Abstract
Allogeneic stem cell transplant for multiple myeloma (MM) is one treatment associated with long-term disease-free survival. The high incidence of treatment-related mortality and relapses, however, are important reasons for controversy about the role of allografting in the management of MM. We reviewed our results of allografting for MM spanning a period of 34 years in order to better define long-term outcomes and identify areas of progress as well as areas requiring improvement. A total of 278 patients received allogeneic marrow or PBSCs after high-dose myeloablative (N=144) or reduced intensity, non-myeloablative (N=134) regimens. In multivariable analysis, adjusting for differences in patient groups, reduced intensity/non-myeloablative transplants were associated with significantly less acute GVHD, lower transplant mortality, better PFS and overall survival. There were no significant differences in relapse, progression or chronic GVHD, when adjusted. In multivariable analysis of patients receiving only non-myeloablative transplants, decreased overall survival and PFS were associated with relapse after a prior autograft and a β2 microglobulin >4.0. Transplant mortality was reduced and only influenced by a prior tandem autograft.
Keywords: multiple myeloma, plasma cell disorders, allogeneic BM transplant, allo-stem cell transplant
Subject terms: Bone marrow transplantation, Stem cells, Myeloma
Introduction
The survival of patients with multiple myeloma (MM) has improved over the last decade as a result of melphalan-based high-dose therapy followed by auto-SCT, the introduction of novel anti-myeloma agents with increased efficacy in relapsed and refractory MM, and improvements in supportive care.1, 2, 3, 4, 5, 6 Registry data indicate an improvement in median survival from 3 to 5 years, primarily among younger patients, as a result of these treatment innovations.7 Despite these new developments, MM remains an incurable disease for the large majority, as all but a few patients will relapse. Allogeneic hematopoietic cell transplantation is currently one treatment with a potential for long-term disease control although its curative potential is debated. This is in part due to the graft-vs-myeloma effect, mediated by immune competent donor lymphocytes, best illustrated by the induction of sustained (molecular) remissions following donor lymphocyte infusions,8 but could also be due in part to absence of contaminating myeloma cells in the donor graft and documented lower levels of residual disease.9, 10
The role of allo-SCT in MM, however, is controversial due to the high mortality and morbidity associated with conventional myeloablative regimens and because convincing evidence for a survival benefit is lacking.11, 12, 13 In the last decade, non-myeloablative allo-SCT has gained in popularity due to significantly reduced TRM.14, 15 Among four reports comparing auto-SCT with allo-SCT, two have shown survival advantages for the non-myeloablative approach when compared with tandem autologous transplantation.16, 17, 18, 19 A recently reported US clinical trial prospectively comparing tandem autologous transplant to autologous followed by non-myeloablative allo-SCTs found no differences in PFS or OS at 3 years.20 In contrast, a European multicenter trial found than tandem autologous, non-myeloablative allo-SCT resulted in superior OS compared with single or tandem auto-SCT.19
Furthermore, at least one registry report comparing conventional ablative with non-myeloabalative/reduced intensity allo-SCTs have shown similar survival outcomes with lower TRM for patients receiving non-ablative transplants yet higher rates of relapse and PFS inferior to ablative allo-SCT.21 We reviewed our results of allo-SCT for patients with MM beginning in 1975 with the aim of identifying factors associated with improvements in disease-free survival and OS as preparative regimens have changed from ablative to non-myeloablative.
Patients and methods
Beginning in 1975, patients with MM were referred to the University of Washington, Fred Hutchinson Cancer Research Center or the Seattle Veterans Hospital for consideration of allo-SCT. Patients were evaluated for suitability for transplant based on treatment protocols in effect at the time. Patient records, laboratory, X-rays and marrow aspirates were reviewed to confirm the diagnosis of MM. To be considered for marrow transplantation, patients had to meet the established criteria for active, symptomatic MM according to Durie and Salmon22 and had to have received at least one cycle of conventional dose chemotherapy. Patients with a Karnofsky score of <50, a pulmonary diffusion capacity of <50% of predicted and symptomatic heart failure were excluded. Non-ablative transplant candidates were allowed to enroll with a diffusion capacity as low as 30%. Standard hematologic and chemistry studies were used to evaluate organ function. A suitable marrow donor was required, which included HLA identical relatives, HLA haplo-identical relatives or an unrelated donor who was phenotypically HLA identical, or single allele or Ag HLA-mismatched at class I with the patient. Transplants occurred between January 1975 and September 2008. The date of last follow-up was August 2011.
