Abstract
Listeria monocytogenes is an intracellular Gram-positive bacterial pathogen that produces many types of surface proteins. To get insights into its intracellular lifestyle, we used high-resolution mass spectrometry to characterize the cell wall proteome of bacteria proliferating within the eukaryotic cell. The relative amount of a few surface proteins was found notoriously different in intracellular bacteria. Internalin A (InlA), which is covalently bound to the peptidoglycan and plays a central role in bacterial entry into non-phagocytic eukaryotic cells, was present in high amounts in the cell wall of intracellular bacteria. Our study also revealed that the actin assembly-inducing protein ActA co-purified with peptidoglycan isolated from intracellular bacteria. Growth of L. monocytogenes in minimal media reproduced the predominance of InlA in the cell wall and the association of ActA with peptidoglycan. Intriguingly, bacteria grown in this condition used ActA for efficient invasion of host cells. These findings suggest that the adaptation of L. monocytogenes to the intracellular lifestyle involves changes in the relative abundance of certain surface proteins and in their mode of association to the peptidoglycan. These alterations, probably promoted by yet-unknown changes in the cell wall architecture, may instruct these proteins to perform different functions outside and inside the host cell.
Keywords: ActA, cell wall, intracellular, Listeria, proteome
Gram-positive bacteria contain a thick multilayered peptidoglycan macromolecule decorated with different types of proteins, teichoic and lipoteichoic acids.1 In infections caused by these bacteria, surface proteins contribute to escape from the host immune attack, to biofilm formation and to promote adhesion/invasion of host cells.2-4 Listeria monocytogenes is a Gram-positive intracellular bacterial pathogen in which surface proteins have been intensively studied.5 This pathogen encodes a large family of surface proteins covalently bound to the peptidoglycan upon recognition of their LPXTG motif.5-7 An important LPXTG surface protein of L. monocytogenes is Internalin A (InlA), an invasin that interacts with E-cadherin to promote bacterial entry into non-phagocytic eukaryotic cells.8
Despite many studies focused on surface proteins, the function of the numerous LPXTG proteins of the genus Listeria remains in most cases unknown. Only seven L. monocytogenes LPXTG proteins have a function assigned,8-14 which contrasts with the more than 40 genes encoding this type of proteins that are found in every Listeria genome sequenced to date. We are also missing a global view of how the entire LPXTG protein family is regulated as a consequence of remodeling in the peptidoglycan structure. In this respect, several lines of evidence support the existence of changes in the peptidoglycan of L. monocytogenes when bacteria are located inside eukaryotic cells. Increased expression of genes encoding different cell wall-associated proteins and enzymes that modify peptidoglycan chemistry has been shown in intracellular bacteria.15,16 Similar findings were obtained in vivo in bacteria collected from mouse organs.17 Cell wall remodeling and host cell colonization by L. monocytogenes seem therefore related events. Modifications in the peptidoglycan structure involving N-deacetylation or O-acetylation reactions are also known to impair L. monocytogenes recognition by the host immune system.18,19 Whether such structural changes occur in the peptidoglycan of intracellular L. monocytogenes has not been addressed yet. Considering that the peptidoglycan is the platform to which all LPXTG proteins anchor, changes in their relative amount or distribution are also conceivable in bacteria residing within the host cell.
Our first gel-less proteomic studies identified 13 LPXTG proteins in cell wall of L. monocytogenes growing in laboratory media.20,21 Using mass spectrometry equipment of higher resolution, we have extended that work to define the LPXTG protein content in bacteria proliferating inside eukaryotic cells.22 Different numbers of peptides for concrete LPXTG proteins were identified in the cell wall of extra- and intracellular bacteria, which denoted changes in their relative abundance. Two examples were Lmo0514, identified in intracellular bacteria but barely detected in extracellular bacteria and Lmo2085, which displayed an opposite trend.
