Abstract
The cell wall integrity (CWI) signal transduction pathway, which has been well-studied in the yeast Saccharomyces cerevisiae, plays an important role in the regulation of cell wall biogenesis. Recently, we characterized the CWI stress sensor orthologs WscA and WscB in the filamentous fungus Aspergillus nidulans. Disruption of the wscA and wscB genes causes a change in the transcriptional levels of agsA and agsB, which encode α-1,3-glucan synthase, resulting in an increase in alkaline soluble cell wall glucan. However, the contribution of these putative sensors to downstream CWI pathway signaling remains unclear because MpkA-RlmA signaling remains active in wscA-wscB double disruptants exposed to cell wall stress associated with exposure to micafungin, a potent inhibitor of β-1,3-glucan synthase. In this addendum, we report the results of further studies involving hypo-osmotic shock as a stressor that suggest WscA and WscB are not essential for MpkA-RlmA signaling. Finally, we describe for the first time other Aspergillus CWI stress sensor candidate Mid2-like protein.
Keywords: Aspergillus, cell wall biogenesis, cell wall integrity, hypo-osmotic stress, micafungin, sensor protein
The cell wall integrity (CWI) signal transduction pathway plays an important role in the regulation of cell wall biogenesis in the yeast Saccharomyces cerevisiae.1,2 Recent progress in genomic studies indicates that the CWI signaling components, including sensor and response regulator proteins, are conserved in fungal species.3,4 While S. cerevisiae is a monocellular fungus that commonly lives on the surface of fruits and flowers in nature, Aspergillus species are multicellular filamentous fungi distributed widely throughout the soil, plant, and indoor environments. Included within this genus are the opportunistic human pathogen A. fumigatus, the industrial citric acid producer A. niger, and Koji molds such as A. oryzae and A. kawachii. Differences in habitat and biology associated with evolutionary history are believed to direct the responses of these species to environmental stimuli.
In S. cerevisiae, five plasma membrane-spanning CWI sensors (Wsc1, Wsc2, Wsc3, Mid2 and Mtl1) that transmit environmental stimuli to downstream signaling pathways to initiate gene expression responses have been identified.1,2,5 At least two putative CWI sensors belonging to the Wsc family have been identified in A. nidulans.3,4,6,7 We recently characterized the putative CWI sensors WscA and WscB in A. nidulans and determined that they are both N- and O-glycosylated and localized on the cell surface.7 We found that wsc-disruptants are characterized by reduced colony size, the formation of fewer conidia, and a high frequency of swollen hyphae in hypo-osmotic YG medium, while osmotic stabilization with KCl restores the normal phenotype. Moreover, transcription of the α-1,3-glucan synthase encoding genes (agsA and agsB) is significantly altered in wsc-disruptant strains, resulting in an increase in the amount of alkali-soluble cell wall glucan, including soluble α-1,3-glucan.
In S. cerevisiae, the activated sensors initiate the signaling cascade that eventually activates a mitogen-activated protein kinase (MAPK, Slt2).1,2 Activation of Slt2 triggers the phosphorylation of the transcriptional regulators Rlm1 and Swi4/Swi6, which regulate the transcription of cell wall synthesis-related genes. On the other hand, MpkA-RlmA signaling, which corresponds to Slt2-Rlm1, induces transient transcriptional upregulation of the agsB gene in response to exposure to the β-1,3-glucan synthase inhibitor micafungin in A. nidulans.6 We therefore compared the transcriptional response of wild type and wsc-disruptant strains to obtain direct evidence that WscA and WscB are located upstream of MpkA-RlmA signaling.7 However, transient transcriptional upregulation of the agsB gene was still observed in the ΔwscA ΔwscB strain, indicating that WscA and WscB are not essential for MpkA-RlmA signaling, at least with respect to stress associated with micafungin exposure. In S. cerevisiae however, the transcriptional response to caspofungin (an echinocandin class anti-fungal drug similar to micafungin) is mediated almost exclusively by Wsc1.8 Phosphorylation of Slt2 is reduced in the wsc1-disruptant after a 2 h caspofungin treatment. Our results therefore confirmed that the stress-sensing spectrum of A. nidulans Wsc proteins differs from that of S. cerevisiae.
