Abstract
Purpose: Molecular breast imaging (MBI) has shown promise as an adjunct screening technique to mammography for women with dense breasts. The demonstration of reliable lesion detection with MBI performed at low administered doses of Tc-99 m sestamibi, comparable in effective radiation dose to screening mammography, is essential to adoption of MBI for screening. The concept of performing low-dose MBI with dual-head cadmium zinc telluride (CZT) gamma cameras has been investigated in phantoms in Part I. In this work, the objectives were to evaluate the impact of the count sensitivity improvement methods on image quality in patient MBI exams and to determine if adequate lesion detection could be achieved at reduced doses.
Methods: Following the implementation of two count sensitivity improvement methods, registered collimation optimized for near-field imaging and energy acceptance window optimized for CZT, MBI exams were performed in the course of clinical care. Clinical image count density (counts/cm2) was compared between standard MBI [740 MBq (20 mCi) Tc-99 m sestamibi, standard collimation, standard energy window] and low-dose MBI [296 MBq (8 mCi) Tc-99 m sestamibi, optimized collimation, wide energy window] in a cohort of 50 patients who had both types of MBI exams performed. Lesion detection at low doses was evaluated in a separate cohort of 32 patients, in which low-dose MBI was performed following 296 MBq injection and acquired in dynamic mode, allowing the generation of images acquired for 2.5, 5, 7.5, and 10 min/breast view with proportionately reduced count densities. Diagnostic accuracy at each count density level was compared and kappa statistic was used to assess intrareader agreement between 10 min acquisitions and those at shorter acquisition durations.
Results: In patient studies, low-dose MBI performed with 296 MBq Tc-99 m sestamibi and new optimal collimation/wide energy window resulted in an average relative gain in count density of 4.2 ± 1.3 compared to standard MBI performed with 740 MBq. Interpretation of low-dose 296 MBq images with count densities corresponding to acquisitions of 2.5, 5, 7.5, and 10 min/view and median lesion size of 1.4 cm resulted in similar diagnostic accuracy across count densities and substantial to near-perfect intrareader agreement between full 10 min-views and lower count density views.
Conclusions: Review of patient studies showed that registered optimized collimation and wide energy window resulted in a substantial gain in count sensitivity as previously indicated by phantom results. This proof of concept work indicates that MBI performed at administered doses of 296 MBq Tc-99 m sestamibi with the applied count sensitivity improvements permits the detection of small breast lesions in patients. Findings suggest that further reductions in acquisition duration or administered dose may be achievable.
Keywords: breast cancer, molecular breast imaging, gamma camera, radiation risk, count sensitivity
INTRODUCTION
Screening mammography is the standard tool for breast cancer screening and the only modality shown to significantly decrease breast cancer mortality.1 However, the sensitivity of mammography in women with radiographically dense breasts is reduced; reported sensitivities range from 80% to 98% in women with entirely fatty breasts versus 29% to 62% in women with extremely dense breasts.2, 3, 4, 5 Compared with film mammography, digital mammography has been shown to offer better sensitivity in the single subgroup of women who are under 50 yr, pre- or perimenopausal and have either heterogeneously or extremely dense breasts, yet, in this subgroup sensitivity remained under 60% for digital mammography (versus 27% for film).6 Compounding the problem of reduced mammographic sensitivity with increasing mammographic density is the fact that density is a significant independent risk factor for developing breast cancer. After controlling for the masking effect of dense tissue, women with extremely dense breasts have a relative risk up to 5 times that of women with almost no density.7, 8, 9
We recently reported the results from the first large-scale evaluation of molecular breast imaging (MBI) performed with a dual-head cadmium zinc telluride (CZT) based gamma camera as a supplemental screening technique in women with dense breasts.10 In that study, MBI was performed using 740 MBq (20 mCi) Tc-99 m sestamibi in 936 asymptomatic women with either heterogeneously dense or extremely dense breasts on past prior mammogram. The sensitivity of mammography with supplemental MBI was 91%, significantly better than 27% for mammography alone (P = 0.016). The addition of supplemental MBI did reduce specificity from 91% for mammography alone, yet remained clinically acceptable at 85%. Despite the promising results from this proof-of-principle study, we recognize that adoption of nuclear medicine techniques in the breast screening setting is limited by the relatively higher radiation doses associated with these procedures compared to mammography. In order for MBI to have an effective dose to the patient and subsequent radiation risk comparable to that of screening mammography, our eventual goal is to perform screening MBI with administered activity of 148 MBq (4 mCi) Tc-99 m sestamibi.11
To work toward this reduction in effective dose, we have implemented two count sensitivity improvement strategies for MBI, including redesign of the detector’s collimation to a registered design, optimized for imaging in the near-field, and the use of a wider energy acceptance window to capture photopeak events registered at lower energies in CZT. These two strategies have been evaluated in phantoms in Part I of this work.
Following implementation of these count sensitivity improvement methods, we reduced the standard dose used for MBI performed at our institution to 296 MBq Tc-99 m sestamibi, and in some research cases, MBI was performed using 148 MBq Tc-99 m sestamibi. An MBI study is acquired using dynamic acquisitions to allow for postprocessing motion correction if necessary. By summing the appropriate number of frames in the 10 min/breast view acquisition, we are able to simulate acquisitions of shorter durations including 2.5, 5, and 7.5 min/view, which have proportionately reduced count densities and allow further testing of count sensitivity improvement strategies.