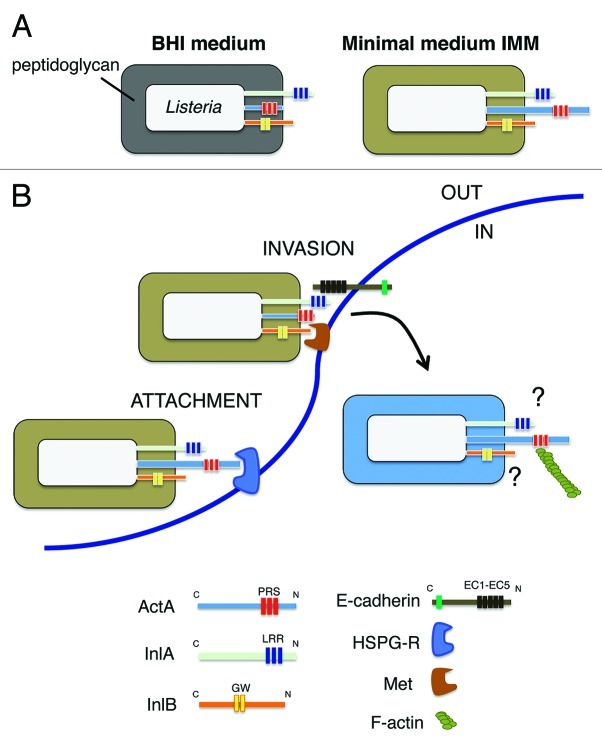
Another novel finding of our study involved the association of the actin-assembly protein ActA with the peptidoglycan. Up to now, ActA was believed to be tethered to the envelope only by its C-terminal transmembrane region. These diverse modes of association could provide functional versatility to ActA and explain why this protein is involved in so varied processes as the formation of actin-tails that propel intracellular bacteria to neighbor cells, the resistance to autophagy, or the invasion of host cells.23-25 Our proteomic data support the idea of ActA adopting these distinct conformations in response to alterations in the cell wall architecture, as it may occur inside the eukaryotic cell. The significance of these hypothetical conformational changes is still unknown, but they could be linked to the ‘exposure’ outside the peptidoglycan lattice that this protein demands to interact with host cell cytoskeletal proteins such as VASP and the Arp2/3 complex. Interestingly, ActA is also associated with peptidoglycan in bacteria grown in minimal defined medium. In this condition, bacteria uses ActA for efficient invasion of epithelial cells together with InlA and InlB.22 An early study reported that ActA could interact with a heparan-sulfate proteoglycan (HSPG) receptor and that such interaction promoted bacterial entry into epithelial cells.26 Based on our current data, we favor a model of invasion in which ActA could play a relevant role in attachment of the bacteria to the host cell surface that could further facilitate the binding of InlA and/or InlB to their respective receptors (Fig. 1). Such hypothetical role in adhesion might rely on the association of ActA with the peptidoglycan, which could be promoted by either a particular structure in this scaffold or by interacting proteins. Importantly, changes in cell wall architecture, as those caused by the absence of the peptidoglycan hydrolase IspC, have been shown to decrease ActA exposure on the cell surface.27
Figure 1. Remodeling of the cell wall architecture in L. monocytogenes when growing extracellularly in two different broth media or inside eukaryotic cells may affect exposure and function of ActA and the invasins InlA and InlB. (A) The different structure of the cell wall in bacteria grown in BHI or minimal media influences ActA association with the peptidoglycan and also probably its degree of exposure on the cell surface; (B) Based on data collected with L. monocytogenes grown in minimal medium, which show that ActA, InlA and InlB are all required for bacterial invasion of epithelial cells,22 a model is proposed in which ActA could promote bacterial early attachment via its interaction with heparan-sulfate proteoglycan receptor (HSPG-R). This stage would be followed by InlA/E-Cadherin and/or InlB/Met interactions ultimately responsible for mediating bacterial entry. Different colors in the peptidoglycan denote changes in the cell wall architecture. Abbreviations: PRS: proline-rich sequences; LRR: leucine-rich repeats; GW: GW-rich domain; EC1-EC5: extracellular immunoglobulin-like domains reported for E-Cadherin. The putative role(s) played by InlA and InlB in intracellular L. monocytogenes remain unknown.
