Abstract
An initial step in platelet shape change is disassembly of actin filaments, which are then reorganized into new actin structures, including filopodia and lamellipodia. This disassembly is thought to be mediated primarily by gelsolin, an abundant actin filament-severing protein in platelets. Shape change is inhibited by VASP, another abundant actin-binding protein. Paradoxically, in vitro VASP enhances formation of actin filaments and bundles them, activities that would be expected to increase shape change, not inhibit it. We hypothesized that VASP might inhibit shape change by stabilizing filaments and preventing their disassembly by gelsolin. Such activity would explain VASP’s known physiological role. Here, we test this hypothesis in vitro using either purified recombinant or endogenous platelet VASP by fluorescence microscopy and biochemical assays. VASP inhibited gelsolin’s ability to disassemble actin filaments in a dose-dependent fashion. Inhibition was detectable at the low VASP:actin ratio found inside the platelet (1:40 VASP:actin). Gelsolin bound to VASP-actin filaments at least as well as to actin alone. VASP inhibited gelsolin-induced nucleation at higher concentrations (1:5 VASP:actin ratios). VASP’s affinity for actin (Kd ~ 0.07 μM) and its ability to promote polymerization (1:20 VASP actin ratio) were greater with Ca++-actin than with Mg++-actin (Kd ~ 1 μM and 1:1 VASP), regardless of the presence of gelsolin. By immunofluorescence, VASP and gelsolin co-localized in the filopodia and lamellipodia of platelets spreading on glass, suggesting that these in vitro interactions could take place within the cell as well. We conclude that VASP stabilizes actin filaments to the severing effects of gelsolin but does not inhibit gelsolin from binding to the filaments. These results suggest a new concept for actin dynamics inside cells: that bundling proteins protect the actin superstructure from disassembly by severing, thereby preserving the integrity of the cytoskeleton.
Keywords: VASP, gelsolin, severing, actin polymerization, poly-L-proline, platelet activation and shape change
INTRODUCTION
During activation, platelets undergo a series of shape changes, first rounding, then projecting filopodia, and finally spreading [Allen et al., 1979]. This shape change apparently begins with disassembly of the existing actin filament network followed by reorganization of the actin into new structures in different locations within the cell [Bearer, 1991, 1995; Hartwig, 1999; Fox and Philips, 1983; Nachmias and Yoshida, 1988]. This actin reorganization is regulated by the interplay between many different actin-binding proteins, of which gelsolin and VASP are among the most abundant [Hartwig, 1999; Laurent et al., 1999]. Although platelets are much smaller than fibroblasts (5–10-fold smaller), fluorescence microscopy has been successful in imaging the actin structures that form in the activated platelet and in identifying specific actin binding proteins localized to these structures [Nachmias and Golla, 1991; Reinhard et al., 1992; Bearer, 1995]. We initially discovered a 46–50 kDa protein doublet that was retained by F-actin columns from PG-E1-inhibited platelets [Bearer, 1995]. Both 46–50 kDa species were subsequently recognized as VASP upon sequencing [Haffner et al., 1995]. VASP localizes to the adhesion plaques in platelets by immunofluorescence [Reinhard et al., 1992; Bearer, 1995], but its distribution during the early stages of spreading has not been reported. Gelsolin has been detected by immunogold electronmicroscopy at the barbed ends of actin filaments [Hartwig, 1992], but its distribution throughout the spread platelet by immunofluorescence has also not been reported.
Gelsolin is thought to be the major mediator of actin filament disassembly during rounding, the first step of shape change in platelets [Hartwig, 1992; Hartwig et al., 1995]. Gelsolin-deficient platelets fail to round, which is a defect attributed to the loss of severing activity [Witke et al., 1995]. Overexpression of gelsolin increases cell migration rates [Cunningham et al., 1991]. In vitro, gelsolin disassembles actin filaments by two mechanisms: it severs them and it caps the fast-growing barbed end [Kwiatkowski, 1999; Stossel, 1994]. Severing can be readily imaged by fluorescence microscopy of rhodamine-phalloidin-labeled actin filaments [Bearer, 1991]. After severing, gelsolin remains bound to the barbed end, preventing re-polymerization by addition of monomer to this fast-growing end of the filament [Yin and Stossel, 1979; Stossel, 1994; Kwiatkowski, 1999]. Filaments capped at the barbed end rapidly shorten if the concentration of actin monomer is lower than the critical concentration for pointed end assembly. Thus, at low actin concentrations, barbed end capping results in loss of monomer from the pointed end.
VASP has effects opposite to those of gelsolin in cells. Platelets from VASP-deficient mice are more easily activated than normal [Aszodi et al., 1999; Hauser et al., 1999] and VASP-deficient fibroblasts migrate more quickly [Bear et al., 2000]. Consistent with this, over-expression of VASP causes slower migration rates [Bear et al., 2000]. Paradoxically, in vitro VASP induces actin polymerization and bundles filaments [Manchester et al., 1998; Bachmann et al., 1999; Huttelmaier et al., 1999; Laurent et al., 1999]. These activities should enhance platelet shape change and cell motility, not inhibit it.
We reasoned that VASP’s inhibition of migration and shape change would be explained if VASP prevented severing and/or depolymerization of actin filaments, a necessary step in actin reorganization [Hartwig, 1999]. In this study, we tested this hypothesis in vitro. Using fluorescence microscopy, pyrene fluorescence, and sedimentation assays, we analyzed the effect of VASP on gelsolin disassembly and nucleation of actin filaments under conditions favorable for gelsolin. We purified VASP from platelets and from recombinant sources with new protocols that allowed us to obtain large amounts of VASP in a single phosphorylation state quickly. We focused on recombinant dephosphorylated VASP because we could obtain it as a homogeneous preparation, whereas VASP purified from platelets or phosphorylated in vitro contains varying ratios of different phosphorylation states [Halbrugge and Walter, 1989; Halbrugge et al., 1990; Butt et al., 1994]. Assays were conducted with Ca++-actin under physiologic conditions most favorable for gelsolin activities. As we initially reported [Manchester et al., 1998], these conditions, different from those others reported [Bachmann et al., 1999; Huttelmaier et al., 1999; Laurent et al., 1999], also enhanced the activity of VASP. We used quantitative sedimentation assays to analyze binding of gelsolin and VASP to actin filaments. As a first step in determining whether these effects of VASP on gelsolin in vitro are indicative of VASP’s physiological role inside the cell, we studied the relative localization of these two proteins inside the spread platelet using double-label immunofluorescence.
This paper addresses the question of how VASP might inhibit platelet shape change while promoting actin polymerization in vitro. Our results lead us to propose a new concept for actin reorganizations: that bundling by such proteins as VASP regulates the effect of severing on the integrity of the actin filament network. This concept proposes a yin-yang relationship between bundling and severing and is likely to apply not only to VASP-gelsolin, but to other pairs of actin-crosslinking and severing proteins.
MATERIALS AND METHODS
Purification of VASP From Human Platelets on Poly-L-Proline Columns
VASP was purified in a new two-step procedure using poly-L-proline affinity chromatography from thrombin-activated human blood platelets (Fig. 1A). Platelet-rich plasma (PRP) was obtained from the Rhode Island Blood Bank within 24 h of outdating, i.e., 6 days after donation. Platelets were washed [Bearer, 1995] and incubated in 10 ml/U of “incubation buffer” (145 mM NaCl, 5 mM KCl, 10 mM HEPES (pH 7.4), 2 mM MgCl2, 0.5 mM Na2HPO4 per Hartwig et al. [1995]) for 1 h at 37°C. To obtain activated platelets, 1 U/ml of thrombin (Sigma Chemical Co., St. Louis, MO) was added 15 s prior to lysis. Lysis was achieved by adding an equal volume of ice cold lysis buffer (2×: 120 mM Pipes, pH 6.5, 50 mM Hepes, pH 7.0, 20 mM EGTA, 4 mM MgCl2, 10 mM glucose 1.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 1/100 dilution of pro-tease inhibitor cocktail) [Bearer, 1995], followed immediately by sonication with two 10 s burst using the microtip of a Branson sonicator.
Fig. 1.
