Abstract
The detection of saccharides in biological media is of great current importance for the monitoring of disease states. We have previously reported that solutions of boronic acid-functionalized macrocycles form acyclic oligomeric materials in situ. The oligomers contain fluorescent xanthene moieties. Current efforts are aimed at modulating the spectroscopic responses of these materials for the analysis of specific sugars. We describe conditions whereby the xanthene boronic acids exhibit high colorimetric fructose selectivity. In contrast, at physiological levels selective glucose monitoring can be achieved via fluorescence. Additionally, we describe a method which exhibits promise for detecting both glucose and fructose at dual wavelengths in the UV-Vis region. Mechanistic rationale for each of these findings is presented.
Keywords: Glucose, fructose, resorcinarene, boronic acid, xanthene
INTRODUCTION
There has been great progress made towards the design, synthesis and evaluation of organic dyes functionalized with boronic acids for sugar detection [1]. There are relatively few studies, however, addressing the simultaneous detection of common sugars, such as glucose and fructose, in mixtures [1,2]. It has been known for decades that boronic acids exhibit relatively high affinity for fructose compared to other saccharides [3]. More recently several glucose-selective chemosensors have been synthesized [1]. Nearly all are based on the key discovery by Shinkai and co-workers that scaffolds containing appropriately spaced bis-boronic acid moieties may selectively chelate glucose [1]. Herein we describe novel methodology which shows promise for the detection of both glucose and fructose via a combination of UV-Vis and fluorescence spectroscopy.
Previously we reported the facile synthesis and isolation of 1 on multi-gram scale via the acid-catalyzed condensation of resorcinol and 4-formyphenylboronic acid [4]. Compound 1 is soluble in aqueous polar aprotic solvents such as DMSO. We observed that colorless DMSO solutions containing 1 (Fig. 1), upon standing or heating at 90°C for 1 min, turned pinkish-purple. These color changes were monitored with UV-Vis spectroscopy via the appearance of new absorptions at 465, 500 and 536 nm [5].
Fig. 1.
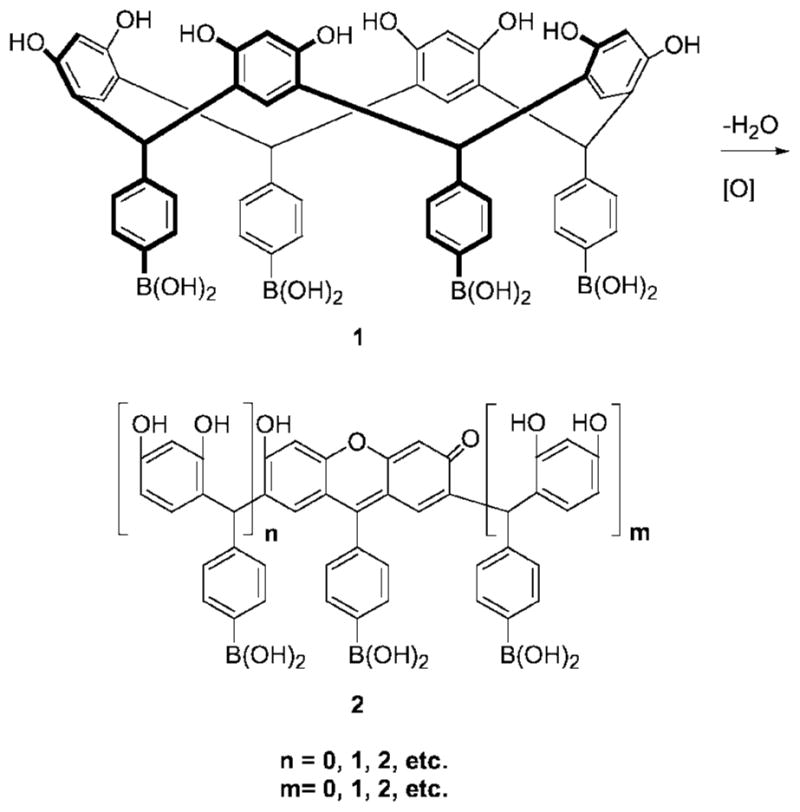
Ring opening of resorcinarene boronic acid macrocycle 1 affords acyclic oligomers containing xanthene moieties.
We found that upon heating eleven aqueous DMSO solutions each containing boronic acid resorcinarene macrocycles and eleven respective saccharides, a different solution color could be observed by visual inspection corresponding to each sugar [5]. The colorimetric responses were rapid, quantifiable and reproducible. More recently, we applied similar methodology towards the colorimetric detection of neutral oligosaccharides [6].
