INTRODUCTION
The overactive bladder syndrome (OAB) is a prevalent problem in clinical practice, diagnosed and managed mainly by patients’ reports1. Pharmacological or conservative treatment has only moderate success, perhaps because the underlying causes or phenotypes are heterogeneous and require different therapies. The central symptom of OAB is urgency2, sometimes accompanied by urine leakage episodes (urgency urinary incontinence – UUI), believed to be associated with or caused by involuntary detrusor contractions2,3. Such contractions, observed during urodynamic examination and either spontaneous or provoked 1, are known as detrusor overactivity (DO). Although the etiology of DO and OAB is frequently unknown and although DO is not always observed in patients with OAB, it is the only unambiguous physiological sign of the lower urinary tract (LUT) dysfunction underlying OAB. Its involuntary character implies, at least in part, an abnormality in CNS function, since the CNS is essential for the regulation of voluntary micturition and continence.4, 5,6
Failure to elicit DO during urodynamics in some patients with OAB may suggest that the underlying functional disorder of continence control is different or less severe than in those with more easily elicitable DO. Recent urodynamic studies support this idea by demonstrating physiological differences at LUT level between OAB patients who exhibit DO on urodynamic study and those who do not.7,8 In a group of 144 such women7, those with urodynamically elicitable DO reported strong desire to void and urgency at smaller volumes. These findings suggested elicitability of DO as a marker of the severity of impairment of continence control within the spectrum of OAB symptoms. Elicitability may differ according to circumstances; e.g. it may be more difficult to elicit DO in the scanner than during regular urodynamics, and more difficult during urodynamics than in daily life. The underlying mechanism of elicitability remains unknown: information about brain activity during DO or during urgency preceding DO would shed light on mechanisms involving the CNS.
Functional brain-imaging studies 9,10,11 have identified a group of brain regions believed to be a part of a network that regulates all phases of the micturition cycle (the ‘brain-bladder control network’) (see provisional working model, Fig. 1). Neuroanatomical and neurophysiological observations6,12 show that voiding is fundamentally regulated by a long-loop, spinobulbospinal (brainstem) reflex. During urine storage bladder afferents ascend via the sacral spinal cord to the midbrain periaqueductal gray (PAG). If they exceed a certain threshold, the reflex is triggered and a signal is sent to the micturition center in the pons (PMC). Thence an efferent signal passes to the sacral cord, where it stimulates both detrusor contraction and, via inhibitory interneurons, striated sphincter relaxation, so that voiding ensues. This voiding reflex thus switches back and forth between the storage and voiding phases 4,6.
Figure 1.
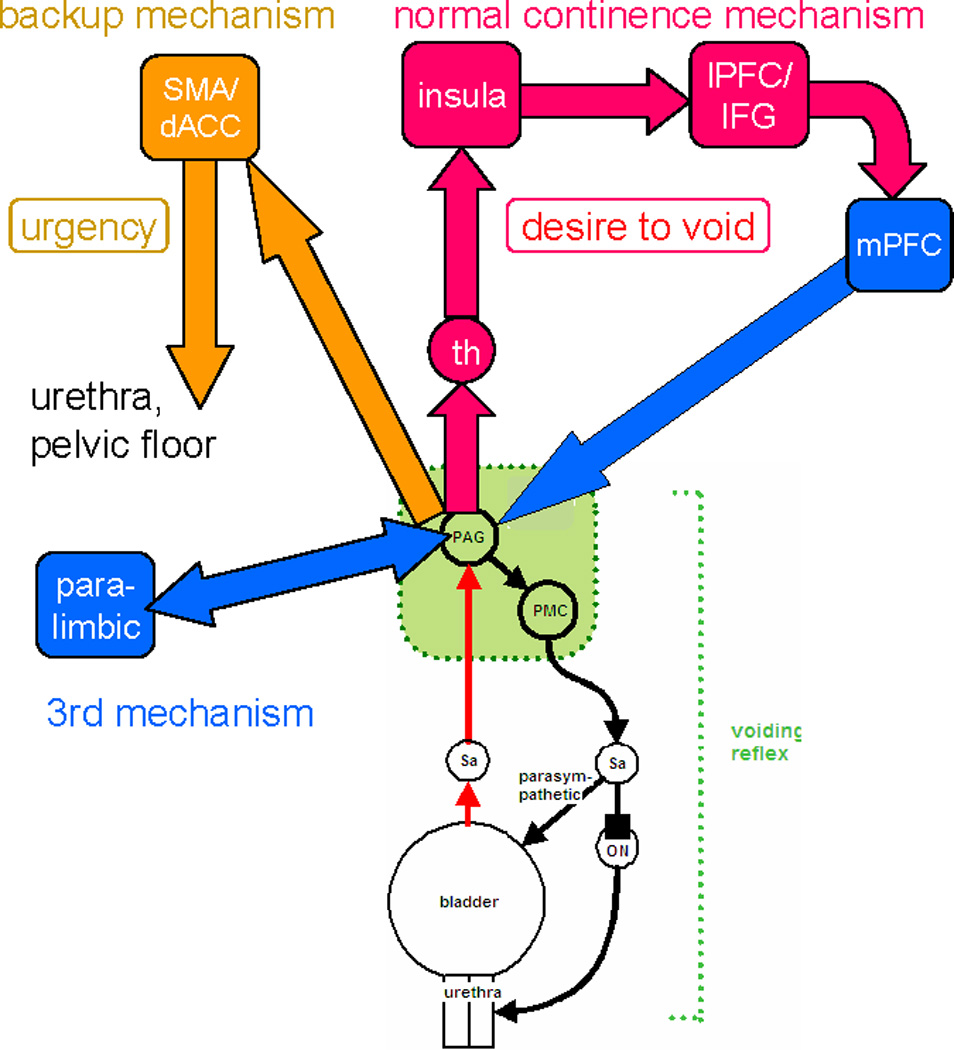
Provisional working model of brain/bladder control4,6,9,12,13,15. Voiding is triggered and coordinated by the voiding reflex shown in simplified form at the bottom of the diagram. Cerebral control is exercised by suppressing the reflex at the PAG during storage, and lifting suppression for voiding. Normal continence mechanism: ascending signals pass from the PAG via the thalamus to the insula, where desire to void is registered. Signals are relayed to excite the inferior frontal gyrus or dorsolateral prefrontal cortex where the voluntary decision to void can be made in light of social propriety. If voiding is not desired the medial prefrontal cortex is deactivated and this signal passes to the PAG and reinforces suppression of the voiding reflex. Backup continence mechanism: If the normal mechanism threatens to fail, as in OAB, the dACC is activated and generates a sensation of urgency together with output that tightens pelvic floor and sphincter muscles and relaxes the bladder, attempting thus to maintain continence. This signal may be relayed via the pontine continence center 5, 11 (not shown). A third continence mechanism involves deactivation of parahippocampal complex, posterior cortex and perhaps hypothalamus. It may be concerned with basic safety.