Initially, patients who had achieved a complete response (CR) to first-line therapy and were without any evidence of disease were excluded from transplantation. This policy changed, however, as non-myeloablative allo-SCT regimens were adopted. Ablative allo-SCT were utilized as stand-alone therapy. In contrast, non-abaltive allo-SCT were performed in the majority of patients, 2–4 months following recovery from a standard auto-SCT utilizing high-dose melphalan. The auto-SCT was utilized to provide cyto-reduction before the non-ablative Allo-SCT, yet allow the patient time to recover from the effects of high-dose therapy used for auto-SCT. Maintenance therapies were not used following allo-SCT.
For purposes of this analysis, patients with at least a 50% reduction in monoclonal proteins in the blood or a 75% reduction in 24 h quantitative Bence Jones protein, to their most recent chemotherapy before allo-SCT or auto-SCT, in the case of tandem transplants, were categorized as having sensitive disease, whereas all other patients were judged to have chemotherapy-resistant disease.
Responses were categorized according to the IMWG criteria.23 If certain data were missing that were required for response categorization, for example immunofixation for CR, the patient was classified as responding in the next lower category. An analysis of OS, PFS, TRM, relapse or progression, acute and chronic GVHD was undertaken. The initial analysis compared outcomes using non-myeloablative conditioning for the allogeneic transplant vs those with myeloablative conditioning. In the analysis of relapse or progression, time-dependent competing risks of treatment failure such as death from TRM were included. Cox regression models for these outcomes were adjusted for patient age (continuous), donor sex, chemotherapy responsive vs resistant disease, related vs unrelated donor, time from diagnosis to transplant (<2.5 years vs >2.5 years), prior radiation, prior number of chemotherapy regimens (continuous), β-2 microglobulin >4.0 either at diagnosis or transplant, and abnormal cytogenetics or FISH either at diagnosis or transplant. Abnormal cytogenetics included multiple abnormalities or any abnormality by conventional cytogenetics other than hyperdiploidy. Abnormal FISH included deletion 13, deletion 17, translocation 4;14, 14;16, or 14;20. Because data were missing for some patients, data available for abnormalities were compared with patients who had no abnormalities and patients with missing data. Subsequent multivariate analyses of risk factors for the same outcomes among patients receiving non-myeloablative conditioning included the factors noted above, plus single allo-SCT vs tandem autologous-allo SCT, and progression after a prior autologous SCT used as stand-alone treatment.
Results
Patient characteristics are shown in Table 1. Patients receiving non-myeloablative allo-SCTs were older by a median of 8 years. There were no important differences in the percentages of patients with advanced Durie Salmon staging, IgG or IgA subtypes, number of prior regimens, or total cycles of chemotherapy. Availability of data on beta-2 microglobulin levels, albumin and cytogenetic data were limited. A higher percentage of patients receiving ablative regimens had been given local radiation therapy, 50% compared with patients receiving non-ablative regimens, 33%. One third of the patients receiving non-ablative conditioning had progressed after an autologous transplant, while only four patients receiving ablative conditioning had progressed after an autologous transplant. A higher percentage of patients receiving ablative regimens were judged to have refractory disease, 77%, (based on less than a partial response to their last salvage chemotherapy), compared with 52% of patients who received non-ablative regimens. Relatively few patients were in remission before allografting; two patients undergoing myeloablative allografts were in 2nd CR, whereas among the non-myeloablative group, three were in first CR and four in 2nd CR.
Table 1.
