Introduction
Levo-tetrahydropalmatine (l-THP) has been clinically and safely prescribed for about 30 years as an analgesic, hypnotic medication in many fields without showing any abuse potential (1–3). Recent preclinical and laboratory behavioral studies have demonstrated great potential for l-THP to treat drug addiction, ameliorate withdrawal symptoms and prevent or inhibit relapse. Although many research efforts have been directed at studying l-THP, even at the molecular level, the region-specific effects of intravenous l-THP infusion on cerebral blood oxygen-level dependent (BOLD) changes in rodents has not yet been investigated.
Pharmacological magnetic resonance imaging (phMRI) tracks signal changes that reflect a central hemodynamic response to acute drug challenges and may be considered representative of changes in the underlying neuronal activity (4,5). In human phMRI studies, drug challenges are typically performed on fully conscious, alert subjects, while phMRI in rodents is most often performed under general anesthesia. Anesthesia improves image quality by reducing head motion artifact and minimizing stress induced by restraint. However, the phMRI response to the drug of interest may be affected by interactions with the anesthetic agent, which consequently complicates the interpretation of the data. Some studies have addressed specific issues related to the use of general anesthesia in phMRI, such as reductions in baseline metabolism and neural activity (6), alterations in regional cerebral blood flow (7,8), uncoupling between blood flow and metabolism (9,10), potential perturbations of cerebrovascular autoregulation following the administration of vasoactive compounds and the effect of specific anesthetics on neurotransmitter function (11). Cocaine induces region-specific activation in the dopaminergic mesocorticolimbic system in the anesthetized (6,12–15) animals, and similar activation patterns were also found in the awake animals (16,17). However, drugs that stimulate the nicotinic cholinergic system produce dose-dependent changes in the BOLD fMRI signal in discrete brain areas in awake rats that are obscured by the use of anesthesia (10). Differences were also found in brain activation patterns between anesthetized and awake rhesus monkeys challenged with apomorphine (17).
The pharmacological mechanism of anesthesia is complex, and the potential interaction of an anesthetic with a second drug under study introduces additional confounding factors that can influence the interpretation of results. Therefore, the choice of anesthetic for each phMRI study has to be carefully considered. The investigation of anesthetic effects becomes particularly important when the compound under investigation possesses additional analgesic or sedative properties, as in the case of l-THP.
To better understand the potential anesthetic confounds, here we compared the effects of three anesthetics —isoflurane, medetomidine and urethane — on l-THP-induced cerebral BOLD changes in naïve rats. We hypothesized, based on the preliminary data, that anesthetics would produce region-specific interactions with the dose-dependent effects of l-THP.
Experimental
Materials and animal preparation
The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Medical College of Wisconsin and was in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 24 naïve male Sprague-Dawley (SD) rats weighing 300–350g were used in this study. All rats were given free access to food and water and kept in a home cage with 12 hours of day/night light alternation for at least one week before the experiment. Rats were evenly randomized into three anesthetic groups (n= 8 each): 1.4–1.6% isoflurane in 3:7 O2 and N2 mixture; 0.1 mg/kg medetomidine subcutaneous bolus injection followed by 0.1 mg/kg/h tail vein infusion; and 1.2 g/kg IP injection of urethane. The eight rats in each anesthetic group were further divided into two sub-groups of l-THP dosage (5mg/kg and 20mg/kg in distilled water). Four out of all eight animals in each anesthetic group received a distilled water (1 ml/kg) injection before the l-THP injection; they served as control animals. The l-THP, isolated from the tuberous roots of Stephania delavayi Diels, was acquired from Guangxi Nanning Baihui Pharmaceutical Group (Nanning, Guangxi, China) via the Beijing Basic Science Research Institute (Beijing, China) and was dissolvable in distilled water instead of saline solution. Therefore, distilled water was chosen as a vehicle for the control group.
On the day of the experiment, the right femoral vein and femoral artery were cannulated with PE-50 polyethylene tubing (Becton-Dickinson, Sparks, MD), flushed previously with heparinized saline for intravenous drug delivery and arterial blood pressure monitoring, respectively. The lungs were ventilated with room air through an intra-tracheal Y-shaped Teflon tube at 50 pushes per minute and 2.40–2.80 ml tidal volume, using a volume-cycled MR-compatible rodent ventilator (CWE, Ardmore, PA). To eliminate spontaneous respirations, and minimize motion artifacts, the muscle relaxant, gallamine (250 mg/kg, IV), was administered at the onset of mechanical ventilation, approximately 15 minutes before the beginning of the scan session. The rats’ core temperature was monitored with a rectal thermometer, and was maintained at 37 ± 1°C by circulating warm water through a custom-built G-10 fiberglass animal holder controlled by a water-pump driven temperature regulator (Medi-therm III, Gaymar Industries, Orchard Park, NY). A small animal monitoring system (Model 1025, SA Instruments, Stony Brook, NY) was applied to monitor arterial blood pressure, core temperature, and respiratory rate. Pulse oximeter (8600V, Nonin Medical, Plymouth, MN), and inspired/expired O2 and CO2 sampler (POET IQ2, Criticare Systems, Waukesha, WI) were also used. The physiological variables were kept within normal ranges, and the signals were continuously recorded with WinDaq Pro recording software (DataQ Instrument, Akron, OH).
