR by decreasing the negative input from AMP kinase 62. During nutrient stress AMPK phosphorylates TSC2 on S and T to increase the GTPase activating protein (GAP) activity of TSC2 toward Rheb, thereby inhibiting mTOR activity 63. AMPK also phosphorylates Raptor on S792 to inhibit the interaction of mTOR with its substrates 64. Lastly, nutrient stress also induces a p38 kinase/p38-regulated kinase (PRAK) pathway that phosphorylates Rheb on S130 to inhibit Rheb nucleotide binding and thus mTOR activity 65. Thus, leucine and other BCAA, by inhibiting AMPK or PRAK, may facilitate the normal activation of Rheb and mTOR to stimulate protein accretion in skeletal muscle.
In contrast to nutrients, growth factors such as IGF-I stimulate mTOR via an IGF-IR, PI3K, Akt pathway that disrupts the TSC1 and TSC2 complex. This protein-protein complex normally represses mTOR signaling but when TSC2 is phosphorylated on multiple serine residues (S939 and S981), TSC2 binds to 14-3-3 proteins that sequester TSC2 away from Rheb. 8
TSC2 is a GAP for Rheb that converts Rheb from an active GTP-bound form to an inactive GDP-bound state. Therefore, the sequestration of TSC2 with 14-3-3 increases the relative amount of GTP bound Rheb and this activates mTOR in muscle cells 66. The importance of TSC1 and TSC2 in the regulation of mTOR and protein synthesis is highlighted by the observation that over expression of TSC1 in skeletal muscle leads to a more stable TSC1/2 complex, inhibition of mTOR activity, and as a result severe muscle wasting 67. Likewise, mutation of the Akt phosphorylation sites on TSC2 to alanine prevents IGF-I from activating Rheb and inhibits mTOR activity 66,68.
The above studies emphasize that amino acids and IGF-I stimulate mTOR via separate but parallel pathways 69. These pathways converge by stimulating both the activity of Rheb and its ability to interact with mTOR. How the binding of Rheb to mTOR activates the mTOR kinase remains controversial. Rheb stimulates phospholipase D1 (PLD1) activity and the synthesis of phosphatidic acid (PA), a known activator of mTOR 70. Conversely, siRNAmediated knockdown of PLD1 prevents Rheb-induced phosphorylation of mTOR substrates.
This picture is complicated by the fact that PA derived from membrane lipids may be different than PA derived from de novo glycerolipid synthesis as the later form of PA actually inhibits mTORc2 activity 71. Likewise, although PA binds directly to mTOR it is not sufficient on its own to stimulate mTOR kinase activity in vitro suggesting that PA needs to interact with other signaling molecules and partners and thus activates mTOR indirectly 72. One possibility is that PA facilitates the recruitment of mTOR substrates 72,73. This would fit with the known ability of Rheb to enhance the binding of mTOR substrates, such as the translation initiation factor 4E-BP1 independent of the intrinsic mTOR kinase activity 74. Recent work would also suggest that PLD9 derived PA stabilizes both the mTORc1 and mTORc2 complexes leading to greater phosphorylation of additional substrates such as Akt on S473 75.
The parallel activation of mTOR via an amino acid induced-interaction of Rheb and mTOR and an insulin/IGF-I- induced increase in GTP bound Rheb suggests that the two pathways allow for the fine tuning of mTOR activity and translation initiation. This is especially relevant to insulin responsive tissues such as the liver that is exposed to relatively high concentrations of amino acids after a protein rich meal which then triggers a rapid release of insulin into the portal circulation. Insulin or amino acids alone only minimally stimulate global hepatic protein synthesis, but in combination they robustly increase mTOR activity and protein synthesis 76. Teleologically it makes sense for muscle to also respond to both amino acid and growth factor stimulation. Indeed, the combination of insulin and amino acids are necessary for the maximal stimulation of muscle protein synthesis observed in healthy adults and together they enhance the anabolic effect of exercise 77,78. Insulin and IGFs in addition to stimulating pathways upstream of mTOR also stimulate amino acid uptake and may therefore replenish intracellular levels of glutamine and leucine when these amino acids become limiting for the activation of mTOR 48,79. In the presence of adequate amino acid availability insulin prevents muscle protein breakdown whereas IGF-I enhances amino acid transport and the amino acid induced increase in muscle protein synthesis 80,81.
Amino acids, growth factors and exercise may also function synergistically to induce muscle hypertrophy by up regulating the expression of JunB 82–84. JunB over-expression increases the relative cross-sectional area of skeletal muscle while increasing protein synthesis in C2C12 myotubes 83. Unlike the mechanisms described above that alter muscle protein 10 homeostasis via Akt and/or mTOR-dependent pathways, JunB increases muscle size independent of mTOR activation 83. Jun B interacts with multiple transcription factors including FoxO3 and these interactions prevent the expression of genes that negatively regulate muscle mass such as the muscle-specific ubiquitin E3 ligase atrogin-1 and the activin receptor type II (ActRII) ligand myostatin 83,85. Thus, muscle tissue appears to have multiple redundant, but independent mechanisms, for regulating muscle mass and its response to growth factors and feeding.
Muscle IGFs and exercise: may the force be with you
Although IGF-I stimulates Akt and mTOR as well as protein synthesis in skeletal muscle 86, the role of IGF-I in the context of exercise-induced hypertrophy is much more tenuous 86. For example, resistance exercise is known to increase IGF-I mRNA expression in skeletal muscle but neither endocrine nor autocrine increases in the peptide have been reliably demonstrated 87–90. Recent studies in humans also imply that the total level of IGF-I peptide is unchanged in muscle microdialysis fluid during and after exercise suggesting no net synthesis of IGF-I occurs in exercised muscle 91. Yet, because the amount of free IGF-I (IGF-I not bound to IGF binding proteins, IGFBPs) is increased in venous blood draining skeletal muscle, it appears that IGF-I could be released from IGFBPs during exercise. Thus, the overall local IGF-I bioactivity could be increased in skeletal muscle during exercise and thus stimulate hypertrophy, although the 11 mechanism by which this may occur remains undetermined 91. Lastly, although immunization of control rats with IGF-I antibody does not prevent the exercise-induced increase in protein synthesis it does in diabetic rats 92. These results suggest that IGF-I can compensate for insulin in hypoinsulinemic rats by enabling an anabolic response after acute resistance exercise.
Using a functional overload model in which the plantaris is forced to do the work of the excised soleus and gastrocnemius, Spangenburg demonstrated that hypertrophy occurs equally well in control mice and mice expressing a kinase dead form of the IGF-IR (MKR mice) 93.
Because the MKR mice exhibited a decreased ability of IGF-I to stimulate Akt phosphorylation but a normal mTOR response to overload it can be concluded that IGF-IR kinase activity is not necessary for muscle hypertrophy in this model. A similar finding has been made by Barton et al in which adeno-associated virus expression of a variety of IGF-I isoforms in the tibialis failed to increase muscle mass in MKR mice 94. Others have also posited that the IGF-I peptide is not necessary for stretch-induced changes in mTOR signaling because conditioned media from stretched C2C12 myotubes was not sufficient to activate the phosphorylation of S6K in naïve myotubes 95. Therefore, it has been concluded that exercise-induced muscle hypertrophy is an intrinsic process independent of hormones and growth factors 96. Yet, in a mouse model in which the tibialis anterior was electrically stimulated via the sciatic nerve, the same authors found that electrical stimulation robustly increased S6K1phosphorylation and this was dramatically reduced in MKR mice, suggesting the IGF-IR tyrosine kinase activity may be necessary for mTOR signaling to its substrates in this model 97. MKR mice also exhibit a reduced capacity to recover from muscle injury implying that the IGF-IR is necessary for adequate myoblast fusion and differentiation during the regeneration process 3.
Although the MKR mouse has been used to determine whether the IGF-IR is necessary for IGF-I signaling in skeletal muscle it must be remembered that MKR mice exhibit many compensatory changes in response to the expression of the kinase-dead receptor including hyperglycemia, insulin resistance, and decreased muscle fatty acid oxidation 98. In addition, despite having smaller muscles the existing muscle exhibits a compensatory hyperplasia and a greater number of type II glycolytic fibers suggesting it may have a greater potential to hypertrophy independent of the IGF-IR tyrosine kinase activity 98,99. MKR muscle also exhibits a basal 2-fold increase in Akt phosphorylation, a response exceeding that observed in wild-type mice over expressing IGF-I94. One interpretation of these data is that Akt and mTOR are already maximally stimulated.
MKR mice also tend to be hypogonadal 100. These and other compensatory changes may influence the final response to functional overload observed by Spangenburg 93. Recent work by Mavailli would suggest that muscle- specific deletion of the IGF-IR might be an additional model for exploring the role of the IGF-IR in muscle hypertrophy independent of the compensatory changes observed in MKR mice 7,101.
MKR mice initially exhibit reduced ERK levels in skeletal muscle compared to wild-type controls, but this is followed by a compensatory increase in ERK phosphorylation as the mice age 99. There is now evidence that IGF-IR tyrosine kinase activity may not be necessary for ERK activation in response to IGF-I in some cell types. For example, ERK continues to be activated in cells treated with IGF-IR tyrosine kinase inhibitors and in cells expressing a kinase dead IGF-IR 102. These data suggest that muscle expressing the MKR mutation could still be signaling via ERK or other pathways that have not previously been appreciated as mediating the IGF-I hypertrophic response 103. Although highly speculative, IGF signaling to ERK in MKR mice could activate PLD and generate PA to stimulate signaling to mTOR via an ERK pathway 13 as recently demonstrated by Winter et al in Rat2 cells 104. However, whether such a mechanism also occurs in hypertrophying muscle fibers remains to be determined 104,105.