Patient and treatment characteristics
| Characteristics | All patients | Myeloablative | Non-myeloablative |
|---|---|---|---|
| No. of patients | 278 | 144 | 134 |
| Date of first transplant | January 1975 | January 1975 | March 1998 |
| Sex, % male | 63 | 62 | 64 |
| Age, median (range) | 49 (20–69) | 45 (20–59) | 53 (25–69) |
| % Durie salmon stage3 | 77 | 79 | 75 |
| Type | |||
| IgG | 156 | 80 | 76 |
| IgA | 64 | 31 | 33 |
| Light chain | 39 | 24 | 15 |
| Nonsecretory | 13 | 5 | 8 |
| IgD | 2 | 1 | 1 |
| IgM | 1 | 1 | |
| Plasma cell leukemia | 7 | 4 | 3 |
| B-2m | |||
| At dx (n=19) | 4.1 (1.3–14.6) | ||
| At tx (n=122) | 2.9 (0.8–24.4), n=52 | 1.8 (0.8–10.3), n=70 | |
| Albumin at tx (n=236) | 3.5 (1.4–4.9) | 3.5 (1.4–4.9), n=118) | 3.5 (2.0–4.4), n=118 |
| Cytogenetics | |||
| Normala | At dx 0, at tx 53 | At dx 15, at tx 96 | |
| Abnormal | At dx 2, at tx 9 | At dx 18, at tx 12 | |
| FISH any abnormality | 11 | 30 | |
| Prior radiation | 72 | 44 | |
| No. of regimens | 2 (1–6) | 2 (1–6) | |
| Total chemotherapy cycles | 7 (1–32) | 6 (3–40) | |
| Tandem auto-allo (%) | 0 | 99 (74) | |
| Relapse after autograft (%) | 4 | 46 (34) | |
| Refractory (%)b | 65 | 77 | 52 |
| Time from diagnosis to transplant, median years | 1.2 (0.1–11.3) | 1.5 (0.3–11.4) | |
| Survivors follow-up, median years | 15.1 (3.6–23.5) | 7.1 (2.9–12.9) | |
Includes hyperdiploidy, numbers at dx=diagnosis, at tx=transplant.
Refractory patients achieved <PR to their last salvage therapy prior to allograft or tandem auto-allograft.
The regimens used for transplant differed significantly by the time periods during which patients were transplanted with almost all ablative allo-SCTs occurring between 1975 and 2000, whereas the non-ablative approach was utilized from 1998 to 2008. (Table 2) The conditioning regimens given to ablative allo-SCT recipients consisted mostly of fractionated TBI 9–12 Gy, plus CY, and/or BU. BU and CY without TBI were utilized for 69 patients. The non-ablative regimens were primarily TBI 2 Gy with or without fludarabine, whereas 14 patients received additional melphalan 100 mg/m2. Most donors were HLA-matched siblings (n=198) for both ablative and non-ablative transplants, however, a greater percentage of non-ablative transplants were performed from unrelated donors. Marrow was the primary stem cell source for most of the patients receiving ablative conditioning whereas PBSCs were used almost exclusively for non-ablative recipients. The majority of regimens for GVHD prophylaxis in ablative recipients consisted of a calcineurin inhibitor with MTX or steroids. Almost all recipients of non-ablative regimens received a calcineurin inhibitor and mycophenolic acid for GVHD prophylaxis.
Table 2.
Patient treatment characteristics
| Characteristics | All patients | Myeloablative | Non- myeloablative |
|---|---|---|---|
| No. of patients | 278 | 144 | 134 |
| Conditioning regimens | |||
| 2 GyTBI | 64 | ||
| Fludarabine, 2 GyTBI | 54 | ||
| L-PAM, fludarabine, 2 GyTBI | 14 | ||
| CY, fludarabine, 2 GyTBI | 2 | ||
| Holmium, fludarabine, 2 GyTBI | 1 | ||
| CY, 12 GyTBI | 16 | ||
| L-PAM, 12 GyTBI | 1 | ||
| BU, CY, modifiedTBI 9 Gy | 44 | ||
| BU, modifiedTBI 9–12 Gy | 8 | ||
| BU, CY | 69 | ||
| BU, L-PAM | 3 | ||
| BEAM | 1 | ||
| DMM, Etoposide, 10 GyTBI | 1 | ||
| Donors | |||
| Sibling-matched | 110 | 88 | |
| Sibling-haploidentical | 4 | 1 | |
| Parent-haploidentical | 2 | ||
| Child | 6 | 2 | |
| Unrelated-matched | 21 | 40 | |
| Unrelated-mismatched | 1 | 3 | |
| Stem cell source | |||
| Marrow | 120 | 118 | 2 |
| PBSCs | 158 | 26 | 132 |
| GVHD prophylaxis | |||
| ATG, steroids | 1 | ||
| CYA | 7 | ||
| CYA, MTX | 84 | ||
| CYA, MMF | 1 | 92 | |
| Tacrolimus, MMF | 37 | ||
| CY, tacrolimus, MMF | 1 | ||
| Tacrolimus. MMF, rapamycin | 4 | ||
| Tacrolimus, MTX | 11 | ||
| CYA, MTX, Steroids | 5 | ||
| CYA, steroids | 24 | ||
| CYA, trimetrexate | 2 | ||
| Monoclonal antibody | 1 | ||
| MTX, steroids | 1 | ||
| MTX | 7 | ||
Response to transplant
Among the 144 ablative transplant recipients, 33 (23%) achieved CRs, 33 (23%) a partial response, 12 (8%) did not respond and 67 (46%) were not evaluable owing to early death. Of 134 patients receiving non-ablative transplants, 51 (38%) achieved a CR, 48 (36%) a partial response, 31 (23%) did not respond, whereas 4 (3%) were not evaluable owing to early death. Patients who achieved a CR (n=84) had 5 and 10 year survivals of 62 and 53% compared with patients who did not achieve a CR (n=132) and excluding patients who were not evaluable owing to early death, who had 5 and 10 year survivals of 28% and 17%, respectively.
