Abstract
XIAP, a potent caspase inhibitor, is highly expressed in acute myeloid leukemia (AML) cells and contributes to chemoresistance. A multi-center phase 1/2 trial of XIAP antisense oligonucleotide AEG35156 in combination with idarubicin/cytarabine was conducted in 56 patients with relapsed/refractory AML. Herein we report the pharmacodynamic studies of the patients enrolled at M. D. Anderson Cancer Center. A total of 13 patients were enrolled in our institution: five in phase 1 (12–350 mg/m2 AEG35156) and eight in phase 2 (350 mg/m2 AEG35156) of the protocol. AEG35156 was administered on 3 consecutive days and then weekly up to a maximum of 35 days. Blood samples were collected from patients on days 1 through 5 and on day 28–35 post-chemotherapy for detection of XIAP levels and apoptosis. AEG35156 treatment led to dose-dependent decreases of XIAP mRNA levels (42–100% reduction in phase 2 patients). XIAP protein levels were reduced in all five samples measured. Apoptosis induction was detected in 1/4 phase 1 and 4/5 phase 2 patients. Importantly, apoptosis was most pronounced in CD34+38− AML stem cells and all phase 2 patients showing apoptosis induction in CD34+38− cells achieved response. We conclude that at 350 mg/m2, AEG35156 is effective in knocking down XIAP in circulating blasts accompanied by the preferential induction of apoptosis in CD34+38− AML stem cells.
Keywords: XIAP, Antisense oligonucleotide AEG35156, Apoptosis, Clinical trial, AML
Introduction
Acute myeloid leukemia (AML) is an aggressive hematological malignancy with generally poor prognosis. Chemotherapies that induce apoptosis are the primary treatment for patients with AML but their effectiveness is limited by evolving chemoresistance. XIAP, a member of the inhibitor of apoptosis family of proteins (IAPs) inhibits initiator caspase-9 and also effector caspases-3 and caspase-7, the convergence point of multiple caspase activation pathways and thus suppresses apoptosis induced by a variety of stimuli. XIAP, the best-characterized IAP has received great attention as a potential therapeutic target because it is the most potent cellular caspase inhibitor [1], is highly expressed in various cancer cells [2, 3], and contributes to chemoresistance [4–6]. Targeting XIAP has been shown to sensitize non-small-cell lung carcinoma to γ-irradiation and human ovarian and prostate cancer cells to chemotherapeutic agents in vitro [7–9]. Inhibition of XIAP with an antisense oligonucleotide (ASO) delayed tumor growth in a lung cancer xenograft model [10].
We have demonstrated that XIAP is highly expressed in AML cells [11, 12] and that inhibition of XIAP by ASO or small molecule antagonists sensitizes HL-60 cells to cytarabine (Ara-C) [11] and promotes apoptosis in both leukemic cell lines and AML blasts [13], suggesting that XIAP is a potential therapeutic target in AML [14].
AEG35156 is a second-generation of XIAP ASO developed by Aegera Therapeutics (Montreal, QC, Canada). Preclinical studies of this ASO showed knockdown of XIAP and sensitization of malignant cells to chemotherapies both in vitro and in murine models [15, 16] and phase 1 studies of AEG35156 in solid tumor have been completed [17, 18]. However, the therapeutic potential of this ASO in relapsed/refractory AML was not known. A multi-center phase 1/2 trial of AEG35156 in combination with idarubicin and Ara-C chemotherapy in patients with relapsed or primary refractory AML was conducted and the results have recently been reported [19]. AEG35156 was administered at a maximum dose of 350 mg/m2 and the combination of XIAP ASO, idarubicin, and Ara-C resulted in complete remission (CR) in 10/11 frontline induction failure AML patients [19]. We performed the associated pharmacodynamic studies of samples obtained from patients treated at MD Anderson Cancer Center and report here that AEG35156 is effective in knocking down XIAP in circulating blasts and preferentially induces apoptosis in CD34+38− AML stem cells.