Nowhere is muscle hypertrophy more evident than in myostatin knockout mice and mice that express endogenous myostatin inhibitors such as follistatin 106. These mice exhibit muscle weights 2–3 times that of wild-type control mice. Follistatin prevents myostatin binding to the type II ActR but the mechanism by which this occurs has not been discerned. Recent work by Kalista et al 107 indicates that the myostatin inhibitor follistatin causes muscle hypertrophy via IGF-IR signaling. Control mice overexpressing follistatin exhibited a 3-fold greater increase in muscle fiber cross-sectional area than mice expressing follistatin on the MKR background 107. The follistatin response was also dependent on signaling pathways downstream of the IGF-IR, including mTORc1 and Akt, as the follistatin response was blunted either moderately by rapamycin or strongly by dominant-negative Akt 107. Thus, follistatin, by increasing muscle IGFs appears to increase both satellite cell activation and muscle hypertrophy via the classical IGF-IR/Akt/mTOR pathway 107,108. Future work will have to determine whether follistatin, working via the IGF-IR, can also maintain both muscle fiber myonuclear domain size and muscle force 109,110.
Recent work by Goodman et al 44 reports that systemic administration of rapamycin can completely block the load-induced hypertrophy of the plantaris in a model where the synergistic muscles (soleus and part of the gastrocnemius) are ablated. Goodman extended these findings by showing that mice expressing a muscle- specific mTOR kinase-dead transgene exhibited 14 virtually no compensatory hypertrophy as evidenced by the unchanged cross-sectional area. These observations suggest that mTOR kinase activity is essential for muscle hypertrophy in this model 111. In contrast, neither rapamycin nor the kinase-dead form of mTOR inhibited muscle hyperplasia due to synergistic muscle ablation. This implies that load-induced changes in muscle mTOR activity induce mainly muscle hypertrophy but not hyperplasia.
It has been posited that because IGF peptides can also bind to IGF binding proteins and integrin receptors, that a signal independent of IGF-IR kinase activity could be generated in the muscle of MKR mice 112. For example, Canonici et al have found that the IGF-IR forms a ternary complex with E-cadherin and the alpha V integrin receptor and that IGF binding initiates signaling by disrupting this complex 113. A similar finding was made by Saegusa who demonstrated direct binding of IGF-I to the alphaV beta 3 integrin 103. In smooth muscle, IGF-dependent ERK signaling is dependent upon direct binding of vitronectin to the beta 3 receptor because disruption of binding with a beta-3 antibody reduced IGF-I signaling 114. The alpha v beta 3 integrin receptor is present on C2C12 cells and in skeletal muscle. Therefore, it has the potential to interact with the IGF-IR and stimulate downstream signaling pathways, such as the activation of focal adhesion kinases (FAK) and the adapter protein paxillin 115–117. Indeed, FAK can transactivate IGF-I receptors in which the receptor autophosphorylation sites have been 15 mutated. In addition, peptides that disrupt IGF-IR/alpha V beta 3 interactions inhibit signaling to Akt and ERK kinases 118,119.
Barton and colleagues have reported that different IGF-I isoforms have different capacities to stimulate gene expression in skeletal muscle 94. IGF-I is transcribed off a single gene, but multiple mRNAs are generated by alternative splicing of exons in the gene. Muscle expresses IGF-IA and IGF-1B in rodents as well as IGF-IC in humans. Although these mRNAs produce identical IGF-I peptides, there is less homology in the E-peptides they produce. Some have suggested that the E peptides (i.e. Ea and Eb) themselves may signal through other receptors or facilitate signaling of the IGF-I peptide 94. Data are available indicating the Epeptides are necessary for muscle hypertrophy because over expression of a viral construct containing the IGF-IA or -B peptide, but not mature IGF-I, stimulated hypertrophy of the tibialis and maintained force generation in the extensor digitorum longus 94. In addition, both the IGFIA and –B forms were inhibited in the MKR mouse. Work by Shavlakadze et al suggests overexpression of the IGF-IA isoform in muscle is most efficacious during periods of active growth, but not in adult mice 120. Yet, Matheny et al would argue that although a stable IGF-I E pro-peptide exists, a final processed EB peptide has not been identified in extracellular fluids or cell culture media 121. One possibility is that the E-peptides anchor the IGF-I molecule in the extracellular matrix where they is more likely to come in contact with the IGF-IR. How the Epeptide influences IGF-I signaling in skeletal muscle remains an open question and should be an area of active research 21. Exercise and adequate nutrition are not only important for the maintenance of muscle mass but also for the prevention of and recovery from muscle disease 122–126. As noted above, a key 16 component of the protective effects of exercise and amino acid supplementation are their ability to stimulate mTOR 40,127,128. In the ensuing sections we will review how various muscle diseases affect muscle mass and function, pursue common defects, and investigate whether nutrition, exercise, and IGFs have potential therapeutic benefits.
The release of glucocorticoids from the adrenal cortex during stress contributes to the regulation of blood pressure, blood glucose, and the inflammatory response. Yet, glucocorticoid excess has a negative effect on muscle mass due to its ability to decrease muscle protein synthesis and increase protein degradation 14. The synthetic glucocorticoid dexamethasone stimulates the expression of a protein regulated in development and DNA responses (REDD1) in skeletal muscle and this protein is a putative mTOR inhibitor 129,130. REDD1 competes with TSC2 for binding to 14-3-3 proteins such that REDD1 over expression drives TSC2 to return Rheb to a GDP-bound form and inhibit mTOR 131. After an 18 h fast, corticosterone up regulates REDD1 protein expression which can be prevented by the glucocorticoid type II receptor antagonist RU486 132.
REDD1 is also up regulated in a number of stress conditions where mTOR and muscle protein synthesis are inhibited, including after endurance exercise, sepsis and acute alcohol intoxication. However, a direct causal role for REDD1 in decreasing mTOR kinase activity in these conditions has not been determined 133–135. REDD1 is also elevated paradoxically by insulin and IGF-I in adipose tissue and skeletal muscle, emphasizing that the expression of 17 REDD1 is not consistently associated with mTOR inhibition 136,137. In skeletal muscle, IGF-Iinduced REDD1 may act in a negative feedback loop to dampen the mitochondrial generation of reactive oxygen species (ROS) since REDD1 localizes to mitochondria where its absence and/or over expression may result in elevated ROS 138,139. REDD1 may have a similar function in glucocorticoid-induced muscle atrophy where ROS are also elevated 140. Although ROS are necessary for IGF-I-induced myocyte hypertrophy in C2C12 myotubes, it is not known if REDD1 is involved in this response 141. In addition, ROS generated after treatment of C2C12 myotubes with lipopolysaccharide and interferon gamma strongly inhibit mTOR activity and protein synthesis. Moreover, both parameters are restored in the presence of IGF-I and antioxidants, suggesting that a normalization of ROS levels is necessary for IGF-I-induced mTOR activity in this model 142. Glucocorticoids can decrease muscle mass and mTOR activity by up regulating both REDD1 and the transcription factor KLF15 via classical glucocorticoid receptor elements (GRE) in the promoters of both genes 143. KLF15 also binds to response elements in atrogenes, such as atrogin-1and muscle-specific ring finger-1, and these ubiquitin ligases target the ubiquitinylation and degradation of critical structural and functional proteins within skeletal muscle including the myosin heavy and light chains 144–146. Glucocorticoids also increase the expression of FoxO transcription factors and FoxO1 and KLF15 cooperate to increase the promoter activity of the atrogenes in skeletal muscle 56,143,147. Autophagy and lysosomal protein degradation pathways are also up regulated in skeletal muscle by FoxO3 resulting in a coordinated effort to mobilize amino acids for hepatic gluconeogenesis 56,148.
Although excess glucocorticoids clearly up regulate FoxO and the translation initiation factor 4E-BP1 these proteins also maintain muscle protein homeostasis by removing protein aggregates during aging where their overexpression can increase muscle strength 149,150. Therefore, a delicate balance of FoxO and 4E-BP1 signaling exists. The importance of FoxO and 4E-BP1 in skeletal muscle and the organism as a whole is underscored by the fact that FoxO signaling in muscle also affects metabolism in other tissues 150. For example, over expression of 4E-BP1 specifically in skeletal muscle, but not in other tissues of Drosophila, increases both the median and maximal lifespan of flies similar to that seen during caloric restriction 150. The FoxOdependent induction of 4E-BP1 in the fat body has also been proposed to be a “metabolic brake” that is activated by stress to prevent excessive “fat burn” during starvation 151. Future studies need to examine whether these proteins play a similar role in vertebrate muscle 152,153. The mitochondrial form of the branched chain amino transferase gene (BCATm) is also up regulated by glucocorticoids in a KLF15-dependent fashion 143. Over expression of KLF15 increases the activity of BCATm resulting in enhanced oxidation of BCAA and decreased mTOR substrate phosphorylation 143. Further, the negative effects of KLF15 can be overcome by excess BCAAs. mTOR activation by BCAA do not alter the translocation of the glucocorticoid receptor (GR) into the nucleus, but do diminish GR-mediated transcriptional activity 143. The positive effects of BCAA therefore appear to be dominant over glucocorticoids because BCAA prevent dexamethasone-induced increases in atrogenes, REDD1, and KLF15 while simultaneously maintaining muscle mass, muscle cross-sectional area, and muscle strength in the gastrocnemius 143,154,155. These data are consistent with previous reports showing that mTOR activation by upstream activators, such as IGF-I and constitutively active Akt or Rheb, 19 overcome the negative effects of dexamethasone on protein synthesis and the phosphorylation of mTOR substrates 14,129,136,155,156.
