Abstract
Gap junctions are multimeric membrane protein channels that connect the cytoplasm of one cell to another. Much information about connexins regards electrophysiology and channel function but relatively little information is known about non-channel functions of connexins. Lens connexins, Cx43, Cx46 and Cx50, have been extensively studied for their role in lens homeostasis. Connexins allow the movement of small metabolically relevant molecules and ions between cells and this action in the lens prevents cataract formation. Interruption of Cx46 channel function leads to cataract formation due to dysregulation of lens homeostasis. The loss of Cx46 upregulates Cx43 in lens cell culture and suppresses tumor growth in breast and retinoblastoma tumor xenografts. Upregulation of Cx46 in hypoxic tissues has been noted and may be due in part to the effects of hypoxia and HIF activators. Here, we report that the Cx46 promoter is regulated by hypoxia and also offer speculation about the role of Cx46 in lens differentiation and solid tumor growth.
Keywords: cellular differentiation, Connexin 43, Connexin 46, connexin promoter, hypoxia, lens, tumors, xenografts
Introduction
Gap junctions are multimeric membrane protein channels that connect the cytoplasm of one cell to another. Six connexin proteins are required to make a hemichannel, or connexon. The extracellular faces of two adjacent connexons dock together to form a gap junction channel.1-3 Although there is much information about connexin electrophysiology and channel function little information is known about non-channel functions of connexins. Connexin 46 (Cx46) has been extensively studied in the mammalian lens, where it is found with Cx43 and Cx50.4 These three connexins are differentially expressed in the various regions that define the lens (Fig. 1). In the outer epithelial layer, Cx43 and Cx50 are the predominate connexins. Immature outer fiber cells express lower amounts of Cx43 than the epithelial cells and they begin to express Cx46 as they mature and progress inward toward the nucleus of the lens. Each cell type expresses roughly the same amount of Cx50 as the other cell types in the lens. As the fiber cells mature, they lose the ability to maintain protein expression and turnover since the organelles are degraded. However, once the cells further differentiate into mature fiber cells they retain functional Cx46 and Cx50 gap junction channels.4-6
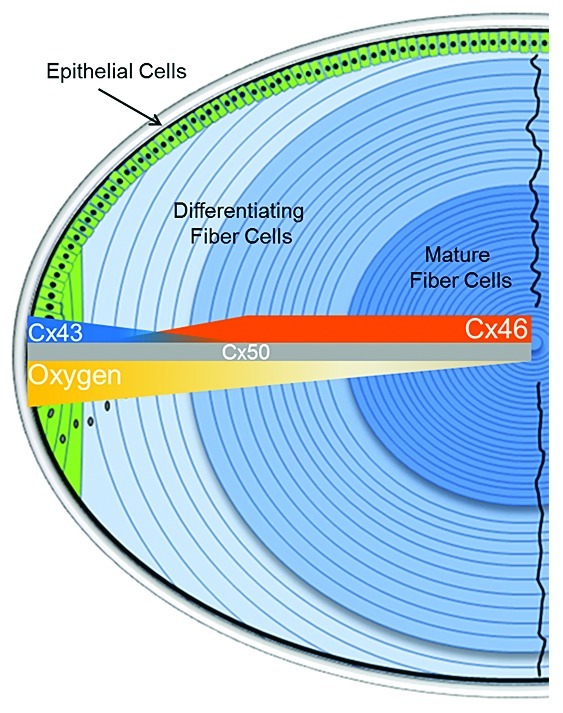
Figure 1. Schematic model of lens connexin expression zones. Cx43 is expressed primarily in the epithelial and short differentiating fiber cells. Cx46 expression begins relatively early in lens differentiation and small amounts can be found in the short differentiating fiber cells. Cx50 expression remains relatively constant throughout lens development. Lens epithelial cells begin to elongate and migrate inward when the transition of Cx43 to Cx46 expression occurs as oxygen levels fall from 10–15 mmHg to ~3–5 mmHg within the lens.