The objectives of this work were to evaluate the impact of the sensitivity improvement methods and a reduction of the injected dose and/or count density on image quality in patient MBI exams and to determine if adequate lesion detection could be achieved at lowered doses.
MATERIALS AND METHODS
MBI clinical protocol
Patient MBI exams used in this work were performed on the LumaGem system [Gamma Medica-Ideas (GMI), Northridge, CA]. This system has previously been described in detail (see Part I). MBI was performed following intravenous injection of Tc-99 m sestamibi. Imaging commenced approximately 2–5 min after injection. The patient was imaged in a seated position with the breast positioned between the two detectors. A typical MBI study consisted of bilateral craniocaudal (CC) and mediolateral oblique (MLO) views acquired for a total of 10 min/view. Light compression was used to limit breast movement and reduce lesion-to-detector distance during imaging; the mean and standard deviation of compressed breast thickness during MBI was previously reported as 6.6 ± 1.4 cm for CC views and 6.7 ± 1.4 cm for MLO views.12
A radiologist thus interpreted a display of eight images comprising bilateral CC and MLO views from two separate detector heads. All MBI exams described in this report were performed under institutional review board (IRB)-approved protocols that allowed the off-label use of reduced administered doses under the direction of the ordering physician, a common practice in nuclear medicine. Written informed consent was obtained from all participants.
Within-patient count density comparison
Between the years 2005 and 2008, approximately 1000 patients had MBI performed as part of a study evaluating the efficacy of screening MBI in women with dense breasts.10 In that study, all patients were imaged using the standard collimation and standard energy acceptance window (126–154 keV), and patients received an injected dose of 740 MBq Tc-99 m sestamibi. In 2010, a second screening study was initiated following implementation of the optimized registered collimation13 and wide energy windows (110–154 keV). In this new ongoing study, patients typically have low-dose MBI with an injected dose of 296 MBq Tc-99 m sestamibi; a 148 MBq injection of Tc-99 m sestamibi has been administered in a small subset of patients.
We identified a cohort of 50 patients who had participated in both the original and low-dose MBI screening studies. A 296 MBq dose of Tc-99 m sestamibi was used in 48 patients and a 148 MBq dose was used in 2 patients. In each MBI image, count density on both the original and low-dose studies was measured using region of interest (ROI) analysis of all breast tissue included in the field of view on the CC and MLO views of each breast. To adjust for differences in administered dose, count density was expressed as counts/cm2/10 min acquisition/MBq.
Another single patient with breast cancer had undergone an MBI study using both the standard and optimal collimation during the same exam. The MBI study was performed with 296 MBq Tc-99 m sestamibi and the wide energy window was used. An acquisition with each collimator was acquired sequentially for 10 min/view. After applying decay correction for time elapsed between acquisition start times, we assessed the gain in count density with the optimal collimator alone.
As performed in the phantom work in Part I, we determined the additional count information provided by widening the energy acceptance window alone, by examining another subset of five patients in whom an MBI study was performed with both standard and wide windows. The MBI studies were performed with 740 MBq Tc-99 m sestamibi and the standard collimation was used. An acquisition with each energy window was acquired sequentially for 10 min/view. We subtracted the standard energy window image from the wide energy window image. This method allowed us to create an image of counts in the 110–125 keV range and assess the spatial information of these additional counts.
Lesion detection study
A reader study was performed to evaluate lesion detection on low-dose MBI with further reduced count densities. Thirty-two low-dose MBI studies were performed using a 296 MBq administered dose of Tc-99 m sestamibi, using optimized registered collimation and wide energy window. All breast views were acquired in dynamic mode, at a frame rate of 1.25 min/frame for 10 min. After acquisition, the appropriate number of frames was summed to create images with count densities corresponding to acquisition durations of 2.5, 5, 7.5, and 10 min/view.
The 32 cases comprised 17 patients with 20 breast lesions (8 cancers, 12 benign) demonstrating positive radiotracer uptake and 15 patients with no diagnosed lesions. The cancer status of each lesion was determined through review of all available imaging studies (mammogram, ultrasound, MBI, and/or MRI) and pathology findings by an expert radiologist specializing in breast imaging. A lesion was classified as malignant if cancer was detected either by pathology findings at core-needle biopsy and/or surgery. A lesion was classified as benign if either no cancer was detected on core-needle biopsy or if in the setting of definitive benign imaging findings on mammogram, ultrasound or MRI, the benign status was confirmed by follow-up imaging at least 12 months post-MBI.
Eight (8/17) patients had breast cancer (one malignant lesion per patient), two of whom also had a benign lesion in the contralateral breast. Eight (8/17) patients had a single benign lesion and one patient had two benign lesions (one in each breast). One (1/17) patient with a single benign lesion had a diagnosis of lobular carcinoma in situ (LCIS), which is a benign finding known to be strongly associated with increased risk of breast cancer. The overall median size of all 20 lesions was 1.4 cm (s.d. = 1.0 cm; range = 0.6–4.3 cm). Median cancer size was 1.0 cm (s.d. = 1.2 cm; range = 0.6–4.3 cm); median size of benign lesions was 1.7 cm (s.d. = 0.8 cm; range 0.8–3.2 cm).
