Abstract
Bone morphogenetic proteins (BMPs) are growth factors that exert important functions in cell proliferation, migration and differentiation. Till date, multiple human tumors have been reported to display a dysregulation of several members of the BMP pathway that is associated with enhanced malignant tumor growth and metastasis. BMPER (BMP endothelial cell precursor-derived regulator) is a direct BMP modulator that is necessary for BMPs to exert their full-range signaling activity. Moreover, BMPER is expressed by endothelial cells and their progenitors, and has pro-angiogenic features in these cells. Here, we describe the expression of BMPER in human specimens of lung, colon and cervix carcinomas and cell lines derived from such carcinomas. In contrast to healthy tissues, BMPER is highly expressed upon malignant deterioration. Functionally, loss of BMPER in the lung tumor cell line A549 impairs proliferation, migration, invasion as well as tumor cell-induced endothelial cell sprout formation. In contrast, stimulation of A549 cells with exogenous BMPER had no further effect. We found that the BMPER effect may be transduced by regulation of the BMP target transcription factor inhibitor of DNA binding 1 (Id1) and matrix metalloproteinases (MMPs) 9 and 2. These facilitators of cell migration are down-regulated when BMPER is absent. To prove the relevance of our in vitro results in vivo, we generated Lewis lung carcinoma cells with impaired BMPER expression and implanted them into the lungs of C57BL/6 mice. In this model, the absence of BMPER resulted in severely reduced tumor growth and tumor angiogenesis. Taken together, these data unequivocally demonstrate that the BMP modulator BMPER is highly expressed in malignant tumors and tumor growth is dependent on the presence of BMPER.
Keywords: BMPER, BMPs, MMPs, tumor progression, tumor angiogenesis
Introduction
The transforming growth factor-beta family of growth factors has been subject of intense research efforts as it becomes more and more evident that perturbation of this pathway is accompanied by the promotion of malignant cell behavior (Hanahan and Weinberg, 2011). Bone morphogenetic proteins (BMPs) form an important subfamily of the transforming growth factor-beta family. Besides their developmental expression, BMPs are expressed in various tumor types including melanoma (Rothhammer et al., 2005), pancreas (Gordon et al., 2009), prostate (Yang et al., 2005; Buijs et al., 2007b), breast (Buijs et al., 2007a; Katsuno et al., 2008), renal (Blish et al., 2008), liver (Maegdefrau et al., 2009; Wu et al., 2011), ovary (Moll et al., 2006), lung (Langenfeld et al., 2006) and colon carcinoma (Deng et al., 2007; Thawani et al., 2010). The role of BMPs in tumor biology is controversially discussed. Several studies have shown that BMPs can either inhibit or promote tumor progression and metastasis (Alarmo and Kallioniemi, 2010). For example, BMP2 has been shown to inhibit tumorigenesis of breast cancer cells (Pouliot and Labrie, 2002), medulloblastoma (Zhao et al., 2008), or prostate cancer cells (Soda et al., 1998). On the contrary, BMP2 was reported to promote tumorigenesis of breast carcinoma (Raida et al., 2005), osteosarcoma (Arihiro and Inai, 2001; Weiss et al., 2006), prostate cancer (Feeley et al., 2005) and lung cancer (Langenfeld et al., 2003, 2006) (for a further review of BMPs and cancer, please refer to Thawani et al., 2010).
BMPs are extracellular proteins that signal through cell-surface complexes of type I and type II serine/threonine kinase receptors (Moser and Patterson, 2005). Upon activation, the receptors mediate intracellular signaling via the Id transcription factors involving the Smad 1/5 transcription factors (Schmierer and Hill, 2007). For instance, BMP2 activates Smad1/5 which is a prerequisite of increased cell proliferation (Langenfeld et al., 2006). Furthermore, BMPs can activate other signaling cascades such as the Erk1/2 pathway to ascertain a closely controlled regulation of cell proliferation and survival (Zhou et al., 2007; Balmanno and Cook, 2009). The impact of BMPs on cell proliferation is also controlled by other pathways, for example, by control of cyclin-dependent kinases (Klose et al., 2011).
BMPs are functionally modulated by extracellular binding proteins, such as Chordin (Piccolo et al., 1996), Noggin (Smith and Harland, 1992), Drm/Gremlin (Stabile et al., 2007), Cerberus/Caronte (Piccolo et al., 1999; Yu et al., 2008), Follistatin (Yamashita et al., 1995; Iemura et al., 1998), uterine sensitization-associated gene-1 (Laurikkala et al., 2003; Yanagita et al., 2004), Sclerostin (Brunkow et al., 2001; Krause et al., 2010), twisted gastrulation (Tsg) (Oelgeschlager et al., 2000) and BMP endothelial cell precursor derived regulator (BMPER) (Moser et al., 2003). (For further review of extracellular BMP modulators please refer to Balemans and Van Hul, 2002; Umulis et al., 2009). BMPER is known to bind and modulate at least three BMPs (BMP-2, -4 and -6), and was originally identified in a screen for differentially expressed proteins in embryonic endothelial precursor cells. BMPER is a secreted glycoprotein that contains five cysteine-rich domains followed by a von Willebrand D domain and a trypsin-inhibitor domain (Moser et al., 2003). We and others recently demonstrated that BMPER may enhance BMP signaling in a concentration-dependent fashion (Heinke et al., 2008; Serpe et al., 2008). Loss-of-function models reveal that BMPER has the ability to act as a pro-BMP modulator most likely by facilitating BMP binding to their respective receptors (Conley et al., 2000; Ikeya et al., 2006; Rentzsch et al., 2006; Heinke et al., 2008; Serpe et al., 2008). Here, we characterize the expression and function of BMPER in the context of malignant disease.
