Abstract
Many aspects of tissue damage following acute or chronic inflammatory reactions can be directly attributed to the concomitant biosynthesis and release of inducible early pro-inflammatory cytokine tumor necrosis factor alpha (TNFα). Conversely, systemic inflammation is impacted by consequences of tissue damage. Dysregulated TNFα contributes to numerous pathophysiological conditions including inflammatory bowel disease (IBD) and arthritis. Inflammatory stimuli trigger proteolytic cleavage and shedding of extracellular domains of TNFα receptors giving rise to two soluble fragments (p55 sTNFR1 and p75 sTNFR2) that block further binding, activity and synthesis of TNFα. We hypothesized that absence of sTNFR inhibitory feedback control would result in accumulated high levels of TNFα and other inflammatory factors promoting the cardinal signs of chronic inflammation and pain.
The present study reports a translational murine model of chronic arthritis precipitated by two consecutive inflammatory insults. The “double hit” procedures provoke a chronic inflammatory response and pain related behaviors in mice that are dually deficient in p55 (TNFR1) and p75 (TNFR2). The inflammation and pain related behaviors are transient in similarly treated wild type (WT) mice. The complete Freund's adjuvant (CFA) method was used initially to induce knee joint inflammation, tactile mechanical and heat hypersensitivity, and gait disturbance. After these transient effects of the insult were resolved, a recrudescence persisting at least through 23 weeks was promoted by gastrointestinal (GI) insult with dilute intra-colonic mustard oil (MO) only in the mutant mice and was reversed by a P2X7 antagonist. Serum Proteome Profiling analysis revealed high levels of serum inflammatory factors TNFα, RANTES, CXCL9 (MIG), CXCL10 (IP-10), and CCL2 (MCP-1).
In conclusion, these data suggest that impaired signaling of TNFα due to deficit of the two protective soluble p55 and p75 sTNFR inhibitory factors plays a pivotal role in re-activation of the immune response to GI insult that can produce recrudescence of inflammatory injury and a chronic pain state through promotion of high levels of serum inflammatory factors.
Keywords: TNFα, recrudescence, autoimmune, arthritis, P2X7
Introduction
Despite current clinical treatments (i.e. anti-TNFα monoclonal antibodies) that effectively diminish inflammation and pain by reducing TNFα and other cytokines, the fact remains that the inflammatory response and pain will likely re-emerge in most patients with autoimmune disease. Therefore, the disease is not completely abrogated by current therapies, suggesting unresolved processes or salvage pathways still active that can re-kindle the inflammatory reaction. Consequently, understanding molecular pathways and neuroregulatory modulation of inflammatory responses will assist in development of adjunct therapy to provide permanent remission of disease processes for more complete abrogation of pain and disability for patients with IBD and other autoimmune conditions.
The etiology of reactive clinical arthritis has been attributed to combined genetic and microenvironmental factors, most notably gastrointestinal (GI) pathogens. Toivanen (2003)1 proposed that even normal GI flora and their degradation components harbor sufficient arthrogenic ability. Activated gut immune cells were shown to home to knee joints of patients suffering from rheumatoid arthritis (RA) never treated for GI disorders1. This clinical trial and others provide evidence that combined genetic polymorphisms and environmental factors such as GI bacterial infections or injuries to the joints play a critical role in the etiology of arthritis. Bacterial pathogens and fragments are frequently found in synovial fluid extracted from inflamed knees of patients, though less so in long term cases. Animal models in which two consecutive inflammatory insults are provided to enhance the immune response are often referred to as “double hit” models and will be used in this study to provoke a chronic inflammatory state.
The “double-hit” hypothesis for inflammatory pathology evolved many years ago as an explanation for transition of the innate immune response into chronic conditions such as arthritis. This hypothesis is based on the ability of soluble non-antibody proteins released from inflammatory cells to prime the immune response so that a subsequent insult elicits an amplified immune response2. Based on this theory and modern gene association studies, we hypothesized that (1) a known genetic polymorphism reportedly inducing high levels of TNFα combined with (2) inflammatory “double hits” would initiate chronic arthritis for study of mechanisms precipitating chronic inflammatory pain. We used mice with genetic deletion of TNFR1 and TNFR2 (TNFR1/R2-/-). Without soluble receptor (sTNFR1/2) inhibitory feedback TNFα levels would accumulate. Inflammatory “double hit” approaches were utilized to aggravate neurogenic mechanisms that would then facilitate the transition to chronic joint inflammation.
In preliminary studies, three methods of experimental arthritis were tested followed weeks later by a gastrointestinal “second hit” with colonic infusion of diluted mustard oil (MO, allylisothiocyanate) for all groups. Arthritis was induced with either (1) the antigen induced autoimmune (AIA) method using incomplete Freund's adjuvant (IFA) and methylated bovine serum albumin (mBSA)3, (2) complete Freund's adjuvant (CFA)4, or (3) the collagen-induced (CIA)5 method. These agents typically evoke only transient hyperalgesic responses in WT mice. MO also promotes transient hypersensitivity responses “referred’ to the footpad6.
Compelling data are presented here finding that a colonic MO “second hit” evokes recrudescence of CFA induced knee joint inflammation that becomes chronic in mice with genetic deletions of TNFR1 and TNFR2. The wild type background, B6129SF2/J mice, recovered fully from all of the insults. Data not shown found that TNFR1/2-/- mice are resistant to the CIA model as previously published5 and thus did not respond with pain related behavior or inflammation. Recrudescence was minimal with the AIA model. This suggests necessity of a pathogen evoked response to provide transition to a chronic inflammatory state. We hypothesize that recrudescence in this model occurs in the TNFR1/R2-/- mice in response to pathogen activated upregulation of TNFα serum levels that enhance inflammation and hypersensitivity that then continue in the absence of sTNFR feedback inhibition.
