Abstract
Actin – 2E4/kaptin – platelet activation – stereocilia – sensory epithelium
Platelet activation, crucial for hemostasis, requires actin polymerization, yet the molecular mechanisms by which localized actin polymerization is mediated are not clear. Here we report the characterization of a novel actin-binding protein. 2E4, originally isolated from human blood platelets and likely to be involved in the actin rearrangements occurring during activation. 2E4 binds to filamentous (F)-actin by F-actin affinity chromatography and is eluted from F-actin affinity columns and extracted from cells with ATP. Its presence at the leading edge of platelets spread on glass and in the lamellipodia of motile fibroblasts suggests a role in actin dynamics. Using localization to obtain clues about function, we stained the sensory epithelium of the embryonic inner car to determine whether 2E4 is at the barbed end of actin filaments during their elongation. Indeed, 2E4 was present at the tips of the elongating stereocilium. 2E4 is novel by DNA sequence and has no identifiable structural motifs. Its unusual amino acid sequence, its ATP-sensitive actin association and its location at sites of actin polymerization in cells suggest 2E4 plays a unique role in the actin rearrangements that accompany platelet activation and stereocilia formation.
Introduction
Platelets rapidly polymerize actin upon stimulation with a variety of pro-coagulants [3, 11, 15. Failure of actin polymerization in platelets results in an inability to form blood clots and fatal hemorrhage. Actin filament structures are regulated and maintained by interactions with many different actin-binding proteins most of which have been found in platelets [14]. These many actin-binding proteins apparently act in concert in an as yet poorly understood choreography to produce four distinct actin-based structures during platelet activation [7]. These four structures represent the major actin-based components of all cells; stress fibers, contractile units, surface ruffles, and filopodia [16]. Each of these structures contains a different complement of actin-binding proteins and mediates a different aspect of platelet function. However, what the proteins are that initiate the formation of each structure remains a mystery.
At least three putative actin-polymerizers are present in platelets; VASP [25], Arp2/3 complex [19, 36, 37], and gelsolin/profilin [4, 30, 39]. While VASP is found in adhesion plaques [25], gelsolin is present at the barbed ends of filaments at the periphery [13] and it is not known where Arp 2/3 complex is in platelets, although it is found in the lamellipodia of cultured fibroblasts [18, 37] and soil amoebae [19].
We have identified a protein from platelets, 2E4, that is likely to be involved in the localized polymerization of actin in the lamellipodia [6, 7, 40, 41]. This protein was identified using filamentous (F)-actin affinity chromatography of proteins solubilized from activated platelets, a procedure that has been successfully used to find actin-binding proteins from other species [2, 10, 21, 31]. Thirty-three individual protein species detected by silver-staining of gels after SDS-PAGE were isolated from platelets, many of which turned out to be actin-binding proteins previously identified by other methods [7]. However, fourteen of these proteins were not identifiable and thus apparently newly discovered by the F-actin affinity column approach.
The localization of protein provides clues as to its cellular function. Highly specialized subcellular structures performing a single function are especially useful for such localization studies, because presence in such a structure indicates participation in relatively few biochemical activities. The stereocilium of the hair cell in the sensory epithelium of the inner ear is such a specialized structure. Since they are composed of a bundle of long actin filaments, 0.8–5.5 µm in length [32, 33, 35], with the barbed ends all at the tip, stereocilia can be used as substrate to determine whether a particular protein is present at the barbed or fast-growing ends of actin filaments inside cells by immunofluorescence microscopy. The actin polymerization machine responsible for the formation of these actin bundles during embryogenesis is thought to be at the tip of the stereocilia [32]. Thus, localization to the tip in the embryo suggests a role in regulated actin filament growth.
In this report, we focus on one of the fourteen new proteins found by F-actin affinity chromatography, protein #32 [7]. We chose this protein because its location in lamellipodia of spreading platelets, migrating fibroblasts and growth cones suggests it plays a role in the events that occur there, including the formation of actin filaments [5, 7]. In this report, the monoclonal antibody, MAb 2E4, generated against this protein [5], is used to study in more detail the location of the protein in spread platelets, in motile fibroblasts, as well as in hair cells of the inner ear. MAb 2E4 is then used to clone the 2E4 cDNA and obtain its sequence.
Materials and methods
Filamentous (F)-actin affinity chromatography
Affinity chromatography was performed as described [7]. Briefly, platelets were obtained from the RI Blood Bank on or before outdate, washed three times in acid citrate dextrose, incubated in Tyrode’s solution (138 mM Nacl, 2.9 mM KCl, 12 mM sodium bicarbonate, 0.36 mM sodium phosphate, 5.5 mM glucose, and 0.4 mM MgCl2, pH 7.4) for one h at 37°C, activated for 15s with ADP, and lysed by a addition of an equal volume of ice-cold lysis buffer (10 mM Tris-HCl (pH 7.5), 1 mM Na, EDTA, 1 mM Na, EGTA, 2% Nikkol monomolecular detergent, 2:100 dilution of a protease inhibitor cocktail containing 0.1 M phenylmethylsulphonyl fluoride, 1 mM benzamidine-HCl, 1 mg/ml each of leupeptin, pepstatin A, phenanthroline and aprotinin in ethanol). Aliquots taken after lysis are referred to as “homogenate”. Immediately after lysing, the platelet suspension was sonicated by four 20s bursts in a Branson sonifier cell disruptor 350 using the microtip set at 4. After sonication, the sonicate was placed on ice and then centrifuged at 10000g for 20 min at 4°C. The resulting supernatant was brought to 50 mM Tris-HCl (pH 7.5), 5 mM DTT and 2 mM sodium pyrophosphate and then clarified by centrifugation at 100000g for 1 h and the resultant supernatant loaded onto an F-actin column. Typically we used 4 units of platelets on a 5 ml column at 1 mg/ml F-actin stabilized with phalloidin, constructed from rabbit skeletal muscle as described [21].