MRI procedures
A 9.4 Tesla MRI system with a 31-cm horizontal bore (Biospec Avance 94/31; Bruker, Karlsruhe, Germany) was employed for scanning. A cylindrical volume coil for radio frequency (RF) transmission and an Insight surface coil (Worcester, MA.) for MR signal receive were used to image the rat brain. After a quick localization scan with the FLASH sequence, a high-resolution scan was acquired with spin-echo rapid acquisitions. A relaxation enhancement (RARE) pulse sequence was used to acquire images in the sagittal plane to accurately localize the anterior commissure and ensure repeatability in slice positioning between different rat brains. After obtaining accurate slice positioning, the same RARE sequence and scanning parameters were employed to acquire six slices with the following acquisition parameters: TR of 5000 ms, TE of 11.3 ms, number of average of 2, RARE factor of 8, FOV of 35 × 35 mm2, Matrix size of 128×128, slice thickness of 2 mm in coronal plane. After the high-resolution anatomical scan, phMRI scans were taken with the same set-up and scanning parameters except the TR of 3000 ms (volume repetition time of 24 s, RARE factor of 8), TE of 12.5 ms, single average and matrix size of 64×64 were employed.
A RARE sequence provides good BOLD contrast and adequate temporal resolution for phMRI, and is less subjected to susceptibility artifacts (18). We applied the RARE sequence instead of an EPI sequence for the phMRI scan to preserve good signal-to-noise ratio for many subcortical and frontal regions.
Figure 1 shows the timeline design of the phMRI design BOLD study. For animals in the control subgroup (n=4 for each anesthetic group), 60 minutes (150 repetitions) of the phMRI scan (Scan I) was first performed with intravenous (IV) administration of distilled water (1 ml/kg injected within 40 s) at the fifth minute into the scan. Later, another session of a 60-minute phMRI scan (Scan II) was carried out with l-THP (1 ml/kg in 5 mg/kg or 20 mg/kg dosages) IV injection at the fifth minute of Scan II. A 20-minute interval was applied between any successive two scans to ensure the least postpone effect from the previous pharmacological interventions (19). For animals in the pure experimental group, only Scan II was performed after anatomical scans.
Figure 1.

Data Analysis
The AFNI software package was applied for major data analysis procedures. PhMRI images of individual rats were first coregistered onto corresponding anatomical ones. Then, the time series of each voxel was temporally smoothed. According to the pharmacological and functional response character, the blood oxygen level-dependent (BOLD) phMRI signal of Scan I and II in each voxel of the individual animal was fitted with a nonlinear Beta model, as shown in Equation 1 (20):
| [1] |
where t0 is the time delay of response after drug injection, tf is the end time of response, k is a scaling coefficient, and α and β are parameters of the β-distribution. The initial values to be fitted for parameters t0, tf, k, α, and β were set to 0–5 min, 5–60 min, 0–100, 0–10, and 0–10, respectively. All initial values of those parameters were chosen loosely enough to accommodate the best fitting of time course data in various locations. Because the k value can be positive or negative, the voxelwise BOLD signal can be either positive or negative.
To compare the differences in phMRI results between the various dosages of l-THP groups and the vehicle (distilled water) group, the percentage change in the area under the curve (AUC %) of all voxels in each l-THP group was compared with those in the vehicle group. Two-tail Student’s t test was employed for statistical analyses of phMRI data. The statistical significance for activated voxels in phMRI data was operationally defined as P < 0.05 with a minimum of cluster size of 19 voxels (simulated result with AlphaSim command in AFNI) and voxel size of 0.55×0.55×2 mm3.
To test whether the different anesthetic agents interacted with l-THP-induced BOLD responses, a two-way ANOVA test was carried out on the voxelwise l-THP-induced AUC% values in all groups. Then, thresholding with clusterwise P < 0.05 and clustering with a minimum cluster size of 2 voxels took place.