Methods
Animals
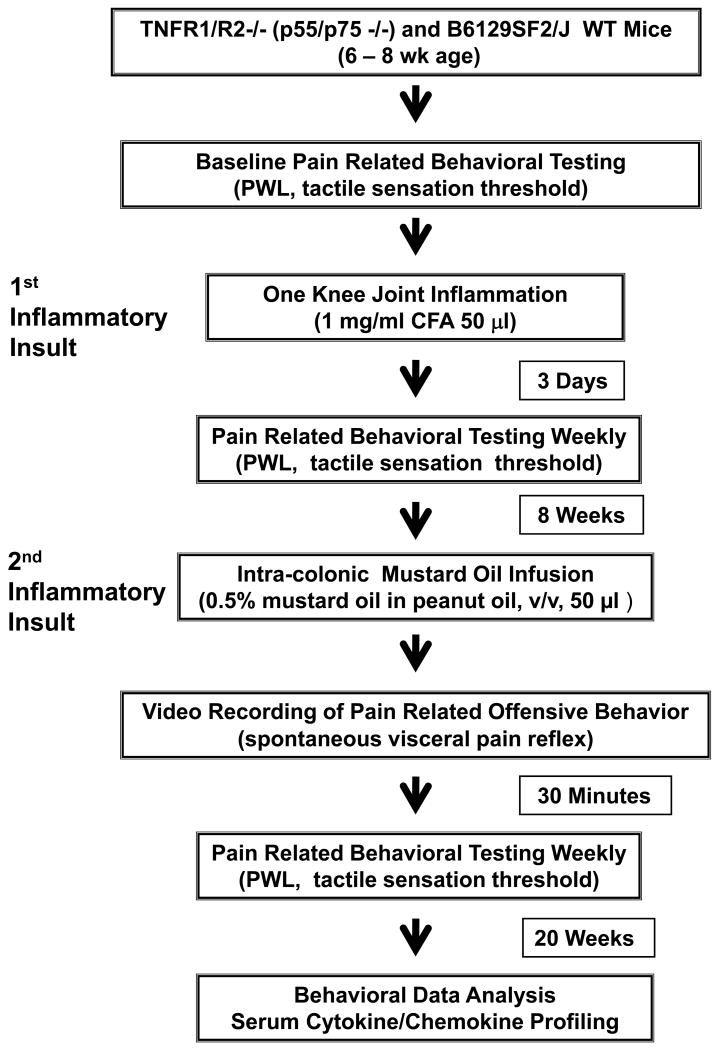
All animal procedures were approved by the University of Kentucky IACUC committee. Mice were monitored daily for continued weight gain and general health. Health Status and Procedures were documented daily on the UK IACUC SOP-102 Post-Operative Evaluation form. Experiments were performed using dually deficient (TNFR1/R2-/-) (20-25 g; Jackson Laboratory) and matched wild type (WT) background mice (B6129SF2/J) inbred at the University of Kentucky animal facilities. Mice were housed in individual cages with a 10h/14h dark/light reversed cycle and allowed access to food and water ad libitum except during testing and dosing. A flowchart for the experimental design is provided in Fig. 1.
Figure 1. Experimental Design.
The experimental timeline for inductions, behavioral testing and antagonist treatment, is shown for the “double hit” chronic inflammation model.
Induction of Chronic Arthritis: “First Hit” Knee Joint Insults
Induction of CFA Monoarthritis
Mice were briefly anaesthetized with 2% isoflourane inhalation. The left knee joint cavity was injected with CFA (1mg/ml, i.e. 50 μg heat killed, desiccated Mycobacterium tuberculosis H37 (DIFCO Laboratories, Detroit, MI) in 50 μl of sterile 1:1 saline:peanut oil) solution. The CFA method was effective in providing chronic knee joint inflammation and pain related behavioral changes in both the WT and the TNFR1/R2-/- mice. Two other inflammatory methods (AIA, CIA) were tested but the results not reported in this study because the inflammation induced was transient in the case of adjuvant induced arthritis or did not occur as in the case of collagen induced arthritis as reported previously5 by others. Several additional controls that did not evoke inflammation included vehicle or injection of IFA alone. Recrudescence did not occur in mice given the “first” or “second hit” only.
Pain-related Behavioral Assessments after Knee Joint CFA
Baseline testing of footpad nociceptive responses to mechanical and heat stimuli with von Frey fiber and paw withdrawal latency (PWL) was determined prior to induction of inflammation9-10. These tests are standards in the field of pain research (411,000+ references). Reflex testing for “referred” secondary mechanical hyperalgesia/allodynia with von Frey fibers was developed by Max von Frey, who in 1896 identified “pain spots” on human skin. Mechanical nociceptive thresholds were assayed using von Frey filaments according to the “up down” algorithm described before10. Paw withdrawal response latencies to noxious thermal stimulation were measured using the method of Hargreaves et al. 8. Withdrawal responses from heat proceeded as in our previous studies and as described in the literature9,11. Pain-related behavior was assessed weekly throughout the study by an observer blinded to group assignment. Gait disturbances (curling toes, limping, guarding, etc) were tallied by an observer blinded to treatment group as in our previous studies12.
Assessment of Secondary Mechanical Allodynia by Testing Paw Withdrawal Threshold
Mice were placed into clear cylindrical plastic enclosures (7 × 4 × 4 cm) on the metal meshed (3 × 3 mm) platform (36 × 29 × 21.5 cm). A mechanical withdrawal threshold testing was done on the plantar surface of both hind paws using a set 8 of von Frey monofilaments [(4.74) 6.0 g; (4.31) 2.0 g; (4.08) 1.0 g; (3.61) 0.4 g; (3.22) 0.16 g; (2.83)0.07; (2.36) 0.02 g; (1.65) 0.008 g]. The von Frey filaments were applied perpendicularly to the plantar surface with sufficient force to bend the monofilament slightly and held for about 5 seconds. A positive response was defined as an abrupt withdrawal (flick response) of the foot during stimulation or immediately after the removal of stimulus. Up/down method was used, i.e. whenever there was a negative or positive response, the next stronger or weaker filament was applied, respectively. Testing proceeded in this manner until four fibers had been applied after the first one caused a withdrawal response, allowing the estimation of the 50% mechanical withdrawal threshold using a curve-fitting algorithm.
Assessment of Secondary Thermal Hyperalgesia
In this assay, mice were placed on a glass platform (2 mm thickness of glass) in a clear plastic enclosure similar to those described above. After 30 min of acclimation, a beam of focused light (5 × 5 mm) was directed through the glass onto the plantar surface of the hind paw. A 15 sec cutoff was used to prevent tissue damage. Prior to the start of the experiment, the light beam intensity was adjusted to 50°C to provide approximately 10 sec baseline latency. Three measurements were made per animal per test session separated by at least 5 min. A shortened PWL response in animals with knee joint inflammation was interpreted as indicative of secondary thermal hyperalgesia. The PWL tests were performed before knee joint injection as baseline, on day 3 and once a week after CFA injection.
