Abstract
Purpose.
The purpose of this study was to determine the effect of histamine and its receptors on goblet cell secretion.
Methods.
Cultured rat and human goblet cells were grown in RPMI 1640. Goblet cell secretion of high molecular weight glycoconjugate was measured by an enzyme-linked lectin assay. Cultured rat goblet cells were homogenized and either RNA was isolated for RT-PCR or proteins were isolated for Western blot analysis for presence of histamine receptors subtypes H1 through H4. The localization of these receptors was determined in rat and human goblet cells by immunofluorescence microscopy.
Results.
Histamine stimulated goblet cell secretion in a concentration- and time-dependent manner. All four histamine receptors were present in cultured rat and human goblet cells. Use of agonists specific to individual histamine receptor subtypes indicated that the rank order of agonist stimulation was H1 = H3 > H4 > H2. Using antagonists specific to individual histamine receptor subtypes determined that H2 and H3, but not the H1 and H4, antagonists, inhibited histamine-stimulated conjunctival goblet cell secretion.
Conclusions.
Rat and human conjunctival goblet cells are a direct target of histamine, which induces secretion. All four histamine receptors are present in rat and human conjunctiva and are active in rat conjunctival goblet cells. These findings suggest that all four histamine receptor subtypes are important for conjunctival goblet cell secretion. Blockage of histamine receptor subtypes could prevent the excess mucus production associated with ocular allergy.
The study demonstrated that all four histamine receptors are present and active in rat conjunctival goblet cells.
Introduction
Ocular allergy can be classified into several subtypes including seasonal allergic conjunctivitis, perennial allergic conjunctivitis, atopic conjunctivitis (AKC), vernal conjunctivitis (VKC), and giant papillary conjunctivitis.1,2 Seasonal and perennial allergic conjunctivitis display a mild presentation, whereas AKC, VKC, and giant papillary conjunctivitis are more serious.2 Seasonal allergic conjunctivitis occurs in response to an allergen. Giant papillary conjunctivitis results from friction or trauma usually induced by contact lens wear. In contrast, the underlying causes of AKC and VKC are unknown, and both can affect the cornea and cause blindness. About 98% of all cases of ocular allergy are from allergic conjunctivitis, and thus it is the most prevalent form of ocular allergy.2
Allergic conjunctivitis is IgE mediated, and its hallmarks are itching, tearing, increased mucus production, and eyelid swelling. These symptoms result from allergen entering the conjunctival stroma and crosslinking with IgE on mast cells or basophils. This crosslinking induces release of histamine, along with other allergic mediators such as lipid mediators and cytokines.3 Histamine can also be produced by antigen processing by dendritic cells and macrophages for presentation to T-helper cells. Histamine in turn can act on a variety of cell types to induce inflammatory cell recruitment and interact with mucosal epithelial cells, smooth muscle cells, and endothelial cells of the blood vessels. These interactions cause many of the symptoms of the allergic response.
Histamine is a major mediator of allergy including allergic conjunctivitis2 and causes an inflammatory response characterized by vasodilation and increased vascular permeability.3 However, histamine can also induce gastric acid secretion; is a neurotransmitter in the central nervous system with a role in sleep–wake cycles, appetite, learning, and memory; and plays a role in the innate immune response.3 These diverse biological effects of histamine are mediated by four different histamine receptors, H1 through H4.3 For example, H1 receptors mediate acute allergic responses; H2 receptors cause gastric acid secretion; H3 receptors mediate the postsynaptic effects of histamine released as a neurotransmitter; and H4 receptors are found predominantly on cells of hematopoietic origin such as dendritic cells, mast cells, eosinophils, monocytes, basophils, and T cells and are immunomodulatory.3
The conjunctival epithelium and its underlying stroma are a major target tissue in ocular allergy. The epithelium consists of two cell types, stratified squamous and goblet cells. Both of these cell types secrete electrolytes and water that could contribute, along with the increased permeability of the blood vessels of the stroma, to the elevation in tearing. In addition, stratified squamous and goblet cells produce mucins. Stratified squamous cells produce membrane-spanning mucins that form the glycocalyx, whereas the goblet cells synthesize and secrete the large, gel-forming mucin MUC5AC. MUC5AC is secreted into the tears and contributes to the soluble mucous portion of the tears. The presence of functioning MUC5AC-containing goblet cells in the conjunctiva is critical for the health of the ocular surface.
The amount of MUC5AC in the tear film is the result of goblet cell exocytotic secretion, which in turn is dependent upon the number of goblet cells present, the amount of MUC5AC synthesized, and the number of goblet cells that respond to a given stimulus. Conjunctival goblet cell mucin secretion and goblet cell proliferation are differentially regulated.4,5 Activation of nerves and exogenous addition of neurotransmitters causes goblet cell secretion when studies are performed in vivo, in tissue pieces, or in cultured cells.6–9 Activation of afferent sensory nerves in the cornea stimulates efferent parasympathetic nerves to induce goblet cell secretion, and the released parasympathetic neurotransmitters acetylcholine and vasoactive intestinal peptide (VIP) stimulate secretion.10,11 Recently the inflammatory mediators leukotriene (LT) B4, LTC4, LTD4, LTE4, and prostaglandin D2 were shown to stimulate goblet cell secretion.6 Thus goblet cell secretion can be induced by activation of nerves using neurotransmitters or by lipid inflammatory mediators released from invading lymphocytic cells.
The major treatment of allergic conjunctivitis is topical or systemic antihistamines that stabilize mast cells, block inflammation, and decrease the effects of histamine.2 The predominant treatments of allergic conjunctivitis are directed at the histamine H1 receptor and include oral antihistamines that target H1 receptors and topical dual-action agents emedastine, epinastine, and ketotifen that are directed at H1, H2, and other targets.12
In the present study the authors determined the effect of histamine and its receptors on goblet cell secretion. The findings showed that histamine using all four histamine receptor subtypes (H1-H4) stimulated goblet cell secretion.
Materials and Methods
Materials
Histamine and the histamine receptor agonists 2-([3-trifluoromethyl]phenyl) histamine dimaleate (H1), amthamine dihydrobromide (H2), and 4-methylhistamine dihydrochloride (H4) were from Sigma-Aldrich (St. Louis, MO), while (R)α-methylhistamine dihydrochloride (H3) was purchased from Tocris Bioscience (Ellisville, MO). The histamine antagonists chlorpheniramine (H1), cimetidine (H2), and JNJ7777120 (H4) were also obtained from Sigma-Aldrich. Conessine, the H3 receptor antagonist, was purchased from Tocris Bioscience.
Animals
Male Sprague-Dawley rats weighing between 125 and 150 g were obtained from Taconic Farms (Germantown, NY). Rats were anesthetized with CO2 for 1 minute and decapitated, and the bulbar and fornical conjunctiva were removed from both eyes. All experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Human Material
Human conjunctival tissue was obtained from patients during ocular surgery using a protocol that adhered to the tenets of the Declaration of Helsinki. The protocol was approved by the Schepens Eye Research Institute Human Studies Internal Review Board. The tissue, which was normally discarded during surgery, was donated by three patients. Tissue was placed in phosphate-buffered saline solution (PBS; 145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 mM NaH2PO4 [pH 7.2]) containing penicillin-streptomycin (300 μg/mL).