Excess BCAA may also enhance the anti-inflammatory effects of endogenous glucocorticoids by decreasing the expression of inflammatory cytokines as occurs in BCATm null mice 157. Other steroid hormones, such as testosterone, may also have beneficial effects on muscle mass due to their ability to prevent glucocorticoid-induced increases in REDD1 158,159.
The age-related decline in muscle mass and function that occurs in humans has long been thought to be due to a coincident decline in hormones and growth factors 160. Serum testosterone concentrations are also positively correlated with muscle mass and muscle fiber cross-sectional area in the elderly 161. Similar to the drop in testosterone during the “andropause”, GH and IGF-I decline during the “somatopause” and their rate of decline correlates with the fall in muscle mass 162. Although GH and testosterone have dramatic effects on muscle mass in the young, replacement therapies that match endogenous hormone levels have only minimal effects on muscle mass and strength during sarcopenia. In addition, exercise-induced changes in GH and testosterone do not influence muscle mass 96,163. These data suggest muscle exhibits resistance to these anabolic factors 164,165. The mechanism behind sarcopenia is therefore multifactorial and consistent with testosterone deficiency and the development of insulin resistance. In addition, leucine resistance develops in castrated rats 166,167. Despite the above, the intrinsic ability of muscle to respond to exercise is largely unchanged during sarcopenia suggesting that a multimodal approach that has exercise as its focal point but includes nutrients and hormones, when necessary, would be most efficacious at preventing muscle loss and/or restoring muscle mass in the peri-sarcopenic period 168.
Studies from rodents have revealed that not all muscles are equally sensitive to androgens due to their differential expression of the androgen receptor 169. In addition, skeletal muscle is capable of converting dehydroepiandrosterone (DHEA) to testosterone but further conversion of testosterone to dihydrotestosterone (DHT) may not be necessary for its anabolic effects 170,171. This finding is promising because testosterone-induced hyperplasia of the prostate can be blocked by 5-alpha reductase inhibitors whereas the anabolic effects of testosterone are not. These data suggest that the benefits of androgens for maintaining muscle mass may be garnered without their inherent cancer risk in males 172. Androgens appear necessary for the development of muscle mass in male but not female mice because genomic knockout of the androgen receptor (AR) decreases muscle mass and force production in a gender-dependent manner 173. The decrease in muscle mass in AR knockout mice is uniform across a number of muscles including the gastrocnemius, soleus, tibialis anterior and the extensor digitorum longus, but not the heart 173. AR knockout was associated with a decrease in maximal force generation in fast-twitch muscle (extensor digitorum longus, EDL) and enhanced fatigue resistance in slow-twitch muscle (soleus). These data are consistent with changes in gene expression in which androgens increase the expression of type-II glycolytic fibers that produce more force, but are highly fatigable. AR null mice exhibit no change in serum or limb muscle IGF-I or IGF-IR mRNA but exhibit a decrease in amino acid and micronutrient transporter mRNAs (Slc38a4/SNAT4) that could influence the co-transport of glutamine and leucine consistent with the leucine resistance observed in castrated rats 167.
Surprisingly, selective ablation of the AR in skeletal myofibers decreases muscle mass in the perineal muscles which contain a high concentration of the AR but not in the limb muscles where the lack of AR signaling does not decrease mass but still affects muscle structural proteins and force production 174. AR knockout postnatally in the perineal muscles also decreased IGFIEa mRNA and this was associated with a decrease in muscle mass 174. In contrast, castration of muscle specific AR null mice decreased both muscle IGF-IEa mRNA and muscle mass suggesting that androgens maintain muscle mass by a myofiber specific and AR-independent mechanism. The AR, in supporting fibroblasts and satellite cells, may therefore ultimately determine autocrine IGF-I levels and muscle mass. In agreement with this is the finding that androgens stimulate the proliferation of human skeletal muscle cells in vitro and this is blocked by both IGF-I neutralizing antibodies and si-RNA mediated knockdown of the IGF-IR 100.
Signal transducers and activators of transcription (Stat) 5a/b, two transcription factors necessary for the maintenance of muscle mass also maintain the expression of the androgen receptor which is transcriptionally induced by GH. Muscle specific knockout of Stat5a/b leads to a muscle fiber type switch from Type II to Type I fibers 175. Inhibition of IGF-IR signaling in the MKR mouse also attenuates testosterone-induced gains in the mass of the perineal muscles by 60% 100. Thus, IGF-IR signaling plays a critical role in the response of muscle to androgens.
Ibebunjo et al have recently discovered a potential link between the andropause and decreased muscle activity and muscle mass. These authors found that castration decreased voluntary running speed and endurance, both of which were restored upon treatment with testosterone 176. Testosterone increased the expression of a number of genes including IGF-I, the IGF-IR, and solute carriers but that the increase in these markers occurred equally well in sedentary and exercised rats suggesting that these genes are only permissive for the observed behavior.
Although testosterone replacement was not associated with dramatic changes in muscle mass it was associated with a transition to Type IIa muscle fibers. Because the transition to Type IIa fibers was greater in the mice that ran than in the sedentary animals, testosterone per se does not mediate the transition to Type II fibers but is obligatory to promote the activity that drives the transition 176.
One scheme for staving off sarcopenia may be the consumption of adequate protein during “middle age”. D'Antona et al found that mice fed a diet supplemented with BCAA exhibited an increase in average lifespan and enhanced mitochondrial biogenesis in skeletal muscle 177. Not surprisingly, the greatest mitochondrial mass was achieved in mice that received both BCAA and exercise and the beneficial effects of BCAA on mitochondrial biogenesis could be blocked by rapamycin 177. It should be noted that many of the beneficial effects of caloric restriction on lifespan can be mimicked by intermittent feeding protocols in which there is little or no reduction in total caloric intake. In C. eleagans the beneficial effects of intermittent feeding require Rheb to be transiently turned on and off and for mTOR to be activated to down regulate the expression of an IGF-like peptide and increase the worms loco motor activity 178.
The above data suggest that hormones, growth factors, nutrients, and exercise cooperate in maintaining muscle mass and function and provide the impetus to overcome sarcopenia. Recent data would suggest that this may occur via a centrally mediated mechanism 176,179. AR knockout in the nervous system generates male mice that exhibit growth retardation, a decrease in “male-typical behavior” and reduced serum levels of IGF-I 180. Although overall activity is not affected by AR knockout in the nervous system of young adult mice (2–5 months) it would be interesting to determine whether these mice are more prone to decreased activity and sarcopenia with aging (> 18 months) 180. AR null mice also lack the normal feedback control regulating glucocorticoid production such that plasma ACTH and corticosterone are chronically elevated 181. Thus, the testosterone deficit seen with aging may be a prerequisite to a glucocorticoid-induced decrease in muscle protein. Conversely, animal studies are promising because they suggest that testosterone and tissue -selective androgen receptor modulators can reverse dexamethasone-induced atrophy of the perineal muscles and repress genes involved in glucocorticoid-induced wasting including REDD1 and FOXO1 158,159,182. In summary, there appear to be significant cooperative effects between amino acids, exercise, the IGF-system, and androgens in the maintenance of muscle mass.
Immobilized muscle: a cast of characters involved in muscle atrophy
Immobilization of the leg muscles induces muscle atrophy and is highly relevant to clinical conditions where patients endure long-standing bed rest 183. During prolonged bed rest, there is a differential rate of atrophy between muscle groups with the anti-gravity muscles and the plantar flexor muscles (medial gastrocnemius and soleus) displaying the greatest atrophy.
The knee extensors, the hip extensors and abductors, and the muscles of the foot and ankle, such as the tibialis posterior, are also greatly affected consistent with the use of these muscles not only for locomotion but also for supporting load 183. In contrast, other muscles such as the toe extensors (anterior tibial muscles) and flexors (flexor digitorum longus) show little or no atrophy 183. These observations suggest that resistance exercise protocols targeting the affected muscles could prevent damage to joints and diminish the risk of injury during the recovery phase.
Immobilization is thought to make skeletal muscle resistant to the positive effects of anabolic amino acids and protein suggesting that nutrition alone is insufficient to mount an anabolic response during bed rest 184. Supplementation of BCAA during bed rest does not affect either muscle protein synthesis or the release of 3-methyl histidine from muscle (a marker of muscle protein breakdown) but does increase the free amino acid pool during the early recovery phase 185. These observations suggest that a combination of amino acids and exercise might enhance muscle recovery. Indeed, recent studies indicate that provision of amino acids and exercise during bed rest increased muscle volume and strength while decreasing the deposition of intramuscular fat compared to amino acid supplementation alone 186. In a follow up study examining potential mechanisms by which the combination of amino acids and resistance exercise restored muscle mass post bed rest, the authors found that only the combination of exercise and amino acids increased IGF-I expression while simultaneously antagonizing the increase in atrogenes 187. Lastly, ectopic expression of IGF-I within the gastrocnemius prevented muscle atrophy by 50% in a mouse model of seven days of hind-limb suspension 188. IGF-I expression from the gastrocnemius also tended to increase the bone mineral content in the lumbar bones by dual-energy x-ray absorptiometry suggesting a beneficial effect of either paracrine IGF-I or the maintenance of muscle function on bone 188.