Normally however the reflex does not operate in isolation. During storage an ascending afferent signal is relayed from the PAG to higher regions of the brain, which assess whether voiding is safe and appropriate, generate bladder sensations, and in return provide output that promotes or suppresses voiding, probably at the PAG13,6. Thus the PAG is the pivotal region via which the reflex influences and is influenced by the cortical parts of the control network.
As suggested in Fig. 1 (and elsewhere13), the primary target of the signal ascending from the PAG is the insula and nearby lateral prefrontal cortex (especially on the right), where it evokes normal bladder sensation (desire to void). Desire to void is a homeostatic emotion 14 that provides motivation as well as appropriate motor output to maintain continence (homeostasis). This appears to be a normal continence mechanism, activated when there is desire to void. Fig. 1 suggests that the insular activity is propagated to the lateral (dlPFC) and medial (mPFC) prefrontal cortex (concerned with executive decision-making and social context) and thence back to the PAG, where it suppresses the voiding reflex. The medial – particularly the ventromedial (vmPFC) – prefrontal cortex appears to be deactivated as part of this mechanism15.
In UUI subjects, the signal ascending from the PAG (provoked in the scanner by further filling of a well-filled bladder) is also relayed to dorsal anterior cingulate gyrus (dACG) and nearby regions such as the supplementary motor area, SMA (Fig. 1). It evokes the abnormal sensation of ‘urgency’ 15–18, a homeostatic emotion. Activation of SMA is known to tighten the pelvic floor and urethral sphincter. This appears to be a back-up continence mechanism, used if the normal mechanism is inadequate.
Finally, bladder filling provokes deactivations in parahippocampal or paralimbic areas19, subcortical changes that suggest evaluation of the safety of voiding, and may provide a third continence mechanism (Fig. 1).
The presence or absence of all these activations and deactivations presumably reflects the ability or inability of the CNS to suppress DO (i.e. reduce DO elicitability) and thus prevent incontinence. To test this assumption, we here present a cohort of older women with OAB/UUI who underwent an experimental protocol set up in the brain imaging scanner, which included bladder filling coupled with simultaneous measurement of regional brain activity (via functional magnetic resonance imaging - fMRI) and urodynamic monitoring. Despite clear signs and symptoms of UUI in all subjects, only one-third of the cohort developed DO and lost continence in the scanner (the ‘DO group’). We therefore set up a secondary analysis to compare brain activity in the DO group with the group that maintained full control of the bladder in the scanner (the ‘no DO group’). These 2 groups apparently differed in DO elicitability and we postulated, a priori, that they would display different patterns of regional brain activity during urgency. Specifically, we made the postulate that the brain responses involved in maintaining continence (see Fig. 1) would be diminished in the DO group, implying: less activation in SMA/dACG (Hypothesis 1a) and less prominent deactivation in vmPFC/mPFC and parahippocampal complex (Hypothesis 1b).