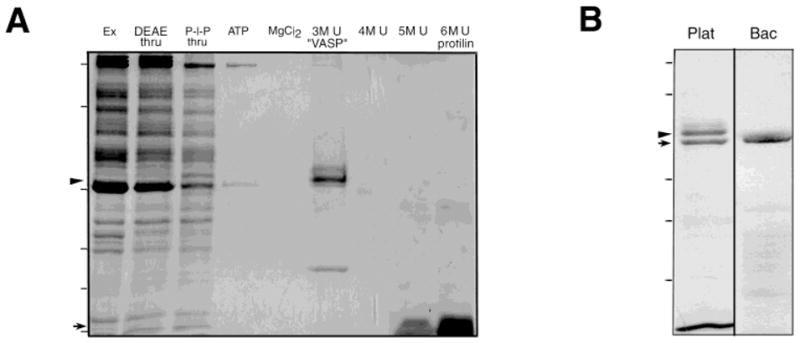
Purification of VASP. A: Poly-L-proline purification of platelet VASP. Coomassie-stained 12% SDS-PAGE gel of a typical purification. Thrombin-activated platelet extract (Ex), was passed through a DEAE column (DEAE thru) and then loaded onto a poly-L-proline column. The poly-L-proline column was eluted with 5 mM ATP (ATP), 0.3 M MgCl2 (MgCl2), and then a stepwise gradient of 3, 4, 5, 6 M urea (lanes: 3M U “VASP”, 4M U, 5M U, and 6M U, respectively). VASP (46/50 kDa doublet, indicated by arrowhead at the left of the gel) elutes with 3M urea, while the bulk of the profilin elutes with 6 M urea (14 kDa, arrow at left of gel). Molecular weight markers indicated by dashes on the left: 200, 98, 45, 31, 21, 14 kDa. B: Purified platelet VASP compared to recombinant human VASP. Coomassie-stained 15% SDS-PAGE gel showing a distinct doublet in the purified platelet VASP (Plat) but a single band in a preparation of an equivalent amount of recombinant VASP (Bac). Peptide sequence of both bands in the platelet preparation demonstrate they contain VASP. The Ser 157 phosphorylated form (arrowhead) migrates at 50 kDa while dephosphoVASP migrates at 46 kDa (arrow). Molecular weight standards 98, 68, 31, 21, and 14 kDa indicated by dashes on the left.
Lysates from 4 U of PRP (~ 5.5 × 1010 platelets per unit) were centrifuged at 10,000g for 15 min, 4°C, the supernatant brought to 5 mM DTT and loaded onto a 10-ml Whatman DEAE anion exchange column pre-equilibrated in Column Buffer (CB), a 1:1 mixture of lysis and incubation buffers. The flow through, known to be enriched in profilin in other cell types [Machesky et al., 1994], was then divided and passed in parallel over either a poly-L-proline-coupled Sepharose column or a control uncoupled Sepharose column using approximately 2 U of PRP for each 5-ml column. Poly-L-proline columns were prepared as previously described [Lindberg et al., 1988; Kaiser et al., 1989]. Columns were washed first with CB with 5 mM Mg-ATP in lysis buffer followed by elution in 0.3 M MgCl2, 1 mM EGTA, 50 mM Tris (pH 7.4) and 100 mM NaCl, described to release filamentous actin from poly-L-proline beads [Lind et al., 1987]. Poly-L-proline and sepharose columns were restored after stripping with 9 M urea, followed by washing with lysis buffer and stored in lysis buffer with 0.5% azide. DEAE columns were stripped with 0.6 M KCl and re-equilibrated for re-use. Columns were re-used a maximum of four times. We also attempted to avoid urea by eluting VASP with DMSO from the poly-L-proline columns as has been successful for the purification of profilin [Lindberg et al., 1988], but this did not apply for VASP.
VASP, profilin, and Grb2 were simultaneously eluted with 6 M Urea in 50 mM Tris-HCl (pH 7.4) and identified by microsequencing of each band performed as we described [Medeiros et al, 1998; Bearer, 2000]. At least two phosphorylated forms of VASP (50 and 46 kDa) [Halbrugge et al., 1990] were present. To separate VASP from profilin, columns were eluted in a stepwise gradient of 3, 4, 5, and 6 M Urea in 50 mM Tris-HCl (pH 7.4). The purification steps are shown in Figure 1A.
Proteins eluted with 3M urea were dialyzed against G-buffer (5 mM Tris-HCl (pH 8.1), 4 mM EGTA, 0.2 mM CaCl2, 0.2 mM ATP, 0.2 mM DTT, and 0.02% azide) for subsequent actin polymerization experiments, or boiled in gel sample buffer (GSB) for SDS-PAGE analysis. Aliquots were removed at each step in the procedure and the distribution of protein species monitored by SDS-PAGE.
Purification of Recombinant Human VASP
To obtain an active VASP in its native conformation and in a uniform phosphorylation state, his-tagged recombinant VASP was synthesized in bacteria and purified on nickel columns using an EDTA elution (Fig 1B, Bac) in Escherichia coli strain BL21(DE3)-pREP4 [Smolenski et al., 1998; Jurchau et al., 1998]. This strategy produced a VASP migrating as a single band on gels and blots with anti-VASP antibodies.
Bacteria were grown overnight (1 L modified 2X YT), induced at an optical density of 0.6–0.7 with 2 mM IPTG for 7 h at RT. Bacteria were harvested by centrifugation and resuspended in 20 ml lysis buffer (50 mM (pH 8.0), 0.5 mM EDTA, 50 mM NaCl, 5% NaPO4 glycerol, 1 mM PMSF, 1 mg/ml lysozyme (Sigma), 1/100 protease inhibitor cocktail [Bearer, 1995]. To solubilize the bacterially expressed VASP, the suspension was frozen in liquid nitrogen and thawed under warm tap water. DNase I (5 mg/ml) and 10 mg/ml RNase (Sigma) were added and the mixture sonicated four times in a Branson sonifier 4 times for 20 sec each burst on ice. Solution was brought to 15 mM imidazole and 0.5% Triton X-100 and centrifuged at 100,000g for 10 min in 42.1 rotor at 33,000 rpm in Beckman ultracentrifuge. The supernatant was removed and brought to 300 mM NaCl.
To purify the bacterially expressed VASP, 4 ml of nickel beads (Qiagen, Valencia, CA) were equilibrated in wash buffer (50 mM NaPO4 (pH 8.0), 300 mM NaCl, 50 mM imidazole (pH 8.0), 0.5% Triton X-100, 1:100 dilution of protease inhibitor cocktail) was added to the suspension and incubated for 1 h at 4°C on a rocker. Beads were collected and washed in five column volumes of wash buffer and incubated for 10 min in 1 column volume (3 ml) of elution buffer (50 mM NaPO4 (pH 7.2), 300 mM NaCl, 100 mM EDTA). The typical yield of VASP from 1 liter of bacteria was 3 mg in 3 ml. Eluate was dialyzed ON at 4°C in 25 mM Hepes (pH 7.4), 75 mM NaCl, 5% glycerol. The final VASP concentration was 1 mg/ml or 20 μM.
Purification of Gelsolin
Gelsolin was purified as described [Kurokawa et al., 1990, with modification per Wen et al., 1996].
Microscopy of Actin Filaments
Rhodamine-phalloidin actin filaments were prepared and imaged as previously described [Bearer, 1991]. Labeled filaments (1/50 dilution of the stock) were incubated in a 1.5-ml Eppendorf and VASP added at either 200 nM or 1.6 nM. After 15–30 min incubation at RT, filaments were either imaged immediately by perfusion into the coverslip chamber, or gelsolin (20 nM) was added and the mixture perfused. Micrographs on TMax ASA 3200 film were taken within 20 min of perfusion. For gelsolin experiments, images were photographed as rapidly as possible (20-s exposures). However, even after an hour, no change in VASP-actin bundles was noted in the presence of gelsolin.
Sedimentation Assays
Polymerized actin at steady state was collected by centrifugation (150,000g for 1 h at 4°C) in a TL100 centrifuge. Supernatants were removed and proteins in them precipitated with 10% trichloroacetic acid. Equivalent amounts of supernatants and pellets were analyzed by SDS-PAGE and Western blot.
Antibodies
Anti-VASP antibodies were generated in rabbits to the peptide KEEIIEAFVQELR according to standard techniques (Berkeley Antibody Company/Covance, Berkeley, CA). Additional VASP antibodies were obtained from Alexis Corp (San Diego, CA). Anti-gelsolin monoclonal antibody was from Sigma. In Western blots, antibody-stained bands were imaged with anti-rabbit-HRP secondary antibodies (Boehringer-Mannheim) followed by chemiluminescence (Tropix, Bedford, MA).
Pyrene Actin Assays of Actin Polymerization
Actin was purified from rabbit skeletal muscle [Pardee and Spudich, 1982] and gel filtered over Sephadex G75 or G200 prior to use to remove contaminating capping proteins or actin oligomers [Gieselmann et al., 1995]. Actin was labeled with pyrene as described previously [Kouyama and Mihashi, 1981; Lamb et al., 1993] resulting in a labeling of 60% based on extinction coefficient. Both labeled and unlabeled actin preparations were stored at −80°C in G-buffer (5 mM Tris-HCl (pH 8.1), 0.2 mM CaCl2, 0.2 mM ATP, 0.2 mM DTT, 0.02% NaN3). Prior to each experiment, aliquots were thawed, diluted, and mixed to give a final concentration of 1 mg/ml 15% pyrene labeled in G-buffer. Actin was then spun for 1 h at 150,000g in TL100 at 4°C to remove oligomers. VASP and gelsolin were also subjected to the same clarifying centrifugation after thawing before polymerization assays.
