Abstract
Four experiments examined the roles of the basolateral amygdala and orbitofrontal cortex in the formation of sensory-specific associations in conditioned flavor preference and conditioned magazine approach paradigms using US devaluation and selective Pavlovian-instrumental transfer procedures in Long Evans rats. Experiment 1 found that pre-training amygdala and orbitofrontal cortex lesions had no detectable effect on the formation or flexible use of sensory-specific flavor-nutrient associations in a US devaluation task, where flavor cues were paired either simultaneously or sequentially with nutrient rewards in water-deprived subjects. In Experiment 2, pre-training amygdala and orbitofrontal cortex lesions both attenuated outcome-specific Pavlovian-instrumental transfer. Experiment 3 indicated that amygdala lesions have no effect on the formation of sensory-specific flavor-nutrient associations in a US devaluation task in food-deprived subjects. Finally, Experiment 4 demonstrated that the outcomes used in Experiment 3 were sufficiently motivationally significant to support conditioned flavor preference. These findings suggest that although both orbitofrontal cortex and amygdala lesions attenuate the acquisition of sensory-specific associations in magazine approach conditioning, neither lesion reduces the ability to appropriately respond to a flavor cue that was paired with a devalued outcome.
Keywords: US devaluation, Pavlovian-to-instrumental transfer (PIT), reward processing, rats
Introduction
Previous research suggests that the basolateral amygdala (BLA) and the orbitofrontal cortex (OFC) structures are involved in the formation of sensory-specific associations in Pavlovian learning. Many of these studies used Pavlovian-instrumental transfer (PIT) and unconditioned stimulus (US) devaluation tasks to assess such learning, in which auditory and visual conditioned stimuli (CS) were paired with nutrient USs in hungry rats (Corbit & Balleine, 2005; Gallagher, McMahan, & Schoenbaum, 1999; Hatfield, Han, Conley, Gallagher, & Holland, 1996; Pickens, Saddoris, Setlow, Gallagher, Holland, & Schoenbaum, 2003). In addition, immediate early gene (IEG) activation studies suggest a role for the BLA, OFC, gustatory cortex (GC) and posterior accumbens shell in sensory-specific associations in Pavlovian learning with auditory CSs (Kerfoot, Agarwal, Lee, and Holland, 2007), as well as a role for the BLA (Desgranges, Ramirez-Amaya, Ricaño-Cornejo, Levy, and Ferreira, 2010) and GC (Saddoris, Geirut, Holland, & Gallagher, 2008; Saddoris, Holland, & Gallagher, 2009) with flavor and odor CSs, respectively.
However, other studies suggest that the BLA is not always involved in the formation of sensory-specific associations (Blundell, Hall, & Killcross, 2003; Dwyer & Killcross, 2006). In their Experiment 2, Blundell et al. (2003) presented thirsty rats with two pairs of taste-taste mixtures (e.g., sucrose + HCL, NaCl + quinine) and then devalued one of the tastes (e.g., HCL) by pairing it with LiCl. The subjects were then given a choice between the two taste associates of the differentially valued tastes (i.e., sucrose vs. NaCl). The results revealed that the BLA-lesioned and the normal subjects both avoided the taste that was previously paired with the devalued taste (e.g., sucrose). These results suggest that sensory-specific associations were formed between the tastes that were paired together and that the BLA lesions failed to disrupt the formation of these associations.
Blundell et al. (2003) attempted to reconcile the conflicting US devaluation results in their task compared to other research (listed above) by suggesting that the BLA may not be involved in the formation of sensory-specific associations in tasks where predictive cues signal motivationally neutral target events, but is involved in tasks where predictive cues signal motivationally significant events. This notion is supported by additional studies that show that BLA lesions have no effect on sensory-preconditioning (Dwyer & Killcross, 2006). For example, Dwyer and Killcross (2006) presented thirsty rats with two compound solutions, each containing one nutritive and one non-nutritive taste-cue. Although the latter procedure included nutritive tastes (5% sucrose and 5% maltodextrin) in each compound, the authors suggested that these nutrients were not sufficiently motivationally-significant to induce BLA-dependent encoding of their sensory characteristics. One potential problem for this interpretation is that these procedures differ from traditional Pavlovian or instrumental conditioning tasks on dimensions other than the degree of motivational significance.
Specifically, a procedure in which the two gustatory stimuli are consumed simultaneously (such as the one implemented by Blundell et al., 2003; Dwyer & Killcross, 2006) may employ different neural structures than procedures in which an auditory or visual event precedes reward delivery, such as the ones used in traditional magazine approach paradigms (Gallagher et al., 1999; Corbit & Balleine, 2005). There is evidence to suggest that learning differs both qualitatively (Higgins & Rescorla, 2004) and quantitatively (Mowrer & Aiken, 1954; Smith & Roll, 1967; Rescorla, 1980) in Pavlovian paradigms where a CS predicts the future presentations of the US (i.e., delay or trace conditioning) as opposed to ones where the CS and US are presented at the same time (simultaneous conditioning). Thus, it is possible that simultaneous and sequential training procedures may result in learning that depends upon different neural mechanisms. Additionally, gustatory-gustatory learning may simply involve different neural substrates than auditory-gustatory or visual-gustatory learning.
Nevertheless, the experiments in this paper were designed to investigate the importance of the BLA and OFC in devaluation situations involving flavor-nutrient learning in which the nutrient USs were clearly motivationally significant. Under these circumstances the hypothesis put forth by Blundell et al. (2003) would suggest that sensory-specific flavor-nutrient associations should be BLA-dependent, and possibly also OFC-dependent. Some research has already demonstrated that BLA lesions impair flavor preference learning (Dwyer, 2011; Touzani & Sclafani, 2005), and these findings suggest that a lesion effect may extend to the learning of sensory-specific associations in such a task, but this issue has not yet been explored and is the point of the studies reported here.
Prior to the beginning of each experiment in the present studies, subjects received bilateral lesions (either BLA, OFC, or Sham). After the subjects recovered from surgery, we established conditioned flavor preferences by pairing two distinct neutral flavor CSs with different motivationally significant USs (Experiments 1 and 3). We then assessed the effect of selective devaluation of one of the nutrients on preference for the two flavor cues. This provided our measure of sensory-specific flavor-nutrient learning. Non-lesioned control rats were expected to avoid the flavor cue that had been associated with the devalued nutrient, but if BLA or OFC lesions undermine the development of sensory-specific flavor-nutrient learning when motivationally significant nutrients are used (see Blundell, et al., 2003) then we expected no US devaluation effect in lesioned rats. Moreover, in order to assess the importance of CS-US pairing method, some rats were trained in the usual way with flavor and nutrients mixed in solution, whereas other rats were trained using a sequential procedure where flavor cues preceded their paired nutrients. In addition, we extended the previous findings of Corbit and Balleine (2005) and Ostlund and Balleine (2007) by using a PIT task with two liquid reinforcers (Experiment 2) to assess the fate of sensory-specific associations in thirsty, as opposed to hungry, rats. Furthermore, this study also extended the findings of Gallagher et al. (1999) and Hatfield et al. (1996) by using a US devaluation technique to assess the fate of sensory-specific associations in a design where two CS-US pairs, as opposed to one, were learned. Finally, we validated the notion that the solutions used in Experiment 3 were in fact motivationally significant to the subjects (Experiment 4). Experiments 1 and 2 were conducted at Brooklyn College (Institutional Animal Care and Use Committee # 222, approved by Brooklyn College), and Experiments 3 and 4 at Johns Hopkins University (Institutional Animal Care and Use Committee # RA07A288 and RA10A162, approved by Johns Hopkins University).
Materials and Methods
Experiment 1
Subjects
Subjects were 96 naïve Long Evans rats (24 male and 72 female), weighing 350-422 g (males) and 251-318 g (females) at the start of the experiment. The subjects were bred at Brooklyn College and derived from rats obtained from Charles River laboratory (Wilmington, Massachusetts, USA). The subjects were individually housed in stainless steel cages (24 cm × 18 cm × 17.5 cm) and maintained on a 14:10 light: dark cycle. Food chow (Lab Diet 5001) was available ad libitum throughout the study, but fluids were restricted to two, 15-min drinking sessions per day, which were always five hours apart, starting four hours after the lights came on in the colony rooms. All sessions were conducted in the rats’ home cages.