Columns were washed with column buffer (50 mM Hepes (pH 7.5), 0.02% Nonidet-P40, 0.5 mM Na3 EDTA, 0.5 mM Na3 EGTA, 5 mM DTT, 1:1000 protease inhibitor cocktail, and 10% glycerol) until the protein in the eluate dropped below 0.01 mg/ml by BioRad Bradford assay. They were then eluted sequentially with (1) 5mM ATP and 10 mM MgCl2 in column buffer and (2) 1 M KCl in column buffer. Columns were eluted no faster than 1 ml per minute to decrease breakage of actin filaments from the column bed. 1.5 ml fractions were collected, analyzed by Bradford protein assay, the peak fractions pooled, protein precipitated in 10% trichloroacetic acid and resuspended in gel sample buffer. Equal elution volumes from parallel F-actin and control columns were loaded in adjacent lanes on 6–12% gradient SDS-gels. Western blots were carried out as described [7], using a 1:1000 dilution of MAb 2E4 ascites followed by goat anti-mouse IgM conjugated to alkaline phosphatase as secondary antibody (Bochringer-Mannheim, IN).
For anion exchange column chromatography, platelet proteins were extracted and solubilized as described above and loaded onto a 10 ml DEAE column prepared according to the manufacturer’s instructions (Whatman BioSystems, Clifton, NJ). The column was eluted with a 120 ml salt gradient from 20 mM NaCl to 1 M NaCl in DEAE column buffer (20 mM Tris (pH 8.1), 20mM NaCl, 1 mM EGTA, 1 mM EDTA, 2 mM Na pyrophosphate). Salt gradient was monitored by conductance. 1.5 ml fractions were collected, mixed with ¼ volume if 4 × gel sample buffer and loaded onto 6–12 % gradient SDS-gels. Blots were performed as described. The Coomassie-stained band corresponding to the band recognized by MAb 2E4 was excised from the gel and subjected to in-gel proteolysis followed by HPLC separation and automated Edman degradative sequencing as we described [20].
Immunofluorescence
Fresh platelets were obtained from healthy volunteers [3] and used on the day they were drawn. Ten microliters of platelet-rich plasma was placed on a clean #1 glass coverslip, mixed 1:1 with 2 × Tyrode’s solution and allowed to spread for 15 min at RT. Spread cells were fixed in 4% paraformaldehyde in Small’s buffer (137 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 2H2O, 0.4 mM KH2PO4, 5.5 mM glucose, 4 mM NaHCO3, 2 mM MgCl2, 2 mM EGTA, 10 mM MES, (pH 6.1) [28] plus 0.25 % Triton X-100 and 1 µg/ml rhodamine phalloidin (Molecular Probes, Eugene, OR) as previously described [7].
Chick embryo fibroblasts were removed from 8–9 day-old chick embryos, plated in Dulbecco’s modified medium supplemented with 10% fetal calf serum, allowed to grow to confluence, and then frozen and stored. Aliquots were thawed, cultured in the same media and used up to the fifth passage. For immunofluorescence, confluent cultures were trypsinized, suspended in growth media, washed, diluted and replated onto UV-irradiation-sterilized coverslips. Cells were allowed to attach to coverslips for 8–36 hours, motility was monitored by phase contrast microscopy, and cells were then fixed by floating the coverslip first in Small’s buffer at 37°C, and then fixed in Small’s buffer at 37°C containing 4% paraformaldehyde, 0.5% Triton X-100.
Coverslips of either fibroblasts or platelets were blocked for 20 min in PBT (phosphate-buffered saline, 1% BSA, 0.1% Triton X-100) and stained fro 20 min with 1:500 dilution of MAb 2E4 in PBT. After three washes in PBT, coverslips were counter-stained with fluorescein-conjugated, affinity-purified goat anti-mouse IgM (Boehringer-Mannheim) in PBT for 20 min, washed in PBS, mounted in 1 mg/ml 4-diazabicyclo [2.2.2]-octane in glycerol (Aldrich Chem. Co. Milwaukee, WI) and viewed in a Nikon SA with a 100 Watt mercury bulb. Platelets were photographed using a 60 × objective with a 1.4 NA, a 1.25 × optivar, and a 5 × projection lens for a final magnification on the film of approximately 375 ×. Fibroblasts were photographed at lower magnifications.
Chick cochlea were obtained by removal of the cochlea from 18-day chick embryos, papain digestion (5 µl/ml of Sigma solution P3125) for 30 min, and subsequent removal of the tectal membrane. After rinsing in Hanks’ buffered saline, isolated cochlea were fixed for 1 h at 22°C, in 3.7% paraformaldehyde, 50 mM phosphate buffer (pH 6.3), and 0.5% Triton X-100. Cochlea were also treated with 1% Triton X-100, 20 mM Pipes (pH 6.8) and 1.5 mM MgSO4 prior to fixation (conditions which preserve the actin bundles of the stecocilia but remove the membrane, [33]).
For immunofluorescence, cochlea were blocked and stained in 20 mM Pipes (pH 6.8), 0.1% Triton X-100, 1.5 mM MgSO4, 1% BSA, MAb 2E4 was used at a 1:1000 dilution. Rhodamine-conjugated goat anti-mouse IgM/IgG (Boehringer-Mannheim) was used as secondary antibody. Anti-tubulin monoclonal IgG and anti-actin monoclonal IgM (Amersham) were used to stain pieces of cochlea from the same bird in parallel with 2E4 as controls for penetration and non-specific effects using the same secondary antibody. In some experiments, actin was also stained with FITC-phalloidin (Sigma, St Louis, MO) at 0.3 µg/ml for 10 min in PBS. Fluorescence and phase-contrast micrographs were photographed on a Nikon Epifluorescence or a Zeiss Axioskop microscope onto hypersensitized Kodak Technical Pan 2514 or Tmax 400 film.
Immunoblots of non-platelet proteins
Chick embryo fibroblasts were harvested by trypsinization from non-confluent cultures, washed briefly in PBS and boiled in sample buffer. Chick intestinal brush border was a gift of Kay Broschat, and growth cones from neonatal mouse brain were from Pate Skene. Chick cochlea were dissected as for immunofluorescence and frozen in liquid nitrogen [34]. All of these samples were then boiled directly in SDS-containing gel sample buffer. Samples were analyzed by Western blotting as described for platelet proteins above.