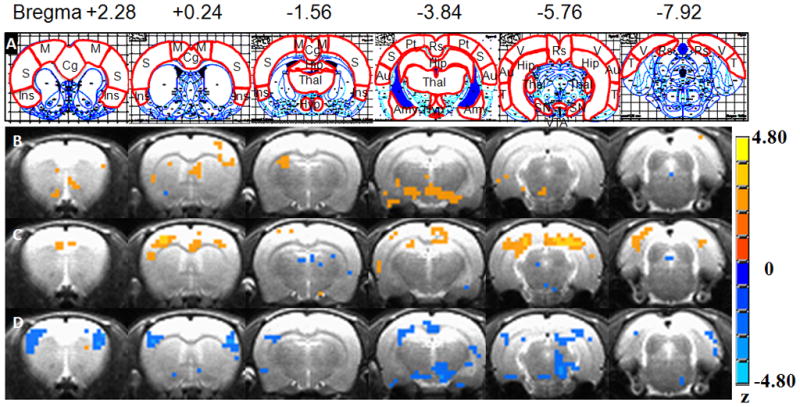
The anatomical image set of one rat was chosen as a reference template. FLIRT program in FSL software (21) was used to register all rats’ AUC% images onto the reference template. MIVA software (Center for Comparative NeuroImaging, Worcester, MA) was employed to segment the reference template images completely into 43 regions of interest ROIs. Regions with positive results were shown in Figure 3 Row A on the atlas (22). All phMRI activation maps, as well as the l-THP and anesthetic agent interaction maps, were overlaid on the corresponding anatomical images to show the localization of neuronal activity.
Figure 3.
Results
Acute l-THP-induced BOLD response in naïve rat brain under three anesthetic conditions
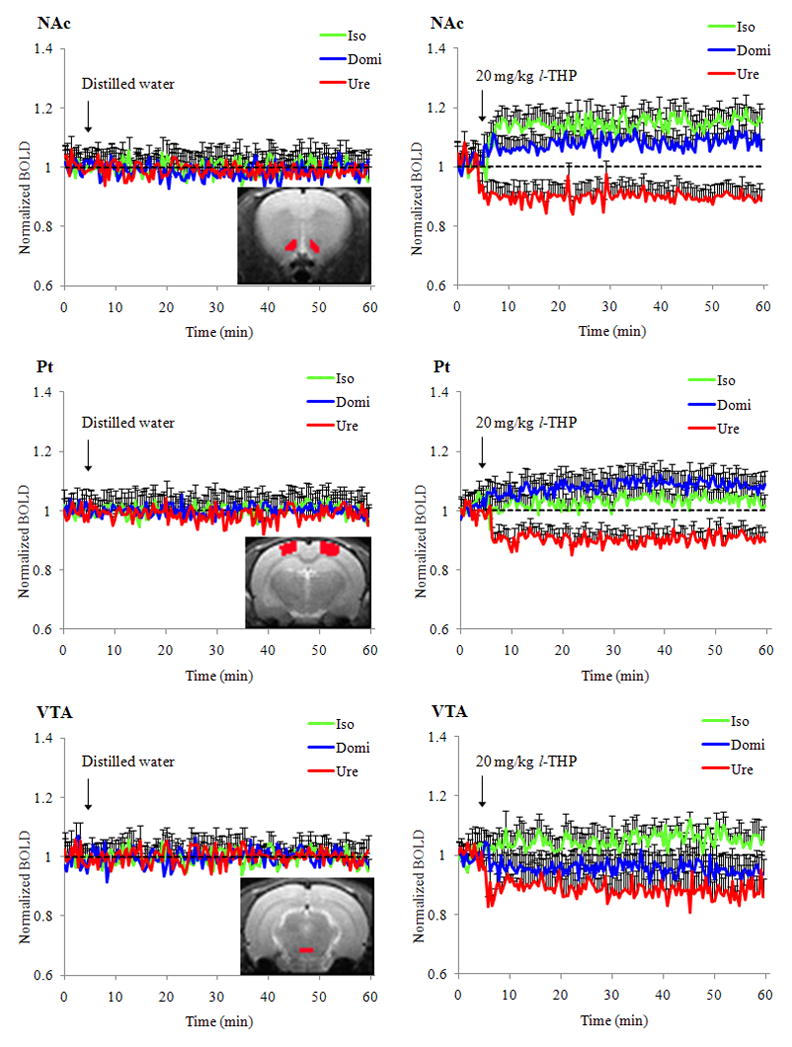
Table 1 shows the physiological parameter changes within five minutes (min) after of 20-mg/kg l-THP infusion. There were mild alterations in mean arterial blood pressure (BP), heart rate (HR), and peripheral oxygen saturation (SPO2) levels after infusion and recovery within three minutes after the introduction of isoflurane, medetomidine or urethane anesthesia. Figure 2 shows the group averaged time courses of regional brain responses to distilled water and 20 mg/kg l-THP infusions during the 60-minute scanning time. Under isoflurane, medetomidine or urethane anesthesia, l-THP induced differential BOLD responses in the nucleus accumbens (NAc), parietal cortex (Pt), and ventral tegmental area (VTA). In NAc and VTA, l-THP induced positive BOLD responses under isoflurane anesthesia. It also induced many negative BOLD responses under urethane anesthesia. In the Pt, the l-THP-induced BOLD change was the most positive when medetomidine anesthesia was introduced and the most negative when urethane anesthesia used. There were no significant BOLD signal changes following distilled water infusion. In respect to the NAc and Pt, regional brain responses to l-THP infusion (20 mg/kg) started within three minutes after infusion, and did not return to baseline level by the end of the 60-minute MRI scan session. PhMRI time-course analysis may reflect the transient pharmacokinetic profile of l-THP. Dr. Jin (2) found that intravenous injections of 50 mg/kg l-THP in rabbit resulted in a peak concentration of the drug in the brain within 1~2 min; this is significantly higher than concentrations found in the kidney, lung and liver. The l-THP blood concentration decreased quickly during 2~15 min, followed by a much slower clearance afterwards until it became negligible four hours after injection. The distribution half-life of l-THP was 12.9± 3.5 min and the elimination half-life was 110.3± 35.5 min.