Among patients who received ablative conditioning, 104 developed acute GVHD; 7 grade 1, 44 grade 2, 34 grade 3 and 19 grade 4. Of patients who received non-ablative conditioning acute GVHD occurred in 90; grade 1 in 6, grade 2 in 72, grade 3 in 8 and grade 4 in 4. The cumulative incidences of chronic extensive GVHD were 27% and 66% for patients receiving ablative and non-ablative conditioning regimens, respectively.
Causes of death varied significantly between patients receiving ablative and non-ablative transplants. (Table 3) Among patients who died after receiving ablative conditioning, major causes included fungal infections (n=20), respiratory failure from diffuse alveolar damage or acute respiratory distress syndrome (n=8), acute GVHD (n=18), multi-organ failure (n=16), viral infections (n=13) and progressive disease (n=39). In contrast, only three patients receiving non-ablative transplants died of any of these causes. The major causes of death among recipients of non-ablative transplants were mostly chronic GVHD (n=12) and progressive disease (n=50). At the time of last follow-up, August 2011, among 144 patients receiving ablative conditioning, 14 were alive a median of 15.1 years (3.6–23.5) post transplant, of whom 6 had relapsed. Among 134 patients receiving non-ablative conditioning, 56 were alive a median of 7.1 years (2.9–12.9) post transplant, of whom 25 had relapsed. At 2 years, the probabilities of non-relapse mortality were 18% and 55% for non-ablative and ablative regimens, respectively. At 6 years, the probabilities of relapse or disease progression were 55% and 34% for non-ablative and ablative regimens, respectively. For patients undergoing ablative allo-SCTs, the probabilities of OS and PFS are 11 and 8% at 15 years. For patients undergoing non-ablative transplants, the probabilities of OS and PFS are 39 and 16% at 10 years. (Figure 1) The best outcomes were found among 88 patients who received an autologous transplant, followed by a non-myeloablative allograft within 4 months of the autologous transplant and who had not progressed after a prior autologous transplant. (Figure 2) Their 10-year OS was 49% and PFS was 27%.
Table 3.
Causes of death
| Cause | Ablative | Nonmyeloablative |
|---|---|---|
| ARDS-Idiopathic pneumonia, DAD | 8 | 1 |
| Fungus | 20 | |
| Aspergillus | 14 | 1 |
| Candida | 3 | |
| Mucormycosis | 1 | |
| Rhizopus | 1 | |
| Torulopsis | 1 | |
| Zygomyces | 1 | |
| Graft failure | 3 | 2 |
| Acute GVHD | 18 | 1 |
| Chronic GVHD | 2 | 12 |
| Hemhorrage | 2 | |
| Multi-organ failure/VOD | 16 | 1 |
| Pneumocystis | 1 | |
| Renal failure | 2 | |
| Sepsis | 5 | 5 |
| E coli | 1 | |
| MRSA | 1 | |
| Pneumococcus | 1 | |
| Pseudomonas | 1 | |
| Unknown | 4 | 2 |
| Stroke | 1 | |
| Virus | 13 | |
| Adenovirus | 1 | |
| CMV | 5 | |
| Hepatitis B | 1 | |
| Herpes simplex/zoster | 2 | |
| Parainfluenza | 1 | |
| Respiratory synctial | 3 | |
| Esophageal cancer | 1 | |
| Lung cancer | 1 | |
| Progressive myeloma | 39 | 50 |
| Pancreatitis | 1 | |
| Polyneuropathy | 1 | |
| Head trauma | 1 |
Bold numerals refer to number of patients for each heading.
Figure 1.