We also explored possible phenotypic differences by analyzing: a) differences between the groups in their response to bladder filling during standard urodynamics (e.g. sensation of filling, desire to void and urgency), and, b) differences in clinical covariates associated with urgency, UUI and continence control such as: age, extent of structural brain changes (white-matter hyperintensities - WMH), cognition, depression history and psychological burden of the disease. In addition, without making specific hypotheses, we planned to look for differences in brain activity between those with and without elicitable DO when the bladder was nearly empty and sensation relatively weak and, thus, without reported urgency.
MATERIALS AND METHODS
Study subjects
30 functional community-dwelling female volunteers aged > 60 years were recruited by newspaper advertising. The majority had a history of past pharmacological treatment but only 4 (2 in each group) were currently on medication for urinary incontinence. All underwent comprehensive clinical evaluation, including detailed history, physical examination (neurological testing and a pelvic exam), a test of cognitive status (Mini-Mental State Examination - MMSE) and comprehensive urodynamics19,20. All subjects had UUI, either pure or with a minor component of stress-related leakage, as determined clinically and by voiding diary and pads. Psychological burden related to incontinence was assessed using the Urge Impact Scale (URIS-24)20.
Women with significant cognitive impairment (MMSE score ≤ 24/30), secondary causes of urinary incontinence (e.g. bladder cancer, spinal cord lesions, multiple sclerosis, pelvic radiation, interstitial cystitis), sphincter implant or significant neurological and vascular disease (history of stroke, neurodegenerative disease or complicated diabetes mellitus), claustrophobia and implanted metal or electromagnetic devices that would preclude performing MRI were excluded. The study was approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent.
Bladder filling protocol
All subjects underwent a brain scanning protocol that included fMRI and simultaneous urodynamic monitoring. Details of our fMRI methods have been described previously 17,19. Bladder filling was simulated by infusion/withdrawal cycles comprising: pause (12 s); infusion (22 ml in 12 s); pause (12 s); withdrawal (20 ml in 12 s). Four cycles formed one measurement block. After 2 measurement blocks, the bladder was filled quickly, without scanning, until the subject signaled compelling desire to void. With the subject’s permission, 1 to 2 further measurement blocks were performed with scanning until subjects stopped the study to void, or developed involuntary bladder muscle contraction (detrusor overactivity/DO) and urine leakage. The BOLD signal acquired in the final measurement block or the last block before DO onset was used for analysis. This measurement block was comparable in different subjects because it was always performed in the same situation: with full bladder and just tolerable sensation. We refer to this situation as ‘urgency’ because it reproduces most aspects of its definition1,21 (compelling desire to void, difficult to defer and associated with fear of leakage). For post hoc analysis at lower bladder volumes, blocks at the beginning of scanning session with nearly empty bladder (<100 ml) were used.
Structural brain imaging (assessment of white-matter hyperintensities – WMH)
We employed a fully automated method for quantifying and localizing WMH on MR images which uses fast-FLAIR images (fast FLuid-Attenuated Inversion Recovery) as reported previously19. The amount of white-matter hyperintensities, measured globally or in specific white matter tracts, is registered in voxels (1 voxel = 4.2 mm3) and then ‘normalized’ for each individual by dividing by total brain volume. 19
fMRI analyses
After image acquisition, pre-processing and further analyses were done using Statistical Parametric Mapping (SPM5; Wellcome Department of Imaging Neuroscience, 2003, http://www.fil.ion.ucl.ac.uk/spm/spm2.html). As previously, first-level analysis was performed in each subject to determine regional brain responses to bladder filling by comparing the fMRI signal during fluid infusion with the baseline during fluid withdrawal16–18. To determine the main effects of bladder filling we combined the single-subject results for the selected measurement block in a standard second-level (random-effects) analysis, displaying statistical maps thresholded at P < 0.01 at voxel level (uncorrected for multiple comparisons). For between-group comparisons , the first-level image for the designated measurement block was selected for each individual and used in 2 independent samples t-tests, generating statistical maps showing voxels significantly differing between groups (more active or less active in one group than the other). For display, results were thresholded at P < 0.05 (uncorrected for multiple comparisons). In all cases, statistical significance was assessed for individual regions at cluster level (corrected or uncorrected) and/or at a more stringent voxel level (see Table II).
Table II.
