Abstract
Properties of the small group of cancer cells called tumor-initiating or cancer stem cells (CSCs) involved in drug resistance, metastasis and relapse of cancers can significantly affect tumor therapy. Importantly, tumor drug resistance seems to be closely related to many intrinsic or acquired properties of CSCs, such as quiescence, specific morphology, DNA repair ability and overexpression of antiapoptotic proteins, drug efflux transporters and detoxifying enzymes. The specific microenvironment (niche) and hypoxic stability provide additional protection against anticancer therapy for CSCs. Thus, CSC-focused therapy is destined to form the core of any effective anticancer strategy. Nanomedicine has great potential in the development of CSC-targeting drugs, controlled drug delivery and release, and the design of novel gene-specific drugs and diagnostic modalities. This review is focused on tumor drug resistance-related properties of CSCs and describes current nanomedicine approaches, which could form the basis of novel combination therapies for eliminating metastatic and CSCs.
Keywords: cancer microenvironment, cancer stem cells, drug efflux transporters, drug resistance, nanomedicine
Since the new concept of cancer stem cells (CSCs) was introduced in late 1990s, it has gradually gained worldwide acceptance and influenced all approaches to cancer research and therapy. The CSCs, which are also accurately called ‘tumor-initiating cells’, represent a small population of cancer cells, sharing common properties with normal stem cells (SCs), that can initiate new tumors following injection into animal models, while the majority of other cancer cells cannot. The reported fractions of CSCs in tumors vary from 0.1 to 30% depending on the type and the advancement of the cancer [1]. In newly developing hierarchic cancer models, tumors are functionally heterogeneous and contain various types of cells (e.g., macrophages and vascular endothelial cells and so on). Among them, only CSCs have tumorigenic ability [2].
Current radio and chemotherapies kill the bulk of cancer cells, but often are not able to eliminate the critical CSCs, which are protected by specific resistance mechanisms. Surviving CSCs give rise to new tumors and metastases, causing relapse of the disease. The recurrent tumors become more malignant, fast spreading and resistant to radiotherapy and previously used drugs, making the prognosis for cancer patients dismal. Thus, the specific survival of CSCs could provide an explanation for many therapeutic failures and highlight new directions for the enhancement of cancer therapy.
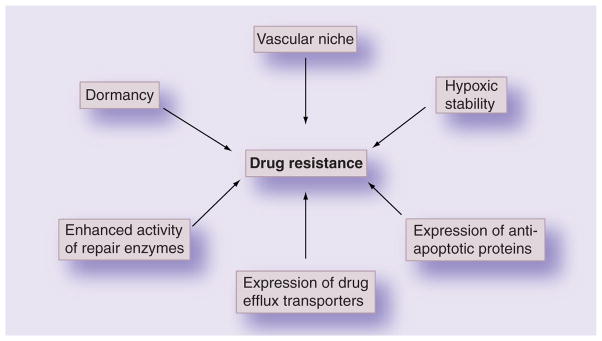
In the traditional view, cancer cells that survived during chemotherapy and acquired drug resistance gave rise to a population of drug-resistant cancer cells. So far, this issue has been discussed based on the mechanisms by which cancer cells become resistant, such as drug inactivation, alterations in cellular targets, suppression of drug accumulation and activation [3–5]. However, according to the CSC concept, drug resistance is mostly caused by the intrinsic or acquired resistance mechanisms of accumulating CSCs (Figure 1). In this review, we will focus on properties closely associated with the resistance of CSCs to therapy and recently developed nanomedicine approaches against drug-resistant cancer cells.
Figure 1.
Major factors enhancing cancer stem cell survival following radio/chemotherapy.
CSC morphology
Survival and accumulation of drug-resistant CSCs following chemo or radiotherapy was used to explain the recurrence of increasingly invasive and malignant tumors. CSCs could be detected as a small subpopulation of cancer cells characterized by specific phenotypes, for example, CD34+/CD38− in leukemia cells, CD44+/CD24− in solid tumors, or CD133+ in other tumors [6]. CD44 binds hyaluronic acid (HA), a major glycosaminoglycan of the extracellular matrix, and can assist the attachment of CSCs to the matrix, contributing to proliferation and migration of malignant tumor cells. The overexpression of CD44 in cancer cells was strongly linked to therapeutic drug resistance [7]. Another marker, CD133+, previously found in abundance in the embryonic epithelium, is also expressed in CSCs of many cancers (e.g., brain tumors with strong resistance to chemotherapy). Therefore, CD44 and CD133 targeting can be applied to deliver drugs to CSCs and kill them.
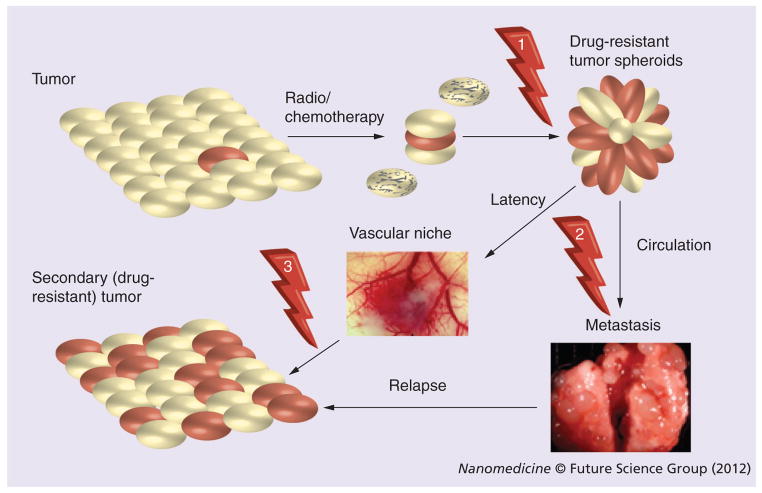
Another important factor of the CSC identity is their strong ability to form multicellular spheroids. Data convened from several reports on CSCs isolated from many ascites and solid tumors demonstrate their enhanced capacity to form chemoresistant multicellular spheroids. These spheroid cancer cells or tumorospheres were able to differentiate into an adherent epithelial lineage, recapitulating the phenotype of the original tumor. Thus, the spheroid-forming ability and morphology in cancer cells could be directly related to their resistance to chemotherapies. Detached tumor spheres enriched with CSCs could also be responsible for tumor metastasis. In metastatic development, a significant number of CSCs undergo epithelial–mesenchymal transition (EMT) and form spheroids, which are able to leave the tumor site and enter the blood or lymph circulation (Figure 2). Locating a convenient microenvironment/niche, they can form metastases and new tumors. Interestingly, the tumor spheroids can differentiate into various cell types, including massively growing cancer cells and aggressively forming neovasculature, step by step becoming tumor tissue. Recently, evidence was found that a large number of endothelial cells in glioblastoma tumors have the same genomic alteration as the major tumor cell population, indicating that at least a subset of them may originate from CSCs [8]. In addition, recent studies demonstrated that a subset of CSCs in human renal cell carcinoma can release microvesicles that stimulate angiogenesis and promote the formation of a vascular niche. These microvesicles were suggested as a contributor to metastasis that activated endothelial cells and induced formation of neovasculature in severe combined immunodeficient mice [9].
Figure 2. Principal steps in the survival of cancer stem cells after tumor treatment, metastasis and tumor relapse following the therapy.
Potential points for targeting of cancer stem cells (CSCs) are shown by arrows and include: post-therapeutic drug delivery or gene therapy affecting residual CSCs/tumorospheres (1); selective removal of CSCs/tumorospheres from circulation by cancer vaccines or vectorized drug conjugates (2); combination cytotoxic and antiangiogenic therapy of vascular niche protecting the surviving CSCs (3). CSCs are shown in red.
3D culturing in cancer studies further supported the hypothesis that nonadherent spheroid-forming cancer cells are more resistant to therapeutic drugs than adherent lineage. Tumor spheroids cultivated in serum-free cultures were shown to be highly tumorigenic and enriched with CSCs [10]. Thus, nonadherent cancer spheroids do possess many properties of CSCs, but the true relationship between them still remains poorly understood.
Drug efflux & detoxification
Drug efflux transporter proteins (or ABC transporters) are generally found to be overexpressed in drug-resistant cancer cells. Multiple transporters have been identified in CSCs, including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and the multidrug resistance-associated proteins (MRP) [11]. P-gp is an ABC transporter with broad substrate specificity, which is currently considered to be the main negative factor in the anticancer therapy of leukemia and many solid tumors [12]. The ABCB5 glycoproteinis notably overexpressed in drug-resistant human malignant melanoma and in circulating melanoma cells. ABCB5 blockade by specific anti-ABCB5 monoclonal antibody (mAb) could restore the sensitivity of melanoma cells to doxorubicin (DOX), suggesting that ABCB5 is a major efflux pump for DOX and can be a therapeutic target for overcoming drug resistance [13]. Expression of MRP1 (ABCC1) and the activity of an apoptosis inhibitor β-livin contributed to a high survival rate for glioblastoma CSCs after etoposide treatment [14]. BCRP (ABCG2) was also found to play an important role in the clinical resistance of CSCs to anticancer drugs [15]. High BCRP levels were linked to increased CD133 expression and the regulation of Akt signaling in drug-resistant cancer cells. Hu et al. suggested that Akt signaling was able to alter the subcellular localization of BCRP, thus regulating drug efflux activity in CSCs. PI3K inhibitors, blocking Akt signaling, not only suppressed cancer cell proliferation, but also enhanced the sensitivity of chemoresistant cells (e.g., BCRP-associated resistance to DOX in hepatocellular carcinoma) [16].
ALDH are a group of enzymes that oxidize aldehydes formed in the process of alcohol metabolism. ALDH1 activity was used as a marker for the identification of high-risk patients with lung and breast cancer. High levels of the detoxifying enzyme ALDH1 were frequently associated with CSCs, and this marker was used for the identification of CSCs [17]. Recently, Honoki et al. evaluated the cancer spheroid subpopulation of cells from human sarcoma with high ALDH1 activity and found that these cells possess strong chemoresistance and detoxifying capability [18]. Treatment with the multikinase inhibitor sorafenib and xenobiotic-processing enzyme inhibitor sulforaphane could reduce the ALDH1 activity in pancreatic cancer cells and, consequently, resulted in tumor growth inhibition in vivo, indicating the potential for a CSC-targeting therapeutic strategy [19].
