Abstract
Although still often considered as simple unicellular organisms, in natural settings yeast cells tend to organize into intricate multicellular communities. Due to specific mechanisms only feasible at the population level, their capacity for social behavior is advantageous for their survival in a harmful environment. Feral Saccharomyces cerevisiae strains form complex structured colonies, which display many properties typical of natural biofilms causing (among others) serious infections in the human body. In our recent paper, we looked inside a growing colony using two-photon confocal microscopy. This allowed us to elucidate its three-dimensional colony architecture and some mechanisms responsible for community protection. Moreover, we showed how particular protective mechanisms complement each other during colony development and how each of them contributes to its defense against attacks from the environment. Our findings broaden current understanding of microbial multicellularity in general and also shed new light on the enormous resistance of yeast biofilms.
Keywords: Saccharomyces cerevisiae, biofilm, drug efflux pumps, extracellular matrix, feral strains, structured colony
Microbial multicellular communities can be found in various (even extreme) environments in the wild.1 Yeast cells can form diverse structures when attached to solid surfaces (e.g., biofilms,2 colonies3), when growing at a liquid/air interface (e.g., cell films on the surface of sherry wine that are called “flors”4) or when they mutually interact in a liquid environment and form cell clumps called “flocs”5. Each of these structures possesses some level of internal cell organization and complexity connected with the formation of differentiated cell subpopulations and also possesses a significant resistance to environmental impacts. Pathogenic yeasts (i.e., of Candida sp.) can colonise various surfaces within the human body, including host tissues and artificial medical devices, and form biofilms that resist otherwise effective drug therapy. Biofilms are thus very difficult to eliminate and serve as a source of serious systemic infections.6,7 The questions of how yeast multicellular populations orchestrate their development and how they achieve their environmental protection are therefore also important in terms of medical care. However, as it is difficult to grow artificial biofilms in the laboratory that have properties similar to those of fully developed natural biofilms, many aspects of biofilm formation are still rather elusive.
Single cells of feral S. cerevisiae strains plated on solid medium retain the ability to develop into structured colonies with typical “fluffy” morphology3,8 within a couple of days of growth. As shown below, such colonies share many properties with natural biofilms, and we therefore call them biofilm colonies. The biofilm colony model enabled us to discover the spatiotemporal localization of specific cell subpopulations with different functions and determine their impact on the protection and survival of the whole colony.
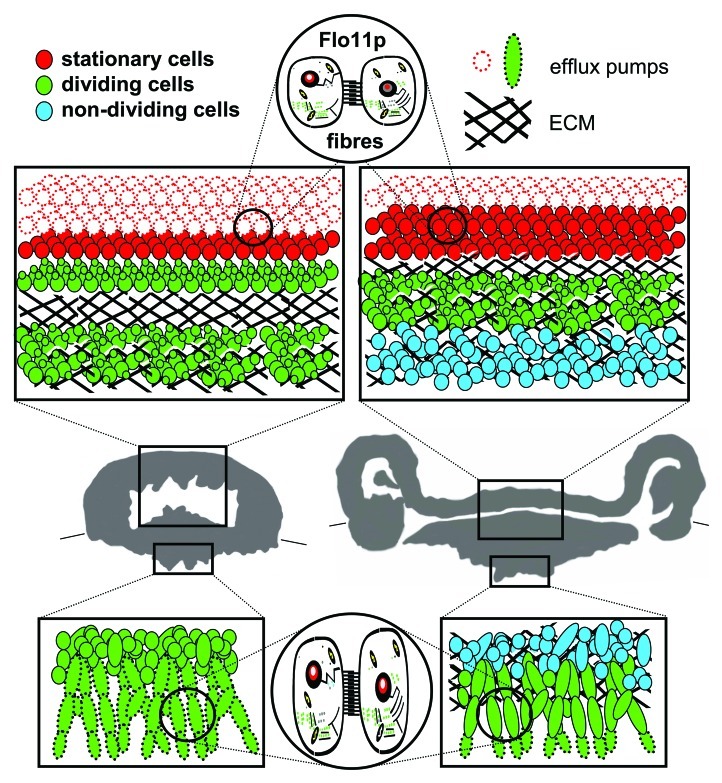
After relatively few cell divisions occur and a simple mound colony is formed, particular cell subpopulations begin to diverge and play different roles. Cells at the colony base form elongated cell chains called pseudohyphae.9 These filaments invade the agar medium, anchoring the structure to the solid substrate. Cells in peripheral layers surrounding the entire colony (including subsurface parts) are equipped with drug-efflux pumps (Pdr5p and Snq2p) localized to the plasma membrane, the expression of which is controlled by Pdr1p together with another, as yet unidentified transcription factor (Fig. 1). These proteins that belong to the family of pleiotropic drug resistance membrane transporters are capable of removing various (including toxic) substances from the cells10 and protect them (and thus also the whole colony) against external attacks. It has been demonstrated that various drug-efflux pumps play a role in yeast biofilm resistance against extracellularly added toxic compounds. However, this has usually been based on the overall change in behavior of mutated strains or expression differences between biofilms and planktonic cells,11,12 without more detailed information on the transporter’s function over the course of community development. In addition to the presence of these pumps, cells at the surface layers of the aerial colony part enter the stationary phase and thus become more resistant to potential environmental