Evaluation of the pain-related posture
The abnormal posture of each animal with an affected hind limb was given a single score using a subjective pain-related behavioral scale (spontaneous pain rating score 0-5) i.e. 0- normal; 1- curling of the toes, 2- eversion of the paw; 3- partial weight bearing; 4- non-weight bearing and guarding; and 5- avoidance of any contact with the hindlimb12.
Induction of Gastrointestinal Irritation: “Second Hit” Colonic Mustard Oil Insult
The “second hit” exposed the mice to their own GI pathogens and inflammatory mediators by infusing diluted MO into the colon in week 8 after CFA.
Intracolonic Infusion of MO
Each mouse was acclimated by placed in the recording chamber 10 min before infusion of MO. Petroleum jelly (Vaseline) was applied in the perianal area to avoid the stimulation of somatic areas by contact with the irritant chemical. Mice were given vehicle or the second inflammatory insult with colonic MO infusion (50 μl, 0.5% in peanut oil, allylisothiocyanate, Sigma-Aldrich, St. Louis, MO). The colonic infusion with MO or vehicle was administered from a syringe connected to a 24 cm soft polyethylene PE 90 tubing (1.27 mm diameter) marked at 4 cm with colored tape to indicate the correct depth for insertion into the colon (and to prevent further entry). The tubing tip was flame melted to form a smooth bulb at the tip and pierced repeatedly along its length for even release of MO in the colon. Immediately after the infusion, the animal was placed in an observation cage for a 25 min recording session to determine if pain related behaviors were displayed.
Spontaneous Visceral Pain Assessment
Animals were placed in the observation chamber 10 min before the colon infusion. Immediately after the infusion, the animal was placed back in the observation chamber for a 25 min recording session. The observation chamber is a 28 × 17.5 × 12.5 cm see-through plastic home cage with one mirrored side located in an isolated room with constant “white noise”. A digital camcorder located 0.5 meter from the chamber with an unobstructed view, was used to record animal spontaneous visceral pain related behaviors. The camcorder was linked to a computer recording program for offline data analysis (Logitech Image Studio). The chamber was washed with a detergent/disinfectant and dried between animals. Postures defined as visceral pain-related behavior in this study included licking of the lower abdomen, stretching the abdomen or hindlimb, lowering the abdomen against the floor and abdomen retractions or arching the back. Recordings were analyzed by an observer blinded to group.
Serum Sample Collection and Measurement
At the end of the study, animals were deeply anesthetized with an overdose of isoflurane inhalant prior to heart puncture to collect blood through an open thorax13.
Cytokine/chemokine profiles
Blood samples were collected in microtainer tubes stored on ice and centrifuged for 5 min (5000 × g). Serum was assayed immediately or stored at -80°C. Cytokine/chemokine profiles of serum samples were determined using Proteomic Profiler kits (Mouse Cytokine Array Panel A array Kit #ARY006, R&D Systems) following manufacture's protocol with modifications. Values were analyzed with NIH Image J and expressed as relative densities (arbitrary unit) of dot blot.
Statistical analysis
All results are expressed as mean and standard error of mean (+/-SEM) unless otherwise stated. Data were analyzed using two-way ANOVA, followed by Bonferroni post hoc test using GraphPad Prism Software, (San Diego, CA), or paired t-test where indicated. Statistical significance was set at p ≤ 0.05.
Results
The timeline for the model induction, treatments, and behavioral testing to determine evidence of hypersensitivity are diagramed in Fig. 1 flowchart. Behavioral testing indicated that pain-related postural changes, secondary mechanical allodynia, and thermal hyperalgesia were present along with the knee joint inflammation. A lowered mechanical threshold and a shortened thermal PWL tested on the footpad indicated hypersensitivity (Fig. 2). Hypersensitivity reached a peak at 3 days after knee joint injection of CFA and persisted over 3 weeks.
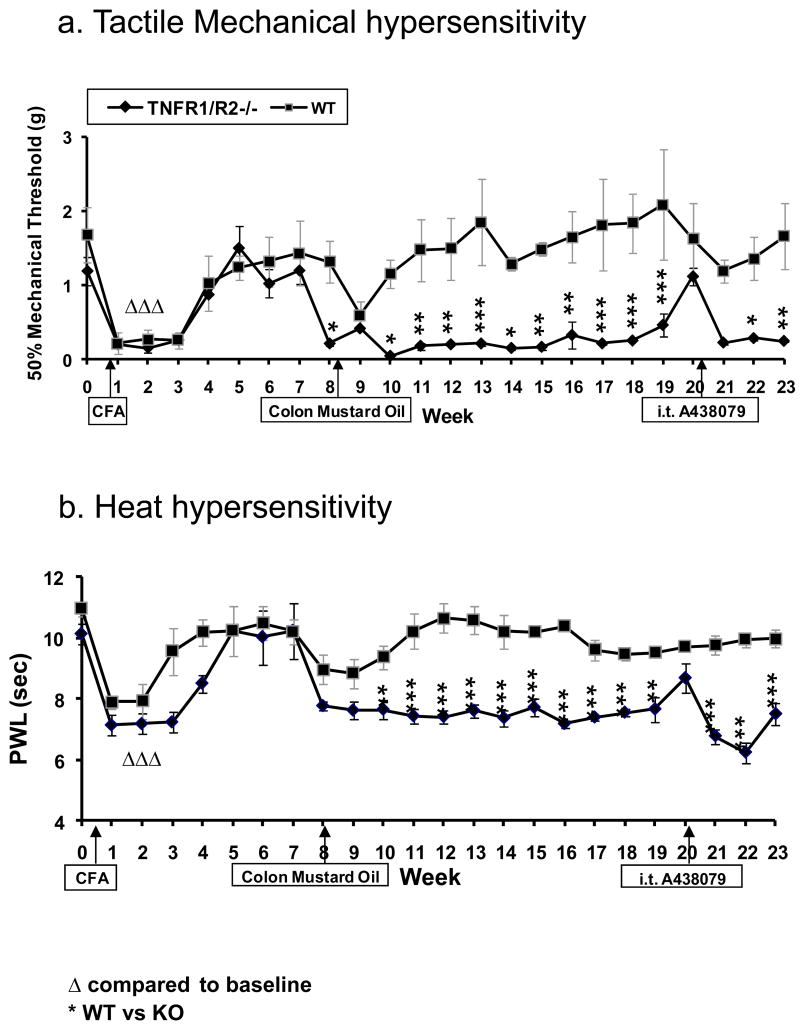
Figure 2. Development of Chronic Arthritis.