Cloning and sequencing of 2E4
A lambda gill library made from cDNA of phorbol ester-treated HEL cells [17] was screened by blotting with MAb 2E4. Thirteen positive clones were identified from screens of 1.5 × 107 clones. Six of these clones were selected at random, plaque purified, and the phage isolated by cesium chloride gradient. EcoR1 digests showed that one of these had an insert large enough to encode the 45 kDa 2E4 antigen, 1.65 kbp and two others had more than one EcoR1 fragment inserted. This 1.65 kbp insert cross-hybridized with 4 of the 5 other clones. This clone was ligated into pGEX in the hopes of obtaining a clone that could be used both for sequencing and for bacterial expression. This pGEX clone was subjected to bidirectional automated sequencing by dideoxy chain termination, beginning with universal plasmid primers, and then by using primers from each successive sequence in a walk. All regions were sequenced in both directions at least twice, and most more than four times. The resultant DNA sequence (GenBank AF105369) was compared to the data bank using Blast which identified several human and mouse ESTs and chromosomal CpG islands (human: 261120, 261121, h28816, z45636, t31890, R21053, R13180, W85777, T08234, AA095563, AA339326, w85778, h08141, z41308; and mouse: AA450505, AA403679, AA403673, AA 174430). Amino acid sequence was predicted using the “translate” function of the GCG package [9].
No protein was expressed by the full-length cDNA in pGEX, probably because of two stop codons in the untranslated region just before the ATG start site. Therefore, to obtain polyclonal antibodies, a 510 bp piece internal to the coding region was obtained by PstI digest (bp 240–750) and ligated into the PstI site of pQE31 (Qiagen, Santa Clara, CA). Bacterially expressed protein was purified by nickel column chromatography, further purified by SDS-PAGE, and then the large band visible by Coomassie excised and submitted to Babco (Berkeley, CA) to generate polyclonal antibodies in rabbits. Serum was titered by Western blot and affinity purified on purified bacterially expressed protein. Immunofluorescence of platelets was performed as described above but with a goat anti-rabbit secondary antibody (Boehringer-Mannheim).
Results
2E4 elutes from F-actin columns with ATP
Filamentous (F)-actin affinity chromatography of proteins in extracts from ADP-activated platelets enriches for a number of different protein species as detected by Coomassie-staining of gels after SDS-PAGE (Fig. 1A. At least four of these proteins are eluted from the affinity column with 5 mM ATP, while the rest, twelve or more additional proteins, are stripped from the column with high salt. Under these conditions, control columns of BSA (Fig. 1A) or insulin (not shown) when loaded, washed and eluted in parallel with the F-actin affinity column retain only small amounts of protein. SDS-PAGE of control column eluates demonstrates that these proteins are of different molecular weights than those eluting from F-actin columns (Fig. 1, BSA lanes). As previously described, one of the effects of thrombin activation is a decrease in the intensity of the top two high molecular weight bands, composed of filamin/actin-binding protein, and talin respectively [8]. In unactivated platelets the top three bands are similar in intensity. The decrease in the top two bands relative to the bank third from the top in the homogenate (Fig. 1, lane H), a sample colleted after ADP treatment of the platelets, confirms that activation has taken place.
Fig. 1.
2E4 elutes from F-actin affinity columns with ATP. A. Coomassie-stained 6–12% gradient SDS-gel of H, ADP-activated platelet homogenate; X, extract of same preparation after clarifying centrifugation which is loaded in parallel onto two columns, one cross-linked with F-actin and the other with albumin (BSA); W, last wash before elution from either column; ATP, fraction containing the peak protein eluted with 5 mM ATP from either column; KC1, peak protein fraction eluted with 1 M KCl from either column. Bars indicate molecular weight standards 200, 98, 68, 45, and 31 kDa. B. Corresponding Western blot of the same samples probed with MAb 2E4. Order of lanes is the same as in (A). Bar indicates 45 kDa standard. Note that a ~45 kDa band appears in homogenate, extract and the ATP elution from the F-actin column only.
The MAb 2E4 antigen elutes from the F-actin column with 5 mM ATP and 10 mM MgCl2, as demonstrated by corresponding Western blots of F-actin column experiments probed with the monoclonal antibody, MAb 2E4 (Fig. 1B. 2E4 is present as a ~45 kDa band in activated platelet homogenates and remains soluble in the extract after clarifying centrifugation. It is not present in the last wash before elution, but reappears in the ATP eluate, which apparently releases all detectable antigen from the column since none is found in the subsequent salt elution. Interaction with F-actin is responsible for this retention, as 2E4 is also absent from both elutions of the BSA control column run in parallel.
Localization by immunofluorescence of 2E4 in spread platelets
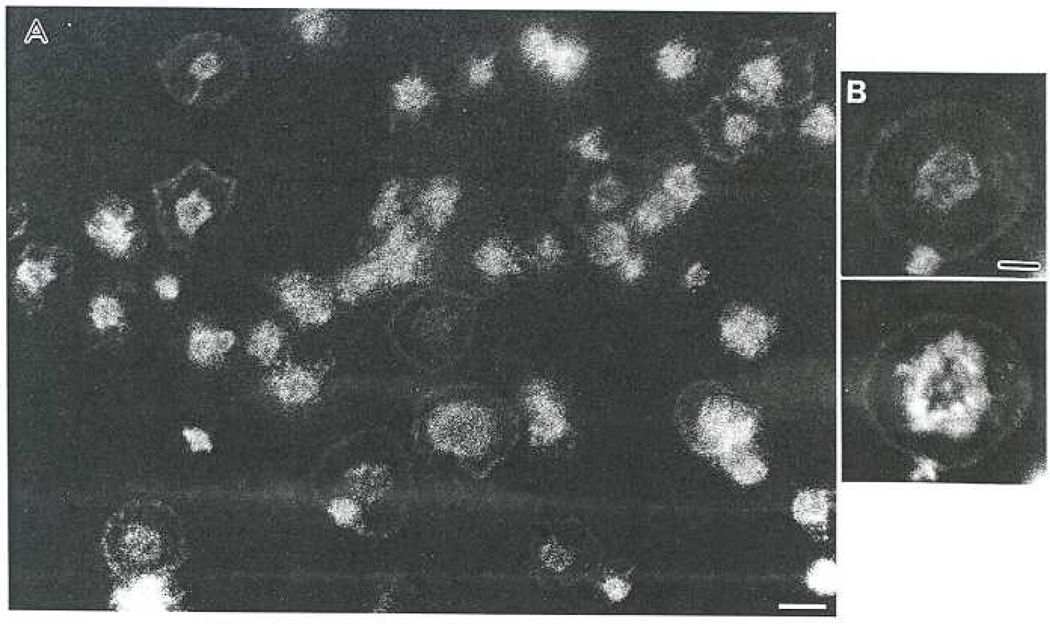
MAb 2E4 stained the outermost edge of platelets spreading on glass (Fig. 2). This is the site where increases in the barbed end of actin filaments occurs [13]. The antibody also stained the hump of cytoplasm in the center of the spreading platelet. The intensity of this stain is too bright to distinguish whether the contractile ring, stress-like fibers or adhesion plaques are also stained. However, in higher magnifications of platelets double-labeled for 2E4 (Fig. 2B, upper) and actin (Fig. 2B, lower) only the peripheral staining by MAb 2E4 is coincident with that of phalloidin staining of actin.