Mechanistic investigations, based on extensive spectroscopic, chromatographic and crystallographic studies, revealed that the color formation in the macrocycle-containing solutions was due to the formation of ring-opened acyclic oligomers possessing xanthene chromophores (2, Fig. 1) [6]. Our studies were greatly facilitated by the pioneering work of Weinelt and Schneider who had earlier described the reversible mechanism of resorcinarene macrocycle genesis in homogeneous solutions [7].
We determined that the sugar-promoted signal transduction arose via the formation of anionic sugar-boronate esters [6], in keeping with the well-known properties of boronic acid-functionalized dyes [1]. This was confirmed by 13C NMR using isotopically labeled sugars [6]. Our results were consistent with the important and useful NMR-based characterizations of related sugar-arylboronates performed by Norrild and co-workers [8].
EXPERIMENTAL
Reagents, solvents and human blood plasma were purchased from Sigma-Aldrich. Lyophilized blood plasma (5.0 mL) was reconstituted with H2O (2.0 mL) and deproteinized by addition of MeCN (3.0 mL). Clear filtrate was used for the glucose detection experiments.
All spectroscopic data was acquired at room temperature. Colored solutions of 1 were produced via preheating DMSO solutions and cooling to room temperature prior to adding water or plasma and analytes. UV-Vis data was obtained using a Spectramax Plus 384 spectrophotometer (Molecular Devices). Fluorescence spectra were recorded with a HR2000 fiber optic spectrometer (Ocean Optics). The excitation wavelength was 470 nm (Ocean Optics LS-450, blue LED) directed into a fluorescence cuvette via a 600 μm entrance fiber, and emission gathered by a 1000 μm optical fiber at λmax 579 nm. Configured for fluorescence, the setup used two mirrored screw plugs positioned at 90° within the cuvette holder for signal enhancement.
RESULTS AND DISCUSSION
Selective Detection of Fructose and the Ratiometric Monitoring of Fructose and Glucose Via UV-Vis Spectroscopy
We find that fructose promotes a striking solution color change in the presence of preheated, colored aryl-boronic acid resorcinarene 1 (from pink-purple) instantly at room temperature. Colorless chemosensor 1 (5.2 mM) is heated at a gentle reflux for 3 min in DMSO (0.9 mL) in air to afford a colored solution containing 1. After cooling to room temperature, fructose (1 equiv) in 0.1 mL H2O is added. A color change from pink-purple to orange-yellow is observed. Glucose, sucrose, maltose, lactose and xylose, 3 equiv each, exhibit no color change within 2 hr at room temperature (Fig. 2).
Fig. 2.
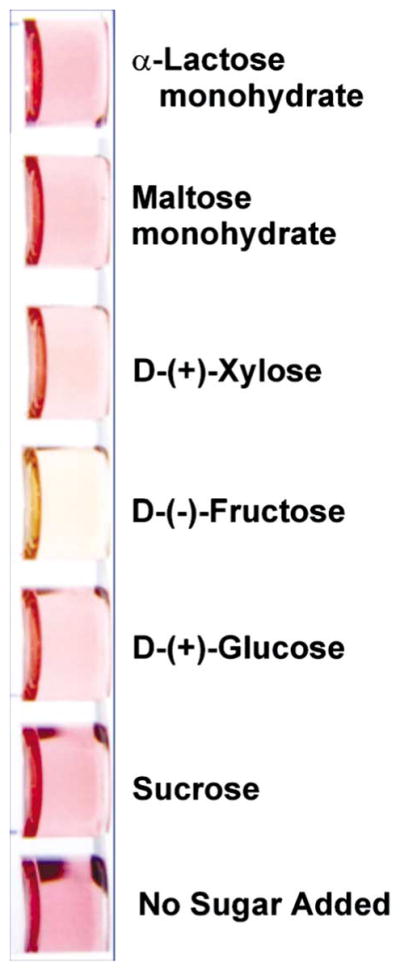
A selective color change promoted by fructose is observed at room temperature upon addition to a colored solution containing 1.