Baseline (a) 50% paw mechanical threshold and (b) paw withdrawal latency (PWL) responses are the same for both TNFR1/R2-/- (p55/p75-/-) and WT mice. After knee joint CFA injection (1st arrow), pain related behaviors develop, peak at 7 – 21 days, and then dissipate. Pain related behaviors re-occur after intra-colonic MO (2nd arrow), but do not regress after the “second hit” in p55/p75 deficient mice. Sensitization, guarding and limping persist at least though the subsequent 23 weeks of testing in the TNFR1R2-/- mice. A highly potent, selective P2X7 receptor antagonist (i.t. A438079) effectively attenuates the secondary thermal hyperalgesia and mechanical allodynia for about 3 hrs at the 20 week time point (3rd Arrow). * p≤0.05; **p≤0.01); ***p≤0.001).
Pain-Related Behavioral Assessments for CFA-Induced Arthritis “First Hit”
Tactile Mechanical Sensitivity
Baseline values for behavioral testing of tactile mechanical sensitivity in both TNFR1/R2-/- mice and WT mice were equivalent. The baseline 50% paw withdraw threshold to von Frey fiber stimuli for both the WT background B6129SF2/J strain mice and TNFR1/R2-/- was 1.28-1.87 g (1.68±0.38 g average) (Fig. 2a). The 50% paw mechanical threshold lowered to 0.15±0.04 g on day 3 after CFA injection, was 0.20±0.05 g on day 7, and persisted for 3 weeks. Mechanical threshold decrease persisted for 3 weeks after CFA injection in WT mice (0.25±0.11g); 4 weeks for KO mice (0.25±0.034g). The mice were all well recovered by week 5 (1.24±0.05 g for WT and 1.50±0.05 g for KO mice).
Heat Sensitivity
The average baseline paw withdrawal latency (PWL) for withdrawal from radiant heat applied to the footpad was 10.23±0.24 sec for all mice (Fig. 2b). The PWL was shortened by about 3 seconds in WT mice with CFA induced arthritis (7.94±0.32 sec) on day 7, remained lowered at week 2 (7.87±0.8 sec), and returned back to baseline at 3 weeks (10.07±0.6 sec) equivalent to responses of wild type mice. In contrast, the PWL on the affected legs of TNF R1/R2-/- mice was 6.85±0.38 sec on day 7 and remained shortened (8.8±0.55 sec) for at least 4-5 weeks. PWL for mice with CFA induced arthritis (both wild type and KO) compared with saline control group or to their own baseline levels (10.24±0.8 sec for WT; 10.02±0.88 sec for KO) was significantly reduced, (p<0.001) and (P<0.01, two way ANOVA test).
Pain-Related Postural Scores
One day after induction of the CFA knee joint inflammation, all arthritic mice displayed gait disturbance and abnormal pain-related posture, consisting of guarding, curling of the toes, eversion of the foot, and decreased weight bearing on the affected limb and lasted about one week. The pain related posture score for each animal at baseline was recorded as zero. Changes after CFA are reflected in increased spontaneous pain rating scores, with a median of 3.0 for CFA arthritic mice persisting for 7days (Table 1).
Table 1.
Spontaneous pain rating score (0-5) evaluated in CFA arthritis mice of both KO and WT. All mice displayed spontaneous pain postures 3 days after CFA knee joint injection and persisting for 1 to 2 weeks. Spontaneous pain-related behavioral scale (0-5) 12: 0- normal; 1- curling of the toes, 2- eversion of the paw; 3- partial weight bearing; 4- non-weight bearing and guarding; and 5- avoidance of any contact with the hindlimb.
| Spontaneous Pain Rating Score (MEDIAN) | |||||
|---|---|---|---|---|---|
| Group\ time point | Week 0 | Day 3 | Week 1 | Week 2 | Week 3 |
| WT (n=6) | 0 | 3 | 1.5 | 0 | 0 |
| KO (n=6) | 0 | 3 | 2.67 | 1.3 | 0.3 |
| ns | ns | ns | ns | ||
Sensitization Induced by Colonic Mustard Oil Infusion after GI “Second Hit”
After the transient CFA-induced tactile sensitization and gait disturbances were resolved, visceral sensitization and pain-related behaviors were promoted with a GI inflammatory “second hit” insult in mice given dilute colonic mustard oil (MO) at week 8. The footpad hypersensitivity was transient for the WT mice. A recrudescence of footpad hypersensitivity occurred in the TNFR1/R2-/- mice. Joint inflammation and gait disturbance reappeared in 30% of the TNFR1/R2-/- mice. Secondary mechanical and heat hyperalgesia (decreased mechanical threshold and shortened PWL) were maintained chronically after the GI insult in the CFA injected limb in TNFR1/R2-/- mice through the remaining experimental period ending at 23 weeks. Specific details are provided below.
Visceral Sensitization
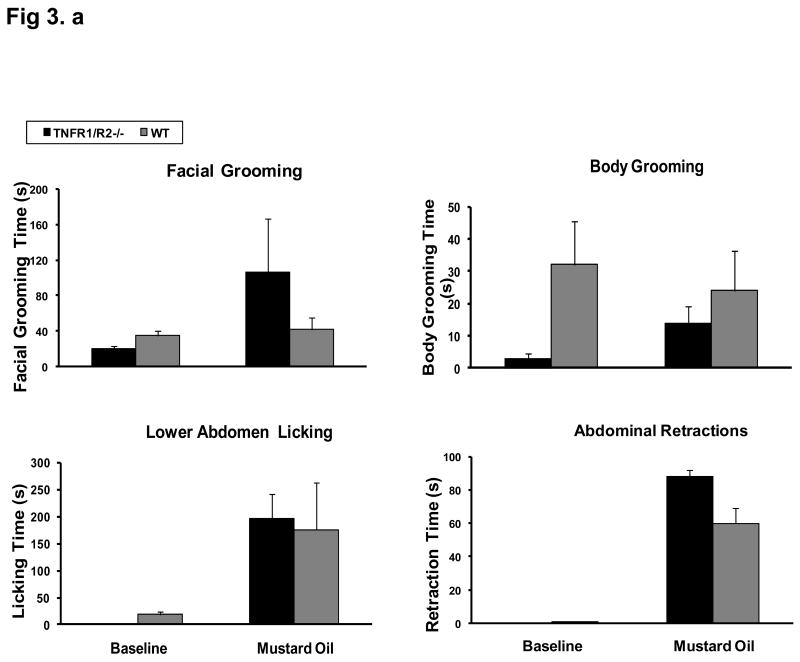
Both WT mice and KO mice developed spontaneous visceral pain related behaviors immediately after colon mustard oil infusion. Abnormal behaviors included increased facial grooming, increased body grooming, frequent lower abdomen licking, and abdomen retraction. (Fig. 3). The spontaneous pain activities lasted for 25–30 min. Subsequently, the animals would remain in a corner of the chamber.