Surgical Procedures
Approximately 2 weeks before behavioral training, some subjects received bilateral BLA lesions (8 males and 24 females), some received bilateral OFC lesions (8 males and 24 females), while the remainder received sham BLA (4 males and 12 females) or sham OFC (4 males and 12 females) lesions. Prior to surgery the subjects were anesthetized with sodium pentobarbital (50 mg/kg; Sigma-Aldrich Laboratories, administered intraperitoneally). Exactly 15 min after receiving the anesthetic, the subjects were treated with atropine sulfate (0.54 mg/kg; Sigma-Aldrich Laboratories, administered intramuscularly) in order to help maintain respiration. The coordinates relative to bregma for the BLA lesioned subjects (see Ostlund & Balleine, 2008) were as follows: −2.3, −3.0 (AP); +/− 5.2 (ML); −7.6 (DV). Neurotoxin (0.25 μl of NMDA; 20 μg/μl mixed in distilled water) was infused into each hole at the rate of 0.10 μl/min using an infusion pump (KD Scientific 53100) and a 1-μl Hamilton syringe. In order to allow for diffusion, the needle stayed in place for an additional 3 min prior to being extracted from the infusion site. The coordinates for the OFC lesioned subjects (see Ostlund & Balleine, 2007) were as follows: +3.5 (AP); +/− 3.2 (ML); −4.7 (DV). NMDA (20 μg/μl) was infused at the rate of 0.12 μl/min, in the amount of 0.50 μl for the injection site. After the injection, the needle remained in place for an additional 5 min before being extracted to allow for diffusion. Sham controls received the same treatment as the lesioned subjects except that after the needle was lowered into the appropriate location, NMDA was not infused.
Histological Procedures
After completing the experiment, the subjects were injected (i.p.) with 1 ml of diluted (10:1) Beuthanasia D (Schering Plough Corporation, Millsboro, DE). Once non-responsive, the rats were perfused transcardially with physiological saline and 10% formalin. The brains were extracted and stored in a refrigerator (3°C) in a solution of 30% sucrose and 10% formalin for 1 week. They were then placed in a Cryostat (Microm HM 505E) and allowed 15 min to freeze (−23°C). The brains were then sectioned (40 μM coronal sections) and mounted onto slides (Corning Frosted Micro Slides), which were pretreated with 2% gelatin (Gelatin Type B, Sigma Aldrich, St. Louis, MO). The tissues were then Nissl stained, and coverslipped (Corning Cover Glass) using Permount histological mounting medium (Sigma Aldrich). The extent of lesion was assessed with a microscope (10, 40, and 100 times zoom) depending on the amount of neuronal loss in the targeted area.
Behavioral Procedures
Solutions
The solutions were presented in 50 ml drinking tubes and were attached to the outside of the cage using a metal spring. The metal spouts protruded approximately 3.5 cm into the cage. The flavor CSs consisted of 1% banana and 1% almond imitation flavor extract (McCormick) mixed with tap water. The nutrient USs were 10% sucrose (Domino) and 10% Polycose (Ross Laboratories) solutions prepared with tap water. These nutrients have been shown to be motivationally significant for thirsty rats since as little as 8% (Albertella & Boakes, 2006) and even 4% (Harris, Shand, Carroll, & Westbrook, 2004) concentrations of sucrose have been found to establish a lasting preference for a flavor paired with sucrose over a flavor not paired with sucrose. These flavors and nutrients were either presented separately (Group Sequential) or mixed together (Group Simultaneous).
Acquisition
The design for this experiment is illustrated in Table 1. In order to familiarize the subjects with the water deprivation schedule, they were given one-bottle water training, which lasted three days. During this phase, the subjects were presented with a single 50-ml tube of tap water for 15 min twice a day, presented 5 hours apart starting 4 hours after the lights came on in the colony rooms. The next day after water training was complete, acquisition training began. During the acquisition phase the subjects were presented with one flavor CS (CSd) paired with a particular nutrient US (USd) and the other flavor CS (CSnd) paired with the other nutrient (USnd). The physical identities of the CSs and USs were counterbalanced. The simultaneous groups (N = 36) received the flavor CS mixed in solution with a nutrient US for 15 min. The sequential groups (N = 60, run in two replications) received the flavor CS for 5 min followed immediately by the appropriate nutrient US for 10 min. One third of the subjects in each set of groups (simultaneous and sequential) were BLA lesioned, one third were OFC lesioned, and the remainder had sham lesions. Each flavor-nutrient pairing was presented once a day for 8 days, five hours apart and was counterbalanced for order across days. Thus, each flavor CS was paired with a distinctive nutrient US on 8 occasions four times in the AM session and four times in the PM session. Intakes were recorded to the nearest 0.1 gram by comparing the weight of the bottles and its solution before and after consumption.
Table 1. Design for Experiment 1.
| Simultaneous | |||
|---|---|---|---|
| Acquisition | Devaluation | Flavor Test | Nutrient Test |
| CSd+USd | USd → LiCl | CSd vs. CSnd | USd vs. USnd |
| CSnd+USnd | Usnd - | ||
| Sequential | |||
|---|---|---|---|
| Acquisition | Devaluation | Flavor Test | Nutrient Test |
| CSd→USd | USd → LiCl | CSd vs. CSnd | USd vs. USnd |
| CSnd→USnd | Usnd - | ||
Note. This table represents the design for Experiment 1 for subjects that received simultaneous (top panel) and sequential training (bottom panel). The CSs were 1% almond and 1% banana McCormick imitation extracts. The USs were 10% sucrose and 10% Polycose. All flavor-nutrient pairings were counterbalanced across subjects. For subjects that received simultaneous training the flavor CSs and the nutrient USs were mixed in a solution. For subjects that received sequential training, the flavor CSs were presented first and was then was immediately followed by the nutrient USs.
Devaluation
On the day following the final acquisition session, the animals entered a 6-day devaluation phase. During the 15-min AM session on Days 1, 3, and 5, the subjects were presented with one of the nutrients (USd) without its associated CS. Immediately following consumption of the USd, they were injected (i.p.) with LiCl (0.3 M, 1% body weight). Subjects were given tap water for 15 min during the PM session of each day. During the AM session on Days 2, 4, and 6, the subjects were presented with the alternate nutrient (USnd), but were not given LiCl. During the PM session on these days the subjects were again given tap water to drink for 15 min.
Testing
The day after the devaluation training was completed, the subjects were given one day of two-bottle water training in order to familiarize them with the testing procedure, where they were presented with two test tubes each containing tap water during the AM session. Two-bottle choice tests throughout all experiments were conducted in the following manner. The subjects were first given the solution on the left to sample. After they had tasted it, the solution was removed and they were allowed to taste the solution on the right. Once they sampled that solution, it was removed and both solutions were then simultaneously presented for 15 min. On each of the two days following the two-bottle water training session, the subjects were given a two-bottle flavor choice test. In these sessions, one bottle contained CSd and the other bottle contained CSnd, and these were pitted against one another without any nutrients for 15 min. On the following day the flavor CS that was presented on the right was presented on the left and vice versa in order to counterbalance the side positions.
On the two days following the completion of the two-bottle flavor CS choice tests, the subjects were given 2, two-bottle nutrient choice tests in order to verify that the nutrients were differentially valued. These tests were conducted exactly like the preceding flavor tests except that the two nutrient USs were pitted against one another (in the absence of the flavor CSs).
Statistical Analysis
Here and throughout this paper, analysis of variance (ANOVA) techniques were used to analyze the data.
Experiment 2
Subjects
Subjects were 36 naïve Long-Evans rats (21 male and 15 female), weighing 356-418 g (males) and 258-320 g (females) at the start of the experiment. Some subjects received a BLA lesion (7 males and 5 females), some received an OFC lesion (7 males and 5 females), while the remainder received a sham BLA (4 males and 2 females) or OFC (3 males and 3 females) lesion. The rats were individually housed and maintained in conditions similar but not identical to those in Experiment 1. In particular, throughout the experiment all subjects were maintained with unlimited access to food, but their water access outside of the experimental session was restricted to 30 min/day (approximately 3 hrs after a given training session).