MBI studies were interpreted by four readers (CMT, ALC, CLT, and RWM) with varying experience levels. Three readers were dedicated breast radiologists who each interpret approximately five MBI studies per week; two of the three readers had over 2 yr experience in reading MBI studies and the third had 1 yr experience. The fourth reader was a radiology resident with no prior experience in reading MBI studies.
Each reader independently and blindly interpreted the low-dose MBI studies in four separate reading sessions: 2.5 min/view studies were interpreted at the first session, 5 min/view studies at the second, 7.5 min/view studies at the third, and the full 10 min/view studies at the fourth. Each session was separated by at least 1 week. Cases interpreted within each session were presented in a random order. Readers interpreted the MBI studies in isolation without access to clinical history or any other imaging results, including mammograms; discussion of cases with other readers was not allowed. Readers examined the MBI studies for the presence of abnormal radiotracer uptake. An assessment score that paralleled the breast imaging reporting and data system (BI-RADS)14 assessments was assigned for each breast using a five point scale, where 1 = negative, 2 = benign, 3 = probably benign, 4 = suspicious, and 5 = highly suggestive of malignancy. A final assessment of 1 or 2 was considered test negative and an assessment of 3 or higher was considered test positive.
ROI analysis was performed to measure the counts/cm2 of all breast tissue included in the field of view for each frame of the four views (bilateral CC and MLO) in order to monitor counts throughout the course of each MBI study. Sensitivity and specificity was calculated and ROC analysis was performed for each count density level. Intrareader agreement between assessments (the 1–5 assessment score scale) of the 296 MBq, 10 min/view studies and each reduced acquisition duration (lowered count density level) was calculated using Cohen’s kappa statistic.15 Kappa statistic provides a measure of the reproducibility of an interpretation. Using the guidelines of Landis and Koch, a kappa value below 0.2 was considered slight agreement; between 0.21 and 0.4 considered fair agreement; 0.41–0.6 considered moderate agreement; 0.61–0.8 considered substantial agreement; and kappa above 0.81 was considered near-perfect agreement.16
RESULTS
Within-patient count statistics
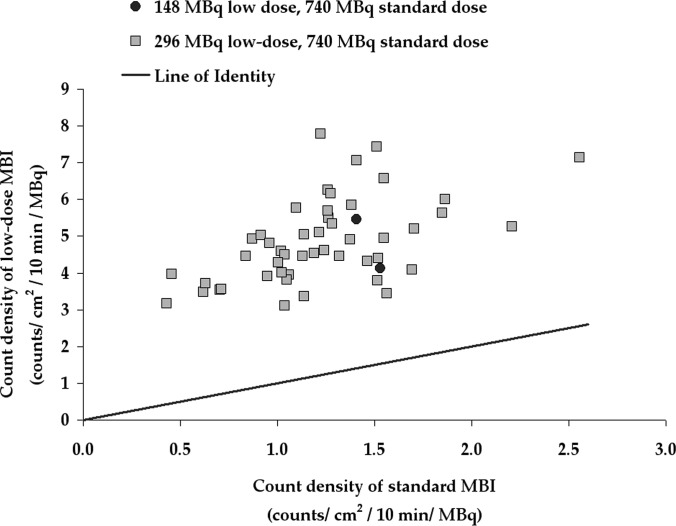
Figure 1 shows the variation in count density in normal breast tissue on standard MBI performed with 740 MBq Tc-99 m sestamibi, standard collimation and standard energy window versus low-dose MBI performed with either 148 MBq (in 2 patients) or 296 MBq (in 48 patients) Tc-99 m sestamibi following implementation of optimized collimation and wide energy window. The median count density for studies performed with 740 MBq and using standard collimation and standard energy window was 1.23 ± 0.41 counts/cm2/10 min/MBq as shown in Fig. 1. With application of these two count sensitivity improvement methods, the median count density for studies performed with 148 or 296 MBq using optimized collimation and wide energy window was 4.60 ± 1.13 counts/cm2/10 min/MBq. The median gain in count density for each individual patient was 4.2 (s.d. = 1.3, range = 2.2–8.8) for low-dose MBI versus the standard MBI. Figure 2 compares MBI studies acquired in a sample of five patients with both the 740 MBq standard acquisitions and either 148 or 296 MBq low-dose acquisition.
Figure 1.
Count density measured in patients who had both standard MBI (performed with a 740 MBq injection, standard collimation, and standard energy window) and low-dose MBI (performed with either 148 or 296 MBq injection, optimized collimation, and wide energy window). Count density (counts/cm2) is expressed per 10 min acquisition per MBq.