Fig. 2.
2E4 is localized in the lamellipodia of platelets spread on glass. A. Low magnification of a field of platelets spread for 15 min on glass and stained with MAb 2E4, Bar = 5 µm. B. An individual platelet shown at higher magnification double-stained for 2E4 (upper) and F-actin with phalloidin (lower). Note that the staining coincides at the periphery but not in the center of the cell. Bar = 2µm.
2E4 antigen is present in other cells with specialized actin structures
Since human blood platelets can be obtained in quantities sufficient for biochemical analysis from the blood bank, they provide a convenient source for human cytoskeletal proteins that would otherwise be hard to obtain. To determine whether the MAb 2E4 antigen is also present in other cells where proteins involved in actin polymerization would be expected, blots of chick intestinal microvilli, chick fibroblasts, and the sensory epithelium of the chick cochlea were probed with the monoclonal antibody (Fig. 3). In all cells but the human red blood cell (not shown) Mab 2E4 detected a ~45 kDa band. In chick, MAb 2E4 recognized a single bank of the same molecular mass, 45 kDa, as in human platelets.
Fig. 3.
MAb 2E4 recognizes ~45 kDa protein in Western blots of other cells with specialized actin structures. Upper panel: Coomassie-stained 10% SDS-gel of specimen as indicated. Molecular weight standards at the left; 200, 98, 45, 31 kDa. Platelets are human and all other samples are chick. Lower panel: Corresponding Western blot of the same samples probed with MAb 2E4. Bands identified by the antibody are the same size, ~45 kDa, in all lanes. In the platelet sample, there is also a faint 55 kDa band as well as some staining at the dye front. Note that the antibody recognizes a single 45 kDa band in chick samples.
MAb 2E4 stained the outermost third of the actin filaments of the leading edge of primary cultures of sub-confluent chick fibroblasts (Fig. 4A and C). As in platelets, it did not stain stress fibers or adhesion plaques, which are easily identified by double-labeling with fluorescent phalloidin (Fig. 4B and D). Just proximal to the leading edge, at the point where the staining of recognizable actin filaments in the ruffle was no longer clear, MAb 2E4 staining was punctuate, suggestive of antigen aggregates (Fig. 4C). In contrast, there is minimal phalloidin staining in this region between the ruffle at the periphery and the intense staining of the stress fibers proximally (Fig. 4D). MAb 2E4 also stained retraction fibers, making them appear as if they are collapsed aggregates of filaments from a ruffle that had accordioned upon itself.
Fig. 4.
2E4 is located in the lamellipodia of chick embryo fibroblasts, Double-labeled images of chick embryo fibroblasts stained with MAb 2E4 (A, C) and for actin with phalloidin (B, D). Arrows in A and B indicate adhesion plaques, visible when stained for actin but not stained by 2E4. Bar = 5µm.
MAb 2E4 stains the tips of the stereocilia in cochlear hair cells
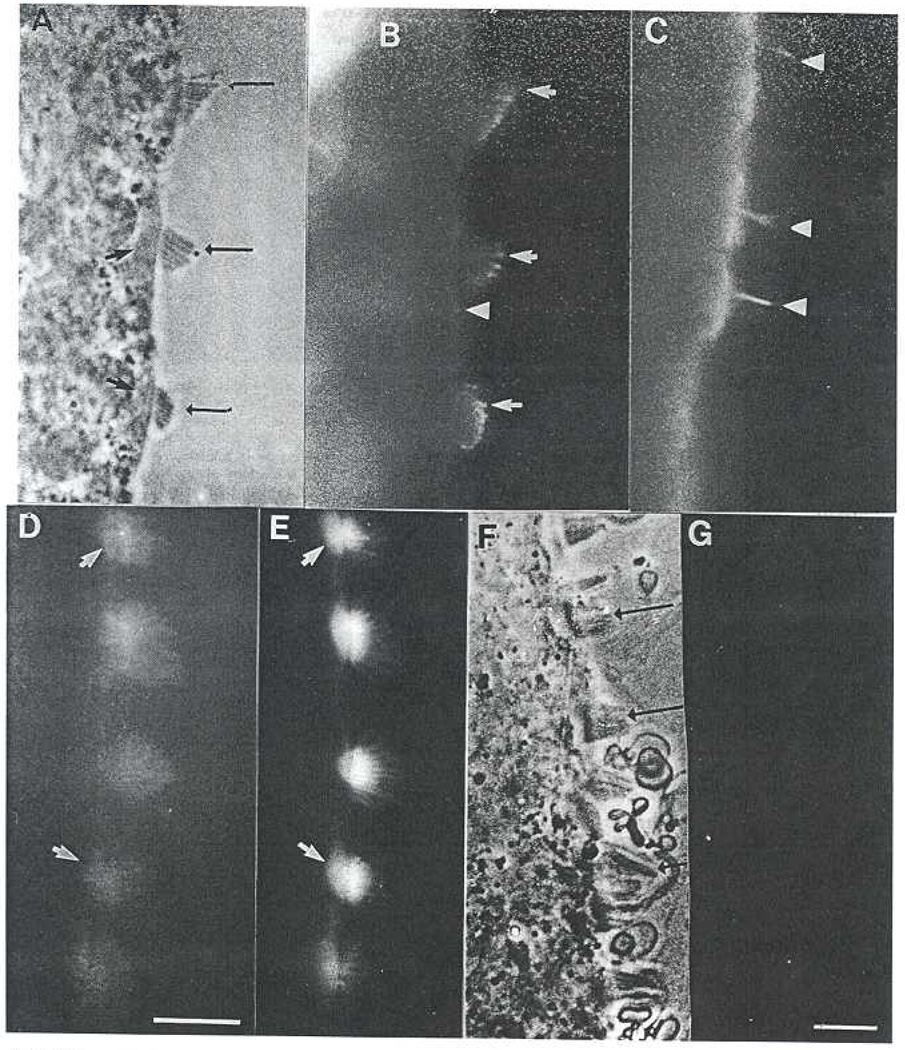
MAb 2E4 stained the outermost (~20%) of stereocilia, Phase-contrast microscopy of a chick embryo cochlea lying on its side reveals the staircase pattern of stereocilia projecting from the surface of the hair cells (Fig. 5A). The same field viewed by immunofluorescence shows that MAb 2E4 staining was confined to the tips of the stereocilia (Fig. 5B). No staining was seen within the hair cell, not even in the actin-rich tectal plate located just beneath the stereocilia in the apical cytoplasm. The taller stereocilia were stained more brightly than shorter stereocilia within a sing staircase. The staircases of stereocilia increase in length from the proximal to the distal end of the cochlea. While all stereocilia throughout the sensory epithelium stained, the staining appeared decreased at the end of the cochlea in which the staircases were shortest.