stress (Fig. 1). Meanwhile, cells in internal colony areas start to produce extracellular polymeric matrix (ECM; Fig. 1) of unknown composition and thus become fully embedded in this matrix. The ECM apparently adopts the role of a protective barrier, because it blocks the penetration of even harmful compounds. ECM is one of the defining components of many yeast multicellular communities including biofilms.2,13,14 Despite the sequestration potential of the ECM in clinical biofilms being implied,15,16 its contribution to biofilm resistance is unclear and sometimes even doubted.7,17 As a colony develops, the area of cells embedded in ECM expands (Fig. 1); in later stages, the ECM encloses almost all colony cells. Complementarily, the layer of cells containing functional drug-efflux pumps surrounding the colony becomes thinner as the transporters are degraded (Fig. 1) and almost completely disappears in an older, fully developed colony. Only the tips of the pseudohyphae in the agar not covered with ECM still maintain functional drug efflux pumps on the membrane, thus enabling the active defense of these exposed cells.
Figure 1. Internal structure of colony of feral Saccharomyces cerevisiae strain. Thirty-six h-old (left) and 72 h-old (right) colony. Boxes in vertical colony cross-sections summarize structure and function of cell subpopulations in upper aerial and bottom subsurface colony parts; the localization of dividing, non-dividing and stationary cells is depicted, as well as cells with active drug efflux pumps Pdr5p and Snq2p. The presence of ECM is marked with black line hatching. Flo11p-dependent fibers interconnect cells in both aerial and subsurface colony parts.
From the early developmental stage (34–42 h), a cell-free cavity in the colony interior is formed. As the timing of its formation correlates with the appearance of ECM, we can speculate that the cavity could be filled with ECM. The ECM may also be involved in the storage of water and possibly nutrients, as the extracellular material isolated from colonies possesses a high water retention capacity.8 Moreover, it may provide a porous nutrient-rich space for dividing cells in the colony interior. Together with the division of internal cells, site-specific ECM production and its subsequent swelling can lead to rapid colony expansion in both the horizontal and vertical direction. As a result, the aerial surface layer undulates and forms ridges containing other cavities, giving the colony its typical “fluffy” appearance.
In addition to ECM that may provide stability to the 3-D colony structure, the flexibility and undulation of the surface colony layer could be dependent on the presence of fibrous interconnections between the cells. These interconnections are observed throughout the entire colony, including subsurface filamentous cells invading the agar (Fig. 1). Cell-cell and cell-substrate adhesion is another feature typical of multicellular communities18,19 and is often ascribed as a function of cell wall adhesive proteins, including the FLO family of S. cerevisiae.19,20 From mutants in individual FLO genes, only those lacking FLO11 are unable to develop a 3D colony architecture and they form smooth and flat colonies.8,21 Δflo11 colonies also lack intercellular connections, suggesting that Flo11p has a unique function in the formation of fibrous cell-cell interconnections in biofilm colonies.
In plentiful and stable laboratory conditions, colonies no longer need the traits described above. They are therefore switched off and energy, otherwise consumed in e.g., the production of an ECM rich in polysaccharides, could be used in a more profitable way.1 Thus, laboratory and domesticated strains (those arising after passages of feral strains on complex media)3 form non-adhesive, smooth flat colonies expanding predominantly in the horizontal direction.3,8,22 Cells within such colonies are tightly packed and neither pores nor cavities can be observed.3,22 In contrast, the strategy of feral strains is to quickly occupy territory ahead of competitors, to build a complex structure protected by several cooperating mechanisms so as to provide a sheltered space for new cell generations. Despite the high energy costs, it is advantageous to build such a complicated structure, because it enables the community to effectively cope with a hostile environment. Thus, a structured biofilm yeast colony should be considered to be a multicellular organism, where everything is subordinated to the success of the community, regardless of individual cell fate.