Results
BMPER is highly expressed in human lung, colon and cervix adenocarcinomas
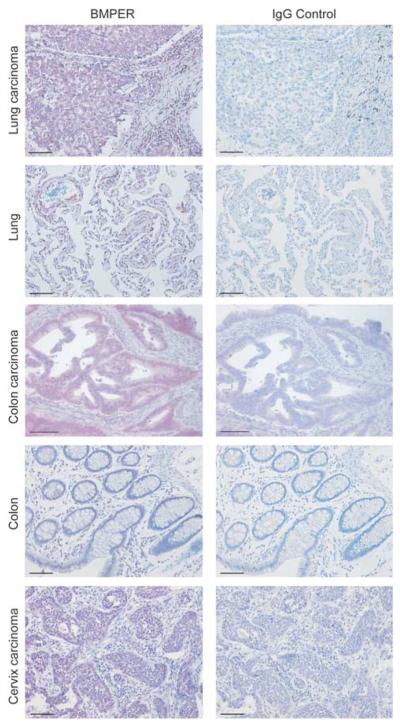
On the basis of reported evidence that BMP signaling is an important regulator of malignant disease, we set out to investigate the expression and function of the BMP modulator BMPER in tumors. As a first step, we analyzed the expression of BMPER in various human carcinomas by immunohistochemistry. As shown in Figure 1, BMPER is strongly expressed in lung, colon and cervix carcinoma specimens, whereas its expression is low in the respective normal tissues. Consistent with our previous data that BMPER is a secreted protein, we found BMPER expression predominantly in the cytoplasm of tumor cells and in the intercellular space. These data show that BMPER is highly upregulated in tumors and encouraged us to investigate BMPER in the context of malignant cell behavior in more detail.
Figure 1.
BMPER is expressed in human lung, colorectal and cervix adenocarcinomas. Tissue specimens of normal epithelium and invasive adenocarcinomas of the lung, colorectum and cervix were analyzed for BMPER expression using immunohistochemistry (left panels). BMPER expression is stained in purple. Cell nuclei were counterstained with hematoxylin (blue). Staining of serial sections with rat IgG was used as negative control (right panels). Scale bar, 100 μm.
BMPER is expressed in human carcinoma cell lines
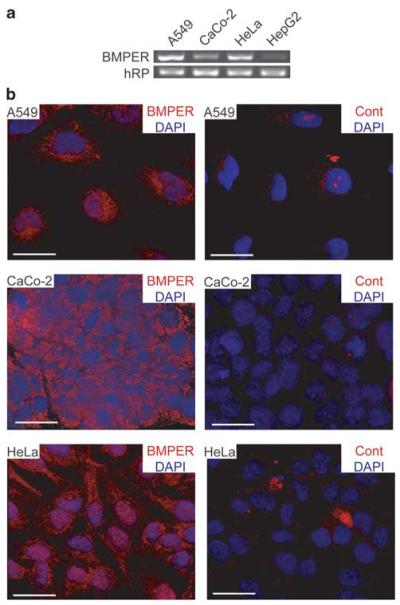
In order to allow for in vitro functional experiments using tumor cell lines, we explored BMPER expression in established tumor cell lines of different tissue origin. The highest levels of BMPER RNA were detected in A549 (lung adenocarcinoma) and HeLa (cervix adenocarcinoma) cells. BMPER RNA was also detectable in CaCo-2 (colon adenocarcinoma) and HepG2 (hepatocellular carcinoma) cells (Figure 2a). Using immunocytochemistry staining, we found that BMPER protein is present in the cytoplasm of cultured tumor cell lines (Figure 2b). This finding is consistent with our immunohistochemistry findings from human specimens. The localization of BMPER expression is also in agreement with reports that BMPER is detectable in late endosomes during embryonic development (Kelley et al., 2009). Thus, BMPER is highly expressed in the cytoplasm of tumor cells.
Figure 2.
BMPER expression and localization in tumor cells. (a) Expression of BMPER mRNA in human tumor cells lines of different origins was analyzed by reverse transcription-PCR. A549 (lung adenocarcinoma), CaCo-2 (colon adenocarcinoma), HeLa (cervix adenocarcinoma) and HepG2 (hepatocellular carcinoma) cells were analyzed using specific primers for BMPER; hRP served as internal control. (b) Localization of BMPER protein by immunocytochemistry in A549, CaCo-2 and HeLa cells (left panel). Rat IgG was used as negative control (right panel). Nuclei were stained with DAPI. Scale bar, 20 μm.
BMPER regulates tumor cell proliferation, cell migration and invasion
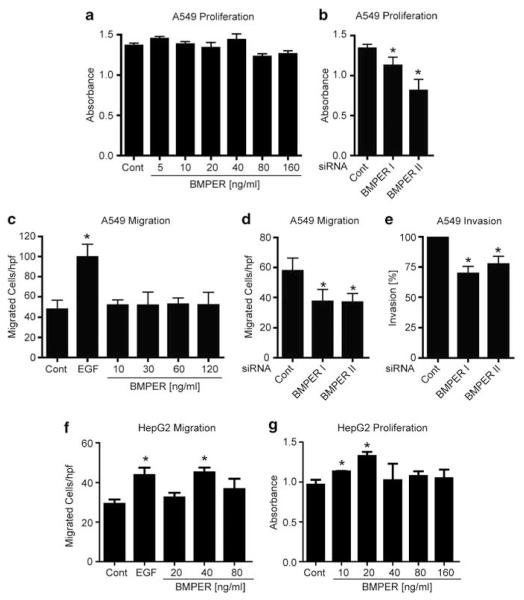
Next, we asked for the functional role of BMPER in tumors. Knowing that BMPs enhance cell proliferation and that BMPER is a crucial regulator of BMP pathway activity in endothelial cells, we investigated if BMPER regulates cell proliferation of tumor cells using the BrdU-incorporation method. When BMPER was added to A549 (Figure 3a) or HeLa cells (Supplementary Figure 1A), no change of proliferation rates occurred. Next, we aimed to investigate if loss of BMPER may influence tumor-cell proliferation. Therefore, BMPER was depleted using two different BMPER-specific siRNAs and proliferation was assessed in A549 cells, which express high levels of BMPER endogenously. In these cells, BMPER-specific siRNA transfection reduced BMPER expression by up to 80% (Supplementary Figure 4A), and resulted in markedly reduced tumor cell proliferation as shown in Figure 3b. This data suggests that both endogenous BMPER expression levels and cell proliferation rates are high in tumor cells even before supplementation with recombinant BMPER, and thus, proliferation cannot be increased beyond this level by supplementation of additional BMPER. But vice versa, when BMPER is depleted tumor cell proliferation is markedly reduced.
Figure 3.