Table 1.
Physiological parameters measurements taken after different time of 20 mg/kg l-THP injection with isoflurane, medetomidine, and urethane anesthesia.
| Anesthetic agent | Isoflurane | Medetomidine | Urethane | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time after l-THP | BP | HR | SPO2 | BP | HR | SPO2 | BP | HR | SPO2 |
| 0 s | 100±17 | 325±28 | 90±5 | 118±22 | 292±35 | 92±5 | 116±18 | 389±33 | 90±6 |
| 30 s | 110±22 | 328±26 | 91±3 | 125±23 | 300±31 | 88±4 | 108±14 | 252±67 | 93±5 |
| 1 min | 103±25 | 335±21 | 89±5 | 120±25 | 310±28 | 93±5 | 112±21 | 189±72 | 92±6 |
| 3 min | 105±18 | 330±29 | 92±6 | 116±20 | 285±36 | 91±6 | 115±22 | 350±38 | 91±5 |
| 5 min | 108±21 | 322±31 | 91±7 | 119±20 | 299±33 | 91±4 | 118±15 | 371±31 | 93±5 |
BP: Mean arterial blood pressure; HR; Hear rate; SPO2: Saturation of peripheral oxygen.
Figure 2.
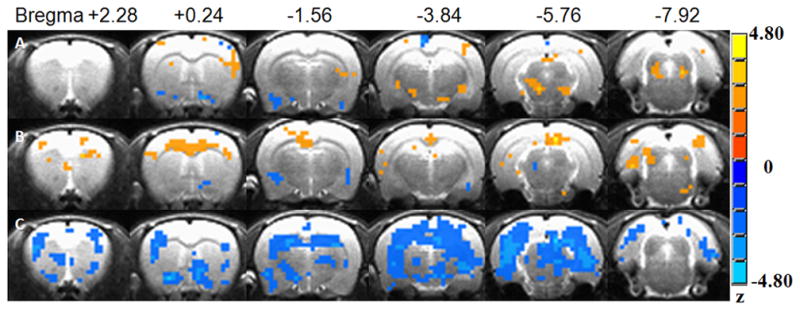
At 5 mg/kg dose (Figure 3), under isoflurane anesthesia, l-THP activated few brain regions (with increased AUC% compared to vehicle), namely the substantia nigra (SN), VTA and somatosensory cortex. The regional mean AUC% changes varied from 0.127 to 0.364. Also, at 5-mg/kg dose level, under medetomidine anesthesia, l-THP activated several positively responded brain regions, including the anterior cingulate cortex (ACC), somatosensory cortex, retrosplenium cortex and parietal cortex, in addition to some spots that responded negatively. The mean AUC% change varied from 0.162 to 0.677. Still, at the lower 5-mg/kg dose level, using urethane anesthesia, acute l-THP generated a regional mean AUC% change, ranging from −0.154 to −0.558. Only negatively responding regions, such as somatosensory cortex, SN, VTA, hypothalamus and visual cortex, were detected.
At a higher dose of 20 mg/kg (as in Figure 4), changes in responses were found under all three anesthetic conditions. With isoflurane, some negatively activated regions, such as the ventral pallidum, and piriform cortex, were detected in addition to the 5mg/kg result. Other brain regions such as the hippocampus and hypothalamus were reduced. In the case of medetomidine anesthesia, the negatively activated insula and part of the Amy were found additionally activated. In using urethane anesthesia, most activation regions were detected, but all of them were uniformly negative ones. These included the insula cortex, parietal cortex, amygdale (Amy), and midbrain.
Figure 4.