Interruption of Cx46 channel function leads to cataract formation in the lens7-13 and cataract formation is the most notable defect in Cx46 null mice,11,14 and Cx46 is the primary functional gap junction in the mature region of the lens.15-17 Our lab has hypothesized that Cx46 is important for cellular survival during hypoxic conditions. We subsequently showed that exogenous Cx46 prolongs survival from hypoxia-induced cell death in mouse N2A cells and is expressed abnormally in solid tumors. Cx46 is upregulated by hypoxia in rabbit lens epithelial cells, which naturally express Cx46.18 We also demonstrated that Cx46 affects tumor growth in two separate human xenograft studies.18,19 In both studies, the xenograft cells were allowed to form sizeable solid tumors which were then treated with high doses of Cx46 siRNA directly injected into the tumor at various positions. Cx46 was upregulated in early growth tumors and knockdown of Cx46 by siRNA resulted in slower growing tumors.18,19 This occurred either by the prevention of Cx46 protein stabilization or by knockdown of existing and ongoing Cx46 mRNA expression. The amount of Cx43 protein found in the tumors was increased when Cx46 expression decreased,19 and this finding could explain why the tumor xenografts slowed their growth when treated with anti-Cx46 siRNA. Cx43 is a known tumor suppressor and a decrease in its expression is linked to tumor progression and metastasis.5,20-23 This mechanism of tumor suppression may contribute to the slow growth of anti-Cx46 siRNA treated tumors.
Methods
Promoter luciferase assay
The predicted upstream human Cx46 promoter was custom synthesized from the NCBI reference sequence NG_016399.1 and provided in pUC57 (GenScript). Base pair numbering begins at the NCBI predicted TSS from mRNA evidence (NM_021954.3) and the first base downstream of the TSS is bp +1. Promoter fragments were produced by PCR amplification from the pUC57-huCx46pro plasmid. The lengths of the fragments were decided by using naturally occurring restriction sites within the promoter. Fragments were cloned into the promoterless vector pMetLuc2-reporter (Clontech) using the HindIII site and pGL4.13 (Promega) was used as the transfection control plasmid. The pTL-HIF1α plasmid (Panomics Products) contains HREs and was used as the hypoxia positive control. A promoterless plasmid without the HREs was used as the hypoxia negative control and pGL4.75 (Promega) was used as a transfection control plasmid. HLEC and N2A cells were transiently transfected with Fugene6 (Roche) and plasmid DNA according to the manufacturer’s suggestions. Ten thousand cells per well in opaque 96-well plates were allowed to transfect for 12 h prior to being placed into the hypoxic growth chamber for either 2 or 12 h. Single 96-well plates were equilibrated to room temperature for 10 min prior to the start of cell lysis and the luciferase assays. The Dual-Glo Luciferase Assay (Promega) was used in conjunction with a Turner Designs Luminometer to obtain the raw light unit numbers. The output of the pTL-HIF1α was used as the positive control and to normalize the data against; the promoterless pTL was used as the negative control and background was subtracted using a media only control. Values for each promoter under hypoxia were set relative to the HIF1α positive control.
Results and Conclusion
Cx46 influences the turnover of Cx43
Interestingly, a reciprocal relationship between Cx43 and Cx46 was observed in vivo during a previous Y79 xenograft study.19 Since Cx43 and Cx46 are lens connexins, we used the lens cell system to study this reciprocal relationship. Current lens connexin studies suggest that phosphorylation of Cx43 by protein kinase C (PKC) regulates the degradation of Cx43 in the mature lens and this alone is sufficient to cause the marked decrease in Cx43 protein.24-26 Although phosphorylation can regulate connexin degradation, we propose that Cx46 plays an additional role in the degradation of Cx43 in the transition region of the lens when Cx43 levels decrease. In the same region of the lens, while Cx46 is being upregulated and/or stabilized, Cx43 is being downregulated and/or destabilized. This would present an opportunity for Cx43 and Cx46 proteins to interact in the endoplasmic reticulum and/or Golgi apparatus prior to trafficking. Recently, in our lens cell culture system, we showed that an interaction between Cx43 and Cx46 occurs and that overexpression of Cx46 has profound effects on the stability of Cx43 prior to the formation of gap junction plaques.27 We also showed, by fluorescence microscopy, that these two proteins interact in the Golgi apparatus. Further, we demonstrated that the soluble carboxyl-terminal domain of Cx46 alone induces the degradation of Cx43 via the proteasome.27 However, the interacting protein partners needed for degradation of Cx43 remain to be identified.