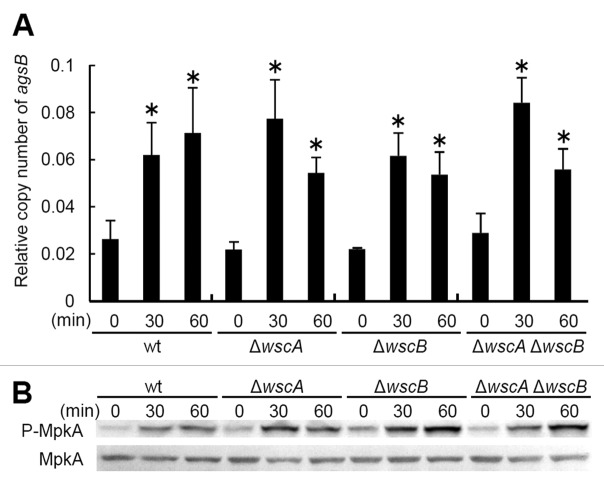
Because the ΔwscA ΔwscB strain exhibits growth defects under hypo-osmotic conditions,7 hypo-osmotic shock was considered an appropriately stressful condition for investigating the relationship between WscA/WscB- and MpkA-RlmA-signaling. For this experiment, we first cultivated each strain in YG liquid medium with 0.6 M KCl, then transferred the cells to YG liquid medium without KCl. We collected the mycelia after 0, 30 and 60 min and quantified the transcriptional levels of the agsB and histone H2B genes using real time-RT-PCR. We also examined the level of MpkA phosphorylation as described previously.7 Our results indicate that phosphorylation of MpkA and transient upregulation of agsB occur even in the ΔwscA ΔwscB strain (Fig. 1A and B). This result supported our previous observation that MpkA-RlmA signaling is functional in the ΔwscA ΔwscB strain. Together with our previous results,7 this suggests that WscA and WscB participate in the tolerance to hypo-osmotic stress in A. nidulans, although their precise roles are unknown, as is the physiological importance of transient MpkA-RlmA signaling in hypo-osmotic stress tolerance.
Figure 1. Response to hypo-osmotic shock in Aspergillus nidulans. (A) Relative transcription of the agsB gene, and (B) phosphorylation of MpkA in the wild type and wsc-disruptant strains. *, statistically significant difference (p < 0.05) relative to the result at 0 min.
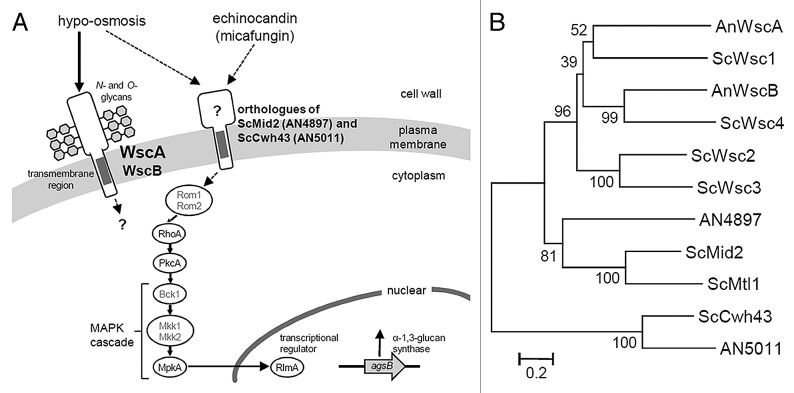
The CWI pathway appears to be different in S. cerevisiae and A. nidulans (Fig. 2A). For example, a protein kinase C (PkcA) is essential for the viability of A. nidulnans,9-11 but the ΔwscA ΔwscB strain was not lethal. This suggests that another CWI stress sensor exists or that there is cross-talk between signaling pathways upstream of PkcA in A. nidulans. This line of reasoning is supported by a report indicating that the central signaling component is well-conserved, whereas the sensors and transcriptional regulators of these modules have diverged significantly.4
Figure 2. (A) A model for CWI signaling in A. nidulans in response to stress associated with micafungin and hypo-osmosis. Dotted lines indicate the unclear relationship derived from the results of this and our previous study.7S. cerevisiae orthologs that have not been functionally characterized in A. nidulans (Rom1, Rom2, Bck1, Mkk1 and Mkk2) are indicated in gray. (B) Phylogenetic tree of putative A. nidulans and S. cerevisiae CWI sensor proteins. The tree was constructed using the neighbor-joining method based on alignment of the amino acid sequences. Bootstrap values are indicated at the tree roots (percentage of 1,000 bootstrap replicates that support the branch). The scale bar represents 0.2 substitutions per amino acid position. An, Aspergillus nidulans; Sc, Saccharomyces cerevisiae.
In S. cerevisiae, Wsc1 and Mid2 act as the primary sensor proteins in the CWI pathway.1,2,5 Although Wsc family proteins have been identified in A. nidulans, no Mid2 ortholog has been reported.3,4,6,7 Recently, we found a Mid2-like protein (systematic name: AN4897) in the genome of A. nidulans during a global analysis of putative O-glycosylated serine/threonine-rich proteins (Figs. 2A and B).12-14 This protein shows a significant Pfam-A match to the Mid2 domain (E-value, 1.9e-05) and it has structural features similar to S. cerevisiae Mid2, including the N-terminal signal sequence, Mid2 domain, a transmembrane region and a putative C-terminal cytoplasmic tail. The homolog of AN4897 is also conserved among Aspergillus species, including A. fumigatus, A. flavus, A. terreus, A. clavatus, A. oryzae, A. niger and A. kawachii. A phylogenetic tree of putative CWI sensor proteins shows AN4897 located in an intermediate position between the Wsc2/Wsc3 branches and Mid2/Mtl1 branches (Fig. 2B). The conserved Mid2 domain is not reflected well in the phylogenetic tree due to the large number of serine/threonine residues. The Aspergillus genome also possesses a homolog of S. cerevisiae putative CWI sensor Cwh43 (systematic name: AN5011) (Fig. 2A and B).6,15 Determining the roles played by potential sensor proteins such as Mid2 and Cwh43 homologs will increase our understanding of CWI signaling in Aspergillus species.