Cell Culture
Goblet cells from rat and human conjunctiva were grown in organ culture as described previously.13,14 Pieces of minced tissue were placed in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 μg/mL penicillin-streptomycin. The tissue plug was removed after nodules of cells were observed. First-passage goblet cells were used in all experiments. Cultured cells were periodically checked by evaluating staining with antibody to cytokeratin 7 (detects goblet cell bodies) and the lectin Ulex europaeus agglutinin (UEA)-1 (detects goblet cell secretory product) to ensure that goblet cells predominated.
Secretion
Cultured goblet cells were plated in 24-well plates and grown to confluence. Cells were serum starved for 2 hours before use, preincubated with antagonists for 30 minutes, and then stimulated with agonists for 0 to 4 hours in the presence of serum-free RPMI 1640 supplemented with 0.5% bovine serum albumin (BSA). Goblet cell secretion was measured using an enzyme-linked lectin assay (ELLA) with the lectin UEA-I. UEA-1 detects high molecular weight glycoconjugates including mucins produced by rat goblet cells. The media were collected and analyzed for the amount of lectin-detectable glycoconjugates, which quantifies the amount of goblet cell secretion. The standards and supernatant were spotted onto Nunc microplates and dried overnight at 60°C. The ELLA was performed according to a protocol from Pierce Biotechnology (Rockford, IL) using UEA-I conjugated to horseradish peroxidase. The UEA-1 was then detected using Amplex Red (Invitrogen, Carlsbad, CA), which, when oxidized by peroxidase in the presence of hydrogen peroxide, produces a highly fluorescent molecule. The fluorescence was quantified on a fluorescent ELISA reader (model FL600; Bio-Tek, Winooski, VT) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. The cells were removed and sonicated, and the cell homogenate was analyzed for the total amount of protein using the Bradford protein assay. Glycoconjugate secretion was normalized to total protein in the homogenate. Bovine submaxillary mucin was used for the standard curve. Glycoconjugate secretion was expressed as fold increase over basal that was set to 1.
RT-PCR
Cultured goblet cells were homogenized in TRIzol (Life Technologies, Grand Island, NY), and total RNA was isolated according to manufacturer's instructions. RNA was also isolated from rat brain to use as a positive control. One microgram of purified total RNA was used for complementary DNA (cDNA) synthesis using the Superscript First-Strand Synthesis system for RT-PCR (Invitrogen). The cDNA was amplified by the polymerase chain reaction (PCR) using primers specific to H1, H2, H3, and H4 receptors using the Jumpstart REDTaq Readymix Reaction Mix (Sigma-Aldrich) in a thermal cycler (Master Cycler; Eppendorf, Hauppauge, NY). Nested PCR was used for H4 amplification. The primers used for histamine receptors were derived from previously published sequences and are listed in the Table. The conditions were as follows: 5 minutes at 95°C followed by 35 cycles of 1 minute at 94°C, 30 seconds at annealing temperature (listed in the Table), and 1 minute at 72°C with a final hold at 72°C for 10 minutes. Samples with no cDNA served as the negative control while the presence of β-actin was the positive control. Amplification products were separated by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining.
Table.
Primers for RT-PCR
|
Receptor |
Primer Sequences 5′-3′ |
Annealing Temp (deg C) |
Fragment Size (bp) |
Source |
|
| HR1 | Sense: | CCTCTACCTTCGAAGACAAG | 60 | 406 | NM_017018 |
| Anti: | GACCAAAGAGATGGCAAC- | ||||
| HR2 | Sense: | ATGGCATTGAAAGTCACC | 60 | 378 | NM_012965.3 |
| Anti: | GACCAAAGAGATGGCAAC | ||||
| HR3 | Sense: | CAGCGTTACCTTCTTCAACC | 60 | 512 | NM_053506.1 |
| Anti: | CAGCTCGGATGATCATTAGG | ||||
| HR4 | Sense: | TAACGATAGGCAATGCTGTG | 60 | 391 | NM_131909.1 |
| Anti: | TCTTCCAAGAATCCGAAGCC | 228 | |||
| Nested Anti: | ACTGTAGACGGATGCTGTGC | ||||
| β-actin | ATGGATGACGATATCGCTG | 59 | 568 | NM_03114.2 | |
| ATGAGGTAGTCTGTCAGGT | |||||
Western Blotting
Pieces of rat conjunctiva and goblet cells cultured in eight-well plates were homogenized in RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and 1 mM EDTA). The homogenate was centrifuged at 2000 g for 30 min at 4°C. Sample buffer (4×) was added to the homogenate and protein separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using a 10% gel and processed for Western blotting as described previously. Primary antibodies for H1 through H4 were the same as those used for immunofluorescence experiments and were diluted to 1:500. Antibodies used included anti-H1, anti-H2, anti-H3, and anti-H4 receptors (all at 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibody was from Millipore (Billerica, MA) and was used at a dilution of 1:5000. Immunoreactive bands were visualized by the enhanced chemiluminescence method. Negative control experiments included omission of the primary antibody.
Immunohistochemistry
For immunofluorescence microscopy of intact conjunctiva, the eyes were enucleated with the lids intact and fixed in 4% formaldehyde in PBS for overnight at 4°C. Eyes were embedded in paraffin, and sections (6 μm) were placed on slides. For immunohistochemistry of cultured cells, first-passage cells were grown on glass coverslips and then fixed in methanol before use. Tissue sections and cultured cells were processed and viewed for immunofluorescence as described previously. Antibodies used included anti-H1, anti-H2, anti-H3, and anti-H4 receptors (all at 1:100 dilution; Santa Cruz Biotechnology). UEA-1 conjugated to FITC (Sigma-Aldrich) was used at a dilution of 1:500 and identified goblet cell secretory product. 4′, 6-diamidino-2-phenylindole (DAPI) was in the mounting medium and indicated cell nuclei. Secondary antibodies were conjugated to either Cy2 or Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA) and were used at a dilution of 1:150. Negative control experiments included incubation with the isotype control antibody.
Statistical Analysis
Results were expressed as the fold increase above basal or the percentage of inhibition of the net stimulation by agonist alone. Results are presented as mean ± SEM. Data were analyzed by Student's t-test. P < 0.05 was considered statistically significant.
