Abstract
Our recent findings indicate that cells exposed to transmembrane (m-CD95L) or metalloprotease-cleaved CD95L (cl-CD95L) undergo a localized Ca2+entry that not only inhibits the initial steps of the CD95-mediated apoptotic signal but also promotes cell motility. Based on recent findings published on the non-apoptotic signals induced by CD95, we discuss how m-CD95L and cl-CD95L diverging by their stoichiometry could both contribute to the immune response by first recruiting activated T lymphocytes in the inflamed area and later by eliminating infected and transformed cells.
Keywords: Apoptosis, calcium, Fas, FasL, lymphocyte, migration, Orai1, PI3K, TNF
Emergent Functions for the Death Receptor CD95
CD95 (also known as Fas) belongs to the TNF (Tumour Necrosis Factor) -receptor superfamily. Fifteen years ago, it has been shown that when exposed to an agonistic anti-CD95 mAb (APO1–3), the aggregated receptor recruits the adaptor protein FADD (Fas-Associated protein with Death Domain), which then binds caspase-8/-10 and ultimately elicits the apoptotic signal and the death of the cell. This complex was designated the DISC for Death Inducing Signaling Complex1 and since numerous factors have been found to modulate the implementation of this complex and thus, the transmission of death receptor-mediated apoptotic signal. The cognate CD95 ligand, CD95L (also known as FasL or CD178) is a transmembrane “cytokine” belonging to the TNF family. CD95L exhibits a restricted expression pattern, being expressed primarily at the surface of activated T lymphocytes and NK cells, where it contributes to the elimination of infected and transformed cells. However, CD95L is also found under inflammatory conditions, at the surface of epithelial cells, macrophages or dendritic cells where its biological function remains elusive. This type II transmembrane protein can be cleaved by metalloproteases such as MMP3,2 MMP7,3 MMP94 or ADAM-10 (A Disintegrin And Metalloproteinase 10)5,6 and released as a soluble ligand into the connective tissue and the bloodstream. Cleaved CD95L (cl-CD95L) was described initially as an inert ligand competing with its membrane-bound and pro-apoptotic counterpart (m-CD95L) for binding to CD95.7,8 More recent studies confirmed that the homotrimeric cl-CD95L fails to trigger cell death but more importantly, they also bring to light that this soluble ligand possesses indeed a biological function by eliciting non-apoptotic signals leading to cell migration9-13 and/or proliferation.14 In this regard, we and others demonstrated that the metalloprotease-processed CD95L actively participates in aggravating inflammation and auto-immunity both in mouse model12 and humans affected by systemic lupus erythematosus (SLE).13 Overall, these findings ascribe non-apoptotic rolesto CD95 through the implementation of different signals (i.e., JNK, PI3K and NF-κB). The role(s) of each CD95-mediated non-apoptotic signal remains however to be finely characterized in pathophysiological contexts.
A Novel Actor in the Initial Events of the CD95 Pathway
Calcium ions (Ca2+) participate in cell signaling as a second messenger that relies on magnitude (cytosolic concentration), temporal parameters (i.e., duration and frequency) and spatial localization to trigger a variety of cellular responses. Following membrane receptor stimulation, Ca2+ responses mainly occur through a biphasic signal caused by activation of IP3 receptors and the release of Ca2+ from the endoplasmic reticulum (ER) followed by a Ca2+ entry across the plasma membrane.15 This store-operated Ca2+ entry (SOCE), mediated in T-lymphocytes by Ca2+ release-activated Ca2+ (CRAC) channels, plays a pivotal role in both the replenishment of the ER store and in cell signaling.16 Recently, STIM1 was identified as the ER-stored Ca2+ sensor that links ER depletion to activation of the plasma membrane CRAC channel formed by Orai1 subunits, allowing Ca2+ to selectively enter the cell.17 Following contact of a T cell with an antigen-presenting dendritic cell, STIM1 and Orai1 colocalize with T cell receptors (TCRs) in the immunological synapse and contribute to a localized Ca2+ influx.18
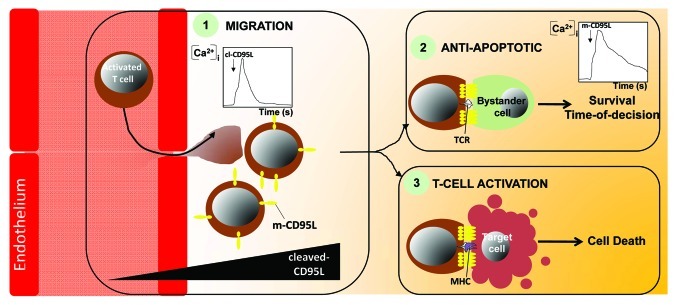
Tissues in which infected or transformed cells are disseminated require the recruitment of immune cells to specifically eliminate these threats. Based on our findings, we surmise that the first line of activated T lymphocytes infiltrating the transformed or infected area expresses high amount of membrane-bound CD95L to trigger cell death in affected cells but also to provide a pool of ligand that will be processed by metalloproteases therefore engendering a cl-CD95L gradient. This gradient would in turn recruit a second wave of activated T cells, which ultimately amplifies if necessary the immune response and leads to the total eradication of the target cells. Of note, we established that the amount of cl-CD95L is dramatically increased in sera of SLE patients and contributes to the endothelial transmigration of activated T cells that accumulate in the damaged organs. We also observed that cl-CD95L evokes a transient and localized SOCE (Fig. 1), which is instrumental in enhancing PI3K activation, actin remodeling and thus migration of activated T cells.13 Seeking for their cellular targets, these migrating T cells may encounter CD95-expressing bystander cells in the inflamed tissue raising the question of how is prevented in these healthy cells an accidental and irreversible activation of the apoptotic signal that will lead to their deleterious elimination. Our recent findings uncovered that engagement of CD95 by the membrane-bound form of CD95L (experimentally replaced by a home-made IgCD95L that mimics the membrane-bound multi-aggregated physiologic ligand) evokes a sustained and localized Ca2+ entry (Fig. 1), which freezes the initial steps of the CD95 apoptotic signal and doing so delays its delivery.19 Although intensity and temporal parameters of the CD95-mediated Ca2+ signal diverge between cells exposed to cl-CD95L and m-CD95L, both ligands implement SOCE through the co-localization of CRAC channel Orai1 with CD95. In addition, we observed that whereas in presence of cl-CD95L, cells undergo the formation of a caspase and FADD-independent Motility-inducing signaling complex (MISC), m-CD95L stimulates the transient and Ca2+-dependent recruitment of PKC-beta2 within DISC that participates in delaying the multiprotein complex formation and the transduction of the apoptotic signal.