Materials and methods
Inhibition of XIAP expression in cells obtained from AML patient samples in vitro
Primary AML patient samples were acquired after informed consent had been obtained according to institutional guidelines. Mononuclear cells were purified by Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO) density-gradient centrifugation and then transfected with AEG35156 or a control oligonucleotide (HyB931A, Hybridon, Cambridge, MA) by electroporation as described previously [11] according to the manufacturer’s instructions (Amaxa Biosystems, Cologne, Germany). Both AEG35156 and the control oligonucleotide have the same backbone of the mix of phosphothio DNA and 2′ O-methyl RNA residues.
Patient eligibility
Patients age >18 with ECOG performance status 0–2 and with relapsed or primary refractory AML except acute promyelocytic leukemia were eligible if they relapsed 6 months or less after their initial CR or if they were refractory to at least one induction chemotherapy regimen. The diagnosis of relapsed or refractory AML required >10% blasts in the marrow or blood or 5–10% blasts in the blood or marrow unrelated to recovery of normal hematopoiesis plus at least one cytopenia. Circulating blasts had to be <50,000/μl and not expected to rise above 50,000/μl in the first 5 days of treatment. Patients were ineligible if they had an ejection fraction <50%, active central nervous system AML, or abnormal organ function. The study was approved by the M. D. Anderson IRB and all patients provided written informed consent before study treatment. The characteristics of patients, whose samples were used in this study, including blast counts, cytogenetics, and previous therapy and responses prior to entering the trial are showing in Table 1.
Table 1.
Characteristics of patients enrolled in the trial
| Patients | Circulating blast (%) | Cytogenetics | Previous treatment and response |
|---|---|---|---|
| 101 | 49 | Pseudodiploid clone46, XX, t(6;9)(p23;q34)[4], Diploid female karyotype 46, XX[16] | CR on IA + ZAENESTRA then relapsed |
| 102 | 95 | Diploid female karyotype 46, XX[22] | Partial remission on IDA + High dose Ara-C |
| 103 | 20 | Pseudodiploid clone 46, XX, inv(7)(q22q34)[20] | CR on ara-C + idarubicin |
| 104 | 24 | Pseudodiploid clone 46, XY, add(2)(q36), del(5)(q13q33), add(6)(q21), −9, −17, +2mar[20] | CR on IA + Zarnestra |
| 105 | 8.5 | Diploid female karyotype 46, XX[20] | Resistant to VNP |
| 106 | 64 | 46, X, −Y, +3, +8, der(12)t(12;17)(p11.2;q11.2), +15, der(15;15)(q10;q10), −17[13] etc. | Resistant to IDA + HDAC |
| 107 | 58 | Diploid female karyotype 46, XX[19] | CR on DAC |
| 109 | NA | 45, XX, del(1)(q12q23), add(6)(q25), t(7;18)(q11.2;q11.2), +8, del(9)(q12q32), add(16)(p13.1), −17, add(18)(p11.2), −22[4], 44–50, XX, del(1)(q12q23), add(6)(q25), t(7;18)(q11.2;q11.2), +8, del(9)(q12q32), add(16)(p13.1), −17, add(18)(p11.2), −22, +2–4mar, 1–3dmin[cp9], Diploid female karyotype 46, XX[3] etc. | Resistant to IDA + HDAC |
| 110 | 4 | Diploid male karyotype 46, XY[20] | Resistant to Clofarabine |
| 111 | 9 | Diploid male karyotype 46, XY[20] | No response to DAC + VA |
| 115 | 31 | Pseudodiploid clone 46, XY, t(6;9)(p23;q34)[19] | Partial remission on idarubicin + Ara-C |
Trial design
Patients received escalating doses of AEG35156 from 12 mg/m2 to 350 mg/m2. AEG35156, in sterile isotonic saline was administered intravenously over 2 h on days 1–3 and 8 and then weekly thereafter until CR/CRp (CR without platelet recovery), disease progression, or day 35, whichever came first. The starting dose was <1/10 of the maximal dose tested in the phase 1 study of AEG35156 as a single agent [17, 18]. Patients received idarubicin 12 mg/m2 by intravenous infusion over 30 min on days 4, 5, and 6 and high-dose Ara-C 1.5 g/m2 by continuous infusion over 24 h for 4 days on days 4, 5, 6, and 7 (patients <65 years) or for 3 days on days 4, 5, and 6 (patients ≤65 years). Patients achieving CR/CRp as previously described [17] could receive up to 4 courses of consolidation chemotherapy with weekly AEG35156 and Ara-C at a daily dose of 0.75 g/m2 using the induction schedule. Initially, AEG35156 was continued weekly beyond day 8 in order to maintain target knockdown. However, due to 2 cases of peripheral neuropathy in the phase 2 portion of the study, the protocol was modified so that the last 8 patients received AEG35156 only on days 1 to 3 and 8 during induction with no additional cases of peripheral neuropathy noted. Patients experiencing improvement or complete remission could proceed to allogenic hematopoietic stem cell transplantation at the discretion of the treating physicians.