Results
Effect of Histamine on Conjunctival Goblet Cell Secretion
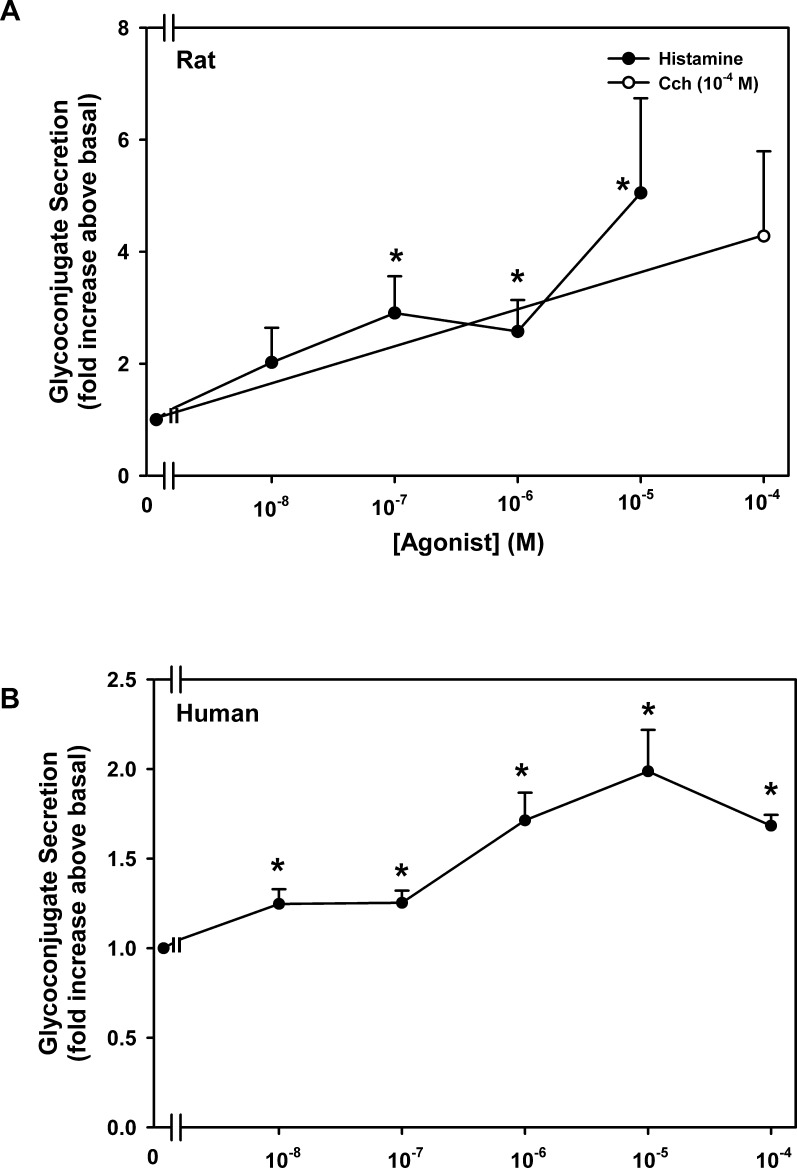
Goblet cells cultured from rat and human conjunctiva were incubated with increasing concentrations of histamine for 2 hours, and high molecular weight glycoconjugate secretion that includes mucin was measured. In rat goblet cells, histamine stimulated goblet cell secretion in a concentration-dependent manner with a maximum increase of 5.0 ± 1.7-fold at 10−5 M (n = 5) (Fig. 1A). In the same experiments, the positive control, the cholinergic agonist carbachol (10−4 M), increased secretion 4.3 ± 1.5-fold. Secretion was significantly increased at 10−7, 10−6, and 10−5 M histamine. When used in human goblet cells, histamine also stimulated secretion with a significant response obtained at 10−8 to 10−4 M (Fig. 1B). Similar to rat cells, secretion was maximum at 10−5 M, but secretion was induced by only 2.0 ± 0.2-fold (n = 3).
Figure 1.
Effect of histamine concentration on goblet cell glycoconjugate secretion. Cultured goblet cells from rats (A) or humans (B) were incubated with histamine (10−8–10−4 M) for 2 hours. Rat cells were also incubated with the cholinergic agonist carbachol (Cch, 10−4 M) for 2 hours as a positive control. Glycoconjugate secretion was measured by enzyme-linked lectin assay. Data are mean ± SEM of three independent experiments. * indicates statistical significance from no addition (0).
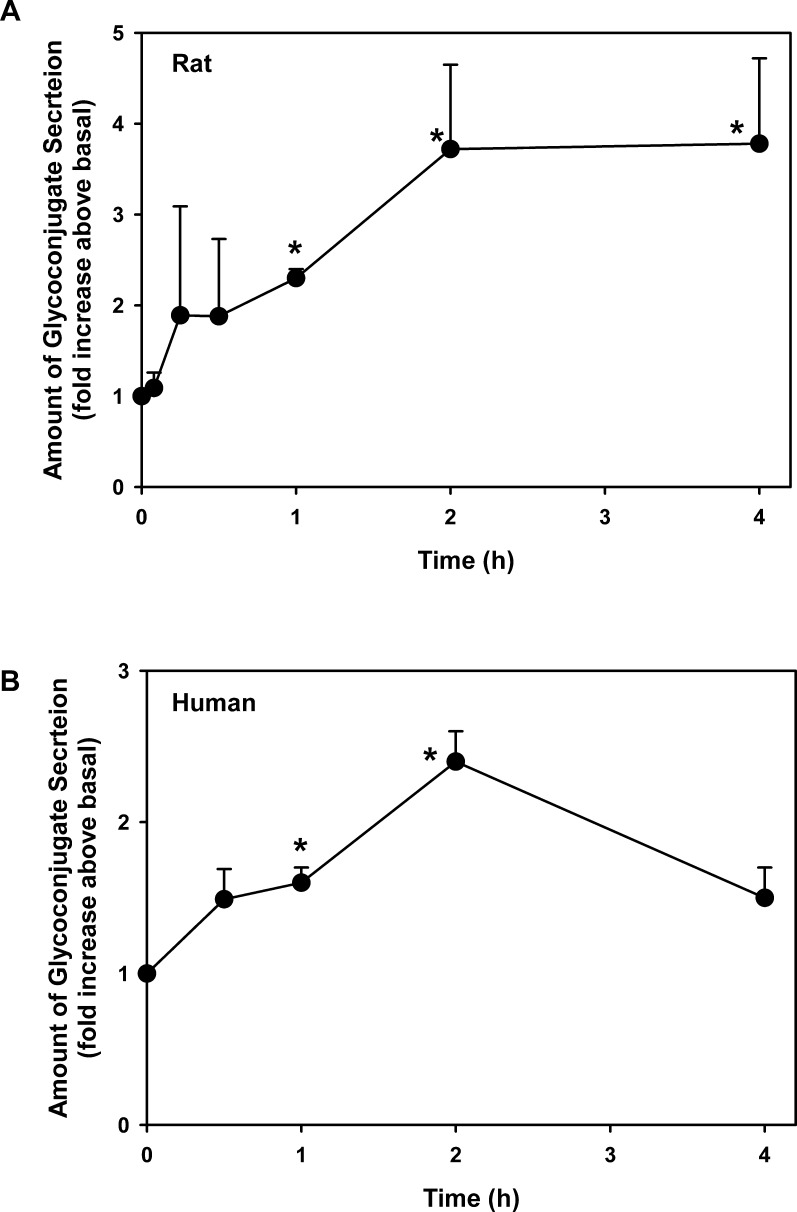
Cultured rat and human conjunctival goblet cells were incubated with histamine at 10−5 M for increasing times from 0 to 4 hours. In rat cells, secretion was increased as soon as 15 minutes after stimulation, but was not significantly increased until 1 hour of incubation (Fig. 2A). At 1 hour, secretion was 2.3 ± 0.1-fold (n = 3). Maximal secretion was obtained at 2 hours of incubation and was 3.7 ± 0.9-fold (Fig. 2A). Secretion remained elevated for 8 hours (data not shown). Histamine stimulated human goblet cell secretion with a time course similar to that for rat goblet cells (Fig. 2B). In human cells, secretion was significantly increased at 1 and 2 hours of incubation, but was decreased at 4 hours. At 2 hours, histamine elevated secretion a maximum of 2.4 ± 0.2-fold (n = 3).
Figure 2.
Effect of time on histamine-stimulated goblet cell glycoconjugate secretion. Cultured goblet cells from rats (A) or humans (B) were incubated with histamine (10−5 M) for 0 to 4 hours. Glycoconjugate secretion was measured by enzyme-linked lectin assay. Data are mean ± SEM of three independent experiments. * indicates statistical significance from t = 0.
Histamine stimulated goblet cell secretion in both rat and human conjunctival goblet cells in culture. Histamine was more effective in rat than human cells, but its potency and time dependence were similar in the two species.