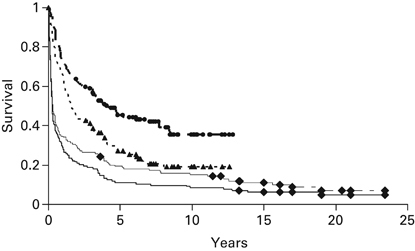
Probabilities of OS and PFS for patients undergoing myeloablative or non-myeloablative allogeneic hematopoietic cell transplants. First line: OS of 134 patients undergoing nonmyeloabaltive allografting; Second line: PFS of 134 patients undergoing non-myeloabaltive allografting; Third line: OS of 144 patients undergoing myeloablative allografting; Fourth line: PFS of 144 patients undergoing myeloablative allografting.
Figure 2.
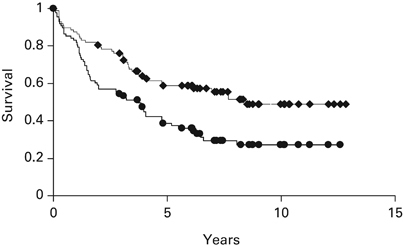
Probabilities of OS and PFS of 88 patients undergoing tandem autologous, non-myeloablative allografting as part of front-line therapy. First line: OS; Secnd line: PFS.
Cox regression analysis of overall mortality, PFS, TRM, relapse or progression and acute or chronic GVHD between non-myeloablative and ablative conditioning regimens are shown in Table 4. When adjusted for patient and donor factors, non-myeloablative conditioning resulted in significantly lower overall mortality HR 0.40 (0.3–0.6), improved PFS HR 0.55 (0.4–0.8) and much lower TRM HR 0.22 (0.1–0.4). The risks of acute GVHD grades 2–4 were also significantly lower with non-myeloablative regimens HR 0.41 (0.3–0.6). The risks of relapse or progression and chronic GVHD when adjusted for competing risks of death and patient and donor factors, were not significantly different between ablative and non-ablative conditioning, despite the almost exclusive use of PBSC for the non ablative recipients.
Table 4.
Hazard ratios for outcomes in patients with multiple myeloma receiving transplants from allogeneic donors, comparing patients receiving non-myeloablative conditioning to those receiving myeloablative conditioning
| HR (95% CI) | P | |
|---|---|---|
| Overall mortality | 0.40 (0.3–0.6) | <0.0001 |
| PFS | 0.55 (0.4–0.8) | 0.0002 |
| TRM | 0.22 (0.1–0.4) | <0.0001 |
| Relapse/prog | 1.20 (0.8–1.9) | 0.43 |
| Acute GVHD | 0.41 (0.3–0.6) | <0.0001 |
| Chronic GVHD | 0.86 (0.5–1.4) | 0.51 |
In a separate multivariable analysis, outcomes of only patients undergoing non-ablative allogeneic transplants were considered. (Table 5) The most important predictors of survival, PFS and relapse or progression were progression after a prior autologous transplant and elevated β2 microglobulin. Mortality HR were 2.51 (95% CI 1.4–5.8) and 2.56 (95% CI 1.2–5.6) for patients who had failed a prior autologous transplant or had β2 microglobulin >4.0, respectively. HR for progression or relapse or death were 2.89 (95% CI 1.4–6.1), 2.45 (95% CI 1.5–3.9) and 2.38 (95% CI 1.1–5.1) for relapse after a prior autologous transplant, chemotherapy-resistant disease and an elevated β2 microglobulin, respectively. Planned tandem autologous/allogeneic transplant was associated with a decreased risk of transplant mortality HR 0.16 (95% CI 0.0–0.6). HR for progression or relapse only, were 5.42 (95% CI 2.2–1.4), 3.18 (95% CI 1.8–5.6), 2.92 (1.2–7.1), 0.50 (0.3–0.9) and 0.77 (0.6–1.0) for prior relapsed autograft, chemoresistant disease, elevated β2 microglobulin, female donor and fewer prior chemotherapy regimens, respectively.
Table 5.
Multivariable analysis of outcomes among patients with multiple myeloma receiving transplants from allogeneic donors following non-myeloablative conditioning
| Variable | Relapsed auto | Risk group a | B2M>4.0 | Tandem auto | Female donor | No. before regimens | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95%CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Survival | 2.51 (1.1–5.8) | 0.03 | NS | 2.56 (1.2–5.6) | 0.02 | NS | NS | NS | ||||
| PFS | 2.89 (1.4–6.1) | 0.005 | 2.45 (1.5–3.9) | 0.0002 | 2.39 (1.1–5.1) | 0.03 | NS | NS | ||||
| TRM | NS | NS | NS | 0.16 (0.0–0.6) | 0.004 | NS | ||||||
| Rel/Prog | 5.42 (2.2–14) | 0.0003 | 3.18 (1.8–5.6) | <0.0001 | 2.92 (1.2–7.1) | 0.02 | NS | 0.50 (0.3–0.9) | 0.01 | 0.77 (0.6–1.0) | 0.04 | |
aChemotherapy-responsive or -resistant disease.
