Abstract
Objective
Clinical use of paclitaxel is limited by variable responses and the potential for significant toxicity. To date, studies of association between variants in candidate genes and paclitaxel effects have yielded conflicting results. We sought to evaluate relationships between global gene expression and paclitaxel sensitivity.
Methods
We utilized well-genotyped lymphoblastoid cell lines derived from the International HapMap Project to evaluate relationships between cellular susceptibility to paclitaxel and global gene expression. Cells were exposed to varying concentrations of paclitaxel to evaluate paclitaxel-induced cytotoxicity and apoptosis. Among the top genes, we identified solute carrier (SLC) genes associated with paclitaxel sensitivity and narrowed down the list to those that had single nucleotide polymorphisms (SNPs) associated with both their expression level of the SLC gene and also with paclitaxel sensitivity. We performed independent validation in an independent set of cell lines and also conducted functional studies using RNA interference.
Results
Of all genes associated with paclitaxel-induced cytotoxicity at p<0.05 (1713 genes), there was a significant enrichment of SLC genes (31 genes). A subset of SLC genes, namely SLC31A2, SLC43A1, SLC35A5, and SLC41A2, were associated with paclitaxel sensitivity and had regulating SNPs that were also associated with paclitaxel-induced cytotoxicity. Multivariate modeling demonstrated that those 4 SLC genes jointly explain 20% of the observed variability in paclitaxel susceptibility. Using RNA interference, we demonstrated increased paclitaxel susceptibility with knockdown of 3 SLC genes, SLC31A2, SLC35A5, and SLC41A2.
Conclusions
Our findings are novel and lend further support to the role of transporters, specifically solute carriers in mediating cellular susceptibility to paclitaxel.
Keywords: paclitaxel, solute carrier genes, transporters, lymphoblastoid cell lines, pharmacogenomics
Introduction
Paclitaxel acts by causing stabilization of tubulin polymerization with consequent induction of apoptosis [1] and early studies demonstrated efficacy in ovarian, breast, non-small-cell lung, and head and neck cancer [2]. In breast cancer, the CALGB 9344 trial demonstrated that sequential paclitaxel, added to standard cyclophosphamide and doxorubicin-based therapy, improved both disease-free and overall survival among women with node-positive disease [3]. Paclitaxel resistance contributes to disease relapse and poor outcomes for patients with several malignancies. Furthermore, peripheral neuropathy is a dose-limiting toxicity that contributes to premature therapy discontinuation and increased morbidity among cancer survivors [4]. A phase II study of paclitaxel in patients with metastatic breast cancer demonstrated overall response rates of 20.8% in a pretreated group and 32% in a chemotherapy-naïve group. In the same study, grade 3 neutropenia, grade 4 neutropenia and grade 3 sensory neuropathy were seen in 36%, 33%, and 8% of patients, respectively [5]. Given the large inter-individual variability in paclitaxel pharmacokinetics and pharmacodynamics, several groups have evaluated polymorphisms in genes encoding paclitaxel metabolizing enzymes for association with outcomes, but findings have been inconclusive [6].
Influx and efflux transporters may act with intracellular metabolizing enzymes to regulate drug effects [7] and reduced therapeutic efficacy may result from transporter inhibition by co-administered drugs [8]. Paclitaxel uptake in liver is mediated by organic anion transporting polypeptides (OATP)1B3 and 1B1 [9, 10] while efflux is mediated by P glycoprotein [11]. Genetic variability in solute carriers involved in paclitaxel transport namely, SLCO1B3 encoding OATP1B3, and SLCO1B1 encoding OATP1B1, has been demonstrated [12] and could potentially alter paclitaxel disposition. Although altered expression of efflux and influx transporters may confer paclitaxel resistance in cancer cell lines [13], studies of functional transporter gene polymorphisms in relation to paclitaxel effects have yielded contradictory results. One study found an association between the ABCB1 G2677T/A polymorphism and antineoplastic effect [14], while another study failed to confirm the association [15]. Sissung et al. demonstrated an increased risk of neutropenia and neuropathy with the ABCB1 G1199T/A polymorphism [16], while another study found no correlation to outcomes among ovarian cancer patients treated with paclitaxel [17]. A single study evaluating the role of three known functional polymorphisms in the SLCO1B3 gene [12] failed to show an association with paclitaxel pharmacokinetics [18]; however, more recently, van de Steeg et al. demonstrated a concomitant increase in systemic exposure to paclitaxel and a decrease in hepatic clearance of the drug using Slco1a/1b knockout mice [19].
The purpose of our work was to identify genetic variants and gene expression signatures associated with paclitaxel-induced cytotoxicity using genome-wide approaches in a cell-based model. We performed the genome-wide studies in a cell-based system for several reasons: 1) Such studies are extremely challenging in a clinical setting because of the need for large, homogeneous patient populations given similar doses of the same drug; 2) Recently, the cell-based model has shown clinical utility with chemotherapeutic associated SNPs identified in the lymphoblastoid cell lines replicated in patient trials with clinical response as the phenotype [20–24] and; 3) Lymphoblastoid cell lines derived from large pedigrees demonstrated that the degree of heritability of paclitaxel-induced cytotoxicity was close to 0.5 [25] providing us with confidence that genetic variants could be identified for paclitaxel-induced cytotoxicity in this system.
Methods
Drug and cell lines
Paclitaxel (NSC 125973) was a gift from the Developmental Therapeutics Program NCI/NIH. HapMap LCLs from a population with Northern and Western European ancestry from Utah, USA (CEU; HAPMAPPT01, n = 78), a Yoruba population in Ibadan, Nigeria (YRI; HAPMAPPT03, n = 87), and an African-American population from the Southwest of the USA (ASW; HAPMAPPT07, ASW, n = 90) were purchased from Coriell Institute for Medical Research (Camden, NJ; http://ccr.coriell.org/), cultured and maintained as described [26].