Recent studies suggest that FoxO and NF-kB transcription factors drive muscle atrophy in the elderly who assume a sedentary lifestyle and in rodent models of muscle immobilization 56,189–191. Indeed, over expression of constitutively active FoxO or NF-kB stimulates the atrophy program in mice 192. Other data suggest that ROS, and in particular hydrogen peroxide, drive changes in muscle mass. Dodd et al have shown that over expression of catalase partially attenuates NFkB/FoxO signalling and the loss of muscle mass during hind limb immobilization is consistent with the ability of some antioxidants to abate immobilization-induced muscle loss and muscle fatigue 193–195. It is noteworthy that the application of intermittent heat during the reloading period also enhanced antioxidant defences and the recovery of muscle mass and mTOR activity 196–198.
Although IGF-I mRNA and protein levels are not altered by immobilization, numerous genes involved in IGF signalling are down regulated in human muscle during voluntary casting 199,200. Therefore investigators have examined whether over expression of IGF-I can prevent immobilization-induced skeletal muscle loss. IGF-I does not inhibit the loss of muscle mass during the atrophy phase but it does enhance muscle weight gain and cross-sectional area during a 3 week reloading period 200,201. This response suggests that casted muscle is IGF-I resistant and that muscle contraction is necessary for IGF-I to have its full biological effect during recovery. Although IGF-I treatment is normally associated with an increase in protein synthesis in control mice the IGF-I induced increase in muscle mass during recovery from casting appears to be independent of changes in muscle protein synthesis and may be related to the ability of IGF-I to stimulate the proliferation and regeneration of muscle stem cells 200. Casting in humans also decreases the expression of genes, such as SLC16A1, involved in the transport of monocarboxylates such as lactate, pyruvate and branched-chain oxo acids derived from leucine 199. SLC16A1, also known as MCT1, is a bidirectional transporter located on the plasma membrane of muscle cells. Genetic defects in SLC16A1 may result in a deficiency in lactate transport out of the cell, intracellular acidification and subsequent muscle degeneration 202. The resulting muscle acidification may also inhibit the subsequent transport of glutamine via acidosis-induced inhibition of the glutamine pump SNAT2 which is obligatory for the transport of BCAA such as leucine, activation of mTOR signaling and protein synthesis 203. Growth factors may be able to overcome intracellular acidification and stimulate protein synthesis by their ability to activate the Na+/H+ antiport system and increase the pH of the intracellular compartment 204,205.
Alcoholics experience a myopathy that selectively affects type II fibers (gastrocnemius >soleus) and appears to result from a decrease in muscle protein synthesis and damage to muscle proteins 206. Similar decrements in muscle protein synthesis are seen in animal models of both acute alcohol intoxication and chronic alcohol abuse 207. Alcohol decreases skeletal muscle IGFI content and makes muscle both IGF-I and insulin resistant at the level of mTOR activity 208,209.
Although skeletal muscle is IGF-I resistant after the acute administration of alcohol, rats fed an alcohol-containing diet for 16 weeks show improved muscle protein synthesis when administered IGF-I as part of a binary complex with IGFBP-3 210. These data suggest that the binary complex may maintain the local concentration of IGF-I in skeletal muscle for a sustained period of time and allow for better recovery of the muscle than a single injection of recombinant IGF-I which is rapidly cleared from the circulation. The binary complex may also be more efficient at restoring plasma amino acids needed to transduce the IGF signal, such as glutamine and alanine 210.
Alcohol also impairs the normal mTOR response to leucine in as little as 2.5 h after an oral gavage of alcohol 211. Although acute alcohol intoxication has a similar effect on mTOR activity in male and female rats, female rats appear to better tolerate the long-term effects of alcohol on muscle. Female, but not male rats, exhibit an increase in markers of muscle protein remodelling such as atrogin-1 and MuRF-1 suggesting female rats may be more efficient at synthesizing new protein and clearing damaged protein than males when placed on a long-term alcohol-containing diet 212. Alcohol also accelerates muscle loss due to unilateral hind limb immobilization (casting a single leg) and alcohol impairs the recovery of muscle mass for at least 5 days after cast removal 213. Following unilateral hind limb immobilization in rats, alcohol accentuates the expression of atrogin-1 and MuRF-1 and muscle recovery is improved by the proteasome inhibitor VelcadeTM. These results indicate a fairly dominant proteasome-mediated muscle proteolysis element exists in the casting model of disuse atrophy and that this can be accentuated by alcohol.
Although the mechanism by which alcohol induces leucine and IGF-I resistance has not been completely elucidated, recent data suggests that mice that have abnormally high plasma leucine levels may be protected from alcohol-induced IGF-I resistance. Mice with a whole-body knockout of the mitochondrial form of the BCAA metabolizing enzyme BCATm exhibit plasma leucine levels 15-fold higher than wild-type mice 157,214. BCAA levels this high do not inhibit the effects of alcohol on muscle protein synthesis pre se but they do sensitize skeletal muscle to IGF-I allowing IGF-I to restore muscle protein synthesis to levels observed in control rats given IGF-I 157. Excess BCAAs may therefore protect the muscle from alcohol-induced changes in IGF-I sensitivity. High doses of leucine have also been shown to ameliorate the negative effects of AMPK signalling on muscle protein synthesis and to increase mitochondrial mass in muscle cells 62,215,216.
Studies by Hong-Brown et al in C2C12 myoblasts suggest that alcohol differentially affects mTORc1 and -2. Alcohol decreases mTORc1 activity while increasing the abundance of mTORc2 components and the phosphorylation of mTORc2 substrates such as Akt on S473 217. These findings suggest that, in myoblasts, alcohol does not directly alter the intrinsic activity of mTOR. These authors have also shown that alcohol disrupts leucine signaling to mTORc1 in myoblasts by suppressing the interaction of Rag A and C with mTOR 218. A constitutively active combination of RagA and C but not A alone overcomes the negative effect of alcohol and maintains the phosphorylation of mTOR substrates such as S6K1 and 4E-BP1 218. This implies that alcohol disrupts mTOR signalling at a step either prior to or directly at the sensing of leucine by the Rag proteins (See Figure).
Summary
The plasticity of skeletal muscle is evidenced by its capacity to double or even triple in size. Overall, muscle volume may be restricted by genetic limits but muscle quantity is primarily regulated by its own use and disuse. Muscle hypertrophy is also influenced by the availability of nutrients and the presence of endocrine and autocrine hormones such as IGF-I. A concerted anabolic signal is generated by leucine which facilitates the translocation of the kinase mTOR to the surface of the lysosome, where it is activated by Rheb, and by IGF-I which relieves the inherent inhibition of mTOR by TSC2. mTOR is also positively regulated by short-term changes in the energy state of individual muscle fibers after a meal and secondarily by prolonged exposure to leucine, glucose and contractile load all of which may influence the long-term energy state by increasing mitochondrial biogenesis. mTOR, when activated, phosphorylates substrates such as the translation initiation factor 4E-BP1 to stimulate muscle protein synthesis and muscle hypertrophy. IGF-I also stimulates the self-renewal of muscle satellite cells to replace myonuclei and rebuild muscle after injury. Exercise, nutrients, and growth factors have evolved together to optimally energize mTOR and overcome the negative impact of catabolic hormones, inflammatory mediators, and muscle damage. Hence, a combination of these mTOR modifiers used in tandem may be efficacious in preventing and/or ameliorating reductions in muscle mass occurring in select pathological conditions.