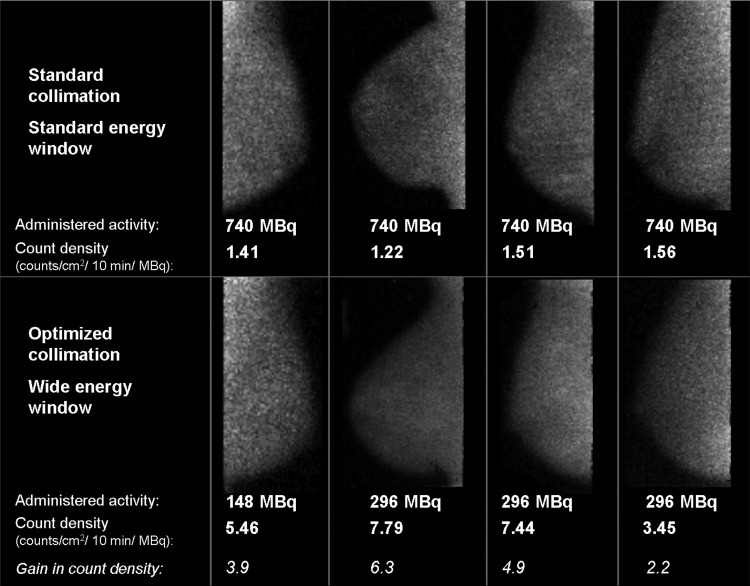
Figure 2.
Example views from four patients undergoing both standard MBI and low-dose screening MBI with approximately 2–3 yr between exams. On the top row, standard 10 min/view MBI was performed with a 740 MBq injection, standard collimator, and standard energy window. On the bottom row, low-dose 10 min/view MBI was performed using either 148 or 296 MBq injection, optimized collimation, and wide energy window. All images were interpreted as negative.
In Fig. 3, the effect of an optimized collimator alone on count density and lesion visibility is illustrated in a patient, where after decay correction for time elapsed between acquisitions, the gain in count density was 2.4 with the optimized collimator. In the five patients who had MBI performed using both the standard and wide energy windows, average gain in count density with the wide window was 1.8 (range 1.5–2.6). Figure 4 displays an example of MBI performed in a patient using both the standard and wide energy windows, where an additional extension of the disease was appreciated in the wide energy window image. The difference image is also shown. As noted in the phantom images previously acquired (see Part I), the image acquired with the wide window is less noisy compared to the standard energy window image, a factor which may improve lesion detection. With the widened energy window, however, more scattered events are present at the chest wall edge of the detector.
Figure 3.
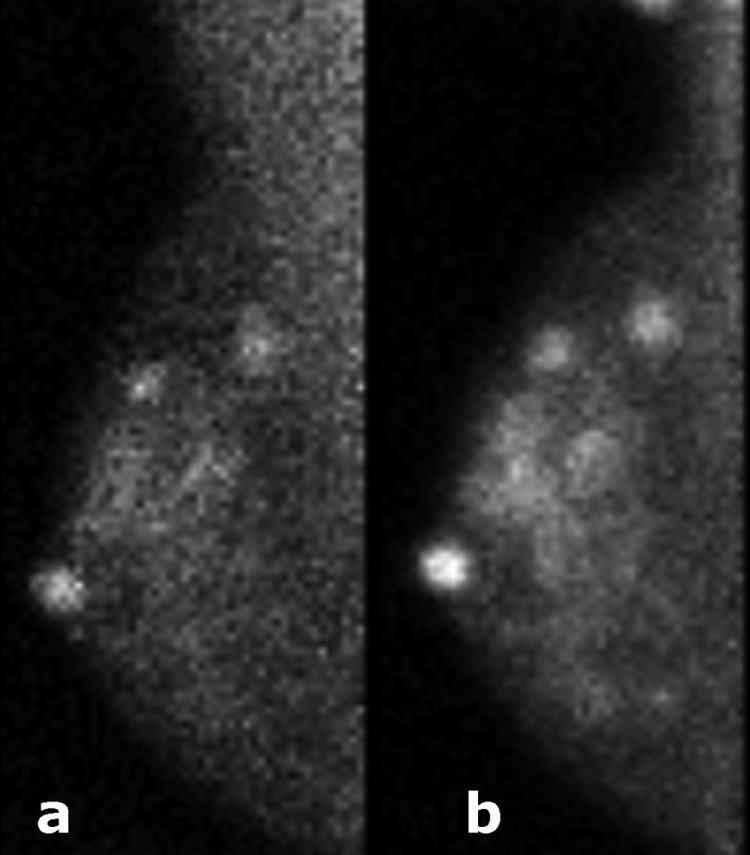
A patient with multifocal invasive lobular cancer and nipple adenoma. MBI was performed following 296 MBq injection Tc-99 m sestamibi and with the wide energy window using the standard collimation [shown in (a)] and the optimized collimation [shown in (b)]. Count density was improved by a factor of 2.4 with the optimized collimation.
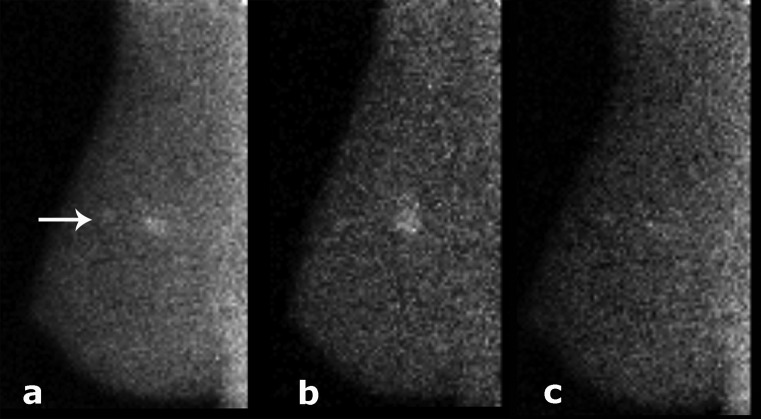
Figure 4.
