Abstract
The insertion of microdialysis probes into the rat striatum disrupts dopaminergic activity near the probe track. The present study suggests that a substantial fraction of DA terminals near the probe track (200 μm) survive the probe implantation itself but that the surviving terminals experience altered presynaptic inhibition. We found that probe implantation did not just alter the amplitude of evoked dopamine responses recorded by voltammetry, but also changed their temporal profile in a fashion similar to that previously observed by quinpirole, an agonist of dopamine D2 autoreceptors. Altered presynaptic inhibition is supported by a hypersensitivity of evoked dopamine responses recorded near to microdialysis probes to raclopride, a D2 antagonist. Further, we found that evoked dopamine release was also hypersensitive to a final dose of the dopamine transporter inhibitor, nomifensine.
Keywords: dopamine, autoinhibition, microdialysis probe, voltammetry, hypersensitivity, injury
INTRODUCTION
The benefits of microdialysis as a method for in vivo neurochemistry are well established. Microdialysis is compatible with a variety of animal models as well as human patients. Sterilized and MRI-compatible probes are available commercially: in a non-clinical setting, probes are easy to make in the laboratory. The dialysate samples are “clean”, so they are compatible with a wide array of analytical techniques, such as chromatography, electrophoresis, mass spectrometry, etc., which has enabled the detection of a vast array of potentially interesting substances in brain dialysates (reviewed by Davies et al., 2000; Zhang and Beyer, 2006; Kennedy, 2007). On-line analysis can produce near real-time results, sometimes with high temporal resolution (e.g. Tucci et al., 1997; Bert et al., 2002). Consequently, microdialysis has made significant contributions to the study of behavior, CNS pathologies, drug discovery, and clinical care (Westerink and Cremers, 2007).
Nevertheless, microdialysis is invasive and the probes cause a penetration injury to brain tissue (Benveniste and Deimer 1987; O'Neill et al., 1991; Clapp-Lilly et al., 1999; Zhou et al., 2001; Bungay et al., 2003; Mitala et al., 2008; Jaquins-Gerstl and Michael, 2009; Jaquins-Gerstl et al., 2011). How the penetration injury affects the outcome of microdialysis experiments remains an open question (Bungay et al., 2003). One reason for this is the limited access to independent measures of the in vivo concentrations of substances of interest, which makes it difficult to validate in vivo results.
In some instances, sensors have been placed in brain tissue next to microdialysis probes for validation purposes (Blaha 1996; Lowry et al., 1998). In the case of dopamine (DA), fast-scan cyclic voltammetry is available as an alternative method for in vivo measurements. Voltammetry is also invasive, as it requires inserting an electrode into brain tissue. But, carbon fiber voltammetric electrodes are tiny in comparison to microdialysis probes. This enables “voltammetry next to a microdialysis probe” as an experimental approach to investigating if and how penetration injury affects in vivo voltammetric DA measurements (Lu et al., 1998; Borland et al., 2005; Yang and Michael, 2007; Mitala et al., 2008).
Direct comparisons of “DA as measured by voltammetry” and “DA as measured by microdialysis” are fraught with difficulties because the two methods operate in such a different manner. A more straightforward approach, and the one pursued here, is to compare identical voltammetric experiments in the presence and absence of a nearby microdialysis probe. Such experiments report on the DA activity in the tissue surrounding the probe track.
Previous “voltammetry next to a microdialysis probe” studies (Lu et al., 1998; Borland et al., 2005; Yang and Michael, 2007; Mitala et al., 2008) have found substantial probe-induced disruption of DA activity: the next questions is, what can be done to prevent this? Recently, we reported that retrodialysis of dexamethasone, a potent anti-inflammatory drug, profoundly decreases gliosis at the probe track (Jaquins-Gerstl et al., 2011). Unfortunately, dexamethasone only modestly affected DA no-net-flux curves measured 1 and 5 days after the probes were implanted. This result indicates that dexamethasone, while highly anti-inflammatory, only partially protected DA terminals.