Impact of BMPER on carcinoma cell proliferation, migration and invasion. (a) Proliferation was determined by BrdU assay. Triplicates of A549 cells were incubated for 24 h with BrdU and increasing concentrations of BMPER. Cells with BrdU and medium served as negative control. (b) A549 cells were transfected in triplicates with either one of two BMPER-specific siRNAs or scrambled-siRNA control. At 48 h post transfection, the BrdU ELISA was performed. (c) For trans-migration, A549 cells were serum-starved overnight and assayed with or without BMPER at indicated concentrations or EGF (50 ng/ml final concentration) as positive control. Triplicates were fixed after 4 h and five random microscopic fields were counted. (d) A549 cells were transfected with either one of two BMPER-specific siRNAs or scrambled siRNA control. Forty-eight hours post transfection tumor cell migration was quantified. (e) At 24 h post transfection of A549 cells, cell invasion into matrix was quantified. Triplicates were fixed after 24 h and five random microscopic fields were counted, respectively. (f) Trans-migration assay of HepG2 cells stimulated with recombinant BMPER protein at indicated concentrations. (g) Proliferation was determined by BrdU assay. Triplicates of HepG2 cells were incubated for 24 h with BrdU and increasing concentrations of BMPER. Cells with BrdU and medium served as negative control. Mean±s.d.; *P<0.05 versus the respective control. hpf = high power field.
Besides cell proliferation, migration and invasive growth capacity of cells are also important features of malignant cell behavior. To investigate the role of BMPER in tumor cell migration and invasion, we performed transmigration experiments using a modified Boyden chamber system. Stimulation of cells with epidermal growth factor (EGF) served as a positive control. A549 tumor cell migration remained unchanged supporting the notion that the effect of endogenous BMPER cannot be further enhanced by addition of exogenous BMPER (Figure 3c). Vice versa and similar to what we found for cell proliferation, when BMPER was depleted from cells, their migratory capacity was reduced by 36% (Figure 3d). To support this hypothesis, we performed additional migration and proliferation assays with the liver-cell carcinoma cell line HepG2, which endogenously expresses low levels of BMPER (Figure 2a). Indeed, HepG2 cells respond to stimulation with exogenous BMPER protein in a concentration-dependent increase of migration (Figure 3f) and proliferation (Figure 3g).
The fact that cell proliferation and migration are dependent on BMPER, led us to ask if the invasive capacity as the final common pathway of malignant cell behavior may be controlled by BMPER as well. To elucidate this question, cells were seeded into a modified Boyden chamber system coated with matrigel to mimic extracellular matrix (ECM). Indeed, when BMPER was silenced by siBMPER, cell invasion was reduced to an average of 73% in A549 cells compared with control siRNA (Figure 3e). To rule out a cell type-specific effect, the experiment was repeated with HeLa cells, and similar results were obtained (Supplementary Figures 1A–E). Mechanistically, we hypothesized that BMPs may be involved in induction of migration and proliferation of cells. Therefore, we performed similar experiments in the presence of the endogenous BMP antagonist Noggin or the small molecule BMP receptor antagonist dorsomorphin. Indeed, proliferation and migration of A549 cells were significantly reduced by both BMP antagonists suggesting that BMP signaling is involved in these cell functions (Supplementary Figures 2A and B). Taken together, exogenous BMPER cannot further enhance cell proliferation and migration in tumor cells that already express high levels of endogenous BMPER. However, cell functions that contribute to malignant cell behavior, such as proliferation, migration and ECM invasion, are dependent on the presence of BMPER.
Tumor cell-derived BMPER regulates endothelial cell function
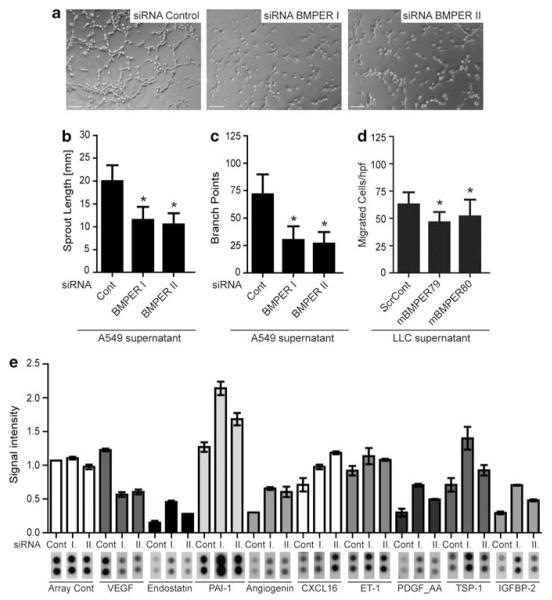
Having found that BMPER controls important tumor cell functions, we next aimed to investigate if tumor cell-derived BMPER may control the function of surrounding endothelial cells and thereby angiogenesis. Human umbilical vein endothelial cells (HUVECs) were analyzed for their capacity to sprout, migrate and form branches, when cultured with conditioned media from tumor cells in which BMPER had been depleted. Under these conditions, HUVEC sprouting was reduced by 48% compared with endothelial cells cultured with conditioned media from control cells (Figures 4a–c). Similarly, endothelial cell migration was significantly inhibited when HUVECs were incubated with conditioned media from BMPER-depleted tumor cells (LLCmBMPER79 or LLCmBMPER80) compared with conditioned media from control cells (LLCscrCont) (Figure 4d). These experiments were repeated with supernatants obtained from HeLa cells and similar results were obtained (Supplementary Figures 1F–G). Thus, when tumor cells express BMPER, their supernatant confers proangiogenic activity to adjacent endothelial cells.
Figure 4.
Impact of BMPER knockdown in tumor cells on endothelial cell function. (a–c) BMPER reduction in tumor cells effects tube formation of endothelial cells. HUVECs were incubated with the supernatant of A549 cells, which were transfected with one of two specific siRNA against BMPER or negative control siRNA, respectively. Thereafter, HUVECs were subjected to matrigel assay. Representative micrographs of HUVECs stimulated with supernatants of siRNA-transfected A549 cells are shown. Scale bar = 200 μm. (b) Cumulative sprout length and (c) branch points of capillary-like structures were measured after 3 h and quantified. Mean±s.d.; *P<0.001 versus control siRNA. (d) HUVEC migration was assayed with the supernatants of LLC shRNA clones in the trans-migration assay. Triplicates were fixed after 4 h and for each five random microscopic fields were counted. Mean±s.d.; *P<0.001 versus ScrCont. (e) ‘Proteome-profiler human-angiogenesis antibody array’ of control and BMPER-knocked down (I. and II.) of A549 cell lysates 48 h post transfection of siRNAs.