In order to discern any association between chronic GVHD and disease progression, we examined this association and its effects on PFS, in a time-dependent fashion among recipients of non-ablative transplants. We found only a weak association between patients with clinical extensive chronic GVHD and reduced rates of progression or relapse HR=0.74 (0.4–1.3), P=0.32. This resulted in no net benefit on PFS HR=0.89 (0.5–1.5), P=0.65.
Discussion
In this retrospective review of allo-SCT for MM going back 34 years, significant improvements were observed in the TRM associated with the introduction of non-myeloablative conditioning. Mortality censored for relapse was 55% among the 144 patients receiving ablative transplants compared with only 18% in the non-myeloablative group. As a result, the survival at 10 years from transplant was significantly superior for non-ablative transplants, 35% compared with 15%. As these two groups were not prospectively studied and were not treated contemporaneously, it is likely that other factors including better anti-infectious prophylaxis and treatment, and the use of PBSCs may have contributed in part to these improvements. Indeed, there were almost no deaths due to viral or fungal pathogens among non-myeloablative recipients; a major cause of mortality among ablative transplant recipients. In addition, there were major differences between the groups in patient age, relapse after prior autologous transplant, and proportion of patients resistant to their last chemotherapy regimen just before transplant. Although the perception is that patients with MM tolerate allografting more poorly than patients with other hematologic malignancies, a recent analysis from the European Group for Blood and Marrow Transplantation suggested that when adjusted for risk factors including age, disease stage, interval from diagnosis to transplant and donor factors, outcomes for patients with MM were similar.24 Additionally, there are now newer drugs available to treat relapse that were not available previously which would certainly affect survival after relapsed disease.
In univariate analysis, non-myeloablative transplants were associated with an apparent greater risk of disease progression or relapse, 55% at 6 years for non-myeloablative compared with 34% for ablative conditioning. When adjusted for competing risks of death due to higher TRM associated with ablative transplants, however, these differences were not statistically significant. Although this result does not appear to agree with the analysis of others such as the EBMT registry data, the study was only a univariate analysis and did not account for competing causes of death, as ours did.25 Nevertheless, the amount of residual disease present at transplant, provides a greater challenge for clearance by the allogeneic donor graft when a non-myeloablative regimen is utilized and is still the primary cause of treatment failure. When comparing the incidences of chronic GVHD, 27% of the ablative recipients developed CGVHD compared with 66% for non-ablative recipients. As the risk of CGVHD is time-dependent, and more non-myeloablative patients survived the early phases of transplant, this did not prove to be significantly higher when adjusted for competing causes of death.
In an attempt to overcome this limitation, many groups have employed a tandem autologous, non-myeloablative allogeneic transplant with the aim of providing major cytoreduction, but an opportunity for the patient to recover from high-dose chemotherapy before the Allo-SCT.14, 16, 26 In multivariable analysis, patients receiving a tandem autologous, non-myeloablative allogeneic transplant had reduced non-relapse mortality, but did not independently affect other outcomes. This analysis also indicated that relapse after a prior autologous transplant is associated with inferior survival as well as other outcome measures. As seen in prior studies, a β-2 microglobulin >4 was also independently associated with increased risk of progression or relapse as well as inferior survival. Female donors were associated with a significantly reduced risk of relapse or progression, consistent with other analyses that have shown more of a graft-vs disease effect from female to male transplants.
These analyses agree with other studies showing prior autograft failure to be one of the major risk factors for disease progression after non-myeloablative allo-SCT.27 The observation that prior autograft failures do poorly with an allo-SCT argues against the recommendation some have made to delay an allo-SCT until disease progression after initial treatment or autologous transplant.7 In some retrospective analyses, a non-myeloablative allograft was able to overcome certain high-risk FISH characteristics such as the 4;14 translocation.28 Our patient population contained too few patients with 4;14 to analyze this separately, however, in the multivariable analysis only high B2 microglobulin and not adverse cytogenetics were associated with inferior outcomes. This does not mean that cytogenetics are not important but merely reflect a limited number of observations in our database to directly address that question.