Figure 3. Visceral Pain Development in Both TNFR1/R2-/- and Wild Type Mice after MO Colonic Infusion.
Spontaneous visceral pain behaviors develop immediately after MO colonic infusion in both TNFR1/R2-/- and wild type mice. Increases in lower body licking and abdominal retractions are accompanied by facial and body grooming.
Mechanical and Heat Sensitization
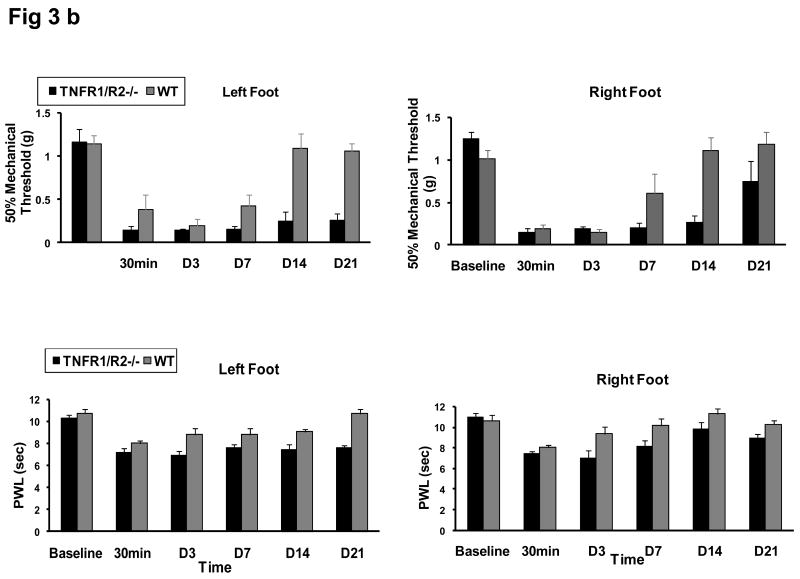
A lowered mechanical threshold and shortened PWL involving both footpads was evident 30 min after the colon MO infusion “second hit” in both groups (Fig. 2). The tactile referred hypersensitization after the visceral insult was evident on both hindpaws, i.e. 50% paw mechanical threshold (0.38±0.17 g for WT; 0.15±0.14 g for KO) and shortened PWL to heat stimulus (8.04±0.19 sec for WT; 7.20±0.32 sec for KO). The lowered mechanical threshold persisted for 2 weeks on both feet (0.17±0.046 g and 0.22±0.080 g, respectively) in WT mice, and 3 weeks in KO mice. Thereafter, the mechanical threshold for WT mice returned back to baseline levels (1.09±0.16 g and 1.11±0.15 g).
Lowered Mechanical Threshold and Heat Paw Withdrawal Latency Persists After “Second Hit” GI Insult in TNFR1/R2-/- mice
Mechanical Sensitivity
The footpad mechanical sensitivity threshold for the leg with the initially inflamed knee joint in TNFR1/R2-/- mice remained lowered (∼0.25±0.08 g) chronically for the remainder of the experiment (23 weeks). The hypersensitive responses in the TNFR1/R2-/- mice were significantly reduced compared to the WT mice (Fig. 2a).
Heat Sensitivity
The significantly shortened PWL in the CFA injected leg remained chronically throughout the 3-4 month experimental period (Fig. 2b). A shortened PWL (6.95 ±0.3 sec - 8.04±0.6 sec) appeared on the uninjected feet of KO mice for 4-5 weeks. After MO infusion, there was not a significant change in the PWL for the WT group (9.78±0.4 sec).
Postural Changes
Spontaneous pain related postures (guarding of the injected leg) re-developed in 30% of the mice 2 weeks after the “second hit”.
P2X7 Antagonist Reverses Chronic Arthritic Symptoms
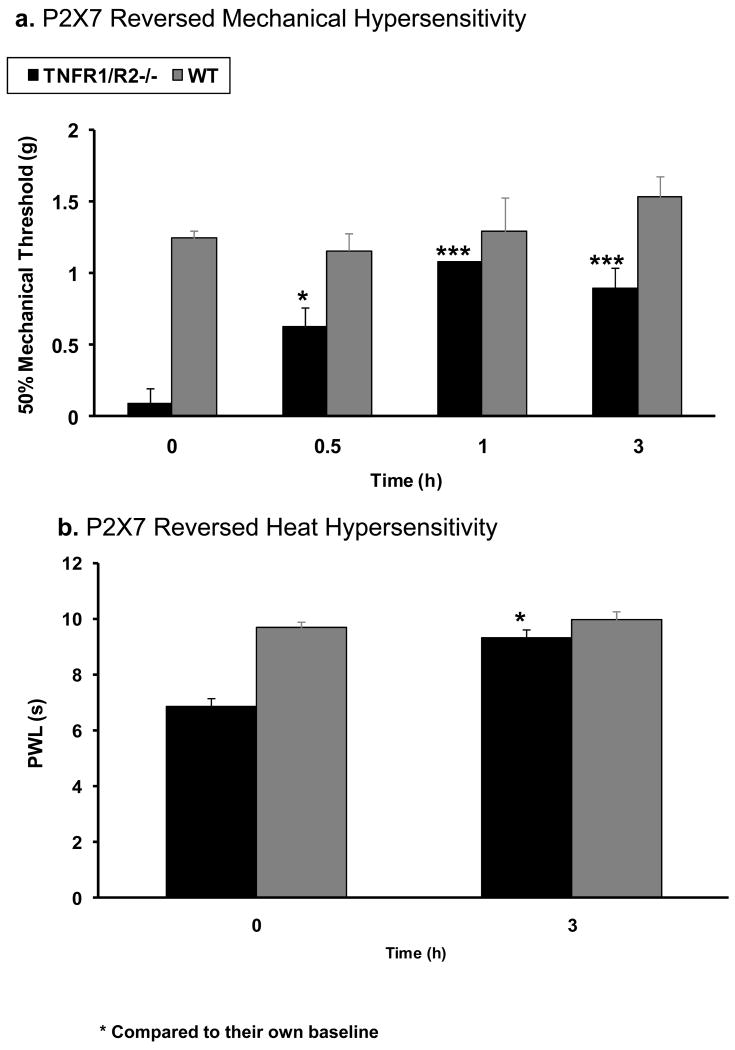
At the 20 week time point, an i.t. injection of a highly potent, selective P2X7 antagonist, A438079 (50 μg/5 μl/per mice) was given to the TNFR1/R2-/- mice with chronic hypersensitivity. Pain-related behaviors, secondary mechanical allodynia (0.09±0.24 g), and thermal hyperalgesia (6.85±0.2 sec) were reversed on the injured leg of TNFR1/R2-/- mice. The reversal effect of A438079 began within 0.5 hour (0.63±0.12 g), reached a peak at 1 hour (1.08±0.24 g), and persisted for at least 3 hours (0.90±0.15 g for 50% mechanical threshold and 9.3±0.3 sec for PWL) (Fig. 4a, b).