Identification and Localization of Histamine Receptor Subtypes in Conjunctival Goblet Cells
RT-PCR, Western blotting analysis, and immunofluorescence microscopy were used to determine the presence and location of the four histamine receptor subtypes.
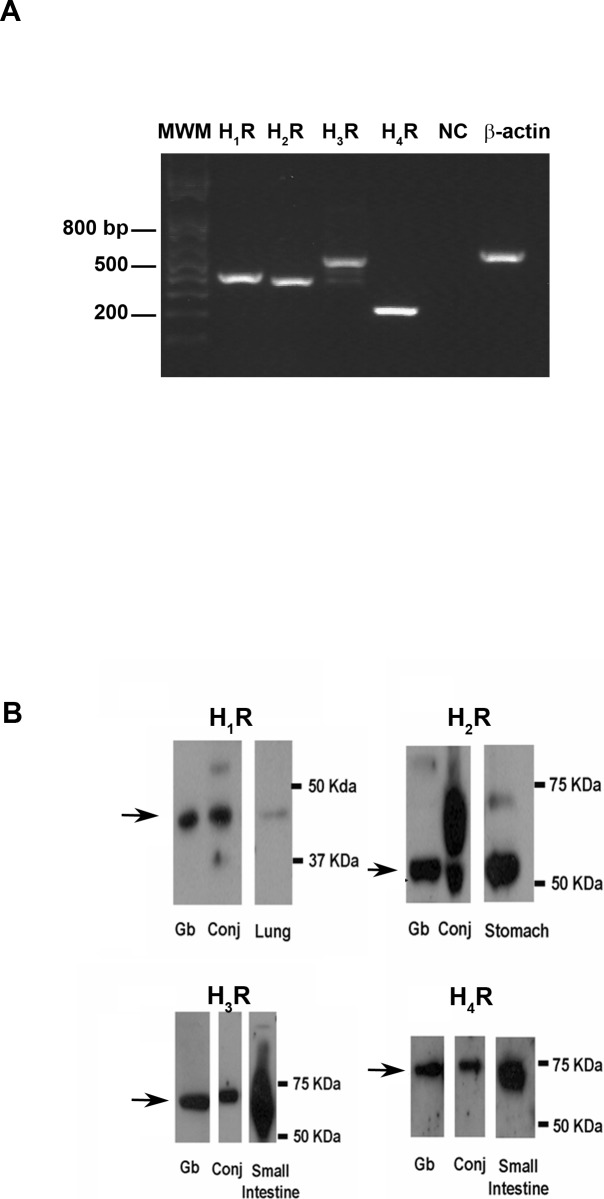
Messenger RNA isolated from cultured rat conjunctival goblet cells was used to determine the presence of message for the four receptor subtypes. As expected, H1 was detected at 406 bp and H2 at 378 bp; H3 was detected at 512 bp, and H4 was detected at 228 bp (Fig. 3A).
Figure 3.
Presence of histamine receptors in rat conjunctiva and cultured goblet cells. Cultured rat goblet cells were homogenized, and RNA isolated for RT-PCR with primers for histamine receptors (A). The blot is representative of three individual animals. Rat conjunctiva and cultured goblet cells were homogenized and Western blot analysis was performed with anti-histamine receptor subtype antibodies, shown in (B). The blot is representative of three individual animals. MWM, molecular weight markers; H1R, histamine receptor type 1; H2R, histamine receptor type 2; H3R, histamine receptor type 3; H4R, histamine receptor type 4; NC, negative control; Gb, cultured goblet cells; Conj, rat conjunctiva.
Western blotting analysis was performed on homogenized cultured conjunctival goblet cells and conjunctival tissue isolated from rats. Rat lung, stomach, and small intestine homogenates were used as positive controls. H1 receptor was detected as a major band at 45 kDa in goblet cells, conjunctiva, and lung, the positive control for the H1 receptor (Fig. 3B). H2 receptor was found in goblet cells and conjunctiva, as well as the positive control for the H2 receptor, stomach. A single band at 59 kDa was apparent in goblet cells. A band at this molecular weight was also detected in conjunctiva and stomach. Conjunctiva had several poorly resolved bands in addition, and stomach had a second, higher molecular weight band. For the H3 receptor, a single major band at 70 kDa was shown in goblet cells. A single major band of slightly higher molecular weight was visible in the conjunctiva. In the small intestine, the positive control for the H3 receptor, bands were not well separated, but did include a positive response at the same molecular weight as in the goblet cells. A single band of 70 kDa was found for the H4 receptor in goblet cells, conjunctiva, and small intestine, the positive control for the H4 receptor. Differential glycosylation of the receptor could account for the multiple bands found in the conjunctiva for the H2 receptor and in the small intestine for the H3 receptor.
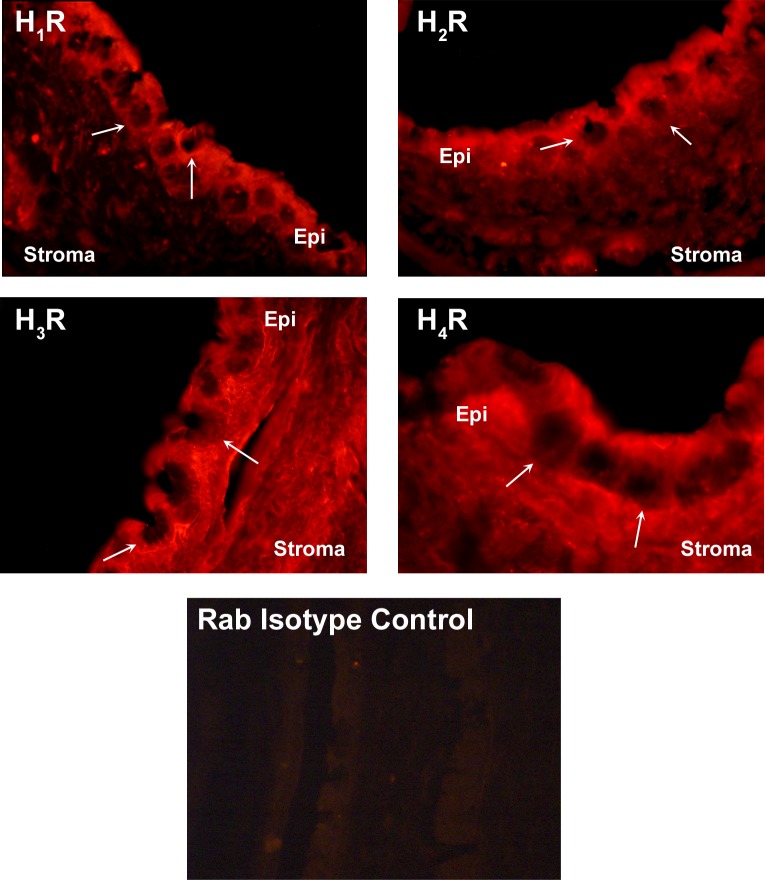
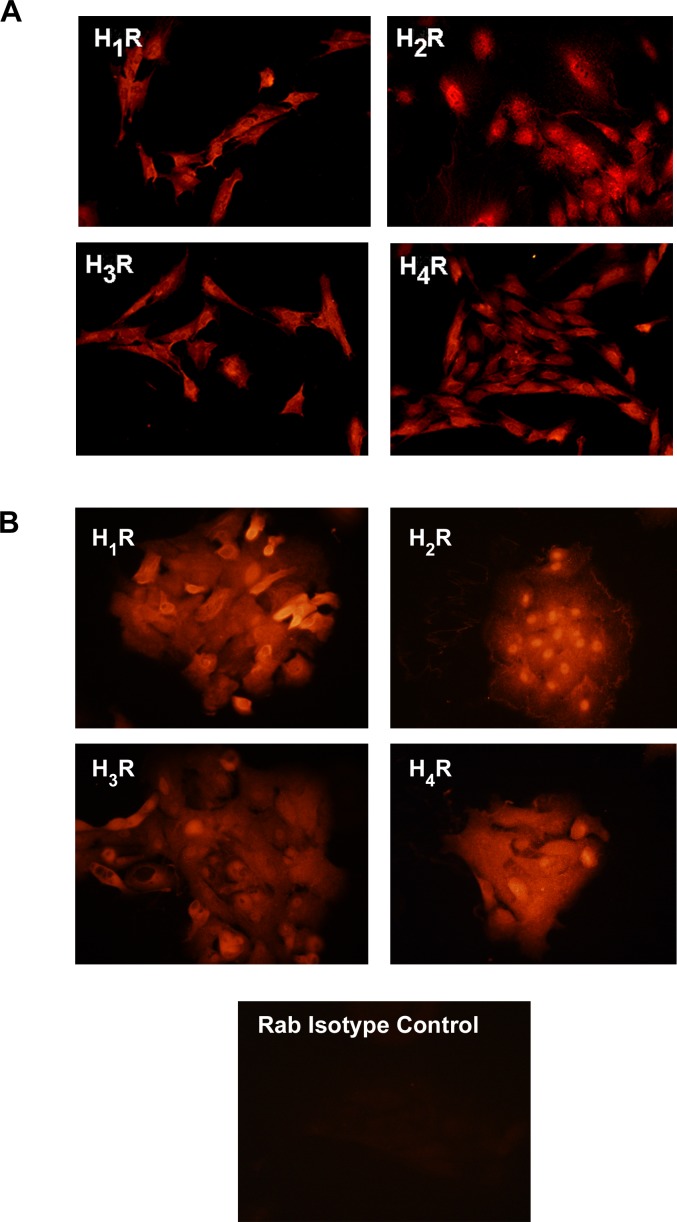
Use of immunofluorescence microscopy showed the presence of all four histamine receptors in the rat conjunctiva, in both stratified squamous and goblet cells (Fig. 4). No major differences in the location of the staining were apparent among the four receptors, except that anti-H3 and anti-H4 immunoreactivity was additionally detected in the stroma. Immunoreactivity for all four histamine receptors was also found in cultured rat and human conjunctival goblet cells (Figs. 5A, 5B). Immunoreactivity with anti-H1 and -H2 receptor antibodies was localized to the plasma membrane and anti-H2 receptor antibody was particulate in the cytoplasm. H1, H3, and H4 immunoreactivity was also diffusely localized in the cytoplasm. Immunoreactivity with antibodies for all four histamine receptors was seen in the cytoplasm of human goblet cells with no difference in localization between the receptors.