Sample preparation
Peripheral blood samples were collected prior to the start of AEG35156 infusions on days 1, 2, and 3 and on days 4 and 5 prior to the start of idarubicin and Ara-C, and on day 28–35 post chemotherapies. For determination of mRNA and protein levels, mononuclear cells were purified by Ficoll-Hypaque (Sigma-Aldrich, St. Louis, Mo) density gradient centrifugation. Leukemic blasts were enriched from the mononuclear cells by depleting CD3+ and CD19+ cells with Miltenyi Microbeads and autoMACS Separator (Miltenyi, Auburn, CA) and frozen at −80°C until use. For apoptosis analysis, total blood was mixed with red blood cell (RBC) lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and then washed with PBS.
RNA isolation and real-time quantitative RT-PCR
Total RNA was extracted from the enriched leukemic blasts and XIAP mRNA levels were determined by real-time RT-PCR and normalized to human 18S RNA or β2 microglobulin mRNA levels as previously described [15]. XIAP mRNA levels at each time points were compared to the levels on day 1 prior to the first dose of AEG35156.
Western blot analysis
To determine XIAP protein levels, we performed western blot analysis as described previously [11]. Briefly, cells were lysed in 2× protein lysis buffer (0.25 M Tris-HCl, 2% SDS, 4% β-mercaptoethanol, 10% glycerol, and 0.02% bromophenol blue). An equal amount of cell lysate was loaded onto a 12% SDS PAGE gel (Bio Rad, Hercules, CA); thereafter, the proteins were transferred to a membrane and then probed with XIAP antibody (Transduction Laboratories and PharMingen, San Diego, CA). After incubation with the second antibody, the membranes were treated with ECL solution (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Signals were detected using a PhosphorImager (Storm 860 Version 4.0; Molecular Dynamics, Sunnyvale, CA) and quantified using ImageJ (NIH, Bethesda, Maryland). β-Actin was included as a loading control and XIAP levels were normalized to β-actin.
Apoptosis analysis
Apoptosis was estimated by measuring phosphatidylserine externalization [20] of cells stained with Annexin V-FLUOS (Roche Applied Science, Indianapolis, IN). All measurements were made using a LSR II FACS analysis (BD Biosciences, San Diego, CA). Apoptosis was assessed in bulk blasts, CD34+38+ cells, and CD34+38− cells using a CD34-phycoerythrin antibody and a CD38-allophycocyanin antibody (both from BD Biosciences).
Statistical analysis
The results were expressed as mean ± SE.
Results
AEG35156 decreases XIAP levels in cells obtained from AML patient samples in vitro
To demonstrate that AEG35156 inhibits XIAP expression in AML cells, we first tested it in vitro. AEG35156 and its control oligonucleotide were transfected into cells obtained from AML patients by electroporation and XIAP levels were determined by western blot. As shown in Fig. 1, AEG35156 was able to effectively decrease XIAP protein levels in AML cells.
Fig. 1.