MBI study acquired for 10 min following 740 MBq injection Tc-99 m sestamibi, using standard collimation, and (a) wide energy window (110–154 keV), (b) standard energy window (126–154 keV). The difference of images in panels (a) and (b) is shown in panel (c). Each image is displayed on the range from its individual minimum to maximum count. Count density in this patient was improved by a factor of 2.6 with the wider energy window, A 1.3 cm × 1.0 cm × 0.9 cm invasive ductal carcinoma was detected, and the wide energy window resulted in improved detection of an extension of ductal carcinoma in situ (see arrow).
Lesion detection study
Results from the lesion detection study comparing full 10 min/view images and reduced-time images for low-dose MBI are shown in Table TABLE I.I . The median count density of all 32 MBI studies was 1254 counts/cm2 for 10-min acquisitions with injected dose of 296 MBq Tc-99 m sestamibi (range 756–2073 counts/cm2). The reduced-time images with acquisition durations of 2.5, 5, and 7.5 min/view had proportionally decreased median count densities of 313, 627, and 940 counts/cm2.
TABLE I.
Results from lesion detection study in which 4 readers interpreted MBI studies from 32 patients with 64 breasts, comprising 8 breasts with breast cancer and 12 breasts with benign lesions. MBI was acquired with 296 MBq injection Tc-99 m sestamibi, and acquisition durations of 2.5–10 min/view.
| Acquisition duration (min/view) | 10 | 7.5 | 5 | 2.5 | ||||
|---|---|---|---|---|---|---|---|---|
| Median count density (counts/cm2) | 1254 | 940 | 627 | 313 | ||||
| Range | Average | Range | Average | Range | Average | Range | Average | |
| Sensitivity | 7/8–8/8 | (97) | 8/8–8/8 | (100) | 7/8–8/8 | (97) | 7/8–8/8 | (97) |
| (88–100) | (100–100) | (88–100) | (88–100) | |||||
| Specificity | 46/56–50/56 | (86) | 43/56–50/56 | (84) | 43/56–52/56 | (86) | 47/56–53/56 | (89) |
| (77–93) | (84–95) | |||||||
| (82–89) | ||||||||
| (77–89) | ||||||||
| AUC | 0.96–0.97 | 0.97 | 0.95–0.98 | 0.97 | 0.96–0.98 | 0.97 | 0.95–1.0 | 0.97 |
| Intrareader agreement with 10 min/view assessments for all 64 breastsa (κ) | — | 0.82–0.91 | 0.86 | 0.69–0.84 | 0.77 | 0.69–0.80 | 0.73 | |
| Proportion of breasts positive at 10 min/view but negative at reduced acquisition durationb | — | 0/17–2/18 | (6) | 1/18–4/13 | (14) | 2/14–6/18 | (24) | |
| (0–11) | (6–31) | (14–33) | ||||||
| Proportion of breasts negative at 10 min/view but positive at reduced acquisition durationb | — | 1/46–4/50 | (5) | 0/50–4/46 | (4) | 1/50–4/47 | (5) | |
| (0–9) | (2–9) | |||||||
| (2–8) | ||||||||
Note: Range and average are of the interpretations by four readers. Numbers in parentheses are percentages, and percentages are rounded.
Intrareader agreement determined by Kappa statistic, κ, where κ below 0.2 = slight agreement; 0.21–0.4 = fair agreement; 0.41–0.6 = moderate agreement; 0.61–0.8 = substantial agreement; >0.81 = near-perfect agreement (Ref. 16).
A per breast final assessment of 3, 4, or 5 was considered positive, while 1 or 2 was considered negative. The number of breasts called positive or negative at 10 min/view varied among the four readers; the ratio corresponding with minimum and maximum percentage is given in the range.
The sensitivity, specificity and area under curve (AUC) of ROC were similar at all count density levels for all readers. Kappa statistics indicated that intrareader agreement between assessments of 296 MBq, 10 min/view MBI and each lower count density level was substantial (>0.6) to nearly perfect (>0.8). However, a comparison of final assessments from interpretations of 296 MBq, 10 min/view MBI versus those at reduced count densities demonstrated that a considerable proportion of breasts interpreted as positive (assessment of 3 or higher) at 10 min/view were interpreted as negative (assessment of 1 or 2) at acquisition duration of 5 and 2.5 min/view. At 5 min/view, an average of 14% of lesions called positive at 10 min/view were called negative; at 2.5 min/view, this number increased to 24%. The lesions with a change from positive to negative at lower count densities were mostly benign lesions with less Tc-99 m sestamibi uptake; hence, the sensitivity of MBI stayed constant with decreasing count density but specificity was highest at 2.5 min/view. A very small percentage (4%–5%) of breasts called negative at 10 min/view had lesions called positive at reduced acquisition duration.