Regional brain activity in response to bladder filling (main effects).
| Region | Coordinates | T- | Z- | Cluster | |||
|---|---|---|---|---|---|---|---|
| x | y | z | value | Value | level | ||
| 1. | A. Activations during reported urgency | ||||||
| Group with DO in the scanner | |||||||
| Precentral gyrus (BA6) | −58 | −6 | 38 | 8.45 | 4.18** | ¶¶¶c | |
| Medial frontal gyrus (BA 6) | 10 | −12 | 56 | 5.82 | 3 54* | ||
| Superior frontal gyrus ( BA6) | −10 | 16 | 58 | 5.66 | 3.49* | ||
| Inferior parietal lobe (BA 40) | −28 | −44 | 54 | 4.75 | 3.19* | ||
| Anterior cingulate gyrus (dorsal, BA 32) | −4 | 24 | 34 | 7.72 | 4.03** | ¶¶¶c | |
| Middle frontal gyrus (BA 10) | 34 | 40 | 10 | 6.84 | 3.82** | ¶¶¶c | |
| Insula (BA 13) | 32 | 20 | 4 | 5.90 | 3.57* | ¶¶¶c | |
| Inferior frontal gyrus (BA 47) | 38 | 30 | 6 | 6.84 | 3.82** | ¶¶¶c | |
| Cuneus (BA 18) | −4 | −88 | 12 | 4.45 | 3.07 | ||
| Cerebellum (posterior lobe) | −16 | −78 | −32 | 7.48 | 3.97** | ¶u | |
| Cerebellum (anterior lobe) | −38 | −48 | −28 | 5.38 | 3.40* | ¶u | |
| Group without DO in the scanner | |||||||
| Medial frontal gyrus (BA 6) | 6 | 2 | 58 | 2.98 | 2.68 | ||
| Superior frontal gyrus (BA 8) | −2 | 18 | 52 | 2.82 | 2.56 | ||
| Insula (BA 13) | 36 | 20 | 8 | 2.72 | 2.48 | ||
| Inferior frontal gyrus (BA 9) | 48 | 8 | 22 | 2.71 | 2.47 | ||
| Middle frontal gyrus (BA 9) | 38 | 22 | 34 | 2.98 | 2.68 | ||
| B. Deactivations during reported urgency | |||||||
| Group without DO in the scanner - | |||||||
| Fusiform gyrus | −26 | −38 | −14 | 4.59 | 3.75** | ¶u | |
| Parahippocampus (BA 19) | −28,28 | −50,−50 | −4,−6 | 3.55,3.55 | 3.09*,3.28* | ¶u, − | |
| Hippocampus | −36 | −12 | −14 | 3.83 | 3.28* | ||
| Occipital gyrus | −40 | −76 | 26 | 3.52 | 3.07 | ||
| Amygdala | −30 | −8 | −14 | 3.18 | 2.83 | ||
| C. Between group differences | |||||||
| Activations in DO group > no DO | |||||||
| Parietal lobe (precuneus, BA 19) | −18 | −78 | 40 | 5.10 | 4.29** | ||
| Fusiform gyrus (BA 37) | −32 | −46 | −12 | 3.70 | 3.33* | ||
| SMA/precentral gyrus (BA 4) | −18 | −20 | 60 | 3.64 | 3.28* | ¶¶¶u | |
| 2. | A. Activations with nearly empty bladder | ||||||
| Group with DO in the scanner | |||||||
| SMA (Superior Frontal gyrus, BA 6) | 14 | −8 | 66 | 6.76 | 3.80** | ||
| Temporal lobe (BA 41) | 52 | −20 | 12 | 4.71 | 3.17* | ||
| Middle frontal gyrus (BA 46) | −40 | 36 | 24 | 4.25 | 2.99 | ||
| Group without DO in the scanner | |||||||
| Postcentral gyrus (BA 2) | −56 | −22 | 32 | 5.97 | 4.27** | ¶c | |
| Claustrum/insula | 36,-36 | 0,−6 | −2,20 | 5.71,4.57 | 4.16**,3.60* | ¶¶c,¶c | |
| Inferior frontal gyrus (BA 47) | 42 | 24 | 0 | 5.24 | 3.94** | ¶¶c | |
| Superior frontal gyrus (BA 9) | −38 | 36 | 26 | 4.76 | 3.70* | ||
| Inferior parietal lobe (BA 40) | 50 | −36 | 48 | 4.56 | 3.60* | ¶c | |
| Medial frontal gyrus (BA 9) | −2 | 46 | 30 | 4.29 | 3.45* | ||
| B. Deactivations with nearly empty bladder | |||||||
| Group with DO in the scanner | |||||||
| ACG (subgenual; BA 24) | 10 | 30 | 14 | 5.26 | 3.37* | ¶¶¶u | |
| Middle frontal gyrus (BA 8) | 20 | 32 | 38 | 5.39 | 3.41* | ¶¶¶u | |
| Superior frontal gyrus (BA 9) | 6,−10 | 50,56 | 28,18 | 4.96,4.06 | 3.26*,2.91 | ¶¶u | |
| C. Between group differences | |||||||
| Activations in DO group > no DO | |||||||
| SMA (Postcentral gyrus, BA 3) | 18 | −36 | 62 | 2.94 | 2.71 | ||
Regional statistic reported as Student’s t-value and Z-value. Coordinates are given in MNI space. All images reported attain significance at threshold P<0.01 uncorrected. Further voxelwise significance:
P<0.001 uncorrected;
P<0.0001 uncorrected. Cluster-level significance:
P< 0.01;
P<0.001;
P<0.0001 (u = uncorrected, c = corrected at cluster level).