AEG35156 inhibits XIAP expression in vitro in cells obtained from AML patients. Mononuclear cells from the bone marrow sample of an AML patient (80% blasts) were transfected with AEG35156 (AS) and a control oligonucleotide (NS) and XIAP protein levels were determined by western blot at 24, 48, and 72 h post-transfection. UD undetectable
AEG35156 infusion results in dose-dependent decrease of XIAP mRNA in circulating AML blasts
The patient characteristics are shown in Table 1. Patients were initially dosed with AEG35156 on days 1–3 prior to chemotherapy commencing on day 4. XIAP mRNA levels were determined by real-time quantitative RT-PCR using RNA samples obtained from the enriched leukemic blasts collected on days 1 through 4 prior to treatment (day 5 and day 28–35 samples were not available in all the patients). XIAP mRNA levels on days 2 through 4 were determined and compared with those on day 1 (before the first AEG35165 infusion). As shown in Fig. 2a and Table 2, there was a dose-dependent reduction in XIAP mRNA levels after treatment with AEG35156, and the phase 2 dose of 350 mg/m2 was very effective in reducing XIAP mRNA levels, with an overall reduction of 47.2% ± 18.7% (n = 6). At this dose, target knockdown was observed in all the day 2 samples, resulting in overall the highest decrease in XIAP mRNA (80% ± 9.2%, Fig. 2b). This was followed by day 4 (36.6% ± 44.6%) and then day 3 (25.0% ± 33.9%) (Fig. 2b), largely because XIAP mRNA levels fluctuated in some day 3 and day 4 samples (Fig. 2a). There was no reduction of XIAP mRNA levels when patient 102 was treated with a dose of 24 mg/m2 AEG35156. However, XIAP mRNA levels were markedly reduced in samples from patient 105 at a dose of 165 mg/m2 AEG35156 and in samples from all the patients treated with 350 mg/m2 at some or all of the time points analyzed. Patients 105, 107, 109, and 110 showed consistent reduction in XIAP mRNA levels over the course of treatment; all but patient 109, who withdrew from the study, achieved CR. Circulating blasts from patients 111 and 115 showed reductions in XIAP mRNA levels on day 2, but increase on day 3. Their XIAP mRNA levels decreased again on day 4, and the patients achieved either CR or CRp (Fig. 2a and Table 2). Samples from patient 106 showed an initial reduction in XIAP mRNA levels on day 2, but the levels increased to above baseline during the following days. This patient did not respond to the treatment.
Fig. 2.
XIAP mRNA levels determined by RT-PCR in circulating blasts of AML patients receiving AEG35156 infusion. a Dose-dependent decrease of XIAP mRNA in AEG35165 treated circulating AML blasts. b XIAP mRNA reduction in AEG35156 treated circulating AML blasts of six phase 2 patients
Table 2.
Decrease in XIAP levels, induction of apoptosis in circulating AML blasts, and patient responses to AEG35156 + idarubicin/Ara-C
| Patient | Dose, mg/m2 | Decrease in
|
Apoptosis induction
|
Responses | |||
|---|---|---|---|---|---|---|---|
| XIAP levels
|
Total blasts | CD34+38+ cells | CD34+38− cells | ||||
| mRNA | Protein | ||||||
| 101 | 12 | NDa | ND | No | No | No | NRb |
| 102 | 24 | No | ND | No | No | No | NR |
| 103 | 48 | ND | ND | No | No | No | NR |
| 104 | 48 | ND | Yes | Yes | Yes | Yes | NR |
| 105 | 165 | Yes | Yes | ND | ND | ND | CRc |
| 106 | 350 | Yes | ND | No | No | No | NR |
| 107 | 350 | Yes | Yes | No | No | Yes | CR |
| 109 | 350 | Yes | ND | ND | ND | ND | Withdrew |
| 110 | 350 | Yes | Yes | No | No | Yes | CR |
| 111 | 350 | Yes | Yes | No | No | Yes | CRpd |
| 115 | 350 | Yes | ND | Yes | Yes | Yes | CR |
Not determined,
No response,
Complete remission, and
Complete remission without platelet recovery
AEG35156 infusion results in a decrease of XIAP protein in circulating AML blasts
Western blot analysis was carried out using lysates from five available patient samples. As shown in Fig. 3, there was a marked time-dependent decrease in XIAP protein levels in circulating blasts from patients 105, 110, and 111, all of whom achieved CR or CRp. For patient 107, although there was a large reduction in XIAP mRNA levels in circulating blasts, XIAP protein levels were only slightly reduced on day 3; nevertheless, this patient achieved CR. Unfortunately no samples on days 2 and 5 were available for protein determination for this patient. For patient 104, a small reduction of XIAP protein levels in circulating blasts was seen when the patient was treated with AEG35156 alone (up to day 4) and the patient was not responsive to the therapy. Of interest, however, the basal XIAP level was extremely low in this patient (Fig. 3).
Fig. 3.