Cytotoxicity and Apoptosis assays
Paclitaxel-induced cytotoxicity was determined using an AlamarBlue (Invitrogen, Carlsbad, CA) cellular growth inhibition assay after 72 h treatment as previously described [26]. Paclitaxel was prepared in 100% DMSO as a stock solution of 2 mM and serially diluted further to concentrations of 0, 6.25, 12.5, 25, 50, and 100nM using a 9:1 mixture of RPMI 1640 media and 100% DMSO. We used a modified area under the curve (referred to as AUC in the rest of the paper) as a measure of drug sensitivity and values were computed by taking the geometric mean of cell survival for paclitaxel concentrations ranging from 0 to 50 nM. In addition to cytotoxicity, we utilized an apoptosis phenotype measured by caspase 3/7 activity following treatment with 12.5nM paclitaxel for both CEU (n=77) and YRI (n=84) populations, as previously described [26]. Mean percent survival and final caspase activity were calculated by averaging a minimum of six replicates from two independent experiments.
Correlation between cytotoxicity and gene expression
An overview of our analytical approach is presented in Figure 1. Genome-wide gene expression data were previously generated in our laboratory for all CEU and YRI LCLs using Affymetrix GeneChip Human Exon 1.0 ST Array [27]. A general linear model was constructed between log2-transformed gene expression level and log2-transformed paclitaxel AUC with population indicator as covariate. A Toeplitz covariance structure with two diagonal bands was used to allow for familial dependencies in the data [28].
Figure 1.
Study Workflow. In the first step, GWAS was conducted between paclitaxel-induced cytotoxicity in CEU/YRI and global gene expression representing 10,748 genes. At a p<0.05, 1713 genes were identified and within these genes, there was an enrichment of SLC genes observed (n=31 out of 181 expressed SLC genes). In a subsequent step, SNPs associated with expression of the 31 SLC genes were identified using the SCAN database, involving GWAS between about 2.5 million testable SNPs (MAF > 0.05). Four SLC genes had SNPs associated with their expression and were associated with paclitaxel cytotoxicity by means of a meta-analysis of QTDT studies in the individual populations (CEU, YRI, and ASW).
SLC gene set enrichment analysis
Enrichment of the top hits for SLC genes was calculated empirically. Of the SLC genes interrogated in this study (from a comprehensive list of 13,080 genes tested), 181 were found to be expressed. We generated 10,000 sets of 181 genes drawn randomly from the genome and counted the number of genes associated with log2-transformed paclitaxel AUC (p<0.05) in each set, generating the null distribution. In order to quantify the level of SLC gene enrichment among our top genes, we compared the number of significant SLC genes (p<0.05) and the empirically generated distribution, as previously described [29].
eQTL association with SLC genes and cytotoxicity
eQTLs associated with the expression of enriched SLC genes were identified using the online SCAN database (http://www.scandb.org) [30]. eQTLs targeting SLC genes (P<10−4) were tested for association with paclitaxel AUC in a meta-analysis combining the CEU, YRI, and ASW populations. Each population was first analyzed separately using the quantitative trait disequilibrium test (QTDT) total association model [31]. To control for population structure in the admixed ASW population, local ancestry at each genotyped SNP locus was used as a covariate in the association tests as previously reported for other drugs [32]. SNP p-values for each population were combined taking into account the sample size of each population and direction of effect (positive or negative beta) using the meta-analysis software METAL [33].
Extent of phenotype explained by 4 SLC genes
A multivariate model was used to compute an adjusted coefficient of determination (adjusted R2) value for the relationship between a linear combination of the expression levels of the chosen SLC genes and cytotoxicity. We conducted 1000 simulations and derived a distribution of adjusted R2 values that would be expected for randomly selected sets of genes of the same size. The observed adjusted R2 value for the relationship between the linear combination of the expression of our chosen SLC genes and cytotoxicity was compared to the distribution to generate an empirical P value.
Evaluation for correlation between gene expression and cytotoxicity in ASW
SLC genes identified above were examined further for association between gene expression measured by quantitative RT-PCR and paclitaxel-induced phenotype in an independent set of LCLs derived from ASW. qRT-PCR was performed for SLC31A2 (Hs00156984_m1), SLC35A5 (Hs00215733_m1), SLC43A1 (Hs00180220_m1) and SLC41A2 (Hs00259767_m1) genes and beta-2-microglobulin (B2M) as an endogenous control using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA,). The reaction was run according to the manufacturers protocol using Fast Advanced Master mix and 1.25 ng/uL cDNA. The Viia7 automatically set the cycle threshold (CT) and baseline for each experiment. A standard curve method was used to obtain the relative SLC expression for each ASW sample. Each qRT-PCR was run at least in duplicate and individual samples were run in triplicate on each plate. Replicate samples with greater than 15% standard deviation from each other were dropped from the analysis.