RESULTS
Study subjects
The study population was representative of older community-dwelling functional women with OAB presenting as moderate to severe urgency incontinence (see Table I). Exclusion of 4 subjects on anticholinergic medication did not substantially alter the results presented below.
Table I.
Clinical characteristics of study participants - phenotypic analyses (DO group, n=9; No DO group, n=21).
| N (Subjects) DO / no DO |
Mean±SD DO / no DO |
p-Value (Two-Sample t-Test) |
||
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age (years) | 9 / 20 | 76.9±6.4 / 70.2±7.3 | 0.026 | |
| UI (#incontinent episodes/24h) | 9 / 20 | 3.6±1.9 / 2.4±1.4 | 0.079 | |
| Chronic conditions | 9 / 20 | 2.8±0.8 / 2.3±1.5 | 0.422 | |
| MMSE (0–30) | 9 / 20 | 29.1±1.4 / 29.3±0.9 | 0.664 | |
| History of depression (%) | 9 / 20 | 33 / 30 | 0.864 | |
| Psychological burden (24–120) | 9 / 19 | 66.8±12.9 / 84.5±18.4 | 0.015 | |
|
N (Subjects) DO / no DO |
Volume (mL) [Mean±SD] |
Mean Volume Difference (mL) |
p-Value (Two Sample t-Test) |
|
| Urodynamic Findings | ||||
| First Sensation of filling | 7 / 16 | 179±123 / 194±145 | 15 | 0.40 |
| First Desire to void | 8 / 21 | 262±125 / 322±151 | 60 | 0.15 |
| Strong Desire/urgency | 8 / 21 | 302±117 / 429±203 | 128 | 0.023 |
| Maximum Capacity | 8 / 21 | 444±186 / 593±198 | 150 | 0.040 |
| “Warning” | 7 / 17 | 254±174 / 457±130 | 203 | 0.011 |
| (Max Capacity - First Sensation) | ||||
|
N (Subjects) DO / no DO |
DO [Mean*] |
No DO [Mean*] |
p-Value (Two-Sample t-Test) |
|
| Functional Brain Imaging | ||||
| Global WMH | 9 / 21 | 0.004448 | 0.001465 | 0.02 |
| ATR (left side) | 9 / 21 | 0.000677 | 0.000292 | 0.14 |
| ATR (right side) | 9 / 21 | 0.001425 | 0.000538 | 0.05 |
| SLFB (left side) | 9 / 21 | 0.000229 | 0.000100 | 0.43 |
| SLFB (right side) | 9 / 21 | 0.000147 | 0.000120 | 0.85 |
ratio calculated by dividing volume of white matter changes by subjects’ brain volume
Abbreviations: DO=subjects with elicitable DO in the scanner; no DO=subjects without DO in the scanner; UI=urge incontinence; MMSE=Mini Mental State Exam; Psychological burden=Urge Impact Scale (24–120)17 (smaller value = more burden); WMH=white matter hyperintensities; ATR=anterior thalamic radiation; SLFB=superior longitudinal fasciculus (entire pathway).
Regional brain activity during self-reported urgency preceding DO (Hypothesis 1)
A. Cohort as a whole (n=30)
Random-effects analysis of the brain responses to bladder filling in the whole cohort of women with UUI showed the expected characteristic pattern of regional activations and deactivations19,22 – namely activations in SMA, SFG, dACG, bilateral insula adjacent to middle frontal gyrus and dlPFC on the right side (Figure 2A – upper part); and deactivations in mPFC and adjacent pregenual ACG and paralimbic areas bilaterally (Figure 2A – lower part).
Figure 2*.