Western blot determination of XIAP protein levels in circulating blast of AML patients treated with AEG35156. UD undetectable
AEG35156 infusion results in apoptosis in circulating AML blasts
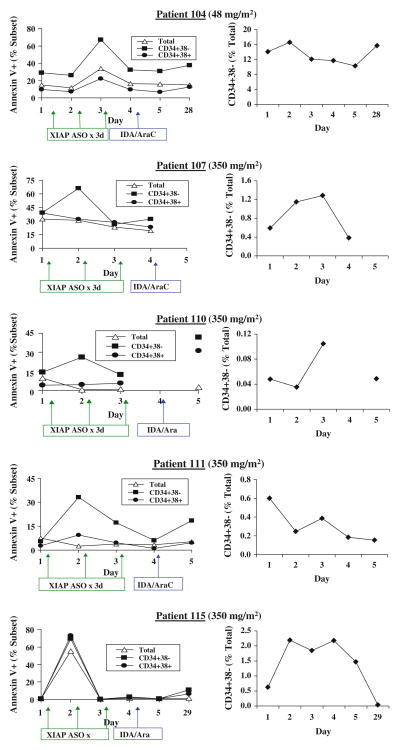
To measure apoptosis induction by AEG35156 infusion, whole blood samples were obtained from patients on days 1 through 5 prior to treatment and on day 28–35 post chemotherapies and lyzed with RBC lysis buffer. Apoptosis in total circulating blasts, CD34+38+ cells, and CD34+38− cells was determined and assessed by increase in annexin V positivity in these cells. As shown in Table 2, apoptosis was analyzed in 9 samples and was detected in 5 (Table 2 and Fig. 4). At a dose of 48 mg/m2 AEG35156, apoptosis was detected in patient 104 in all cell compartments tested. However, a higher degree of apoptosis was detected in CD34+38− cells. For patient 115, treated with 350 mg/m2 AEG35156, apoptosis was induced in all the cell populations analyzed. However, for patients 107, 110, and 111, this effect was observed only in CD34+38− cells. The induction of apoptosis in CD34+38− cells correlated in most cases with the decrease in the percentage of this cell population (Fig. 4). For patients 104, 110, and 111, decrease in % of CD34+38− cells were found on the same day that annexin V + cells were detected. For patient 107, decrease in % of CD34+38− cells occurred 2 days after their annexin V positivity, probably because of delayed clearance of CD34+38− cells in this patient. For patient 115, we did not see a decrease in % of CD34+38− cells although a high rate of apoptosis was observed in this cell population. This may be explained by high rates of apoptosis in all cell populations. Importantly, except for patient 104, all patients who showed apoptosis induction in CD34+38− cells achieved CR or CRp even when no annexin V positivity was detected in bulk blasts and CD34+38+ cells. Table 2 summarizes the effects of AEG35156 treatment on XIAP mRNA and protein levels and apoptosis induction, as well as the responses of patients with relapsed/refractory AML to the AEG35156/Ara-C/idarubucin therapy.
Fig. 4.
Apoptosis analysis of AML patient samples and the percentages of CD34+38− cells in these samples treated with AEG35156 in vivo
Discussion
Two of the objectives of this phase 1/2 trial were to establish proof-of concept for AEG35156-mediated XIAP knockdown in AML blast cells and to identify in vivo biomarkers of XIAP knockdown in patients with relapsed/refractory AML. We demonstrate here that AEG35156 infusion resulted in reduction of both XIAP mRNA and protein levels, which was associated with induction of apoptosis in circulating CD34+38− cells and clinical response (Table 2).
AEG35156 infusion resulted in dose-dependent reduction of XIAP mRNA levels in circulating AML blasts, and at 350 mg/m2, it was effective in reducing XIAP mRNA levels. Of importance, western blot analysis demonstrated a reduction in XIAP protein levels in all five samples analyzed (two from phase 1 of the study and three from phase 2). Four out of five of these samples also showed reduction in XIAP mRNA levels. Patients 105 and 110 (both achieved CR) and 111 (who achieved CRp) had a substantial reduction in XIAP mRNA and protein levels. For patient 107 (who achieved CR), XIAP mRNA levels were markedly reduced at all assessed time points, but protein levels were only marginally reduced on day 3. This discrepancy between protein and RNA levels could have been due to the prolonged half-live of the XIAP protein in this patient’s blasts. For patient 104, although slight decreases in XIAP protein levels and apoptosis induction were detected, the patient was resistant to therapy. This patient received a dose of only 48 mg/m2 AEG35156, which may not have been enough to achieve a clinical benefit. It is important to point out that patient 104 had extremely low baseline XIAP protein level and leukemic cells in this patient may rely more on other antiapoptotic proteins for survival, which is in agreement with our previous finding that the response of AML blasts to XIAP antagonists in vitro correlates with their endogenous XIAP levels [13].