The modest neuroprotective effect of dexamethasone might be a sign of severe loss of DA terminals near the probe track, possibly so severe as to overwhelm potential neuroprotective strategies. So, the objective of the present study was to investigate whether DA terminals near the probe track survive the penetration injury per se. We placed carbon fiber electrodes 200 μm from microdialysis probes and recorded evoked DA responses 2 hr after probe implantation. The probes decreased the maximum amplitude of evoked DA responses by ca 80%. However, after blockade of DA autoinhibition (with raclopride) and DA reuptake (with nomifensine), the decrease in maximum amplitude was only 50%, which suggests that a change in the presynaptic regulation of DA terminals contributes to the probe-induced loss of response amplitude. Based on the response amplitude, it appears that at least 50% of the DA terminals (200 μm from the probe, 2 hrs after implantation) survive probe implantation, which is useful information.
MATERIALS AND METHODS
Voltammetric electrodes and techniques
A borosilicate glass capillary (0.75 mm I.D., 1 mm O.D., Sutter Instruments, Co., Novato, CA) containing a single carbon fiber (7 μm diameter, Thornell Carbon Fiber, T300 3K, Amoco Performance Products, Inc., Greenville, SC) was pulled to a fine tip with a vertical puller (Narishige, Tokyo, Japan). The tip was sealed with epoxy (Spurr, Polysciences, Inc., Warrington, PA) and the exposed fiber was cut to a length of 400 μm. The fiber was connected to a wire with a small drop of mercury. The microelectrodes were pretreated (Feng et al., 1987) in room temperature, N2-purged artificial cerebrospinal fluid (aCSF: 145 mM Na+, 1.2 mM Ca2+, 2.7 mM K+, 1.0 mM Mg2+, 152 mM Cl- and 2.0 mM phosphate, pH 7.4) with a triangular potential waveform (0-2 V vs. Ag/AgCl at 200V/s for 1 s).
Fast scan cyclic voltammetry (FSCV) (Baur et al., 1988) was performed with an EI400 potentiostat (Ensman Instrumentation, Bloomington, IN) and software developed in-house. The rest potential was 0 V vs. Ag/AgCl: the FSCV waveform was a linear sweep to 1 V, then to –0.5 V, and back to 0 V vs. Ag/AgCl at 300 V/s. Scans were performed at 7.5 Hz to maintain synchrony with the stimulus waveform (45 Hz, see below). Dopamine oxidation current was monitored between 0.5 and 0.7 V on the first sweep and converted to DA concentration by calibration after the in vivo experiments (post-calibration).
Microdialysis probes
Concentric-style microdialysis probes with an outer diameter of 220 μm and an active length of 4 mm were constructed with hollow fiber membrane (6000 Da MWCO, Specta/Por RC, Spectrum, Rancho Dominguez). The inlet tubing was PE-20 (0.38 mm I.D., 1.09 mm O.D., ~ 40 cm long, Becton Dickinson, Franklin Lakes, NJ) and the outlet tubing was fused silica (70 μm I.D., 145 μm O.D. and ~ 20 cm long, Polymicro Technologies, Phoenix). The inlet tubing was connected to a 250 μL gas-tight syringe (Hamilton) filled with aCSF driven by a microliter syringe pump (Harvard, Holliston, MA) at a perfusion rate of 0.586 μL/min. This particular perfusion rate, which corresponds to one of the gear stops on our syringe pump, is that used in several prior studies from our lab (Lu et al., 1998; Borland et al., 2005; Yang and Michael 2007) and the Justice lab (Bungay et al 2003). This perfusion rate, which is relatively slow, is expected to minimize disturbance of the tissue via the extraction of substances from the tissue by the probe. Although the probe was perfused continuously throughout these experiments, microdialysate samples were not collected or analyzed because we have previously demonstrated that electrically evoked DA release, as studied here, minimally affects the dialysate DA concentration (Qian et al, 1999).