Possessing a selective advantage, drug-resistant CSCs can enrich the major cancer cell population during long-term treatment and induce poor response to chemotherapy. This explains the difficulties in the treatment of recurrent cancers. With this in mind, the application of efficient therapies targeting CSCs immediately after general cancer therapy would be the most promising treatment option.
Antiapoptotic signaling pathways
Self-renewal and maintenance in quiescence are important properties of normal SCs and CSCs. Different signaling pathways and genes are involved in the maintenance of CSCs in the tumor microenvironment. Recent studies have pointed to the important role of Hedgehog (Hh) signaling in the development of some cancers. Active Hh signaling was demonstrated in human leukemia, especially in CD34+ primary leukemic cells. The anti-Hh monoclonal antibody 5E1 could induce apoptosis of acute myeloid leukemia cells resistant to cytarabine [20]. In addition, the constitutive expression of smoothened, a Hh pathway receptor, resulted in multiplication of the CSCs and acceleration of the chronic myeloid leukemia disease.
Wnt signaling is another well-known pathway, playing a major role in embryogenesis and cancer development. In normal SCs, the Wnt proteins serve as growth factors, which contribute to the maintenance and proliferation of SCs. Activation of Wnt signaling enhanced the drug resistance of CSCs from lung and colon cancers. Silencing of Wnt activity by siRNA against β-catenin was able to effectively inhibit the proliferation and drug resistance of lung cancer cells [21]. Similarly, blocking the Wnt pathway in CD133+ colon cancer cells resulted in the reversal of their resistance to 5-fluorouracil [22].
The Notch pathway is another conservative signaling system of multicellular organisms that was associated with self-renewal and the survival of CSCs. Wang et al. found the Notch pathway to be linked to the metastasis-forming ability of CSCs. Notch-2 and its ligand Jagged-1 were overexpressed in gemcitabine-resistant pancreatic cancer cells, which is consistent with the role of Notch signaling in the EMT and acquisition of the CSC phenotype. EMT is considered as a potential process leading to CSC circulation from tumor lesions into blood, while the reverse process of mesenchymal–epithelial transition was assumed as the major mechanism of CSC invasion into healthy organs. Downregulation of Notch signaling by siRNA led to a partial reversal of the EMT phenotype [23]. Thus, activation of Notch signaling and the increase of EMT potential can be mechanistically linked to the gemcitabine resistance of pancreatic CSCs. Blocking the Notch pathway is a potentially effective strategy for overcoming drug resistance and metastasis.
This list is evidently far from complete. So far, we have illustrated that antiapoptotic signaling pathways are involved in CSC-mediated drug resistance. Thus, nanodelivery of selective genetic inhibitors, such as miRNA and siRNA, and small inhibitor molecules can provide a potential combination strategy for suppressing the drug resistance and viability of CSCs.
Cancer microenvironment
The concept of a ‘niche’ for maintenance of CSCs has attracted significant attention because the specific protective microenvironment is one of the intrinsic properties of CSCs, which potentially allows them to hide in a quiescent state in tissues and avoid the consequences of chemotherapy. Briefly, the niche is defined as the microenvironment where CSCs are located, and where they interact with other types of cells. The normal SC niche functions in specific vascular locations that regulate SC activity during tissue generation, maintenance and repair. The niche-associated vasculature can provide physical and physiological protection from SC depletion. Evidently, the CSC niche is a dynamic supportive system with specific anatomic and functional features that contains a variety of cell types, cytokines and signaling pathways [24].
Currently, only minimal evidence exists regarding the location and fate of CSC niches in vivo. In leukemia, hematopoietic SCs and progenitor cells are localized in the vicinity of bone marrow vasculature. Vascular factors can directly interact with CSCs or their precursors. Considering the possibilities that vascular endothelial cells of the niche could maintain the stem-like state of brain CSCs, Folkins et al. introduced antiangiogenic therapy combined with chemotherapy in order to treat glioma. This combination therapy was able to significantly reduce the number of CSCs or CSC-associated spheroids. This work highlighted the role of the tumor microenvironment and provided a potential strategy against tumor resistance to chemotherapy by targeting the vascular niche rather than treating tumors directly [25]. The role of cytokines and signaling pathways in niche maintenance has been the subject of recent studies. Hovinga et al. recommended selective targeting of endothelial cells of the CSC niche in order to treat drug-resistant brain tumors. They also demonstrated that the Notch pathway plays a critical role in the connection between angiogenesis and self-renewal of CSCs and, therefore, can be considered as a potential therapeutic target [26]. Inflammation can also maintain a supportive environment for CSCs by recruiting immune cells expressing cytokines. Therefore, anti-inflammatory drugs could serve as potential components of CSC-focused therapies affecting vascular niches. For example, continuous treatment with IFN-β was able to disrupt the vascular niche of glioma CSCs. In this experiment, an adenoviral vector was used to express IFN-β in human glioma xenografts. The treatment significantly increased isolation of CSCs from endothelial cells, creating a barrier consisted of perivascular cells [27]. Niche-associated resistance of CSCs to therapy strongly depends on the adhesion of tumor cells to extracellular matrix via integrins. Overexpression of integrin β1 receptors as niche protectors contributed to chemoresistance of cancer cells. Morozevich et al. showed that DOX-resistant MCF-7 cells express an increased amount of integrin α5β1 receptors compared with wild-type MCF-7 cells [28]. The siRNA silencing of the expression of the α5β1 receptor efficiently restored cancer cell sensitivity [28]. Targeting integrin receptors has been a major aspect of developing tumor diagnostics and vectorized drug delivery systems (DDS) over the last two decades, so CSC niche-targeting may become an important component of future anticancer strategies.
Hypoxia
Depletion of oxygen levels in tissue (hypoxia) has long been considered as a major feature of the tumor microenvironment and a potential contributor to the enhanced tumorigenicity of CSCs. Transcriptional factors that respond to hypoxia are called hypoxia-inducible factors (HIFs). These factors serve to prevent cell differentiation, promoting blood vessel formation and regulating apoptosis. The emerging evidence links hypoxia to chemo/radiotherapeutic resistance of tumors, which can be increased by hypoxia. HIFs activate repair enzymes for dsDNA breaks and induce the development of tumor cells resistant to DNA-targeting therapeutic agents. Angiogenesis, one of the driving forces in tumor development, can also be regulated by the hypoxic environment. Aberrant angiogenesis in hypoxia or the activation of HIF-1-related pathways can potentially lead to the formation of CSC niches [29]. Piret et al. found that a hypoxia-stimulated AP-1 induced protection against etoposide-induced apoptosis and suggested that hypoxia confers resistance upon CSCs through some antiapoptotic mechanisms [30]. Targeting hypoxic factors with siRNA or topoisomerase inhibitors showed effectiveness in overcoming the drug resistance in preclinical studies [31,32]. Schwartz et al. reported that a radiotherapy enhancer, PX-478, could potentially inhibit tumor development by blocking HIF-1 signaling and, thus, reducing the innate resistance of hypoxic tumor cells to radiation [33]. Hypoxic conditions in many tumors can be potentially used for the development of nanocarriers with redox-specific labile bonds, which would be able to selectively target this microenvironment and increase drug accumulation and efficacy.
Nanomedicine-based treatment strategies
Drug resistance to current cancer therapies resulting in the imminent accumulation of CSCs, the relapse of the disease in treated tumors, and widespread metastasis during the late stages of cancer make new research for effective ways to overcome these burdens a priority. Novel combination therapies targeting different parts of the tumor microenvironment are critical in order to completely eradicate drug-resistant cancer cells and CSCs. Despite great success in understanding drug resistance mechanisms, the CSC concept added unforeseen dimensions to the problem. Although various methods exist for inhibiting drug efflux transporters, novel therapies must be developed for the eradication of CSCs in their protective niches. Thus, we are entering a new epoch of combination therapies and treatment protocols for cancer diseases. Nanomedicine significantly extends the range of existing anticancer drugs and treatment strategies and, therefore, can be useful in targeting CSCs. In later sections we will discuss major nanomedicine approaches to CSC-targeting using recent examples of therapeutic strategies applied against drug resistance and CSCs. We divided all currently available strategies into three major sections: drug delivery targeting CSCs, subdivided into various nanocarriers such as nanoparticles (NPs), liposomes, micelles, nanotubes and nanogels; targeting genes of drug resistance; and destruction of CSCs/niche (Table 1). Future CSC-targeting strategies will be critically analyzed in a dedicated section.
Table 1.