Singular expression of either Cx43 or Cx46 is not observed in lens cell cultures as it is observed in lenses in vivo. Therefore, there must be other factors that drive the specific expression of Cx46 in the lens in vivo. These factors are unknown at this time. In addition to the reciprocal location of Cx43 and Cx46 in the lens, the level of oxygen is steadily decreasing in the same regions of the lens (Fig. 1). A key question to ask is whether the increase in hypoxia plays a role in Cx46 expression in the lens and other tissues, such as solid tumors which use hypoxia to their growth advantage.
Hypoxia influences Cx46 promoter activity in lens cells
Of the many tissue types present in the human body, only the lens must express Cx46 to remain fully functional and disease free. Other labs have previously identified the expression of Cx46 in ROS 17/2.8 osteosarcoma cells, UMR 106–01 osteosarcoma cells, primary rat calvarial osteoblastic cells,28 and in neoplastic bone tissue.29 This is an interesting observation because osteosarcomas originate in hypoxic bone tissue, an environment similar to the hypoxic environment found in the lens and in early solid tumors. Additionally, Koval et al.28 and Sanchez et al.29 observed intracellular and perinuclear localization of Cx46 as well as gap junction plaque localized expression of Cx43, both of which we also observed in our lens cell culture system.27 These cells, and presumably the tissues they originated from, have the ability to survive hypoxic growth conditions and must be able to adapt to the tissue environment. Cx46 alone did have a pro-survival effect in N2A cells when challenged with 1% oxygen.18 Thus, a critical question is whether the Cx46 promoter is regulated by hypoxia in lens cells.
When the human promoter of Cx46 was challenged with 1% oxygen in human lens cells and normalized to a HIF1α-responsive control promoter, a transient ~17-fold increase in activity was observed with the 482 bp promoter (Fig. 2). HREs are most likely located within the proximal promoter region of less than 500 bp upstream of the TSS. The Cx46 promoter also showed sustained responses with shorter promoter fragments, suggesting that transcriptional regulation of Cx46 gene expression is tightly controlled by one or more positive and/or negative regulatory elements present beyond the proximal promoter region. The basal promoter still remains to be identified, but given the expression profile of the promoter fragments, it is likely located in the first 200 bp upstream of the TSS. Of course, the possibility of long-range transcriptional regulatory effects remains open.
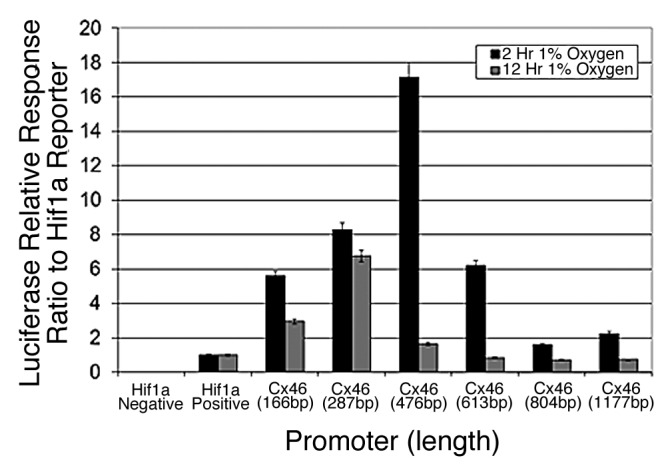
Figure 2. The promoter of human Connexin 46 is transiently responsive to 1% oxygen in human lens cells. Various fragments 5′ to the predicted transcription start site of the human Cx46 promoter were cloned into a promoterless luciferase reporter vector and tested for responsiveness to 1% oxygen in human lens epithelial cells. The varying activity correlated with the length of the promoter indicates the presence of regulatory elements encoded within the promoter. The promoter did not respond to hypoxia in N2A cells in the same assay (unpublished data). Error bars represent standard error of the mean (n = 6).
Hypoxia may play an active role in the regulation of Cx43/Cx46 that occurs naturally in the lens. Tight transcriptional regulation of Cx46 expression is not surprising since Cx46 is expressed primarily in the lens. Other lens proteins, such as crystallins, have been found in solid tumors indicating that the conditions for lens protein expression exist in other tissues.30,31 The possibility that there is overlap in the available gowth factors, nutrients, or other receptor ligands is open; however, given the uncommon expression of Cx46 in other tissues beyond the lens, the agonist and antagonist interactions that lead to Cx46 expression may have minimal overlap in both lens and solid tumor tissues. Hypoxia plays an important role in regulating gene expression in both the lens and in solid tumor tissues as well as a key role in wound healing, ischemia/reperfusion injury, and prenatal development. Here there are unique global and local hypoxic environments that are present and influence cellular responses to stimuli and differentiation.32-39 Since Cx46 was thought to only be a lens-specific protein, Cx46 expression has not been studied throughout any of these processes. Cx46 may play an important role in the cellular response to hypoxic environments; however, this hypothesis still remains to be tested but offers interesting speculation into the unique role that Cx46 may play in physiology, independent of gap junction channel activity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
NIH R01-EY13421 to D.J.T.; Kansas State University Research Foundation Doctoral Scholarship to S.A.M.