Animals and surgical procedures
All procedures involving animals were carried out with the approval of the Institutional Animal Care and Use Committee of the University of Pittsburgh. Male Sprague-Dawley rats (Hilltop, Scottdale, PA) (250-375 g) were anesthetized with chloral hydrate (400 mg/kg i.p.) and were kept unconscious with additional doses of chloral hydrate (~50 mg/kg i.p.). While anesthetized, rats were wrapped in a homoeothermic blanket (EKEG Electronics, Vancouver, BC) with their body temperature monitored and maintained at 37°C. The rats were placed in a stereotaxic frame (David Kopf, Tujunga, CA) with the incisor bar 5 mm above the interaural line (Pellegrino et al., 1979). The scalp was shaved and cleaned before a midline incision exposed the skull. Holes were drilled through the skull and the dura was dissected to allow for placements of devices with minimal disruption to any surrounding blood vessels.
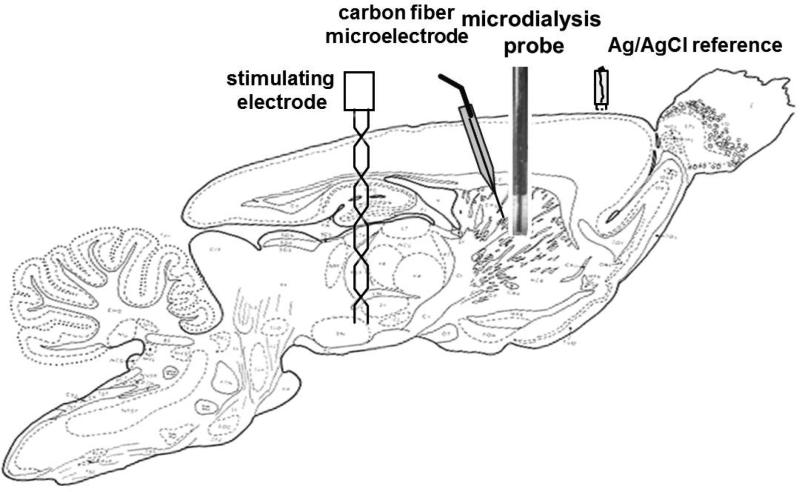
Fig 1 provides a schematic to show how the devices involved in these experiments were arranged in the rat brain. Electrical contact between the brain tissue and a reference electrode (Ag/AgCl) was established by means of a salt bridge fashioned from a plastic pipet tip plugged with tissue paper and filled with aCSF. A bipolar stimulating electrode (MS303/a; Plastics One, Roanoke, VA, USA) was lowered toward the medial forebrain bundle (MFB, 2.2 mm posterior from bregma, 1.6 mm lateral from midline and 7.5 mm below dura) and a voltammetric electrode was lowered into the ipsilateral striatum (2.5 mm anterior from bregma, 2.5 mm lateral from midline, 4.5 mm below dura). The microelectrode was angled 10° from the coronal plane. The stimulating electrode was lowered in small increments until the stimulus waveform (optically isolated, biphasic, constant-current square wave with a pulse duration of 2 ms, pulse height of 280 μA, and frequency of 45 Hz) evoked a DA response in the striatum. Once a stable and robust evoked response of at least 30 nA was established, neither the stimulating nor the recording electrodes were moved for the remainder of the experiment.
Figure 1.
A sagittal section of the rat brain showing schematically the approximate positions of the stimulating electrode, the carbon fiber microelectrode, the Ag/AgCl reference electrode, and the microdialysis probe (sagittal slice from Paxinos and Watson (2005) rotated showing tooth bar was 5 mm above the interaural line as described in Methods).
In experiments involving voltammetry near a microdialysis probe, the electrode and probe were pre-aligned on the stereotax before either was lowered into the striatum to establish a 200-μm gap between them. The probe and the electrode were in the same sagittal plane, with the electrode posterior with respect to the probe. The probes were lowered slowly, taking approximately 30 min for the probe tip to reach a depth of 7 mm below dura.