Polymerization was measured by pyrene fluorescence with excitation at 365 and emission 386 nm in a Perkin Elmer Luminescence Spectrometer Model LS50B. Culture tubes (Kimble-Kimax borosilicate glass 60 × 50 mm, VWR) were prepared with G-buffer calculated to give a final volume of 300 μL after addition of protein and polymerization buffer. Monomeric actin was added first, then aliquots of VASP and/or other proteins, and the mixture vortexed. After measuring the baseline, 15 μL of 20× salt was added (20× salt: 40 mM MgCl2, 3 M KCl, 200 mM Tris, pH 7.8) and polymerization monitored at 1-s intervals in the fluorimeter for 20 min at RT. Controls of actin alone, storage buffer alone, or preparations of bacterial extract that mimicked the “purification” but from strains containing the plasmid lacking the VASP insert, demonstrated no increased activity. Experiments with Mg++-actin were performed as described [Laurent et al., 1999].
Data was collected on a Dell computer, transferred to a PowerMac, and graphs generated in MS-Excel or in Kaleidograph for analysis and to generate graphs for publication. Fluorescence change is presented in arbitrary units (a.u.) as is standard in the field.
Immunofluorescence
Platelet-rich plasma was prepared from blood drawn from healthy volunteers and used on the day of draw. Platelets were spread on glass, fixed, and stained for immunofluorescence as previously described [Bearer, 1995]. We used a fixation containing detergent and phalloidin to prevent cross-linking of soluble proteins to the actin filaments, an artifact that has been suggested as leading to previous conflicting results when staining for gelsolin [Cooper et al., 1988]. That the gelsolin staining is only coincident with a subset of filaments (which we also stained in separate experiments with phalloidin), does not stain stress fibers or overlap with VASP, indicates that such fixation artifact has not occurred here. Double-labeling with VASP and gelsolin used a FITC-conjugated anti-rabbit secondary antibody and a TRIC-conjugated anti-mouse secondary antibody (Boehringer-Mannheim, Roche Diagnostics, Indianapolis, IN). Images were collected either separately with an analog camera onto black and white 35-mm TMax 400 film or digitally with a single filter cube with a Spot, RT slider camera from Diagnostics, Inc. (purchased from MVI, Avon, MA). Images were processed for publication with Adobe Photoshop.
RESULTS
VASP-Bundled Actin Filaments Are Resistant to Gelsolin
Long thick bundles of actin filaments induced by VASP can be observed by fluorescence microscopy of rhodamine-phalloidin-labeled actin incubated with increasing amounts of VASP (Fig. 2, left panels). At low concentrations of VASP (1:20 VASP:actin ratio), isolated bundles appear in a background of fine filaments, suggesting that VASP binding and bundling is cooperative. At high concentrations of VASP (1:2 VASP:actin ratio), only bundles of filaments are found. The thickness of the bundles remained constant regardless of the concentration of VASP. No sheets of filaments were found.
Fig. 2.
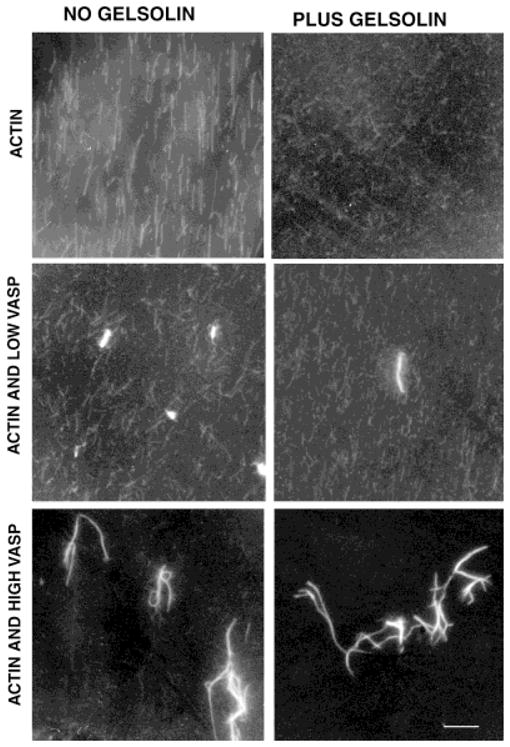
VASP-actin bundles resist gelsolin severing. Actin filaments (10 μM) were labeled with rhodamine phalloidin, imaged by fluorescence microscopy and photographed on standard film. Addition of low VASP concentrations (1:20 VASP to actin ratios) results in formation of some bundles of brighter, thicker filaments, even after short (1–2 min) incubations, while many finer filaments were also present. High concentrations of VASP (1:2 VASP to actin ratios) results in only large, thick, bright bundles and no fine individual filaments. Gelsolin (20 nM) rapidly severs naked actin filaments as previously reported [Bearer, 1991], including the individual filaments in preparations containing VASP, but gelsolin has no detectable effect on the VASP-actin bundles. Bar = 10 μm.
Gelsolin rapidly severs individual filaments whether or not VASP is present, but gelsolin does not detectably affect the VASP-induced bundles (Fig. 2, right panels). As previously reported [Bearer, 1991], gelsolin causes rapid disappearance of individual rhodamine-phalloidin-labeled actin filaments. The individual filaments present at low VASP concentrations are rapidly severed by gelsolin as they are in preparations containing only actin. In contrast, the bundles appear unaffected by gelsolin: no decrease in their number, size, or brightness can be detected. No severed filament ends were seen to detach along the length of the bundle even at low VASP concentration. Increasing the amount of VASP results in fewer gelsolin-sensitive fine filaments in the background and more gelsolin-resistant bundles.
Gelsolin Binds But Does Not Disassemble VASP-Actin Filaments
We used quantitative sedimentation assays of actin filaments at steady state to measure gelsolin’s binding to and disassembly of VASP-actin filaments.
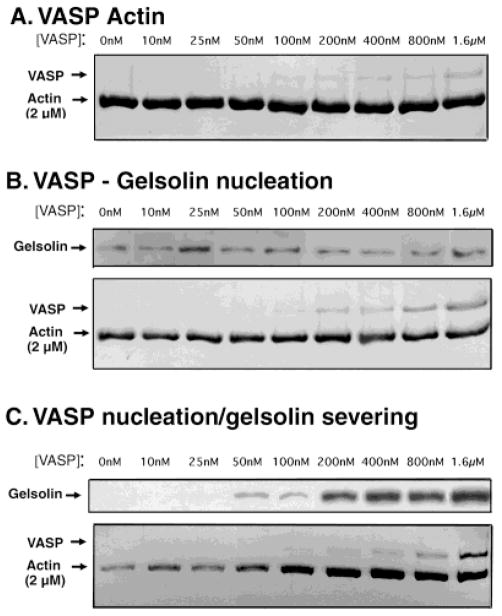
As expected, control experiments demonstrate that VASP binds to Ca++-actin filaments (Fig. 3A). Filaments were polymerized in the presence of increasing amounts of VASP, allowed to reach steady state, and collected by high-speed centrifugation. Analysis of the pellets by SDS-PAGE demonstrates that VASP co-sediments with filamentous (F) actin. Quantitative analysis of binding constants was performed by scanning similar gels of supernatants as well as of pellets from these experiments. We determined that 80% of the actin (2 μM) sedimented as filaments and 67% of the VASP was bound to it. This correlates to an apparent equilibrium dissociation constant (Kd) of ~ 0.25 μM, fourfold higher than reported for Mg++-actin [Laurent et al., 1999), indicating that dephosphoVASP binds more tightly to Ca++-actin than Mg++-actin. We confirmed that our recombinant dephosphoVASP behaved as previously reported for platelet VASP with Mg++-actin, with an apparent Kd ≈ 1 μM (for equations and a comparison of binding constants, see Table I). The maximum concentration of VASP possible was 1.6 μM because of solubility and the procedure for the assay.
Fig. 3.
VASP-stabilized filament networks resist gelsolin disassembly. A: VASP binds F-actin. Actin filaments (2 μM) polymerized in the presence of increasing amounts of recombinant VASP (as indicated) were collected by centrifugation and the pellet analyzed by Coomassie-stained SDS-PAGE as shown here. Note that the amount of actin in the pellet is not detectably affected by the amount of VASP. The amount of VASP in the pellet increases with the amount of VASP added. Supernatants were analyzed by SDS-PAGE in parallel (not shown). B: Actin filaments bind both VASP and gelsolin during polymerization. Actin polymerized with both gelsolin (20 nM) and increasing amounts of VASP (as indicated) were collected by centrifugation and the pellets analyzed for gelsolin by Western blot and for VASP and actin by Coomassie-stained gel. Note that more VASP is bound to less actin in the presence of gelsolin (compare the VASP bands in 3A with those in 3B). C: VASP-actin filaments bind gelsolin but are not solubilized by gelsolin. Actin filaments (2 μM) polymerized with increasing amounts of VASP (as indicated) were allowed to reach steady state and then treated with gelsolin (20 nM) under conditions in which severing occurs. The remaining intact filaments were collected by centrifugation and the pellet analyzed as before for gelsolin, actin, and VASP. Note that actin in the pellet increases with increasing amounts of VASP. Furthermore, increasing VASP also increases the amount of gelsolin in the pellet.
