Abstract
Importin α is recognized as a classical nuclear localization signal (cNLS) receptor which mediates nucleocytoplasmic transport. However, it rapidly accumulates in the nucleus in response to cellular stresses, including oxidative stress, causing a blockade of the classical nuclear import pathway. We set out to determine whether importin α performs roles in the nucleus after cellular exposure to stresses and discovered that it can act directly to modulate gene expression. With remarkable selectivity, importin α2 can access the promoter of Serine/threonine kinase 35 (STK35) and increase the levels of this transcript without requirement for importin β1. The nuclear accumulation of importin α occurred following exposure to stresses which decreased intracellular ATP levels and was followed by non-apoptotic cell death. Hence the gene regulatory function of nuclear importin α can direct cell fate. There are now several reports of nuclear-localized importin α proteins in diverse cellular states, including cancer. Here we discuss the physiological significance of this novel functional capacity of nuclear importin α relationship to a variety of cellular states and fates.
Keywords: ATP, importin α, KPNA2, non-apoptotic cell death, nuclear transport, STK35, stress
A Novel Role for Importin α Proteins in Gene Regulation
We found that HeLa cells overexpressing nuclear importin α2 exhibited downregulation of transcripts encoded by 62 genes, including 22 encoding replication-dependent histones, as well as selective upregulation of only two transcripts, including Serine/threonine kinase 35 (STK35).1 The contrast between the large numbers of downregulated mRNAs with the small number identified as upregulated suggested to us that importin α2 can effectively suppress gene expression through chromatin binding. We hypothesize that this occurs through importin α interaction with the cNLSs in karyophilic proteins such as transcription factors, since the cNLS has been shown to overlap with DNA binding regions in some cargo proteins.2-4 Thus we predict that, in circumstances when importin α accumulates in the nucleus, certain transcription factors interact with importin α via their cNLS and this binding compromises or changes their transcriptional activities. Because the apparent numerical difference between the number of up- and downregulated genes suggests that nuclear importin α generally acts as a suppressor for transcription factors, and in case of STK35, it operates by suppressing the activity of some protein that inhibits transcription. In support of this, we found that the region ≥ 1 kbp upstream of the first exon of Stk35 served a repressor function for the core promoter.1 These data suggest that importin α inhibits the suppressor for the STK35 core promoter, resulting in its enhanced activity.
A New Perspective on Non-Apoptotic Cell Death Associated with Nuclear Importin α
The physiological significance of changes of nucleocytoplasmic transport under stress conditions has been linked to perturbed protein shuttling within signaling cascades, structural modifications of transport machinery, including the nuclear pore complex (NPC), and evocation of cell death by apoptosis.5-7 However, there is relatively little discussion about the mechanisms by which changes in cellular metabolism arising from stress, its associated alterations in nucleocytoplasmic transport, and the subsequent impact on cell fate.8,9 We previously reported that intracellular ATP levels decreased following exposure to all tested stresses: UV-irradiation, heat shock and hydrogen peroxide, and this caused the Ran gradient to collapse.10 This finding is in agreement with a previous report that cellular ATP depletion following exposure to 2-deoxyglucose and sodium azide leads to a decrease in free RanGTP which is followed by nuclear accumulation of importin α.11 Depletion of cellular ATPs itself has been known to induce necrosis or caspase-independent cell death, but not apoptosis, because of the high levels of ATP required for caspase activation.12,13 In addition, apoptosis requires active nuclear transport mediated by importin α and is dependent upon a Ran gradient and intact NPCs.14 Thus several observations support our hypothesis that blocking the classical nuclear transport pathway, including by induced nuclear accumulation of importin α under conditions of ATP depletion, results in the inhibition of apoptosis and promotion of non-apoptotic cell death. Taken together with the ability of STK35 to enhance caspase-independent cell death under oxidative stress,1 it becomes evident that the combined outcomes of both deficient classical nuclear transport and transcriptional modulation by nuclear-localized importin α direct cell fate toward a cell death pathway that bypasses apoptosis, such as necrosis, upon stress exposure. These findings reveal a new mechanistic approach to understanding how non-apoptotic cell death is elicited by a decrease in intracellular ATP in cells under stress, through the re-distribution of importin α into the nucleus (Fig. 1).