Two-way analysis of variance (ANOVA) was carried out to test the different anesthetic agents and dose interactions on the voxelwise l-THP-induced AUC% values in all anesthetic groups with the threshold of clusterwise P <0.05 and a minimum cluster size of two voxels (as in Fig. 5). The l-THP dose showed its main effect in the hypothalamus, CPu, insula, retrosplenium and temporal cortex cortices (as Row A in Fig. 5). Anesthetic agents showed their main effects in the cingulate cortex, insula, hypothalamus, CPu, globus pallidus, Amy, retrosplenium, and parietal cortex, VTA and Raphe nucleus (as Row B in Fig. 5). The anesthetic agent and l-THP dose showed an interaction effect in the broader regions of the cingulate cortex, insula, somatosensory cortex, hypothalamus, thalamus, CPu, Amy, retrosplenium, and parietal cortex, midbrain and Raphe nucleus (as Row C in Figure 5). Voxel AUC% values with significant drug-anesthetic interaction within each segmented ROI were averaged to obtain the regional averaged AUC% values and significant voxel number count (as in Table 2). Therefore, anesthetics and l-THP dose did demonstrate interactions in l-THP neuronal substrates.
Figure 5.
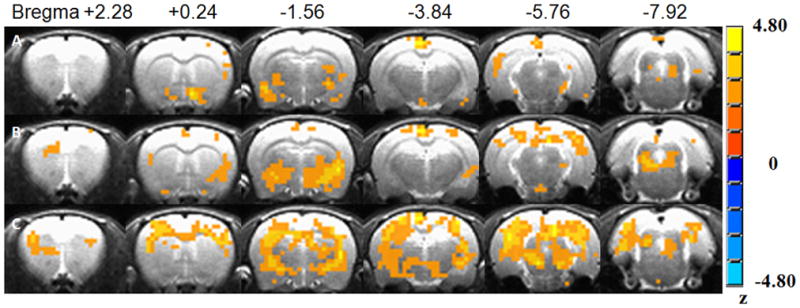
Table 2.
A list of 15 regions, showing significant interaction in three anesthetic agents (isoflurane, medetomidine, and urethane) and two dose levels of acute l-THP administration induced BOLD responses. Regional z value and significant voxel number were obtained from two-way ANOVA test as in Figure 5 C, with threshold of cluster-wise P < 0.01.
| ROI | Z score | voxel number |
|---|---|---|
| ventral tegm. area | 2.49±0.29 | 2 |
| substantia nigra | 2.70±0.23 | 3 |
| cingulate cortex | 2.63±0.32 | 3 |
| insular cortex | 2.29±0.44 | 4 |
| auditory cortex | 2.45±0.54 | 5 |
| amygdala | 2.40±0.58 | 5 |
| motor cortex | 2.12±0.43 | 7 |
| visual cortex | 2.67±0.49 | 8 |
| temporal cortex | 2.64±0.72 | 8 |
| hypothalamus | 2.26±1.36 | 16 |
| parietal cortex | 2.75±0.81 | 20 |
| retrosplenium | 3.05±0.64 | 21 |
| thalamus | 2.40±0.54 | 21 |
| somatosensory cortex | 2.72±1.56 | 65 |
| hippocampus | 2.88±1.61 | 89 |
Discussion and Conclusion
In characterizing the individual anesthesia effects of isoflurane, medetomidine and urethane on the phMRI results of acute l-THP administration, our study proves to be highly significant. For the first time, BOLD-based phMRI methods are used to investigate l-THP pharmacological mechanisms on naïve rats under these three anesthetic conditions. The results showed that acute administration of l-THP induced significant bilateral region-specific BOLD signal responses. The BOLD activation regions are consistent with those of the effective regions for the sedative and hypnotic effects of l-THP. These include most of the DA, adrenergic, and serotonin receptors distribution and projection regions (23).
The differential BOLD responses may result from individual effects on the central nervous system (CNS), as well as the cardiovascular system of each anesthetic agent. Information about these agents has already been discussed at the cellular and molecular level.
Isoflurane, like other inhaled anesthesia, allows better control over the length and depth of anesthesia, as opposed to injectable anesthetics. It is believed to be safe and easy to maintain with a quick and uneventful recovery (24). It has been reported that isoflurane can inhibit the release of the excitatory neurotransmitter, glutamate from synaptosomes harvested from the cortex, but not hippocampus or striatum. Isoflurane binds to gamma-aminobutyric acid (GABA) receptors, glutamate receptors and glycine receptors, and also inhibits conduction in activated potassium channels (25). Isoflurane also reduces phosphorylation of spinophilin at S94, but oppositely regulates phosphorylation of presynaptic (tyrosine hydroxylase) and postsynaptic (DARPP-32) markers of dopaminergic (DA) neurotransmission in striatum (26). The common modulation of dopaminergic neurotransmission of isoflurane and l-THP may result in the drug-anesthetic interaction found in our study.
Medetomidine has recently received increasing interest as a safe anesthetic for longitudinal use in fMRI studies. It is an alpha2 adrenergic receptor agonist and effective as a sedative agent. The agent may have a proconvulsant or anticonvulsant action. It has been used for rapid opioid detoxification and heroin withdrawal. Anesthesia with medetomidine can be easily reversed with antipamezole. Through endogenous hypnotic pathways, medetomidine can decrease in histamine in cortical and subcortical projections, and increase growth hormone secretion. It inhibits iron conductance in L and P type calcium channels, thereby facilitating voltage-gated, calcium-activated potassium channels. Unlike other opioids, it causes no hyperalgesia or allodynia after withdrawal,. Intravenous administration of medetomidine decreases heart rate, vascular resistance and sometimes cerebral blood flow (CBF) in some regions as well (27,28).