TABLE I.
Comparison of VASP-Actin Interactions Under Different Conditions*
| VASP:actin ratio for maximal effect on polymerization | Inhibition by 100 mM KCl | Kd for F-actin (μM) | |
|---|---|---|---|
| DephosphoVASP | |||
| Ca++-actin | 1:20 | No | 0.25 |
| Mg++-actina | 1:1 | Yes | 1.0 |
| Ca++-actin with gelsolin | 1:5b | No | 0.07 |
| PhosphoVASP | |||
| Mg++-actinc | 1:1 | Yes | 0.05 |
Dissociation constants were calculated on the assumption that one VASP binds one actin monomer according to the equation: Kd = [free F-actin] × [free VASP]/[VASP-F-actin] where free F-actin represents unliganded F-actin subunits, derived from the difference between total F-actin and the amount of F-actin bound to VASP, measured in the pellet. Free VASP was determined from scans of the supernatants.
Measured both in this study and in Laurent et al. [1999].
VASP’s effect on gelsolin polymerization (−) is to repress the rate of assembly.
Measured using a mixed population of phosphorylation states in Laurent et al. [1999].
Gelsolin binds but does not disassemble VASP-actin bundles over a wide range of VASP concentrations (Fig. 3B,C). We performed experiments with gelsolin in two ways: (1) actin was polymerized in the presence of both gelsolin and VASP, a situation in which gelsolin primarily binds to the barbed ends of the nascent filaments (Fig. 3B); or (2) actin was polymerized only with VASP, and then gelsolin was added later, after polymerization had reached steady state (Fig. 3C). In this second situation, gelsolin is expected to bind the sides of filaments and sever them. We kept the gelsolin concentration constant and tested its activity over a wide range of VASP concentrations. The results were analyzed by collecting filaments by high-speed centrifugation followed by analysis of VASP and actin by SDS-PAGE and of gelsolin by Western blot analysis of supernatants and pellets.
When actin is polymerized in the presence of gelsolin, gelsolin is expected to bind to the barbed ends of the filaments. Under these conditions, a portion of the gelsolin is found in the high-speed pellet along with the filamentous actin (Fig. 3B). Addition of VASP together with the gelsolin at the start of polymerization does not appreciably affect the amount of gelsolin co-sedimenting with actin in the pellet, which remains constant over a wide range of VASP concentrations (0.05–1.6 μM). VASP also binds these filaments. The ratio of VASP to actin in the pellet in the presence of gelsolin is higher than for VASP alone, although as expected there is less actin in the presence of gelsolin (see below). Control experiments show that VASP and gelsolin do not sediment either alone or together in the absence of F-actin (not shown). These results show that VASP does not inhibit gelsolin from binding to the ends of actin filaments.
Gelsolin-nucleated filaments may have a higher affinity for VASP. As expected, gelsolin causes a decrease of 30% in the final F-actin concentration at steady state compared to F-actin alone (compare the left lanes of Fig. 3A with the left lanes in Fig. 3B). However, a high proportion, 67%, of the total VASP is bound to this lesser amount of F-actin, giving a fourfold lower apparent Kd for VASP-actin in the presence of gelsolin (<0.07 μM) than for VASP-actin alone (~ 0.25 M). Indeed, gelsolin-nucleated filaments have been reported to have a different conformation than actin alone, which could alter binding affinities [Orlova et al., 1995].
In the second set of experiments, actin was first polymerized in the presence of VASP, followed by addition of gelsolin after polymerization had reached steady state (Fig. 3C). With this sequence of events, gelsolin is expected to disassemble the pre-formed filaments. VASP protected the filaments from this disassembly, as the increasing amount of actin in the pellet shows. This increase in F-actin was directly proportional to the amount of VASP after treatment with gelsolin (Fig. 3C). As the VASP:actin ratio approaches 1:1, the amount of actin in the pellet reaches the amount observed without gelsolin (compare the actin bands in the right lanes in Fig. 3A with the right lanes in Fig. 3C).
VASP does not inhibit gelsolin from binding to actin filaments. In fact, more gelsolin sediments with VASP-actin than with actin alone. Surprisingly, the amount of gelsolin in the pellet increases as a function of total VASP (Fig. 3C). Even at a very low VASP:actin ratio (1:40 VASP:actin), slightly more gelsolin binds to VASP-actin filaments than to actin alone. Thus, VASP does not inhibit gelsolin from binding to these filaments but may even increase gelsolin’s affinity for actin. Because of gelsolin’s ability to bind actin monomer [Bryan, 1988], its affinity for actin cannot be determined precisely based on these experiments.
VASP Affects Gelsolin Nucleation
In addition to severing filaments, gelsolin also nucleates actin filament assembly [Bryan, 1988; Stossel, 1994; Kwiatkowski, 1999]. This activity is quantitatively measured by the pyrene-actin assay of polymerization. To study the combined effects of VASP and gelsolin on actin polymerization in vitro, we used Ca++-actin in physiological salt because these are the conditions in which gelsolin is most active [Yin and Stossel, 1979]. These conditions are quite different from those previously reported for VASP nucleation [Bachmann et al., 1999; Huttelmaier et al., 1999; Laurent et al., 1999].
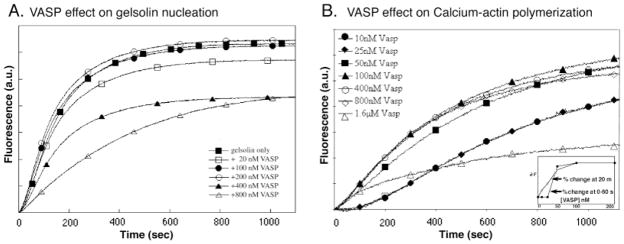
At 1:5 molar ratio with actin, VASP depresses the rate of gelsolin-induced nucleation (Fig. 4A). But gelsolin-nucleated polymerization is unaffected by VASP at concentrations of VASP (50–200 nM, 1:40–1:10 VASP: actin) that have maximal effects on actin alone (Fig. 4B). At higher concentration (>0.4 μM), as for VASP alone, the rate of polymerization is significantly depressed.
Fig. 4.
VASP depresses gelsolin nucleation. A: VASP, at concentrations as indicated, was added at the same time as gelsolin to pyrenyl Ca++-actin (2 μM, 15% labeled). Polymerization was initiated by addition of salt. Change in fluorescence was measured over time (a.u.: arbitrary units). B: VASP on its own at concentrations of 50 nM (filled squares) and higher increased the initial rate of polymerization. VASP alone, at various concentrations as indicated, was added to pyrenyl Ca++-actin (2 μM, 15% labeled) in G-buffer and change in fluorescence monitored over time. Note that at 1.6 μM (open triangles), VASP increased the initial rate, but slowed the elongation rate such that the amount of polymerization after 10 min was less than with actin alone. The curve obtained for 10 and 25 nM VASP are super-imposable (filled circles and filled diamonds). (a.u.: arbitrary units). Inset: A graph of the relationship of per centile change in fluorescence with VASP concentration at 0–60 sec (black squares) and at 20 min (open squares).VASP does not affect gelsolin nucleation.
This effect on gelsolin nucleation was not due to the conditions of the assay. In fact, VASP enhances Ca++- actin polymerization even better than it enhances polymerization of Mg++- actin (Fig. 4) [Manchester et al., 1998; reviewed in Machesky, 2000]. Low concentrations of VASP (1:40 VASP to actin ratios) enhance polymerization of Ca++-actin in physiological salt, with maximal activity at a 1:20 VASP to actin ratio (100 nM VASP) (Fig. 4A). Higher concentrations of VASP (up to 800 nM) have only slightly greater effects. VASP markedly increases the rate of polymerization during the early lag phase (60–100 sec), and also produces small increases in the final concentration of filaments at 20 min. At high concentrations (1.6 μM), VASP’s effect on the early lag phase is indistinguishable from that at 100–800 nM, but the polymerization (elongation) rate at later time points is slowed to below that of actin alone.
A plot of fluorescence change vs. VASP concentration (Fig. 4B, inset) shows that during the initial phases of polymerization (20 sec), VASP has an all-or-none effect on Ca++-actin, consistent with highly cooperative binding to actin. At later time points, the data give a high affinity titration curve. We obtained similar although not identical results with VASP purified from platelets [Manchester et al., 1997] (data not shown). These results are different from previous reports (see Table I for comparisons).