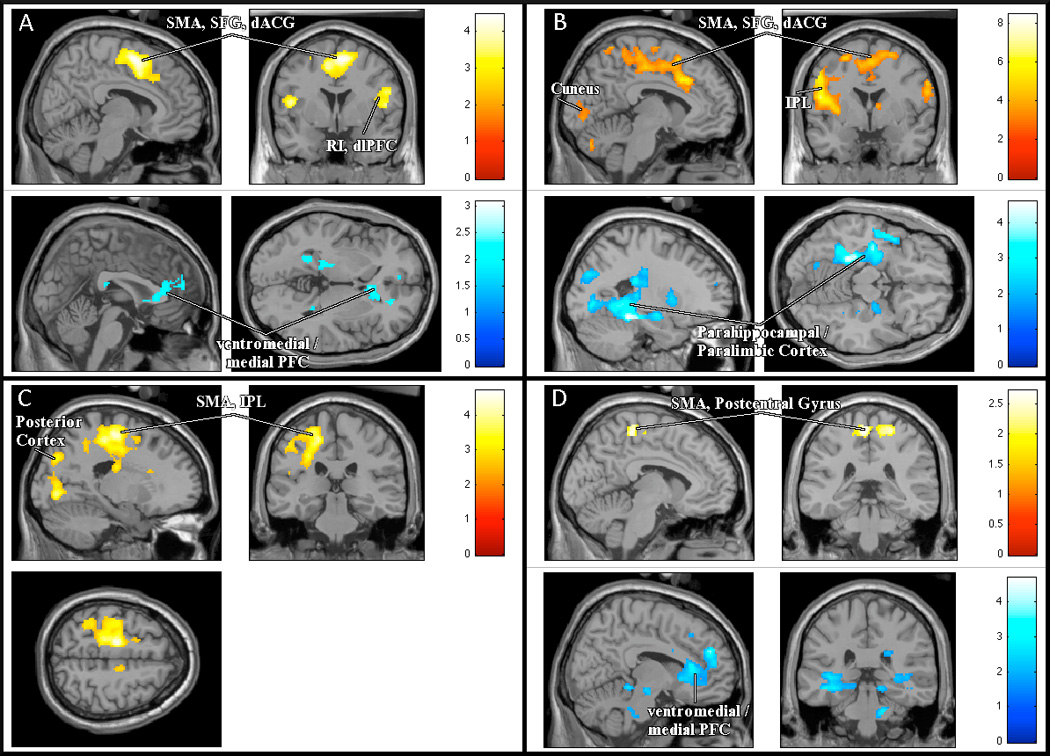
Images related to regional brain activity during scanning procedure (activations and deactivations during bladder filling and self-reported urgency). (A) Upper part (yellow): Group as a whole (n=30) - activations (P < 0.01 uncorrected); Lower part (blue): Group as a whole - deactivations (P < 0.05 uncorrected). (B) Upper part (red): DO group (n=9) - activations (P <0.01); Lower part (blue): No DO group (n=21) - deactivations (P < 0.05). (C) DO group: areas with significantly stronger activity during reported urgency compared to No DO group. (D) Upper part (yellow): DO group - areas with significantly stronger activity at lower bladder volumes compared to No DO group (P < 0.05 uncorrected); Lower part (blue): DO group - areas with significantly weaker activity at lower bladder volumes compared to No DO group (P < 0.05 uncorrected).* some results displayed at lower threshold level (P<0.05) for figure only (SMA=supplemental motor area; SFG=superior frontal gyrus; dACG=dorsal anterior cingulate gyrus; RI=right insula; dlPFC=dorsolateral prefrontal cortex; PFC=prefrontal cortex; IPL=inferior parietal lobule.)
B. Subgroups - DO group (n=9) and no DO group (n=21)
The DO group showed significant activations in similar regions – SMA/SFG adjacent to dACG, and bilateral insula adjacent to inferior frontal gyrus (IFG) (Table II.1A and Figure 2B-upper part). In addition there were significant activations in cerebellum, inferior parietal lobule (IPL) and precentral gyrus. Regional deactivations in this subgroup were relatively weak, just failing to reach the predetermined significance level (P < 0.01) in vmPFC/mPFC (pre-and sub-genual ACG and medial frontal gyrus) (data not shown).
In the no DO group, activations were generally weaker although in similar locations: SMA/SFG adjacent to dACG, bilateral insula adjacent to middle frontal gyrus and dlPFC (on the right side) (Table II.1A). Conversely, regional deactivations were significant, mainly in paralimbic system and posterior cortex: hippocampus, parahippocampal complex, and fusiform gyrus (Table II.1B and Figure 2B-lower part).
Between-groups comparisons confirmed that, in areas adjacent to SMA and posterior cortex (parahippocampus, fusiform gyrus), activation was significantly greater in the DO group than the no DO group (Table II.1C and Figure 2C). (For the posterior cortex this implies weaker deactivation.)
Post hoc analyses – regional brain activity at lower bladder volumes
Even with nearly empty bladder (<100 ml), with no reported urgency, there were differences between the groups in the response to bladder filling. Just as during urgency, the DO group showed activations in areas adjacent to SMA (Table II.2A), which were significantly stronger than in the no DO group (Table II.2C and Figure 2D-upper part), while activations in other regions were not as pronounced. The no DO group had significant activations in different areas (Table II.2A). Surprisingly however, the DO group showed significantly greater deactivation in vmPFC/mPFC than the no DO group (who had no deactivation in this area) (see Table II.2B and Figure 2D-lower part).