To investigate if this effect is conferred by BMPER contained in the media, we analyzed the activity of BMP-responsive signaling cascades in HUVECs. However, there was no activation of Smad1/5 or Erk1/2 signaling pathways under these conditions (data not shown). A second possibility is that tumor cells produce angiogenic factors that are reduced in the absence of BMPER from the tumor cells. To test this hypothesis, we decided to perform a ‘proteome-profiler human angiogenesis antibody array’ of control and BMPER-knocked-down A549 cells (Figure 4e). Indeed, loss of BMPER decreases vascular endothelial growth factor (VEGF) expression to 50% of control levels. Moreover, expression of anti-angiogenic proteins, such as Endostatin, PAI-1 and Thrombospondin-1 is increased in the absence of BMPER.
Together these data demonstrate that BMPER affects the angiogenic proteome of tumor cells that in turn forces endothelial cells to acquire a less angiogenic phenotype.
BMPER regulates the expression of matrix-degrading enzymes
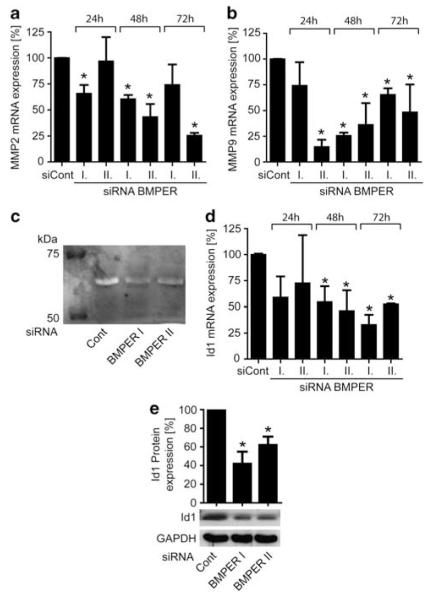
To investigate a potential mechanism for increased tumor cell migration and sprouting, we asked if BMPER might modulate the expression of matrix metalloproteinases (MMPs). Generally, MMPs are required to facilitate migratory and invasive processes during tumor growth (Egeblad and Werb, 2002). When BMPER was silenced in A549 cells, MMP-9 and MMP-2 were indeed downregulated, whereas MMPs-1, -3, and -13 remained unaffected (Figures 5a and b and data not shown). Consequently, MMP-2 activity in cell culture supernatants of BMPER silenced A549 cells was reduced as quantified by zymography (Figure 5c). To rule out a cell line-specific effect in A549 cells, we repeated these experiments in HeLa cells and found similar results (Supplementary Figures 3A–C). Thus, loss of BMPER is accompanied by reduced MMP activity.
Figure 5.
Lack of BMPER in A549 carcinoma cells modulates expression of MMPs and expression of the BMP target gene Id1. (a, b and d) mRNA expression of MMP-2, MMP-9 and Id1 in A549 cells after 24, 48 and 72 h post transfection with siRNA BMPER I or II was analyzed compared with scrambled siRNA control. mRNA content was quantified by real-time (q)PCR using specific primers for MMP-2, MMP-9, Id1 and hRP as internal control. Knock-down efficiency was calculated using ΔΔCT method. Mean±s.d.; *P<0.05 versus control. (c) Representative gelatin zymography assay of conditioned media collected from siRNA-transfected A549 cells 72 h post transfection. MMPs degrade gelatin and produce a white band in the coomassie-stained gelatin SDS gel. (e) Western blot analysis for Id1 was performed 48 h post transfection. GAPDH served as loading control. Densitometric analysis of Id protein expression for three independent experiments is shown. Mean±s.d.; *P<0.01 versus control.
Loss of BMPER reduces BMP pathway activity as reflected by Id1 expression
BMPER is a known modulator of BMP pathway activity in endothelial cells. We have shown earlier that BMPER may exert pro- or anti- BMP effects in a dose-dependent fashion. Now, we aimed to elucidate in which direction BMPER modulates BMP activity in tumor cells. We chose the transcription factor Id1, a well-characterized read-out for BMP pathway activity (Miyazono and Miyazawa, 2002; Fidler, 2003; ten Dijke et al., 2003), to assess BMPER effect on BMP pathway activity in tumor cells. When BMPER was silenced, Id1 expression was significantly reduced as detected on the RNA and protein levels (Figures 5d, e and Supplementary Figures 3D and E). Thus, loss of BMPER results in inhibition of BMP pathway activity, indicating that endogenous BMPER behaves as a pro-BMP mediator.
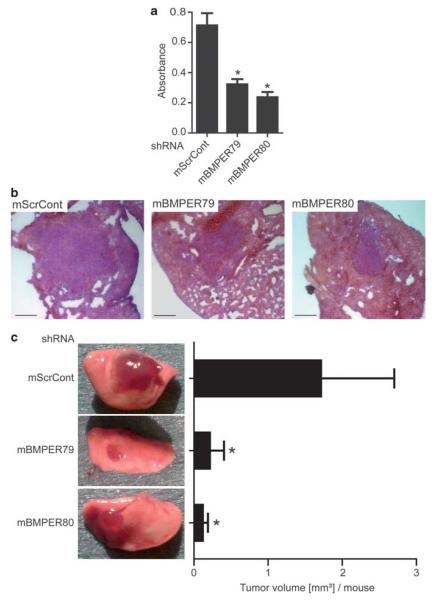
Loss of BMPER results in reduced tumor growth in vivo
Given our in vitro results that tumor cell proliferation and invasion are dependent on the presence of BMPER, our next goal was to investigate if these modifications in cell behavior result in changes in tumor growth in vivo. Therefore, we generated three different Lewis lung carcinoma (LLC) cell lines that stably express either of two different shRNAs specifically targeted against BMPER (mBMPER79 or mBMPER80) or a scrambled control shRNA (ScrCont) using lentiviral transduction. After clonal selection, significant inhibition of BMPER protein was confirmed (Supplementary Figure 4C). First, we functionally assessed these cell lines in vitro. Indeed, LLCmBMPER79 and LLCmBMPER80 cells behaved as expected from our previous experiments using siRNA transfections in A549 cells: Id1 expression was reduced, and proliferation was inhibited in LLCmBMPER79 and LLCmBMPER80 compared with in LLCScrControl cells (Supplementary Figure 4D and Figure 6a). To address the effect of loss of BMPER on tumor growth in vivo, 1 × 104 LLC cells were implanted into the left lung of C57BL/6 mice, respectively. After 14 days, mice were euthanized and tumor sizes were quantified. We observed significant differences in tumor size between tumors derived from BMPER-depleted LLCs (LLCmBMPER79, LLCmBMPER80) and tumors derived from LLCScrControl cells. Volumetric quantification revealed that control tumors were about eightfold larger than BMPER-depleted tumors (Figures 6b and c). Taken together, depletion of BMPER results in reduction of tumor growth in vivo.