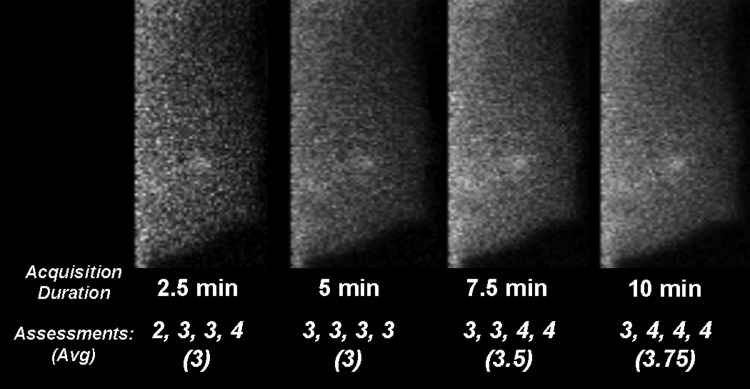
Figure 5 shows an example of an MBI performed with administration of 296 MBq Tc-99 m sestamibi, acquired in eight dynamic frames, and subsets of frames summed to represent images with acquisition durations of 2.5, 5, 7, and 10 min/view. In this example, the average assessment improved with increasing count density level, however, all readers interpreted the lesion as positive (score of 3 or higher) at acquisition durations of ≥5 min/view.
Figure 5.
In this patient with a 0.8 cm tubular carcinoma, low-dose MBI was performed with 296 MBq injection Tc-99 m sestamibi and acquired in eight dynamic frames. Images representing acquisition durations of 2.5, 5, 7.5, and 10 min/breast view were simulated by summing counts from either two, four, six, or eight frames, respectively. Images were acquired with optimized collimation and wide energy window. The average final assessment and individual assessments from four readers are provided, where an assessment of 3 or higher is considered positive.
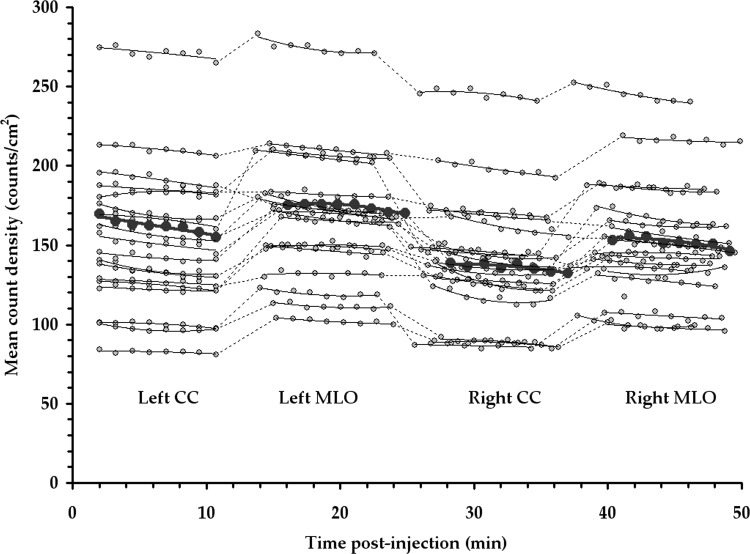
Figure 6 demonstrates the count density observed over the course of an MBI exam, which lasts approximately 50 min from the time of injection of Tc-99 m sestamibi. Measurements from 19 of the 32 patients interpreted for the lesion detection study are shown and were selected for count density analysis because views were acquired in the same order (left CC, left MLO, right CC, and right MLO). Small fluctuations in count rate were observed between breast views, with some individual patient variability across right and left breasts and a generally higher count density in MLO views compared to CC views, due to increased uptake in the pectoralis muscle included on MLO views. However, count rate was generally steady throughout the duration of the exam, showing only a slight downward trend consistent with decay of the Tc-99 m (t1/2 = 6 h). The median count density observed across all views for the 19 patients evaluated was 156 counts/cm2 acquired per 1.25 min frame per 296 MBq injected activity (=1247 counts/cm2 per total 10 min view), with a standard deviation of 39 and range of 95–259 counts/cm2.
Figure 6.
The count density (counts/cm2 in a 1.25 min frame) measured in 19 MBI clinical studies as a function of time postinjection of 296 MBq Tc-99 m sestamibi. Studies were performed with optimized collimation and wide energy window. The patient with the median count density is highlighted in bold.
DISCUSSION AND CONCLUSION
We investigated the effects of two count sensitivity improvement strategies, collimation optimized for count sensitivity and wide energy acceptance window, on MBI performed in patients. With these methods, we found the median gain of over 4 times the count sensitivity in patient studies, permitting a proportional reduction in the necessary administered dose of Tc-99 m sestamibi. Tests performed in phantoms in Part I of this work indicated that spatial resolution is slightly degraded with the count sensitivity improvement methods but still allows adequate resolution of tumors as small as ∼0.6 cm using a dual-head MBI detector. In Part II of this work, small breast cancers (0.6–1.0 cm included in this study) were likewise detected following the count sensitivity improvement methods.