II Phenotypic analyses
A. LUT function on standard urodynamic exam (Table I)
The DO group reported strong desire to void and urgency at significantly lower bladder volumes during bladder filling in standard urodynamic settings and had a significantly smaller volume increment between first sensation of bladder filling and maximum capacity (‘warning’) compared to the no DO group. All subjects who exhibited DO in the scanner also showed DO during standard urodynamic examination, while less than half (n=10/21) of the no DO group exhibited DO during standard urodynamics.
B. Clinical covariates (Table I)
Subjects in the DO group were older than the no DO subjects and had a greater burden of structural changes in the brain’s white matter (Table I). They were more incontinent on bladder diary and also suffered greater psychological burden of incontinence. In our cohort, there were no significant differences in co-morbidities, cognitive function or prevalence of depression history.
DISCUSSION
In general agreement with hypothesis 1, women who exhibited DO in the scanner (the DO group) also exhibited different brain activity during bladder filling compared to those who remained continent during this procedure. In detail, however, contrary to hypothesis 1a, during urgency the DO group showed significantly stronger activations in areas of SMA adjacent to dACG than the no DO group. In parahippocampal regions the no DO group showed significant deactivations while the DO group did not, consistent with hypothesis 1b. In vmPFC however there was no significant difference in deactivation (thus failing to support hypothesis 1b). The study findings therefore did not confirm hypothesis 1a but showed an opposite trend, while hypothesis 1b received partial support. Findings at lower bladder volumes confirmed that even with nearly empty bladder and minimal sensation there were different brain responses in the 2 groups.
These findings suggest new inferences about potential CNS mechanisms underlying impaired continence control. For example, activations in a functional complex around SMA (linked to control of pelvic floor muscles and urethra) and dACC are stronger in subjects who subsequently develop DO - suggesting that increased activity in this region is not a cause of incontinence but represents a compensatory reaction to a failure occurring elsewhere; presumably neuroplasticity enables this region to work harder at maintaining continence even at an early stage of the storage phase. Increased dACC activation further implies a stronger sensation of urgency. Such an emotion may potentially lead to widespread changes in activity 16, based in part on learnt experience of previous incontinence episodes. The brain regions causally linked to failure may be in PFC. Although we do not see a difference in vmPFC/mPFC activity between subgroups during urgency, at small bladder volumes there are significant deactivations in this region in the DO group (not present in the no DO group), perhaps suggesting early engagement of a normal continence mechanism to compensate for insufficiency of this region. Structural imaging of brain’s white matter supports this idea as it shows more white matter damage in the DO group, in particular in the pathway (right anterior thalamic radiation - ATR) that connects the prefrontal regions with regions in thalamus and brain stem known to be involved in continence control.4
The association of deactivations in parahippocampal areas with preserved continence (no DO), consistent with hypothesis 1b, suggests that the premise underlying the hypothesis may be correct: i.e., parahippocampal deactivation might be part of a normal continence mechanism that is damaged in more severely impaired subjects (the DO group). The further physiological significance of this region remains to be elucidated, but it may imply a role for memory retrieval or encoding in the suppression of urgency.
Phenotypic studies
Phenotypic studies confirm the differences between the 2 subgroups. Subjects in the DO group had less tolerance to bladder filling on regular urodynamics as they reported strong desire to void and urgency at smaller bladder volumes, consistent with previous studies on the elicitability of DO7,8,23,24. The DO group also had greater scores in clinical correlates of urgency and UUI such as age and structural damage in the brain’s white matter (both globally and in right ATR). This is in accordance with epidemiological findings in community-dwelling elderly that relate symptoms of urgency and UUI to functional decline at advanced age25 and age-related accumulation of white matter changes26,27,19.
CONCLUSIONS
CNS activity in women with OAB/UUI is different in those who subsequently develop DO and lose bladder control, compared to those who remain continent during an experimental protocol that involves bladder filling and simultaneous brain imaging and urodynamic monitoring. The difference in activity involves brain regions linked to control of pelvic floor muscles and urethra and, thus, may represent a response to compensate for failure of executive control in the prefrontal cortex.
The functional differences observed in this cohort suggest that OAB is a heterogeneous clinical syndrome with a spectrum of functional severity and/or different phenotypes. The greater age and greater burden of white matter damage in subjects with more easily elicitable DO suggest that there is a phenotype with more severe functional impairment, attributable partly to cumulative age-related structural changes in the brain’s white matter.
Coupling of functional brain imaging with urodynamic studies in subjects with OAB and UUI or other LUTS may reveal regional brain activity that could serve in clinical studies as a marker of impaired continence control and its underlying CNS correlates.
ACKNOWLEDGEMENTS
Supported by: NIH: K23AG031916-01; 2R01AG020629-06; John A. Hartford Center of Excellence in Geriatric Medicine. We are especially thankful to Mary Alyce Riley for her help with study subjects during the course of the study.