Summary of nanotechnology developments for targeting of drug-resistant and cancer stem cells.
| Cancer type | Nanodelivery approach | Tumor targeting | Ref. |
|---|---|---|---|
| Drug delivery | |||
| Brain | Curcumin-loaded nanoparticles | None | [36] |
| Breast | Nanomicelles plus paclitaxel | None | [52] |
| Breast | Triblock polymeric micelles with doxorubicin | None | [51] |
| Breast | Stealth liposomal daunorubicin + tamoxifen | None | [45] |
| Breast | Stealth liposomes with all-trans retinoic acid | None | [44] |
| Breast | Poly(benzylglutamate)-hyaluronan polymerosomes with doxorubicin | CD44 | [39] |
| Ovarian | Paclitaxel–hyaluronan bioconjugate | CD44 | [37] |
| Skin (squamous) | Hyaluronic acid-nanoparticles | CD44 | [38] |
| Colon | mAb-modified lipid nanocapsules | CD133 | [42] |
| Ovarian/uterine | mAb-vectorized PLGA nanoparticles loaded with doxorubicin | HER2 receptor | [49] |
| Breast/ovarian | Targeted PEG–PLGA nanoparticles of paclitaxel/lonidamine | EGF receptor | [47] |
| Breast | Biotin-treated nanoparticles with paclitaxel + tariquidar | Biotin receptor | [48] |
| Cervical | Notch signaling GSI-loaded mesoporous silica nanoparticles | Folate receptor | [50] |
| Leukemia | Drug delivery using synthetic low-density lipoprotein particles | Apolipoprotein B receptor | [43] |
| Breast | Liposomes with dequalinium + daunorubicin + quinacrine | Mitochondria | [46] |
| Targeting genes active in CSCs | |||
| Colon | Lipid nano complex of anti-P-gp siRNA + paclitaxel | None | [55] |
| Brain | Liposomes with anti-MGMT siRNA for oral temozolomide therapy | None | [58] |
| Ovarian | Liposome-polycation formulations of anti-P-gp siRNA | None | [56] |
| Pancreatic | Anti-TG-2 siRNA in neutral DOPC liposomes + gemcitabine | VEGF & TG2 receptors | [57] |
| Breast | Biotin-treated nanoparticles for anti-P-gp siRNA + paclitaxel therapy | Biotin receptor | [54] |
| Destruction of CSCs/niche | |||
| Breast | Drug-loaded nanoparticles and pulsed ultrasound treatment | None | [65] |
| Breast | Alginate-PVA nanoparticles for chemo- and photodynamic therapy | None | [61] |
| Melanoma | Ag–Au core–hyaluronan shell nanogels for photothermal treatment | CD44 | [40,41] |
| Leukemia | Indocyanine green-silicate nanoparticles for photodynamic therapy | CD71, gastrin and Tf receptors | [62] |
| Brain | mAb-vectorized SWNT for hypothermic treatment | CD133 | [63] |
| Leukemia | mAb-functionalized carbon nanotubes | P-gp | [64] |
| Pancreatic | Targeted gold nanoparticles and radio frequency-based therapy | HER2 and PAM4 receptors | [60] |
Ag: Silver; Au: Gold; CSC: Cancer stem cell; DOPC: Di-oleoylphosphatidylcholine; GSI: γ-secretase inhibitor; mAb: Monoclonal antibody; MGMT: O-6-methylguanine-DNA-methyltransferase; P-gp: P-glycoprotein; PEG: Polyethylene glycol; PLGA: Poly(lactic-co-glycolic acid); PVA: Poly(vinyl alcohol); SWNT: Single-walled carbon nanotube; Tf: Transferrin; TG2: Tissue transglutaminase.
Drug delivery targeting CSCs
In recent years, the rapid advent of nanotechnology has stimulated the development of many novel drug delivery strategies. Nanosized DDS can potentially overcome known shortcomings of many anticancer drugs, such as low aqueous solubility and stability and high nonspecific toxicity, while at the same time increasing the circulation time and bioavailability of encapsulated drugs. Controlled drug release, rational design for the specific targeting of cancer cells, and additional diagnostic modalities included in DDS could significantly benefit the cancer treatment.
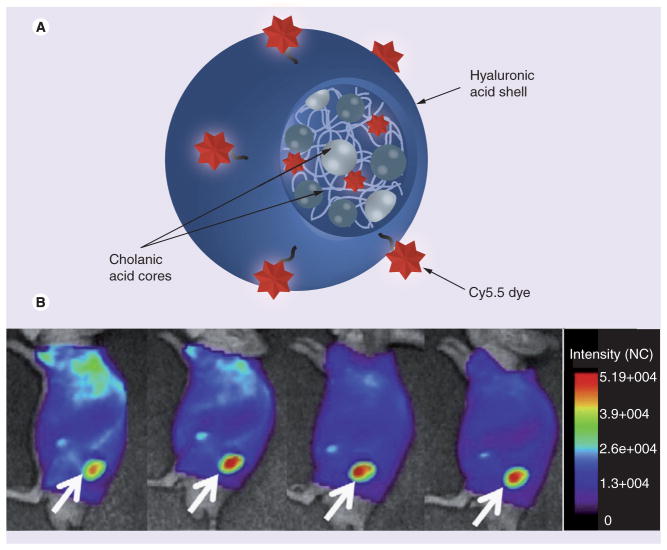
Currently, only a handful of drugs demonstrated selective high efficacy against CSCs. In one example, a veterinary antibiotic, the polyether ionophore salinomycin, was found to possess strong breast CSC-killing capabilities compared with many other drugs tested in the combinatorial study [34]. Due to the prohibitive drug toxicity in humans, only targeted nanoformulations of salinomycin may be potentially devoid of toxicity and efficient in the treatment of CSCs and drug-resistant tumors. Another example, the common Indian spice curcumin, recently attracted great attention in clinical oncology due to its preventive, anticancer and radiotherapy/chemotherapy-sensibilizing activities in aggressive and recurrent tumors. Novel synthetic curcumin analogs and potential nanotechnology-based curcumin formulations have recently been highlighted in the review by Mimeault et al. [35]. Curcumin-loaded Nano-Curc™ (SignPath Pharmaceuticals, Inc., PA, USA; 1.5% curcumin content) NPs were able to significantly suppress anchorage-independent clonogenic growth and reduce the CD133+ CSC population in medulloblastoma and glioblastoma [36]. Curcumin was encapsulated during the synthesis of NPs by the polymerization of N-isopropylacrylamide, vinylpyrrolidone and acrylic acid in the presence of a cross-linker, N,N′-methylenebisacrylamide. This nanoformulation caused a dose-dependent growth inhibition of various brain tumor cell cultures and neurospheres, effectively blocking the CSC-associated Hh pathway, but not the Notch pathway, and reducing IGF and STAT3 levels. Adding negative charge to the NPs increased their dispersion stability, while the thermosensitive N-isopropylacrylamide component enhanced the efficacy of drug release in vivo. These NPs are nanogel-type carriers, swelling in aqueous media, with low retention and fast-release kinetics for low-molecular-weight drugs. The CSC targeting can be potentially applied via surface carboxylic groups. Many CSC-associated surface biomarkers, such as CD44 and CD133, could be utilized for targeting purposes in anti-cancer therapies. Several reported approaches exploited HA as a CD44-specific tumor-targeting drug carrier. Banzato et al. prepared and evaluated pharmacologic and biological properties of a paclitaxel–hyaluronan bioconjugate (ONCOFID™-P, 20% drug content) against human ovarian cancer IGROV-1 and OVCAR-3 xenografts following intraperitoneal administration [37]. The conjugate formed NPs with a hydrophobic core of paclitaxel surrounded by a HA shell in an aqueous solution and entered cancer cells through CD44 receptor-mediated endocytosis. It exerted a concentration-dependent inhibitory effect against tumor cell growth and was more effective than paclitaxel therapy. Another type of HA-based NP formed by self-assembly of amphiphilic 5β-cholanic acid–HA derivatives with controlled substitution rates of between two and ten per 100 sugar residues [38]. HA-NPs (230–400-nm diameter) actively accumulated in CD44-overexpressing squamous cell carcinoma SCC7 tumors after intravenous administration, with the efficacy depending on the hydrophobic core content (HA-NP10 > HA-NP6 > HA-NP2) (Figure 3). As a targeting moiety, HA was also applied to modification of the surface of NPs or liposomes. Upadhyay et al. reported preparation of DOX-loaded poly(γ-benzyl-L-glutamate)-block-HA polymersomes (PolyDOX) using a solvent nanoprecipitation method [39]. NPs with an average diameter of 200 nm demonstrated CD44-dependent cellular accumulation, but suboptimal drug release kinetics during degradation (50% during the first 12 h, and another 30% for 10 days). PolyDOX induced reactive oxygen species-related cytotoxicity in CD44+ breast cancer MCF-7 cells and demonstrated much lower cardiotoxicity than DOX alone. Single intravenous injection of PolyDOX in a 7,12-dimethylbenz(a) anthracene (DMBA)-induced rat mammary tumor model resulted in significant suppression of tumor growth, so that animals demonstrated no tumor-related mortality during the 60-day observation period (100% mortality in the control group). In another application, HA-linked polyethylene glycol (PEG)-nanogels (<100-nm diameter) with a Ag–Au bimetallic core have been synthesized and assayed for bioimaging and combined chemo and photothermal therapies of cancer [40,41]. An almost fourfold increase of the in vitro drug release could be observed from temozolomide or curcumin-loaded nanogels after the near-infrared (NIR) irradiation of the Ag–Au core due to the temperature rising up to 41°C. In summary, CD44-targeting HA-based DDS are simple and potentially effective drug carriers for accessing CD44+ CSCs, and may serve as a component of future combination therapies.
Figure 3. Targeting of CD44-rich tumors.
(A) Structure of Cy5.5-labeled hyaluronic acid nanoparticles in aqueous solution, and (B) fluorescent bioimaging 1, 8, 24 and 48 h after intravenous injection of hyaluronic acid nanoparticles in SCC7 tumor-bearing athymic nude mice. Arrows indicate tumor locations.
Reproduced with permission from [38].
Similarly, CD133+ CSCs can be targeted by vectorized nanocarriers. AC133, an epitope of the CD133/prominin-1 protein associated with glycosylation, is one of the best documented CSC markers. Recently, Bourseau-Guilmain et al. reported preparation of lipoprotein-like nanocapsules (LNCs) with a narrow distribution and size that can be adjusted from 20 to 100 nm [42]. These LNCs could be modified with DSPE–PEG2000-maleimide and coupled with thiolated anti-CD133 mAb, obtaining efficient mAb-LNCs (126–188-nm diameter) with 40 mAb per particle introduced. Caco-2 cells, constitutively expressing a high level of CD133, demonstrated enhanced particle uptake and cell detachment from the surface (effect not observed with mAb alone). As potential nanocarriers, LNCs could deliver various anti-cancer drugs such as paclitaxel, etoposide and radiopharmaceuticals within cancer cells.