Figure 6.
Mouse in vivo model of BMPER knockdown in LLC-2 cells. (a) Triplicates of three murine LLC clones stable expressing either a scrambled shRNA (ScrCont) or one of two different shRNAs targeted against BMPER (mBMPER79 or 80) were incubated for 24 h with BrdU in serum-free medium. Afterwards, the BrdU ELISA was performed. Mean±s.d.; *P<0.01 versus the ScrCont. (b, c) LLC shRNA clones were implanted into the left lung of 8-week old female C57BL/6 mice and tumor growth examined 2 weeks later. (b) Hematoxylin and Eosin (HE)-stained representative cryosections of LLC shRNA clone tumors in mouse lungs. Scale bar, 500 μm. (c) Macroscopic pictures and quantitative analysis of the tumor volume (ScrCont and mBMPER79 n = 9/group, mBMPER80 n = 6/group; mean±s.d.; *P<0.01 versus ScrCont).
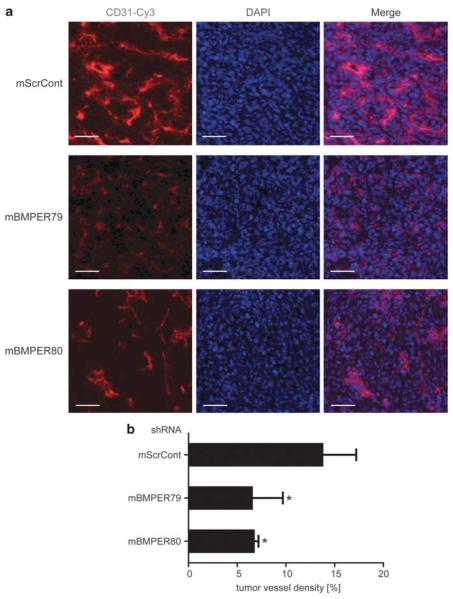
Loss of BMPER results in reduced tumor vessel density in vivo
Given our in vitro results that endothelial cell sprouting and branching are dependent on the presence of BMPER in tumor cells, our next goal was to investigate changes in tumor-derived angiogenesis in vivo. Tumor vascularization was determined at the time of autopsy on day 14 after implantation of LLC. Endothelial cells were visualized in tumor sections by immunofluorescence using an antibody directed against CD31 (Figure 7). Indeed, in tumors derived from LLCmBMPER79 and LLCmBMPER80, vessel density is significantly decreased to 50% compared with LLCScrControl. Thus, loss of BMPER in tumor cells results in inhibition of tumor angiogenesis in vivo.
Figure 7.
Vessel density of BMPER knock down LLC cells-derived tumors in vivo. (a) Immunohistofluorescence of CD31 (red— endothelial cells) and DAPI (blue—nuclei) stainings of representative cryosections from LLC shRNA tumors in mouse lungs. Scale bar = 50 μm. (b) Quantitative analysis of tumor vessel density (n=5/group; mean±s.d.; *P<0.01 versus ScrCont).
Discussion
In the present work, we found that tumor cell growth is strongly reduced in the absence of the extracellular BMP modulator BMPER, in vitro and in vivo. Mechanistically, we demonstrate that tumor cell growth and proliferation, BMP pathway activity, MMP expression and tumor angiogenesis are inhibited when BMPER is lacking.
As outlined in the introduction, the effect of BMPs on cancer cells is complex. On one hand, it is well documented that activation of the BMP pathway is associated with enhanced tumor progression; however, on the other hand, there are also reports that BMP pathway activation may have the potential to inhibit tumor cell proliferation, migration and angiogenesis. Certainly, dose effects and the respective local signaling context are variables that need to be taken into account to explain these discrepancies. Generally, the activity of BMP signaling can be regulated by extracellular modulators (Balemans and Van Hul, 2002). This mechanism is also in place in tumors and has consequences on tumor cell growth. For instance, the classical BMP antagonist Chordin is almost absent in epithelial ovarian cancers compared with normal tissues allowing for a dominant proliferative effect of BMPs. Vice versa, when Chordin is added to malignant cells in vitro, their invasive and migratory behavior is attenuated (Moll et al., 2006). Similarly, stable overexpression of Chordin leads to reduced migration and invasion of melanoma cells and consequently, to reduced tumor growth in vivo (Rothhammer et al., 2007). Along the same line of argument, when Noggin, another extracellular BMP antagonist, is added to lung carcinoma cells it bears anti-proliferative potential (Langenfeld et al., 2003). Several groups have shown that the presence of the downstream transcription factor of the BMP-pathway Id1 is associated with more aggressive, invasive cancer phenotypes (Ling et al., 2005; Langenfeld et al., 2006; Gautschi et al., 2008) and several tumors are dependent on the presence of Id1 or Id3 to grow at all (Lyden et al., 1999). Altogether, these findings suggest that in several malignant tumors the balance between pro- and anti-BMP factors is disturbed. One may speculate that the reconstitution of normal BMP signaling will at least inhibit tumor growth and may furthermore contribute to antagonize the malignant phenotype (Bailey et al., 2007).