Footnotes
Study presented at 2011 SUFU Winter Meeting (Phoenix, Arizona, March 1–5th) as ‘The Best Clinical Essay’ Prize.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Brubaker L. Urgency: the corner stone symptom of overactive bladder. Urology. 2004;64(suppl 6A):12–16. doi: 10.1016/j.urology.2004.10.073. [DOI] [PubMed] [Google Scholar]
- 3.Michel MC, Chapple CR. Basic mechanisms of urgency: preclinical and clinical evidence. Eur Urol. 2009;56:298–308. doi: 10.1016/j.eururo.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- 6.Blok BFM, Holstege G. The central nervous system control of micturition in cats and humans. Behav Brain Res. 1998;92:119–125. doi: 10.1016/s0166-4328(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 7.Guralnick ML, Grimsby G, Liss M, Szabo A, O’Connor RC. Objective differences between overactive bladder patients with and without urodynamically proven detrusor overactivity. Int Urogynecol J. 2010;21:325–329. doi: 10.1007/s00192-009-1030-7. [DOI] [PubMed] [Google Scholar]
- 8.Naoemova I, De Wachter S, Wuyts FL, Wyndaele JJ. Do sensation-related bladder diaries differ between patients with urodynamically confirmed and non-objectivised urinary incontinence? Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:213–216. doi: 10.1007/s00192-007-0420-y. [DOI] [PubMed] [Google Scholar]
- 9.Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29:49–55. doi: 10.1002/nau.20740. [DOI] [PubMed] [Google Scholar]
- 10.Blok B, Sturms L, Holstege G. Brain activation during micturition in women. Brain. 1998;121:2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- 11.Blok B, Willemsen T, Holstege G. A PET study of brain control of micturition in humans. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Holstege G. The emotional motor system and micturition control. Neurourol Urodyn. 2010;29:42–48. doi: 10.1002/nau.20789. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths DJ. Use of functional imaging to monitor central control of voiding in humans. In: Andersson K-E, Michel MC, editors. Urinary Tract, Handbook of Experimental Pharmacology 202. Springer-Verlag Berlin Heidelberg; 2011. pp. 81–97. [DOI] [PubMed] [Google Scholar]
- 14.Craig AD. A new view of pain as a homeostatic emotion. Trends in Neurosciences. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths D, Tadic SD. Bladder control, urgency and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27:466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005;174:1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tadic SD, Griffiths D, Schaefer W, Resnick NM. Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. NeuroImage. 2008;39:1647–1653. doi: 10.1016/j.neuroimage.2007.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadic SD, Griffiths D, Murrin A, Schaefer W, Aizenstein H, Resnick NM. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. NeuroImage. 2010;51:1294–1302. doi: 10.1016/j.neuroimage.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tadic SD, Zdaniuk B, Griffiths D, Rosenberg L, Schaefer W, Resnick NM. Effect of biofeedback on psychological burden and symptoms in older women with urge urinary incontinence. J Am Geriatr Soc. 2007;55:2010–2015. doi: 10.1111/j.1532-5415.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 21.Abrams P, Blaivas JG, Stanton S, et al. The standardization of terminology of lower urinary tract function. Neurourol Urodyn. 1988;7:403–426. [Google Scholar]
- 22.Tadic SD, Griffiths D, Schaefer W, Cheng CI, Resnick NM. Brain activity measured by functional magnetic resonance imaging fMRI) is related to patient-reported severity of urgency incontinence. J Urol. 2010;183:221–228. doi: 10.1016/j.juro.2009.08.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haylen BT, Chetty N, Logan V. Is sensory urgency part of the same spectrum of bladder dysfunction as detrusor overactivity? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:123–128. doi: 10.1007/s00192-006-0165-z. [DOI] [PubMed] [Google Scholar]
- 24.Fall M, Geirsson G, Lindstrom S. Toward a new classification of overactive bladders. Neurourol Urodyn. 1995;14:635–646. doi: 10.1002/nau.1930140605. [DOI] [PubMed] [Google Scholar]
- 25.Pantoni L, Poggesi A, Basile AM, Pracucci G, Barkhof F, Chabriat H, Erkinjuntti T, Ferro JM, Hennerici M, O’Brien J, Schmidt R, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D LADIS Study Group. Leucoaraiosis predicts hidden global functioning impairment in nondisabled older people: the LADIS (Leukoaraiosis And DISability in the Elderly) J Am Geriatr Soc. 2006;54:1095–1101. doi: 10.1111/j.1532-5415.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 26.Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A, Hennerici M, Langhorne P, O’Brien J, Scheltens P, Visser MC, Crisby M, Waldemar G, Wallin A, Inzitari D, Pantoni L Leukoaraiosis And DISability Study Group. Urinary complaints in nondisabled elderly people with age-related white matter changes: The Leucoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc. 2008;56:1638–1643. doi: 10.1111/j.1532-5415.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuchel GA, Moscufo N, Guttman CR, Zeeve N, Wakefield D, Schmidt J, DuBeau CE, Wolfson L. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:902–909. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]