In order to overcome the significant clinical problem of CSC persistence in chronic myeloid leukemia, these cells must be exposed to much higher cellular drug concentrations than could be achieved with standard oral dosing. Leukemic CSCs overexpress Bcr–Abl tyrosine kinase and respond to imatinib by a reversible arrest of proliferation without significant apoptosis. As a result, patients are unlikely to be cured during the therapy, because the leukemic CSCs are capable of initiating relapse. Zhou et al. demonstrated that the resistance of leukemic CSCs may be overcome by targeted drug delivery using small synthetic low density lipoprotein particles (sLDL; ~20-nm diameter) [43]. The sLDL particles were prepared from a mixture of phosphatidylcholine, triolein, cholesterol and cholesteryl oleate in the molar ratio 3:2:1:1 using a solvent evaporation method. LDL receptor-specific lipophilic synthetic peptide, a stearate/cholesterol ester of apoB fragment, was used for chronic myeloid leukemia targeting of sLDL particles. Binding and uptake specificity in leukemic cells was confirmed by using the LDL receptor inhibitor, suramin. Results with targeted particles showed an increased and preferential uptake of the carrier by Bcr–Abl-positive cells in comparison to Bcr–Abl-negative cell lines.
Multifunctional DDS offer new modalities in the development of combination therapies focused on overcoming drug-resistant cancer cells. For example, the inhibitory effect of vinorelbine, a plant alkaloid and anticancer drug, on CSCs is limited since it is a cell cycle-specific drug inhibiting cell growth during metaphase, while most CSCs are quiescent cells. Li et al. reported the preparation of negatively charged PEGylated stealth liposomes (~80-nm diameter) loaded with the most effective tumor-inhibiting all-trans isomer of retinoic acids (ATRA) [44]. The liposomes could slowly release ATRA, with an average rate of 9% per 24 h. CD44 +/CD24− human breast cancer MCF-7 and MDA-MB-231 cells were the source of breast CSCs (4 and 1%, respectively), which were resistant to vinorelbine and had a stronger capability for proliferation and differentiation. The in vivo evaluation of ATRA liposomes was performed on the newly established relapse model using the breast CSCs. The inhibitory effect of ATRA stealth liposomes was more potent in CSCs than in proliferating cancer cells, and the most effective combination was vinorelbine plus ATRA liposomes, which induced the arrest of breast CSCs in the G0/G1 phase and differentiation of breast CSCs. Using a similar stealth liposomal system, daunorubicin plus tamoxifen were delivered to eradicate human breast cancer cells together with CSCs [45]. The mean particle size of the negatively charged stealth liposomes was approximately 100 nm and drug encapsulation efficiency was 90–95%. Inhibitory effect was measured in bulk MCF-7 cells, the sorted side population of MCF-7 (typically CSCs), and the sorted nonside population of MCF-7 cells. Liposomal daunorubicin plus tamoxifen demonstrated significantly increased (~ninefold) inhibitory effects in both breast cancer cells and CSCs in a dose-dependable manner compared with liposomal daunorubicin. These liposomes could passively target MCF-7 xenograft tumors in mice, owing to the enhanced permeability and retention effect, and showed the synergistic effects of two drugs and potent anti-tumor activity. Recently, Zhang et al. demonstrated the strong therapeutic potential of mitochondria-targeting liposomes in the treatment of relapsed breast cancer arising from CSCs [46]. Dequalinium (DQA) was used as a mitochondria-specific molecule, and DQA-PEG2000-DSPE-containing liposomes (98 nm in diameter) were formulated with daunorubicin and quinacrine. These liposomes could selectively accumulate in mitochondria, activating the pro-apoptotic Bax protein and affecting other mitochondrial functions, and induced apoptosis of CD44+/CD24− MCF-7 cells cultured in serum-free medium (i.e., CSCs). Efficacy testing was performed on MCF-7 CSCs, mammospheres and relapsed tumors after mouse xenografting with MCF-7 CSCs. These liposomes exhibited significant anti-tumor efficacy by penetrating deeply into the core of the tumor spheroids. The observed strong inhibition of the relapsed tumor growth opens an interesting new direction of mitochondria-specific treatment using dual-drug liposomal formulations.
Biodegradable NPs have been in various clinical trials over the years. They offer the advantages of lower toxicity and sustained drug release. Milane et al. recently evaluated the EGF receptor (EGFR)-targeted nanocarriers in combination therapy against drug-resistant cancers [47]. The delivery system consisted of the EGFR-specific peptide-modified PEG–poly(lactic-co-glycolic acid) (PLGA) or PEG–polycaprolactone NPs loaded with paclitaxel and lonidamine, an inhibitor of aerobic glycolysis in cancer cells. The drug-blended NPs were prepared in sizes between 123 and 137 nm with a negative zeta-potential. Full drug release was achieved at 9 days for paclitaxel and 4–7 days for lonidamine at pH 7.4. These NPs targeted drug-resistant (P-gp+) cells with a high level of EGFR expression; however, the difference between targeted and nontargeted particles was significant only at low incubation times (up to 30 min). Hypoxia usually increased EGFR expression in SKOV3 and MDA-MB-231 cell lines, and the hypoxic breast cancer cells were 70-times more resistant to paclitaxel, showing overexpression of the anti-apoptotic Bcl-2 protein. Lonidamine attacked the Bcl-2 protein and enhanced the effect of paclitaxel in combination therapy. In general, the treatment was more efficient with drug-loaded targeted NPs than drug combinations, which were in turn more efficient than single drugs. The different combination of paclitaxel with the third generation P-gp inhibitor tariquidar was recently evaluated for using biotin-modified PLGA NPs [48]. Paclitaxel accumulation in drug-resistant cancer cells is usually hampered by the overexpression of P-gp; as a result, both the drug and the inhibitor may need to be temporally colocalized in cancer cells for optimal synergy. Biotin–PEG–PLGA NPs were prepared from biotin–PEG–PLA and PLGA, with an average content of 35 biotin moieties per particle. The obtained particles (220–240 nm in diameter) contained up to 17% paclitaxel and 3% tariquidar. The drug release as a result of NP degradation was expended over 15 (tariquidar) or 30 days (paclitaxel). Biotin modification significantly increased (six- to 13-fold) delivery of NPs into drug-resistant cells. The biotinylated dual-drug NPs were also the most efficient in terms of tumor growth inhibition and the extension of survival for mice with mammary tumors in vivo. NPs could potentially be modified with CSC-specific mAbs using the biotin–streptavidine high-affinity binding strategy. Lei et al. reported a different method of preparation of mAb-vectorized PLGA NPs loaded with DOX (DNPs) [49]. In this approach, the carboxyl groups on the DNP surface were activated using the ethyl(dimethylaminopropyl) carbodiimide/N-hydroxy succinimide (EDC/NHS) method and then conjugated with the HER2 mAb. HER2–DNPs demonstrated an average size of 213 nm and slightly negative zeta-potential compared with nonmodified DNPs with a size of 163 nm and highly negative zeta-potential. Improved cellular uptake and cytotoxic effect of DNPs on cancer cell growth compared with free DOX was observed in cancer cell lines overexpressing P-gp, though the influence of HER2-modification of DNPs was found to be statistically significant only in human uterine sarcoma MES-SA cells.
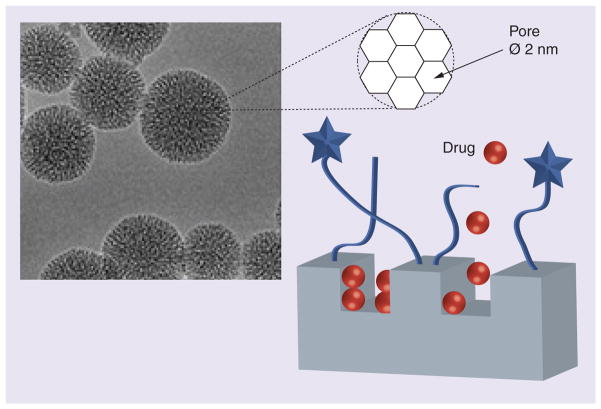
Mamaeva et al. recently described the application of another type of nanocarriers, mesoporous silica NPs (MSNPs), for targeted delivery of γ-secretase inhibitors (GSIs) of Notch signaling, which are potentially effective against CSCs [50]. However, the clinical use of GSIs is restricted by severe toxicity. MSNPs with a diameter of 50 nm were covered with a polyethylenimine (PEI) layer and modified with PEG and folic acid for targeted delivery to the folate receptor-enriched tumors (Figure 4). Drug release from MSNPs can occur either while they are still intact or through matrix decomposition. These MSNPs showed efficient targeting and accumulation in cervical xenograft tumors after systemic administration. The authors reported enhanced tumor retention of GSI–MSNPs and efficient Notch inhibition in vivo, for example in intestinal SCs after oral delivery. These biocompatible and biodegradable MSNPs provide an attractive platform for DDS encapsulating low-molecular-weight drugs and for the further development of CSC-targeted therapies.
Figure 4.
Mesoporous silica nanoparticles with a size of 50 nm covered with a folic acid–polyethylene glycol-modified polycationic layer were used as vehicles for the targeted delivery of γ-secretase inhibitors to block Notch signaling in cancer stem cells.
Among clinically advanced nanocarriers, polymeric micelles constitute a broad variety of self-assembled DDSs. They demonstrate many attractive properties, such as the solubilization of poorly soluble drugs, prolonged circulation, low toxicity and high cellular permeation. In this regard, drug-loaded micelles may also provide the advantage of more efficient penetration in tumors. Recently, Kim et al. reported an enhanced penetration efficacy of DOX-loaded micelles consisting of a PEG–poly-(R)-3-hydroxybutyrate–PEG triblock copolymer (PEG–PHB–PEG) through multicellular cancer spheroids [51]. The PEG–PHB–PEG copolymer formed very small drug-loaded micelles (37-nm diameter) with a long circulation time, which released 50% of the drug load after 1 day and an additional 25% after 3 days-incubation. The micellar drug demonstrated a twofold higher penetration in human cervical carcinoma SiHa spheroids and equal to DOX growth inhibition, and showed significantly lower toxicity in murine xenografted tumors following multiple intravenous injections. Other mitochondria-specific paclitaxel-loaded phospholipid micelles (15-nm diameter) were found to be effective against drug-resistant tumors after oral administration [52]. These micelles were prepared from vitamin E-TPGS, PEG-DSPE and DQA, and were loaded with paclitaxel (~2.5% content). They strongly increased cellular uptake and mitochondrial accumulation of paclitaxel, and were able to penetrate deeply into MCF-7 cancer spheroids, causing a significant reduction in spheroid size. Together with the observed permeability across Caco-2 cell monolayers, this micellar nanocarrier demonstrated enhanced anti-tumor efficacy in xenograft models after oral administration. In summary, the CSC-targeted DDS discussed above offer evident advantages over free drugs and form a strong basis for further development of efficient nanoformulations to eliminate drug-resistant tumors.