Functional siRNA knockdown studies
Variability in SLC gene expression and effects on phenotype were studied using RNA interference to knockdown expression of SLC genes in 2 LCLs (GM19119 and GM18916). 24 hrs after seeding the LCLs at 5×105 cells/ml, the cells were nucleofected using Lonza’s Cell Line 96-well Nucleofector Kit SF (Lonza Inc, Basel, Switzerland). The reaction was performed according to protocol using 1×106 cells and 2000 nM final concentration of AllStars Negative Control siRNA labeled with AlexaFluor488 (Qiagen Inc., Valencia, CA,) or a pool of Hs_SLC31A2 (GS1318), Hs_SLC35A5 (GS55032), Hs_SLC41A2 (GS84102), or Hs_SLC43A1 (GS8501) FlexiTube siRNA (Qiagen). The cells were nucleofected using the DN-100 program. After a 10 min rest and replating, cells were treated with paclitaxel for cytotoxicity and apoptosis, 5 h and 24 h post nucleofection, respectively. Cells were also pelleted at 5, 24, and 48 h post nucleofection and total RNA was extracted using the RNeasy Plus Mini kit (Qiagen). Subsequently, mRNA was reverse transcribed to cDNA using Applied Biosystems High Capacity Reverse Transcription kit. The cDNA (50 ng/uL) was then used to perform qRT-PCR, as described above, to confirm the knockdown of the 4 SLC genes via siRNA.
Statistical Considerations
In order to assess the size and significance of the effect of the knockdown on paclitaxel response (survival for cytotoxicity assay and caspase activity for apoptosis assay) we fit the following linear mixed model: response ~ knockdown + dose + (1|id) + (1|experiment), where knockdown is 1 if the gene was knocked down and 0 if scrambled. Cell line id (denoted by id) and experiment were used as random effects to properly account for correlation between responses. In order to increase precision we pooled the data from both cell lines and all doses. The mixed effects model was fit using lme4 package in the R Statistical package (http://cran.r-project.org/). The goodness of fit of the model is assessed by examining the residuals. Normality of the residuals is assessed using Shapiro Wilk test using the R statistical software. Log transformation of the response was used in order to achieve approximate normality.
Results
Variability in sensitivity to paclitaxel-induced cytotoxicity
LCLs (255 total) derived from three populations including 78 LCLs from individuals of Northern and Western European ancestry from Utah, USA (CEU), 87 LCLs from individuals from a Yoruba population in Ibadan, Nigeria (YRI), and 90 LCLs from individuals from an African-American population from the Southwest of the USA (ASW) were exposed to increasing concentrations of paclitaxel. The mean (SD) log2 AUC was 5.86 (± 0.27 [%•μM]). There were no statistically significant inter-ethnic differences in log2 AUC with 5.88 ± 0.3 %•μM for CEU; 5.88 ± 0.28 %•μM for YRI; and 5.81 ± 0.25 %•μM for ASW. Figure 1 inset illustrates the box plots of CEU and YRI.
Correlation of gene expression with cytotoxicity
We utilized a genome-wide approach to identify genes for which baseline gene expression levels were significantly associated with cytotoxicity; however because whole gene expression data using the Affymetrix Exon Array was only available for CEU and YRI LCLs [27], only those populations were evaluated. Figure 1 illustrates our workflow. We found 1713 genes at p < 0.05 correlated to paclitaxel-induced cytotoxicity with the strongest association between gene expression and cytotoxicity observed for SLC31A2 (P = 4.3 × 10−8). Supplemental Table 1 lists the top 30 significant correlations between gene expression and paclitaxel-induced cytotoxicity.
Enrichment of 31 SLC genes in CEU/YRI
Gene expressions associated with cytotoxicity at p<0.05 were evaluated to see if they were disproportionately more likely to be SLC genes. Table 1 lists 31 SLC genes significantly associated with cytotoxicity (p < 10−4). Furthermore, we observed SLC gene enrichment among the top genes associated with cytotoxicity (Figure 2A; enrichment p = 0.036).
Table 1.
Association between SLC gene transcript level and cytotoxicity.
| Gene | Beta | P value |
|---|---|---|
| SLC31A2 | 5.75 | 4.34 × 10−8 |
| SLC25A37 | −4.49 | 1.34 × 10−5 |
| SLCO4A1 | −3.32 | 0.0016 |
| SLC25A44 | 3.21 | 0.0016 |
| SLC43A1 | −3.02 | 0.003 |
| SLC35A4 | 3.00 | 0.003 |
| SLC35A5 | 2.99 | 0.0032 |
| SLC9A9 | 2.98 | 0.0033 |
| SLC32A1 | 2.96 | 0.0035 |
| SLC41A2 | 2.93 | 0.0039 |
| SLC25A12 | 2.81 | 0.0055 |
| SLC35E3 | 2.78 | 0.006 |
| SLC37A2 | −2.66 | 0.0086 |
| SLC37A1 | 2.60 | 0.010 |
| SLC1A1 | 2.57 | 0.011 |
| SLC29A1 | −2.53 | 0.012 |
| SLC29A2 | −2.53 | 0.012 |
| SLC26A11 | −2.52 | 0.013 |
| SLC25A45 | 2.50 | 0.013 |
| SLC30A6 | 2.48 | 0.014 |
| SLC30A4 | 2.44 | 0.016 |
| SLC15A3 | −2.30 | 0.022 |
| SLC1A5 | −2.27 | 0.024 |
| SLC44A1 | 2.25 | 0.026 |
| SLC25A4 | 2.20 | 0.029 |
| SLC7A1 | −2.16 | 0.032 |
| SLC25A1 | −2.14 | 0.034 |
| SLC35D2 | 2.12 | 0.035 |
| SLC35B3 | 2.12 | 0.035 |
| SLC38A9 | 2.06 | 0.041 |
| SLC9A8 | 1.99 | 0.048 |
Figure 2.
The final list consisted of 4 SLC genes for which expression level is associated with cytotoxicity, and for which regulating eQTL genotypes are also associated with cytotoxicity. A. SLC gene set enrichment. With 10,000 simulations, there was a significant enrichment of SLC genes among the top genes significantly associated with cytotoxicity (p<0.05). The null distribution is shown and the observed SLC gene count of 31 (black dot) translates to an enrichment P value of 0.036. B. Regulation of paclitaxel susceptibility by four SLC genes. SLC31A2, SLC43A1, SLC35A5, and SLC41A2 collectively explain more of the variability in log2 paclitaxel AUC compared to a randomly chosen set of four genes. The null distribution (empirically generated from 1000 simulations) is shown and compared to the observed adjusted R2 of 0.20 for the four SLC genes (empirical P < 0.001).