Fig. 5.
2E4 is located at the distal tips of stereocilia of hair cells in the inner ear. A. Phase-contrast image of sensory epithelium from the chick cochlea. Long arrows indicate staircase of stereocilia projecting from the apical surface of the hair cells. Short arrows indicate position of tectal plate. B. Same field as in (A) imaged by fluorescence microscopy to show 2E4 staining, which is limited to the outer 1/3 of the stereocilia (arrows). Note possible weak staining of short microvilli on support cell (arrowhead) and no staining of tectal plate. C. A different sensory epithelium stained for microtubules, Arrowheads indicate the primary cilium, a microtubule-rich structure. D. Another specimen, stained with an IgM anti-actin monoclonal antibody. Note that the entire stereocilium is stained, as well as the tectal plate in the apical cytoplasm of the hair cell. Bar = 10 µm. E. Another specimen labeled with phalloidin for F-actin. Note that the distribution of staining matches that of anti-actin. F. Phase-contrast image of a cochlear sensory epithelium that was extracted with 5 mM ATP and detergent before fixation. Note that the stereocilia are intact (arrows) although there is substantial membranous debris. G. Same fields as (F), imaged with fluorescence for 2E4. No staining is detected. Bar shown in (G) indicates magnification for A–C, E–G and corresponds to 10 µm.
Staining of another sensory epithelium from the cochlea with anti-tubulin antibodies using the same secondary antibody as that shown in Fig. 5B demonstrates that restriction of staining to the tip is not an artefact of the secondary antibody (Fig. 5C). The primary cilium, a microtubule-rich structure, was brightly stained by the anti-tubulin antibodies as is the apex of the hair cell, while the stereocilia were not stained. Failure to stain the more proximal actin filaments in the stereocilia could be due to failure of MAb 2E4 to penetrate the densely packed filaments in the bundle. This is not the case, however, since sensory epithelia stained in parallel with an anti-actin IgM antibody are stained along their length and the tectal plate was stained as well (Fig. 5D). This anti-actin IgM staining corresponds to the staining pattern observed with phalloidin, a very small and readily permeable molecule (Fig. 5E). Thus, IgM can indeed penetrate the actin bundles along their length and therefore stains if the antigen is present there.
It has previously been demonstrated that 2E4 is extracted from cells with detergent and ATP [5]. When the cochlear sensory epithelium was extracted with detergent and ATP in a buffer that preserves intact stereocilia (Fig. 5F), MAb 2E4 staining is absent (Fig. 5G). This extraction together with its molecular weight in Western blots (Fig. 4) supports the conclusion that the antigen stained by MAb 2E4 in stereocilia is similar biochemically to the platelet antigen, and thus likely to be the same protein as that originally identified in platelets.
Partial purification of the 2E4 antigen and determination of primary amino acid sequence
Platelet proteins solubilized by sonication and detergent are separated by anion exchange chromatography, as demonstrated by SDS-PAGE (Fig. 6A). Actin, a major platelet protein, elutes between 0.2 and 0.3 M salt with a peak in fraction 19 readily visible on a Coomassie-stained gel, migrating at 43 kDa. In contrast, MAb 2E4 detects a band eluting at lower salt in fraction 7, well before the major actin peak (Fig. 6B, western blot). A band at the bottom of the blot is possibly a result of proteolytic breakdown.
Fig. 6.
Semi-purification of 2E4 by anion exchange chromatography. A. Coomassie-stained gel of peak fractions from a DEAE column loaded with activated platelet extract and eluted with a 0.02–1.0 M salt gradient. Note that each fraction is enriched with a different set of protein species. Molecular weights: 200, 98, 68, 45, 31 kDa. B. Corresponding Western blot loaded with the same samples as (A) and probed with MAb 2E4. Arrows in A and B indicate 45 kDa. Note the ~45 kDa band in fraction 7 in B. Molecular weight standards: 200, 98, 68, 45 kDa.
Peptide sequencing of the ~45 kDa band from the Coomassie-stained gel corresponding to the band recognized by the monoclonal antibody produced two amino acid sequences: RGLVVGITFIK, and LQFNYIVDA, Fasta and Blast searches of the databank produced no significant matches for either of these sequences.
2E4 is a novel protein
Platelets have no nucleus and do not synthesize protein. Hence, they have little mRNA. However, a few platelet proteins have been successfully cloned using leukemie cell lines that can be induced to synthesize platelet proteins. One such library, made from phorbol ester-induced HEL cells, has produced full-length clones of important platelet adhesion molecules [17]. Screening of 1.5 × 107 plaques of this lambda phage gt11 library with MAb 2E4 yielded 13 clones. After purifying phage DNA from 6 of these clones, one was found to be the right length, ~1.65 kbp, to encode a full-length 45 kDa protein. Northern blot analysis of six different human cell lines using this clone as probe revealed that the endogenous mRNA in human cells was also ~1.65 kb (data not shown).