Targeting genes active in CSCs
Novel gene-targeting approaches against CSC-mediated drug resistance are based on gene silencing by specific RNA inhibitors. For example, silencing the MDR1 gene in drug-resistant tumors can reduce the expression and efficacy of P-gp transporters and make chemotherapy more efficient. Since low stability and cell accumulation efficiency hamper direct therapeutic applications of free RNA, significant efforts have been made in recent years in order to design DDS for siRNA molecules [53]. Here, we will illustrate the targeting genes active in CSCs with several recent examples of siRNA nanodelivery. As an example, anti-MDR1 siRNA was administered in PEG–PLGA NPs as a part of a paclitaxel–siRNA combination therapy for drug-resistant tumors [54]. Using PEI for complexation, the siRNA was securely encapsulated in NPs (~230-nm diameter), which could induce approximately 50% reduction of P-gp expression in drug-resistant murine mammary cancer JC cells following treatment. Biotinylated dual-drug NPs demonstrated enhanced tumor accumulation and more efficient growth inhibition in a mouse tumor model compared with nonmodified NPs. Using another nanocarrier platform, lipid nanocomplexes obtained from PEGylated cationic PEI and biodegradable bifunctional lipid-modified linker molecules, anti-MRP1 siRNA was encapsulated in nanocomplexes with a diameter of 300–400 nm [55]. Lipid composition in the nanocomplex played a critical role in successful transfection. Optimization of physicochemical properties of the nanocomplex was also necessary to achieve efficient P-gp knockdown. Activity analysis performed in CD133-enriched human colon cancer HT-29 cells demonstrated that nanocomplexes with the PEI-lipid ratio 1:16 demonstrated the best gene silencing. The resulting P-gp inhibition increased the sensitivity of colon CSCs to paclitaxel by more than twofold.
Chen et al. developed two preclinical formulations of siRNA – cationic and anionic liposome-polycation-DNA NPs (LPD and LPD-II, respectively) – for systemic codelivery of DOX and siRNA to drug-resistant tumors [56]. DOX was encapsulated in the intercalated form in calf thymus DNA, which was a part of both NPs. A guanidinium-containing lipid, DSAA, served as a P-gp inhibitor and siRNA condensor in LPD. NPs were targeted specifically to the cancer cells by modification with anisamide, a ligand of σ-receptor that is overexpressed in many tumors. LPD-II was tested in codelivery of DOX and anti-c-Myc siRNA, while LPD code-livered DOX, DSAA and angiogenesis inhibitor anti-VEGF siRNA. Vectorized LPD and LPD-II were able to reverse P-gp activity in drug-resistant NCI-ADR/RES cells, and improved cellular penetration and uptake of DOX. Both types of LPD and LPD-II significantly inhibited the growth of drug-resistant xenograft tumors, despite the low in vitro transfection efficacy of LPD-II. In addition, LPD-II had a stronger advantage over the LPD carrier: much higher DOX entrapment efficacy and significantly lower toxicity.
Targeting of CSC regulatory pathways by siRNA inhibitors was also evaluated for the treatment of aggressive cancers. Verma et al. reported that nanodelivery of specific siRNA could efficiently downregulate the activity of TG2 associated with increased cancer cell invasiveness and survival, and directly linked to cancer patients morbidity [57]. Anti-TG2 siRNA was incorporated in lyophilizable neutral di-oleoylphosphatidylcholine liposomes. Treatment of human pancreatic cancer (Panc-28) xenografts with this liposomal formulation showed medium therapeutic effect compared with gemcitabine alone, however, it significantly enhanced the efficacy of gemcitabine therapy and very efficiently inhibited the spread of metastases.
To date, a combination of radiotherapy and chemotherapy using an oral temozolomide (TMZ) has been the first-line therapy for glioma. However, the efficacy of chemotherapy for treating glioblastoma multiforme (GBM) has been very limited, partly because of the high activity of DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) in brain tumors, creating a resistant phenotype. In an effort to improve the therapeutic efficacy of TMZ, Kato et al. showed that siRNA-based downregulation of MGMT could enhance the sensitivity of malignant gliomas to treatment [58]. TMZ-resistant glioma CSCs with increased levels of DNA repair and drug efflux capabilities have been efficiently transfected with anti-MGMT-siRNA using LipoTrust™, a novel liposomal formulation for siRNA delivery. Intratumoral injection of LipoTrust–siRNA resulted in efficient siRNA distribution in tumor volume and reduced MGMT activity in 93% of cells. Glioma CSCs could be sensitized to TMZ in both in vitro and in vivo tumor models. Continuous drug administration via osmotic pump resulted in sixfold tumor growth inhibition and extended the lifespan of xenografted mice twofold.
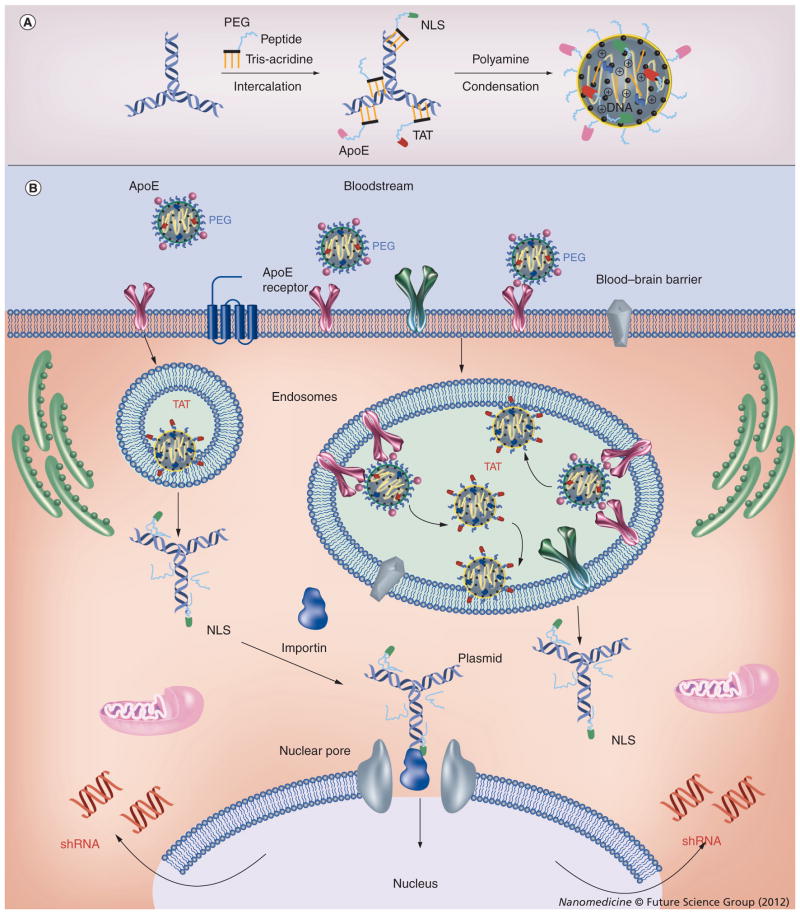
Overexpressed drug efflux transporters limit many drugs entering the brain and, thus, render chemotherapy ineffective. Recently, we developed a novel targeted strategy of transient silencing of the MRP4 transporter that is responsible for the efflux of nucleoside analogs from the brain [59]. The plasmid expressing anti-MRP4 shRNA was encapsulated in a multifunctional nanocarrier containing intercalating PEG conjugates of nuclear localization signal, transactivator of transcription and brain-targeting (e.g., ApoE) peptides and biodegradable polyamine. The plasmid-formulated nanocarrier could efficiently introduce shRNA-expressing DNA constructs into slowly dividing/nondividing cells, and was able to suppress MRP4 expression in brain capillary endothelial by more than 50%. Thus, nanoformulations could penetrate several cellular barriers using specific cellular targeting and endocytosis, transactivator of transcription peptide-mediated endosomal escape and efficient nuclear targeting by the nuclear localization signal peptide (Figure 5). This nanoformulation was then evaluated in vivo for transient silencing of MRP4 pump overexpressed in the blood–brain barrier. Following the MRP4 silencing we observed a fourfold increase of nucleoside drug accumulation in the brain following intravenous injection. Silencing of CSC-specific genes can be a valuable strategy either by itself or by enhancing the efficacy of other cancer drugs, assuming that special DDS overcoming multiple obstacles to RNA delivery will be successfully developed.
Figure 5. Delivery of anti-MRP4 shRNA-expressing plasmid in murine brain vascular endothelial cells using a nanoformulation of polyamine-packed DNA modified with multifunctional peptide-intercalator conjugates.
(A) DNA formulation with intercalating conjugates of functional peptides and polyamine. (B) Initially, the nanoformulation binds and enters cells via ApoE receptor-mediated endocytosis. The TAT peptide and biodegradation of polyamine then assist in the transfer of plasmid DNA across the cellular membrane. The next step of cytoplasmic translocation of DNA is performed by the nuclear localization signal peptide, and the plasmids enter nuclei through nuclear pores. Finally, active plasmids express anti-MRP4 shRNA that exits the nuclei and, in the complex with the RNA-induced silencing complex, suppress the activity of the MRP4 drug efflux transporter.
PEG: Polyethylene glycol
Destruction of CSCs in niches
Various physicochemical methods are currently being investigated for specific destruction of CSCs and the CSC-supporting environment (niche). Most approaches include local delivery of vectorized NPs (empty or drug loaded) and subsequent body irradiation for thermoablation or photodynamic therapy (PDT). Glazer et al. used the efficient heating rates of gold NPs (AuNPs) under irradiation at a nonionizing radiofrequency (13.56 MHz) in this way [60]. AuNPs (10 and 20 nm in diameter) were modified with EGFR1-specific cetuximab or MUC1-specific PAM4 mAb, respectively. Irradiation of AuNPs increased the local temperature up to 60°C, which could result in the rapid destruction of the AuNP-accumulating cancer cells. A total of 36 h after intraperitoneal treatment of human pancreatic Panc-1 and Capan-1 cancer xenografts with mAb-conjugated AuNPs, the mice were exposed to radiofrequency irradiation. Tumors significantly shrank after 6 weeks of weekly treatments and developed visible necrosis. There was no evidence of injury in bystander organs after the therapy. This study demonstrated the amazing potential of noninvasive AuNP-based therapy inducing cellular hyperthermia.