To test whether this higher affinity and stronger activity was due to the conditions of the assay or to our preparation of VASP, we analyzed our VASP preparation for activity with Mg++-actin under reported conditions [Laurent et al., 1999]. We also found lower VASP activity with Mg++-actin (data not shown). A 20-fold larger amount of VASP is required for the maximal activity with Mg++-actin as compared to Ca++-actin and physiological salt inhibits VASP activity with Mg++-actin but not with Ca++-actin (data not shown). Thus, our procedure represents more optimal conditions for VASP activity. Furthermore, these results suggest that small divalent cations may affect VASP-actin interactions.
VASP and Gelsolin Co-Localize in Filopodia and at the Leading Edge
If VASP affects gelsolin’s activity in cells, then VASP must be in the same place at the same time as gelsolin. As a first step in determining potential interactions, we used double label immunofluorescence to examine whether the distribution of VASP and gelsolin coincided in the same cell at two stages of platelet activation: filopodial projection and spreading. These are major foci of actin reorganizations in the platelet.
VASP and gelsolin co-localize in the filopodia and in the leading edge of the platelet during spreading (Fig. 5). In the filopodia that form early in activation, gelsolin appears slightly more concentrated at the tips while VASP is more concentrated along the lengths of the bundles (Fig. 5A, left panels, arrows). Filopodia are difficult to image by fluorescence microscopy as they occur in suspension and are three-dimensional.
Fig. 5.
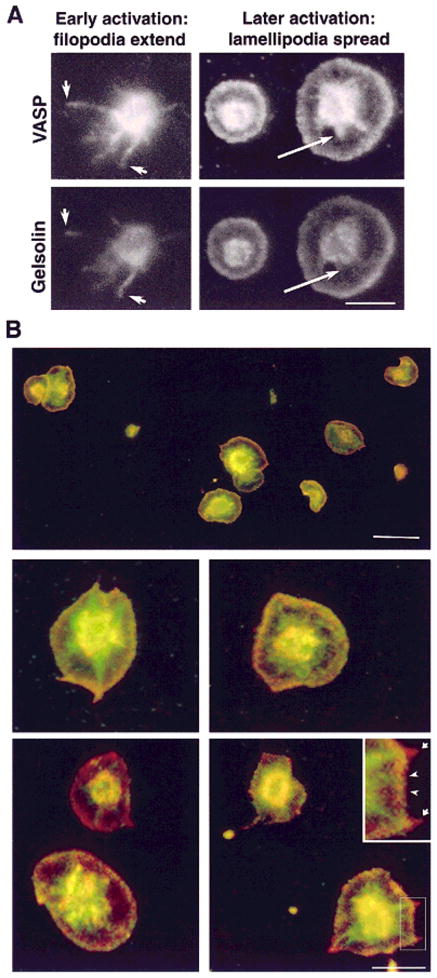
Gelsolin and VASP colocalize in filopodia and lamellipodia but not in stress fibers in the spread platelet. A: Filopodia extend early in platelet activation. Two representative platelets imaged for VASP (top) and gelsolin (bottom) at early and late stages of spreading. There is significant but not identical overlap of staining patterns. Gelsolin staining is more pronounced at the tips of filopodia (left panels, arrows) and VASP staining is brighter in the proximal arm, although their three-dimensional shape makes imaging difficult. Later in activation (right panels), lamellipodia spread between filopodia on the glass surface and are relatively easy to observe. Both VASP and gelsolin are at the edge of the lamella, whereas only VASP staining appears more centrally in the nascent adhesion plaques (right panel, arrows) and stress fibers. Bar = 5 μm. B: Double-label of spread platelets stained for both VASP and gelsolin. Virtually all spread platelets display similar morphology and staining pattern: VASP (green) appears in the nascent adhesion plaques, while gelsolin (red) appears at the tips of former filopodia (see inset, arrows). Both proteins are present at the edge of the lamellipodia (appearing yellow; inset, arrowheads) and in the central dome of cytoplasm. Bar (top) = 10 μm, (bottom) = 5 μm.
As spreading proceeds, a lamellipodia advances between the filopodia, along the coverslip surface with a frill of actin filaments at its outermost edge. Both gelsolin and VASP are present at the edge of this lamellipodia (Fig. 5A, right panels). Both VASP and gelsolin are also concentrated in the dome of cytoplasm, the hyalomere that contains the bulk of the soluble components, granules, and other organelles. This may represent a soluble component. As spreading continues, adhesion plaques and stress-like fibers composed of actin bundles appear centrally. VASP but not gelsolin is found in these stress fibers (Fig. 5A, right panels, arrows).
Double exposure to image both labels (Fig. 5B, top micrograph) demonstrates the uniformity of staining among a field of spread platelets. The overlap of VASP and gelsolin at the edge appears yellow, and the unique staining of stress-like fibers with VASP appears green. At higher magnification (Fig. 5B, lower panels and inset), gelsolin staining (red) remains at the tip of former filopodia while the proximal portion of what appears to be a continuous actin bundle stains only with VASP (green) (Fig. 5B).
DISCUSSION
A central question in platelet physiology is how actin is reorganized to mediate shape change. VASP and gelsolin are both involved in this process. VASP-deficient platelets activate more readily, suggesting that VASP inhibits actin reorganizations, although the molecular mechanisms of this inhibition are unknown. We hypothesized that VASP could inhibit shape change by preventing the disassembly of actin filaments present in the resting platelet. Severing of these filaments by gelsolin is thought to be primarily responsible for this disassembly [Witke et al., 1995; Hartwig, 1992, 1999; Stossel, 1994; Kwiatkowski, 1999]. We, therefore, tested whether VASP could inhibit gelsolin activity in vitro. We found that VASP inhibited the effect of gelsolin severing on actin filaments but did not inhibit gelsolin binding to these filaments. This inhibition of gelsolin was detected even at very low VASP concentrations. Thus, gelsolin binds but does not disassemble VASP-actin bundles. Co-localization of VASP and gelsolin during two stages of activation provides evidence that VASP may also prevent disassembly by gelsolin inside cells as well. Such an inhibitory role for VASP on severing would explain its known ability to restrain shape change and decrease cellular motility.
VASP Modulates Gelsolin’s Effect
By two independent approaches, we show here that VASP, even at low concentrations, protects filaments from disassembly by gelsolin. Our biochemical analysis shows that gelsolin can indeed bind to these VASP-stabilized filaments. Thus, VASP protection is not a result of competitive binding. However, by the assays we used, we cannot definitively know whether individual filaments in a bundle have actually been severed. Cross-linking could hold the bundle together even if the filaments are fragmented. Whether or not filaments in the bundle are actually severed in some places may not be functionally important, since by both structural and biochemical assays the bundles remain intact. Thus, even if gelsolin has severed the filaments within the bundle, the integrity of the actin structure is not lost, and reorganization into another structure would be difficult. Furthermore, as long as gelsolin remains attached to the barbed ends, they cannot act as centers for additional polymerization.
Although we cannot determine definitively that VASP blocks severing, we predict that VASP induces a conformational change in the filaments that renders them resistant to breakage by gelsolin. We have previously reported that severing by gelsolin is readily observed by direct observation of rhodamine-phalloidin-labeled filaments [Bearer, 1991]. In these preparations at low VASP concentrations, we also readily observe severing of the individual filaments in the background. Detection of severing in this assay requires that the filament ends be free to move far enough apart for separate signals to be resolved. At low concentrations of VASP, there should be long lengths of filaments within a bundle that are not cross-linked and thus free enough to move apart from the bundle after they are severed. Thus, if filaments in a bundle were severed, we should have seen them detaching from the bundle and snaking along beside it. This was never seen. However, higher resolution will be required to test this further. Even with electron microscopy, it might be difficult to image single severing events within a bundle.
VASP increases the amount of actin filaments after gelsolin treatment. Under normal conditions, gelsolin causes a decrease in the amount of F-actin at steady state by capping the barbed end after severing the filament [Harris and Weeds, 1984]. Barbed end capping blocks treadmilling, a process that maintains actin filament length by coupling addition of monomer to the barbed end with loss of monomer from the pointed end. Thus, gelsolin-capped barbed ends cannot replace pointed end monomer loss, and filaments shorten. The increase in F-actin we observe after gelsolin severing would occur if VASP stabilized filaments and decreased pointed end off rate. This would be consistent with previous reports that VASP decreases the critical concentration of F-actin, suggesting it stabilizes filaments [Laurent et al., 1999].