This 1641 bp cDNA was sequenced (GenBank AF105369). Several nucleotide sequences almost identical to 2E4 were found by a Blastn search in mouse and human EST and genomic libraries (see Materials and methods for list of accession numbers of matching clones). Alignments of the DNA sequence of 2E4 with these nucleotide sequences was used to confirm the nucleotide sequence obtained by bidirectional automated sequencing of the 2E4 cDNA. Tissues from which ESTs were identified included human infant and fetal brain, human fetal liver-spleen and fetal heart, and mouse mammary gland. None of these databank sequences were full length - most were 400 bp or less. DNA sequence identities between human ESTs and 2E4 ranged from 95–100%, and between mouse and 2E4 from 82–95% in the coding region. There were three or more sequences matching 2E4 in all places except for a stretch from bp 970–1084, which had only one other match. For the 3′ non-coding region there were only mouse sequences in the databank, and these were not similar to 2E4 and could not be used to confirm our sequence. The coding sequence reported here contains some differences from the other human sequences reported in the databank. These could be a result of allelic variation or sequencing errors. They produce no frame shifts.
The predicted amino acid sequence begins on bp 40 with two tandem ATG start sites and is 496 aa long (Fig. 7) with a predicted size of 55 kDa. It contains the peptide sequences obtained from the original antigen at aa 68–77 and aa 92–100 (Fig. 7, underline), confirming definitively the reading frame and the identity of the clone. Fasta and Blast searches of this predicted full-length sequence failed to find any significant matches. Searches for amino acid sequence motifs using the motif program in the GCG software package [9] also failed to find any identifiable functional domains.
Fig. 7.
Amino acid sequence of 2E4. Peptide sequences obtained from the 45 kDa band recognized by MAb 2E4 in fraction 7 of anion exchange separation of platelet proteins are underlined. No motifs or homologous proteins were found in the data bank, although ESTs that matched the DNA sequence were found in both mouse and human libraries. Accession number of 2E4; (GenBank AF105369).
To confirm that the cloned cDNA encoded the 2E4 antigen, a variety of tests were applied. First, His-tagged protein expressed in bacteria from the 2E4 cDNA was purified on nickel columns and probed by Western blot with the MAb 2E4 (Fig. 8). The monoclonal antibody recognized the over-expressed full-length protein as well as the 500 bp piece, but not Arp2 (Arp14D) prepared under similar conditions and probed on the same blot (Fig. 8). Although the size of the protein encoded by the 2E4 cDNA is predicted to be 55 kDa, the protein migrates at 45–49 kDa. Polyclonal antibodies raised against the cloned protein recognized a ~45 kDa band in Western blots of platelet extracts (Fig. 8, pAb). When the fractions of the DEAE column were probed with the polyclonal antibody, a single band of 45 kDa was recognized in fraction 7 (data not shown). This anti-2E4 polyclonal antibody also stained spread platelets in the same unique pattern as the monoclonal (Fig. 9A, polyclonal antibody, and 9B the same cells stained for phalloidin). The outer rim of the spreading lamellipodia is stained by polyclonal anti-2E4 and by phalloidin (Fig. 9B. In contrast, anti-2E4 polyclonal staining in the central cytoplasm is different from that of phalloidin; 2E4 stains diffusely while phalloidin stains distinct structures – stress-like fibers and the contractile ring. This unique staining pattern is the same as that seen in platelets with the monoclonal, MAb 2E4 (compare Fig. 2 with Fig. 9).
Fig. 8.
Monoclonal antibody recognizes bacterially expressed 2E4 protein, and polyclonal antibodies recognize a 45 kDa band in platelet homogenates. Coomassie-stained 12% SDS gel (Gel) loaded with bacterially expressed full-length 2E4 protein purified by nickel column (2E4) and bacterially expressed full-length Arp2 also isolated by nickel column (Arp2), 45 kDa band indicated by arrow at left. A parallel blot probed with MAb 2E4 (MAb Blot) shows that MAb 2E4 recognizes the 2E4 protein (2E4) but not Arp 2 (Arp 2) pAb shows a Coomassie-stained 8.5% SDS gel of platelet homogenates (gel) and corresponding Western blot (blot) probed with affinity-purified polyclonal anti-bodies raised against bacterially expressed cloned 2E4. Molecular weight markers in pAb indicated to the left: 200, 98, 68, 45, 31 kDa.
Fig. 9.
Polyclonal antibodies raised against the cloned protein stain the lamellipodia of spreading platelets. A. Platelets spread on glass stained with polyclonal anti-2E4. B. Same two platelets, double-labeled with phalloidin, Bar = 2.5 µm.
Discussion
In this paper we identify a novel ATP-sensitive actin-associated protein, 2E4, which can be isolated from human platelets and is present in a wide variety of other cells. By immunofluorescence, this protein is present in the leading edge of platelets and fibroblasts, a site of rapid actin filament polymerization [13]. It is also present at the tips of the stereocilia in the hair cells of the inner ear of the chick embryo at a time when actin filaments in the stereocilia are elongating.
2E4 and actin
Retention on the F-actin column and localization at sites of actin polymerization inside cells suggest 2E4 might bind actin and be involved in actin dynamics. Since binding to F-actin in the column occurs in the presence of other proteins, this may not be a direct interaction. However, immunoflouorescence shows that 2E4 co-localizes with actin, though only with a subset of filaments. Other proteins that bind directly to actin have been identified using the F-actin affinity column approach in four other systems: Drosophila, yeast, C. elegans and Xenopus [2, 10, 21, 31]. Up to this point, the sequence of 2E4 has not yielded information about its biochemical behavior or cellular function. Thus, although a direct interaction between 2E4 and actin is most likely, it will require additional in vitro biochemical experiments to determine definitively whether 2E4 associates directly with actin filaments, and if so what effect such binding has on actin dynamics.
Solubilization by ATP and detergent suggest that 2E4 might be a membrane-associated “motor”, as some myosins are similarly extracted from cells. Furthermore elution with ATP supports a myosin-like ATP-sensitive association with F-actin. This elution is unlikely to be due to the 10 mM salt in the elution buffer, since the protein is loaded in 70 mM salt (½ × Tyrode’s). However, 45 kDa is too small to be a myosin, which requires at least 70 kDa to accommodate the myosin head domain, nor does 2E4 have any of the myosin consensus sequences. Only one other ATP-sensitive actin-binding protein has been described [1] and this is a gelation factor. But again, like the interaction with F-actin, 2E4's ATP-sensitive cytoskeletal association is in the presence of other proteins. Thus, it is possible that ATP does not directly affect either 2E4 or actin. The lack of any ATPase consensus sequence in the 2E4 supports this interpretation.