Funding Sources
Dr. Lang: NIH Grants GM038032 and AA11290
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
Conflict of Interest Dr. Lang: None Dr. Frost: None
References
- 1.Porter Abate J, Blackwell TK. Life is short, if sweet. Cell Metab. 2009;10:338. doi: 10.1016/j.cmet.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spanier B, Rubio-Aliaga I, Hu H, et al. Altered signalling from germline to intestine pushes daf-;pept-1 Caenorhabditis elegans into extreme longevity. Aging Cell. 9:636. doi: 10.1111/j.1474-9726.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- 3.Heron-Milhavet L, Mamaeva D, LeRoith D, et al. Impaired muscle regeneration and myoblast differentiation in mice with a muscle-specific KO of IGF-IR. J Cell Physiol. 225:1. doi: 10.1002/jcp.22218. [DOI] [PubMed] [Google Scholar]
- 4.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 224:7. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- 5.Liu JP, Baker J, Perkins AS, et al. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59. [PubMed] [Google Scholar]
- 6.Liu JL, LeRoith D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology. 1999;140:5178. doi: 10.1210/endo.140.11.7151. [DOI] [PubMed] [Google Scholar]
- 7.Mavalli MD, DiGirolamo DJ, Fan Y, et al. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 120:4007. doi: 10.1172/JCI42447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemmons DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. 2009;20:349. doi: 10.1016/j.tem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Stratikopoulos E, Szabolcs M, Dragatsis I, et al. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci U S A. 2008;105:19378. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Sun H, Yakar S, et al. Elevated levels of insulin-like growth factor (IGF)-I in serum rescue the severe growth retardation of IGF-I null mice. Endocrinology. 2009;150:4395. doi: 10.1210/en.2009-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han B, Zhu MJ, Ma C, et al. Rat hindlimb unloading down-regulates insulin like growth factor-1 signaling and AMP-activated protein kinase, and leads to severe atrophy of the soleus muscle. Appl Physiol Nutr Metab. 2007;32:1115. doi: 10.1139/H07-102. [DOI] [PubMed] [Google Scholar]
- 12.Gehrig SM, Ryall JG, Schertzer JD, et al. Insulin-like growth factor-I analogue protects muscles of dystrophic mdx mice from contraction-mediated damage. Exp Physiol. 2008;93:1190. doi: 10.1113/expphysiol.2008.042838. [DOI] [PubMed] [Google Scholar]
- 13.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol. 2007;103:378. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 14.Schakman O, Gilson H, Kalista S, et al. Mechanisms of muscle atrophy induced by glucocorticoids. Horm Res. 2009;72(Suppl 1):36. doi: 10.1159/000229762. [DOI] [PubMed] [Google Scholar]
- 15.Inder WJ, Jang C, Obeyesekere VR, et al. Dexamethasone administration inhibits skeletal muscle expression of the androgen receptor and IGF-1--implications for steroid-induced myopathy. Clin Endocrinol (Oxf) 73:126. doi: 10.1111/j.1365-2265.2009.03683.x. [DOI] [PubMed] [Google Scholar]
- 16.Muscaritoli M, Bossola M, Aversa Z, et al. Prevention and treatment of cancer cachexia: new insights into an old problem. Eur J Cancer. 2006;42:31. doi: 10.1016/j.ejca.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Crown AL, Cottle K, Lightman SL, et al. What is the role of the insulin-like growth factor system in the pathophysiology of cancer cachexia, and how is it regulated? Clin Endocrinol (Oxf) 2002;56:723. doi: 10.1046/j.1365-2265.2002.01540.x. [DOI] [PubMed] [Google Scholar]
- 18.Frost RA, Lang CH. Regulation of insulin-like growth factor-I in skeletal muscle and muscle cells. Minerva Endocrinol. 2003;28:53. [PubMed] [Google Scholar]
- 19.Schertzer JD, Lynch GS. Comparative evaluation of IGF-I gene transfer and IGF-I protein administration for enhancing skeletal muscle regeneration after injury. Gene Ther. 2006;13:1657. doi: 10.1038/sj.gt.3302817. [DOI] [PubMed] [Google Scholar]
- 20.Demonbreun AR, Fahrenbach JP, Deveaux K, et al. Impaired muscle growth and response to insulin-like growth factor 1 in dysferlin-mediated muscular dystrophy. Hum Mol Genet. 20:779. doi: 10.1093/hmg/ddq522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinciguerra M, Musaro A, Rosenthal N. Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol. 694:211. doi: 10.1007/978-1-4419-7002-2_15. 32. [DOI] [PubMed] [Google Scholar]
- 22.Schertzer JD, Gehrig SM, Ryall JG, et al. Modulation of insulin-like growth factor (IGF)-I and IGFbinding protein interactions enhances skeletal muscle regeneration and ameliorates the dystrophic pathology in mdx mice. Am J Pathol. 2007;171:1180. doi: 10.2353/ajpath.2007.070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zdanowicz MM, Teichberg S. Effects of insulin-like growth factor-1/binding protein-3 complex on muscle atrophy in rats. Exp Biol Med (Maywood) 2003;228:891. doi: 10.1177/153537020322800804. [DOI] [PubMed] [Google Scholar]
- 24.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713. doi: 10.1097/00000658-199905000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Fang Y, Chen J, et al. Activation of mTOR signaling by novel fluoromethylene phosphonate analogues of phosphatidic acid. Bioorg Med Chem Lett. 2004;14:1461. doi: 10.1016/j.bmcl.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Long YC, Cheng Z, Copps KD, et al. Insulin Receptor Substrates Irs1 and Irs2 Coordinate Skeletal Muscle Growth and Metabolism via the Akt and AMPK Pathways. Mol Cell Biol. 31:430. doi: 10.1128/MCB.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy JJ, Esser KA. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr Opin Clin Nutr Metab Care. 13:230. doi: 10.1097/MCO.0b013e32833781b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Risson V, Mazelin L, Roceri M, et al. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol. 2009;187:859. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bentzinger CF, Romanino K, Cloetta D, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Setia S, Sridhar MG. Changes in GH/IGF-1 axis in intrauterine growth retardation: consequences of fetal programming? Horm Metab Res. 2009;41:791. doi: 10.1055/s-0029-1231026. [DOI] [PubMed] [Google Scholar]
- 31.Kappeler L, De Magalhaes Filho C, Leneuve P, et al. Early postnatal nutrition determines somatotropic function in mice. Endocrinology. 2009;150:314. doi: 10.1210/en.2008-0981. [DOI] [PubMed] [Google Scholar]
- 32.Christoforidis A, Maniadaki I, Stanhope R. Growth hormone / insulin-like growth factor-1 axis during puberty. Pediatr Endocrinol Rev. 2005;3:5. [PubMed] [Google Scholar]
- 33.Moller N, Vendelbo MH, Kampmann U, et al. Growth hormone and protein metabolism. Clin Nutr. 2009;28:597. doi: 10.1016/j.clnu.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Dreyer HC, Drummond MJ, Pennings B, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 93:402. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heron-Milhavet L, Haluzik M, Yakar S, et al. Muscle-specific overexpression of CD36 reverses the insulin resistance and diabetes of MKR mice. Endocrinology. 2004;145:4667. doi: 10.1210/en.2003-1543. [DOI] [PubMed] [Google Scholar]
- 37.Gingras AA, White PJ, Chouinard PY, et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol. 2007;579:269. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rennie MJ. Exercise- and nutrient-controlled mechanisms involved in maintenance of the musculoskeletal mass. Biochem Soc Trans. 2007;35:1302. doi: 10.1042/BST0351302. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe RR. Skeletal muscle protein metabolism and resistance exercise. J Nutr. 2006;136:525S. doi: 10.1093/jn/136.2.525S. [DOI] [PubMed] [Google Scholar]
- 40.Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 41.de Oliveira CA, Latorraca MQ, de Mello MA, et al. Mechanisms of insulin secretion in malnutrition: modulation by amino acids in rodent models. Amino Acids. 40:1027. doi: 10.1007/s00726-010-0716-y. [DOI] [PubMed] [Google Scholar]
- 42.Apro W, Blomstrand E. Influence of supplementation with branched-chain amino acids in combination with resistance exercise on p70S6 kinase phosphorylation in resting and exercising human skeletal muscle. Acta Physiol (Oxf) 200:237. doi: 10.1111/j.1748-1708.2010.02151.x. 33. [DOI] [PubMed] [Google Scholar]
- 43.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptordependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman CA, Miu MH, Frey JW, et al. A phosphatidylinositol 3-kinase/protein kinase Bindependent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell. 21:3258. doi: 10.1091/mbc.E10-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long X, Ortiz-Vega S, Lin Y, et al. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem. 2005;280:23433. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 46.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suryawan A, Davis TA. Regulation of protein synthesis by amino acids in muscle of neonates. Front Biosci. 16:1445. doi: 10.2741/3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDowell HE, Christie GR, Stenhouse G, et al. Leucine activates system A amino acid transport in L6 rat skeletal muscle cells. Am J Physiol. 1995;269:C1287. doi: 10.1152/ajpcell.1995.269.5.C1287. [DOI] [PubMed] [Google Scholar]
- 49.Avruch J, Long X, Ortiz-Vega S, et al. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296:E592. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sancak Y, Bar-Peled L, Zoncu R, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 141:290. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoncu R, Bar-Peled L, Efeyan A, et al. mTORC1 senses lysosomal amino acids through an insideout mechanism that requires the vacuolar H(+)-ATPase. Science. 334:678. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagne C, Agulhon C, Ravassard P, et al. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc Natl Acad Sci U S A. 2001;98:7206. doi: 10.1073/pnas.121183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goberdhan DC. Intracellular amino acid sensing and mTORC1-regulated growth: new ways to block an old target? Curr Opin Investig Drugs. 11:1360. [PMC free article] [PubMed] [Google Scholar]
- 55.Heublein S, Kazi S, Ogmundsdottir MH, et al. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 29:4068. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J, Brault JJ, Schild A, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Iida RH, Kanko S, Suga T, et al. Autophagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for aging. Mol Cell Biochem. 348:89. doi: 10.1007/s11010-010-0642-z. [DOI] [PubMed] [Google Scholar]