In this context, our findings that BMPER is expressed at high levels in tumors in vitro and in vivo (Figures 1 and 2) are of particular interest because BMPER has been shown earlier to act as a BMP modulator (Heinke et al., 2008; Serpe et al., 2008). BMPER is part of a positive feedback loop that contributes to enhance local BMP activity, as shown in zebrafish and Drosophila (Rentzsch et al., 2006; Serpe et al., 2008). Here, we show that BMPER also plays a functional role in tumor cell biology. BMPER controls features of malignant cell behavior, such as cell proliferation and migration, as shown in loss-of-function experiments (Figures 3, 4 and 6). These results hold true in all tumor cell lines that we have investigated, suggesting a general role of BMPER in malignant cell behavior. Interestingly, it has been reported that in immune-resistant tumor cells BMPER expression is highly upregulated confirming our notion that BMPER expression is a feature of a highly malignant tumor phenotype (Lin et al., 2007). The functional role of BMPER in tumor cells is similar to its role in endothelial cells. In these cells, BMPER has an activating effect and controls the activity of the prototypical BMP agonist BMP4 (Heinke et al., 2008). The data presented here support the notion that endogenous BMPER is a general BMP enhancer not only in endothelial but also in tumor cells (Figure 5).
Malignant cell behavior downstream of BMP signaling is conferred—at least in part—by modulation of MMP activity. To enable detachment of tumor cells for invasion and metastasis, ECM is subjected to degradation by MMPs (Fidler, 2003; Thiery and Sleeman, 2006). Moreover, MMPs contribute to angiogenesis by exposing cryptic pro-angiogenic integrin-binding sites in the ECM and by releasing ECM-bound angiogenic growth factors such as VEGF (Bergers et al., 2000). In pancreatic tumor cells, upregulation of MMP-2 through Smad signaling induces their invasiveness (Gordon et al., 2009). In contrast, inhibition of BMP4 impairs MMP expression in melanoma cells (Rothhammer et al., 2008). Here we report a regulatory relationship between BMPER and MMPs that may contribute to explain the effect of BMPER on tumor cell behavior. When BMPER is silenced in tumor cells, MMP-9 and MMP-2 are downregulated (Figure 5). As a consequence, ECM cannot be processed properly to allow for tumor growth and metastasis explaining impaired tumor cell migration and invasion in the absence of BMPER.
Another important mechanism of tumor cell growth is angiogenesis (Hanahan and Weinberg, 2011). BMPs do not only enhance tumor proliferation but are also necessary to establish a vascular network for the growing tumor (Bailey et al., 2007; Rothhammer et al., 2007; Goumans et al., 2009). Along the same line of argument, we and others have shown that BMPs promote angiogenesis and capillary sprouting during embryonic development and in adulthood (Valdimarsdottir et al., 2002; Langenfeld and Langenfeld, 2004; Zhou et al., 2007). Recently, we reported that BMPER is a key regulator of BMPs in endothelial cells during angiogenesis in vitro and in vivo (Heinke et al., 2008). It has pro-angiogenic functions and is necessary for BMP4 to exert its activating role on endothelial cells (Heinke et al., 2008). Here, we show that tumor cells that have been depleted from BMPER are less potent to induce angiogenesis, most likely by shifting the balance of pro- and antiangiogenic factors expressed by these cells (Figure 4). VEGF is downregulated whereas anti-angiogenic proteins such as Endostatin, PAI-1 and Thrombospondin-1 are upregulated. These findings are functionally in line with reports that BMPs may upregulate VEGF (Deckers et al., 2002). Another mechanism of modulation of angiogenesis by BMPER is its modulatory role on MMPs, which are necessary not only for tumor cell invasiveness but also for angiogenesis (Orlichenko and Radisky, 2008; Huang et al., 2009).
Summary and conclusion
Taken together, BMPER is expressed in adenocarcinomas of the lung, colorectum and cervix and is necessary for their invasive behavior. Regarding tumor progression, BMPER is important in two ways: for the tumor cells themselves to promote growth and malignant cell behavior and also for stimulation of tumor-associated angiogenesis. Thus, BMPER may be a promising target for future anti-tumor therapy.
Materials and methods
Further details and additional methods are provided as Supplementary Information.
Cell culture and reagents
Detailed descriptions of cell culture and patient samples are provided in the Supplementary Materials and Methods.
RNA interference
BMPER-siRNAs I and II were purchased from Invitrogen, Karlsruhe, Germany. The sequences are available in the Supplementary Material and Methods Online. Scrambled negative control-Alexa Fluor 488 nm was purchased from Qiagen, Hilden, Germany. For transfection, a final concentration of 100 nmol/l siRNA together with Lipofectamine RNAiMAX was used according to the manufacturer’s reverse transfection protocol (Invitrogen).
RNA extraction and reverse transcription
DNA-free total RNA was extracted from A549, CaCo-2, HeLa and HepG2 cells using the Aurum RNA Mini Kit (Bio-Rad, Munich, Germany). Reverse transcriptions were performed with iScript cDNA-Kit applying 1 μg RNA following the manufacturer’s protocol (Bio-Rad).
Semiquantitative and real-time PCR
Reverse transcription-PCR analysis was performed as described previously (Heinke et al., 2008).
Quantitative real-time PCR analysis following RNA interference was performed using IQ SybrGreen 2 × Supermix and the iCycler real-time PCR detection system (Bio-Rad). Primer sequences are available in the Supplementary Material and Methods. Quantification was performed using MyiQ light-cycler software (Bio-Rad). Knockdown efficiency was calculated using the ΔΔCT method (Wong and Medrano, 2005). The housekeeping gene hRP was used for internal normalization.
Western blot analysis
Antibodies against BMPER (monoclonal rat ab; R&D systems, Wiesbaden, Germany), Id1 (polyclonal rabbit ab; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and GAPDH (polyclonal rabbit ab; Millipore, Schwalbach, Germany) were used and western blot analysis performed as previously described (Heinke et al., 2008).
Gelatin zymography
The method was performed as previously described by Gordon et al. (2009). Details are given in the Supplementary Materials and Methods.
Proteome profiler human angiogenesis antibody array
Protein expression analysis of A549 cells treated with siRNA control or BMPER siRNA (I and II) was performed 48 h post transfection using the R&D Systems proteome profiler human angiogenesis antibody array following the manufacturer’s instructions.