A larger overall gain in count sensitivity was observed in patients (4.2×) compared to the gain previously observed in phantoms (2.8–3.6×) with implementation of both optimized collimation and wide energy window. When considering the collimation optimization alone, the gain in count sensitivity was similar in patients compared to phantom results. However, the use of a wider energy acceptance window led to greater gains in count sensitivity in patients than observed in phantoms. This difference is likely because the phantom geometry only modeled the breast, and in patient imaging, additional counts from the patient’s torso can be present in the image. We did note that although use of a wider energy window allowed a substantial number of additional photopeak events from the breast, it also increased the amount of scatter at the chest wall edge of the image. Williams et al. previously demonstrated the varying scatter contribution in the energy spectra as a function of distance from the chest wall.17 Although not explored in this work, it may be beneficial to apply a standard energy window at the chest wall and the wider energy window for pixels away from the chest wall edge.
The lesion detection study demonstrated similar sensitivity, specificity, and a high level of agreement in reader’s assessment scores, as determined by kappa statistic, between interpretations of the 296 MBq, 10 min/view low-dose MBI images and those interpreted from images with shorter acquisition durations. It should be noted, however, that although kappa statistic as performed in this study gives a measure of reproducibility of interpretations, it is not sensitive to important diagnostic implications of assessment scores. For instance, an assessment scores of 2, considered negative and a score of 3, considered positive, may yield a high level of agreement according to the kappa calculation.
These preliminary findings may indicate that MBI could be performed with either acquisition durations lower than 10 min/view or count densities corresponding to doses of Tc-99 m sestamibi lower than 296 MBq while maintaining a high sensitivity and specificity. If high diagnostic accuracy can be subsequently demonstrated using a 148 MBq administered dose of Tc-99 m sestamibi, this may represent the optimal dose in the setting of screening MBI at 2-yr intervals, as the effective dose to the patient would be comparable to that of annual screening mammography. A trial is currently in progress at our institution to evaluate the efficacy of low-dose screening MBI in women with mammographically dense breasts performed with injected doses of 296 and 148 MBq Tc-99 m sestamibi.
As we implement these technical enhancements in clinical practice, a key assumption in this work is that a reduction in image acquisition duration is equivalent to a proportional reduction in the administered dose of Tc-99 m sestamibi. This implies that a change in the absolute injected amount of the drug sestamibi does not elicit a change in its biodistribution. This assumption is consistent with the radiotracer principle, with the known behavior of sestamibi in the body,18 and with the absence of any reported finding of a dose-dependent effect from over 15 yr of clinical use of sestamibi in oncology or cardiology. Hence, the demonstration of high diagnostic accuracy of low-dose MBI obtained with 296 MBq Tc-99 m sestamibi and 5 min/view acquisitions, as found in this study, would indicate that high diagnostic accuracy should also be obtained with an administered dose of 148 MBq Tc-99 m sestamibi and 10 min/view. We have demonstrated that the uptake of Tc-99 m sestamibi occurs rapidly and remains steady throughout the course of ∼50 min exam time (Fig. 6), indicating a lack of any significant uptake and washout effects of the tracer in this time period. This finding is consistent with previous studies that have shown rapid uptake in breast tissue (1–3 min) after injection,19 and long term stability of sestamibi uptake in breast tumor cells.20
Although we have shown that adequate MBI image quality can be obtained with count densities corresponding to 296 MBq administered dose and 5 min/view acquisitions, we have yet to extensively demonstrate the feasibility of generating acceptable clinical images with administered doses of 148 MBq Tc-99 m sestamibi. In this work, two patients who received a 148 MBq injection of Tc-99 m sestamibi were included (Fig. 1). In both patients, count densities greater than 800 counts/cm2 in 10 min-views were achieved, giving excellent image quality. As this administered activity is much lower than that typically used for other nuclear medicine procedures, use of such a low dose in clinical practice makes it important that additional measures are taken to ensure accurate calibration of the dose to imaging time and to minimize residual activity in the needle and injection set-up following injection.
We conclude that low-dose MBI performed with an administered dose of 296 MBq Tc-99 m sestamibi, a dual-head CZT-based MBI system, and the optimized registered collimation and wider acceptance window designed for this type of system, can provide adequate diagnostic image quality for detection of small breast cancers. Our preliminary findings also suggest that similar diagnostic accuracy may be achievable at even lower count densities, achieved either through further reductions in administered dose or acquisition duration.
ACKNOWLEDGMENTS
This research was supported by Mayo Clinic Foundation and grants from Komen for the Cure (BCTR0504394) and National Institutes of Health (R21R33 CA 128407). The Mayo Foundation and three authors of this work (C. B. Hruska, A. L. Weinmann, and M. K. O’Connor) obtain royalties from licensing arrangements between the Mayo Foundation and Gamma Medica-Ideas.