Surgery and Histology
The surgery and histology methods were identical to those used in Experiment 1.
Apparatus
The apparatus consisted of two sets of eight identical conditioning chambers, which were encased in lightproof and soundproof wooden shells. The dimensions of the conditioning chambers were 30.5 cm long × 24.0 cm wide × 25.0 cm deep. The end walls were made from aluminum and the ceiling and the sidewalls were made from clear Plexiglass. The food magazine measured 3.0 cm long × 3.6 cm wide × 2.0 cm deep and was located at the center of one of the end walls. The USs (0.1 ml droplet of 10% sucrose or 10% Polycose) was delivered into a well on the bottom of the food magazine. The floor was made from stainless steel rods (0.6 cm in diameter, 2.0 cm apart). An infrared detector and emitter were mounted on the magazine walls (and positioned at the entrance) to record magazine entry behavior. A response lever was 4 cm wide and was located 3 cm to the right of the food magazine and 8 cm above the floor level. While the lever was permanently mounted in the chamber, access to it was only available during appropriate instrumental training or test sessions. At other times a sheet metal covering prevented contact with the lever. A chain manipulandum was located 3 cm to the left of the magazine. Unless used during the instrumental training and test sessions, the chain was not present in the chamber. A tone CS (1500 Hz) was produced by a speaker located approximately 22 cm behind the front wall of the conditioning chamber. This tone measured 4 dB above background noise levels. A flashing light CS (6-W light bulb) was mounted on the bottom sidewall of the outer chamber and the light flashed with approximately equal on-off pulse durations at the approximate rate of 2/s. Background noise and ventilation was provided by a fan, which was attached to the outer shell. Background noise was measured at 78 dB. The equipment was controlled and data were recorded by a personal computer and interfacing equipment (Alpha Products), which was located in the same room as the experimental chambers.
Behavioral Procedures
Magazine training
The design for this experiment is illustrated in Table 2. For two days thirsty subjects were placed in individual conditioning chambers and were taught to approach the food magazine. During each 40-min training session the subjects were given 20 presentations of one kind of US during the first 20 min of the session and this was followed by 20 presentations of the other US during the second half of the session. The USs were presented on a variable time 60-s schedule. The two USs were delivered to different wells, adjacent to each other in the food magazine. On each of the two days, the order of US presentations within the session was counterbalanced. Access to the lever manipulandum was prevented with a sheet metal covering and the chain manipulandum was withdrawn during the magazine and Pavlovian training phases. The tone and flash CSs were not presented during the magazine training phase.
Table 2. Design for Experiment 2.
| PIT Task | |||
|---|---|---|---|
| Pavlovian | Instrumental | Retraining | PIT Test |
| CS1 → US1 | R1 → US1 | CS1 → US1 | CS1: R1 vs. R2 |
| CS2 → US2 | R2 → US2 | CS2 → US2 | CS2: R1 vs. R2 |
Note. The CSs consisted of tone (1500 Hz) and flash (6-W bulb). The USs were 10% sucrose and 10% Polycose. The instrumental responses were lever press and chain pull. All conditions were counterbalanced across subjects. During each of the two Pavlovian-instrumental transfer (PIT) tests, the subjects received 16 presentations of each CS and were allowed to make an instrumental response in either manipulandum without any USs present.
Pavlovian training
For eight days following the completion of magazine training, the subjects were taught to associate the tone CS with one US (e.g., sucrose) and the flash CS with the other US (e.g., Polycose). Half of the subjects in each lesion condition (BLA, OFC, and Sham) had tone paired with sucrose and light paired with Polycose and the rest of the subjects received the alternate pairings. Each conditioning session was 1 hr and 7 min long, during which there occurred 6 presentations of each CS-US pair. The order of CS presentations was varied irregularly across days with the constraint that no single CS could occur three or more times in a row. Each CS lasted 30 s and the appropriate US was delivered immediately at the offset of the CS. The intertrial intervals (ITIs) varied between 180 and 420 s and averaged 300 s. The subjects were removed from the chambers 1 min following the final conditioning trial. The number of magazine approach responses was recorded 30 s before, during, and 20 s after each trial.
Instrumental training
On the day immediately following the completion of Pavlovian training, the subjects were taught to press the lever to earn one outcome (e.g., sucrose) and pull the chain for the other outcome (e.g., Polycose) on a continuous reinforcement schedule (CRF). Half of the subjects in each lesion condition (BLA, OFC, and Sham) received lever paired with sucrose and chain paired with Polycose and the rest of the subjects received the alternate pairings. These assignments were orthogonal to the particular Pavlovian CS-US combinations. Each subject was trained on the CRF schedule until they made 50 responses on each manipulandum. After completing CRF training, all subjects were trained on steadily increasing variable interval schedules: VI 10 s for 1 day, VI 30 s for 2 days, and VI 60 s for 2 days. On each day, subjects were given two 20-min training sessions, one with lever and one with chain. The order of these training sessions was counterbalanced across days. The number of responses was recorded during each session.
Pavlovian-instrumental transfer test
Two Pavlovian retraining sessions were given to all the subjects on the two days following the completion of instrumental training. The first transfer test occurred the day after Pavlovian retraining. Both instrumental manipulanda were present at this time but no USs were presented during this test. The test session lasted for 34 min, with alternating periods of 30 s CS on and 30 s CS off periods. Each PIT test began with a 2-min extinction period in order to familiarize the animals with the choice procedure as well as to lower the overall levels of responding before the transfer trials began. Each CS was presented 16 times and the sequence was as follows: FTTFTFFTTFFTFTFTTFTFTFTFTFTFTFFT. A second transfer test was conducted the day after two additional instrumental (VI 60) retraining sessions were given with each response. The second PIT test was identical to the first one. Instrumental as well as magazine approach responses were measured 30 s before and during each trial.
Experiment 3
Subjects
Subjects were 24 male Long-Evans rats (Charles River Laboratories, Raleigh, North Carolina, USA), weighing 363-617 g prior to the experiment. Prior to the experiment, 14 subjects received BLA lesions and the remainder received sham lesions. After surgery, the rats were involved in an appetitive-conditioning experiment in which they were all food deprived and exposed to audio and visual stimuli as well as a sucrose reward. After that experiment, the subjects were returned to an ad lib diet for two weeks. After two weeks, the rats’ weights were recorded, and they were gradually food deprived to 85% of their ad libitum body weight.
Surgery and Histology
The procedure for making BLA lesions differed slightly from that employed in Experiments 1 and 2, although the resultant lesions were similar. Neurotoxic lesions were made using NMDA (Sigma-Aldrich, St. Louis, MO) at a concentration of 10.0 mg/ml in Dulbecco’s saline (Sigma-Aldrich, St. Louis, MO) infused at a rate of 0.1 μl/min. Injections were made 2.8-mm posterior to bregma and 5.1-mm from the midline, and at 8.4-mm (0.08 μl) and 8.7-mm (0.16 μl) ventral from the skull surface at bregma. These microinjections were made using a 2.0-μl Hamilton syringe. The histology methods were similar, with no relevant differences.
Acquisition and Devaluation
Acquisition and devaluation phases were similar to those used for the simultaneously trained subjects in Experiment 1 (see Table 1, top panel) with a few exceptions. The nutrient USs consisted of 16% (w/v) maltodextrin and a 7.6% (v/v) corn oil (Foodhold U.S.A., Landover, MD) emulsion prepared with 0.38% (w/v) soy lecithin (Swanson Health Products, Fargo, ND). Subjects received three 10-min pairings of each flavor-nutrient compound. In addition, the devaluation phase consisted of only 1 devaluation cycle.
Testing Procedures
The flavor test consisted of one 30-min two-bottle choice test where the two flavors (i.e., almond and banana) were pitted against one another. Nutrient tests consisted of two 10-min, one-bottle consumption tests where on one day the subjects were given one nutrient to drink (e.g., maltodextrin) and the next day they were given the other nutrient (e.g., corn oil).
Experiment 4
Subjects
The subjects were six naïve Long Evans rats weighing between 388-535g. Approximately a week before any training, the subjects were food deprived to 85% of their free-feeding body weight.