It is clear that reduced intensity allo-SCT regimens can result in reliable donor engraftment with a relatively low mortality compared with high-dose regimens. The immunologic effect of the allograft is, however, relatively modest requiring a prior autologous transplant for cytoreduction. Even with the tandem auto-non-myeloablative allo-SCT approach, relapses beyond 3–5 year continue to occur, making disease recurrence the primary cause of treatment failure after tandem auto, non-myeloablative allo-SCT.
Future studies of allo-SCT in MM should focus on regimens that are less toxic but able to preserve anti-tumor effects such as radioisotopes linked to antibodies that target myeloma cells or other marrow-based cells. It should be relatively easy to combine targeted radiotherapy with a non-myeloablative regimen to create a more tolerable cytoreductive protocol. It is also worth reconsidering more myeloablative regimens, as supportive care has improved greatly in the past 20 years. As previously noted, when younger patients are transplanted earlier from initial diagnosis, TRM is reduced.
Another strategy to make non-myeloablative regimens more effective would be to combine the donor graft with infusions of allogeneic donor lymphocytes or subsets of lymphocytes in the form of ‘engineered grafts’, for example CD4 lymphocytes, which may have a graft vs myeloma effect without increasing GVHD.29 It may also be possible to exploit killer-Ig-like mismatching between donor and recipient, which has been shown to result in improved PFS due to a reduced rate of relapse.30, 31 Maintenance strategies, which have been shown to delay disease progression after auto-SCT may also be effective after allo-SCT.32, 33 Finally, it may be worthwhile to exploit monoclonal antibodies targeting myeloma cells such as the CD40 Ag or CS-1 Ag, in order to increase the ability of donor allogeneic cells to eliminate residual host disease.34 In any case, due to the substantial morbidity and mortality associated with allografting as well as the uncertain benefits, future approaches to allografting for myeloma should only be performed within well-designed clinical trials.
Acknowledgements
This work was supported by the grants: CA78902, CA 155911, CA 18029 and CA 15704 (Core) from the Jose Carreras Foundation Against Leukemia. We would like to thank Katie Ahlgren for assistance in the preparation of this manuscript.
Competing interests
The authors declare no conflict of interest.
References
- 1.Anderson KC. The role of immunomodulatory drugs in multiple myeloma (Review) Semin Hematol. 2003;40:23–32. doi: 10.1053/j.seminhematol.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 3.Lacy M, Gertz M, Dispenzieri A, Hayman S, Geyer S, Zeldenrust S. Lenalidomide plus dexamethasone (Rev/Dex) in newly diagnosed myeloma: response to therapy, time to progression, and survival. Blood. 2006;108(Part 1):239a. [Google Scholar]
- 4.Attal M, Harousseau J-L, Stoppa A-M, Sotto J-J, Fuzibet J-G, Rossi J-F. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 5.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 6.Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 7.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 8.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. [PubMed] [Google Scholar]
- 9.Corradini P, Voena C, Tarella C, Astolfi M, Ladetto M, Palumbo A. Molecular and clinical remissions in multiple myeloma: role of autologous and allogeneic transplantation of hematopoietic cells. J Clin Oncol. 1999;17:208–215. doi: 10.1200/JCO.1999.17.1.208. [DOI] [PubMed] [Google Scholar]
- 10.Kroger N, Badbaran A, Lioznov M, Schwarz S, Zeschke S, Hildebrand Y. Post-transplant immunotherapy with donor-lymphocyte infusion and novel agents to upgrade partial into complete and molecular remission in allografted patients with multiple myeloma. Exp Hematol. 2009;37:791–798. doi: 10.1016/j.exphem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Bensinger WI, Buckner CD, Anasetti C, Clift R, Storb R, Barnett T. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88:2787–2793. [PubMed] [Google Scholar]
- 12.Gahrton G, Svensson H, Cavo M, Apperley J, Bacigalupo A, Björkstand B. Progress in allogeneic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983–93 and 1994–98 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113:209–216. doi: 10.1046/j.1365-2141.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KC. Who benefits from high-dose therapy for multiple myeloma? (editorial; comment) J Clin Oncol. 1995;13:1291–1296. doi: 10.1200/JCO.1995.13.6.1291. [DOI] [PubMed] [Google Scholar]