References
- U.S. Preventive Services Task Force, “Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement,” Ann. Intern. Med. 151, 716–726 (2009). [DOI] [PubMed] [Google Scholar]
- Mandelson M. T., Oestreicher N., Porter P. L., White D., Finder C. A., Taplin S. H., and White E., “Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers,” J. Natl. Cancer Inst. 92, 1081–1087 (2000). 10.1093/jnci/92.13.1081 [DOI] [PubMed] [Google Scholar]
- Kolb T. M., Lichy J., and Newhouse J. H., “Comparison of the performance of screening mammography, physical examination, and breast ultrasound and evaluation of factors that influence them: An analysis of 27,825 patient evaluations,” Radiology 225, 165–175 (2002). 10.1148/radiol.2251011667 [DOI] [PubMed] [Google Scholar]
- Carney P. A., Miglioretti D. L., Yankaskas B. C., Kerlikowske K., Rosenberg R., Rutter C. M., Geller B. M., Abraham L. A., Taplin S. H., Dignan M., Cutter G., and Ballard-Barbash R., “Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography,” Ann. Intern. Med. 138, 168–175 (2003). [DOI] [PubMed] [Google Scholar]
- Buist D. S. M., Porter P. L., Lehman C., Taplin S. H., and White E., “Factors contributing to mammography failure in women aged 40-49 years,” J. Natl. Cancer Inst. 96, 1432–1440 (2004). 10.1093/jnci/djh269 [DOI] [PubMed] [Google Scholar]
- Pisano E. D., Hendrick R. E., Yaffe M. J., Baum J. K., Acharyya S., Cormack J. B., Hanna L. A., Conant E. F., Fajardo L. L., Bassett L. W., D’Orsi C. J., Jong R. A., Rebner M., Tosteson A. N. A., and C. A. Gatsonis for DMIST Investigators Group, “Diagnostic accuracy of digital versus film mammography: Exploratory analysis of selected population subgroups in DMIST,” Radiology 246, 376–383 (2008). 10.1148/radiol.2461070200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N. F., Guo H., Martin L. J., Sun L., Stone J., Fishell E., Jong R. A., Hislop G., Chiarelli A., Minkin S., and Yaffe M. J., “Mammographic density and the risk and detection of breast cancer,” N. Engl. J. Med. 356, 227–236 (2007). 10.1056/NEJMoa062790 [DOI] [PubMed] [Google Scholar]
- McCormack V. A. and dos Santos Silva I., “Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis,” Cancer Epidemiol. Biomarkers Prev. 15, 1159–1169 (2006). 10.1158/1055-9965.EPI-06-0034 [DOI] [PubMed] [Google Scholar]
- Byrne C., Schairer C., Wolfe J., Parekh N., Salane M., Brinton L. A., Hoover R., and Haile R., “Mammographic features and breast cancer risk: Effects with time, age, and menopause status,” J. Natl. Cancer Inst. 87, 1622–1629 (1995). 10.1093/jnci/87.21.1622 [DOI] [PubMed] [Google Scholar]
- Rhodes D. J., Hruska C. B., Phillips S. W., Whaley D. H., and O’Connor M. K., “Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts,” Radiology 258, 106–118 (2011). 10.1148/radiol.10100625 [DOI] [PubMed] [Google Scholar]
- O’Connor M. K., Li H., Rhodes D. J., Hruska C. B., Clancy C. B., and Vetter R. J., “Comparison of radiation exposure and associated radiation-induced cancer risks from mammography and molecular imaging of the breast,” Med. Phys. 37, 6187–6198 (2010). 10.1118/1.3512759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska C. B., Phillips S. W., Whaley D. H., Rhodes D. J., and O’Connor M. K., “Molecular breast imaging: Use of a dual-head dedicated gamma camera to detect small breast tumors,” AJR, Am. J. Roentgenol. 191, 1805–1815 (2008). 10.2214/AJR.07.3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann A. L., Hruska C. B., and O’Connor M. K., “Design of optimal collimation for dedicated molecular breast imaging systems,” Med. Phys. 36, 845–856 (2009). 10.1118/1.3077119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- College of Radiology American, Breast Imaging Reporting and Data System, BI-RADS: Mammography, 4th ed. (American College of Radiology, Reston, VA, 2003). [Google Scholar]
- Crewson P. E., “Reader agreement studies,” AJR, Am. J. Roentgenol. 184, 1391–1397 (2005). [DOI] [PubMed] [Google Scholar]
- Landis J. R. and Koch G. G., “The measurement of observer agreement for categorical data,” Biometrics 33, 159–174 (1977). 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- Williams M. B., Narayanan D., More M. J., Goodale P. J., Majewski S., and Kieper D. A., “Analysis of position-dependent Compton scatter in scintimammography with mild compression,” IEEE Trans. Nucl. Sci. 50, 1643–1649 (2003). 10.1109/TNS.2003.817297 [DOI] [Google Scholar]
- Piwnica-Worms D. and Holman B. L., “Noncardiac applications of hexakis (alkylisonitrile) Technetium-99m complexes,” J. Nucl. Med. 31, 1166–1167 (1990). [PubMed] [Google Scholar]
- Schillaci O., Scopinaro F., Danieli R., Tavolaro R., Picardi V., Cannas P., and Colella A. C., “99Tcm-sestamibi scintimammography in patients with suspicious breast lesions: Comparison of SPET and planar images in the detection of primary tumours and axillary lymph node involvement,” Nucl. Med. Commun. 18, 839–845 (1997). 10.1097/00006231-199709000-00007 [DOI] [PubMed] [Google Scholar]
- Arbab A. S., Koizumi K., Toyama K., Arai T., and Araki T., “Ion transport systems in the uptake of 99Tcm-tetrofosmin, 99Tcm-MIBI and 201Tl in a tumour cell line,” Nucl. Med. Commun. 18, 235–240 (1997). 10.1097/00006231-199703000-00007 [DOI] [PubMed] [Google Scholar]