Our proteomic study also revealed an unexpected predominance of the invasin InlA in the cell wall of intracellular L. monocytogenes several hours after entry. The other well-known invasin of this pathogen, Internalin B (InlB), was also identified by proteomics in membrane fractions of intracellular bacteria. Early studies claimed low expression of the inlAB locus in intracellular bacteria compared with those grown in broth media.28 However, two subsequent transcriptomic studies listed inlA and inlB as genes induced inside macrophages and epithelial cells.15,16 Interestingly, one of these studies described a ‘late-induction’ of inlA since it seems to be upregulated at 6 h post-infection.16 These observations are in concordance with our cell wall proteome data. However, none of these studies explain why intracellular bacteria anchor to the cell wall so large amounts of invasins used by extracellular bacteria to bind to plasma membrane receptors. InlA interacts via its leucine-rich repeat (LRR) domains with the ‘extracellular’ EC1 immunoglobulin-like domain of the E-cadherin molecule8,29 but this EC1 domain should be ‘invisible’ to intracellular bacteria. A similar rationale could be made for InlB. So, it is tempting to speculate on yet-unknown interaction(s) of bacteria-associated InlA and InlB with alternate host molecule(s) inside the infected cell. Release of InlA and/or InlB from the bacterial surface as a result of the cell wall turnover could also potentially signal the infected cell from the ‘inside’ based on interactions with host molecules located either in the cytosol or internal membranes facing to this compartment. Future work is clearly needed to dissect whether InlA and/or InlB could play different roles outside and inside the host cell, as it occurs with ActA.
Acknowledgments
Work in our laboratories is supported by grants BIO2010–18962 (to M.G.P.) and PIM2010EPA-00714 (to F.G.-P.) from the Spanish Ministry of Science and Innovation.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18678
References
- 1.Vollmer W, Seligman SJ. Architecture of peptidoglycan: more data and more models. Trends Microbiol. 2010;18:59–66. doi: 10.1016/j.tim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Löfling J, Vimberg V, Battig P, Henriques-Normark B. Cellular interactions by LPxTG-anchored pneumococcal adhesins and their streptococcal homologues. Cell Microbiol. 2011;13:186–97. doi: 10.1111/j.1462-5822.2010.01560.x. [DOI] [PubMed] [Google Scholar]
- 3.Cabanes D, Dehoux P, Dussurget O, Frangeul L, Cossart P. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 2002;10:238–45. doi: 10.1016/S0966-842X(02)02342-9. [DOI] [PubMed] [Google Scholar]
- 4.Marraffini LA, Dedent AC, Schneewind O. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol Mol Biol Rev. 2006;70:192–221. doi: 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierne H, Cossart P. Listeria monocytogenes surface proteins: from genome predictions to function. Microbiol Mol Biol Rev. 2007;71:377–97. doi: 10.1128/MMBR.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hain T, Steinweg C, Chakraborty T. Comparative and functional genomics of Listeria spp. J Biotechnol. 2006;126:37–51. doi: 10.1016/j.jbiotec.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 7.Doumith M, Cazalet C, Simoes N, Frangeul L, Jacquet C, Kunst F, et al. New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. Infect Immun. 2004;72:1072–83. doi: 10.1128/IAI.72.2.1072-1083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonazzi M, Lecuit M, Cossart P. Listeria monocytogenes internalin and E-cadherin: from structure to pathogenesis. Cell Microbiol. 2009;11:693–702. doi: 10.1111/j.1462-5822.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 9.Cabanes D, Sousa S, Cebrí A, Lecuit M, García-del Portillo F, Cossart P. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J. 2005;24:2827–38. doi: 10.1038/sj.emboj.7600750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Personnic N, Bruck S, Nahori MA, Toledo-Arana A, Nikitas G, Lecuit M, et al. The stress-induced virulence protein InlH controls interleukin-6 production during murine listeriosis. Infect Immun. 2010;78:1979–89. doi: 10.1128/IAI.01096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dortet L, Mostowy S, Samba-Louaka A, Gouin E, Nahori MA, Wiemer EA, et al. Recruitment of the major vault protein by InlK: a Listeria monocytogenes strategy to avoid autophagy. PLoS Pathog. 2011;7:e1002168. doi: 10.1371/journal.ppat.1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popowska M, Markiewicz Z. Characterization of Listeria monocytogenes protein Lmo0327 with murein hydrolase activity. Arch Microbiol. 2006;186:69–86. doi: 10.1007/s00203-006-0122-8. [DOI] [PubMed] [Google Scholar]