Behavioral Procedures
This study proceeded as Experiment 3 with three exceptions. Only one CS (CS+) was presented in solution with a US, while the other CS (CS-) was presented alone. Half of the subjects received the corn-oil US whereas the other half received maltodextrin. In addition, there was no devaluation phase (the nutrient was never paired with LiCl), and two 10-min single-bottle flavor solution consumption tests followed the two-bottle test. It was expected that the subjects would prefer CS+ to CS-.
Results
Experiment 1
Histology
Figures 1 and 2 display the maximum and minimum damage as a result of BLA (Figure 1) and OFC (Figure 2) lesions for Experiment 1 (top panel), Experiment 2 (second panel), and Experiment 3 (bottom panel for BLA only) of subjects that were included in the analysis. The black areas represent minimum lesion and the grey areas represent the areas of maximum lesions. The lesion was considered to be acceptable if significant (i.e., > 95%) neuronal loss was found in the target area and was accompanied by gliosis (i.e., proliferation of astrocytes). BLA lesions typically caused neuronal loss in the anterior, posterior and ventral basolateral amygdala with some sparing of the tissue in ventro-medial lateral and dorso-lateral amygdala. OFC lesions extended to the ventral orbital, medial orbital, and the lateral orbital cortexes. Subjects with unilateral lesions or with lesions outside of the target areas were excluded from the analysis.
FIG 1.
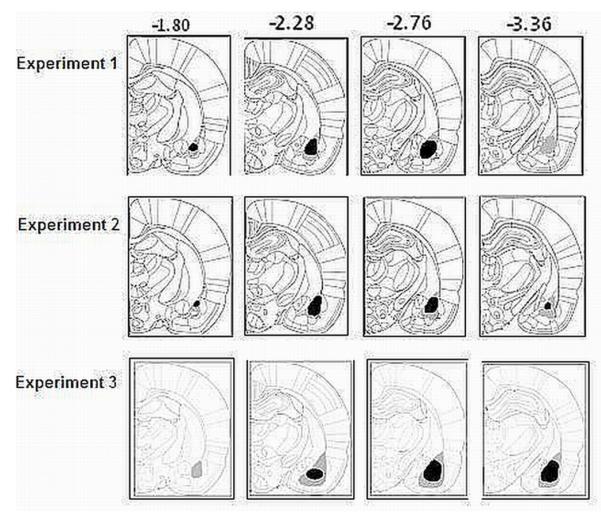
BLA histology results for Experiments 1 (top panel), 2 (middle panel), and 3 (bottom panel). Diagrams of coronal sections (40 ’M slices) illustrating the extent of the BLA lesions ranging from −1.80 to −3.36 mm (posterior to bregma). The drawings illustrate the approximate extent of the lesions. The black areas represent minimum lesion and the grey areas represent the areas of maximum lesions. Images adapted from Paxinos and Watson.
FIG 2.
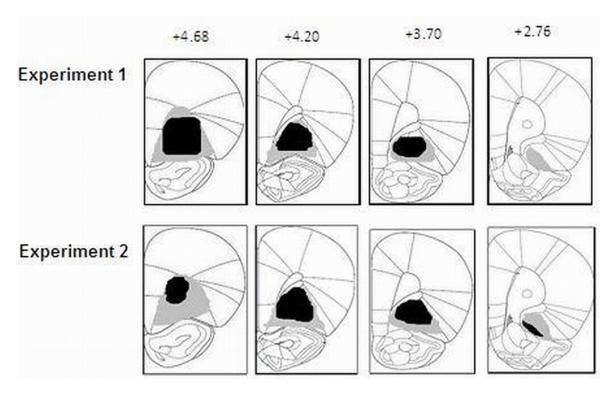
OFC histology results for Experiments 1 (top panel) and 2 (bottom panel). Diagrams of coronal sections (40 $M slices) illustrating the extent of the OFC lesions ranging from 4.68 to 2.76 mm (anterior to bregma). The drawings illustrate the approximate extent of the lesions. The black areas represent minimum lesion and the grey areas represent the areas of maximum lesions. Images adapted from Paxinos and Watson
After histological analysis, 4 BLA subjects in the simultaneous condition and 5 BLA subjects in the sequential condition were excluded due to unacceptable lesions. In addition, 5 additional BLA subjects and 2 OFC subjects from the sequential condition were excluded as the tissue became damaged during histology rendering it impossible to analyze. The data presented here include only the remaining subjects whose lesions were analyzed to be appropriate rendering the following number of subjects given simultaneous training: n = 8 for BLA (1 male and 7 females), n = 12 for OFC (4 males and 8 females), and n = 12 for Group Sham (4 males and 8 females). For the subjects given sequential training the final counts were as follows: n = 10 for BLA (1 male and 9 females), n = 18 for OFC (4 males and 14 females) and n = 20 for Sham (4 males and 16 females).
Preliminary analyses found no differences between males and females throughout the experiment in any measure, so the data presented here were collapsed across sex. In addition, the BLA, OFC, or Sham lesioned subjects did not differ in the acquisition or devaluation intake.
Flavor tests
The data of most interest are shown in Figure 3, which illustrate the results of the 2, two-bottle Flavor Tests combined in the subjects given simultaneous training (top panel) and those given sequential training (bottom panel). Preliminary analyses did not find a difference between subjects devalued on sucrose and those devalued on the Polycose US, so the data were collapsed across the devalued nutrient factor. The figure illustrates the mean intakes of CSd and CSnd for each lesion condition. The results show that all subjects despite lesion condition or training procedure consumed more of the CSnd solution than of CSd. Separate Flavor (CSd vs. CSnd) × Lesion (BLA, OFC, or Sham) ANOVAs performed on the simultaneously and sequentially trained animals revealed a significant main effect of Flavor for the groups given simultaneous conditioning (F1, 29 = 67.72, P < 0.001) as well as for the groups given sequential conditioning (F1,45 = 10.20, P = 0.003), but in neither case did these interact with Lesion (F2,29 = 0.79, P = 0.46, for the group given simultaneous and F2,47 = 0.65, P = 0.53, for sequential conditioning. Finally, the Lesion main effect was not significant for simultaneous (F2,29 = 0.16, P = 0.85) or sequential (F2, 47 = 0.86, P = 0.43) conditions.
FIG 3.
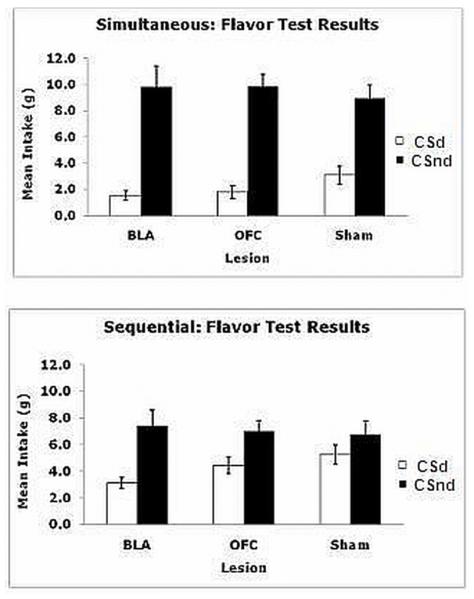
Experiment 1, flavor test results. Mean intakes of the flavor CS paired with the devalued nutrient (CSd) and flavor CS paired with the nondevalued nutrient (CSnd) collapsed across the two 2-bottle choice flavor tests, where the flavor CSs (i.e., 1% almond and 1% banana extracts) were pitted against each other without any nutrients present. The top panel illustrates the results of the two-bottle flavor choice tests for subjects with simultaneous training, whereas the bottom panel illustrates the results from the two-bottle flavor choice test for subjects with sequential training.
Nutrient tests
The nutrient test results were collapsed across tests 1 and 2 for the groups given simultaneous and sequential training. The mean intakes and standard errors of USd and USnd for each lesion condition are reported in Table 3. Since preliminary analyses did not find significant differences between subjects devalued on sucrose and those devalued on Polycose USs, the data were collapsed across this factor. The results found that all the subjects consumed significantly more of the nondevalued than the devalued solution. Separate Nutrient (USd vs. USnd) × Lesion (BLA, OFC, or Sham) ANOVAs yielded a significant main effect of Nutrient for the groups given simultaneous training (F1, 29 = 180.79, P < 0.001) and for the groups given sequential training (F1, 45 = 280.22, P < 0.001) but no Lesion main effect or Nutrient × Lesion interaction, (maximum F2,47 = 1.46, P = 0.24).