Urethane is widely used as an anesthetic in animal studies, because of its minimal effects on cardiovascular and respiratory systems, and maintenance of spinal reflexes. Hara and Harris (29) found that urethane potentiates the functions of neuronal nicotinic GABA A, and glycine receptors, and it inhibits N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5--methyl-4-isoxazole propionic acid receptors in a concentration-dependent manner. At concentrations close to an anesthetic 50% effective concentration, urethane had modest effects on all above channels, suggesting the lack of a single predominant target for its action. This may account for its usefulness as a veterinary anesthetic. However, a large concentration of urethane exerts marked effects on all those channels. Urethane anesthesia showed no effect on cardiac output, while there was a tendency of decreased blood flow rate and percentage of cardiac output in each tissue, other than muscle tissue, in which they increased as a counterbalance, in normal and tumor-bearing rats. Brain blood flow rate was about half of that in the conscious rats (30). This decrease in blood flow and multichannel blocking effects may impose additional negativity for l-THP induced BOLD responses.
Gallamine, a non-depolarizing muscle relaxant, was applied in the current study to minimize motion artifacts. It is found to be a competitive blocker of the nicotinic acetylcholine receptors at the neuromuscular junction. It causes a slower onset (>5min) of a relatively long-lasting (about 2hrs) paralyzing effect with no initial muscle fasciculation (31). Gallamine has a parasympatholytic effect on the cardiac vagus nerve, which causes tachycardia and occasionally hypertension. After IV injection, gallamine may pass the blood brain barrier in small amounts (32) and competitively block to the M2 and M4 muscarinic receptors in the CNS. Both receptors act via a Gi type receptor, which causes a decrease in cAMP in the cell, inhibition of voltage-gated Ca2+ channels, and efflux of K+, in general, leading to inhibitory-type effects. Recent studies demonstrated that muscarine enhances spontaneous GABA release and inhibits electrically evoked GABAergic synaptic transmission via the M1/M3 instead of M2/M4 receptors (33). Gallamine also did not block the enhancement of neuronal GABA-activated current induced by muscarine invitro (34). Besides, neither of the M2 or M4 receptors has been found to interact with the central DA, NE, or 5-HT receptors. Therefore, we expect minimal confounding from the interactions of gallamine with l-THP or any of the three anesthetic agents used in current study.
Differential results were obtained under three anesthetic conditions. Isoflurane revealed the smallest BOLD activation areas. Urethane had the most, although they were negative. Medetomidine showed mixed positive and negative activations. The relatively small BOLD responses under isoflurane may be partly due to the lower evoked field potential and/or higher baseline CBF under isoflurane anesthesia during stimulation and its dose-dependent modulation in neurovascular coupling particularly in the cerebral cortex (35). Increased baseline blood flow (CBF) level may be associated with decreased cerebral vascular reactivity and subsequent decreased CBF increase (10). Even with comparable stimulation-induced CBF increase, the higher baseline CBF under isoflurane anesthesia will still serve to decrease the relative CBF changes. As oxygen consumption should be similar with the same stimulation, the increased baseline CBF therefore may lead to decreased amplitude of l-THP induced BOLD responses. This is consistent with previous findings in the literature that indicate that there is decreased BOLD responses to forepaw stimulation and hypercapnia in isoflurane-anesthetized rats compared to those in the awake state (36–38). Combined positive and negative BOLD responses were found in our study, as well as several other fMRI studies carried out under medetomidine anesthesia (39,40). In using urethane, all negative l-THP-induced BOLD responses were revealed. Given the decreased blood flow and multichannel blocking effects of urethane, we speculate that the negative l-THP-induced BOLD responses found in our study may be partially related to the anesthetic effect of urethane.
Drug-anesthetic interactions in our phMRI study may be the result of anesthetic modulation in the neurotransmitter pathways shared with the drug the anesthetic modulation in cardiovascular reactivity and baseline cerebral blood flow; and also the anesthetic altered neurovascular coupling. As previously demonstrated, l-THP selectively acts on mesocorticolimbic dopaminergic, serotonergic and noradrenergic networks (3,41,42). The anesthetics of isoflurane, medetomidine, as well as urethane, act either directly or indirectly on these same circuits. This may explain the interaction of l-THP-induced BOLD responses with these anesthetic agents in specific brain regions.
In conclusion, isoflurane, medetomidine, and urethane have differential effects on acute l-THP-induced BOLD responses in naïve rats. There is region-specific drug-anesthetic interaction of l-THP with these anesthetic agents. In this study, we prefer urethane anesthesia, because of its sensitive responses to l-THP administration, although caution should be taken when interpreting results. To minimize the possible interactions between anesthetic agents and drug doses, an awake animal model should be adapted with acclimating procedures as previously studied (43).