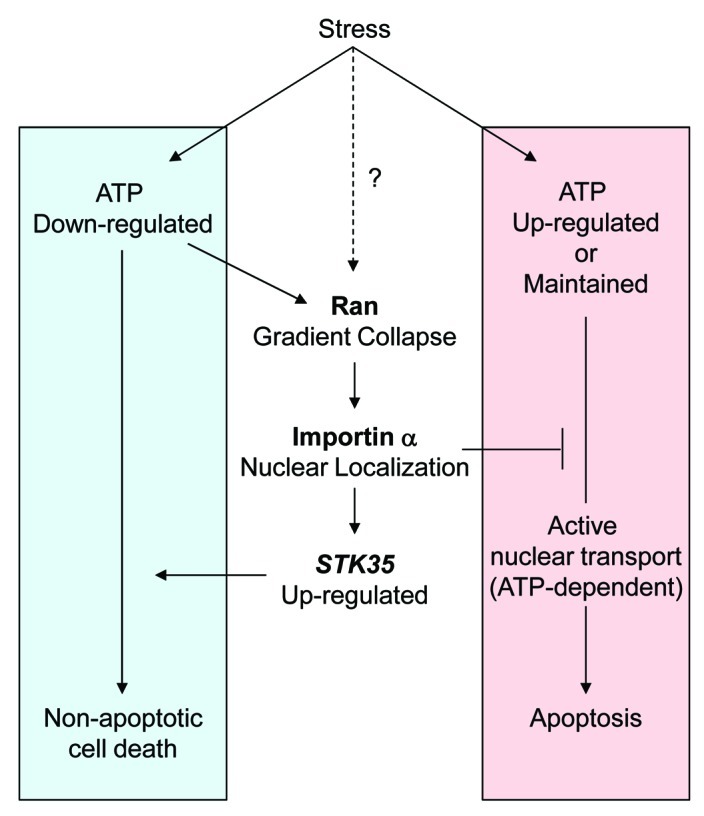
Figure 1. Schematic model for mode of cell death induced by stress in response to depleted intracellular ATP. Cellular stresses which deplete intracellular ATP induce a Ran gradient collapse and importin α accumulates in the nucleus. This leads to a block in classical nucleocytoplasmic protein transport via the importin α/β1 pathway. Nuclear importin α functions to elevate STK35 transcription and promotes non-apoptotic cell death in oxidative stress. The Ran gradient collapse may be induced by both ATP depletion and through modulated activity of Ran-related proteins, such as RCC1, in some stress conditions.9
Additional Physiological Importance of Importin α Nuclear Localization
Is the nuclear accumulation of importin α restricted to stress conditions? C. elegans importin α proteins, particularly IMA-1 and -2, were detected in the nucleoplasm of germ cells.15 In Drosophila, all three importin αs exhibit nuclear accumulation in a stage-specific manner during spermatogenesis.16 In mammals, importin α4, but not its close subfamily member importin α3, is predominantly nuclear in the adult testis, with a striking nuclear signal evident in pachytene spermatocytes and round spermatids.17,18 In addition, the importin α4 protein exhibits nuclear localization in the murine embryonic stem (mES) cells in undifferentiated, but not differentiated, stages.19 These observations suggest that nuclear-localized importin α proteins serve key roles in cell fate choice between maintenance of pluripotency and differentiation.
Recently, a novel importin α family member was identified, referred to as karyopherin α7 (KPNA7) in human, mouse and cattle.20-22 KPNA7 is closely related to importin α2 and localized in the nucleus in mouse oocytes and zygotes as well as in HeLa cells.20,21 Interestingly, a mutant Kpna7 gene caused abnormal expression of chromatin modification-associated genes and also induced epigenetic modification of histone H3K27me3.21 These observations bear a striking correlation to our finding that nuclear importin α2 causes downregulation of mRNAs encoding replication-dependent histones1 and highlight the need to gain a precise understanding of the genomic and chromatin-associated modifications effected by nuclear importin α in the nucleus.
Of direct relevance to human disease, breast cancer cells exhibit the remarkable expression and nuclear localization of human karyopherin α2 (KPNA2, ortholog of mouse importin α2), and this may be significantly associated with patient survival rates.23-26 High expression and nuclear localization of KPNA2 was also observed in lung tumor tissues,27 esophageal squamous cell carcinoma,28 bladder cancer29 and prostate cancer.30 Moreover, increased expression and elevated nuclear accumulation of importin α5 and importin α7 have been reported in tubular and glomerular cells of diabetic rats.31
Collectively these reports highlight the potential contribution of nuclear importin α to various cellular events, each of which might involve a different substrate specificity, reflect cell-specific expression patterns and effect distinct transcriptional outcomes. Our findings should encourage investigations of additional functions for importin α in a variety of cellular states and fates.