Table 3. Mean Intakes and Standard Errors of the Mean for the Nutrient Tests for Experiment 1.
| Group | Mean USd | SEM USd | Mean USnd | SEM USnd |
|---|---|---|---|---|
| Simultaneous Training | ||||
| BLA | 1.50 | 0.21 | 15.70 | 1.99 |
| OFC | 2.60 | 0.92 | 12.50 | 1.07 |
| Sham | 1.10 | 0.44 | 12.50 | 1.24 |
| Sequential Training | ||||
| BLA | 0.80 | 0.18 | 14.20 | 1.46 |
| OFC | 1.60 | 0.74 | 12.90 | 1.07 |
| Sham | 1.00 | 0.28 | 15.10 | 1.20 |
Note. USd = devalued nutrient; USnd = nondevalued nutrient; SEM = standard error of the mean. This table represents the means and standard errors of the mean for the flavor tests for Experiment 1. Subjects received either simultaneous or sequential training and the subjects within each training condition received either basolateral amygdala (BLA), orbitofrontal cortex (OFC), or Sham lesions. The flavor tests show that subjects in all conditions alike consumed more of the flavor paired with the non-devalued nutrient than the flavor paired with the devalued nutrient.
Experiment 2
Histology
The inclusion criteria were the same as that in Experiment 1. After histological analysis, 4 BLA subjects and 3 OFC subjects were excluded from the analysis due to inappropriate lesions. The data presented on the second from the top panel on Figures 1 and 2 include only the remaining subjects whose lesions were analyzed to be appropriate rendering the following number of subjects in each group: n = 8 for BLA (3 males and 5 females), n = 9 for OFC (5 males and 4 females), and n = 12 for Group Sham (7 males and 5 females).
Preliminary analysis found no differences between males and females throughout the experiment, so the data presented here are collapsed across sex. The acquisition results showed that the rats across all lesion conditions did not differ in their acquisition rates in magazine approach, instrumental or devaluation training. At the end of training the mean and SEM magazine response rates (CS-Pre) for BLA, OFC, and Sham respectively, were 5.95 (±1.08), 8.04 (±0.99), and 7.08 (±0.84).
PIT tests
The results from the two PIT tests combined are illustrated in Figure 4. Depicted there are the mean rates of instrumental responding expressed in terms of a CS - Pre CS difference score collapsed across the two PIT tests for each of the lesion conditions (i.e., BLA, OFC, and Sham). The “same” response was defined as the instrumental response that was previously reinforced with the same outcome as that signaled by the presented CS, whereas the “diff” response was defined as the instrumental response that was reinforced with the alternative outcome. The results showed that all subjects made less instrumental responding during the CSs than they did during the Pre-CS periods, presumably due to competition from magazine approach responses. However, the Sham subjects were less suppressed (i.e., made more instrumental responses) on the “same” manipulandum than on the “diff” manipulandum. A Lesion (BLA, OFC or Sham) × Response (same vs. different) ANOVA yielded a significant interaction, (F2, 26 = 3.01, P < 0.05). Furthermore, a separate Fmax test applied to the “same” – “different” difference scores showed that the homogeneity of variance assumption was violated, Fmax (3, 11) = 4.96, P < 0.05 due to increased variability in Group BLA (σ2 = 2.73) compared to the other groups (σ2 = 0.55 for OFC and σ2 = 0.55 for Sham). Because of these unequal variances, we performed separate analyses directly comparing “same” vs. “different” responses for each of the three groups using each group’s own error term rather than pooling over the three groups’ error terms. These follow-up tests revealed that the subjects in Group Sham made significantly more “same” than “diff” instrumental responses, (F1, 11 = 7.73, P = 0.02) but that there were no corresponding differences in Group BLA (F1,7 = 0.28, P = 0.61) or Group OFC (F1,8 = 3.48, P = 0.09).
FIG 4.
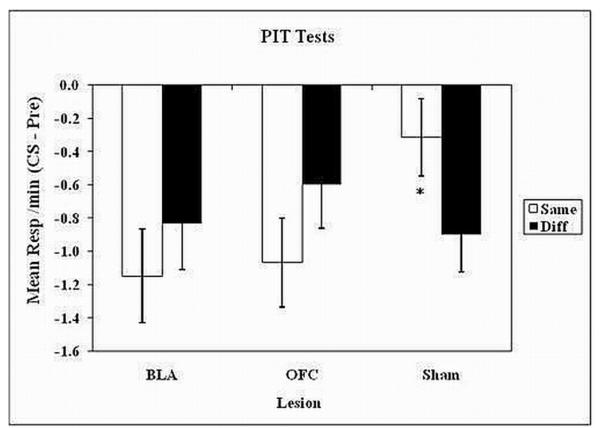
Experiment 2, PIT test results. Mean instrumental responses/min (CS-Pre) collapsed across the two Pavlovian-instrumental transfer tests for BLA, OFC and Sham lesioned subjects. During these tests, both manipulanda (i.e., lever and chain) were available but no USs could be earned. The instrumental responses on each manipulanda were measured before, during and after each CS (i.e. tone and flash) were presented. The “Same” responses were considered to be the instrumental responses, which shared an outcome with the presented CS, whereas the “Diff” responses were the instrumental responses, which did not share an outcome with the presented CS. The asterisks indicate instrumental response types, the rates of which were significantly different from the other response type for that group
Experiment 3
Histology
Eight BLA lesioned subjects were not included in the analyses because they had insufficient bilateral damage. Thus, 6 BLA lesioned subjects and 10 sham-lesioned subjects remained.
Consumption tests
Consumption of the flavor-nutrient compounds during the conditioning phase was similar in the two groups. During the devaluation phase, the BLA lesioned rats consumed 19.45 (±1.67) ml of USd and 10.82 (±1.47) ml of USnd. Sham rats did not exhibit this difference, consuming 14.91 (±1.34) and 15.49 (±1.55) ml of USd and USnd, respectively. Figure 5 shows the consumption of the two flavor solutions during the two-bottle test. In both groups, there was a strong preference for CSnd, suggesting that the flavor that was paired with the devalued nutrient evoked an updated (aversive) representation of the devalued nutrient. Finally, both groups consumed more of USnd than USd during the nutrient tests, with BLA lesioned rats consuming 0.93 (±0.18) ml of USd and 16.58 (±1.34) ml of USnd, and Sham rats consuming 1.11 (±0.23) ml of USd and 15.10 (±1.78) ml of USnd. The data were all analyzed with mixed ANOVAs with a between-subjects factor of group (lesion vs. sham) and a within-subjects factor that compared consumption of the two solutions present in each phase of the experiment. The analysis of the Phase 2 data showed that subjects consumed more USd than USnd, F1, 14 = 6.80, P = 0.02, and that this greater consumption was influenced by the BLA lesion, F1, 14 = 8.89, P < 0.01, but no main effect of lesion, F1, 14 < 0.01, P = 0.97. It is not uncommon for this difference to arise because subjects that had a USd→LiCl pairing before USnd exposure can show a level of generalized bait shyness. But there is no reason to expect this to be any greater in rats that have damage to the BLA. The analysis of the flavor choice test indicated that both groups of subjects preferred CSnd to CSd, F1, 14 = 21.56, P < 0.01 but there was no effect or interaction involving group, Fs < 1.00. Finally, the analysis of the nutrient tests revealed greater consumption of USnd than USd, F1, 14 = 154.26, P < 0.01 but no other effect or interaction, Fs < 0.23. It is not clear why the BLA lesioned rats consumed more of USd than USnd during the devaluation phase while the shams showed no such difference, but any lesion differences in consumption during the devaluation phase did not affect learning about the USs as assessed in the flavor and nutrient tests.
FIG 5.