Experimental design
All electrical stimuli were delivered to the MFB with a frequency of 45 Hz and duration of 10 s (other parameters as listed above). Two groups of rats (n=4 each) were assigned randomly to experiments with and without a microdialysis probe. In experiments without a microdialysis probe, an initial pre-drug evoked response was recorded, raclopride (2 mg/kg, i.p.) was administered 5 min later, and a post-raclopride was response was recorded 15 min after that. Nomifensine (20 mg/kg, i.p.) was administered 40 min after raclopride and a final, post-nomifensine response was recorded 15 min later. Please note, for clarity the final response will be called the post-nomifensine response, but the animal received raclopride prior to nomifensine. In experiments with a microdialysis probe, an initial pre-probe response was recorded. Then, the microdialysis probe was inserted and, after a 2 hr post-implantation wait period, experiments were performed in identical fashion to those just described without a microdialysis probe.
Statistics
Two-way repeated measures ANOVA was performed on the evoked DA responses in Figure 4. Three-way repeated measures ANOVA was performed on the normalized evoked DA responses in Figure 5.
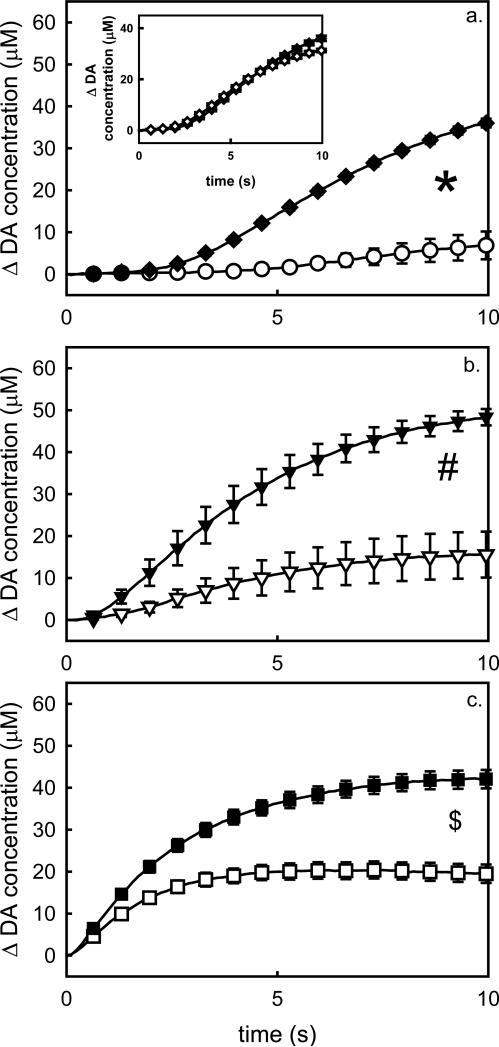
Figure 4.
Comparison of absolute evoked DA responses recorded in the rat striatum in the absence (solid symbols) and presence (open symbols) of microdialysis probes a) before drug administration, b) after raclopride, and c) after a final dose of nomifensine. The inset in panel a compares the initial pre-drug/pre-probe responses from the two groups, which are not statistically different. *, p<0.00004; #, p=0.005; $, p<0.05.
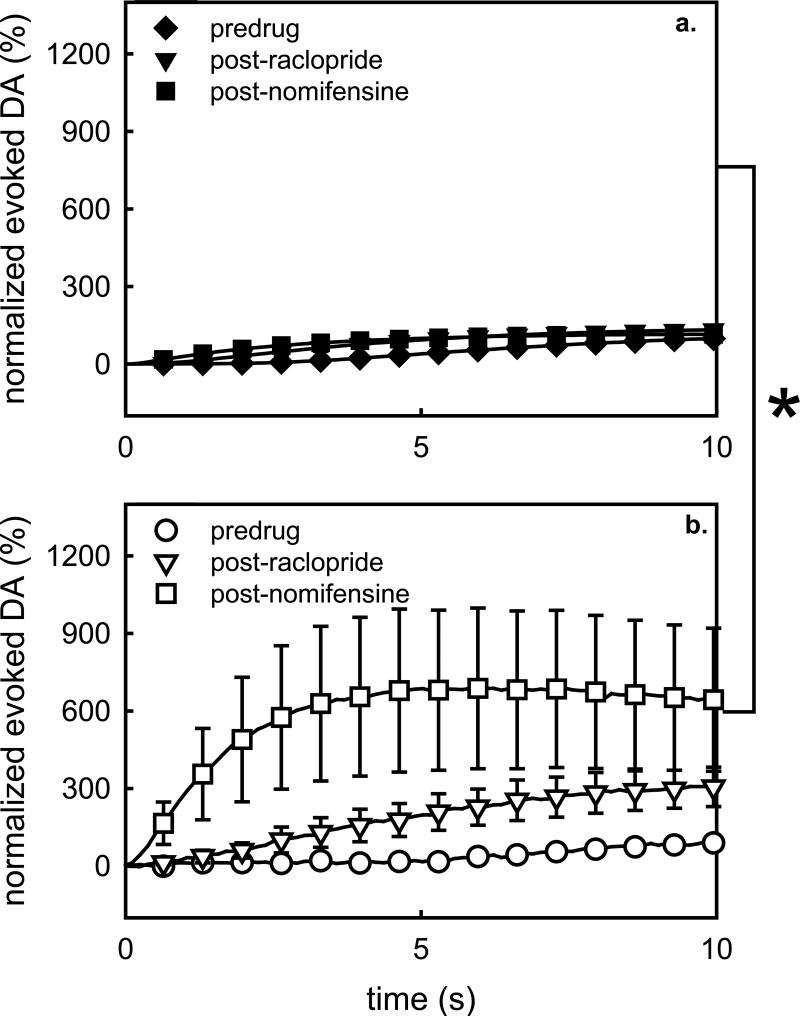
Figure 5.
Normalized evoked responses recorded in the a) absence and b) presence of microdialysis probes. The responses were normalized with respect to the maximum DA concentration observed during the initial (pre-drug and pre-probe) stimulus. *, p<0.05.