Figure 4.
Localization of histamine receptors in rat conjunctiva. Rat conjunctiva was removed and fixed, and immunofluorescence experiments were performed with antibodies against the H1 receptor (H1R), H2 receptor (H2R), H3 receptor (H3R), and H4 receptor (H4R). Magnification 200×. Each micrograph is representative of three independent experiments. Arrows indicate the location of goblet cells. Epi, conjunctival epithelium.
Figure 5.
Localization of histamine receptors in cultured rat and human goblet cells. Goblet cells from rat (A) and human (B) conjunctiva were grown and fixed, and immunofluorescence experiments were performed with antibodies against the H1 receptor (H1R), H2 receptor (H2R), H3 receptor (H3R), and H4 receptor (H4R). Magnification 200×. Each micrograph is representative of three separate experiments.
Use of three different methods identified all four histamine receptors in conjunctival goblet cells, as well as in the stratified squamous cells. All four receptors were present in both rat and human goblet cells.
Effect of Histamine Receptor Agonists on Conjunctival Goblet Cell Secretion
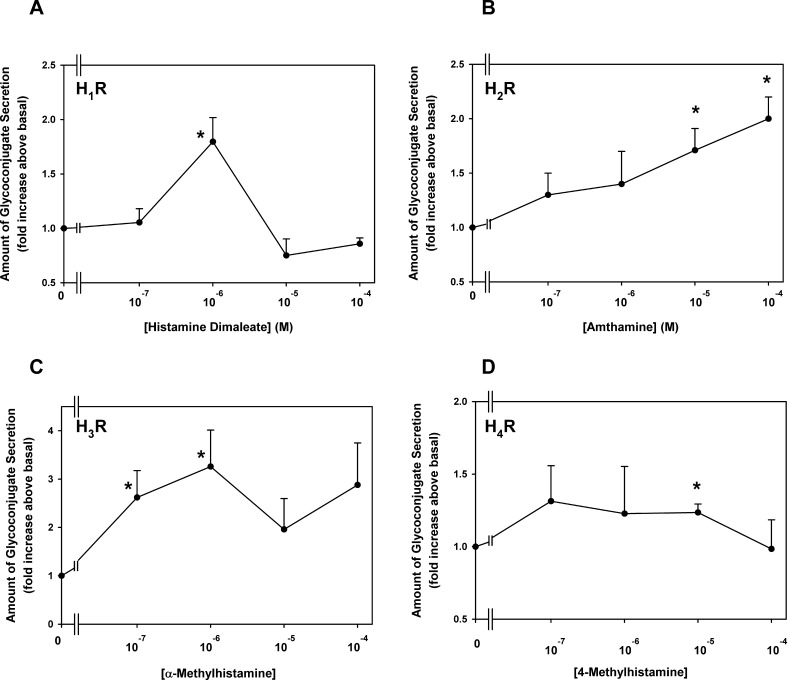
An agonist for each of the four histamine receptor subtypes was used to determine which receptor subtypes caused conjunctival goblet cell secretion. The H1 receptor agonist histamine dimaleate used at 10−7 to 10−4 M significantly stimulated goblet cell secretion (Fig. 6A). Secretion was maximum at 10−6 M and was a 1.8 ± 0.2-fold increase. Increasing the concentration of histamine dimaleate caused a lower response. Amthamine, which activates H2 receptors, was used at 10−7 to 10−4 M. It increased secretion a maximum of 2.0 ± 0.2-fold at 10−4 M (Fig. 6B, n = 5). Secretion increased with increasing amthamine concentration, with 10−5 and 10−4 M reaching statistical significance. α-Methylhistamine was chosen as the H3 agonist (Fig. 6C). This agonist increased secretion a maximum of 3.3 ± 0.8-fold at 10−6 M and caused a biphasic secretory response (n = 5). The H4 agonist used was 4-methylhistamine (Fig. 6D). Secretion was maximum at 10−7 M and was increased 1.3 ± 0.2-fold (n = 4). The secretory response was decreased with increasing 4-methylhistamine concentration.
Figure 6.
Effect of agonists specific to histamine receptor subtypes (H1R, [A]; H2R, [B]; H3R, [C]; and H4, [D]) on goblet cell glycoconjugate secretion. Cultured rat goblet cells were incubated with agonists specific to histamine receptor subtypes (10−7–10−4 M) for 2 hours. Glycoconjugate secretion was measured by enzyme-linked lectin assay. Data are mean ± SEM of four (4-methylhistamine) or five (histamine dimaleate, amthamine, and α-methylhistamine) independent experiments. * indicates statistical significance from no addition (0).