Khdair et al. investigated PDT using negatively charged alginate NPs (40–73-nm diameter) with DOX loading approximately 7% and nonoptimal in vitro drug release: an initial significant burst DOX release was observed [61]. In the presence of methylene blue, a PDT enhancer agent and also a P-gp inhibitor, the NPs-associated PDT enhanced tumor sensitivity to DOX and induced necrosis. Invivo studies, using a P-gp-rich murine JC tumor model, demonstrated that this approach has a strong potential against drug-resistant cancers [61].
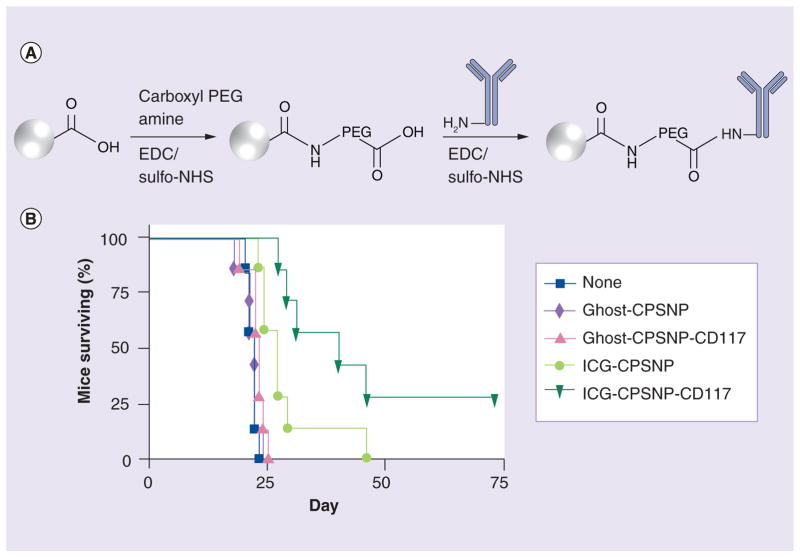
In another example, nontoxic and non-aggregating calcium phosphosilicate NPs (CPSNPs, 20–40 nm in diameter) loaded with a near-infrared (NIR) probe indocyanine green (ICG) have been developed for diagnostic imaging, drug delivery and PDT of drug-resistant cancers (Figure 6) [62]. The efficacy of leukemia therapy can be severely limited in many cases by the presence of drug-resistant CSCs. In order to generate vectorized PEGylated CPSNPs, a multistep conjugation approach was employed using sulfo-NHS-activated ester, which was then reacted at neutral pH with anti-CD117 mAbs abundantly expressed in leukemia SCs. Vectorized ICG-CPSNPs demonstrated enhanced accumulation in cancer cells and dramatically improved PDT efficacy against a murine leukemia cell line and human leukemia. Furthermore, the in vivo efficacy of ICG-CPSNPs in a murine leukemia model resulted in 29% disease-free survival [62].
Figure 6. Targeted photodynamic nanotherapy.
(A) Synthesis of calcium phosphosilicate nanoparticles modified with CD117 mAb (CPSNP-CD117). (B) Survival of mice with myeloid leukemia established with 32D-p210-GFP cells, was monitored after the treatment with phosphate-buffered saline, empty (ghost)-CPSNPs, CD117-targeted ghost-CPSNPs, ICG-CPSNPs, or CD117-targeted ICG-CPSNPs followed by near infrared laser treatment of the spleen. A log rank test indicated significance (p < 0.05) between the curves (group of seven).
CPSNP: Calcium phosphosilicate nanoparticle; EDC: Ethyl(dimethylaminopropyl) carbodiimide; ICG: Indocyanine green; NHS: N-hydroxysuccinimide; PEG: Polyethylene glycol.
Reproduced with permission from [62].
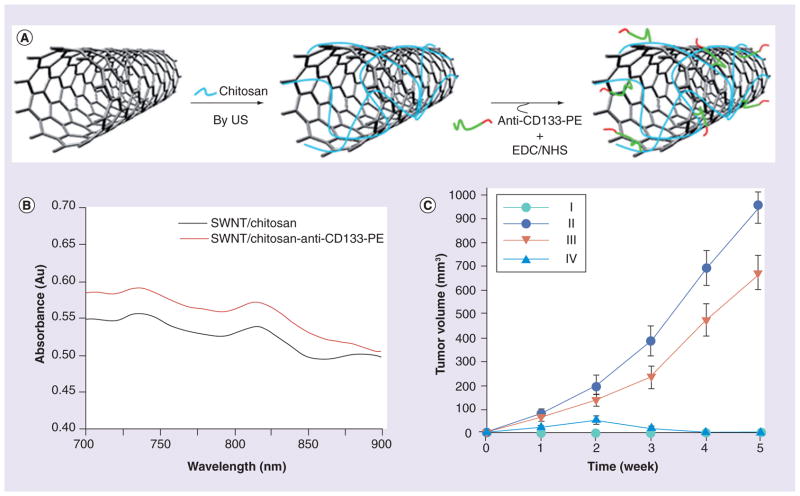
Recently, Wang et al. designed anti-CD133 mAb-conjugated single-walled carbon nanotubes, which could selectively target CD133+ glioblastoma cells and assist in their photothermal destruction by a NIR laser (Figure 7) [63]. Single-walled carbon nanotubes (SWNTs) were initially covered with chitosan and then coupled with EDC/NHS-activated anti-CD133-PE, resulting in NPs with an average diameter of 233 nm. GBM-CD133 + and GBM-CD133− cells mixed in various ratios were challenged with these conjugated SWNTs and then irradiated with NIR light (808 nm). The in vitro tumorigenic and self-renewal capability of GBM-CD133+ cells was significantly blocked due to the localized hyperthermia. Information about the toxicity of carbon nanotubes (CNTs) has been controversial until now, due to the application of different cytotoxicity assays, the use of CNTs with or without surface modifications and the residual heavy metals in CNTs. Actually, many functionalized carbon nanotubes (CNTs), especially SWNTs, which possess excellent biocompatibility and contain no residual heavy metals, are suggested to be non-toxic at cellular levels. Anti-P-gp mAb-functionalized oxidized (i.e., containing carboxyl groups for derivatization) SWNTs loaded with DOX (DOX/Ap-SWNTs) have been synthesized for challenging drug-resistant K562 human leukemia cells [64]. These Ap-SWNTs could not only specifically recognize the multidrug-resistant human leukemia cells (K562R vs K562S binding was more than 20-times higher), but also demonstrated effective loading and controlled release of DOX in targeted K562R cells after the exposure to NIR. Drug loading was 4% and drug release was approximately 33% for 3 days without irradiation, or 70% with NIR. Potentially this system can not only destroy CSCs, but also target and inhibit growth of tumor metastases.
Figure 7. Targeted tumor ablation therapy.
(A) Preparation of single-walled carbon nanotubes conjugated with CD133 mAb antibody (CDSWNTs). (B) UV spectrum of CDSWNTs showing a near infrared maximum at 815 nm. (C) Tumor growth rate and volumes of tumor-bearing nude mice for four different groups: (I) glioblastoma multiforme (GBM)-CD133− cells; (II) GBM-CD133+ cells; (III) GBM-CD133+ cells pretreated with CDSWNTs only; and (IV) GBM-CD133+ cells pretreated with CDSWNTs combined with near infrared laser irradiation. Data shown were the mean ± standard deviation of three experiments (p < 0.001).
EDC: Ethyl(dimethylaminopropyl) carbodiimide; NHS: N-Hydroxysuccinimide; SWNT: Single-walled carbon nanotube.
Reproduced with permission from [63].
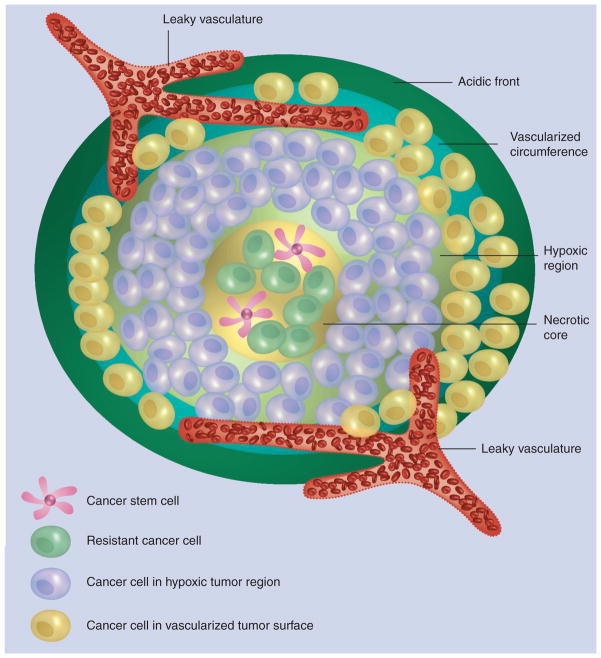
Ultrasound is another physical treatment that can potentially enhance CSC-directed therapy and destruction of the CSC niche. In the following example, pulsed ultrasound was applied in order to enhance the penetration of drug-loaded polymeric and lipid NPs into breast cancer spheroids (300–350 μm diameter) [65]. Effective treatment of solid tumors requires homogeneous distribution of anticancer drugs within the entire tumor volume to deliver lethal concentrations to drug-resistant cancer cells and CSCs. However, penetration of drug-loaded polymeric and lipid particles into the hypoxic and necrotic regions of solid tumors with high concentration of CSCs remains a significant challenge (Figure 8). In this article, penetration of fluorescent NPs into breast cancer spheroids was investigated as a function of particle size and charge. During pulsed sonication for 30, 60 or 90 s in the presence of microbubbles, the penetration of small NPs (20 nm) could be increased six- to 20-times. Anionic NPs demonstrated the highest efficacy in penetrating MCF-7 spheroids in vitro compared with cationic or neutral NPs, or NPs not exposed to ultrasound. Increase in particle diameter to 40 and 100 nm resulted in less effective penetration into the spheroid’s core (nine and threefold, respectively). These results clearly demonstrate the feasibility of utilizing pulsed ultrasound in order to increase penetration of NPs into 3D spheroids mimicking tumor tissue.