- 58.Bartke A. Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol Metab. 2006;17:33. doi: 10.1016/j.tem.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Yu L, McPhee CK, Zheng L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 465:942. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Settembre C, Zoncu R, Medina DL, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. Embo J. 31:1095. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saci A, Cantley LC, Carpenter CL. Rac1 Regulates the Activity of mTORC1 and mTORC2 and Controls Cellular Size. Mol Cell. 42:50. doi: 10.1016/j.molcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saha AK, Xu XJ, Lawson E, et al. Downregulation of AMPK accompanies leucine- and glucoseinduced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 59:2426. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inoki K, Ouyang H, Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955. doi: 10.1016/j.cell.2006.06.055. 34. [DOI] [PubMed] [Google Scholar]
- 64.Avruch J, Lin Y, Long X, et al. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67. doi: 10.1097/00075197-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Zheng M, Wang YH, Wu XN, et al. Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat Cell Biol. 13:263. doi: 10.1038/ncb2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyazaki M, McCarthy JJ, Esser KA. Insulin like growth factor-1-induced phosphorylation and altered distribution of tuberous sclerosis complex (TSC)1/TSC2 in C2C12 myotubes. Febs J. 277:2180. doi: 10.1111/j.1742-4658.2010.07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan M, Wu X, Guan KL, et al. Muscle atrophy in transgenic mice expressing a human TSC1 transgene. FEBS Lett. 2006;580:5621. doi: 10.1016/j.febslet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 68.Avruch J, Hara K, Lin Y, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Proud CG. mTORC1 signaling: what we still don't know. J Mol Cell Biol. doi: 10.1093/jmcb/mjq038. [DOI] [PubMed] [Google Scholar]
- 70.Sun Y, Fang Y, Yoon MS, et al. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci U S A. 2008;105:8286. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang C, Wendel AA, Keogh MR, et al. Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc Natl Acad Sci U S A. 109:1667. doi: 10.1073/pnas.1110730109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 40:310. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fang Y, Vilella-Bach M, Bachmann R, et al. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 74.Sato T, Nakashima A, Guo L, et al. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009;284:12783. doi: 10.1074/jbc.M809207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toschi A, Lee E, Xu L, et al. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29:1411. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of the mammalian target of rapamycin complex 1 (mTORC1) by insulin and amino acids. J Biol Chem. doi: 10.1074/jbc.M110.209171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drummond MJ, Bell JA, Fujita S, et al. Amino acids are necessary for the insulin-induced activation of mTOR/S6K1 signaling and protein synthesis in healthy and insulin resistant human skeletal muscle. Clin Nutr. 2008;27:447. doi: 10.1016/j.clnu.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tipton KD, Rasmussen BB, Miller SL, et al. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. 2001;281:E197. doi: 10.1152/ajpendo.2001.281.2.E197. [DOI] [PubMed] [Google Scholar]
- 79.Wasa M, Wang HS, Tazuke Y, et al. Insulin-like growth factor-I stimulates amino acid transport in a glutamine-deprived human neuroblastoma cell line. Biochim Biophys Acta. 2001;1525:118. doi: 10.1016/s0304-4165(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 80.Fryburg DA, Jahn LA, Hill SA, et al. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J Clin Invest. 1995;96:1722. doi: 10.1172/JCI118217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henriksen EJ, Louters LL, Stump CS, et al. Effects of prior exercise on the action of xsinsulin-like growth factor I in skeletal muscle. Am J Physiol. 1992;263:E340. doi: 10.1152/ajpendo.1992.263.2.E340. [DOI] [PubMed] [Google Scholar]
- 82.Coletta DK, Balas B, Chavez AO, et al. Effect of acute physiological hyperinsulinemia on gene expression in human skeletal muscle in vivo. Am J Physiol Endocrinol Metab. 2008;294:E910. doi: 10.1152/ajpendo.00607.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raffaello A, Milan G, Masiero E, et al. JunB transcription factor maintains skeletal muscle mass and promotes hypertrophy. J Cell Biol. 191:101. doi: 10.1083/jcb.201001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trenerry MK, Carey KA, Ward AC, et al. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol. 2007;102:1483. doi: 10.1152/japplphysiol.01147.2006. [DOI] [PubMed] [Google Scholar]
- 85.Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol. 2007;292:C188. doi: 10.1152/ajpcell.00542.2005. 35. [DOI] [PubMed] [Google Scholar]
- 86.Bark TH, McNurlan MA, Lang CH, et al. Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol. 1998;275:E118. doi: 10.1152/ajpendo.1998.275.1.E118. [DOI] [PubMed] [Google Scholar]
- 87.Stokes KA, Sykes D, Gilbert KL, et al. Brief, high intensity exercise alters serum ghrelin and growth hormone concentrations but not IGF-I, IGF-II or IGF-I bioactivity. Growth Horm IGF Res. 20:289. doi: 10.1016/j.ghir.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Nindl BC, Pierce JR. Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med Sci Sports Exerc. 42:39. doi: 10.1249/MSS.0b013e3181b07c4d. [DOI] [PubMed] [Google Scholar]
- 89.Heinemeier KM, Olesen JL, Schjerling P, et al. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. 2007;102:573. doi: 10.1152/japplphysiol.00866.2006. [DOI] [PubMed] [Google Scholar]
- 90.Vendelbo MH, Jorgensen JO, Pedersen SB, et al. Exercise and fasting activate growth hormonedependent myocellular signal transducer and activator of transcription-5b phosphorylation and insulin-like growth factor-I messenger ribonucleic acid expression in humans. J Clin Endocrinol Metab. 95:E64. doi: 10.1210/jc.2010-0689. [DOI] [PubMed] [Google Scholar]
- 91.Berg U, Gustafsson T, Sundberg CJ, et al. Interstitial IGF-I in exercising skeletal muscle in women. Eur J Endocrinol. 2007;157:427. doi: 10.1530/EJE-07-0141. [DOI] [PubMed] [Google Scholar]
- 92.Fedele MJ, Lang CH, Farrell PA. Immunization against IGF-I prevents increases in protein synthesis in diabetic rats after resistance exercise. Am J Physiol Endocrinol Metab. 2001;280:E877. doi: 10.1152/ajpendo.2001.280.6.E877. [DOI] [PubMed] [Google Scholar]
- 93.Spangenburg EE, Le Roith D, Ward CW, et al. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586:283. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barton ER, DeMeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol. 108:1069. doi: 10.1152/japplphysiol.01308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hornberger TA, Armstrong DD, Koh TJ, et al. Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: implications for mechanotransduction. Am J Physiol Cell Physiol. 2005;288:C185. doi: 10.1152/ajpcell.00207.2004. [DOI] [PubMed] [Google Scholar]
- 96.West DW, Burd NA, Staples AW, et al. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 42:1371. doi: 10.1016/j.biocel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Witkowski S, Lovering RM, Spangenburg EE. High-frequency electrically stimulated skeletal muscle contractions increase p70s6k phosphorylation independent of known IGF-I sensitive signaling pathways. FEBS Lett. 584:2891. doi: 10.1016/j.febslet.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vaitheesvaran B, LeRoith D, Kurland IJ. MKR mice have increased dynamic glucose disposal despite metabolic inflexibility, and hepatic and peripheral insulin insensitivity. Diabetologia. 53:2224. doi: 10.1007/s00125-010-1827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernandez AM, Dupont J, Farrar RP, et al. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest. 2002;109:347. doi: 10.1172/JCI13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serra C, Bhasin S, Tangherlini F, et al. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology. 152:193. doi: 10.1210/en.2010-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 1:4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perrault R, Wright B, Storie B, et al. Tyrosine kinase-independent activation of extracellularregulated kinase (ERK) 1/2 by the insulin-like growth factor-1 receptor. Cell Signal. doi: 10.1016/j.cellsig.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 103.Saegusa J, Yamaji S, Ieguchi K, et al. The direct binding of insulin-like growth factor-1 (IGF-1) to integrin alphavbeta3 is involved in IGF-1 signaling. J Biol Chem. 2009;284:24106. doi: 10.1074/jbc.M109.013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Winter JN, Fox TE, Kester M, et al. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am J Physiol Cell Physiol. 299:C335. doi: 10.1152/ajpcell.00039.2010. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarbassov DD, Jones LG, Peterson CA. Extracellular signal-regulated kinase-1 and -2 respond differently to mitogenic and differentiative signaling pathways in myoblasts. Mol Endocrinol. 1997;11:2038. doi: 10.1210/mend.11.13.0036. [DOI] [PubMed] [Google Scholar]
- 106.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kalista S, Schakman O, Gilson H, et al. The type 1 insulin-like growth factor receptor (IGF-IR) pathway is mandatory for the follistatin-induced skeletal muscle hypertrophy. Endocrinology. 153:241. doi: 10.1210/en.2011-1687. [DOI] [PubMed] [Google Scholar]
- 108.Gilson H, Schakman O, Kalista S, et al. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab. 2009;297:E157. doi: 10.1152/ajpendo.00193.2009. [DOI] [PubMed] [Google Scholar]
- 109.Qaisar R, Renaud G, Morine K, et al. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level? Faseb J. 26:1077. doi: 10.1096/fj.11-192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kota J, Handy CR, Haidet AM, et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009;1:6ra15. doi: 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 23:1896. doi: 10.1016/j.cellsig.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stewart CE, Pell JM. Point:Counterpoint: IGF is/is not the major physiological regulator of muscle mass. Point: IGF is the major physiological regulator of muscle mass. J Appl Physiol. 108:1820. doi: 10.1152/japplphysiol.01246.2009. [DOI] [PubMed] [Google Scholar]