Inhibition of Disassembly May Be a Common Theme for Bundling Proteins
VASP bundling activity is similar to that of the side-binding class of actin-binding proteins. These proteins stabilize individual filaments by binding along their lengths. They include high molecular weight tropomyosin and caldesmon, which also inhibit gelsolin severing [Dabrowska et al., 1996]. Unlike the small severing factors, cofilin and ADF, gelsolin severs phalloidin-stabilized filaments [Bearer, 1991; Verkhovsky et al., 1984; Allen and Janmey, 1996]. Thus, the ability of VASP to stabilize actin filaments in bundles that resist gelsolin could serve the valuable function of restraining gelsolin inside cells.
It will be of interest to determine whether other bundling proteins such as alpha-actinin also permit binding but not severing by gelsolin. Alpha-actinin is also found in platelets [Puszkin et al., 1985] and has long been known to bundle filaments [reviewed in Otto, 1994]. Alpha-actinin affects the activity of small severing factors like cofolin/actophorin. Actophorin promotes the formation of rigid actin bundles in the presence of alpha-actinin [Maciver et al., 1991].
It will also be interesting to test whether VASP inhibits severing by the small severing factors, since cofilin, is also found in platelets [Davidson and Haslam, 1994]. There exists some evidence for synergy between cofilin and bundling. Overexpression of cofilin in Dictyostelium stimulates actin bundle formation but the bundling protein responsible for this activity has not been identified [Aizawa et al., 1996]. Cofilin, like Vasp and alpha-actinin, is present in the actin tail induced by Listeria bacteria in platelet extracts [Theriot, 1997; David et al., 1998]. Of these, only cofilin is required for reconstituted bacterial motility in vitro [Loisel et al., 1999]. A VASP effect on cofilin activity could explain VASP’s enhancement of motility in these reconstitution assays.
VASP phosphorylation by antagonists of platelet activation was its original identifying feature [Halbrugge and Walter, 1989]. Three residues are phosphorylated in VASP, but the pattern of phosphorylation in platelets is not yet known [Butt et al., 1994; Smolenski et al., 1998]. Very recently, phosphorylation has been shown to affect VASP’s affinity for actin and its ability to enhance polymerization [Laurent et al., 1999; Lambrechts et al., 2000; Harbeck et al., 2000). It is attractive to speculate that phosphorylation on particular sites might regulate the relative stability of VASP-actin bundles against severing.
How Might VASP/Gelsolin Interactions Affect the Platelet Actin?
Biochemical in vitro studies always raise the question of whether activities measured in vitro are relevant to the cell. Several lines of evidence suggest that the VASP inhibition of gelsolin we report here may indeed be significant in platelet physiology. First, as described here, VASP and gelsolin co-localize in dynamic actin structures during platelet activation by immunofluorescence. Second, VASP and gelsolin have high relative concentrations in the platelet. Finally, VASP and gelsolin perform reciprocal functions in vivo. Each of these points is discussed in more detail below.
Co-localization by immunofluorescence is a first step in determining whether VASP could actually be present on a filament inside the platelet when gelsolin tries to sever it. Double-label immunogold electronmicroscopy will be needed to determine exactly where each of these proteins is in relationship to single filaments inside the cell. However, the actin bundles in platelets are large enough to suggest that the immunofluorescence results reported here represent the presence of both proteins in a single bundle [Bearer, 1990; Hartwig, 1992]. Platelets are anucleate and thus not amenable to transfection. Thus, there is no reliable method to observe these proteins in the living platelet. That gelsolin is indeed present in filopodia is supported by studies in fibroblasts and neurons from gelsolin-deficient mice. Filopodia are abnormally stable in growth cones from mice lacking gelsolin [Lu et al., 1997], suggesting that gelsolin is not only present there but required for normal filopodial dynamics.
The concentration of VASP that produced effects in vitro was similar to concentrations found in the platelet. More importantly, the ratios of VASP, gelsolin, and actin that had maximal effects in our experiments were also analogous to those ratios present in the platelet. Actin is 0.5 mM actin in platelets, 40% of which is polymerized into 1.1-μm-long filaments in the resting cell [Hartwig, 1999] while the remainder of the actin monomer pool is sequestered by thymosinβ4 [Nachmias et al., 1993]. Upon activation, the filaments are severed and their number increases while their length initially decreases. Both gelsolin and VASP are abundant in platelets, each at approximately 5 μM [Hartwig, 1999; Laurent et al., 1999] Thus, there is a 1:100 ratio with total actin, and a 1:40 ratio with F-actin in the resting cell [Hartwig, 1999; Laurent et al., 1999]. In our experiments, this ratio of VASP to actin (1:40) inhibited gelsolin severing and solubilization of F-actin. Using the dissociation constants derived here, we can calculate that 3.5 μM of F-actin would be bound to VASP in the resting cell. This would be a small proportion of the monomer in filaments. However, VASP-actin interactions are likely to be cooperative as our experiments suggest and as has been suggested for other actin bundling proteins [Sherman et al., 1999]. Thus, we would expect that VASP would be concentrated on a subset of filaments and thus bundle them. This is just as we have observed in vitro at low VASP concentrations where bundles appear in a background of individual filaments. Such a subset of bundled filaments in the platelet would be resistant to disassembly, and thus provide a stable actin network within the cell.
Elegant physiological studies in living cells support our hypothesis that VASP bundles and stabilizes actin filament in vivo. Overexpression of VASP/Mena in fibroblasts decreases cell motility [Bear et al., 2000]. Conversely, VASP-deficient platelets are more readily activated, and VASP-deficient fibroblasts migrate more quickly. That VASP and gelsolin activities are physiologically linked is also supported by reports that their expression is coordinated in endothelial cells during capillary morphogenesis [Salazar et al., 1999]. This type of coordinated expression of actin binding proteins within the cell is not unique to gelsolin and VASP, and has also been described between capping protein and gelsolin [Barkalow et al., 1996].
Listeria Motility as a Model for VASP Activity
The presence of VASP at the Listeria-actin interface and its ability to increase the speed of Listeria motility has lead to a variety of hypotheses linking VASP to actin polymerization [Chakraborty et al., 1995; Southwick and Purich, 1996; Purich and Southwick, 1997; Theriot, 1997; Niebuhr et al., 1997; Laurent et al., 1999; Loisel et al., 1999]. Such a role for VASP seems contradictory to the requirement for VASP in dNTP-mediated inhibition of platelet shape change [Aszodi et al., 1999; Hauser et al., 1999]. Our results show that VASP promotes stability by inhibiting filament disassembly in vitro. This activity would also make sense for its role in Listeria motility if it were not for the fact that VASP has only been detected at the bacterial-actin interface and not along the length of the actin tail. In light of the results we present, VASP distribution within the actin tail should be re-evaluated. Failure to detect VASP within the actin tail using immunofluorescence could be because the actin filaments in the tail are too tightly packed to allow the antibody to bind. Indeed, it was initially even difficult to label them with the S1 fragment of myosin, a 110 kD protein smaller than antibodies [Tilney et al, 1992; Zhukarev et al., 1995]. Alternatively, VASP may not serve to stabilize the Listeria-induced actin filaments in the tail, since alpha-actinin also found there could serve this role in this system [Dold et al., 1994; Nanavati et al., 1994].
How VASP Might Work Inside the Platelet
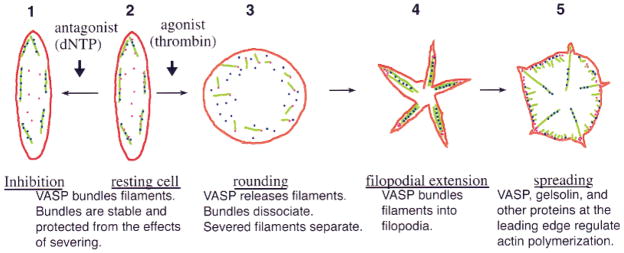
We propose a hypothetical scenario based on our results to show how VASP might inhibit actin reorganization in platelets (Fig. 6). In this scenario, VASP plays a dynamic role both in the resting cell and in shape change. This scenario is described in detail in the legend to Figure 6. In summary, our model proposes that VASP binds to and stabilizes actin filaments in the resting platelet, preventing them from being disassembled by severing. Upon activation, VASP releases the filaments, which are then rapidly severed, causing the platelet to lose its discoid shape and round up. As activation proceeds, VASP binds to new and severed filaments, reorganizing them into new bundles, leading to the formation of filopodia. Gelsolin remains associated with the filaments it has severed and thus remains present in these new filopodial bundles. As activation proceeds and the lamellipodia form, VASP and gelsolin, along with actin nucleators, such as kaptin [Bearer and Abraham, 1999] and Arp2 [Bearer, 1995; reviewed in Welch, 1999] also induce the formation of more new filaments at the membrane surface.
Fig. 6.