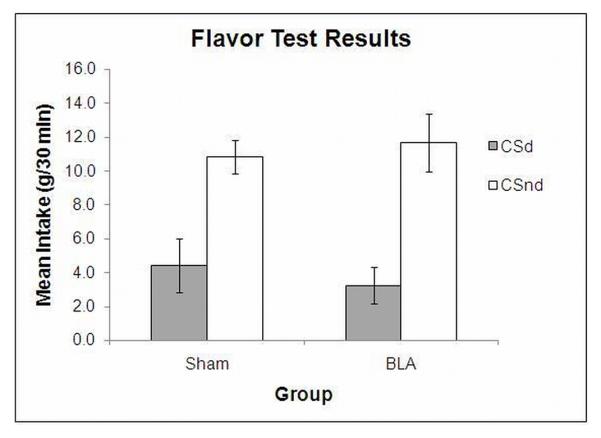
Experiment 3, flavor test results. Mean intakes of the flavor CS paired with the devalued nutrient (CSd) and flavor CS paired with the nondevalued nutrient (CSnd) collapsed across the two 2-bottle choice flavor tests, where the flavor CSs (i.e., 1% almond and 1% banana extracts) were pitted against each other without any nutrients present.
It is worth noting that in typical devaluation studies, there is some measure of conditioning in the first phase of training. Because the consumption of the flavors is confounded with consumption of the nutrients in Phase 1 of Experiment 3, there is no evidence of conditioning, and no evidence that consumption of CSnd at test is augmented because it was previously paired with a motivationally significant nutrient US. To differentiate this procedure from simple sensory preconditioning, in which two motivationally neutral cues are used, it is important to show that the nutrients are, in fact, motivationally significant reinforcers.
Experiment 4
Consumption tests
Regardless of whether corn oil or maltodextrin served as the US, mean (± standard error) consumption of CS+US solution (corn oil = 8.00 ± 0.57 ml; maltodextrin = 8.13 ± 0.32 ml) and CS-(corn oil = 1.43 ± 0.47 ml; maltodextrin = 0.98 ± 0.53 ml) collapsed across the three pairings indicated greater consumption of the CS+US solution. When the flavors were tested in a two-bottle test, rats showed a preference for CS+ (corn oil = 6.17 ± 0.60 ml; maltodextrin = 4.20 ± 1.15 ml) over CS-(corn oil = 0.6 ± 0.40 ml; maltodextrin = 0.97 ± 0.07 ml). This pattern was also reflected in the one-bottle consumption tests, in which rats consumed more CS+ solution (corn oil = 4.90 ± 1.04 ml; maltodextrin = 3.70 ± 0.64 ml) than CS-(corn oil = 1.6 ± 0.82 ml; maltodextrin = 1.03 ± 0.34 ml). These observations were supported by a series of mixed ANOVAs that revealed significant differences in consumption during training (F1, 4 = 593.70, P < .001), the two-bottle test (F1, 4 = 45.14, P < .003), and the two single-bottle tests (F1, 4 = 31.85, P < .005), but no effects or interactions involving US identity (ps > .14). The preference for CS+ indicates that the maltodextrin and corn-oil nutrients used in Experiment 3 were sufficiently rewarding to support appetitive conditioning in hungry rats. Without flavor-nutrient conditioning, consumption of the flavor solution was minimal, even in the one-bottle test.
Discussion
The experiments presented here demonstrated that pre-training BLA and OFC lesions did not impair the formation of sensory-specific flavor-nutrient associations and that these results did not depend on whether the CSs and the USs were trained simultaneously or sequentially. In addition, BLA lesions did not impair such associations regardless of the subjects’ motivational state (i.e., food-deprived or water-deprived). On the other hand, it was found that BLA and OFC lesions disrupted selective Pavlovian-instrumental transfer. These findings suggest that the roles of the BLA and OFC in learning are constrained by the nature of the stimuli involved.
While the results from Experiments 1 and 3 appear to be consistent with other studies (Blundell et al., 2003, Experiment 2; Dwyer & Killcross, 2006, Experiment 1a), they are in disagreement with common interpretations of such findings (Dwyer & Killcross, 2006; Hatfield et al., 1996). For example, Blundell et al. (2003) and Dwyer and Killcross (2006) suggested that the BLA was not critical in their designs because the devalued solutions lacked motivational significance. Here we show that even nutrient USs with demonstrable motivational significance (as seen in Experiment 4; also Albertella & Boakes, 2006) produced a similar null result regardless of the subjects’ motivational state. As is the case with the OFC, it is more likely that other characteristics of the stimuli used in these studies limit the importance of BLA functioning in this sort of devaluation task.
The PIT test in Experiment 2 indicated that both BLA and OFC lesions were sufficient to disrupt the ability of a Pavlovian cue to selectively transfer to an instrumental response, which had been trained with the same outcome. It is worth noting that the selective PIT effect seen in Group Sham showed up as a selective depression of “diff” responding rather than a selective increase in “same” responding. This effect is not uncommonly observed in the literature (e.g., see Delamater & Holland, 2008), and very likely reflects competition from the conditioned magazine approach response. It is important to realize that selective PIT whether it reflects a selective increase or decrease reveals learning of sensory-specific CS-US associations. One common explanation for the selective PIT effect comes from Pavlov’s bidirectional hypothesis (Pavlov, 1932). This assumes that the CS associates with the specific US with which it was trained, and that the instrumental responses also associate with their specific USs. When the CS is presented during the test session, it is assumed that it activates a specific representation of its associated US that, in turn, activates the relevant instrumental response through its bidirectional association with that US. This account would predict that “same” responding should be greater than “diff” responding so long as these US representations were specific in their sensory content even though the CSs and instrumental responses were never trained together. The reason why this selective effect may, in some circumstances, show up as a selective increase or decrease relative to baseline is very likely related to the fact that in addition to these selective processes, the two CSs will activate a conditioned magazine approach response that may generally compete with instrumental responding to different degrees in different laboratories. The main conclusion here is that we were able to replicate prior findings that BLA and OFC lesions undermine learning of sensory-specific CS-US associations when auditory and visual cues are used. This strengthens our finding that under similar motivational conditions these very same lesions do not affect such learning when flavor cues are used.
The discrepancy between our results as well as those of Blundell et al. (2003) and Dwyer and Killcross (2006) from the findings of Hatfield et al. (1996), Gallagher et al. (1999), and Corbit and Balleine, (2005) can be explained by the fact that perhaps there is something special about flavor-nutrient learning, which causes it to use different learning mechanisms than the magazine approach paradigm. More specifically, it is possible that these structures (i.e., BLA, and OFC), are not absolutely necessary for the formation of sensory-specific associations in a task where the CSs and the USs are both from olfactory and gustatory modalities but are only critical in tasks where auditory/visual stimuli are used to predict gustatory rewards. In the past, BLA lesions were found to impair the devaluation effect in magazine approach or instrumental tasks in rats (Johnson, Gallagher, & Holland, 2009; Blundell et al., 2003; Balleine, Killcross, & Dickinson, 2003) and monkeys (Malkova, Gaffan, & Murray, 1997). Furthermore, BLA lesions were also found to disrupt the formation of sensory-specific associations in a mediated conditioning task with contextual CSs but not in a sensory preconditioning task with flavor CSs (Dwyer & Killcross, 2006). It is possible that outcome devaluation in a flavor preference paradigm, which employs flavor/nutrient rather than auditory/visual stimuli, is unique in its utilization of neural structures when compared to magazine approach or instrumental learning tasks.
There are theoretical reasons to suppose that an outcome devaluation procedure with flavor-nutrient stimuli might yield a different sort of learning that engages alternative neural structures as compared to training with auditory/visual-nutrient stimuli. For instance, according to Kehoe, Horne, Horne, and Macrae (1994; see also Trost & Batsell, 2004), when conditioned and unconditioned stimuli are from similar modalities (e.g., both flavor CSs and nutrient USs have olfactory and gustatory components), the representations of both events become integrated as a configural whole, whereas when the stimuli are from different modalities (e.g., audio-visual CSs and olfactory-gustatory USs), these events are represented elementally. This interpretation suggests that lower consumption of the CSd in Experiments 1 and 3 might be due to generalization from the devalued stimulus configuration to CSd rather than an associatively activated sensory-specific representation of the devalued nutrient. Our findings that OFC and BLA lesions impaired the formation of sensory-specific associations in Experiment 2 but not in a flavor conditioning task in Experiments 1 and 3 are consistent with this view if it is assumed that configural processes are uniquely engaged in Experiments 1 and 3.