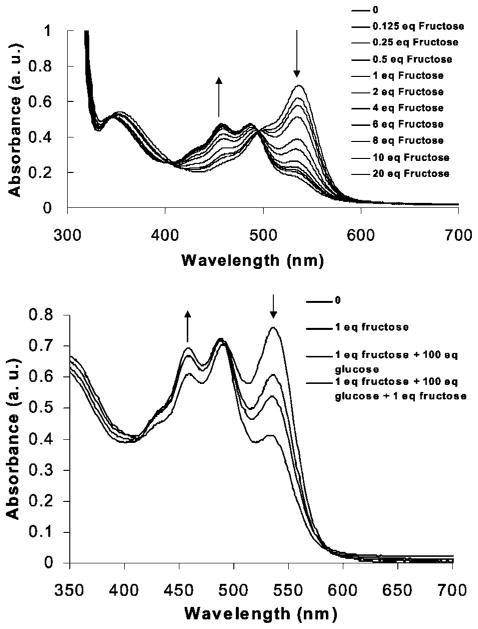
The color change is monitored by observing the ratiometric absorbance intensity decrease at 536 nm and increase at 464 nm (Fig. 3). The absorbance changes exhibit a linear dependence with fructose concentration (R = 0.9079 and 0.9419 at each of the two wavelengths, respectively).
Fig. 3.
Upper: Addition of fructose to a preheated (3.0 min at reflux) solution of 1 (5.2 × 10−3 M) in DMSO at room temperature affords concentration-dependent absorbance changes at 464 and 536 nm. Lower: UV-Vis spectra of a 9:1 DMSO:H2O solution containing 1 (5.2 × 10−3 M) pre-heated (1.5 min at reflux) (i) alone, (ii) upon addition of 1 equiv fructose at room temperature which produces an absorbance increase at 464 nm and a corresponding decrease at 536 nm, (iii) upon addition of 100 equiv glucose which produces no absorbance change at 464 nm but a decrease at 536 nm and (iv) upon addition of a second equivalent of fructose which affords a further absorbance increase at 464 nm and decrease at 536 nm.
The ratio of glucose to fructose in blood plasma is ca. 100:1. Addition of 1 equivalent of fructose to a colored solution containing 1 at room temperature results in an 8.6% increase in the absorbance at 464 nm. Addition of 100 equivalents of glucose to the fructose/1 solution results in no detectable change in the fructose/1 absorbance at 464 nm. Addition of a second equiv of fructose to this latter solution results in a readily observable absorbance increase of 3.3%.
At 536 nm, the absorbance of solutions of 1 is lowered by 20% upon addition of 1 equiv fructose. Subsequent addition of 100 equivalents of glucose lowers the absorbance further by 12%. Addition of another equivalent of fructose again lowers the absorbance by 24% (Fig. 3).
This result indicates the potential feasibility of determining fructose, in the presence of excess of glucose, by monitoring fructose concentrations at 464 nm, where an absorbance change is not produced in response to glucose. In addition, one should be able to concurrently determine the glucose present in a sample via analysis of the ratio of the absorbance at 536 nm (at which wavelength both glucose and fructose promote signal changes) to the absorbance at 464 nm, for instance, after the fructose concentration is determined at 464 nm.
The selectivity for fructose appears consistent with our previous binding constant studies of neutral sugars [6]. Fructose binds monoboronic acids with a greater affinity than glucose due to a favorable configuration of hydroxyls [1]. Additionally, we had determined that anionic fructoseboronate formation lowers the pKa of the colored xanthenes (2), resulting in absorbance changes [6]. It is well-known that xanthene dyes show an increase in absorbance at their shorter visible wavelength and a decrease in absorbance at their longer visible wavelength in response to a lowering of solution pH. Thus, we can ascribe the ratiometric responses observed at 464 and 536 nm in the current studies as due, in large part, to a lowering of xanthene pKa upon sugar binding.
The UV-Vis studies described above would require higher concentrations of sugars than typically found in most naturally-occurring biological samples in order to generate useful signals. We are investigating the synthesis and study of new functional xanthene dyes with enhanced sensitivity in order to avoid concentration steps in sample monitoring. Since the xanthenes (2) are present in colored solutions only at micromolar levels [6], their synthesis and/or isolation as discreet compounds should afford materials with higher colorimetric sensitivity, since a significant amount of sugar binds to excess colorless boronic acid 1. They (2) are attained readily via the highly facile synthesis of 1 [6]. The presence of an excess of visually inactive binding sites appears to diminish sensitivity; however, high fructose selectivity is attained. The proportion of boronic acid binding sites functioning as signal induction-promoting moieties can also favorably influence selectivity in these systems in fluorescence experiments, as described below. Colored solutions containing 1 show promise for monitoring glucose levels in the range of physiological levels via fluorescence spectroscopy.