Acknowledgments
This work was supported by the Grant Agency of the Czech Republic 204/08/0718 and the Ministry of Education LC531, LC06063; MSM0021620858 and AV0Z50200510.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18912
References
- 1.Palková Z. Multicellular microorganisms: laboratory versus nature. EMBO Rep. 2004;5:470–6. doi: 10.1038/sj.embor.7400145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9:588–94. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Kuthan M, Devaux F, Janderová B, Slaninová I, Jacq C, Palková Z. Domestication of wild Saccharomyces cerevisiae is accompanied by changes in gene expression and colony morphology. Mol Microbiol. 2003;47:745–54. doi: 10.1046/j.1365-2958.2003.03332.x. [DOI] [PubMed] [Google Scholar]
- 4.Ibeas JI, Lozano I, Perdigones F, Jimenez J. Dynamics of Flor Yeast Populations During the Biological Aging of Sherry Wines. Am J Enol Vitic. 1997;48:75–9. [Google Scholar]
- 5.Smukalla S, Caldara M, Pochet N, Beauvais A, Guadagnini S, Yan C, et al. FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell. 2008;135:726–37. doi: 10.1016/j.cell.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–6. doi: 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 7.d’Enfert C. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr Drug Targets. 2006;7:465–70. doi: 10.2174/138945006776359458. [DOI] [PubMed] [Google Scholar]
- 8.St’ovíček V, Váchová L, Kuthan M, Palková Z. General factors important for the formation of structured biofilm-like yeast colonies. Fungal Genet Biol. 2010;47:1012–22. doi: 10.1016/j.fgb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Gancedo JM. Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2001;25:107–23. doi: 10.1111/j.1574-6976.2001.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 10.Wolfger H, Mamnun YM, Kuchler K. Fungal ABC proteins: pleiotropic drug resistance, stress response and cellular detoxification. Res Microbiol. 2001;152:375–89. doi: 10.1016/S0923-2508(01)01209-8. [DOI] [PubMed] [Google Scholar]
- 11.Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–80. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–40. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beauvais A, Schmidt C, Guadagnini S, Roux P, Perret E, Henry C, et al. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell Microbiol. 2007;9:1588–600. doi: 10.1111/j.1462-5822.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- 14.Zara G, Zara S, Pinna C, Marceddu S, Budroni M. FLO11 gene length and transcriptional level affect biofilm-forming ability of wild flor strains of Saccharomyces cerevisiae. Microbiology. 2009;155:3838–46. doi: 10.1099/mic.0.028738-0. [DOI] [PubMed] [Google Scholar]
- 15.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55:999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 16.Nett JE, Sanchez H, Cain MT, Andes DR. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J Infect Dis. 2010;202:171–5. doi: 10.1086/651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8:1382–91. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 18.Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, et al. Complementary adhesin function in C. albicans biofilm formation. Curr Biol. 2008;18:1017–24. doi: 10.1016/j.cub.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brückner S, Mösch HU. Choosing the right lifestyle: adhesion and development in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2012;36:25–58. doi: 10.1111/j.1574-6976.2011.00275.x. [DOI] [PubMed] [Google Scholar]
- 20.Verstrepen KJ, Klis FM. Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol. 2006;60:5–15. doi: 10.1111/j.1365-2958.2006.05072.x. [DOI] [PubMed] [Google Scholar]
- 21.Vopálenská I, St’ovícek V, Janderová B, Váchová L, Palková Z. Role of distinct dimorphic transitions in territory colonizing and formation of yeast colony architecture. Environ Microbiol. 2010;12:264–77. doi: 10.1111/j.1462-2920.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- 22.Váchová L, Chernyavskiy O, Strachotová D, Bianchini P, Burdíková Z, Fercíková I, et al. Architecture of developing multicellular yeast colony: spatio-temporal expression of Ato1p ammonium exporter. Environ Microbiol. 2009;11:1866–77. doi: 10.1111/j.1462-2920.2009.01911.x. [DOI] [PubMed] [Google Scholar]