However, the result of Experiment 1 casts some doubt on the role of configural processing in the devaluation effects observed here. In Experiment 1, half of the rats were given a simultaneous flavor-nutrient compound whereas the other half were given a serial flavor-nutrient compound. Intuitively, serial compounds are much less prone to configural conditioning because two unique stimuli are unlikely to be spontaneously treated as a single stimulus if they are never presented at the same time. This notion is supported by studies that show that training with simultaneous and serial compounds lead to different forms of learning in “feature discrimination learning” tasks (e.g., Holland, 1989). From this, one would expect the serial condition in Experiment 1 to show less configuring. In contrast to this expectation, the BLA and OFC lesions were equally ineffective in attenuating devaluation in either condition. However, we cannot rule out the possibility that configuring might occur even when flavors and nutrients are presented serially.
Another important distinction between a conventional magazine-approach outcome devaluation task and one in a flavor-nutrient learning situation relates to the associative characteristics of the CSs used. A flavor CS differs from an auditory or visual CS both in terms of the way that conditioned responding is measured, and the potential relationship between the CS and the ultimate devaluing agent. For example, it is possible that the reduced consumption of CSd in Experiments 1 and 3 is the result of mediated learning rather than devaluation of the nutrient. Mediated learning (e.g., Holland, 1981) occurs when an associatively-activated representation of a CS (in this case CSd) enters into a direct association with a US (in this case illness) when the US physically is paired with an associate of the stimulus (in this case, the nutrient). This differs from devaluation because the flavor itself is devalued, and an updated sensory-specific representation of the nutrient is unnecessary to express that devaluation during the testing phase. In traditional devaluation designs, this possibility is very unlikely because the auditory and visual CSs used are not easily associated with illness (Garcia & Koelling, 1966) and in some cases, illness is not used in the devaluation procedure. However, in the present case, such a mechanism becomes a viable alternative, and could explain why the BLA and OFC may be less critical in sensory-specific learning involving flavor cues than with auditory/visual cues.
Even if the cognitive nature of the associations formed in Experiments 1 and 2 are the same, however, there could still be different physiological underpinnings. For example, an intact hippocampus is not required for the expression of sensory-preconditioning in a taste-aversion situation (Ward-Robinson, Coutureau, Good, Honey, Killcross, & Oswald, 2001), but other researchers have observed that hippocampus lesions attenuate sensory preconditioning in different tasks, such as eye-blink (Port & Patterson, 1984; Port, Beggs, & Patterson, 1987) and fear conditioning (Talk, Gandhi, & Matzel, 2002). It is commonly accepted that the neural circuitry of different types of associative learning must differ at least at the perceptual level, and in many circumstances it is likely that these differences extend to further stages of the learning process. Although OFC neurons do encode the relationship between olfactory cues and gustatory outcomes (e.g., Schoenbaum, Chiba, & Gallagher, 1998), it appears that processing in other areas is sufficient to support appropriate responding to a flavor cue after nutrient devaluation.
Another physiology-based explanation for the failure to detect BLA or OFC lesion effects on the formation of sensory-specific flavor-nutrient associations in our studies is that the sensory reward representations might be more distributed as a function of training given prior to selective nutrient devaluation. There is evidence to suggest that while amygdala lesions abolish learning and memory of fear conditioning in subjects given minimal training, they do not prevent fear conditioning when subjects are extensively trained (Maren, 1999). These results suggest that when the subjects receive extensive training, it can result in structures outside the amygdala participating in fear conditioning. It seems possible that this might reflect a more general principle in the nervous system, i.e., that neural networks for learning become more widely distributed with additional training. It is then possible that with extended training the sensory-specific flavor-nutrient associations assessed in this paper become so widely distributed that pre-training lesions to any one particular structure is without effect. In Experiments 1 and 3 subjects received eight and three exposures to each of two flavor-nutrient compound stimuli, respectively. Since other work in our lab has established that the US devaluation effect can be seen after a single flavor-nutrient pairing, the amount of training given here could be considered extensive.
If the BLA and OFC are not necessary for the behaviors described in Experiments 1 and 3, then other structures must be involved. Evidence for this notion arises from the work of Kerfoot, et al. (2007), as well as the Balleine lab. Additional structures thought to be involved in the formation of sensory-specific associations include the nucleus accumbens shell (Kerfoot et al., 2007, Corbit, Muir, & Balleine, 2001), nucleus accumbens core (Corbit et al., 2001), and dorsomedial striatum (Corbit & Janak, 2007, 2010; Yin, Ostlund, Knowlton & Balleine, 2005). Hence, it is possible that one or more of these structures compensated for the loss of BLA or OFC structures. For example, Kerfoot and colleagues (2007) found higher c-Fos expression in the BLA, OFC, GC and posterior accumbens shell in subjects that were tested with a sucrose-paired cue following a US devaluation treatment compared to controls. These results imply that more than one structure is involved in this type of learning.
Regardless of the null results reported in Experiments 1 and 3, it is unreasonable to conclude that the amygdala is irrelevant for all flavor or odor-nutrient learning situations. Indeed, other studies (Dwyer, 2011; Touzani & Sclafani, 2005) have shown that localized lesions of the BLA attenuate basic conditioned flavor preference discrimination learning. This attenuation is not evident in Experiments 1 or 3, but we did not use the appropriate design to investigate this issue (i.e., there was no unpaired CS-flavor cue to compare to the CS+). Instead, we are able to focus on the sensory-specific aspect of learning, whereas the flavor preference studies typically do not, thus failing to distinguish the formed associations between the flavor cue and any combination of the post-ingestive effects of the nutrient, the positive hedonic responses to the nutrient, the sensory properties of the nutrient, or some combination of these. It is possible that the amygdala lesions impair only the hedonic or post-ingestive aspects of associative learning, which can only be observed with motivationally-significant outcomes. Also, those previous studies of conditioned preference use two flavor cues with a common taste element, which might make the initial discrimination learning more difficult by enhancing generalization. Thus, while the BLA does not appear to be critical for the expression of outcome devaluation in learning tasks involving flavor-nutrient learning, it is involved in other olfactory and gustatory learning phenomena such as conditioned preference, conditioned aversion (see Reilly & Bornovalova, 2005 for review), and taste-potentiated odor aversion (e.g., Hatfield, Graham, & Gallagher, 1992). It is entirely possible that the BLA is engaged by the learning tasks in Experiments 1 and 3, but that other brain areas are sufficient to produce devaluation.
One final point concerns our lack of a BLA or OFC lesion effect compared to other research showing that olfactory cue learning does entail physiological changes in these structures. Schoenbaum, et al. (1998), for instance, have provided electrophysiological evidence to suggest that BLA and OFC cells alter their reactivity during a task where olfactory cues signal gustatory stimuli (e.g., sucrose and quinine). The specific temporal parameters of such procedures and ours differ in many ways that could contribute to our finding that these structures do not participate in sensory-specific learning. In addition, it is important to note that it is equally unknown whether the electrophysiological recording data seen in these studies is at all relevant to US devaluation tasks used in the present study. Future work will be required to address these issues.
In summary, the present data demonstrate that while neither of the structures we analyzed, the BLA or the OFC, were found to be critical in the encoding of sensory-specific associations in a conditioned flavor preference paradigm where motivationally significant USs were used, we found that both the BLA and the OFC are critical for the formation of these associations in the magazine approach paradigm. While there is still much to learn about the nature of sensory-specific associations in Pavlovian learning paradigms and the neural mechanisms they involve, the present studies provide some additional information about the complicated mechanisms involved in this type of learning. In particular, the data suggest that different neural circuits may be recruited when flavor cues and exteroceptive cues are used to signal motivationally significant nutrient rewards.
Acknowledgement
This research was supported by grants from the National Institute of Mental Health (ARD: RO1 065947), and the National Institute of Neurological Disorders and Stroke (DSW: NS 061587).