Drugs and solutions
Chloral hydrate, raclopride tartrate, and nomifensine were used as received from Sigma (St. Louis, MO, USA). Chloral hydrate was dissolved to 0.2 g/mL in phosphate buffered saline (PBS: 155 mM NaCl, 100 mM phosphate, pH 7.4). Raclopride and nomifensine were dissolved in 1 mL of PBS. All drugs were administered by intraperitoneal injections (i.p.). All solutions were prepared with ultrapure water (NANOPure; Barnstead, Dubuque, IA, USA).
RESULTS
Voltammetry in the striatum: no microdialysis probe
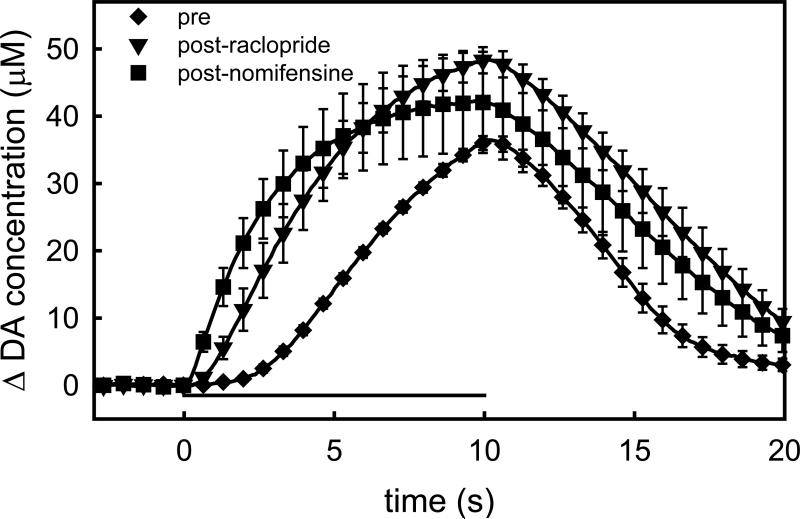
In this study, the placement of the electrodes was not optimized, as is often done to locate ‘hot spots’ of evoked DA release (Kawagoe et al., 1992; Robinson et al., 2003; Venton et al., 2003). Without optimization, evoked responses usually exhibit the slow-type characteristics evident in Fig 2 (diamonds) and discussed in detail by Moquin and Michael (2009), Wang et al (2010), and Moquin and Michael (2011). The response begins slowly but becomes faster (i.e. exhibits short-term facilitation) as the stimulus continues. After the stimulus ends the DA signal returns towards the baseline with no noticeable delay. The initial segment of the descending phase of the response is approximately linear, a sign of zeroth-order clearance associated with saturation of the DAT (Moquin and Michael, 2011). The prolonged delay in the onset of evoked DA release when the stimulus starts is due to autoinhibition of DA terminals at the recording site (Moquin and Michael, 2009; Wang et al., 2010). Consistent with our recent reports (Moquin and Michael, 2009; Wang et al., 2010), administration of raclopride, a D2R antagonist, abolishes both the initial delay in evoked release and the short-term facilitation (Fig 2, triangles). The final dose of nomifensine had a relatively minor further impact on the evoked response (Fig 2, squares).