Apoptosis induction was observed in five of the nine patient samples analyzed. Interestingly, apoptosis induction seemed to be more prominent in the CD34+38− population, which may explain the relative long remission duration observed in the patients treated with XIAP ASO/idarubicin/Ara-C [19]. For patients 107, 110, and 111, apoptosis was observed only in the CD34+38− compartment, but not in bulk blasts or CD34+38+ cells, although there was marked reduction of XIAP protein levels in total blasts. The reason for this discrepancy is unclear although it may suggest that leukemia stem cells are more dependent on XIAP for survival. Importantly, all of the phase 2 patients whose samples demonstrated induction of apoptosis in the CD34+38− population achieved either CR or CRp.
In our previous report [19], only the maximal knockdown of XIAP mRNA for each patient was demonstrated. In this study, XIAP mRNA levels on days 2 through 4 were determined and compared with those on day 1. Overall, the maximal reduction of XIAP mRNA was found on day 2, followed by day 4, and then day 3, largely due to the fluctuation of XIAP mRNA levels in the later days. The reason for this fluctuation was not understood. One possibility is a compensatory mechanism that may be overcome in part by repeating AEG35156 infusion as observed in patients 111 and 115. Analysis of XIAP mRNA levels daily and correlating the results with patient’s clinical responses suggest that reduction of XIAP mRNA at the start of chemotherapy (day 4) is important for patients to achieve clinical responses.
Unfortunately, no samples from other participating centers were available for XIAP protein measurement and apoptosis analysis. Nevertheless, although the sample size was small, we were able to observe an association of clinical responses with the reduction of XIAP levels and induction of apoptosis particularly in the CD34+38− cell population based on the samples we obtained from the patients enrolled at M. D. Anderson Cancer Center (Table 2, bold and bold italic). The data might support the contention that the decrease in XIAP and induction of apoptosis, particularly in the stem cell compartment are necessary, even though may not be sufficient, for the clinical response.
In summary, AEG35156, at a dose of 350 mg/m2, is effective in reducing XIAP levels in circulating myeloid leukemic blasts. This effect was accompanied by induction of apoptosis, preferentially in the CD34+38− stem cell compartment, which may explain the relatively long remission achieved in this clinical trial. A randomized phase 2 clinical study of re-induction chemotherapy with or without AEG35156 in AML patients with frontline induction failures has been initiated.
Acknowledgments
The work was supported in part by grants from the National Institutes of Health (P01 CA55164 and CA16672) to M.A and Aegera Therapeutic Inc. to B.Z.C. We would like to thank Wenjing Chen for collecting patient information and Bradley S. Tadlock and Kate J. Newberry for helping with the manuscript preparation.
Footnotes
Conflicts of interest B.Z.C received financial support from Aegera for this study, S.J.M works for Aegera, M.A. holds Aegera stocks, and others indicated no conflicts of interests.
Contributor Information
Bing Z. Carter, Department of Stem Cell Transplantation and Cellular Therapy, Unit 448, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA
Duncan H. Mak, Department of Stem Cell Transplantation and Cellular Therapy, Unit 448, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA
Stephen J. Morris, Aegera Therapeutics Inc., Montreal, QC, Canada
Gautam Borthakur, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA.
Elihu Estey, Seattle Cancer Care Alliance, University of Washington, Seattle, WA, USA.
Anna L. Byrd, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA
Marina Konopleva, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA.
Hagop Kantarjian, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA.
Michael Andreeff, Email: mandreef@mdanderson.org, Department of Stem Cell Transplantation and Cellular Therapy, Unit 448, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA. Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA.