- 113.Canonici A, Steelant W, Rigot V, et al. Insulin-like growth factor-I receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha-catenin. Int J Cancer. 2008;122:572. doi: 10.1002/ijc.23164. [DOI] [PubMed] [Google Scholar]
- 114.Maile LA, Busby WH, Sitko K, et al. Insulin-like growth factor-I signaling in smooth muscle cells is regulated by ligand binding to the 177CYDMKTTC184 sequence of the beta3--subunit of alphaVbeta3. Mol Endocrinol. 2006;20:405. doi: 10.1210/me.2005-0241. [DOI] [PubMed] [Google Scholar]
- 115.Svendsen OS, Liden A, Nedrebo T, et al. Integrin alphavbeta3 acts downstream of insulin in normalization of interstitial fluid pressure in sepsis and in cell-mediated collagen gel contraction. Am J Physiol Heart Circ Physiol. 2008;295:H555. doi: 10.1152/ajpheart.00161.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sinanan AC, Machell JR, Wynne-Hughes GT, et al. Alpha v beta 3 and alpha v beta 5 integrins and their role in muscle precursor cell adhesion. Biol Cell. 2008;100:465. doi: 10.1042/BC20070115. [DOI] [PubMed] [Google Scholar]
- 117.Furundzija V, Fritzsche J, Kaufmann J, et al. IGF-1 increases macrophage motility via PKC/p38-dependent alphavbeta3-integrin inside-out signaling. Biochem Biophys Res Commun. 394:786. doi: 10.1016/j.bbrc.2010.03.072. [DOI] [PubMed] [Google Scholar]
- 118.Andersson S, D'Arcy P, Larsson O, et al. Focal adhesion kinase (FAK) activates and stabilizes IGF-1 receptor. Biochem Biophys Res Commun. 2009;387:36. doi: 10.1016/j.bbrc.2009.06.088. [DOI] [PubMed] [Google Scholar]
- 119.Zheng D, Kurenova E, Ucar D, et al. Targeting of the protein interaction site between FAK and IGF-1R. Biochem Biophys Res Commun. 2009;388:301. doi: 10.1016/j.bbrc.2009.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shavlakadze T, Chai J, Maley K, et al. A growth stimulus is needed for IGF-1 to induce skeletal muscle hypertrophy in vivo. J Cell Sci. 123:960. doi: 10.1242/jcs.061119. [DOI] [PubMed] [Google Scholar]
- 121.Matheny RW, Jr., Nindl BC, Adamo ML. Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology. 151:865. doi: 10.1210/en.2009-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsatsoulis A, Fountoulakis S. The protective role of exercise on stress system dysregulation and comorbidities. Ann N Y Acad Sci. 2006;1083:196. doi: 10.1196/annals.1367.020. [DOI] [PubMed] [Google Scholar]
- 123.Braith RW, Magyari PM, Pierce GL, et al. Effect of resistance exercise on skeletal muscle myopathy in heart transplant recipients. Am J Cardiol. 2005;95:1192. doi: 10.1016/j.amjcard.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 124.Morley JE, Argiles JM, Evans WJ, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 11:391. doi: 10.1016/j.jamda.2010.04.014. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ahola-Erkkila S, Carroll CJ, Peltola-Mjosund K, et al. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum Mol Genet. 19:1974. doi: 10.1093/hmg/ddq076. [DOI] [PubMed] [Google Scholar]
- 126.Muscaritoli M, Costelli P, Aversa Z, et al. New strategies to overcome cancer cachexia: from molecular mechanisms to the `Parallel Pathway'. Asia Pac J Clin Nutr. 2008;17(Suppl 1):387. [PubMed] [Google Scholar]
- 127.Hornberger TA, Sukhija KB, Chien S. Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle. 2006;5:1391. doi: 10.4161/cc.5.13.2921. [DOI] [PubMed] [Google Scholar]
- 128.Sakuma K, Yamaguchi A. Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr Aging Sci. 3:90. doi: 10.2174/1874609811003020090. [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Kubica N, Ellisen LW, et al. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem. 2006;281:39128. doi: 10.1074/jbc.M610023200. [DOI] [PubMed] [Google Scholar]
- 130.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280:9769. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- 131.DeYoung MP, Horak P, Sofer A, et al. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McGhee NK, Jefferson LS, Kimball SR. Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor, REDD1. J Nutr. 2009;139:828. doi: 10.3945/jn.108.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lang CH, Frost RA, Vary TC. Acute alcohol intoxication increases REDD1 in skeletal muscle. Alcohol Clin Exp Res. 2008;32:796. doi: 10.1111/j.1530-0277.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 134.Murakami T, Hasegawa K, Yoshinaga M. Rapid induction of REDD1 expression by endurance exercise in rat skeletal muscle. Biochem Biophys Res Commun. 405:615. doi: 10.1016/j.bbrc.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 135.Kumari R, Willing LB, Jefferson LS, et al. REDD1 (regulated in development and DNA damage response 1) expression in skeletal muscle as a surrogate biomarker of the efficiency of glucocorticoid receptor blockade. Biochem Biophys Res Commun. 412:644. doi: 10.1016/j.bbrc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frost RA, Huber D, Pruznak A, et al. Regulation of REDD1 by insulin-like growth factor-I in skeletal muscle and myotubes. J Cell Biochem. 2009;108:1192. doi: 10.1002/jcb.22349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Regazzetti C, Bost F, Le Marchand-Brustel Y, et al. Insulin induces REDD1 expression through hypoxia-inducible factor 1 activation in adipocytes. J Biol Chem. 285:5157. doi: 10.1074/jbc.M109.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Horak P, Crawford AR, Vadysirisack DD, et al. Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci U S A. 107:4675. doi: 10.1073/pnas.0907705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ellisen LW, Ramsayer KD, Johannessen CM, et al. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol Cell. 2002;10:995. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 140.Orzechowski A, Ostaszewski P, Wilczak J, et al. Rats with a glucocorticoid-induced catabolic state show symptoms of oxidative stress and spleen atrophy: the effects of age and recovery. J Vet Med A Physiol Pathol Clin Med. 2002;49:256. doi: 10.1046/j.1439-0442.2002.00443.x. [DOI] [PubMed] [Google Scholar]
- 141.Handayaningsih AE, Iguchi G, Fukuoka H, et al. Reactive Oxygen Species Play an Essential Role in IGF-I Signaling and IGF-I-Induced Myocyte Hypertrophy in C2C12 Myocytes. Endocrinology. doi: 10.1210/en.2010-0981. [DOI] [PubMed] [Google Scholar]
- 142.Frost RA, Pereyra E, Lang CH. Ethyl pyruvate preserves IGF-I sensitivity toward mTOR substrates and protein synthesis in C2C12 myotubes. Endocrinology. 152:151. doi: 10.1210/en.2010-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shimizu N, Yoshikawa N, Ito N, et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 13:170. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 144.Fielitz J, Kim MS, Shelton JM, et al. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest. 2007;117:2486. doi: 10.1172/JCI32827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clarke BA, Drujan D, Willis MS, et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376. doi: 10.1016/j.cmet.2007.09.009. 38. [DOI] [PubMed] [Google Scholar]
- 146.Cohen S, Brault JJ, Gygi SP, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185:1083. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Waddell DS, Baehr LM, van den Brandt J, et al. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab. 2008;295:E785. doi: 10.1152/ajpendo.00646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu Y, Dentin R, Chen D, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao J, Brault JJ, Schild A, et al. Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy. 2008;4:378. doi: 10.4161/auto.5633. [DOI] [PubMed] [Google Scholar]
- 150.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 143:813. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Teleman AA, Chen YW, Cohen SM. 4E-BP functions as a metabolic brake used under stress conditions but not during normal growth. Genes Dev. 2005;19:1844. doi: 10.1101/gad.341505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chiba T, Kamei Y, Shimizu T, et al. Overexpression of FOXO1 in skeletal muscle does not alter longevity in mice. Mech Ageing Dev. 2009;130:420. doi: 10.1016/j.mad.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 153.Zhang D, Contu R, Latronico MV, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 120:2805. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goodman CA, Mabrey DM, Frey JW, et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. Faseb J. 25:1028. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shah OJ, Anthony JC, Kimball SR, et al. Glucocorticoids oppose translational control by leucine in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1185. doi: 10.1152/ajpendo.2000.279.5.E1185. [DOI] [PubMed] [Google Scholar]
- 156.Schakman O, Gilson H, de Coninck V, et al. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology. 2005;146:1789. doi: 10.1210/en.2004-1594. [DOI] [PubMed] [Google Scholar]
- 157.Lang CH, Lynch CJ, Vary TC. BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice. Am J Physiol Regul Integr Comp Physiol. 299:R935. doi: 10.1152/ajpregu.00297.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu Y, Zhao W, Zhao J, et al. REDD1 is a major target of testosterone action in preventing dexamethasone-induced muscle loss. Endocrinology. 151:1050. doi: 10.1210/en.2009-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qin W, Pan J, Wu Y, et al. Protection against dexamethasone-induced muscle atrophy is related to modulation by testosterone of FOXO1 and PGC-1alpha. Biochem Biophys Res Commun. 403:473. doi: 10.1016/j.bbrc.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 160.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 161.Verdijk LB, Snijders T, Beelen M, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc. 58:2069. doi: 10.1111/j.1532-5415.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 162.Perrini S, Laviola L, Carreira MC, et al. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 205:201. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 163.West DW, Phillips SM. Anabolic processes in human skeletal muscle: restoring the identities of growth hormone and testosterone. Phys Sportsmed. 38:97. doi: 10.3810/psm.2010.10.1814. [DOI] [PubMed] [Google Scholar]
- 164.Thomas DR. Sarcopenia. Clin Geriatr Med. 26:331. doi: 10.1016/j.cger.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 165.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 166.Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673. doi: 10.1038/nrendo.2009.212. 39. [DOI] [PubMed] [Google Scholar]