Figure 2.
Voltammetric recordings of evoked DA release in the rat striatum in the absence of microdialysis probes. Each trace is the mean ± SEM (n=4) of the individual responses recorded pre-drug (diamonds), after raclopride (triangles), and after nomifensine (squares). The horizontal bar shows the timing of the 10-s stimulus. Note the delay in the onset of the pre-drug response: this delay is a hallmark of the slow responses in the striatum (see text).
Voltammetry near a microdialysis probe in the striatum
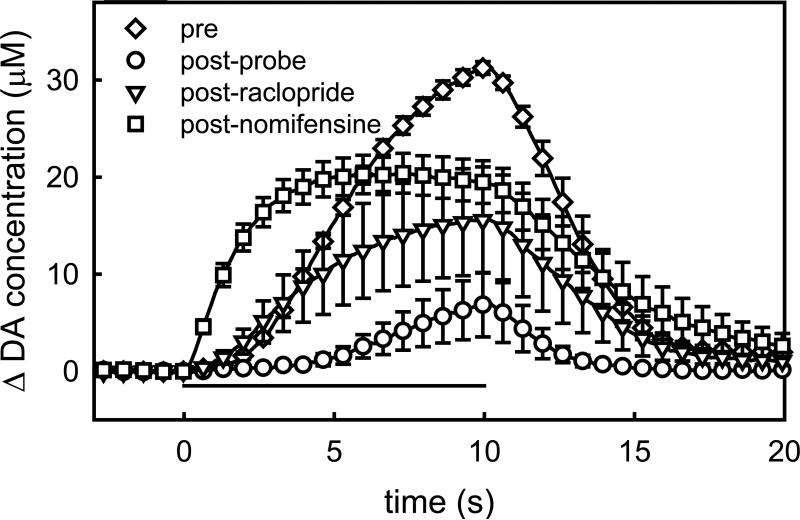
In the second group of rats, the pre-probe response was again slow-type (Fig 3, diamonds). After the pre-probe stimulus, the probe was lowered to a location 200 μm from the electrode: however, the precise spacing was not confirmed by histology because the microelectrodes tracks are too small to visualize by light microscopy (Peters et al., 2004). The next stimulus response, recorded 2 hrs after implanting the probe (post-probe), was substantially reduced in amplitude (Fig 3, circles). The post-probe response also had a different temporal profile: there was more delay in the onset of DA release after the stimulus began. This is very similar to the effect of quinpirole, a D2R agonist (Moquin and Michael, 2009; Wang et al., 2010), and raises the possibility that some of the impact of probe implantation on the evoked response is associated with an increased autoinhibitory tone on DA terminals after probe implantation. Consistent with this idea, both the effect of raclopride (Fig 3, triangles) and the final dose of nomifensine (Fig 3, squares) were substantially increased in presence of the probe (statistics are discussed in the following sections).
Figure 3.
The impact of a nearby microdialysis probe on evoked DA responses in the rat striatum. These traces are the mean ± SEM, (n=4) of responses recorded before (diamonds) and after probe implantation (circles). After probe implantation, additional responses were recorded after the administration of raclopride (triangles) and nomifensine (squares).