Proliferation assay
Proliferation was assessed using a colorimetric BrdU-incorporation ELISA (Roche, Penzberg, Germany). At 24 h after siRNA transfection, cells were cultured in fresh BrdU-containing medium for another 24 h. The colorimetric ELISA for BrdU quantification was performed following the manufacturer’s instruction.
Migration assay
Cell migration assay was performed as previously described (Heinke et al., 2008).
Invasion assay
Cell invasion was examined using the BD tumor invasion system following the manufacturer’s instructions (BD, Heidelberg, Germany).
Tumor cell supernatant-induced endothelial cell sprouting assay
At 24–48 h after siRNA transfection, A549 and HeLa cells were incubated with 1% fetal bovine serum endothelial basal media. Subsequently, HUVECs were pretreated with these tumor cell supernatants for 16–18 h and subjected to a matrigel assay as described previously (Heinke et al., 2008).
Orthotopic lung tumor implantation
The study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and was performed after securing appropriate institutional approval. Log-phase cell cultures of LLC shRNA clones were harvested, washed three times with PBS and resuspended at a cell density 6.6 × 105/ml. Mice were anesthetized, 1 × 104 LLCs were mixed with 15 μl of matrigel and implanted into the lung parenchyma, as previously described (Doki et al., 1999). At 14 days after tumor implantation, animals were euthanized through cardiac perfusion. To estimate the tumor volume as well as for histology, the lungs were removed and snap frozen in liquid nitrogen-cooled N-methylbutane until further analysis.
Estimation of tumor volume and vascularization
Details are given in the Supplementary Materials and Methods.
Immunocytochemistry
A549, CaCo-2 and HeLa cells grown on Falcon four-well chamber slides (BD) and were fixed in ice-cold methanol/acetone at −20 °C for 10 min. Afterwards, cells were incubated over night at 4 °C with the monoclonal BMPER antibody (1:250; R&D Systems) or with control ratIgG (Vector Laboratories, Burlingane, CA, USA), respectively. The staining was completed with goat-anti rat-Cy3 (1:500; Chemicon International, Millipore). For visualization of nuclei, slides were treated with DAPI (1:30 000; Sigma, Deisenhofen, Germany). All photographs were taken with Zeiss Apotome and analyzed with Zeiss Axiovision Rel. 4.7, Göttingen, Germany.
Immunohistochemistry
Details are given in the Supplementary Materials and Methods.
Statistical analysis and quantification
Statistical analysis was performed using GraphPad Prism 4.0, Graph Pad Software, La Jolla, CA, USA. Data are presented as mean±s.d. and comparisons were calculated by Student’s t-test (two-sided, unpaired). All experiments were repeated at least three times in triplicates. Results were considered statistically significant for P<0.05. For in vivo experiments one-way analysis of variance was performed.
Supplementary Material
Acknowledgements
We thank Bianca Engert, Anja Schöpflin and Ute Wering for excellent technical assistance. Work in MM’s laboratory is supported by German Research Foundation (DFG) SFB-TR23 (A1), DFG Mo973/5-1 and DFG Mo973/6-1. Work in the laboratory of SL and MW is supported by the German Research Foundation/DFG (SFB850 TP C5, TP Z1), the LandesStiftung Baden-Württemberg and the Mushett Family Foundation (Chester, NJ, USA) and the Robert-Bosch-Stiftung.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Alarmo EL, Kallioniemi A. Bone morphogenetic proteins in breast cancer: dual role in tumourigenesis? Endocr Relat Cancer. 2010;17:R123–R139. doi: 10.1677/ERC-09-0273. [DOI] [PubMed] [Google Scholar]
- Arihiro K, Inai K. Expression of CD31, Met/hepatocyte growth factor receptor and bone morphogenetic protein in bone metastasis of osteosarcoma. Pathol Int. 2001;51:100–106. doi: 10.1046/j.1440-1827.2001.01164.x. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem. 2007;102:829–839. doi: 10.1002/jcb.21509. [DOI] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blish KR, Wang W, Willingham MC, Du W, Birse CE, Krishnan SR, et al. A human bone morphogenetic protein antagonist is down-regulated in renal cancer. Mol Biol Cell. 2008;19:457–464. doi: 10.1091/mbc.E07-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R, et al. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007a;67:8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- Buijs JT, Rentsch CA, van der Horst G, van Overveld PG, Wetterwald A, Schwaninger R, et al. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol. 2007b;171:1047–1057. doi: 10.2353/ajpath.2007.070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, et al. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- Deng H, Makizumi R, Ravikumar TS, Dong H, Yang W, Yang WL. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp Cell Res. 2007;313:1033–1044. doi: 10.1016/j.yexcr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Doki Y, Murakami K, Yamaura T, Sugiyama S, Misaki T, Saiki I. Mediastinal lymph node metastasis model by orthotopic intrapulmonary implantation of Lewis lung carcinoma cells in mice. Br J Cancer. 1999;79:1121–1126. doi: 10.1038/sj.bjc.6690178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Feeley BT, Gamradt SC, Hsu WK, Liu N, Krenek L, Robbins P, et al. Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J Bone Miner Res. 2005;20:2189–2199. doi: 10.1359/JBMR.050802. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Gautschi O, Tepper CG, Purnell PR, Izumiya Y, Evans CP, Green TP, et al. Regulation of Id1 expression by SRC: implications for targeting of the bone morphogenetic protein pathway in cancer. Cancer Res. 2008;68:2250–2258. doi: 10.1158/0008-5472.CAN-07-6403. [DOI] [PubMed] [Google Scholar]
- Gordon KJ, Kirkbride KC, How T, Blobe GC. Bone Morphogenetic Proteins Induce Pancreatic Cancer Cell Invasiveness through a Smad1-dependent Mechanism that Involves Matrix Metalloproteinase Protein-2. Carcinogenesis. 2009;30:238–248. doi: 10.1093/carcin/bgn274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, et al. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103:804–812. doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- Huang SC, Sheu BC, Chang WC, Cheng CY, Wang PH, Lin S. Extracellular matrix proteases—cytokine regulation role in cancer and pregnancy. Front Biosci. 2009;14:1571–1588. doi: 10.2741/3325. [DOI] [PubMed] [Google Scholar]
- Iemura S, Yamamoto TS, Takagi C, Uchiyama H, Natsume T, Shimasaki S, et al. Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci USA. 1998;95:9337–9342. doi: 10.1073/pnas.95.16.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, et al. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development. 2006;133:4463–4473. doi: 10.1242/dev.02647. [DOI] [PubMed] [Google Scholar]
- Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27:6322–6333. doi: 10.1038/onc.2008.232. [DOI] [PubMed] [Google Scholar]
- Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, et al. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose A, Waerzeggers Y, Monfared P, Vukicevic S, Kaijzel EL, Winkeler A, et al. Imaging bone morphogenetic protein 7 induced cell cycle arrest in experimental gliomas. Neoplasia. 2011;13:276–285. doi: 10.1593/neo.101540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJ, van Bezooijen RL, et al. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem. 2010;285:41614–41626. doi: 10.1074/jbc.M110.153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P, Langenfeld J. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis. 2003;24:1445–1454. doi: 10.1093/carcin/bgg100. [DOI] [PubMed] [Google Scholar]
- Langenfeld EM, Kong Y, Langenfeld J. Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene. 2006;25:685–692. doi: 10.1038/sj.onc.1209110. [DOI] [PubMed] [Google Scholar]
- Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- Laurikkala J, Kassai Y, Pakkasjarvi L, Thesleff I, Itoh N. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol. 2003;264:91–105. doi: 10.1016/j.ydbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Lin KY, Lu D, Hung CF, Peng S, Huang L, Jie C, et al. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer Res. 2007;67:1832–1841. doi: 10.1158/0008-5472.CAN-06-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling MT, Lau TC, Zhou C, Chua CW, Kwok WK, Wang Q, et al. Overexpression of Id-1 in prostate cancer cells promotes angiogenesis through the activation of vascular endothelial growth factor (VEGF) Carcinogenesis. 2005;26:1668–1676. doi: 10.1093/carcin/bgi128. [DOI] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Maegdefrau U, Amann T, Winklmeier A, Braig S, Schubert T, Weiss TS, et al. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol. 2009;218:520–529. doi: 10.1002/path.2563. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE. 2002;2002:pe40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- Moll F, Millet C, Noel D, Orsetti B, Bardin A, Katsaros D, et al. Chordin is underexpressed in ovarian tumors and reduces tumor cell motility. FASEB J. 2006;20:240–250. doi: 10.1096/fj.05-4126com. [DOI] [PubMed] [Google Scholar]
- Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, et al. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Patterson C. Bone morphogenetic proteins and vascular differentiation: BMPing up vasculogenesis. Thromb Hae-most. 2005;94:713–718. doi: 10.1160/TH05-05-0312. [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot F, Labrie C. Role of Smad1 and Smad4 proteins in the induction of p21WAF1,Cip1 during bone morphogenetic protein-induced growth arrest in human breast cancer cells. J Endocrinol. 2002;172:187–198. doi: 10.1677/joe.0.1720187. [DOI] [PubMed] [Google Scholar]
- Raida M, Clement JH, Ameri K, Han C, Leek RD, Harris AL. Expression of bone morphogenetic protein 2 in breast cancer cells inhibits hypoxic cell death. Int J Oncol. 2005;26:1465–1470. [PubMed] [Google Scholar]
- Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–811. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene. 2007;26:4158–4170. doi: 10.1038/sj.onc.1210182. [DOI] [PubMed] [Google Scholar]
- Rothhammer T, Braig S, Bosserhoff AK. Bone morphogenetic proteins induce expression of metalloproteinases in melanoma cells and fibroblasts. Eur J Cancer. 2008;44:2526–2534. doi: 10.1016/j.ejca.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65:448–456. [PubMed] [Google Scholar]
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, et al. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14:940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Soda H, Raymond E, Sharma S, Lawrence R, Cerna C, Gomez L, et al. Antiproliferative effects of recombinant human bone morphogenetic protein-2 on human tumor colony-forming units. Anticancer Drugs. 1998;9:327–331. doi: 10.1097/00001813-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Stabile H, Mitola S, Moroni E, Belleri M, Nicoli S, Coltrini D, et al. Bone morphogenic protein antagonist Drm/gremlin is a novel proangiogenic factor. Blood. 2007;109:1834–1840. doi: 10.1182/blood-2006-06-032276. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Thawani JP, Wang AC, Than KD, Lin CY, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;66:233–246. doi: 10.1227/01.NEU.0000363722.42097.C2. discussion 246. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, et al. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106:2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- Weiss KR, Cooper GM, Jadlowiec JA, McGough RL, III, Huard J. VEGF and BMP expression in mouse osteosarcoma cells. Clin Orthop Relat Res. 2006;450:111–117. doi: 10.1097/01.blo.0000229333.98781.56. [DOI] [PubMed] [Google Scholar]
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- Wu JB, Fu HQ, Huang LZ, Liu AW, Zhang JX. Effects of siRNA-targeting BMP-2 on the abilities of migration and invasion of human liver cancer SMMC7721 cells and its mechanism. Cancer Gene Ther. 2011;18:20–25. doi: 10.1038/cgt.2010.55. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, et al. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita M, Oka M, Watabe T, Iguchi H, Niida A, Takahashi S, et al. USAG-1: a bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem Biophys Res Commun. 2004;316:490–500. doi: 10.1016/j.bbrc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhong C, Frenkel B, Reddi AH, Roy-Burman P. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005;65:5769–5777. doi: 10.1158/0008-5472.CAN-05-0289. [DOI] [PubMed] [Google Scholar]
- Yu X, He F, Zhang T, Espinoza-Lewis RA, Lin L, Yang J, et al. Cerberus functions as a BMP agonist to synergistically induce nodal expression during left-right axis determination in the chick embryo. Dev Dyn. 2008;237:3613–3623. doi: 10.1002/dvdy.21769. [DOI] [PubMed] [Google Scholar]
- Zhao H, Ayrault O, Zindy F, Kim JH, Roussel MF. Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes Dev. 2008;22:722–727. doi: 10.1101/gad.1636408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Heinke J, Vargas A, Winnik S, Krauss T, Bode C, et al. ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc Res. 2007;76:390–399. doi: 10.1016/j.cardiores.2007.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.