Extent of cytotoxicity and apoptosis phenotypes explained by 4 SLC genes
Four SLC genes, SLC31A2, SLC43A1, SLC35A5 and SLC41A2 collectively explain more of the phenotypic variability in cytotoxicity than a randomly chosen set of four genes (including SLC genes), as shown in Figure 2B (P < 0.001). See supplemental table 2 for the baseline expression levels of these four genes in CEU and YRI. For comparison, supplemental table 3 is a table of GeneCards queries for the relative expression levels of the four genes in a variety of tissues, as quantified by Affymetrix GeneChips HG-U95A-E (GeneNote) and by HG-U133A in normal human tissues (GNF Normal) and NCI60 cancer cell lines (GNF Cancer) [34]. Multivariate analysis in CEU/YRI yielded an adjusted R2 value of 0.20. Using Pearson correlation, we found modest correlations in expression between SLC41A2 and SLC31A2 (r = 0.04; p = 5×10−2), and between SLC41A2 and SLC35A5 (r = 0.24; p = 1×10−4). We also evaluated to what extent these four genes explained variation in a separate population, the ASW samples; the adjusted R2 for the 4 genes versus cytotoxicity is 0.085, with an overall p-value of 0.065 that is trending significant.
Genetic regulation of SLC genes by cytotoxicity-associated eQTLs
To further evaluate whether the 4 SLC genes were associated with genetic variants, we used publicly available information from the SCAN database (http://www.scandb.org) [30]. We identified eQTLs regulating SLC gene expression in the YRI and CEU populations at p ≤ 1 × 10−4. All SNPs are trans-acting eQTLs of their respective SLC gene in the YRI population as evidenced by a different SNP chromosomal location compared to the respective regulated SLC gene. We further filtered this set of genes by evaluating the relationship between these eQTLs and paclitaxel-induced cytotoxicity using a meta-analysis in CEU, YRI, ASW (Table 2, Figure 3). For example, rs6935238 was significantly associated with both SLC31A2 gene expression (p = 8 × 10−5) and with paclitaxel-induced cytotoxicity in CEU and YRI populations (p = 1.5 × 10−6). One eQTL (rs13419945) was significantly associated with cytotoxicity (p = 4.6 × 10−5) and gene expression of both SLC35A5 and SLC41A2. SLC35A5 was also associated with a second SNP, rs11835712, which also associates with cytotoxicity (Fig. 3, Table 2).
Table 2.
Significance of association between eQTL genotype, gene transcript level and cytotoxicity as well as p-values for gene expression and cytotoxicity and apoptosis for four SLC genes.
| SLC gene | eQTL/Host gene | MAF (CEU/YRI) | P value (eQTL genotype and gene expression in YRI) | P value (eQTL genotype and cytotoxicity in meta of CEU, YRI, ASW) | P value (gene expression and cytotoxicity in CEU/YRI) | P value (gene expression and apoptosis in CEU/YRI) |
|---|---|---|---|---|---|---|
| SLC31A2 | rs6935238 | 0.15/0.27 | 8 × 10−5 | 1.5 × 10−6 | 4.3 × 10−8 | 1.8 × 10−5 |
| SLC43A1 | rs4973907/ZNF620 | 0.44/0.13 | 8 × 10−5 | 9.8 × 10−5 | 0.003 | 0.0012 |
| SLC35A5 | rs11835712 | 0/0.38 | 0.0001 | 1.3 × 10−4 | 0.0032 | 0.00029 |
| SLC35A5 | rs13419945 | 0.075/0.1 | 7 × 10−5 | 4.6 × 10−5 | 0.0032 | 0.00029 |
| SLC41A2 | rs13419945 | 0.075/0.1 | 0.0001 | 4.6 × 10−5 | 0.0039 | 0.039 |
Figure 3.
Relationships between eQTL genotype, SLC gene expression, and paclitaxel-induced cytotoxicity. Five eQTLs were identified in the YRI population for which genotype was correlated with both SLC gene expression and cytotoxicity. Four relationships shown are: (A) rs6935238 genotype, SLC31A2 expression, and paclitaxel-induced cytotoxicity; (B) rs4973907 genotype, SLC43A1 expression, and paclitaxel-induced cytotoxicity; (C) rs11835712 genotype, SLC35A5 expression, and paclitaxel-induced cytotoxicity; and (D) rs13419945 genotype, SLC41A2 expression, and paclitaxel-induced cytotoxicity. Not shown is an illustration of the relationship between rs13419945 genotype and SLC35A5 expression (p = 7 ×10−5) and paclitaxel-induced cytotoxicity (4.6 × 10−5).
Correlation between gene expression and cytotoxicity in ASW
Using an independent set of ASW LCLs, we quantified the expression levels of the four SLC genes by qRT-PCR. Figure 4 illustrates correlations between expression of SLC31A2 and cytotoxicity (CEU/YRI: p = 4.3. × 10−8; ASW: p = 1.4 × 10−2) and apoptosis (CEU/YRI: p = 1.8.×.10−5; ASW: p = 6.4 × 10−2). In addition, SLC43A1 and cytotoxicity (CEU/YRI: p = 3 × 10−3; ASW: p = 1.7 × 10−3) and apoptosis (CEU/YRI: p = 1.2.×.10−3; ASW: p = 1.5 × 10−2). These data provide evidence that in all populations the level of gene expression is correlated with sensitivity to paclitaxel as measured by both cytotoxicity and apoptosis.