- 167.Jiao Q, Pruznak AM, Huber D, et al. Castration Differentially Alters Basal and Leucine-Stimulated Tissue Protein Synthesis in Skeletal Muscle and Adipose Tissue. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00473.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pennings B, Koopman R, Beelen M, et al. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 93:322. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 169.Johansen JA, Breedlove SM, Jordan CL. Androgen receptor expression in the levator ani muscle of male mice. J Neuroendocrinol. 2007;19:823. doi: 10.1111/j.1365-2826.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 170.Sato K, Iemitsu M, Aizawa K, et al. Testosterone and DHEA activate the glucose metabolismrelated signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E961. doi: 10.1152/ajpendo.00678.2007. [DOI] [PubMed] [Google Scholar]
- 171.Aizawa K, Iemitsu M, Maeda S, et al. Acute exercise activates local bioactive androgen metabolism in skeletal muscle. Steroids. 75:219. doi: 10.1016/j.steroids.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 172.Borst SE, Lee JH, Conover CF. Inhibition of 5alpha-reductase blocks prostate effects of testosterone without blocking anabolic effects. Am J Physiol Endocrinol Metab. 2005;288:E222. doi: 10.1152/ajpendo.00305.2004. [DOI] [PubMed] [Google Scholar]
- 173.MacLean HE, Chiu WS, Notini AJ, et al. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. Faseb J. 2008;22:2676. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- 174.Chambon C, Duteil D, Vignaud A, et al. Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci U S A. 107:14327. doi: 10.1073/pnas.1009536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Klover P, Chen W, Zhu BM, et al. Skeletal muscle growth and fiber composition in mice are regulated through the transcription factors STAT5a/b: linking growth hormone to the androgen receptor. Faseb J. 2009;23:3140. doi: 10.1096/fj.08-128215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ibebunjo C, Eash JK, Li C, et al. Voluntary running, skeletal muscle gene expression, and signaling inversely regulated by orchidectomy and testosterone replacement. Am J Physiol Endocrinol Metab. 300:E327. doi: 10.1152/ajpendo.00402.2010. [DOI] [PubMed] [Google Scholar]
- 177.D'Antona G, Ragni M, Cardile A, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 12:362. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 178.Honjoh S, Yamamoto T, Uno M, et al. Signalling through RHEB-1 mediates intermittent fastinginduced longevity in C. elegans. Nature. 2009;457:726. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- 179.Szulc P, Duboeuf F, Marchand F, et al. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 180.Raskin K, de Gendt K, Duittoz A, et al. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29:4461. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miyamoto J, Matsumoto T, Shiina H, et al. The pituitary function of androgen receptor constitutes a glucocorticoid production circuit. Mol Cell Biol. 2007;27:4807. doi: 10.1128/MCB.02039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jones A, Hwang DJ, Narayanan R, et al. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology. 151:3706. doi: 10.1210/en.2010-0150. [DOI] [PubMed] [Google Scholar]
- 183.Belavy DL, Miokovic T, Armbrecht G, et al. Differential atrophy of the lower-limb musculature during prolonged bed-rest. Eur J Appl Physiol. 2009;107:489. doi: 10.1007/s00421-009-1136-0. [DOI] [PubMed] [Google Scholar]
- 184.Murton AJ, Greenhaff PL. Muscle atrophy in immobilization and senescence in humans. Curr Opin Neurol. 2009;22:500. doi: 10.1097/WCO.0b013e32832f15e1. [DOI] [PubMed] [Google Scholar]
- 185.Stein TP, Donaldson MR, Leskiw MJ, et al. Branched-chain amino acid supplementation during bed rest: effect on recovery. J Appl Physiol. 2003;94:1345. doi: 10.1152/japplphysiol.00481.2002. 40. [DOI] [PubMed] [Google Scholar]
- 186.Brooks N, Cloutier GJ, Cadena SM, et al. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J Appl Physiol. 2008;105:241. doi: 10.1152/japplphysiol.01346.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brooks NE, Cadena SM, Vannier E, et al. Effects of resistance exercise combined with essential amino acid supplementation and energy deficit on markers of skeletal muscle atrophy and regeneration during bed rest and active recovery. Muscle Nerve. 42:927. doi: 10.1002/mus.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alzghoul MB, Gerrard D, Watkins BA, et al. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. Faseb J. 2004;18:221. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- 189.Cai D, Frantz JD, Tawa NE, Jr, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 190.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Buford TW, Cooke MB, Manini TM, et al. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci. 65:532. doi: 10.1093/gerona/glp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Reed SA, Senf SM, Cornwell EW, et al. Inhibition of IkappaB kinase alpha (IKKalpha) or IKKbeta (IKKbeta) plus forkhead box O (Foxo) abolishes skeletal muscle atrophy. Biochem Biophys Res Commun. 405:491. doi: 10.1016/j.bbrc.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dodd SL, Gagnon BJ, Senf SM, et al. Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve. 41:110. doi: 10.1002/mus.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reid MB. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic Biol Med. 2008;44:169. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 195.Arbogast S, Smith J, Matuszczak Y, et al. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J Appl Physiol. 2007;102:956. doi: 10.1152/japplphysiol.00538.2006. [DOI] [PubMed] [Google Scholar]
- 196.Selsby JT, Rother S, Tsuda S, et al. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol. 2007;102:1702. doi: 10.1152/japplphysiol.00722.2006. [DOI] [PubMed] [Google Scholar]
- 197.Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol. 2005;289:R134. doi: 10.1152/ajpregu.00497.2004. [DOI] [PubMed] [Google Scholar]
- 198.Kakigi R, Naito H, Ogura Y, et al. Heat stress enhances mTOR signaling after resistance exercise in human skeletal muscle. J Physiol Sci. 61:131. doi: 10.1007/s12576-010-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen YW, Gregory CM, Scarborough MT, et al. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genomics. 2007;31:510. doi: 10.1152/physiolgenomics.00115.2006. [DOI] [PubMed] [Google Scholar]
- 200.Stevens-Lapsley JE, Ye F, Liu M, et al. Impact of viral-mediated IGF-1 gene transfer on skeletal muscle following cast immobilization. Am J Physiol Endocrinol Metab. 299:E730. doi: 10.1152/ajpendo.00230.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Criswell DS, Booth FW, DeMayo F, et al. Overexpression of IGF-I in skeletal muscle of transgenic mice does not prevent unloading-induced atrophy. Am J Physiol. 1998;275:E373. doi: 10.1152/ajpendo.1998.275.3.e373. [DOI] [PubMed] [Google Scholar]
- 202.Merezhinskaya N, Fishbein WN, Davis JI, et al. Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve. 2000;23:90. doi: 10.1002/(sici)1097-4598(200001)23:1<90::aid-mus12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 203.Evans K, Nasim Z, Brown J, et al. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol. 2007;18:1426. doi: 10.1681/ASN.2006091014. [DOI] [PubMed] [Google Scholar]
- 204.Chambard JC, Pouyssegur J. Intracellular pH controls growth facto-induced ribosomal protein S6 phosphorylation and protein synthesis in the G0----G1 transition of fibroblasts. Exp Cell Res. 1986;164:282. doi: 10.1016/0014-4827(86)90029-7. 41. [DOI] [PubMed] [Google Scholar]
- 205.Woods DJ, Soden J, Tomlinson S, et al. Transmembrane Na+/H+ exchange in the rat thyroid cell strain FRTL-5: a possible role in insulin-like growth factor-I-mediated proliferation. J Mol Endocrinol. 1990;4:177. doi: 10.1677/jme.0.0040177. [DOI] [PubMed] [Google Scholar]
- 206.Fernandez-Sola J, Preedy VR, Lang CH, et al. Molecular and cellular events in alcohol-induced muscle disease. Alcohol Clin Exp Res. 2007;31:1953. doi: 10.1111/j.1530-0277.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 207.Lang CH, Kimball SR, Frost RA, et al. Alcohol myopathy: impairment of protein synthesis and translation initiation. Int J Biochem Cell Biol. 2001;33:457. doi: 10.1016/s1357-2725(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 208.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E917. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- 209.Lang CH, Fan J, Lipton BP, et al. Modulation of the insulin-like growth factor system by chronic alcohol feeding. Alcohol Clin Exp Res. 1998;22:823. [PubMed] [Google Scholar]
- 210.Lang CH, Frost RA, Svanberg E, et al. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab. 2004;286:E916. doi: 10.1152/ajpendo.00554.2003. [DOI] [PubMed] [Google Scholar]
- 211.Lang CH, Frost RA, Deshpande N, et al. Alcohol impairs leucine-mediated phosphorylation of 4EBP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab. 2003;285:E1205. doi: 10.1152/ajpendo.00177.2003. [DOI] [PubMed] [Google Scholar]
- 212.Lang CH, Frost RA, Vary TC. Skeletal muscle protein synthesis and degradation exhibit sexual dimorphism after chronic alcohol consumption but not acute intoxication. Am J Physiol Endocrinol Metab. 2007;292:E1497. doi: 10.1152/ajpendo.00603.2006. [DOI] [PubMed] [Google Scholar]
- 213.Vargas R, Lang CH. Alcohol accelerates loss of muscle and impairs recovery of muscle mass resulting from disuse atrophy. Alcohol Clin Exp Res. 2008;32:128. doi: 10.1111/j.1530-0277.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 214.Lang CH, Lynch CJ, Vary TC. Alcohol-induced IGF-I resistance is ameliorated in mice deficient for mitochondrial branched-chain aminotransferase. J Nutr. 140:932. doi: 10.3945/jn.109.120501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sun X, Zemel MB. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab (Lond) 2009;6:26. doi: 10.1186/1743-7075-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Du M, Shen QW, Zhu MJ, et al. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci. 2007;85:919. doi: 10.2527/jas.2006-342. [DOI] [PubMed] [Google Scholar]
- 217.Hong-Brown LQ, Brown CR, Navaratnarajah M, et al. Alcohol-induced modulation of rictor and mTORC2 activity in C2C12 myoblasts. Alcohol Clin Exp Res. 35:1445. doi: 10.1111/j.1530-0277.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hong-Brown LQ, Brown CR, Kazi AA, et al. Rag GTPases and AMPK/TSC2/Rheb Mediate the Differential Regulation of mTORC1 Signaling in Response to Alcohol and Leucine. Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]