There remains some ambiguity as to whether the cloned protein is the 2E4 antigen since peptide sequence was not obtained from highly purified protein. Evidence supporting the identity of the cDNA as the MAb 2E4 antigen includes the result that MAb 2E4 recognizes purified bacterially expressed cloned protein but not another His-tagged bacterially-expressed actin-binding protein, Arp2; and that antibodies to the cloned protein recognize the platelet protein in homogenates, and detect the antigen only in the same fraction after DEAE chromatography as the band recognized by MAb 2E4 from which two peptide sequences were obtained both of which match the cloned cDNA. More importantly, antibodies to the cloned protein also stain the leading edge. Thus, the cloned protein has the same interesting localization as the original antigen, and is therefore worth further study regardless of whether it can be unambiguously identified as the 2E4 antigen.
2E4 in lamellipodia
Localization in the outer third of the leading edge suggests that 2E4 plays a role in the polymerization and/or reorganizations of membrane-associated actin that occurs in this place in the cell. Other candidates also found in platelets and proposed to mediate this process include VASP [25], Arp 2/3 complex [36] and interactions between gelsolin and profiling [13, 30]. Since actin polymerization is a crucial process required for a range of cellular behaviors including cytokinesis, it is certain to be mediated by redundant pathways, each with its own complement of proteins. Although in platelets gelsolin is in the periphery and at the barbed ends of actin filaments, platelets from transgenic mice deficient for gelsolin have only small decreases in F-actin content and assembly rates [38], demonstrating that other proteins, like 2E4, must also perform these functions.
Initiation of actin polymerization from different molecules could serve as the basis for the functional and biochemical differences between the four distinct actin structures in the spread platelet [7]. If the actin filaments in each structure were nucleated by a different protein, the filaments could differ enough structurally to recruit preferentially a subset of actin-binding proteins. Indeed, gelsolin-nucleated filaments differ structurally from filaments polymerized with salt alone [23]. Recent evidence demonstrates that formation of lamellipodia, stress fibers and filopodia is initiated selectively by a different G-protein [26, 27]. This supports the notion that each actin structure is formed by a different polymerization machine.
2E4 in stereocilia of the hair cell
The location of 2E4 at the tips of all stereocilia in the late embryo is highly suggestive that 2E4 promotes actin filament elongation. Although towards the end of embryogenesis, there is a progressive cessation of elongation, beginning with the shorter stereocilia [33], at the embryonic stage examined here (17–19d), most stereocilia would be expected to be elongating. The impression that staining is decreased in the shorter stereocilia lends more support to a possible role in actin dynamics than to a role for 2E4 in the other functions also performed at the tip, such as mechanosensory transduction [12] and tip-to-tip attachments [24].
Because the amount of protein that can be obtained from the cochlea is prohibitively small – there are only two cochlea in an animal, and they are very tiny, containing only a few hundred hair cells each – it is thus difficult to use them as a source for even analytical biochemistry. Thus, immunofluorescence is one of the few ways to identify the proteins present in the hair cell [34], although genetic approaches have also contributed towards identifying those molecules that are uniquely required for hearing [12, 22, 29]. Thus, a side benefit of using the cochlea as an experimental model to determine whether 2E4 is located at the barbed end of actin filaments is that the results also provide information about the hair cell. It would be of great benefit to discover what the proteins are that determine the length and number of actin filaments in a stereocilium, since the size and number of each stereocilium is different, and it is this unique combination of length and number that determines the pitch to which each hair cell responds.
Acknowledgments
We thank Lew Tilney for showing us how to dissect chick cochlea and his vigorous discussions of our work. The method for immunofluorescence of cochlea was developed by E. L. Bearer in Tilney’s lab at Marine Biological Laboratories, Woods Hole, MA, USA, the summer of 1988. We thank Howard Jaffe (NINDS) for peptide sequencing and Judith Nutkis for DNA constructs. Leslie Hunter prepared the bacterial protein for polyclonal antibody generation. We thank Robert Lundsten for critical reading of the manuscript. Many undergraduate students at Brown University have contributed in one way or another to this project. They include Alex Yang, Ruth Bodner, Saman Kanangara, John Morrow, Kate Matsutani, and Kyung Yu, David Stanford (Penn. State, Hershey) helped with sequence alignment. We also thank Bruce M. Alberts for his sustained support and encouragement of this project.