The cartoon diagrams how VASP might affect platelet actin dynamics based on our in vitro results. Interactions between VASP, gelsolin, and actin are shown during five stages of platelet shape change (numbered 1–5 in the diagram), from inhibition to spreading. In the untreated resting cell (stage 2), VASP (dark blue dots) binds actin filaments (green), bundling them and preventing severing by gelsolin (pink dots). Antagonists such as dNTP increase this inhibitory effect of VASP probably by phosphorylating it (stage 1). Upon agonist stimulation, VASP releases actin filaments (stage 3). As VASP-actin bundles come apart, severing proceeds and severed filaments separate. Gelsolin remains bound to the barbed ends of severed filaments, preventing growth by the addition of monomer to the barbed end. Filopodia form as VASP re-associates and bundles the new and severed filaments into new configurations. As spreading proceeds, VASP and gelsolin, together with other actin-binding proteins, participate in the ongoing actin reorganizations occurring at the leading edge (stage 5).
Acknowledgments
We acknowledge the technical contributions to this work by Becky Blumenthal and Steve Dunaway, under-graduate and graduate students at Brown University, respectively. Peptide sequences were obtained by Howard Jaffe, NINDS. T. Jarchau and U. Walter generously provided the his-tag VASP clone. VASP purification and nucleation activity was presented, in preliminary form, at the annual meetings of ASCB in 1997 and 1998. This work was supported in part by NIH GM47368 (E.L.B.), GM 57256 (P.A.), and Salomon Research Award from Brown University to E.L.B.
Contract grant sponsor: NIH; Contract grant numbers: GM 47368, GM 57256.
References
- Aizawa H, Sutoh K, Yahara I. Overexpression of cofilin stimulates bundling of actin filaments, membrane ruffling, and cell movement in Dictyostelium. J Cell Biol. 1996;132:335–344. doi: 10.1083/jcb.132.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PG, Janmey PA. Gelsolin displaces phalloidin from actin filaments: a new fluorescence method shows that both Ca2+ and Mg2+ affect the rate at which gelsolin severs F-actin. J Biol Chem. 1994;269:32916–32923. [PubMed] [Google Scholar]
- Allen RD, Zacharski LR, Widirstky ST, Rosenstein R, Zaitlin LM, Burgess DR. Transformation and motility of human platelets: details of the shape change and release reaction observed by optical and electron microscopy. J Cell Biol. 1979;83:126–142. doi: 10.1083/jcb.83.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszodi A, Pfeifer A, Ahmad M, Glauner M, Zhou XH, Ny L, Andersson KE, Kehrel B, Offermanns Fassler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999;18:37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–23557. doi: 10.1074/jbc.274.33.23549. [DOI] [PubMed] [Google Scholar]
- Barkalow K, Witke W, Kwiatkowski DJ, Hartwig J. Coordinated regulation of platelet actinfilament barbed ends by gelsolin and capping protein. J Cell Biol. 1996;134:389–399. doi: 10.1083/jcb.134.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Louriero JJ, Libova I, Fassler R, Wehland J, Gertler FB. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- Bearer EL. Platelet membrane skeleton revealed by quick-freeze deep-etch. Anat Rec. 1990;227:1–11. doi: 10.1002/ar.1092270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL. Direct observation of actin filament severing by gelsolin and binding by CapZ and gCap39. J Cell Biol. 1991;115:1629–1638. doi: 10.1083/jcb.115.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL. Role of actin polymerization in cell locomotion: molecules and models. Am J Respir Cell Mol Biol. 1993;8:582–591. doi: 10.1165/ajrcmb/8.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL. Cytoskeletal domains in the activated platelet. Cell Motil Cytoskeleton. 1995;30:50–66. doi: 10.1002/cm.970300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer EL. Obtaining peptide sequences from myosins for the design of PCR primers. In: Gavin R, editor. Methods in molecular biology: the cytoskeleton. New York: Humana Press; 2000. in press. [Google Scholar]
- Bearer EL, Abraham MT. 2E4 (kaptin): a novel actin-associated protein from human blood platelets found in lamellipodia and the tips of the stereocilia of the inner ear. Eur J Cell Biol. 1999;78:117–126. doi: 10.1016/S0171-9335(99)80013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. Gelsolin has three actin-binding sites. J Cell Biol. 1988;106:1553–1562. doi: 10.1083/jcb.106.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- Chakraborty T, Ebel F, Domann E, Niebuhr K, Gerstel B, Pistor S, Temm-Grove CJ, Jockusch BM, Reinhard M, Walter U. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 1995;14:1314–1321. doi: 10.1002/j.1460-2075.1995.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Loftus DJ, Frieden C, Bryan J, Elson EL. Localization and mobility of gelsolin in cells. J Cell Biol. 1988;106:1229–1240. doi: 10.1083/jcb.106.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CC, Stossel TP, Kwiatkowski DJ. Enhanced motility of NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991;251:1233–1251. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- Dabrowska R, Hinssen H, Galazkiewicz B, Nowak E. Modulation of gelsolin-induced actin -filament severing by caldesmon and tropomyosin and the effect of these proteins kn actin activity of myosin Mg(2+)-ATPase activity. Biochem J. 1996;315:753–759. doi: 10.1042/bj3150753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V, Gouin E, Troys MV, Grogan A, Segal AW, Ampe C, Cossart P. Identification of cofilin, coronin, Rac and capZ in actin tails using a Listeria affinity approach. J Cell Sci. 1998;111:2877–2884. doi: 10.1242/jcs.111.19.2877. [DOI] [PubMed] [Google Scholar]
- Davidson MM, Haslam RJ. Dephosphorylation of cofilin in stimulated platelets: roles for a GTP-binding protein and Ca2+ Biochem J. 1984;301:41–47. doi: 10.1042/bj3010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dold FG, Sanger JM, Sanger JW. Intact alpha-actinin molecules are needed for both the assembly of actin into the tails and the locomotion of Listeria monocytogenes inside infected cells. Cell Motil Cytoskeleton. 1994;28:97–107. doi: 10.1002/cm.970280202. [DOI] [PubMed] [Google Scholar]
- Fox EB, Phillips D. Polymerization and organization of actin filaments within platelets. Semin Hematol. 1983;20:243–260. [PubMed] [Google Scholar]
- Gieselmann R, Kwiatkowski DJ, Janmey PA, Witke W. Distinct biochemical characteristics of the two human profilin isoforms. Eur J Biochem. 1995;229:621–628. doi: 10.1111/j.1432-1033.1995.tb20506.x. [DOI] [PubMed] [Google Scholar]
- Haffner C, Jarchau T, Reinhard M, Hoppe J, Lohmann SM, Walter U. Molecular cloning, structural analysis and functional expression of the proline-rich focal adhesion and microfilament-associated protein VASP. EMBO J. 1995;14:19–27. doi: 10.1002/j.1460-2075.1995.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbrugge M, Walter U. Purification of a vasodilator-regulated phosphoprotein from human platelets. Eur J Biochem. 1989;185:41–50. doi: 10.1111/j.1432-1033.1989.tb15079.x. [DOI] [PubMed] [Google Scholar]
- Halbrugge M, Friedrich C, Eigenthaler M, Schanzenbacher P, Walter U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem. 1990;265:3088–3093. [PubMed] [Google Scholar]
- Harbeck B, Huttelmaier S, Schluter K, Jockusch BM, Illenberger S. Phosphorylation of the vasodilator-stimulated phosphoprotein (VASP) regulates its interaction with actin. J Biol Chem. 2000 Jul 5; doi: 10.1074/jbc.M005066200. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- Harris HE, Weeds AG. Plasma gelsolin caps and severs actin filaments. FEBS Lett. 1984;177:184–188. doi: 10.1016/0014-5793(84)81280-6. [DOI] [PubMed] [Google Scholar]
- Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig JH. Platelet morphology. In: Loscalzo J, Schafer AI, editors. Thrombosis and Hemorrhage. 2. Baltimore: Williams and Wilkins; 1999. pp. 207–227. [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Hauser W, Knobeloch KP, Eigenthaler M, Gambaryan S, Krenn V, Geiger J, Glazova M, Rohde E, Horak I, Walter U, Zimmer M. Megakaryocyte hyperplasia and enhanced agonist-induced platelet activation in vasodilator-stimulated phosphoprotein knockout mice. Proc Natl Acad Sci USA. 1999;96:8120–8125. doi: 10.1073/pnas.96.14.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttelmaier S, Harbeck B, Steffens O, Messerschmidt T, Illenberger S, Jockusch BM. Characterization of the actin binding properties of the vasodilator-stimulated phosphoprotein VASP. FEBS Lett. 1999;1451:68–74. doi: 10.1016/s0014-5793(99)00546-3. [DOI] [PubMed] [Google Scholar]