Acknowledgments
This work was supported in part by Grants from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan, JST, CREST and Takeda Science Foundation (to Y. Y.), from a National Health and Medical Research Council of Australia Fellowship (#545917 to K. L.) and from the Australian Research Council (Discovery Project; DP0878102 to Y. M., K. L., and Y. Y.).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/19194
References
- 1.Yasuda Y, Miyamoto Y, Yamashiro T, Asally M, Masui A, Wong C, et al. Nuclear retention of importin a coordinates cell fate through changes in gene expression. EMBO J. 2012;31:83–94. doi: 10.1038/emboj.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaCasse EC, Lefebvre YA. Nuclear localization signals overlap DNA- or RNA-binding domains in nucleic acid-binding proteins. Nucleic Acids Res. 1995;23:1647–56. doi: 10.1093/nar/23.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cokol M, Nair R, Rost B. Finding nuclear localization signals. EMBO Rep. 2000;1:411–5. doi: 10.1093/embo-reports/kvd092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair R, Carter P, Rost B. NLSdb: database of nuclear localization signals. Nucleic Acids Res. 2003;31:397–9. doi: 10.1093/nar/gkg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrando-May E. Nucleocytoplasmic transport in apoptosis. Cell Death Differ. 2005;12:1263–76. doi: 10.1038/sj.cdd.4401626. [DOI] [PubMed] [Google Scholar]
- 6.Fahrenkrog B. The nuclear pore complex, nuclear transport, and apoptosis. Can J Physiol Pharmacol. 2006;84:279–86. doi: 10.1139/y05-100. [DOI] [PubMed] [Google Scholar]
- 7.Kodiha M, Stochaj U. Nuclear transport: a switch for the oxidative stress-signaling circuit? J Signal Transduct 2012; 2012:208650; PMID:22028962; DOI:10.1155/2012/208650. [DOI] [PMC free article] [PubMed]
- 8.Grote P, Schaeuble K, Ferrando-May E. Commuting (to) suicide: an update on nucleocytoplasmic transport in apoptosis. Arch Biochem Biophys. 2007;462:156–61. doi: 10.1016/j.abb.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Kelley JB, Paschal BM. Hyperosmotic stress signaling to the nucleus disrupts the Ran gradient and the production of RanGTP. Mol Biol Cell. 2007;18:4365–76. doi: 10.1091/mbc.E07-01-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda Y, Miyamoto Y, Saiwaki T, Yoneda Y. Mechanism of the stress-induced collapse of the Ran distribution. Exp Cell Res. 2006;312:512–20. doi: 10.1016/j.yexcr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Schwoebel ED, Ho TH, Moore MS. The mechanism of inhibition of Ran-dependent nuclear transport by cellular ATP depletion. J Cell Biol. 2002;157:963–74. doi: 10.1083/jcb.200111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Leist M, Single B, Castoldi AF, Kühnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–6. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasuhara N, Eguchi Y, Tachibana T, Imamoto N, Yoneda Y, Tsujimoto Y. Essential role of active nuclear transport in apoptosis. Genes Cells. 1997;2:55–64. doi: 10.1046/j.1365-2443.1997.1010302.x. [DOI] [PubMed] [Google Scholar]
- 15.Adam SA. The nuclear transport machinery in Caenorhabditis elegans: A central role in morphogenesis. Semin Cell Dev Biol. 2009;20:576–81. doi: 10.1016/j.semcdb.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Giarrè M, Török I, Schmitt R, Gorjánácz M, Kiss I, Mechler BM. Patterns of importin-alpha expression during Drosophila spermatogenesis. J Struct Biol. 2002;140:279–90. doi: 10.1016/S1047-8477(02)00543-9. [DOI] [PubMed] [Google Scholar]
- 17.Hogarth CA, Jans DA, Loveland KL. Subcellular distribution of importins correlates with germ cell maturation. Dev Dyn. 2007;236:2311–20. doi: 10.1002/dvdy.21238. [DOI] [PubMed] [Google Scholar]