Absolute effects of drugs on evoked DA responses
Fig 4 compares the evoked responses recorded with (open symbols) and without (solid symbols) the probe under the three conditions studied here: pre-drug (Fig 4a), post-raclopride (Fig 4b), and post-nomifensine (Fig 4c, recall that nomifensine was administered after raclopride). The initial evoked responses in each group (pre-drug and pre-probe) are compared in the inset in Fig 4a. Each data set in Fig 4 was subjected to 2-way ANOVA with repeated measures: the two factors were group (probe, no-probe) and time (the repeated measure). There is no significant difference between the initial responses (Fig 4a inset, F(1,6)=0.305, p=0.6). Here, the group factor is of primary interest: in each other case, the group factor was significant (Fig 4a F(1,6)=119.016, p<0.00004; Fig 4b F(1,6)=18.542, p=0.005; Fig 4c F(1,6)=6.592, p<0.05). Thus, the nearby probe significantly affected the responses under all three conditions examined here.
Proportional effects of drugs evoked DA responses
In vivo results are often reported after being normalized with respect to the control, or baseline, condition. Fig 5 shows the results of Figs 2 and 3 after normalizing the responses with respect to the maximum amplitude of the pre-drug response in each group. In this normalized format, the probe substantially increased the effects of raclopride and nomifensine (after raclopride) on the evoked responses. The data in Fig 5 were subjected to a 3-way ANOVA with repeated measures: the factors were the group (probe, no probe), the drug treatment (pre-drug, after raclopride, and after nomifensine), and time (the repeated measure). The group effect is of prime interest here (F(1,18)=4.657, p<0.05). Thus, the probe caused a significant hypersensitivity to these pharmacological manipulations.
DISCUSSION
The implantation of microdialysis probes into brain tissue causes injury and triggers a tissue response (Benveniste and Diemer 1987; O'Neill et al., 1991; Clapp-Lilly et al., 1999; Zhou et al., 2001; Mitala et al., 2008; Jaquins-Gerstl and Michael, 2009). This issue is not unique to microdialysis probes, as the subject of the tissue response to brain implants has become a central issue in the brain machine interface field (Szarowski et al., 2003; Winslow and Tresco, 2010). A tissue response is also an issue in subcutaneous microdialysis (Mou et al., 2011), although subcutaneous immune processes are very different from those in brain. So, it is important to understand if and how the tissue response affects the function and properties of neurochemical systems. In the case of DA, voltammetry offers an alternative for in vivo measurements, which provides a useful approach to investigating this issue. A concern also exists over the penetration injury caused by microelectrodes, but published evidence suggests that microelectrodes are substantially less injurious than larger devices, such as microdialysis probes and neuroprosthetic devices (Peters et al., 2004; Jaquins-Gerstl and Michael, 2009). For example, in a recent immunocytochemical study we were unable to visualize astrocytic activation by voltammetric electrodes, whereas astrocytes were clearly activated by microdialysis probes (Jaquins-Gerstl and Michael, 2009). This contrast justifies the experimental approach of ‘voltammetry near a microdialysis probe’, since any injury associated with the microelectrode does not appear to add substantially to that associated with the probe.
Prior studies with voltammetry near a microdialysis probe show that the probes significantly alter electrically evoked DA responses (Lu et al., 1998; Borland et al., 2005; Yang and Michael, 2007). The general interpretation of this is that implanting the probe has a traumatic effect on DA release in the tissue layer surrounding the probe. This has a major effect on the microdialysis recovery of DA, apparently because DA uptake is less affected by the trauma than DA release (Bungay et al., 2003; Borland et al., 2005). Consequently, DA appears likely to be taken up from the extracellular space before reaching the microdialysis probe. Exactly how trauma separately alters DA release and uptake is not exactly known. DA terminals are the only known element within the striatum to have the capacity to synthesize DA and store it in synaptic vesicles. DA terminals are also the only element that expresses the DAT. So, at first glance trauma might be expected to similarly affect release and clearance. But, studies show that the serotonin transporter (Schmidt and Lovenberg, 1985) and the organic cation transporter (Eisenhofer, 2001) can also remove DA from the extracellular space. So, the possibility exists that DA release and clearance involve different cellular components. Because the interplay between release and uptake are unique to different substances, the impact of trauma on microdialysis recovery appears to be substance-specific. In the case of glucose (Lowry et al., 1998), for example, microdialysis and implanted sensors report similar glucose concentrations.