- 13.Sabet C, Lecuit M, Cabanes D, Cossart P, Bierne H. LPXTG protein InlJ, a newly identified internalin involved in Listeria monocytogenes virulence. Infect Immun. 2005;73:6912–22. doi: 10.1128/IAI.73.10.6912-6922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis O, Sousa S, Camejo A, Villiers V, Gouin E, Cossart P, et al. LapB, a novel Listeria monocytogenes LPXTG surface adhesin, required for entry into eukaryotic cells and virulence. J Infect Dis. 2010;202:551–62. doi: 10.1086/654880. [DOI] [PubMed] [Google Scholar]
- 15.Joseph B, Przybilla K, Stühler C, Schauer K, Slaghuis J, Fuchs TM, et al. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J Bacteriol. 2006;188:556–68. doi: 10.1128/JB.188.2.556-568.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee SS, Hossain H, Otten S, Kuenne C, Kuchmina K, Machata S, et al. Intracellular gene expression profile of Listeria monocytogenes. Infect Immun. 2006;74:1323–38. doi: 10.1128/IAI.74.2.1323-1338.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camejo A, Buchrieser C, Couvé E, Carvalho F, Reis O, Ferreira P, et al. In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathog. 2009;5:e1000449. doi: 10.1371/journal.ppat.1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aubry C, Goulard C, Nahori MA, Cayet N, Decalf J, Sachse M, et al. OatA, a peptidoglycan O-acetyltransferase involved in Listeria monocytogenes immune escape, is critical for virulence. J Infect Dis. 2011;204:731–40. doi: 10.1093/infdis/jir396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boneca IG, Dussurget O, Cabanes D, Nahori MA, Sousa S, Lecuit M, et al. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci U S A. 2007;104:997–1002. doi: 10.1073/pnas.0609672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvo E, Pucciarelli MG, Bierne H, Cossart P, Albar JP, García-Del Portillo F. Analysis of the Listeria cell wall proteome by two-dimensional nanoliquid chromatography coupled to mass spectrometry. Proteomics. 2005;5:433–43. doi: 10.1002/pmic.200400936. [DOI] [PubMed] [Google Scholar]
- 21.Pucciarelli MG, Calvo E, Sabet C, Bierne H, Cossart P, García-del Portillo F. Identification of substrates of the Listeria monocytogenes sortases A and B by a non-gel proteomic analysis. Proteomics. 2005;5:4808–17. doi: 10.1002/pmic.200402075. [DOI] [PubMed] [Google Scholar]
- 22.García-del Portillo F, Calvo E, D’Orazio V, Pucciarelli MG. Association of ActA to peptidoglycan revealed by cell wall proteomics of intracellular Listeria monocytogenes. J Biol Chem. 2011;286:34675–89. doi: 10.1074/jbc.M111.230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Súrez M, González-Zorn B, Vega Y, Chico-Calero I, Vázquez-Boland JA. A role for ActA in epithelial cell invasion by Listeria monocytogenes. Cell Microbiol. 2001;3:853–64. doi: 10.1046/j.1462-5822.2001.00160.x. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M, et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol. 2009;11:1233–40. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- 25.Lambrechts A, Gevaert K, Cossart P, Vandekerckhove J, Van Troys M. Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol. 2008;18:220–7. doi: 10.1016/j.tcb.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Domínguez C, Vázquez-Boland JA, Carrasco-Marín E, López-Mato P, Leyva-Cobín F. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun. 1997;65:78–88. doi: 10.1128/iai.65.1.78-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Lin M. A novel cell wall-anchored peptidoglycan hydrolase (autolysin), IspC, essential for Listeria monocytogenes virulence: genetic and proteomic analysis. Microbiology. 2008;154:1900–13. doi: 10.1099/mic.0.2007/015172-0. [DOI] [PubMed] [Google Scholar]
- 28.Bubert A, Sokolovic Z, Chun SK, Papatheodorou L, Simm A, Goebel W. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol Gen Genet. 1999;261:323–36. doi: 10.1007/PL00008633. [DOI] [PubMed] [Google Scholar]
- 29.Schubert WD, Urbanke C, Ziehm T, Beier V, Machner MP, Domann E, et al. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell. 2002;111:825–36. doi: 10.1016/S0092-8674(02)01136-4. [DOI] [PubMed] [Google Scholar]