- 18.Whiley PA, Miyamoto Y, McLachlan RI, Jans DA, Loveland KL. Changing subcellular localization of nuclear transport factors during human spermatogenesis. Int J Androl. 2011;5:158–69. doi: 10.1111/j.1365-2605.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- 19.Young JC, Major AT, Miyamoto Y, Loveland KL, Jans DA. Distinct effects of importin α2 and α4 on Oct3/4 localization and expression in mouse embryonic stem cells. FASEB J. 2011;25:3958–65. doi: 10.1096/fj.10-176941. [DOI] [PubMed] [Google Scholar]
- 20.Kelley JB, Talley AM, Spencer A, Gioeli D, Paschal BM. Karyopherin alpha7 (KPNA7), a divergent member of the importin alpha family of nuclear import receptors. BMC Cell Biol. 2010;11:63. doi: 10.1186/1471-2121-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Wang F, Yuan Y, Zhu X, Wang Y, Zhang Y, et al. Novel importin-alpha family member Kpna7 is required for normal fertility and fecundity in the mouse. J Biol Chem. 2010;285:33113–22. doi: 10.1074/jbc.M110.117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tejomurtula J, Lee KB, Tripurani SK, Smith GW, Yao J. Role of importin alpha8, a new member of the importin alpha family of nuclear transport proteins, in early embryonic development in cattle. Biol Reprod. 2009;81:333–42. doi: 10.1095/biolreprod.109.077396. [DOI] [PubMed] [Google Scholar]
- 23.Dahl E, Kristiansen G, Gottlob K, Klaman I, Ebner E, Hinzmann B, et al. Molecular profiling of laser-microdissected matched tumor and normal breast tissue identifies karyopherin alpha2 as a potential novel prognostic marker in breast cancer. Clin Cancer Res. 2006;12:3950–60. doi: 10.1158/1078-0432.CCR-05-2090. [DOI] [PubMed] [Google Scholar]
- 24.Dankof A, Fritzsche FR, Dahl E, Pahl S, Wild P, Dietel M, et al. KPNA2 protein expression in invasive breast carcinoma and matched peritumoral ductal carcinoma in situ. Virchows Arch. 2007;451:877–81. doi: 10.1007/s00428-007-0513-5. [DOI] [PubMed] [Google Scholar]
- 25.Gluz O, Wild P, Meiler R, Diallo-Danebrock R, Ting E, Mohrmann S, et al. Nuclear karyopherin alpha2 expression predicts poor survival in patients with advanced breast cancer irrespective of treatment intensity. Int J Cancer. 2008;123:1433–8. doi: 10.1002/ijc.23628. [DOI] [PubMed] [Google Scholar]
- 26.Noetzel E, Rose M, Bornemann J, Gajewski M, Knüchel R, Dahl E. Nuclear transport receptor karyopherin-α2 promotes malignant breast cancer phenotypes in vitro. Oncogene. 2011 doi: 10.1038/onc.2011.403. In press. [DOI] [PubMed] [Google Scholar]
- 27.Wang CI, Wang CL, Wang CW, Chen CD, Wu CC, Liang Y, et al. Importin subunit alpha-2 is identified as a potential biomarker for non-small cell lung cancer by integration of the cancer cell secretome and tissue transcriptome. Int J Cancer. 2011;128:2364–72. doi: 10.1002/ijc.25568. [DOI] [PubMed] [Google Scholar]
- 28.Sakai M, Sohda M, Miyazaki T, Suzuki S, Sano A, Tanaka N, et al. Significance of karyopherin-alpha 2 (KPNA2) expression in esophageal squamous cell carcinoma. Anticancer Res. 2010;30:851–6. [PubMed] [Google Scholar]
- 29.Jensen JB, Munksgaard PP, Sørensen CM, Fristrup N, Birkenkamp-Demtroder K, Ulhøi BP, et al. High expression of karyopherin-α2 defines poor prognosis in non-muscle-invasive bladder cancer and in patients with invasive bladder cancer undergoing radical cystectomy. Eur Urol. 2011;59:841–8. doi: 10.1016/j.eururo.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 30.Mortezavi A, Hermanns T, Seifert HH, Baumgartner MK, Provenzano M, Sulser T, et al. KPNA2 expression is an independent adverse predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res. 2011;17:1111–21. doi: 10.1158/1078-0432.CCR-10-0081. [DOI] [PubMed] [Google Scholar]
- 31.Köhler M, Buchwalow IB, Alexander G, Christiansen M, Shagdarsuren E, Samoilova V, et al. Increased importin alpha protein expression in diabetic nephropathy. Kidney Int. 2001;60:2263–73. doi: 10.1046/j.1523-1755.2001.00069.x. [DOI] [PubMed] [Google Scholar]