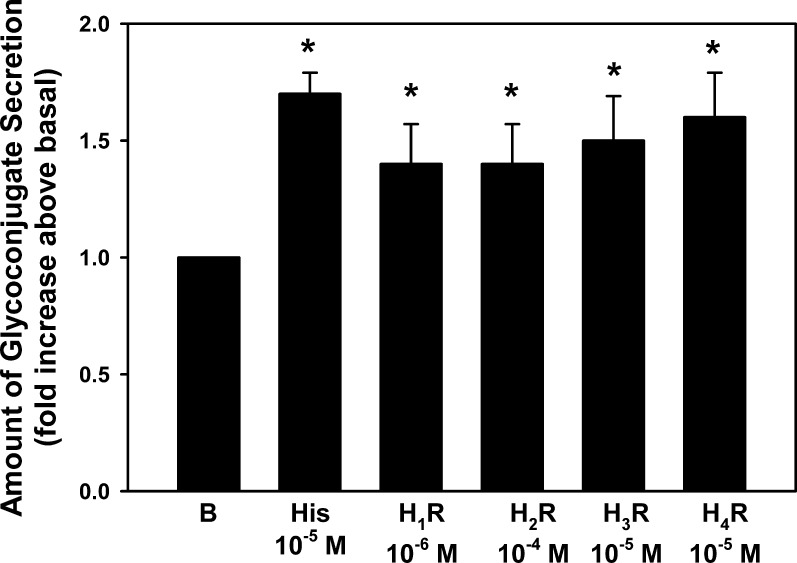
To compare the effectiveness of the four histamine receptor agonists and histamine, experiments were performed using the concentration of each agonist (histamine and H1-H4 receptor subtype agonists) that caused maximum secretion (Figs. 6A–D) in the same experiment (Fig. 7). Histamine at 10−5 M stimulated secretion 1.70 ± 0.09-fold (n = 7). Histamine dimaleate (H1R, 10−6 M), amthamine (H2R, 10−4 M), α-methylhistamine (H3R, 10−6 M), and 4-methylhistamine (H4R, 10−5 M) each significantly stimulated secretion and to the same level as histamine.
Figure 7.
Effect of agonists specific to histamine receptor subtypes on goblet cell glycoconjugate secretion. Cultured goblet cells from rats were incubated with the concentration of agonists that caused maximum secretion. The agonists used were histamine (10−5 M), the H1 receptor agonist histamine dimaleate (10−6 M), the H2 receptor agonist amthamine (10−4 M), the H3 receptor agonist α-methylhistamine (10−5 M), and the H4 receptor agonist 4-methylhistamine (10−5 M) for 2 hours. Glycoconjugate secretion was measured by enzyme-linked lectin assay. Data are mean ± SEM of seven independent experiments. * indicates statistical significance from no addition (0).
Not only were all four histamine receptors present in conjunctiva goblet cells both in situ and in culture, but activation of all four histamine receptors caused goblet cell secretion. The rank order of efficacy stimulation of secretion was H1 = H3 > H4 > H2.
Effect of Histamine Receptor Antagonists on Conjunctival Goblet Cell Secretion
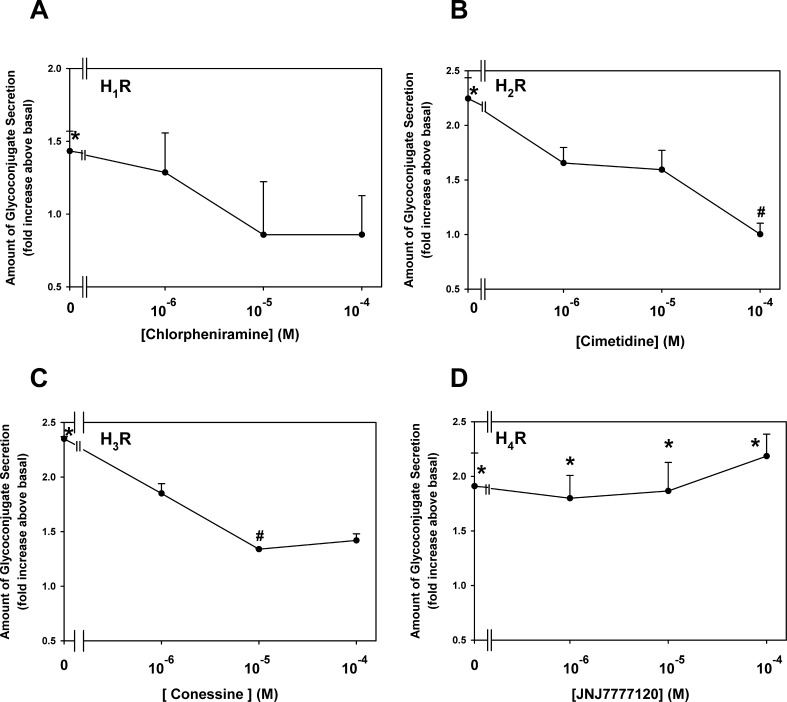
Conjunctival goblet cells were preincubated for 30 minutes with a histamine receptor antagonist, and then secretion was stimulated by histamine at 10−5 M for 2 hours. For the H1 receptor, the antagonist chlorpheniramine was used from 10−6 to 10−4 M. Chlorpheniramine at 10−4 M decreased, but not significantly, secretion to 0.9 ± 0.4-fold from 1.4 ± 0.1, the response to histamine alone (Fig. 8A, n = 3). Cimetidine was the H2 antagonist and was used at 10−6 to 10−4 M. Cimetidine at 10−4 M significantly decreased histamine-stimulated secretion, from 2.2 ± 0.2-fold to 1.0 ± 0.1-fold (Fig. 8B). For the H3 antagonist, conessine was chosen. In these experiments, histamine stimulated secretion 2.4 ± 0.0-fold, and conessine at 10−5 M significantly inhibited secretion to 1.3 ± 0.0-fold (Fig. 8C, n = 3). Finally, JNJ7777120 was chosen as the H4 antagonist. The histamine-stimulated secretion of 1.9 ± 0.3-fold was not altered by any concentration of JNJ7777120 (Fig. 8D, n = 3). In contrast to the agonists for the four histamine receptor subtypes that all stimulated goblet cell secretion, only the H2 and H3, not the H1 and H4, antagonists significantly inhibited conjunctival goblet cell secretion.
Figure 8.
Effect of antagonists specific to histamine receptor subtypes (H1R, [A]; H2R, [B]; H3R, [C]; and H4, [D]) on goblet cell glycoconjugate secretion. Cultured goblet cells from rats were incubated with antagonists specific to histamine receptor subtypes (10−6–10−4 M) for 30 minutes prior to stimulation with histamine (10−5 M) for 2 hours. Glycoconjugate secretion was measured by enzyme-linked lectin assay. Data are mean ± SEM of three independent experiments. * indicates statistical significance from no addition (0); # indicates statistical significance from histamine alone.
Discussion
Allergic conjunctivitis is characterized by histamine release from mast cells and neutrophils.15 Histamine is one of the primary compounds that mediate the symptoms of allergic conjunctivitis, and inhibition of its release and its action are major targets for pharmaceutical treatments. Results from the present study show that histamine directly stimulates secretion from conjunctival goblet cells and that all four histamine receptors are present, but that only H2 and H3 receptors are most consistently functional. These findings do not support the suggestion of Woodward et al.16 that conjunctival goblet cell depletion in vivo (analogous to goblet cell secretion) is secondary to eosinophil infiltration, as goblet cells can be directly activated by histamine. The results of the present study are in agreement with those on colonic and airway goblet cells in studies in which histamine stimulated goblet cell secretion.17,18 Thus modulation of goblet cell secretion should be added to the targets for inhibition of histamine function in allergic conjunctivitis.