- Jarchau T, Mund T, Reinhard M, Walter U. Purification and assays of vasodilator-stimulated phosphoprotein. Methods Enzymol. 1998;298:103–113. doi: 10.1016/s0076-6879(98)98012-0. [DOI] [PubMed] [Google Scholar]
- Kaiser DA, Goldschmidt-Clermont PJ, Levine BA, Pollard TD. Characterization of renatured profilin purified by urea elution from poly-L-proline agarose columns. Cell Motil Cytoskeleton. 1989;14:251–262. doi: 10.1002/cm.970140211. [DOI] [PubMed] [Google Scholar]
- Kouyama T, Mihashi K. Fluorimetry study of N-(1-pyrenyl)-iodoacetamide-labelled F-actin. Eur J Biochem. 1981;114:33–38. [PubMed] [Google Scholar]
- Kurokawa H, Fujii W, Ohmi K, Sakurai T, Nonomura Y. Simple and rapid purification of brevin. Biochem Biophys Res Commun. 1990;168:451–457. doi: 10.1016/0006-291x(90)92342-w. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ. Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol. 1999;11:103–108. doi: 10.1016/s0955-0674(99)80012-x. [DOI] [PubMed] [Google Scholar]
- Lamb JA, Allen PG, Tuan BY, Janmey PA. Modulation of gelsolin function. Activation at low pH overrides Ca2+ requirement. J Biol Chem. 1993;268:8999–9004. [PubMed] [Google Scholar]
- Lambrechts AI, Kwiatkowski A, Lanier LM, Bear JE, Vandekerck-hove J, Ampe C, Gertler FB. PKA phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3-domains. J Biol Chem. 2000 doi: 10.1074/jbc.M006274200. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- Laurent V, Loisel TP, Harbeck B, Wehman A, Grobe L, Jockusch BM, Wehland J, Gertler FB, Carlier MF. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J Cell Biol. 1999;144:1245–1258. doi: 10.1083/jcb.144.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind SE, Janmey PA, Chaponnier C, Herbert TJ, Stossel TP. Reversible binding of actin to gelsolin and profilin in human platelet extracts. J Cell Biol. 1987;105:833–842. doi: 10.1083/jcb.105.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U, Schutt CE, Hellsten E, Tjader AC, Hult T. The use of poly(L-proline)-Sepharose in the isolation of profilin and profilactin complexes. Biochim Biophys Acta. 1988;967:391–400. doi: 10.1016/0304-4165(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Loisel TP, Boujemaa R, Pantaloni D, Carlier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- Lu M, Witke W, Kwiatkowski DJ, Kosik KS. Delayed retraction of filopodia in gelsolin null mice. J Cell Biol. 1997;138:1279–1287. doi: 10.1083/jcb.138.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Atkinson SJ, Ampe C, Vanderkerckhove J, Pollard TD. Purification of cortical complexes containing two unconventional actins from acanthamoeba by affinity chromatography on profilin-agarose. J Cell Biol. 1994;127:107–115. doi: 10.1083/jcb.127.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM. Putting on the brakes: a negative regulatory function for Ena/VASP proteins in cell migration. Cell. 2000;101:685–688. doi: 10.1016/s0092-8674(00)80879-x. [DOI] [PubMed] [Google Scholar]
- Maciver SK, Wachsstock DH, Schwarz WH, Pollard TD. The actin filament severing protein actophorin promotes the formation of rigid bundles of actin filaments crosslinked with alpha-actinin. J Cell Biol. 1991;115:1621–1628. doi: 10.1083/jcb.115.6.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester R, Allen PG, Bearer EL. Affects of VASP and profilin on actin polymerization. Mol Biol Cell. 1998;9:141a. [Google Scholar]
- Medeiros NA, Reese TS, Jaffe H, Degiorgis JA, Bearer EL. Primary peptide sequences from squid muscle and optic lobe myosin IIs: a strategy to identify an organelle myosin. Cell Biol Int. 1998;22:161–173. doi: 10.1006/cbir.1998.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias VT, Golla R. Vinculin in relation to stress fibers in spread platelets. Cell Motil Cytoskeleton. 1991;20:190–202. doi: 10.1002/cm.970200303. [DOI] [PubMed] [Google Scholar]
- Nachmias VT, Yoshida K. The cytoskeleton of the blood platelets: A dynamic structure. Adv Cell Biol. 1988;2:181–211. [Google Scholar]
- Nachmias VT, Cassimeris L, Golla R, Safer D. Thymosin beta 4 (T beta 4) in activated platelets. Eur J Cell Biol. 1993;61:314–320. [PubMed] [Google Scholar]
- Nanavati D, Ashton FT, Sanger JM, Sanger JW. Dynamics of actin and alpha-actinin in the tails of Listeria monocytogenes in infected PtK2 cells. Cell Motil Cytoskeleton. 1994;28:346–358. doi: 10.1002/cm.970280408. [DOI] [PubMed] [Google Scholar]
- Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl UD, Walter U, Gertler FB, Wehland J, Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997;16:5433–5444. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova A, Prochniewicz E, Egelman EH. Structural dynamics of F-actin: II. Cooperativity in structural transitions. J Mol Biol. 1995;245:598–607. doi: 10.1006/jmbi.1994.0049. [DOI] [PubMed] [Google Scholar]
- Otto JJ. Actin-bundling proteins. Curr Opin Cell Biol. 1994;6:105–109. doi: 10.1016/0955-0674(94)90123-6. Review. [DOI] [PubMed] [Google Scholar]
- Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85:164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Purich DL, Southwick FS. ABM-1 and ABM-2 homology sequences: consensus docking sites for actin-based motility defined by oligoproline regions in Listeria ActA surface protein and human VASP. Biochem Biophys Res Commun. 1997;231:686–691. doi: 10.1006/bbrc.1997.6158. [DOI] [PubMed] [Google Scholar]
- Puszkin EG, Jenkins CS, Ores-Carton C, Zucker MB. Platelet cytoskeleton alpha-actinin in normal and thrombasthenic platelets: distribution and immunologic characterization. J Lab Clin Med. 1985;105:52–62. [PubMed] [Google Scholar]
- Reinhard M, Halbrugge M, Scheer U, Wiegand C, Jockusch BM, Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 1992;11:2063–2070. doi: 10.1002/j.1460-2075.1992.tb05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar R, Bell SE, Davis GE. Coordinate induction of the actin cytoskeletal regulatory proteins gelsolin, vasodilator-stimulated phosphoprotein, and profilin during capillary morphogenesis in vitro. Exp Cell Res. 1999;249:22–32. doi: 10.1006/excr.1999.4460. [DOI] [PubMed] [Google Scholar]
- Sherman MB, Jakana J, Sun S, Matsudaira P, Chiu W, Schmid MF. The three-dimensional structure of the Limulus acrosomal process: a dynamic actin bundle. J Mol Biol. 1999;294:139–149. doi: 10.1006/jmbi.1999.3222. [DOI] [PubMed] [Google Scholar]
- Smolenski A, Bachmann C, Reinhard K, Honig-Liedl P, Jarchau T, Hoschuetzky H, Walter U. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273:20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- Southwick FS, Purich DL. Intracellular pathogenesis of listeriosis. N Engl J Med. 1996;334:770–776. doi: 10.1056/NEJM199603213341206. [DOI] [PubMed] [Google Scholar]
- Stossel TP. The machinery of cell crawling. Sci Am. 1994;271:54–55. 58–63. doi: 10.1038/scientificamerican0994-54. [DOI] [PubMed] [Google Scholar]
- Theriot JA. Accelerating on a treadmill: ADF/cofilin promotes rapid actin filament turnover in the dynamic cytoskeleton. J Cell Biol. 1997;136:1165–1168. doi: 10.1083/jcb.136.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, DeRosier DJ, Tilney MS. How Listeria exploits host cell actin to form its own cytoskeleton. I. Formation of a tail and how that tail might be involved in movement. J Cell Biol. 1992;118:71–81. doi: 10.1083/jcb.118.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky AB, Surgucheva IG, Gelfand V. Phalloidin and tropomyosin do not prevent actin filament shortening by the 90 kD protein-actin complex from brain. Biochem Biophys Res Commun. 1984;123:596–603. doi: 10.1016/0006-291x(84)90271-7. [DOI] [PubMed] [Google Scholar]
- Welch MD. The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 1999;9:423–427. doi: 10.1016/s0962-8924(99)01651-7. [DOI] [PubMed] [Google Scholar]
- Wen D, Corina K, Chow EP, Miller S, Janmey PA, Pepinsky RB. The plasma and cytoplasmic forms of human gelsolin differ in disulfide structure. Biochemistry. 1996;35:9700–9709. doi: 10.1021/bi960920n. [DOI] [PubMed] [Google Scholar]
- Witke W, Sharpe AH, Hartwig JH, Azuma T, Stossel TP, Kwiat-kowski DJ. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995;81:41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- Yin HL, Stossel TP. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979;281:583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Zhukarev V, Ashton F, Sanger JM, Sanger JW, Shuman H. Organization and structure of actin filament bundles in Listeria-infected cells. Cell Motil Cytoskeleton. 1995;30:229–246. doi: 10.1002/cm.970300307. [DOI] [PubMed] [Google Scholar]