Figure 4. Chronic Arthritis Induced Hypersensitization is Reversed by P2X7 Antagonist.
Intrathecal injection of a selective P2X7 receptor antagonist, A438079 (50μg/5μl/mice) reverses the tactile hypersensitivity characterized as secondary thermal hyperalgesia and mechanical allodynia in TNFR1/R2-/- mice with chronic arthritis. Sensitization begins within 0.5 hour and persists for over 3 hours. Significant differences compared to the baseline values (two-way ANOVA. * p≤0.05; ***p≤0.001).
Cytokine/Chemokine Proteome Profiles
High levels of pro-inflammatory cytokine TNFα and other inflammatory mediators were prominent at 23 weeks (5 months), including RANTES, CCL2 (MCP-1), CXCL9 (MIG), CXCL10 (IP-10). Relative dot blot densities of these mediators are shown in Table 2.
Table 2.
Cytokine/chemokine profile analyses at 23 weeks found high levels of TNFα and several other inflammatory mediators in TNFR1/R2-/- mice with arthritis contributing to the chronic inflammatory hypersensitivity. Relative levels in serum of TNFR1/R2-/- mice were compared to wild type mice and expressed as relative dot blot intensity (arbitrary units, a.u.).
| TNFR1/R2-/- (a.u.) |
WT (a.u.) |
Fold Increases TNFR1/R2-/- vs. Controls | |
|---|---|---|---|
| TNFα | 5911.39±851.78 | 705.94±49.6 | 8.37 * |
| CCL5 (RANTES) | 4192.16±1000.73 | 1780.85±158.94 | 2.35 * |
| CCL2 (MCP-1) | 4263±289.09 | 2091.52±310.0 | 2.04 * |
| CXCL10 (IP-10) | 4922.79±652.51 | 2394.71±628.45 | 2.06 * |
| CXCL1 | 5587.85±628.34 | 3526.53±190.43 | 1.58 * |
| CXCL9 (MIG) | 4840.78±1594.37 | 1946.71±1946.71 | 2.49 |
indicates p≤0.05.
Discussion
The data indicate that chronic arthritis develops in TNFR1/R2-/- mice with CFA knee joint injections when followed by the gastrointestinal “second hit” provided by MO inflammation, while WT mice recover. Our studies determined that mice with genetic alteration of both TNFRs have maladaptive high levels of TNFα and other specific inflammatory mediators present along with tactile mechanical and heat hypersensitivity referred to the footpad. A selective antagonist for P2X7 ion channels acutely reduced the pain-related behaviors and mobility issues precipitated by chronic knee joint inflammation.
These studies demonstrate that combined genetic deletions of TNFR1 and TNFR2 receptors facilitate emergence of an altered immune state with chronic joint inflammation, hypersensitivity, and gait disturbance in animals exposed to an inflammatory “double hit” regimen. The chronic inflammatory state was induced with injection of CFA followed weeks later by a mild colitic insult with MO. We have noted previously that intracolonic MO promotes ulceration and evidence of cFos activation in the spinal cord with recovery within 4-8 hours14. The CFA arthritis is a well established in vivo model with some features of human chronic inflammatory disease and rheumatoid pathology, i.e. increased nociceptive responses, edema, and gait disturbances3,15 (Arthritis Foundation, http://www.arthritis.org). In preliminary studies, the TNFR1/R2-/- mice were resistant to induction of a chronic pain state with the collagen + CFA (CIA) method, and the IFA + mBSA (AIA) method produced only transient effects (data not shown). The severity and chronic persistence of the pain related behaviors was significant in mice with CFA induced arthritis.
Further investigation of the cytokine/chemokine profile determined that high levels of serum TNFα and other inflammatory mediators [RANTES, CCL2 (MCP-1), CXCL9 (MIG), CXCL10 (IP-10)] were prominent at 5 months in TNFR1/R2-/- mice with chronic arthritis compared to the recovered WT mice. Gene mutations that produce increases in TNFα initiate cascades of other inflammatory cell-to-cell mediators including RANTES (Regulated upon Activation Normally T-cell Expressed and Secreted), MIP-1α (macrophage inflammatory protein-1alpha) and IL-8. This has been demonstrated in TNFα-treated primary cultures of synovial fibroblasts obtained from patients with inflamed joints16-17. Inflammatory stimuli provoke elevations of TNFα in both serum and synovial fluid of adults with arthritis18. Locally in the joint, inflammation occurs when TNFα binds and activates its receptors causing synoviocytes to proliferate and thicken the synovial lining (pannus). Proliferation of the synoviocyte pannus layer further amplifies local production of inflammatory mediators19. Dysregulation of TNFα and sTNFR1/R2 has been shown by others to contribute to joint pain and bone destruction20-21. High levels of TNFα in synovial fluid are correlated with increased pain experienced by RA patients in response to mechanical and thermal stimuli22. TNFα itself sensitizes neuronal network provoking pain23. High levels of TNFα are reported in numerous other pathophysiological conditions, including, cerebrospinal fluid (CSF) of patients with complex regional pain syndrome24, in Schwann cells from patients with painful neuropathies25, in blood of patients with long-term pain26, in LPS induced systemic inflammatory syndrome27 and in CSF of headache patients28. Dysregulated TNF also contributes to inflammatory bowel disorder (IBD)13,29-30, but functional involvement of TNFRs in IBD is not fully discovered. The mechanism for the high levels of TNFα that promotes the chronic pain related behavior in the absence of regulating TNFR fragments is unknown and under investigation. To date there are no other known pathways through which TNF can act. However, biochemical studies are typically done in control animals rather than animals with ongoing persistent pain related behaviors that likely have undergone plastic changes.