Figure 4.
Correlation between SLC gene expression and log2 paclitaxel AUC in CEU/YRI and also in ASW. Positive correlation was observed for SLC31A2 indicative of greater paclitaxel sensitivity with lower gene expression and an inverse relationship for SLC43A1 indicating heightened paclitaxel sensitivity with increased gene expression. Affymetrix exon array was used for CEU/YRI LCLs (n = 165) and qRT-PCR for ASW (n = 90).
Functional siRNA knockdown studies
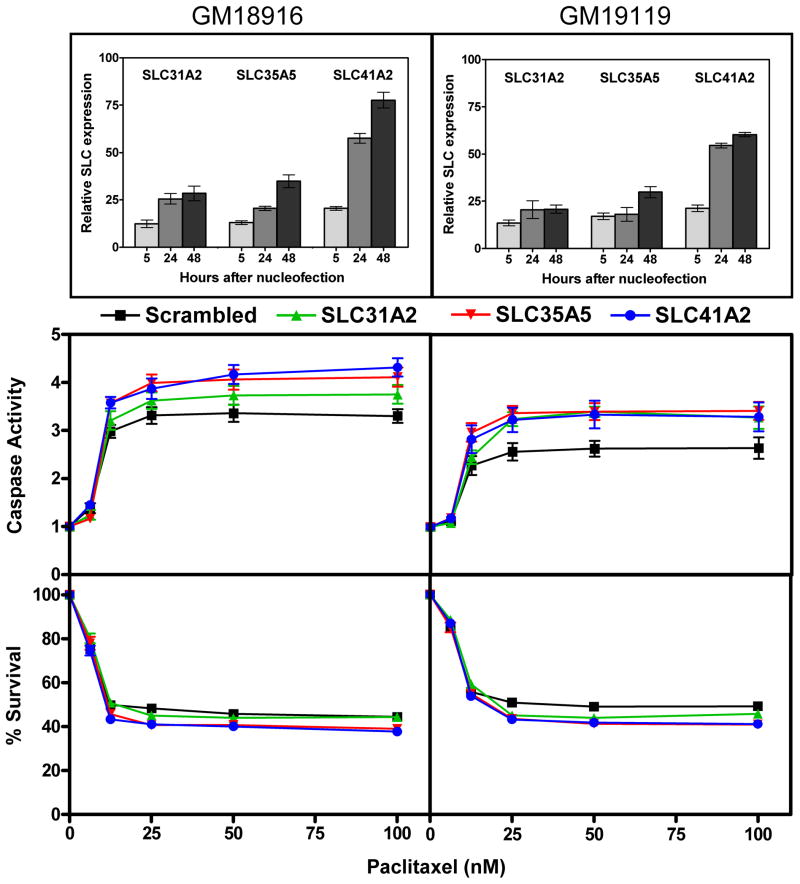
Using RNA interference, we carried out gene knockdown in YRI LCLs and studied changes in paclitaxel-induced cytotoxicity and apoptosis. When we pooled data from both cell lines and across all dose levels and accounted for correlations within cell line and experiments via a mixed effects model, knockdown of SLC31A2 SLC35A5 and SLC41A2 resulted in a significant increase in caspase activity and a decrease in cell survival (Figure 5). The change for SLC31A2 in apoptosis was 9.8% (p=6.5×10−6) and cytotoxicity was −1.8% (p=1.3×10−1). The change in apoptosis and cytotoxicity for SLC35A5 was 16.3% (p=3.4×10−13) and −8.1% (p=4.7×10−12), respectively. The change in apoptosis and cytotoxicity for of SLC41A2 was 17.7% (p=6.7×10−15) and −8.8% (p=3.3×10−14), respectively. Changes in cellular susceptibility with variable SLC gene expression were consistent with the previously described positive correlation between gene expression and paclitaxel-induced cytotoxicity (i.e. high expression confers resistance to drug) for SLC31A2, SLC35A5, SLC41A2. Furthermore, greater changes were seen for apoptosis (5–18%) than for cytotoxicity (2–9%). For SLC43A1, satisfactory gene knockdown was not achieved and although significant in the pooled analysis, changes in cellular paclitaxel susceptibility were inconsistent with the expectation of increased cell survival with gene knockdown.
Figure 5.
Functional studies in YRI. Knockdown of SLC genes in two YRI cell lines, GM18916 and GM19119, resulted in increased cellular susceptibility to paclitaxel as demonstrated by reduced overall cell survival and increased caspase activity. The change in apoptosis and cytotoxicity for SLC31A2 (green, triangle) was 9.8% (5.4%, 14.4%, p=6.5×10−6) and −1.8% (−4.2%, +0.5%, p=1.3×10−1), respectively. The change in apoptosis and cytotoxicity for SLC35A5 (red, reverse triangle) was 16.3% (11.7%, 21.2%; p=3.4×10−13) and −8.1% (−10.3%, −5.9%; p=4.7×10−12), respectively. The change in apoptosis and cytotoxicity for of SLC41A2 (blue, circle) was 17.7% (13.0% 22.6%; p=6.7×10−15) and −8.8% (−11.0%, −6.6%; p=3.3×10−14), respectively. Real-time expression results show two replicates across at least two independent experiments with standard error of the mean. Caspase activity and percent survival curves show a minimum of six replicates from two independent experiments also with standard error of the mean.