We would like to extend a very special thank you to Khalid Touzani for his assistance and suggestions with this paper. Also, we would like to thank Stephen Chang for his technical assistance and Peter C. Holland for his valuable advice and access to his facilities. Experiments 1 and 2 were performed at Brooklyn College, whereas experiment 3 and 4 were performed at Johns Hopkins University. The data presented in experiments 1 and 2 were discussed at the Society for Neuroscience Conference in San Diego, CA, and appear in the conference proceedings: “Pretraining BLA, OFC, or GC lesions fail to impair the formation of sensory-specific flavor-nutrient associations, but BLA and OFC lesions disrupt sensory-specific Pavlovian-instrumental transfer in thirsty rats,” by J. Scarlet and A. R. Delamater, 2010.
Janina Scarlet is currently at the Department of Psychology, Alliant International University, 10455 Pomerado Rd, San Diego, CA 92131, and Daniel Wheeler is at the Department of Biomedical Sciences, Marquette University, Milwaukee, WI 53233.
Abbreviations
- ANOVA
analysis of variance
- AP
anterior/posterior
- BLA
basolateral amygdala
- C
Celsius
- cm
centimeter
- CR
conditioned response
- CRF
continuous reinforcement schedule
- CS
conditioned stimulus
- CSd
conditioned stimulus paired with the devalued nutrient
- CSnd
conditioned stimulus paired with the nondevalued nutrient
- dB
decibel
- diff
different
- DV
dorsal/ventral
- F
flash
- g
gram
- GC
gustatory cortex
- HCL
hydrochloric acid
- hr
hour
- Hz
Hertz
- IEG
immediate early gene
- i.p.
intraperitoneal
- ITI
intertrail interval
- kg
kilogram
- LiCl
lithium chloride
- μg
microgram
- μl
microliter
- μM
micrometer
- M
mole
- mg
milligram
- min
minute
- ML
medial/lateral
- ml
milliliter
- mm
millimeter
- NaCl
sodium chloride
- NMDA
NMethyl-D-aspartate
- OFC
orbitofrontal cortex
- PIT
Pavlovian-instrumental transfer
- R
instrumental response
- s
second
- SEM
standard error of the mean
- T
tone
- US
unconditioned stimulus
- USd
devalued nutrient
- USnd
nondevalued nutrient
- VI
variable interval schedule
- VT
variable time
- v/v
volume/volume
- W
Watt
- w/v
weight/volume
References
- Albertella L, Boakes RA. Persistence of conditioned flavor preferences is not due to inadvertent food reinforcement. J. Exp. Psychol. Anim. Behav. Process. 2006;32:386–395. doi: 10.1037/0097-7403.32.4.386. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J. Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Preserved sensitivity to outcome value after lesions of the basolateral amygdala. J. Neurosci. 2003;23:7702–7709. doi: 10.1523/JNEUROSCI.23-20-07702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central Amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J. Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J. Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Inactivation of the lateral but not medial dorsal striatum eliminates the excitatory impact of Pavlovian stimuli on instrumental responding. J. Neurosci. 2007;27:13977–13981. doi: 10.1523/JNEUROSCI.4097-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. Eur. J. Neurosci. 2010;31:1312–1321. doi: 10.1111/j.1460-9568.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Holland PC. The influence of CS-US interval on several different indices of learning in appetitive conditioning. J. Exp. Psychol. Anim. Behav. Process. 2008;34:202–222. doi: 10.1037/0097-7403.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges B, Ramirez-Amaya V, Ricaño-Cornejo I, Levy F, Ferreira G. Flavor preference learning increases olfactory and gustatory convergence onto single neurons in the basolateral amygdala but not in the insular cortex in rats. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DM. Lesions of the basolateral, but not central, amygdala impair flavor-taste learning based on fructose or quinine reinforcers. Beh. Brain Res. 2011;220:349–353. doi: 10.1016/j.bbr.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Killcross S. Lesions of the basolateral amygdala disrupt conditioning based on the retrieved representations of motivationally significant events. J. Neurosci. 2006;26:8305–8309. doi: 10.1523/JNEUROSCI.1647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of the incentive value in associative learning. J. Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychon. Sci. 1966;4:123–124. [Google Scholar]
- Harris JA, Shand FL, Carroll LQ, Westbrook RF. Persistence of preference for a flavor presented in simultaneous compound with sucrose. J. Exp. Psychol. Anim. Behav. Process. 2004;30:177–189. doi: 10.1037/0097-7403.30.3.177. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Graham PW, Gallagher M. Taste-potentiated odor aversion learning: Role of amygdaloid basolateral complex and central nucleus. Behav. Neurosci. 1992;106:286–293. doi: 10.1037//0735-7044.106.2.286. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland PC. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J. Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Acquisition of representation-mediated conditioned food aversions. Learn. Motiv. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Acquisition and transfer of conditional discrimination performance. J. Exp. Psychol. Anim. Behav. Process. 1989;15:154–165. [Google Scholar]
- Higgins T, Rescorla RA. Extinction and retraining of simultaneous and successive flavor conditioning. Learn. Behav. 2004;32:213–219. doi: 10.3758/bf03196022. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC. The basolateral amygdala is critical to the expression of Pavlovian and instrumental outcome-specific reinforcer devaluation effects. J. Neurosci. 2009;29:696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe EJ, Horne AJ, Horne PS, Macrae M. Summation and configuration between and within sensory modalities in classical conditioning of the rabbit. Anim. Learn. Behav. 1994;22:19–26. [Google Scholar]
- Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: A FOS and behavioral analysis. Learn. Mem. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory reinforcement but interfere with reinforcer devaluation effects in Rhesus monkeys. J. Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren SU. Neurotoxic bilateral amygdala lesions impair learning and memory but not the performance of conditioned fear in rats. J. Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer OH, Aiken EG. Contiguity vs. drive-reduction in conditioned fear: Temporal variations in conditioned and unconditioned stimulus. Am. J. Psychol. 1954;67:26–38. [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J. Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J. Neurosci. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. The reply of a physiologist to psychologists. Psychol. Rev. 1932;39:91–127. [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J. Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port RL, Beggs AL, Patterson MM. Hippocampal substrate of sensory associations. Physiol. Behav. 1987;39:643–647. doi: 10.1016/0031-9384(87)90167-3. [DOI] [PubMed] [Google Scholar]
- Port RL, Patterson MM. Fimbrial lesions and sensory preconditioning. Behav. Neurosci. 1984;98:584–589. doi: 10.1037//0735-7044.98.4.584. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: A critical review. Neurosci. Biobehav. R. 2005;29:1067–1088. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Simultaneous and successive associations in sensory preconditioning. J. Exp. Psychol. Anim. Behav. Process. 1980;6:207–216. doi: 10.1037//0097-7403.6.3.207. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Geirut DJ, Holland PC, Gallagher M. Society for Neuroscience. Washington, D. C.: 2008. Representations of expected taste outcomes reactive primary sensory taste ensembles in gustatory cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Holland PC, Gallagher M. Associatively learned representations of taste outcomes activate taste-encoding neural ensembles in gustatory cortex. J. Neurosci. 2009;29:15386–15396. doi: 10.1523/JNEUROSCI.3233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basoltaeral amygdala encode expected outcomes during learning. Nat. Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Smith JC, Roll DL. Trace Conditioning with X-Rays as an aversive stimulus. Psychon. Sci. 1967;9:11–12. [Google Scholar]
- Talk AC, Gandhi CC, Matzel LD. Hippocampal function during behaviorally silent associative learning: Dissociation of memory storage and expression. Hippocampus. 2002;12:648–656. doi: 10.1002/hipo.10098. [DOI] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats. Eur. J. Neurosci. 2005;22:1767–1774. doi: 10.1111/j.1460-9568.2005.04360.x. [DOI] [PubMed] [Google Scholar]
- Trost CA, Batsell WR., Jr. Taste + odor interactions in compound aversion conditioning. Learn. Behav. 2004;32:440–453. doi: 10.3758/bf03196040. [DOI] [PubMed] [Google Scholar]
- Ward-Robinson J, Coutureau E, Good M, Honey RC, Killcross AS, Oswald CJP. Excitotoxic lesions of the hippocampus leave sensory preconditioning intact: Implications for models of hippocampal function. Behav. Neurosci. 2001;115:1357–1362. doi: 10.1037//0735-7044.115.6.1357. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]