Glossary
Abbreviations:
- Cx
Connexin
- N2A
mouse neuro2a neuroblastoma cells
- HLEC
human lens epithelial cell
- siRNA
small interfering RNA
- HIF1α
hypoxia inducible factor 1 alpha transcription factor
- TSS
transcription start site
- HRE
hypoxia response element
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18715
References
- 1.Ahmad S, Martin PEM, Evans WH. Assembly of gap junction channels: mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur J Biochem. 2001;268:4544–52. doi: 10.1046/j.1432-1327.2001.02380.x. [DOI] [PubMed] [Google Scholar]
- 2.Harris AL, Locke D. Connexins: A Guide. New York, NY: Springer, 2009. [Google Scholar]
- 3.Griffith TM. Which connexins connect? Circ Res. 2007;101:1219–21. doi: 10.1161/CIRCRESAHA.107.165670. [DOI] [PubMed] [Google Scholar]
- 4.Mathias RT, White TW, Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev. 2010;90:179–206. doi: 10.1152/physrev.00034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winterhager E. Gap Junctions in Development and Disease. Berlin: Springer, 2005. [Google Scholar]
- 6.Xia CH, Liu H, Cheung D, Cheng C, Wang E, Du X, et al. Diverse gap junctions modulate distinct mechanisms for fiber cell formation during lens development and cataractogenesis. Development. 2006;133:2033–40. doi: 10.1242/dev.02361. [DOI] [PubMed] [Google Scholar]
- 7.Dunia I, Cibert C, Gong X, Xia CH, Recouvreur M, Levy E, et al. Structural and immunocytochemical alterations in eye lens fiber cells from Cx46 and Cx50 knockout mice. Eur J Cell Biol. 2006;85:729–52. doi: 10.1016/j.ejcb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Ebihara L, Tong JJ, Vertel B, White TW, Chen TL. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Invest Ophthalmol Vis Sci. 2011;52:882–9. doi: 10.1167/iovs.10-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckert R. pH gating of lens fibre connexins. Pflugers Arch. 2002;443:843–51. doi: 10.1007/s00424-001-0760-2. [DOI] [PubMed] [Google Scholar]
- 10.Fleschner CR. Connexin 46 and connexin 50 in selenite cataract. Ophthalmic Res. 2006;38:24–8. doi: 10.1159/000088527. [DOI] [PubMed] [Google Scholar]
- 11.Gong X, Baldo GJ, Kumar NM, Gilula NB, Mathias RT. Gap junctional coupling in lenses lacking alpha3 connexin. Proc Natl Acad Sci U S A. 1998;95:15303–8. doi: 10.1073/pnas.95.26.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, et al. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–64. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, Beyer EC, et al. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am J Physiol Cell Physiol. 2000;279:C596–602. doi: 10.1152/ajpcell.2000.279.3.C596. [DOI] [PubMed] [Google Scholar]
- 14.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, et al. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–43. doi: 10.1016/S0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 15.White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–20. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- 16.Xia CH, Cheng C, Huang Q, Cheung D, Li L, Dunia I, et al. Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Exp Eye Res. 2006;83:688–96. doi: 10.1016/j.exer.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Xia CH, Cheung D, DeRosa AM, Chang B, Lo WK, White TW, et al. Knock-in of alpha3 connexin prevents severe cataracts caused by an alpha8 point mutation. J Cell Sci. 2006;119:2138–44. doi: 10.1242/jcs.02940. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee D, Gakhar G, Madgwick D, Hurt A, Takemoto D, Nguyen TA. A novel role of gap junction connexin46 protein to protect breast tumors from hypoxia. Int J Cancer. 2010;127:839–48. doi: 10.1002/ijc.25107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burr DB, Molina SA, Banerjee D, Low DM, Takemoto DJ. Treatment with connexin 46 siRNA suppresses the growth of human Y79 retinoblastoma cell xenografts in vivo. Exp Eye Res. 2011;92:251–9. doi: 10.1016/j.exer.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. Connexins and their channels in cell growth and cell death. Cell Signal. 