Figure 8. The schematic drawing shows solid tumor organization with the characteristic acidic front, vascularized circumference, hypoxic region and necrotic core.
Resistant cancer cells and cancer stem cells are typically sequestered in the tumor’s hypoxic and necrotic regions.
Reproduced with permission from [65].
Future perspective
Nanomedicine has a strong potential to accelerate the development of effective approaches to the treatment of drug-resistant and recurrent cancers. However, despite the significant progress made in the development of DDS and other nanoplatform-based approaches, serious limitations have also been identified in applications of these therapies in vivo. A comprehensive review of major problems encountered during the application of nanocarriers for cancer treatment can be found in the literature [66]. Here, we highlight several important limitations of nanocarriers (mostly liposomes and NPs), such as ineffective uptake and distribution in tumor tissue, retention in bypassing organs and by macrophages of the reticuloendothelial system after systemic administration, and limited oral availability. In addition, variations in DDS due to overplaying of various factors (e.g., architecture, size, charge, shape, surface modification and type of vector) may affects the properties and even the efficacy of nanoformulated drugs. A solution to these problems will require substantial and systematic studies in conditions closely related to drug administration routes.
Currently, the main directions in the treatment of drug-resistant cancer cells and CSC-targeting are associated with the following areas: design of novel gene-targeting therapies (e.g., siRNA, miRNA and antisense oligos) against the proteins responsible for the intrinsic drug resistance and survival of CSCs, such as drug efflux transporters, antiapoptotic proteins and members of underlying signaling pathways; development of novel efficient small drug molecules and inhibitors, polymeric drug conjugates and nanocarriers, which are able to reach CSCs in their niche; development of sensitive bioimaging approaches, including theranostics, for the precise location of CSCs; and potential application of methods of physical destruction such as thermoablation, PDT, laser therapy, surgery and so on. Advanced nanoformulations targeting CSCs and other drug-resistant cells must be composed of the following components: a targeting ligand that selectively binds CSC-specific surface receptors; a nanocarrier with high drug-loading capacity; supporting drug molecules, such as inhibitors of P-gp or proapoptotic proteins and pathways, which are capable of sensitizing CSCs and other drug-resistant cells to therapeutic drugs; drug molecules effective against mitosis-inactive CSCs (e.g., salinomycin, or other cell cycle-independent compounds); and conventional cytotoxic therapeutic agent(s) for elimination of the dominant tumor mass. The multimodal drug delivery strategies for overcoming tumor drug resistance and some implications of CSC properties have been summarized in a recently published review [67]. As part of these efforts, our group and other investigators recently developed hydrophilic nanogel carriers that could deliver activated nucleoside analogs, or other cancer drugs, both hydrophobic and hydrophilic, or gene-targeting molecules such as siRNA against drug-resistant cancer cells [68–70]. Nanogels-encapsulated drugs sometimes demonstrated more than 100-fold efficacy against drug-resistant lymphomas, breast and prostate cancers compared to free drugs. Diagnostic elements, which are able to make dispersed cancer cells visible by using bioimaging technologies in vivo, could also be part of nanocarriers with the ability to cure metastatic disease. Evidently, only proper combination of all these elements in future drug nanoformulations could render them effective against drug-resistant cancer cells and CSCs. Whatever the fate of the CSC hypothesis is, the main idea of striking more competitive cells with low proliferative rates and resistance to radio/chemotherapy in order to prevent tumor relapse remains unchanged, as well as simultaneous targeting of different tumor properties in combination cancer therapy that must become like the now standard multidrug HIV treatment. Overall, although the full potential of nanomedicine is not yet completely recognized and its competitiveness against chemotherapeutic or immunological approaches remains to be established, we accept the major thesis that development of proper combinations of chemo and nanotherapies, including novel gene-silencing, drug efflux-inhibiting and CSC-targeting strategies, would be the most effective investment into treatment of drug-resistant and aggressive tumors.
Executive summary.
Cancer stem cell-associated properties
Tumor-initiating or cancer stem cells (CSCs) represent a therapy-resistant cell population that, evidently, is the cause of cancer relapse and the failure of many treatment protocols.
These CSCs demonstrate enhanced survival potential associated with adopting a unique morphology (tumor spheroids), protection by a supportive niche (vascular niche), dormancy and activation of protective antihypoxic, antiapoptotic and drug efflux mechanisms.
CSC-targeted therapies
Eradication of CSCs is currently one of the major challenges of cancer therapy, and can be achieved by using drugs or gene-specific molecules that target CSC-specific pathways, or bind CSC-specific receptors (CD44 and CD133), or deliver drugs in the form of polymer conjugates or nanocarriers.
Special attention is focused on drug-resistant cancers, overexpressing drug efflux transporters (P-glycoprotein, breast cancer resistance and multidrug resistance-associated proteins) and sharing the property with CSCs, and current approaches to their inhibition in order to enhance the therapeutic efficacy of anticancer drugs.
Various combination therapies, including nanomedicine approaches and bioimaging, which form a background for the development of future effective drug formulations against drug-resistant cancers and CSCs, are discussed.
Acknowledgments
We are grateful to J Vetro (UNMC, Omaha, NE, USA) for helpful discussion and help in the preparation of thisreview.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The work was supported by NIH grant R01 CA136921 (SV Vinogradov) and Chinese Scholarship Council (X Wei). The authors also appreciate the support to X Wei from State Key Laboratory of Biotherapy, Sichuan University, China. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008;68:4018–4021. doi: 10.1158/0008-5472.CAN-07-6334. [DOI] [PubMed] [Google Scholar]
- 2▪.Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol. 2009;27(1):44–46. doi: 10.1038/nbt0109-44. The review discusses cancer stem cell (CSC) targeting. [DOI] [PubMed] [Google Scholar]
- 3.Ewald B, Sampath D, Plunkett W. Nucleoside analogs: molecular mechanisms signaling cell death. Oncogene. 2008;27(50):6522–6537. doi: 10.1038/onc.2008.316. [DOI] [PubMed] [Google Scholar]
- 4.Wilson TR, Johnston PG, Longley DB. Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer Drug Targets. 2009;9(3):307–319. doi: 10.2174/156800909788166547. [DOI] [PubMed] [Google Scholar]
- 5.Rochat B. Importance of influx and efflux systems and xenobiotic metabolizing enzymes in intratumoral disposition of anticancer agents. Curr Cancer Drug Targets. 2009;9(5):652–674. doi: 10.2174/156800909789056999. [DOI] [PubMed] [Google Scholar]
- 6.Schatton T, Frank NY, Frank MH. Identification and targeting of cancer stem cells. Bioessays. 2009;31:1038–1049. doi: 10.1002/bies.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong SP, Wen J, Bang S, et al. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 8.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 9.Grange C, Tapparo M, Collino F, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung pre-metastatic niche. Cancer Res. 2011;71(15):5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Cheng W, Lai D, Huang Y, Guo L. Characterization of primary ovarian cancer cells in different culture systems. Oncol Rep. 2010;23(5):1277–1284. doi: 10.3892/or_00000761. [DOI] [PubMed] [Google Scholar]
- 11▪.Shervington A, Lu C. Expression of multidrug resistance genes in normal and cancer stem cells. Cancer Invest. 2008;26(5):535–542. doi: 10.1080/07357900801904140. Expression of multidrug resistance proteins in cancer cells and CSC is discussed. [DOI] [PubMed] [Google Scholar]
- 12.Nobili S, Landini I, Giglioni B, et al. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7(7):861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- 13.Frank NY, Margaryan A, Huang Y, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65(10):4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 14.Jin F, Zhao L, Guo YJ, et al. Influence of etoposide on anti-apoptotic and multidrug resistance-associated protein genes in CD133 positive U251 glioblastoma stem-like cells. Brain Res. 2010;1336:103–111. doi: 10.1016/j.brainres.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Shigeta J, Katayama K, Mitsuhashi J, et al. BCRP/ABCG2 confers anticancer drug resistance without covalent dimerization. Cancer Sci. 2010;101(8):1813–1821. doi: 10.1111/j.1349-7006.2010.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu C, Li H, Li J, et al. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis. 2008;29(12):2289–2297. doi: 10.1093/carcin/bgn223. [DOI] [PubMed] [Google Scholar]
- 17.Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Curr Stem Cell Res Ther. 2008;3:237–246. doi: 10.2174/157488808786734006. [DOI] [PubMed] [Google Scholar]
- 18.Honoki K, Fujii H, Kubo A, et al. Possible involvement of stem-like populations with elevated ALDH1 in sarcomas for chemotherapeutic drug resistance. Oncol Rep. 2010;24(2):501–505. doi: 10.3892/or_00000885. [DOI] [PubMed] [Google Scholar]
- 19.Rausch V, Liu L, Kallifatidis G, et al. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010;70(12):5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- 20.Kobune M, Takimoto R, Murase K, et al. Drug resistance is dramatically restored by Hedgehog inhibitors in CD34+ leukemic cells. Cancer Sci. 2009;100(5):948–955. doi: 10.1111/j.1349-7006.2009.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng Y, Wang X, Wang Y, et al. Wnt/β-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392(3):373–379. doi: 10.1016/j.bbrc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Deng YH, Pu XX, Huang MJ, et al. 5-fluorouracil upregulates the activity of Wnt signaling pathway in CD133-positive colon cancer stem-like cells. Chin J Cancer. 2010;29(9):810–815. doi: 10.5732/cjc.010.10134. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Li Y, Kong D, et al. Acquisition of epithelial–mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69(6):2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪▪.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. Discusses the importance of CSC niches. [DOI] [PubMed] [Google Scholar]