Enhanced Glucose Selectivity Via Fluorescence Detection in the Range of Physiological Concentrations and in Human Blood Plasma
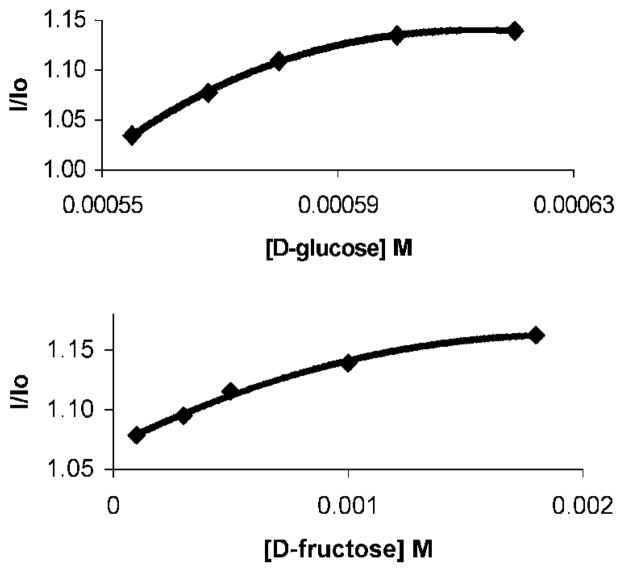
The fluorescence emission spectra of colored solutions containing 1 and added glucose or fructose are shown in Fig. 4. As the sugar concentration is increased we observe concomitant emission increases promoted by fructose and glucose. The significant signaling generated by glucose is in contrast to the UV-Vis studies (vide supra) in which relatively much weaker absorbance responses were promoted by glucose as compared to fructose.
Fig. 4.
Upper: Fluorescence emission changes produced upon addition of D-glucose to a colored solution (DMSO:H2O 9:1) containing 1 (5.0 × 10−3 M) at room temperature. The glucose concentration was increased from 0 to 6.2 × 10−4 M. Lower: Fluorescence emission changes produced upon addition of D-fructose to a colored solution (DMSO:H2O 9:1) containing 1 (5.0 × 10−3 M) at room temperature. The fructose concentration is increased from 0 to 1.8 × 10−3 M. The fluorescence intensity increases (corresponding to the increase of glucose or fructose concentration) are 13.9 and 18.2%, respectively. The emission changes are obtained at glucose levels that are smaller than those of fructose.
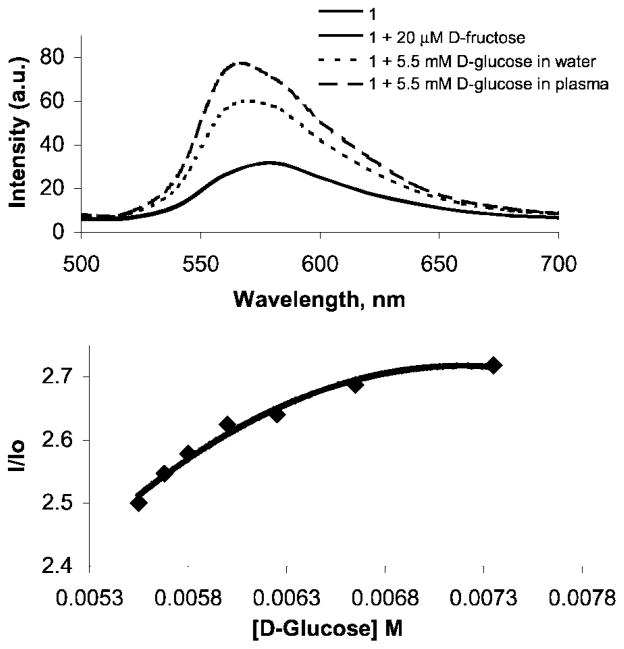
The normal level of D-fructose in human blood plasma is ca. 50 μM. Fluorescence emission spectra of colored solutions containing 1 and added fructose, obtained over the fructose concentration range of 20 to 100 μM, exhibit no detectable fructose-promoted emission. Healthy levels of D-glucose are ca. 5 mM. The emission spectra of glucose (5.5 × 10−3 M) and 1 (1.0 × 10−3 M) in DMSO:H2O, 9:1, as well as in a 9:1 DMSO:plasma solution, are shown in Fig. 5. Emission increases due to the presence of glucose are observed in both cases. A dependence of fluorescence emission intensity on increased glucose levels in plasma is observed (Fig. 5).
Fig. 5.
Upper: Fluorescence emission spectra produced by (i) a preheated (3 min at reflux) colored solution (DMSO:H2O 9:1) containing 1 (5.0 × 10−3 M) at room temperature, (ii) the same conditions but in the presence of 20 μM D-fructose, added at room temperature, which affords no observable change in emission, (iii) the same conditions as (i) but with added D-glucose (5.3 μM) which promotes an emission increase and (iv) same conditions as (iii) but in deproteinized human blood plasma instead of H2O, which exhibits an emission increase in response to added glucose. Lower: Concentration-dependent emission changes produced via room temperature additions of D-glucose to a 9:1 DMSO:plasma solution containing 1.