Discussion
There is growing evidence that transporter function may be responsible for a large component of chemotherapeutic drug responses. We utilized a hypothesis-generating genome-wide approach involving the use of LCLs to study the relationship between SLC gene expression and paclitaxel susceptibility. Our findings suggest that OAT transporters which are encoded by SLC genes may have a role in transcellular paclitaxel transport, and that variability in SLC gene expression may be relevant to the clinically observed inter-individual variability in paclitaxel-related effects. We identified SNPs associated with cellular susceptibility to paclitaxel acting through their effect on baseline expression of SLC genes.
The organic anion (OAT) and organic cation (OCT) transporter family of genes are encoded by solute carrier (SLC) genes, but members are structurally different from OATP transporters. OATP transporters, encoded by the SLCO (or SLC21) family of solute carrier genes, are primarily involved in transport of large, lipophilic organic anions. There have been reports of association between SLC gene expression and sensitivity to platinum compounds [35] and irinotecan [36], as well as reports of a relationship between expression of OCT genes (members of the SLC22 family) and outcomes in patients with chronic myeloid leukemia (CML) treated with imatinib [37]. Some groups have identified paclitaxel as a substrate of OAT2, a transporter encoded by the SLC22A7 gene [38] and SLC22 genes are expressed in NCI-60 cancer cell lines [39]. Furthermore, studies have shown widespread expression of SLC genes in the neurologic system [40], B lymphocytes [41], and mammary gland [42]. Given the relationship between SLCO and other SLC genes, it is plausible that SLC genes are involved in paclitaxel transport in humans thus partly mediating the clinical effects of paclitaxel.
Interestingly, the top gene in our analysis, SLC31A2 (also known as CTR2), has been described as a determinant of cisplatin- and carboplatin-induced cytotoxicity in mouse embryo fibroblasts, and expression levels are significantly correlated with cisplatin sensitivity in ovarian carcinoma cell lines [35]. SLC31A2 baseline gene expression is also associated with cisplatin (YRI p = 8.4 × 10−5, CEU p = 4.6 × 10−3) and carboplatin (YRI p = 2.1 × 10−5, CEU p = 7.5 × 10−4) cytotoxicity in LCLs (data not shown). In our study, we observed an association between high gene expression and paclitaxel resistance for SLC31A2, SLC35A5, and SLC41A2; and paclitaxel sensitivity for SLC43A1. We validated the roles of SLC31A2, SLC35A5, and SLC41A2 in paclitaxel resistance through siRNA knockdown resulting in greater sensitivity to drug. OAT transporters may serve as bidirectional transporters [43] with some mediating drug influx and others mediating drug efflux.
We identified genetic variants that regulate paclitaxel susceptibility through their association with baseline SLC gene expression. Previously, functional polymorphisms have been identified in the SLCO1B3 gene [12], although conclusive evidence of an effect on outcomes in patients treated with paclitaxel is lacking. Among patients with CML treated with imatinib, a significant relationship has been demonstrated between polymorphisms in OCT-1 and outcomes. For example, rs693369 genotype is associated with loss of response or treatment failure [44], while rs34130495 genotype is associated with a higher rate of major molecular response [45]. In our study, genetic variants associated with SLC genes were only observed in YRI implying that other factors may contribute to variability in SLC gene expression. Indeed, it has been reported that epigenetic mechanisms may be involved in OAT transporter abundance [46]. Of the four SLC genes studied, gene expression and phenotype relationships were also seen for two in ASW, namely SLC31A2 and SLC43A1 and here, population-specific factors may be involved. Paclitaxel, like many other microtubule agents, acts by inducing apoptosis [1], such that evaluation of this second phenotype enhances robustness of our findings and increases chances of relevance to the in vivo situation.
We have gone further to demonstrate increased cellular susceptibility to paclitaxel with SLC gene knockdown. Increased paclitaxel susceptibility with knockdown of SLC31A2, SLC35A5, and SLC41A2 suggests a role in drug efflux, possibly with lower gene expression resulting in greater intracellular drug accumulation, enhanced apoptosis, and cytotoxicity. Greater changes in apoptosis, compared to cytotoxicity, may be related to the predominantly apoptotic mechanism of cell death from paclitaxel. Additionally, other cellular mechanisms may attenuate overall cell death, despite marked changes in apoptosis resulting from SLC gene knockdown. For SLC43A1, changes in cellular susceptibility were not consistent across all dose levels and the two cell lines possibly resulting from multiple interacting cellular mechanisms, sample size and technical issues.
An unbiased functional pathway enrichment using Database for Annotation, Visualization, and Integrated Discovery (DAVID) software v6.7 (http://david.abcc.ncifcrf.gov) software identified enrichment of genes in a single pathway involved in ‘ER-to-Golgi’ transport (p = 9 × 10−4) among the top 500 genes associated with cytotoxicity (p<0.006). ER-to-Golgi pathway is a reasonable expectation for paclitaxel given its microtubule inhibitory effects [47] and may be applicable to other microtubule inhibiting agents.
Multiple preclinical and clinical studies have shown improved oral pharmacokinetics of paclitaxel with co-administration of P glycoprotein inhibitors [48]. Although the role of solute carriers in paclitaxel transport remains undisputed, studies to date have not addressed SLC modulation as a means of improving paclitaxel bioavailability. A (GeneCards-based) comparison of gene expression patterns for the four SLC genes in normal and cancer tissues shows that SLC genes are widely expressed. In a variety of paclitaxel-susceptible tumors (breast, lung, ovarian) GeneCards indicate expression of SLC31A2, SLC35A5 and SLC43A1, therefore paclitaxel transport by these transporters may be relevant for response. Concerns have been raised about applicability to the in vivo situation; however, several studies in LCLs have demonstrated the feasibility of integrating genotypic data with cytotoxicities of anticancer agents [49].