- 25.Folkins C, Man S, Xu P, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67(8):3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 26.Hovinga KE, Shimizu F, Wang R, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28(6):1019–1029. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams RF, Sims TL, Tracey L, et al. Maturation of tumor vasculature by interferon-β disrupts the vascular niche of glioma stem cells. Anticancer Res. 2010;30(9):3301–3308. [PubMed] [Google Scholar]
- 28.Morozevich GE, Kozlova NI, Ushakova NA, Preobrazhenskaia ME, Berman AE. Implication of integrin α5β 1 in human breast carcinoma apoptosis and drug resistance. Biomed Khim. 2011;57(1):77–84. doi: 10.18097/pbmc20115701077. [DOI] [PubMed] [Google Scholar]
- 29▪▪.Harada H, Kizaka-Kondoh S, Li G, et al. Significance of HIF-1-active cells in angiogenesis and radioresistance. Oncogene. 2007;26(54):7508–7516. doi: 10.1038/sj.onc.1210556. Authors reviewed the resistance to hypoxia in cancer cells. [DOI] [PubMed] [Google Scholar]
- 30.Piret JP, Cosse JP, Ninane N, et al. Hypoxia protects HepG2 cells against etoposide-induced apoptosis via a HIF-1-independent pathway. Exp Cell Res. 2006;312(15):2908–2920. doi: 10.1016/j.yexcr.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Wirthner R, Wrann S, Balamurugan K, et al. Impaired DNA double-strand break repair contributes to chemoresistance in HIF-1 α-deficient mouse embryonic fibroblasts. Carcinogenesis. 2008;29(12):2306–2316. doi: 10.1093/carcin/bgn231. [DOI] [PubMed] [Google Scholar]
- 32.Choi YJ, Rho JK, Lee SJ, et al. HIF-1α modulation by topoisomerase inhibitors in non-small cell lung cancer cell lines. J Cancer Res Clin Oncol. 2009;135(8):1047–1053. doi: 10.1007/s00432-009-0543-2. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz DL, Bankson JA, Lemos R, et al. Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer. Mol Cancer Ther. 2010;9:2057–2067. doi: 10.1158/1535-7163.MCT-09-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mimeault M, Batra SK. Potential applications of curcumin and its novel synthetic analogs and nanotechnology-based formulations in cancer prevention and therapy. Clin Med. 2011;6:31. doi: 10.1186/1749-8546-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Lim KJ, Bisht S, Bar EE, et al. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther. 2011;11(5):464–473. doi: 10.4161/cbt.11.5.14410. Authors describe the application of curcumin-loaded nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banzato A, Bobisse S, Rondina M, et al. Apaclitaxel-hyaluronan bioconjugate targeting ovarian cancer affords a potent in vivo therapeutic activity. Clin Cancer Res. 2008;14(11):3598–3606. doi: 10.1158/1078-0432.CCR-07-2019. [DOI] [PubMed] [Google Scholar]
- 38.Choi KY, Chung H, Min KH, et al. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31(1):106–114. doi: 10.1016/j.biomaterials.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Upadhyay KK, Bhatt AN, Mishra AK, et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(γ-benzyl L-glutamate)-b-hyaluronan polymersomes. Biomaterials. 2010;31(10):2882–2892. doi: 10.1016/j.biomaterials.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 40▪.Wu W, Shen J, Banerjee P, et al. Core-shell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemophotothermal treatment. Biomaterials. 2010;31(29):7555–7566. doi: 10.1016/j.biomaterials.2010.06.030. Describes the application of thermoablative hybrid nanogels. [DOI] [PubMed] [Google Scholar]
- 41.Wu W, Shen J, Banerjee P, Zhou S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials. 2011;32(2):598–609. doi: 10.1016/j.biomaterials.2010.08.112. [DOI] [PubMed] [Google Scholar]
- 42.Bourseau-Guilmain E, Bejaud J, Griveau A, et al. Development and characterization of immunonanocarriers targeting the cancer stem cell marker AC133. Int J Pharm. 2011;423(1):93–101. doi: 10.1016/j.ijpharm.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhou P, Hatziieremia S, Elliott MA, et al. Uptake of synthetic low density lipoprotein by leukemic stem cells – a potential stem cell targeted drug delivery strategy. J Control Release. 2010;148(3):380–387. doi: 10.1016/j.jconrel.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Li RJ, Ying X, Zhang Y, et al. All-trans retinoic acid stealth liposomes prevent the relapse of breast cancer arising from the cancer stem cells. J Control Release. 2011;149(3):281–291. doi: 10.1016/j.jconrel.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Guo J, Zhou J, Ying X, et al. Effects of stealth liposomal daunorubicin plus tamoxifen on the breast cancer and cancer stem cells. J Pharm Pharm Sci. 2010;13(2):136–151. doi: 10.18433/j3p88z. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Yao HJ, Yu Y, et al. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials. 2012;33(2):565–582. doi: 10.1016/j.biomaterials.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 47.Milane L, Duan Z, Amiji M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol Pharm. 2011;8(1):185–203. doi: 10.1021/mp1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009;136(1):21–29. doi: 10.1016/j.jconrel.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 49.Lei T, Srinivasan S, Tang Y, et al. Comparing cellular uptake and cytotoxicity of targeted drug carriers in cancer cell lines with different drug resistance mechanisms. Nanomedicine. 2011;7(3):324–332. doi: 10.1016/j.nano.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamaeva V, Rosenholm JM, Bate-Eya LT, et al. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of notch signaling in cancer. Mol Ther. 2011;19(8):1538–1546. doi: 10.1038/mt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim TH, Mount CW, Gombotz WR, et al. The delivery of doxorubicin to 3-D multicellular spheroids and tumors in a murine xenograft model using tumor-penetrating triblock polymeric micelles. Biomaterials. 2010;31(28):7386–7397. doi: 10.1016/j.biomaterials.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Yao HJ, Ju RJ, Wang XX, et al. The anti-tumor efficacy of functional paclitaxel nanomicelles in treating resistant breast cancers by oral delivery. Biomaterials. 2011;32(12):3285–3302. doi: 10.1016/j.biomaterials.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 53▪.Pan X, Thompson R, Meng X, et al. Tumor-targeted RNA-interference: functional non-viral nanovectors. Am J Cancer Res. 2011;1(1):25–42. Application of siRNA against genes of drug resistance is discussed. [PMC free article] [PubMed] [Google Scholar]
- 54.Patil YB, Swaminathan SK, Sadhukha T, et al. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31(2):358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Zhao G, Liu J, et al. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Control Release. 2009;140(3):277–283. doi: 10.1016/j.jconrel.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Bathula SR, Li J, et al. Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. J Biol Chem. 2010;285(29):22639–22650. doi: 10.1074/jbc.M110.125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verma A, Guha S, Diagaradjane P, et al. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res. 2008;14(8):2476–2483. doi: 10.1158/1078-0432.CCR-07-4529. [DOI] [PubMed] [Google Scholar]
- 58.Kato T, Natsume A, Toda H, et al. Efficient delivery of liposome-mediated MGMT-siRNA reinforces the cytotoxity of temozolomide in GBM-initiating cells. Gene Ther. 2010;17(11):1363–1371. doi: 10.1038/gt.2010.88. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Gerson T, Varney ML, et al. Multifunctional peptide-PEG intercalating conjugates: programmatic of gene delivery to the blood–brain barrier. Pharm Res. 2010;27(12):2528–2543. doi: 10.1007/s11095-010-0256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60▪.Glazer ES, Zhu C, Massey KL, et al. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin Cancer Res. 2010;16(23):5712–5721. doi: 10.1158/1078-0432.CCR-10-2055. Authors describe killing cancer cells by radiofrequency-activated gold nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khdair A, Chen D, Patil Y, et al. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance. J Control Release. 2010;141(2):137–144. doi: 10.1016/j.jconrel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barth BM, Altlnoglu EI, Shanmugavelandy SS, et al. Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia. ACS Nano. 2011;5(7):5325–5337. doi: 10.1021/nn2005766. [DOI] [PubMed] [Google Scholar]
- 63.Wang CH, Chiou SH, Chou CP, et al. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. 2011;7(1):69–79. doi: 10.1016/j.nano.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 64.Li R, Wu R, Zhao L, et al. P-glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells. ACS Nano. 2010;4(3):1399–1408. doi: 10.1021/nn9011225. [DOI] [PubMed] [Google Scholar]
- 65▪.Grainger SJ, Serna JV, Sunny S, et al. Pulsed ultrasound enhances nanoparticle penetration into breast cancer spheroids. Mol Pharm. 2010;7(6):2006–2019. doi: 10.1021/mp100280b. Authors introduce ultrasound-enhanced nanodelivery of anticancer drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪.Bae YH, Park K. Targeted drug delivery to tumors: myth, reality and possibility. J Control Release. 2011;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. Discusses problems associated with the tumoral delivery of nanocarriers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪▪.Milane L, Ganesh S, Shah S, et al. Multimodal strategies for overcoming tumor drug resistance: hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. J Control Release. 2011;155(2):237–247. doi: 10.1016/j.jconrel.2011.03.032. An excellent review of drug delivery strategies to overcome drug resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galmarini CM, Warren G, Senanayake MT, Vinogradov SV. Efficient overcoming of drug resistance to anticancer nucleoside analogs by nanodelivery of active phosphorylated drugs. Int J Pharm. 2010;395(1–2):281–289. doi: 10.1016/j.ijpharm.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ambardekar VV, Han HY, Varney ML, et al. The modification of siRNA with 3′ cholesterol to increase nuclease protection and suppression of native mRNA by select siRNA polyplexes. Biomaterials. 2011;32(5):1404–1411. doi: 10.1016/j.biomaterials.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senanayake TM, Warren G, Vinogradov SV. Novel anticancer polymeric conjugates of activated nucleoside analogs. Bioconjug Chem. 2011;22(10):1983–1993. doi: 10.1021/bc200173e. [DOI] [PMC free article] [PubMed] [Google Scholar]