References
- 1.Deveraux QL, Reed JC. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 2.Fong WG, Liston P, Rajcan-Separovic E, St Jean M, Craig C, Korneluk RG. Expression and genetic analysis of XIAP-associated factor 1 (XAF1) in cancer cell lines. Genomics. 2000;70:113–122. doi: 10.1006/geno.2000.6364. [DOI] [PubMed] [Google Scholar]
- 3.Levkau B, Garton KJ, Ferri N, et al. xIAP induces cell-cycle arrest and activates nuclear factor-kappaB: new survival pathways disabled by caspase-mediated cleavage during apoptosis of human endothelial cells. Circ Res. 2001;88:282–290. doi: 10.1161/01.res.88.3.282. [DOI] [PubMed] [Google Scholar]
- 4.Notarbartolo M, Cervello M, Poma P, Dusonchet L, Meli M, D’Alessandro N. Expression of the IAPs in multidrug resistant tumor cells. Oncol Rep. 2004;11:133–136. [PubMed] [Google Scholar]
- 5.McManus DC, Lefebvre CA, Cherton-Horvat G, et al. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene. 2004;23:8105–8117. doi: 10.1038/sj.onc.1207967. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Jian Z, Xia K, et al. XIAP is related to the chemo-resistance and inhibited its expression by RNA interference sensitize pancreatic carcinoma cells to chemotherapeutics. Pancreas. 2006;32:288–296. doi: 10.1097/01.mpa.0000218314.67111.fb. [DOI] [PubMed] [Google Scholar]
- 7.Holcik M, Yeh C, Korneluk RG, Chow T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene. 2000;19:4174–4177. doi: 10.1038/sj.onc.1203765. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki H, Sheng Y, Kotsuji F, Tsang BK. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659–5666. [PubMed] [Google Scholar]
- 9.Amantana A, London CA, Iversen PL, Devi GR. X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol Cancer Ther. 2004;3:699–707. [PubMed] [Google Scholar]
- 10.Hu Y, Cherton-Horvat G, Dragowska V, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9:2826–2836. [PubMed] [Google Scholar]
- 11.Carter BZ, Milella M, Tsao T, et al. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia. 2003;17:2081–2089. doi: 10.1038/sj.leu.2403113. [DOI] [PubMed] [Google Scholar]
- 12.Carter BZ, Kornblau SM, Tsao T, et al. Caspase-independent cell death in AML: caspase inhibition in vitro with pan-caspase inhibitors or in vivo by XIAP or Survivin does not affect cell survival or prognosis. Blood. 2003;102:4179–4186. doi: 10.1182/blood-2003-03-0960. [DOI] [PubMed] [Google Scholar]
- 13.Carter BZ, Gronda M, Wang Z, et al. Small-molecule XIAP inhibitors derepress downstream effector caspases and induce apoptosis of acute myeloid leukemia cells. Blood. 2005;105:4043–4050. doi: 10.1182/blood-2004-08-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samudio I, Konopleva M, Carter BZ, Andreeff M. Apoptosis in leukemias: regulation and therapeutic targeting. In: Nagarajan L, editor. Acute myelogenous leukemia: genetics, biology and therapy. 1. Springer; 2009. pp. 197–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaCasse EC, Cherton-Horvat GG, Hewitt KE, et al. Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res. 2006;12:5231–5241. doi: 10.1158/1078-0432.CCR-06-0608. [DOI] [PubMed] [Google Scholar]
- 16.Shaw TJ, LaCasse EC, Durkin JP, Vanderhyden BC. Downregulation of XIAP expression in ovarian cancer cells induces cell death in vitro and in vivo. Int J Cancer. 2008;122:1430–1434. doi: 10.1002/ijc.23278. [DOI] [PubMed] [Google Scholar]
- 17.Dean E, Jodrell D, Connolly K, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J Clin Oncol. 2009;27:1660–1666. doi: 10.1200/JCO.2008.19.5677. [DOI] [PubMed] [Google Scholar]
- 18.Jolivet J, Dean E, Ward TH, et al. A Phase I Trial of AEG35156 (XIAP antisense) administered as 2-hour intravenous infusions in patients with advanced tumours. J Clin Oncol. 2008 May 20;26(suppl):3541. [Google Scholar]
- 19.Schimmer AD, Estey EH, Borthakur G, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27:4741–4746. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin S, Reutelingsperger C, McGahon A, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and ABL. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]