Histamine stimulates goblet cell secretion or proliferation in the other goblet cell–containing tissues, the intestine and the lung. Histamine stimulates rat colonic goblet cells to secrete mucin and in rabbit ileum disrupts the goblet cell intercellular junctions.17,19 In the lung, activation of mast cells, known to release histamine upon degranulation, induces goblet cell hyperplasia and mucus secretion.20 In guinea pig conjunctiva, topical histamine caused depletion of filled conjunctival goblet cells, which is consistent with stimulation of secretion that empties the entire goblet cell contents.16
An alteration of goblet cell mucin production (synthesis and secretion) has been identified in different types of ocular allergy. In AKC there is a loss of mucin-filled goblet cells and a decrease in MUC5AC mRNA.21,22 In a mouse model of allergic conjunctivitis, there was a transient decrease in the number of filled goblet cells and MUC5AC mRNA.23 A decrease in the number of filled goblet cells with a decrease in mRNA suggests that the goblet cells have released their secretory product, as goblet cells secrete all their granules together. In addition, the decrease in mRNA along with the increase in secretion suggests that the goblet cell synthetic and secretory mechanisms have been exhausted from chronic stimulation preventing resynthesis of mucin. In VKC there is conflicting evidence about the alteration in the number of filled goblet cells. One study found a decrease in mucin-filled goblet cells and MUC5AC mRNA.24 In contrast, several studies found an increase in filled goblet cell number.25–28 An increase in filled goblet cells suggests either increased goblet cell proliferation or a block in goblet cell secretion that is preventing the goblet cells from releasing their mucin, hence an increase in filled goblet cells. Measurement of tear MUC5AC levels could help determine the role of goblet cells in the different types of ocular allergy.
The presence of goblet cells filled with secretory product (filled goblet cells) in the conjunctiva is critical to the health of the ocular surface. In the literature, filled goblet cells were usually detected by Alcian blue/periodic acid Schiff's (AB/PAS) staining. Ocular surface diseases are described as having a decrease or increase in filled goblet cells. Diseases with a decrease in filled goblet cells include late dry eye, cicatricial pemphigoid, vitamin A deficiency, anesthetic cornea, thermal burns, and chemical burns.11,29–31 Diseases with an increase in filled goblet cells include allergic conjunctivitis, AKC, VKC, giant papillary conjunctivitis, and mucus fishing syndrome.21,23,32–34 That an increase or a decrease in filled goblet cells leads to disease suggests that (1) goblet cell mucus production is tightly regulated and (2) there is an optimum amount of mucus production necessary for a healthy ocular surface. Either a decrease or an increase in mucus production is detrimental to the ocular surface and leads to disease, with a decrease in production being the most devastating. In ocular allergy it would be beneficial to block goblet cell mucus secretion; thus blocking the histamine receptor subtypes could be an effective treatment of allergy. It is important to point out that mucus production is multifactorial and that goblet cell proliferation, mucus synthesis, and mucus secretion all contribute to the amount of mucus on the ocular surface. A thorough study of the regulation of mucus production has not been performed in any of the ocular surface diseases. Such knowledge could benefit the treatment of ocular surface diseases including ocular allergy.
Most treatments of allergic conjunctivitis, including loratadine, desioratadine, and levocabastine, are aimed at H1 receptors; the exception is one drug, emedastine, which is aimed at H2 receptors.12 Four histamine receptors are now cloned, and many cell types contain at least three different histamine receptors.3 Dendritic and CD4+ T cells contain H1, H2, and H4 receptors.3 H1, H2, and H3 receptors were detected in a bronchial epithelial cell line.35 All four histamine receptors were detected in detrusor smooth muscle cells.36 H1, H2, and H4 receptors were identified on the same nasal mucosal nerves.37 A retrospective study on human conjunctiva detected three histamine receptors, H1, H2, and H3, with H1 receptors causing puritis and H2 receptors causing vasodilation.38 The presence of H4 receptors was not evaluated. The current study also demonstrated multiple histamine receptor subtypes on the conjunctiva, but additionally yielded evidence of the presence of the H4 receptor. Furthermore, all four receptor subtypes were found on both goblet cell and stratified squamous cells.
The histamine receptor subtypes can each activate specific signaling pathways depending on the cell type studied. Thus, activation of H1 receptors in acute allergic reactions increases [Ca2+]i; activation of H2 receptors in gastric acid secretion elevates cAMP levels; in neurotransmitter modulation, activation of H3 receptors lowers cAMP levels; and stimulation of H4 receptors for immunodulation raises [Ca2+]i.3 Activation of histamine receptor subtypes can also activate several additional cellular pathways. H3 receptor activation can also stimulate phospholipase A2, mitogen-activated protein kinase, and phosphatidylinositol-3 kinase (PI3K) and increase [Ca2+]i.39 In conjunctival goblet cells, all four histamine receptors are active. As each histamine receptor subtype can connect with multiple signaling pathways, further study of additional signaling pathways activated by these subtypes, to determine if the signaling pathways for these receptors diverge or are similar, is warranted.
In the present study, goblet cells were cultured from both rat and human conjunctiva. The effect of histamine was the same on secretion from goblet cells from both species, and the same histamine receptor subtypes were present in both species. This finding is consistent with previous results from this group showing human and rat goblet cells to be similar in the effect of cholinergic agonists,8 epidermal growth factor (EGF),5 leukotrienes, and resolvins.6 Thus evidence continues to be generated that rat conjunctival goblet cells are an excellent model for the human cells even when the human cells are obtained from older individuals.
Conjunctival goblet cells appear to be well endowed with signaling machinery to respond to challenges from the external environment. This is in contrast to the cornea, whose capacity to respond is limited by its need to maintain transparency. The conjunctiva contains the cellular apparatus to vigorously respond in order to protect the ocular surface and the rest of the eye. For example, all four histamine receptors are present in goblet cells, as are both cysteinyl leukotriene receptors,6 all four EGF receptors,4 four Toll-like receptors (unpublished data), and three of four muscarinic receptors.8 Furthermore, all these receptors are active in causing goblet cell secretion. Many of these receptors are present in the stratified squamous cells as well.
In conclusion, rat and human conjunctival goblet cells are a direct target of histamine, which induces secretion. All four histamine receptors are present in rat and human conjunctiva and are active in rat conjunctival goblet cells. These findings suggest that all four histamine receptor subtypes are important for conjunctival goblet cell secretion. Blockage of histamine receptor subtypes could prevent the excess mucus production associated with ocular allergy.
Footnotes
Supported by National Institutes of Health Grant EY019470.