2006;18:592–600. doi: 10.1016/j.cellsig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan-Dagli ML, Omori Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III. 1999;322:151–9. doi: 10.1016/S0764-4469(99)80038-9. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Sinovas A, Cabestrero A, López D, Torre I, Morente M, Abellán A, et al. The modulatory effects of connexin 43 on cell death/survival beyond cell coupling. Prog Biophys Mol Biol. 2007;94:219–32. doi: 10.1016/j.pbiomolbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Mesnil M. Connexins and cancer. Biol Cell. 2002;94:493–500. doi: 10.1016/S0248-4900(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 24.Das S, Wang H, Molina SA, Martinez-Wittinghan FJ, Jena S, Bossmann LK, et al. PKCγ, role in lens differentiation and gap junction coupling. Curr Eye Res. 2011;36:620–31. doi: 10.3109/02713683.2011.573899. [DOI] [PubMed] [Google Scholar]
- 25.Akoyev V, Das S, Jena S, Grauer L, Takemoto DJ. Hypoxia-regulated activity of PKCepsilon in the lens. Invest Ophthalmol Vis Sci. 2009;50:1271–82. doi: 10.1167/iovs.08-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saleh SM, Takemoto LJ, Zoukhri D, Takemoto DJ. PKC-gamma phosphorylation of connexin 46 in the lens cortex. Mol Vis. 2001;7:240–6. [PubMed] [Google Scholar]
- 27.Banerjee D, Das S, Molina SA, Madgwick D, Katz MR, Jena S, et al. Investigation of the reciprocal relationship between the expression of two gap junction connexin proteins, connexin46 and connexin43. J Biol Chem. 2011;286:24519–33. doi: 10.1074/jbc.M110.217208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koval M, Harley JE, Hick E, Steinberg TH. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol. 1997;137:847–57. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanches DS, Pires CG, Fukumasu H, Cogliati B, Matsuzaki P, Chaible LM, et al. Expression of connexins in normal and neoplastic canine bone tissue. Vet Pathol. 2009;46:846–59. doi: 10.1354/vp.08-VP-0263-S-FL. [DOI] [PubMed] [Google Scholar]
- 30.Nakatsugawa M, Hirohashi Y, Torigoe T, Asanuma H, Takahashi A, Inoda S, et al. Novel spliced form of a lens protein as a novel lung cancer antigen, Lengsin splicing variant 4. Cancer Sci. 2009;100:1485–93. doi: 10.1111/j.1349-7006.2009.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng M, Chen P, Xie S, Zhao J, Gong L, Liu J, et al. The small heat shock protein αA-crystallin is expressed in pancreas and acts as a negative regulator of carcinogenesis. Biochimica et Biophysica Acta 2010; 1802:621-31 [DOI] [PubMed]
- 32.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183:152–6. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med. 2010;10:653–66. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, et al. Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci. 2010;1192:322–6. doi: 10.1111/j.1749-6632.2009.05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10:821–32. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 36.Shui YB, Arbeit JM, Johnson RS, Beebe DC. HIF-1: an age-dependent regulator of lens cell proliferation. Invest Ophthalmol Vis Sci. 2008;49:4961–70. doi: 10.1167/iovs.08-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edsjö A, Holmquist L, Påhlman S. Neuroblastoma as an experimental model for neuronal differentiation and hypoxia-induced tumor cell dedifferentiation. Semin Cancer Biol. 2007;17:248–56. doi: 10.1016/j.semcancer.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Provot S, Schipani E. Fetal growth plate: a developmental model of cellular adaptation to hypoxia. Ann N Y Acad Sci. 2007;1117:26–39. doi: 10.1196/annals.1402.076. [DOI] [PubMed] [Google Scholar]
- 39.Xie XJ, Wang JA, Cao J, Zhang X. Differentiation of bone marrow mesenchymal stem cells induced by myocardial medium under hypoxic conditions. Acta Pharmacol Sin. 2006;27:1153–8. doi: 10.1111/j.1745-7254.2006.00436.x. [DOI] [PubMed] [Google Scholar]