Translational Significance
These data supported the concept that maladaptative genetic mutation conditions that promote chronic overexpression of TNFα can contribute to the establishment of chronic inflammatory hypersensitivity, and gait disturbance. The data also imply that aberrations in TNFR1 and TNFR2 function are factors relevant to chronic arthropathies and pain in patients with polymorphic TNFR defects. It is speculated that the TNFR1/R2-/- mice develop chronic hypersensitivity through complex interactions involving local immune cells, high levels of circulating inflammatory mediators as a result of the TNFR1/R2 genetic deletions, and the neurogenic responses of the peripheral and/or central nervous system since administration of a selective P2X7 antagonist abrogated the mechanical hypersensitivity in these mice. Holmes and colleagues31 reported that TNFR1 but not TNFR2 is found in nerve fibers innervating the spinal cord dorsal horn of WT mice. TNFR2 immunoreactivity is reportedly present in the central canal ependymal cells, motor neurons, and vascular endothelial cells. A recent study has shown in mice with single deletions of TNFR1 or TNFR2, that both TNFR1 and TNFR2 play distinct roles in regulating different phases of inflammation induced sensitization32. TNFR1 is ubiquitously expressed on the surface of most cell types. TNFR2 is primarily found on lymphocytes33.
Literature review finds no single mechanism or pharmacological agent can effectively reduce chronic pain and inflammation long term. Previous studies using two consecutive immune challenges to prime pain behavioral responses serve as reminders of the complexity of the immune response which involves activation of the innate immune response of cells residing within tissues as well as the adaptive immune response34. However, activation of microglia, the local immune cells resident in the spinal cord, is a likely contributor to the increased vulnerability to a “second hit” similar to macrophage priming peripherally33. Since the P2X7 channels are present on microglia the reduction of the behavioral hypersensitivity by the P2X7 antagonist implies that this mechanism of microglial priming may be involved. The reversal of pain-related behaviors in TNFR1/R2-/- mice by the P2X7 antagonist indicates involvement of this ion channel in the arthritis model likely through sensitization of the peripheral nerves and/or the spinal cord dorsal horn ganglia. P2X7 has been found in neuronal fibers of patients with neuropathic pain35. It is also known that P2X7 is elevated in spinal cord microglia and in synoviocytes after LPS initiating release of IL-1β36–37. In addition, P2X7 stimulation of keratinocytes with ATP in vitro has been shown to increase CCL2 chemokine expression acutely38.
The data provide experimental evidence that combined genetic polymorphisms and environmental factors such as GI bacterial infections or injuries to the joints play a critical role in the etiology of chronic arthritis as proposed by Toivanen (2003)1. The double hit model has provided us with an effective experimental tool for study of the transition from acute to chronic inflammatory pain states. Understanding molecular pathways and neuroregulatory modulation of inflammatory responses will assist in development of adjunct therapy to provide permanent remission of disease processes for more complete abrogation of pain and disability in patients.
Conclusions
Induction of CFA knee joint inflammation followed by a colitic “double hit” with MO evokes a recrudescence of the initial knee joint inflammatory response in a mouse strain genetically deficient in the TNFR1/R2 subunits promoting chronic elevations of TNFα levels. Chronic hypersensitivity and gait disturbance persist through at least 23 weeks of trials. High levels of serum TNFα, and other inflammatory mediators, such as RANTES, CCL2 (MCP-1), CXCL9 (MIG), CXCL10 (IP-10) are also prominent in this chronic inflammatory and pain model. Pain-related behaviors, gait disturbances, remain chronically (>5 months) in TNFR1/R2-/- mice while WT background mice recover. Other non-pathogen activated methods of knee joint inflammation (AIA, CIA) did not produce chronic behavioral hypersensitivity responses in preliminary studies. This suggests that combined genetic polymorphisms and environmental factors such as GI bacterial infections or injuries to the joints play a critical role in the etiology of chronic arthritis.
Acknowledgments
These studies were supported by start-up dollars (KNW) from the University of Kentucky Dean of Medicine and NIH grants NINDS NS39041 (KNW) and NIDCR DE19177 (HSO).
Abbreviations
- AIA
Antigen induced Arthritis
- CCL2 (MCP-1)
Chemokine (C-C motif) ligand 2
- CFA
complete Freund's adjuvant
- CIA
Collagen-induced arthritis
- CXCL9 (MIG)
Chemokine (C-X-C motif) ligand 9
- CXCL10 (IP-10)
Chemokine (C-X-C motif) ligand 10
- DRG
Dorsal root ganglia
- IACUC SOP
Institution Animal Care and Use Committee Standard Operating Procedure
- IFA
Incomplete Freund's adjuvant
- mBSA
Methylated bovine serum albumin
- MIP-1α
Macrophage inflammatory protein-1alpha
- MO
Mustard oil
- PFA
Paraformaldehyde in phosphate buffered saline
- PWL
Paw withdrawal latency
- sTNF-R
Soluble TNF receptor
- TNFα
Tumor necrotic factor
- RA
Reactive arthritis
- RANTES
Regulated upon Activation Normally T-cell Expressed and Secreted
- WT
Wild type
Footnotes
The authors confirm that no potential conflicts of interest exist. All authors have read the journal's policy on disclosure of potential conflicts of interest.