Disclosure: D. Hayashi, None; D. Li, None; C. Hayashi, None; M. Shatos, None; R.R. Hodges, None; D.A. Dartt, None
References
- 1. Calder VL. Molecular and cellular mechanisms in allergic conjunctiva. : Dartt D. Encyclopedia of the Eye. Oxford, UK: Elsevier; 2010:30–36 [Google Scholar]
- 2. del Cuvillo A, Sastre J, Montoro J, et al. Allergic conjunctivitis and H1 antihistamines. J Investig Allergol Clin Immunol. 1999;19 (suppl 1):11–18 [PubMed] [Google Scholar]
- 3. Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53 [DOI] [PubMed] [Google Scholar]
- 4. Gu J, Chen L, Shatos MA, et al. Presence of EGF growth factor ligands and their effects on cultured rat conjunctival goblet cell proliferation. Exp Eye Res. 2008;86:322–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horikawa Y, Shatos MA, Hodges RR, et al. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2535–2544 [DOI] [PubMed] [Google Scholar]
- 6. Dartt DA, Hodges RR, Li D, et al. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011;186:4455–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dartt DA, Kessler TL, Chung EH, et al. Vasoactive intestinal peptide-stimulated glycoconjugate secretion from conjunctival goblet cells. Exp Eye Res. 1996;63:27–34 [DOI] [PubMed] [Google Scholar]
- 8. Kanno H, Horikawa Y, Hodges RR, et al. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003;284:C988–998 [DOI] [PubMed] [Google Scholar]
- 9. Rios JD, Zoukhri D, Rawe IM, et al. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40:1102–1111 [PubMed] [Google Scholar]
- 10. Dartt DA, McCarthy DM, Mercer HJ, et al. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res. 1995;14:993–1000 [DOI] [PubMed] [Google Scholar]
- 11. Kessler TL, Mercer HJ, Zieske JD, et al. Stimulation of goblet cell mucous secretion by activation of nerves in rat conjunctiva. Curr Eye Res. 1995;14:985–992 [DOI] [PubMed] [Google Scholar]
- 12. Schultz BL. Pharmacology of ocular allergy. Curr Opin Allergy Clin Immunol. 2006;6:383–389 [DOI] [PubMed] [Google Scholar]
- 13. Shatos MA, Rios JD, Horikawa Y, et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2477–2486 [DOI] [PubMed] [Google Scholar]
- 14. Shatos MA, Rios JD, Tepavcevic V, et al. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001;42:1455–1464 [PubMed] [Google Scholar]
- 15. Lightman S. Therapeutic considerations: symptoms, cells and mediators. Allergy. 1995;50 (21 suppl):10–13, discussion 34–38 [DOI] [PubMed] [Google Scholar]
- 16. Woodward DF, Spada CS, Nieves AL, et al. Platelet-activating factor causes goblet cell depletion in the conjunctiva. Eur J Pharmacol. 1989;168:23–30 [DOI] [PubMed] [Google Scholar]
- 17. Neutra MR, O'Malley LJ, Specian RD. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol. 1982;242:G380–387 [DOI] [PubMed] [Google Scholar]
- 18. Sakai N, Tamaoki J, Kobayashi K, et al. Adenosine potentiates neurally- and histamine-induced contraction of canine airway smooth muscle. Int Arch Allergy Appl Immunol. 1989;90:280–284 [DOI] [PubMed] [Google Scholar]
- 19. Porvaznik M, Baker W, Walker RI. Disruption of the goblet cell intercellular junction following histamine infusion of the rabbit ileum. Experientia. 1983;39:514–518 [DOI] [PubMed] [Google Scholar]
- 20. Casale TB, Marom Z. Mast cells and asthma. The role of mast cell mediators in the pathogenesis of allergic asthma. Ann Allergy. 1983;51 (1 pt 1):2–6 [PubMed] [Google Scholar]
- 21. Dogru M, Matsumoto Y, Okada N, et al. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy. 2008;63:1324–1334 [DOI] [PubMed] [Google Scholar]
- 22. Dogru M, Okada N, Asano-Kato N, et al. Atopic ocular surface disease: implications on tear function and ocular surface mucins. Cornea. 2005;24 (8 suppl):S18–S23 [DOI] [PubMed] [Google Scholar]
- 23. Kunert KS, Keane-Myers AM, Spurr-Michaud S, et al. Alteration in goblet cell numbers and mucin gene expression in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2001;42:2483–2489 [PubMed] [Google Scholar]
- 24. Hu Y, Matsumoto Y, Dogru M, et al. The differences of tear function and ocular surface findings in patients with atopic keratoconjunctivitis and vernal keratoconjunctivitis. Allergy. 2007;62:917–925 [DOI] [PubMed] [Google Scholar]
- 25. Aragona P, Romeo GF, Puzzolo D, et al. Impression cytology of the conjunctival epithelium in patients with vernal conjunctivitis. Eye (Lond). 1996;10 (pt 1):82–85 [DOI] [PubMed] [Google Scholar]
- 26. Bonini S, Coassin M, Aronni S, et al. Vernal keratoconjunctivitis. Eye (Lond). 2004;18:345–351 [DOI] [PubMed] [Google Scholar]
- 27. Foster CS, Rice BA, Dutt JE. Immunopathology of atopic keratoconjunctivitis. Ophthalmology. 1991;98:1190–1196 [DOI] [PubMed] [Google Scholar]
- 28. Roat MI, Ohji M, Hunt LE, et al. Conjunctival epithelial cell hypermitosis and goblet cell hyperplasia in atopic keratoconjunctivitis. Am J Ophthalmol. 1993;116:456–463 [DOI] [PubMed] [Google Scholar]
- 29. Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol. 1984;102:1049–1051 [DOI] [PubMed] [Google Scholar]
- 30. Sommer A. Effects of vitamin A deficiency on the ocular surface. Ophthalmology. 1983;90:592–600 [DOI] [PubMed] [Google Scholar]
- 31. Kuckelkorn R, Schrage N, Redbrake C, et al. Autologous transplantation of nasal mucosa after severe chemical and thermal eye burns. Acta Ophthalmol Scand. 1996;74:442–448 [DOI] [PubMed] [Google Scholar]
- 32. Dogru M, Asano-Kato N, Tanaka M, et al. Ocular surface and MUC5AC alterations in atopic patients with corneal shield ulcers. Curr Eye Res. 2005;30:897–908 [DOI] [PubMed] [Google Scholar]
- 33. Allansmith MR, Baird RS, Greiner JV. Density of goblet cells in vernal conjunctivitis and contact lens-associated giant papillary conjunctivitis. Arch Ophthalmol. 1981;99:884–885 [DOI] [PubMed] [Google Scholar]
- 34. McCulley JP, Moore MB, Matoba AY. Mucus fishing syndrome. Ophthalmology. 1985;92:1262–1265 [DOI] [PubMed] [Google Scholar]
- 35. Muller T, Myrtek D, Bayer H, et al. Functional characterization of histamine receptor subtypes in a human bronchial epithelial cell line. Int J Mol Med. 2006;18:925–931 [PubMed] [Google Scholar]
- 36. Neuhaus J, Weimann A, Stolzenburg JU, et al. Histamine receptors in human detrusor smooth muscle cells: physiological properties and immunohistochemical representation of subtypes. World J Urol. 2006;24:202–209 [DOI] [PubMed] [Google Scholar]
- 37. Nakaya M, Takeuchi N, Kondo K. Immunohistochemical localization of histamine receptor subtypes in human inferior turbinates. Ann Otol Rhinol Laryngol. 2004;113:552–557 [DOI] [PubMed] [Google Scholar]
- 38. Bielory L, Ghafoor S. Histamine receptors and the conjunctiva. Curr Opin Allergy Clin Immunol. 2005;5:437–440 [DOI] [PubMed] [Google Scholar]
- 39. Leurs R, Bakker RA, Timmerman H, et al. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov. 2005;4:107–120 [DOI] [PubMed] [Google Scholar]