Acknowledgments
This work was supported by National Institute of Health grants: R01 EB001820 (SJL) and R01 GM-56398 (AGH).
We thank Ms. Carrie M. O’Connor, M.A., for editorial assistance, Mr. Douglas Ward, M.S., for discussions on the statistical analysis. This work was supported by National Institute of Health grants: R01 EB001820 (SJL) and R01 GM-56398 (AGH).
References
- 1.Jin GZ, Zhu ZT, Fu Y. (−)-Stepholidine: a potential novel antipsychotic drug with dual D1 receptor agonist and D2 receptor antagonist actions. Trends Pharmacol Sci. 2002;23(1):4–7. doi: 10.1016/s0165-6147(00)01929-5. [DOI] [PubMed] [Google Scholar]
- 2.Jin GZ. Discoveries in the voyage of corydalis research. 2001. [Google Scholar]
- 3.Jin G-Z. (−)-Tetrahydropalmatine and its analogues as new dopamine receptor antagonists. Trends in Pharmacological Sciences. 1987;8(3):81–82. [Google Scholar]
- 4.Martin C, Sibson NR. Pharmacological MRI in animal models: A useful tool for 5-HT research? Neuropharmacology. 2008;55(6):1038–1047. doi: 10.1016/j.neuropharm.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Wise RG, Tracey I. The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23(6):862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt KF, Febo M, Shen Q, Luo F, Sicard KM, Ferris CF, Stein EA, Duong TQ. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI. Psychopharmacology (Berl) 2006;185(4):479–486. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah N, Long C, Marx W, DiResta GR, Arbit E, Mascott C, Mallya K, Bedford R. Cerebrovasculr response to CO2 in edematous brain during either fentanyl or isoflurane anesthesia. J Neurosurg Anesthesiol. 1990;2(1):11–15. doi: 10.1097/00008506-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res. 2007;1135(1):186–194. doi: 10.1016/j.brainres.2006.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu M, Ramani R, Swetye M, Constable RT. Spatial nonuniformity of the resting CBF and BOLD responses to sevoflurane: In vivo study of normal human subjects with magnetic resonance imaging. Hum Brain Mapp. 2008;29(12):1390–1399. doi: 10.1002/hbm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23(4):472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kida I, Smith AJ, Blumenfeld H, Behar KL, Hyder F. Lamotrigine suppresses neurophysiological responses to somatosensory stimulation in the rodent. Neuroimage. 2006;29(1):216–224. doi: 10.1016/j.neuroimage.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz AJ, Zocchi A, Reese T, Gozzi A, Garzotti M, Varnier G, Curcuruto O, Sartori I, Girlanda E, Biscaro B, Crestan V, Bertani S, Heidbreder C, Bifone A. Concurrent pharmacological MRI and in situ microdialysis of cocaine reveal a complex relationship between the central hemodynamic response and local dopamine concentration. Neuroimage. 2004;23(1):296–304. doi: 10.1016/j.neuroimage.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz AJ, Reese T, Gozzi A, Bifone A. Functional MRI using intravascular contrast agents: detrending of the relative cerebrovascular (rCBV) time course. Magn Reson Imaging. 2003;21(10):1191–1200. doi: 10.1016/j.mri.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Scherfler C, Donnemiller E, Schocke M, Dierkes K, Decristoforo C, Oberladstatter M, Kolbitsch C, Zschiegner F, Riccabona G, Poewe W, Wenning G. Evaluation of striatal dopamine transporter function in rats by in vivo beta-[123I]CIT pinhole SPECT. Neuroimage. 2002;17(1):128–141. doi: 10.1006/nimg.2002.1158. [DOI] [PubMed] [Google Scholar]
- 15.Mandeville JB, Jenkins BG, Kosofsky BE, Moskowitz MA, Rosen BR, Marota JJ. Regional sensitivity and coupling of BOLD and CBV changes during stimulation of rat brain. Magn Reson Med. 2001;45(3):443–447. doi: 10.1002/1522-2594(200103)45:3<443::aid-mrm1058>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology. 2005;30(5):936–943. doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology (Berl) 2000;148(3):299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- 18.Nitz WR. Fast and ultrafast non-echo-planar MR imaging techniques. Eur Radiol. 2002;12(12):2866–2882. doi: 10.1007/s00330-002-1428-9. [DOI] [PubMed] [Google Scholar]