References
- 1.Toivanen P. Normal intestinal microbiota in the aetiopathogenesis of rheumatoid arthritis. Ann Rheum Dis. 2003;62:807–11. doi: 10.1136/ard.62.9.807. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin AS, Coons AH. Specific heterologous enhancement of immune responses IV. Specific generation of a thymus-derived enhancing factor. J Exp Med. 1972;136:1501–1517. doi: 10.1084/jem.136.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staite ND, Cutshaw LG, Dunn CJ. The effects of sensitization protocols on arthritic responses in antigen-induced arthritis. Agents Actions. 1989;27(3-4):338–340. doi: 10.1007/BF01972816. [DOI] [PubMed] [Google Scholar]
- 4.Chou LW, Wang J, Chang PL, Hsieh YL. Hyaluronan modulates accumulation of hypoxia-inducible factor-1 alpha, inducible nitric oxide synthase, and matrix metalloproteinase-3 in the synovium of rat adjuvant-induced arthritis model. Arthritis Res Ther. 2011;13:R90. doi: 10.1186/ar3365. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagari T, Doi H, Shimozato T. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002;169:1459–66. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]
- 6.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92(3):335–42. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 7.Kojima F, Kapoor M, Yang L, Fleishaker EL, Ward MR, Monrad SU, Kottangada PC, Pace CQ, Clark JA, Woodward JG, Crofford LJ. Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice. J Immunol. 2008;180:8361–8. doi: 10.4049/jimmunol.180.12.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosloniec EF, Cremer M, Kang AH, Myers LK, Brand DD. Collagen-induced arthritis. Curr Protoc Immunol. 2010 Apr;Chapter 15(Unit 15.5):1–25. doi: 10.1002/0471142735.im1505s89. [DOI] [PubMed] [Google Scholar]
- 9.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 10.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1984;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 11.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 12.Sluka KA, Jordan HH, Westlund KN. Reduction in joint swelling and hyperalgesia following post-treatment with a non-NMDA glutamate receptor antagonist. Pain. 1994;59:95–100. doi: 10.1016/0304-3959(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 13.Oz HS, Chen T, Nagasawa H. Comparative efficacies of two cysteine prodrugs and a glutathione delivery agent in a colitis model. Transl Res. 2007;150:122–129. doi: 10.1016/j.trsl.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Westlund KN. Effects of baclofen on colon inflammation-induced Fos, CGRP and SP expression in spinal cord and brainstem. Brain Res. 2001;889(1-2):118–30. doi: 10.1016/s0006-8993(00)03124-3. [DOI] [PubMed] [Google Scholar]
- 15.Vermeirsch H, Biermans R, Salmon PL, Meert TF. Evaluation of pain behavior and bone destruction in two arthritic models in guinea pig and rat. Pharmacol Biochem Behav. 2007;87:349–59. doi: 10.1016/j.pbb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol. 2001;167:5381–5385. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- 17.Ogura N, Tobe M, Sakamaki H, Nagura H, Abiko Y, Kondoh T. Tumor necrosis factor-alpha increases chemokine gene expression and production in synovial fibroblasts from human temporomandibular joint. J Oral Pathol Med. 2005;34:357–63. doi: 10.1111/j.1600-0714.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaneyama K, Segami N, Nishimura M, Suzuki T, Sato J. Importance of proinflammatory cytokines in synovial fluid from 121 joints with temporomandibular disorders. Br J Oral Maxillofac Surg. 2002;40:418–423. [PubMed] [Google Scholar]
- 19.Pap T, Muller-Ladner U, Gay RE, Gay S. Fibroblast biology. The role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arth Res. 1989;2:361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alstergren P, Kopp S. Insufficient endogenous control of tumor necrosis factor-alpha contributes to temporomandibular joint pain and tissue destruction in rheumatoid arthritis. J Rheumatol. 2006;33:1734–9. [PubMed] [Google Scholar]
- 21.Kim H, Chun S, Ku SY, Suh CS, Choi YM, Kim JG. Association between polymorphisms in tumor necrosis factor (TNF) and TNF receptor genes and circulating TNF, soluble TNF receptor levels, and bone mineral density in postmenopausal Korean women. Menopause. 209;16:1014–20. doi: 10.1097/gme.0b013e3181a039c8. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RR, Wasan AD, Bingham CO, 3rd, Bathon J, Haythornthwaite JA, Smith MT, Page GG. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11:R61. doi: 10.1186/ar2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst. 2000;5:96–100. doi: 10.1046/j.1529-8027.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 24.Alexander GM, Van Rijn MA, Van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116:213–219. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Empl M, Renaud S, Erne B, Fuhr P, Straube A, Schaeren-Wiemers N, Steck AJ. TNF-alpha expression in painful and nonpainful neuropathies. Neurology. 2001;56:1371–1377. doi: 10.1212/wnl.56.10.1371. [DOI] [PubMed] [Google Scholar]
- 26.Koch A, Zacharowski K, Boehm O, Stevens M, Lipfert P, Von Giesen HJ, Wolf A, Freynhagen R. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm Res. 2007;56:32–37. doi: 10.1007/s00011-007-6088-4. [DOI] [PubMed] [Google Scholar]
- 27.Oz HS, Chen T, Neuman M. Nutritional intervention against systemic inflammatory syndrome. J Parenteral Enteral Nutrition. 2009;33:380–389. doi: 10.1177/0148607108327194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47:1050–1055. doi: 10.1111/j.1526-4610.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 29.Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants a novel therapy in a murine model of colitis. J Nutri Biochem. 2005;16:297–304. doi: 10.1016/j.jnutbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Neuman MG. Immune dysfunction in inflammatory bowel disease. Transl Res. 2007;149:173–86. doi: 10.1016/j.trsl.2006.11.009. Review. [DOI] [PubMed] [Google Scholar]
- 31.Holmes GM, Hebert SL, Rogers RC, Hermann GE. Immunocytochemical localization of TNF type 1 and type 2 receptors in the rat spinal cord. Brain Res. 2004;1025(1-2):210–219. doi: 10.1016/j.brainres.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152:419–27. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xanthoulea S, Curfs DM, Hofker MH, de Winther M. Nuclear factor kappa B signaling in macrophage function and atherogenesis. Curr Opin Lipidol. 2005;16:536–42. doi: 10.1097/01.mol.0000180167.15820.ae. [DOI] [PubMed] [Google Scholar]
- 34.Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, Taylor FR, Harrison JA, Martin TJ, Eisenach JC, Maier SF, Watkins LR. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain. 2010;11:1004–14. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CBA, Casula MA, Yiangou Y, Birch R, Anand P, Gary N, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Caporali F, Capecchi PL, Gamberucci A, Lazzerini PE, Pompella G, Natale M, Lorenzini S, Selvi E, Galeazzi M, Laghi Pasini F. Human rheumatoid synoviocytes express functional P2X7 receptors. J Mol Med. 2008;86:937–49. doi: 10.1007/s00109-008-0365-8. [DOI] [PubMed] [Google Scholar]
- 37.Clark AK, Staniland AA, Marchand F, Kaan TKY, McMahon SB, Malcangio M. P2X7-Dependent Release of Interleukin-1β and Nociception in the Spinal Cord following Lipopolysaccharide. J Neuroscience. 2010;30:573–582. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pastore S, Mascia F, Gulinelli S, Forchap S, Dattilo C, Adinolfi E, Girolomoni G, Di Virgilio F, Ferrari D. Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J Invest Dermatol. 2007;127:660–7. doi: 10.1038/sj.jid.5700591. [DOI] [PubMed] [Google Scholar]