References
- 1.Albanesi JP, Lynch TJ, Fujosaki H, Bowerts B, Korn ED. Purification and characterization of an ATP-sensitive actin gelation factor protein from Acanthamoeba castellani. J. Biol. Chem. 1987;262:3404–3408. [PubMed] [Google Scholar]
- 2.Aroian RV, Field C, Pruliere G, Kenyon C, Alberts BM. Isolation of actin-associated proteins from Caenorhabditis elegans oocytes and their localization in the early embryo. EMBO J. 1997;16:1541–1549. doi: 10.1093/emboj/16.7.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bearer EL. Platelet membrane skeleton revealed by quick freeze-deep etch. Anat. Rec. 1990;227:1–11. doi: 10.1002/ar.1092270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearer EL. Direct observation of actin filament severing by gelsolin and binding by gCap 39 and CapZ. J. Cell Biol. 1991;115:1629–1638. doi: 10.1083/jcb.115.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearer EL. An actin-associated protein present in the microtubule organizing center and the growth cones of PC-12 cells. J. Neurosci. 1992;12:750–761. doi: 10.1523/JNEUROSCI.12-03-00750.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bearer EL. Role of actin polymerization in cell locomotion; Molecules and models. Am. J. Resp. Cell Mol. Biol. 1993;8:582–591. doi: 10.1165/ajrcmb/8.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearer EL. Cytoskeletal domains in the activated platelet. Cell Motil. Cytoskeleton. 1995;30:50–66. doi: 10.1002/cm.970300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckerle MC, O'Halloran T, Burridge K. Demonstration of a relationship between talin and P235, a major substrate of the calcium-dependent protease in platelets. Cell. Biochem. 1986;30:259–270. doi: 10.1002/jcb.240300307. [DOI] [PubMed] [Google Scholar]
- 9.Devereaux J, Haeberli L, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drubin DG, Miller KG, Botstein D. Yeast actin-binding proteins; evidence for a role in morphogenesis. J. Cell Biol. 1988;107:2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox JEB, Philips DR. Polymerization and organization of actin filaments within platelets. Sem. Hematol. 1983;20:243–260. [PubMed] [Google Scholar]
- 12.Garcia-Anoveros J, Corey DP. The molecules of mechanosensation. Annu. Rev. Neurosci. 1997;20:567–594. doi: 10.1146/annurev.neuro.20.1.567. [DOI] [PubMed] [Google Scholar]
- 13.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J. Cell. Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartwig J. Platelet Morphology. In: Loscalzo J, Schafer AI, editors. Thrombosis and Hemorrhage. Williams and Wilkins: Baltimore; 1998. pp. 207–228. [Google Scholar]
- 15.Jennings LK, Fox JE, Edwards HH, Phillips DR. Changes in the cytoskeletal structure of human platelets following thrombin activation. J. Biol. Chem. 1981;254:6927–6932. [PubMed] [Google Scholar]
- 16.Karlsson R, Lassing I, Hoglund AS, Lindberg U. The organization of microfilaments in spreading platelets; a comparison with fibroblasts and glial cells. J. Cell. Physiol. 1984;121:96–113. doi: 10.1002/jcp.1041210113. [DOI] [PubMed] [Google Scholar]
- 17.Lopez J, Chung D, Fujikawa K, Hagen F, Papayannopoulou T, Roth G. Cloning of the alpha chain of human platelet glycoprotein 1b: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc. Natl. Acad. Sci. USA. 1987;84:5615–5619. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machesky LM, Reeves E, Wientjes F, Mattheyse F, Grogan A, Totty NF, Burlingame AL, Hsuan JS, Segal AW. Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionarily conserved proteins. Biochem. J. 1997;328:105–112. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machesky LM, Arkinson SJ, Ampe C, Vanderkerckhove J, Pollard TD. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J. Cell Biol. 1994;127:107–115. doi: 10.1083/jcb.127.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medeiros NA, Reese TS, Jaffe H, DeGiorgis JA, Bearer EL. Primary peptide sequences from a squid muscle ad optic lobe myosin IIs: A strategy to identify an organelle myosin. Cell Biol. Int. 1998;22:1–9. doi: 10.1006/cbir.1998.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller KG, Alberts BM. F-actin affinity chromatography; Technique for isolating previously unidentified actin-binding proteins. Proc. Nat. Acad. Sci. USA. 1989;86:4808–4812. doi: 10.1073/pnas.86.13.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolson T, Ruesch A, Friederich RW, Granato M, Rupperberg JP, Nuesslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation; the Zebrafish cicler mutants. Neuron. 1998;20:271–283. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 23.Orlova A, Prochniewicz E, Egelman EH. Structural dynamics of F-actin: II. Cooperativity in structural transitions. J. Mol. Biol. 1995;245:598–607. doi: 10.1006/jmbi.1994.0049. [DOI] [PubMed] [Google Scholar]
- 24.Pickles JO, Corey DP. Mechanoelectrical transduction by hair cells. Trends Neurosci. 1992;15:254–259. doi: 10.1016/0166-2236(92)90066-h. [DOI] [PubMed] [Google Scholar]
- 25.Reinhard M, Halbrugge M, Scheer U, Wiegand C, Jockusch B, Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 1992;11:2063–2070. doi: 10.1002/j.1460-2075.1992.tb05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridley A, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 27.Ridley A, Paterson H, Johnston C, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 28.Rinnerthaler G, Geiger B, Small JV. Contact formation during fibroblast locomotion; involvement of membrane ruffles and microtubules. J. Cell Biol. 1988;106:747–760. doi: 10.1083/jcb.106.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steel KP, Brown SDM. Genes and deafness. Trends Genet. 1994;12:428–435. doi: 10.1016/0168-9525(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 30.Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1093. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 31.Terasaki A, Ohnuma M, Mabuchi I. Identification of actin-binding proteins from sea urchin eggs by F-actin affinity column chromatography. J. Biochem. 1997;122:226–236. doi: 10.1093/oxfordjournals.jbchem.a021733. [DOI] [PubMed] [Google Scholar]
- 32.Tilney LG, Tilney MS. The actin filaments content of hair cells of the bird cochlea is nearly constant even though the length, width, and number of stereocilia vary depending on the hair cell location. J. Cell Biol. 1988;107:2563–2574. doi: 10.1083/jcb.107.6.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilney LG, Tilney MS, Cotanche D. Actin filaments, stereocilia, and hair cells of the bird cochlea V. How the staircase pattern of stereociliary length is generated. J. Cell Biol. 1988;107:2563–2574. doi: 10.1083/jcb.106.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilney LG, Tilney MS, Stephens RE, Merte C, Drenckhahn D, Cotanche DA, Bretsher A. Preliminary biochemical characterization of the stereocilia and cuticular plate of hair cells of the chick cochlea. J. Cell Biol. 1989;109:1711–1723. doi: 10.1083/jcb.109.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilney LG, Contanche DA, Tilney MS. Actin filaments, stereocilia, and hair cells of the bird cochlea, VI. How the number and arrangement of stereocilia are determined. J. Cell Biol. 1988;116:213–226. doi: 10.1242/dev.116.1.213. [DOI] [PubMed] [Google Scholar]
- 36.Welch MD, Iwamatsu A, Mitchison T. Actin polymerization is induced by Arp2/3 protein complex at the surface or Listeria monocytogenes. Nature. 1997;385:265–268. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- 37.Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J. Cell Biol. 1997;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witke W, Sharpe AH, Hartwig JH, Azuma T, Stossel TP, Kwiatkowski DJ. Haemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995;81:41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- 39.Yin HL, Stossel TP. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979;281:583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- 40.Bearer EL, Miller KG, Alberts BM. Changes in platelet actin-binding proteins with activation detected by F-actin affinity chromatography. J. Cell Biol. 1987;105:195a. [Google Scholar]
- 40.Bearer EL, Alberts BM. Kaptin, a 43 kD protein isolated from platelets and present at sites of actin polymerization in many other cells. J. Cell Biol. 1988;107:173a. [Google Scholar]