- 19.Luo F, Xi ZX, Wu G, Liu C, Gardner EL, Li SJ. Attenuation of brain response to heroin correlates with the reinstatement of heroin-seeking in rats by fMRI. Neuroimage. 2004;22(3):1328–1335. doi: 10.1016/j.neuroimage.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Garren ST, Smith RL, Piegorsch WW. On a likelihood-based goodness-of-fit test of the beta-binomial model. Biometrics. 2000;56(3):947–950. doi: 10.1111/j.0006-341x.2000.947_1.x. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. xliii. Amsterdam; Boston: Elsevier Academic Press; 2005. p. 166. [Google Scholar]
- 23.Bian CF, Xing SH, Xu PC. The interactions between dihydroetorphine, L-tetrahydropalmatine, B-7601 and diazepam. Yao Xue Xue Bao. 1986;21(8):561–565. [PubMed] [Google Scholar]
- 24.Flecknell PA, Cruz IJ, Liles JH, Whelan G. Induction of anaesthesia with halothane and isoflurane in the rabbit: a comparison of the use of a face-mask or an anaesthetic chamber. Lab Anim. 1996;30(1):67–74. doi: 10.1258/002367796780744910. [DOI] [PubMed] [Google Scholar]
- 25.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322(5903):876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder GL, Galdi S, Hendrick JP, Hemmings HC., Jr General anesthetics selectively modulate glutamatergic and dopaminergic signaling via site-specific phosphorylation in vivo. Neuropharmacology. 2007;53(5):619–630. doi: 10.1016/j.neuropharm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Weber R, Ramos-Cabrer P, Wiedermann D, van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage. 2006;29(4):1303–1310. doi: 10.1016/j.neuroimage.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Ganjoo P, Farber NE, Hudetz A, Smith JJ, Samso E, Kampine JP, Schmeling WT. In vivo effects of dexmedetomidine on laser-Doppler flow and pial arteriolar diameter. Anesthesiology. 1998;88(2):429–439. doi: 10.1097/00000542-199802000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94(2):313–318. doi: 10.1097/00000539-200202000-00015. table of contents. [DOI] [PubMed] [Google Scholar]
- 30.Sakaeda T, Fukumura K, Takahashi K, Matsumura S, Matsuura E, Hirano K. Blood flow rate in normal and tumor-bearing rats in conscious state, under urethane anesthesia, and during systemic hypothermia. J Drug Target. 1998;6(4):261–272. doi: 10.3109/10611869808996834. [DOI] [PubMed] [Google Scholar]
- 31.Rang HP, Dale MM. Rang and Dale’s pharmacology. xiii. Edinburgh: Churchill Livingstone; 2007. p. 829. [Google Scholar]
- 32.Burke W, Ramzan I. Myoclonus in the decerebrate cat produced by gallamine. Brain Res. 1992;580(1–2):189–196. doi: 10.1016/0006-8993(92)90944-5. [DOI] [PubMed] [Google Scholar]
- 33.Guo J, Chiappinelli VA. Distinct muscarinic receptors enhance spontaneous GABA release and inhibit electrically evoked GABAergic synaptic transmission in the chick lateral spiriform nucleus. Neuroscience. 2001;104(4):1057–1066. doi: 10.1016/s0306-4522(01)00152-x. [DOI] [PubMed] [Google Scholar]
- 34.Hu HZ, Shao M, Li ZW. Enhancement of GABA-activated current by muscarine in rat dorsal root ganglion neurons. Neuroscience. 1999;89(3):883–890. doi: 10.1016/s0306-4522(98)00329-7. [DOI] [PubMed] [Google Scholar]
- 35.Masamoto K, Fukuda M, Vazquez A, Kim SG. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci. 2009;30(2):242–250. doi: 10.1111/j.1460-9568.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25(3):850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52(2):277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sommers MG, van Egmond J, Booij LH, Heerschap A. Isoflurane anesthesia is a valuable alternative for alpha-chloralose anesthesia in the forepaw stimulation model in rats. NMR Biomed. 2009;22(4):414–418. doi: 10.1002/nbm.1351. [DOI] [PubMed] [Google Scholar]
- 39.Pawela CP, Biswal BB, Hudetz AG, Schulte ML, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage. 2009;46(4):1137–1147. doi: 10.1016/j.neuroimage.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39(1):248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xing SH, Ge XQ, Yan M, Bian CF. Effects of dl-tetrahydropalmatine on blood pressure and norepinephrine and epinephrine contents in peripheral tissues. Zhongguo Yao Li Xue Bao. 1994;15(1):92–96. [PubMed] [Google Scholar]
- 42.Liu GQ, Algeri S, Garattini S. D-L-tetrahydropalmatine as monoamine depletor. Arch Int Pharmacodyn Ther. 1982;258(1):39–50. [PubMed] [Google Scholar]
- 43.Luo F, Li Z, Treistman SN, Kim YR, King JA, Fox GB, Ferris CF. Confounding effects of volatile anesthesia on CBV assessment in rodent forebrain following ethanol challenge. J Magn Reson Imaging. 2007;26(3):557–563. doi: 10.1002/jmri.21083. [DOI] [PubMed] [Google Scholar]