Acknowledgments
We thank Dr. Shuichiro Tagane for a helpful discussion regarding phylogenetic analysis. This work was supported in part by a Ministry of Education, Science, Sports and Culture Grant-in-Aid for Scientific Research (C) (no. 21580096, to M.G.) and a Grant-in-Aid for Young Scientists (B) (no. 23780084, to T.F.). The cost of publication was covered in part by a Research Grant for Young Investigators from the Faculty of Agriculture, Kyushu University.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/18993
References
- 1.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–91. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesage G, Bussey H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2006;70:317–43. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rispail N, Soanes DM, Ant C, Czajkowski R, Grünler A, Huguet R, et al. Comparative genomics of MAP kinase and calcium-calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet Biol. 2009;46:287–98. doi: 10.1016/j.fgb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Nikolaou E, Agrafioti I, Stumpf M, Quinn J, Stansfield I, Brown AJ. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol Biol. 2009;9:44. doi: 10.1186/1471-2148-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodicio R, Heinisch JJ. Together we are strong--cell wall integrity sensors in yeasts. Yeast. 2010;27:531–40. doi: 10.1002/yea.1785. [DOI] [PubMed] [Google Scholar]
- 6.Fujioka T, Mizutani O, Furukawa K, Sato N, Yoshimi A, Yamagata Y, et al. MpkA-Dependent and -independent cell wall integrity signaling in Aspergillus nidulans. Eukaryot Cell. 2007;6:1497–510. doi: 10.1128/EC.00281-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Futagami T, Nakao S, Kido Y, Oka T, Kajiwara Y, Takashita H, et al. Putative stress sensors WscA and WscB are involved in hypo-osmotic and acidic pH stress tolerance in Aspergillus nidulans. Eukaryot Cell. 2011;10:1504–15. doi: 10.1128/EC.05080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermejo C, García R, Straede A, Rodríguez-Peña JM, Nombela C, Heinisch JJ, et al. Characterization of sensor-specific stress response by transcriptional profiling of wsc1 and mid2 deletion strains and chimeric sensors in Saccharomyces cerevisiae. OMICS. 2010;14:679–88. doi: 10.1089/omi.2010.0060. [DOI] [PubMed] [Google Scholar]
- 9.Teepe AG, Loprete DM, He Z, Hoggard TA, Hill TW. The protein kinase C orthologue PkcA plays a role in cell wall integrity and polarized growth in Aspergillus nidulans. Fungal Genet Biol. 2007;44:554–62. doi: 10.1016/j.fgb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Ronen R, Sharon H, Levdansky E, Romano J, Shadkchan Y, Osherov N. The Aspergillus nidulans pkcA gene is involved in polarized growth, morphogenesis and maintenance of cell wall integrity. Curr Genet. 2007;51:321–9. doi: 10.1007/s00294-007-0129-y. [DOI] [PubMed] [Google Scholar]
- 11.Ichinomiya M, Uchida H, Koshi Y, Ohta A, Horiuchi H. A protein kinase C-encoding gene, pkcA, is essential to the viability of the filamentous fungus Aspergillus nidulans. Biosci Biotechnol Biochem. 2007;71:2787–99. doi: 10.1271/bbb.70409. [DOI] [PubMed] [Google Scholar]
- 12.Oka T, Hamaguchi T, Sameshima Y, Goto M, Furukawa K. Molecular characterization of protein O-mannosyltransferase and its involvement in cell-wall synthesis in Aspergillus nidulans. Microbiology. 2004;150:1973–82. doi: 10.1099/mic.0.27005-0. [DOI] [PubMed] [Google Scholar]
- 13.Goto M. Protein O-glycosylation in fungi: diverse structures and multiple functions. Biosci Biotechnol Biochem. 2007;71:1415–27. doi: 10.1271/bbb.70080. [DOI] [PubMed] [Google Scholar]
- 14.Goto M, Harada Y, Oka T, Matsumoto S, Takegawa K, Furukawa K. Protein O-mannosyltransferases B and C support hyphal development and differentiation in Aspergillus nidulans. Eukaryot Cell. 2009;8:1465–74. doi: 10.1128/EC.00371-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Yken H, Dagkessamanskaia A, De Groot P, Ram A, Klis F, François J. Saccharomyces cerevisiae YCRO17c/CWH43 encodes a putative sensor/transporter protein upstream of the BCK2 branch of the PKC1-dependent cell wall integrity pathway. Yeast. 2001;18:827–40. doi: 10.1002/yea.731. [DOI] [PubMed] [Google Scholar]