The results of the fluorescence studies show that the boronic acid-functionalized xanthenes (2) may promote the detection of glucose in blood plasma with negligible interference from fructose. New UV-Vis and near-IR absorbing congeners of 2, possessing functionality for greater potential glucose signaling ability, are currently being designed in our lab to address potential fluorescence interference issues in blood plasma.
The enhanced fluorescence emission promoted by glucose, as compared to the its relatively weaker UV-Vis responses (e.g., relative to fructose), may be attributed to chelation by neighboring boronic acids of 2. It is well-known that glucose can be chelated by bis-boronic acids. This results in chemosensor scaffold rigidification effects, which have been previously demonstrated to afford fluorescence emission enhancement [1,9]. An excess of boronic acid binding sites relative to glucose should promote chelation. The large excess of non-absorbing 1 compared to responsive fluorophores (2) should thus facilitate glucose-promoted emission by competing for glucose binding to 2.
CONCLUSION
We have presented evidence that oligomeric xanthene dye-functionalized boronic acids, which form in situ from tetraarylboronic acid resorcinarene macrocycles, show promise for the selective detection of glucose and fructose. The fluorescence studies indicate that selectivity for glucose over fructose at physiological levels in plasma may be achieved. Via UV-Vis spectroscopy, we observe high fructose selectivity. Additionally, ratiometry may also be used to simultaneously measure fructose and glucose levels, at proportionally higher concentrations. The mechanistic insights gained from these results will aid us in designing improved xanthene-derived chemosensors with tuneable properties. The synthesis of water soluble and surface-bound congeners of the molecules described herein is also in progress.
Acknowledgments
We are very grateful to the National Institutes of Health for supporting this research via grant 8R01EB002044-03.
References
- 1.James TD, Shinkai S. Artificial receptors as chemosensors for carbohydrates. Top Curr Chem. 2002;218:159–200. [Google Scholar]; (b) Wang W, Gao X, Wang B. Boronic acid-based sensors. Curr Org Chem. 2002;6(14):1285–1317. [Google Scholar]
- 2.Arimori S, Bell ML, Oh CS, Farimat KA, James TD. Modular fluorescence sensors for saccharides. Chem Commun. 2001:1836–1837. [PubMed] [Google Scholar]
- 3.Lorand JP, Edwards JD. Polyol complexes and structure of the benzeneboronate ion. J Org Chem. 1959;24:769–774. [Google Scholar]
- 4.Lewis PT, Davis CJ, Saraiva M, Treleaven D, McCarley T, Strongin RM. Tetraarylboronic acid resorcinarene stereoisomers. Versatile new substrates for divergent polyfunctionalization and molecular recognition. J Org Chem. 1997;62(18):6110–6111. [Google Scholar]
- 5.Davis CJ, Lewis PT, McCarroll ME, Read MW, Cueto R, Strongin RM. Simple and rapid visual sensing of saccharides. Org Lett. 1999;1(2):331–334. doi: 10.1021/ol990105a. [DOI] [PubMed] [Google Scholar]
- 6.He M, Johnson RJ, Escobedo JO, Beck PA, Kim KK, St Luce NN, Davis CJ, Lewis PT, Fronczek FR, Melancon BJ, Mrse AA, Treleaven WD, Strongin RM. Chromophore formation in resorcinarene solutions and the visual detection of mono- and oligosaccharides. J Am Chem Soc. 2002;124(18):5000–5009. doi: 10.1021/ja017713h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinelt F, Schneider H-J. Mechanisms of macrocycle genesis. The condensation or resorcinol with aldehydes. J Org Chem. 1991;56(19):5527–5535. [Google Scholar]
- 8.Norrild JC, Eggert H. Boronic acids as fructose sensors. Structure determination of the complexes involved using (1)J(CC) coupling constants. J Chem Soc, Perkin Trans. 1996;2(12):2583–2588. [Google Scholar]
- 9.(a) Takeuchi M, Yoda S, Imada T, Shinkai S. Chiral sugar recognition by a diboronic-acid-appended binaphtyl derivative through rigidification effect. Tetrahedron. 1997;53(25):8335–8348. [Google Scholar]; (b) Takeuchi M, Mizuno T, Shinmori H, Nakashima M, Shinkai S. Fluorescence and CD spectroscopic sugar sensing by a cyanine-appended diboronic acid probe. Tetrahedron. 1996;52(4):1195–1204. [Google Scholar]