Figure 1. Role of the Orai1-driven Ca2+ entry in T lymphocytes challenged with the different forms of CD95L. When exposed to the two forms of CD95L, cleaved and membrane-bound, activated T lymphocytes, target cells (infected or transformed cells) or bystander cells undergo an Orai1-driven Ca2+ entry that modulates differently the CD95-mediated signaling pathway. The Ca2+ traces obtained with cells exposed to cleaved-CD95L (1) or membrane-bound CD95L (2) are depicted. MHC: Major Histocompatibility Complex.
Accordingly, these findings support the hypothesis that a non-specific T lymphocyte/bystander cell contact would transiently engage the CD95receptor and achieve a Ca2+-dependent Time-Of-Decision (TOD) preventing the transmission of the CD95-mediated apoptotic signal in the target cell. In contrast, the selective recognition of the MHC/peptide by cytotoxic T lymphocytes may provide a sustained interaction that overrides the Ca2+-driven TOD allowing the selective elimination of infected or malignant cells. Numerous questions still remain to be addressed. First, whether cl-CD95L induces cell motility of activated T cells in general or only on specific T-cell subpopulations involved in the etiology of the autoimmune disorders is still unknown. Second, how two ligands only distinguishable by their divergent stoichiometries, are able to evoke such different intracellular Ca2+ patterns and biological outcomes using the same receptor is very puzzling, and finally, the molecular ordering leading to the activation of the Ca2+ signal remain to be identified.
Acknowledgments
This work was supported by Agence Nationale de la Recherche (ANR JC07_183182), INCa (projets libres recherche biomédicale), Cancéropole GO, Région Bretagne, Rennes Métropole, Université de Rennes-1 and by Ligue Contre le Cancer (Comités d’Ille-et-Vilaine/du Morbihan/des Côtes d’Armor/du Maine et Loire/des Landes). N.K. is supported by Region Bretagne (ARED).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18888
References
- 1.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuno H, Yudoh K, Watanabe Y, Nakazawa F, Aono H, Kimura T. Stromelysin-1 (MMP-3) in synovial fluid of patients with rheumatoid arthritis has potential to cleave membrane bound Fas ligand. J Rheumatol. 2001;28:22–8. [PubMed] [Google Scholar]
- 3.Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys. 2002;408:155–61. doi: 10.1016/S0003-9861(02)00525-8. [DOI] [PubMed] [Google Scholar]
- 4.Kiaei M, Kipiani K, Calingasan NY, Wille E, Chen J, Heissig B, et al. Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2007;205:74–81. doi: 10.1016/j.expneurol.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Kirkin V, Cahuzac N, Guardiola-Serrano F, Huault S, Lückerath K, Friedmann E, et al. The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells. Cell Death Differ. 2007;14:1678–87. doi: 10.1038/sj.cdd.4402175. [DOI] [PubMed] [Google Scholar]
- 6.Schulte M, Reiss K, Lettau M, Maretzky T, Ludwig A, Hartmann D, et al. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007;14:1040–9. doi: 10.1038/sj.cdd.4402101. [DOI] [PubMed] [Google Scholar]
- 7.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–13. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–50. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnhart BC, Legembre P, Pietras E, Bubici C, Franzoso G, Peter ME. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004;23:3175–85. doi: 10.1038/sj.emboj.7600325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogwater FJ, Nijkamp MW, Smakman N, Steller EJ, Emmink BL, Westendorp BF, et al. Oncogenic K-Ras turns death receptors into metastasis-promoting receptors in human and mouse colorectal cancer cells. Gastroenterology. 2010;138:2357–67. doi: 10.1053/j.gastro.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–48. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 12.O’Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–63. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauzin S, Chaigne-Delalande B, Selva E, Khadra N, Daburon S, Contin-Bordes C, et al. The naturally processed CD95L elicits a c-yes/calcium/PI3K-driven cell migration pathway. PLoS Biol. 2011;9:e1001090. doi: 10.1371/journal.pbio.1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, et al. CD95 promotes tumour growth. Nature. 2010;465:492–6. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–8. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian D, Weiss A. T cell antigen receptor signal transduction. Curr Opin Cell Biol. 1997;9:205–12. doi: 10.1016/S0955-0674(97)80064-6. [DOI] [PubMed] [Google Scholar]
- 17.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–77. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, et al. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A. 2008;105:2011–6. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khadra N, Bresson-Bepoldin L, Penna A, Chaigne-Delalande B, Ségui B, Levade T, et al. CD95 triggers Orai1-mediated localized Ca2+ entry, regulates recruitment of protein kinase C (PKC) β2, and prevents death-inducing signaling complex formation. Proc Natl Acad Sci U S A. 2011;108:19072–7. doi: 10.1073/pnas.1116946108. [DOI] [PMC free article] [PubMed] [Google Scholar]