In conclusion, we have utilized a cell-based model in a genome-wide study to identify genes for which transcript levels are correlated with paclitaxel-induced cytotoxicity. We have demonstrated significant enrichment of SLC genes among those genes, identified trans-regulating eQTLs of four SLC genes, and demonstrated increased cellular paclitaxel susceptibility with knockdown of SLC31A2, SLC35A5, and SLC41A2 genes. Our findings are novel and in addition to contributing to unraveling of paclitaxel pharmacogenomics, they lend further support to a role for solute carriers in mediating variability in chemotherapeutic drug responses.
Supplementary Material
Acknowledgments
Source of Funding: This Pharmacogenetics of Anticancer Agents Research Group (http://pharmacogenetics.org) study was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants UO1GM61393, P50 CA125183]. In addition, U.O.N. was supported by National Institutes of Health National Cancer Institute [GrantT32 CA009566].
The authors thank Kenneth Hecht and Bonnie LaCroix for technical support and help with thawing and maintaining the cell lines, Marleen Welsh for instruction and assistance with cytotoxicity assays, and Amy Stark for instruction and assistance with functional studies.
Footnotes
Conflicts of Interest: None declared
Disclaimers: None declared
References
- 1.Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82(15):1247–59. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 2.Rowinsky EK, Wright M, Monsarrat B, Donehower RC. Clinical pharmacology and metabolism of Taxol (paclitaxel): update 1993. Ann Oncol. 1994;5 (Suppl 6):S7–16. [PubMed] [Google Scholar]
- 3.Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–83. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 4.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 Suppl):2577–92. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidman AD, Tiersten A, Hudis C, Gollub M, Barrett S, Yao TJ, et al. Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol. 1995;13(10):2575–81. doi: 10.1200/JCO.1995.13.10.2575. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Antona C. Pharmacogenomics of paclitaxel. Pharmacogenomics. 2010;11(5):621–3. doi: 10.2217/pgs.10.32. [DOI] [PubMed] [Google Scholar]
- 7.Giacomini KMSY. In: Membrane transporters and drug response. Brunton LLPK, editor. New York: McGraw-Hill; 2006. [Google Scholar]
- 8.Zhang L, Huang SM, Lesko LJ. Transporter-mediated drug-drug interactions. Clin Pharmacol Ther. 2011;89(4):481–4. doi: 10.1038/clpt.2010.359. [DOI] [PubMed] [Google Scholar]
- 9.Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4(8):815–8. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- 10.Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, et al. Effect of pregnane × receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584(1):57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997;94(5):2031–5. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letschert K, Keppler D, Konig J. Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8) Pharmacogenetics. 2004;14(7):441–52. doi: 10.1097/01.fpc.0000114744.08559.92. [DOI] [PubMed] [Google Scholar]
- 13.Takano M, Otani Y, Tanda M, Kawami M, Nagai J, Yumoto R. Paclitaxel-resistance conferred by altered expression of efflux and influx transporters for paclitaxel in the human hepatoma cell line, HepG2. Drug Metab Pharmacokinet. 2009;24(5):418–27. doi: 10.2133/dmpk.24.418. [DOI] [PubMed] [Google Scholar]
- 14.Green H, Soderkvist P, Rosenberg P, Horvath G, Peterson C. mdr-1 single nucleotide polymorphisms in ovarian cancer tissue: G2677T/A correlates with response to paclitaxel chemotherapy. Clin Cancer Res. 2006;12(3 Pt 1):854–9. doi: 10.1158/1078-0432.CCR-05-0950. [DOI] [PubMed] [Google Scholar]
- 15.Marsh S, Paul J, King CR, Gifford G, McLeod HL, Brown R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol. 2007;25(29):4528–35. doi: 10.1200/JCO.2006.10.4752. [DOI] [PubMed] [Google Scholar]
- 16.Sissung TM, Mross K, Steinberg SM, Behringer D, Figg WD, Sparreboom A, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42(17):2893–6. doi: 10.1016/j.ejca.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green H, Soderkvist P, Rosenberg P, Horvath G, Peterson C. ABCB1 G1199A polymorphism and ovarian cancer response to paclitaxel. J Pharm Sci. 2008;97(6):2045–8. doi: 10.1002/jps.21169. [DOI] [PubMed] [Google Scholar]
- 18.Smith NF, Marsh S, Scott-Horton TJ, Hamada A, Mielke S, Mross K, et al. Variants in the SLCO1B3 gene: interethnic distribution and association with paclitaxel pharmacokinetics. Clin Pharmacol Ther. 2007;81(1):76–82. doi: 10.1038/sj.clpt.6100011. [DOI] [PubMed] [Google Scholar]
- 19.van de Steeg E, van Esch A, Wagenaar E, van der Kruijssen CM, van Tellingen O, Kenworthy KE, et al. High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin Cancer Res. 2011;17(2):294–301. doi: 10.1158/1078-0432.CCR-10-1980. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler HE, Dolan ME. Lymphoblastoid cell lines in pharmacogenomic discovery and clinical translation. Pharmacogenomics. 2012;13(1):55–70. doi: 10.2217/pgs.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang RS, Johnatty SE, Gamazon ER, Im HK, Ziliak D, Duan S, et al. Platinum sensitivity-related germline polymorphism discovered via a cell-based approach and analysis of its association with outcome in ovarian cancer patients. Clin Cancer Res. 2011;17(16):5490–500. doi: 10.1158/1078-0432.CCR-11-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziliak D, O’Donnell PH, Im HK, Gamazon ER, Chen P, Delaney S, et al. Germline polymorphisms discovered via a cell-based, genome-wide approach predict platinum response in head and neck cancers. Transl Res. 2011;157(5):265–72. doi: 10.1016/j.trsl.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitra AK, Crews KR, Pounds S, Cao X, Feldberg T, Ghodke Y, et al. Genetic variants in cytosolic 5′-nucleotidase II are associated with its expression and cytarabine sensitivity in HapMap cell lines and in patients with acute myeloid leukemia. J Pharmacol Exp Ther. 2011;339(1):9–23. doi: 10.1124/jpet.111.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maini CL, Turco GL, Castellano G, Liboni W, Podio V, Chianale G, et al. Cerebral blood flow and volume in symptom-free migraineurs: a SPECT study. Nuklearmedizin. 1990;29(5):210–4. [PubMed] [Google Scholar]