- 14.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 15.Einsele H, Schafer HJ, Hebart H, Bader P, Meisner C, Plasswilm L. Follow-up of patients with progressive multiple myeloma undergoing allografts after reduced-intensity conditioning. Br J Haematol. 2003;121:411–418. doi: 10.1046/j.1365-2141.2003.04299.x. [DOI] [PubMed] [Google Scholar]
- 16.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 17.Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–3480. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 18.Rosiñol L, Pérez-Simón JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3593. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 19.Bjorkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011;29:3016–3022. doi: 10.1200/JCO.2010.32.7312. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan A, Pasquini MC, Ewell M, Stadtmauer EA, Alyea EP, III, Antin JH. Tandem autologous hematopoietic stem cell transplants (AuHCT) with or without maintenance therapy (auto-auto) versus single AuHCT followed by HLA matched sibling non- myeloablative allogeneic HCT (auto-allo) for patients with standard risk (SR) multiple myeloma (MM); results from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 trial. Blood. 2010;116:24–25. [Google Scholar]
- 21.Crawley C, Iacobelli S, Björkstrand B, Apperley JF, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood. 2007;109:3588–3594. doi: 10.1182/blood-2006-07-036848. [DOI] [PubMed] [Google Scholar]
- 22.Durie BGM, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::AID-CNCR2820360303>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K. International uniform response criteria for multiple myeloma [Erratum appears in Leukemia. 2007 May;21(5):1134] [Erratum appears in Leukemia. 2006 Dec;20(12):2220] Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 24.Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115:4715–4726. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 25.Gahrton G, Iacobelli S, Bandini G, Bjorkstrand B, Corradini P, Crawley C. Peripheral blood or bone marrow cells in reduced-intensity or myeloablative conditioning allogeneic HLA identical sibling donor transplantation for multiple myeloma. Haematologica. 2007;92:1513–1518. doi: 10.3324/haematol.11353. [DOI] [PubMed] [Google Scholar]
- 26.Kroger N, Schwerdtfeger R, Kiehl M, Sayer HG, Renges H, Zabelina T. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100:755–760. doi: 10.1182/blood-2002-01-0131. [DOI] [PubMed] [Google Scholar]
- 27.Kröger N, Perez-Simon JA, Myint H, Klingemann H, Shimoni A, Nagler A. Relapse to prior autograft and chronic graft-versus-host disease are the strongest prognostic factors for outcome of melphalan/fludarabine-based dose-reduced allogeneic stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2004;10:698–708. doi: 10.1016/j.bbmt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Schilling G, Hansen T, Shimoni A, Zabelina T, Simon-Perez JA, Gutierrez NC. Impact of genetic abnormalities on survival after allogeneic hematopoietic stem cell transplantation in multiple myeloma. Leukemia. 2008;22:1250–1255. doi: 10.1038/leu.2008.88. [DOI] [PubMed] [Google Scholar]
- 29.Klyuchnikov E, Sputtek A, Slesarchuk O, Lioznov M, Stübig T, Bacher U. Purification of CD4(+) T cells for adoptive immunotherapy after allogeneic hematopoeitic stem cell transplantation. Biol Blood Marrow Transplant. 2010;17:374–383. doi: 10.1016/j.bbmt.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Rizzieri DA, Storms R, Chen DF, Long G, Yang Y, Nikcevich DA. Natural killer cell-enriched donor lymphocyte infusions from A 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1107–1114. doi: 10.1016/j.bbmt.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol. 2008;143:641–653. doi: 10.1111/j.1365-2141.2008.07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attal M, Harousseau JL, Marit G, Caillot D, Stoppa AM, Benboubker L. Lenalidomide after autologous transplantation for myeloma: first analysis of a prospective, randomized study of the Intergroupe Francophone Du Myelome (IFM 2005 02) Blood. 2009;114:220–221. [Google Scholar]
- 33.McCarthy PL, Owzar K, Stadtmauer EA, Giralt S, Hurd DD, Hassoun H. Phase III intergroup study of lenalidomide (CC-5013) versus placebo maintenance therapy following single autologous stem cell transplant for multiple myeloma (CALBG 100104): initial reprot of patient accrual and adverse events. Blood. 2009;114:1327. [Google Scholar]
- 34.Jakubowiak AJ, Bensinger W, Siegel D, Zimmerman TM, Van Tornout JM, Zhao C. Phase 1/2 study of elotuzumab in combination with bortezomib in patients with multiple myeloma with one to three prior therapies: interim results. Blood. 2009;114:1491–1492. [Google Scholar]