- 25.Peters EJ, Motsinger-Reif A, Havener TM, Everitt L, Hardison NE, Watson VG, et al. Pharmacogenomic characterization of US FDA-approved cytotoxic drugs. Pharmacogenomics. 2011;12(10):1407–15. doi: 10.2217/pgs.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen Y, Gorsic LK, Wheeler HE, Ziliak DM, Stephanie Huang R, Eileen Dolan M. Chemotherapeutic-induced apoptosis: a phenotype for pharmacogenomics studies. Pharmacogenet Genomics. 2011;21(8):476–88. doi: 10.1097/FPC.0b013e3283481967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Duan S, Kistner EO, Bleibel WK, Huang RS, Clark TA, et al. Evaluation of genetic variation contributing to differences in gene expression between populations. Am J Hum Genet. 2008;82(3):631–40. doi: 10.1016/j.ajhg.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamazon ER, Huang RS, Cox NJ, Dolan ME. Chemotherapeutic drug susceptibility associated SNPs are enriched in expression quantitative trait loci. Proc Natl Acad Sci U S A. 2010;107(20):9287–92. doi: 10.1073/pnas.1001827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamazon ER, Duan S, Zhang W, Huang RS, Kistner EO, Dolan ME, et al. PACdb: a database for cell-based pharmacogenomics. Pharmacogenet Genomics. 2010;20(4):269–73. doi: 10.1097/FPC.0b013e328337b8d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamazon ER, Zhang W, Konkashbaev A, Duan S, Kistner EO, Nicolae DL, et al. SCAN: SNP and copy number annotation. Bioinformatics. 2010;26(2):259–62. doi: 10.1093/bioinformatics/btp644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66(1):279–92. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheeler HE, Gorsic LK, Welsh M, Stark AL, Gamazon ER, Cox NJ, et al. Genome-wide local ancestry approach identifies genes and variants associated with chemotherapeutic susceptibility in African Americans. PLoS One. 2011;6(7):e21920. doi: 10.1371/journal.pone.0021920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebhan M, Chalifa-Caspi V, Prilusky J, Lancet D. GeneCards: a novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics. 1998;14(8):656–64. doi: 10.1093/bioinformatics/14.8.656. [DOI] [PubMed] [Google Scholar]
- 35.Blair BG, Larson CA, Safaei R, Howell SB. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of Cisplatin and Carboplatin. Clin Cancer Res. 2009;15(13):4312–21. doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramirez J, Relling M, et al. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol. 2009;27(16):2604–14. doi: 10.1200/JCO.2008.20.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE. hOCT 1 and resistance to imatinib. Blood. 2005;106(3):1133–4. doi: 10.1182/blood-2005-02-0694. author reply 4. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]) J Pharm Pharmacol. 2005;57(5):573–8. doi: 10.1211/0022357055966. [DOI] [PubMed] [Google Scholar]
- 39.Okabe M, Szakacs G, Reimers MA, Suzuki T, Hall MD, Abe T, et al. Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol Cancer Ther. 2008;7(9):3081–91. doi: 10.1158/1535-7163.MCT-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447(5):756–9. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- 41.Sahni J, Nelson B, Scharenberg AM. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem J. 2007;401(2):505–13. doi: 10.1042/BJ20060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel OV, Casey T, Dover H, Plaut K. Homeorhetic adaptation to lactation: comparative transcriptome analysis of mammary, liver, and adipose tissue during the transition from pregnancy to lactation in rats. Funct Integr Genomics. 2011;11(1):193–202. doi: 10.1007/s10142-010-0193-0. [DOI] [PubMed] [Google Scholar]
- 43.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim DH, Sriharsha L, Xu W, Kamel-Reid S, Liu X, Siminovitch K, et al. Clinical relevance of a pharmacogenetic approach using multiple candidate genes to predict response and resistance to imatinib therapy in chronic myeloid leukemia. Clin Cancer Res. 2009;15(14):4750–8. doi: 10.1158/1078-0432.CCR-09-0145. [DOI] [PubMed] [Google Scholar]
- 45.Bazeos A, Marin D, Reid AG, Gerrard G, Milojkovic D, May PC, et al. hOCT1 transcript levels and single nucleotide polymorphisms as predictive factors for response to imatinib in chronic myeloid leukemia. Leukemia. 2010;24(6):1243–5. doi: 10.1038/leu.2010.86. [DOI] [PubMed] [Google Scholar]
- 46.Wu W, Dnyanmote AV, Nigam SK. Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol Pharmacol. 2011;79(5):795–805. doi: 10.1124/mol.110.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaughan KT. Microtubule plus ends, motors, and traffic of Golgi membranes. Biochim Biophys Acta. 2005;1744(3):316–24. doi: 10.1016/j.bbamcr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27(1):17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Welsh M, Mangravite L, Medina MW, Tantisira K, Zhang W, Huang RS, et al. Pharmacogenomic discovery using cell-based models. Pharmacol Rev. 2009;61(4):413–29. doi: 10.1124/pr.109.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.