In the present study, implanting a microdialysis probe approximately 200 μm from the voltammetric electrode (Fig 1) significantly reduced the amplitude of a stimulus response recorded 2 hrs later (Fig 3). However, the probe also altered the temporal profile of the response, specifically by increasing the delay in the onset of evoked release at the beginning of the stimulus. This effect is very similar to that caused by quinpirole (Moquin and Michael, 2009; Wang et al., 2010), which raises the possibility that some of the loss in amplitude could be due to an alteration in presynaptic regulation of the DA terminals, in this case by an increase in autoinhibition. An alternative explanation for the delayed onset of the response could be diffusion across a gap between the electrode and nearby DA terminals. But, as we have pointed out before, such a diffusion gap is expected to also delay DA clearance (Moquin and Michael, 2009), assuming that DA molecules must diffuse away from the electrode in order to reach the DATs responsible for reuptake. However, there is no obvious delay in DA clearance in the post-probe response (Fig 3), which suggests that DATs, and thereby surviving DA terminals, are present in the vicinity of the electrode.
The pharmacological manipulations reported herein were undertaken to investigate the possibility that some of the probe-induced loss in evoked response amplitude might be due to altered presynaptic regulation, i.e. not simply a loss of DA terminals. Fig 4 shows clearly that after each drug evoked DA responses recorded near a microdialysis probe are reduced in amplitude compared to those recorded without a probe. These absolute comparisons are justified on the grounds of the absence of any statistical difference between the initial responses in the two groups of animals (Fig 4a), consistent with our prior experience that the slow evoked responses are highly reproducible between subjects (Moquin and Michael, 2009; Wang et al., 2010). Even though the responses recorded near the probe (Fig 4 open symbols) are of lower amplitude than those recorded without a probe (Fig 4 closed symbols), it is also the case that both drug treatments (first raclopride and then nomifensine) decreased the difference between the responses. In the pre-drug condition, the probe decreased the maximum amplitude of the response by 80% (Fig 4a) but the decrease was reduced to 50% after the final dose of nomifensine (Fig 4c).
The study of Bungay et al. (2003) provides some precedent for this finding of a partial DA lesion associated with microdialysis probes. Their analysis of DA uptake near probes found only a partial loss, of similar magnitude to that we report here.
This is an encouraging result because it suggests that at least part of the probe-induced loss in amplitude of the evoked responses is not due to an outright loss of terminals, but rather that the activity of the surviving terminals is altered. Autoinhibition and uptake are well known to limit the amplitude of evoked responses, especially in the case of slow domains. So, these were the presynaptic mechanisms selected for study here.
At first glance, the decrease in reduction from 80% (pre-drug) to 50% (post-nomifensine) might not seem all that impressive. However, there is another way to view this effect. As mentioned in the Introduction, direct comparisons of ‘DA as measured by microdialysis’ with ‘DA as measured by voltammetry’ are difficult because the two procedures are so different. Nevertheless, ‘back of the envelope’ comparisons are reasonable. The commonly reported basal DA concentration in striatal dialysate is 5 nM (e.g. Westerink and De Vries, 1988; Smith and Justice, 1994). Although the results are somewhat controversial, our voltammetry-based estimate of the basal level is ~1 μM (Kulagina et al., 2001; Borland and Michael, 2004; Mitala et al., 2008). Thus, there is a 200-fold difference between these measures of basal DA. In contrast, however, there is only a 2-fold difference in the DA measures obtained by voltammetric recording 200 μm from the probe, 2 hrs after probe insertion, after the combined blockade of autoinhibition and reuptake. This amounts to a 100-fold reduction in the disparity between these two comparisons of measured DA, which is quite interesting.
Our overall interpretation of these comparisons is that the DA terminal lesion caused by the probe penetration injury is sensitive to both time and position relative to the probe. Previous studies, for example, showed a complete loss of the evoked responses if the voltammetric electrode was placed on the probe's outer surface or if the wait time after implantation was extended to 16 hrs (Yang and Michael, 2007). This evidence of a progression of the DA lesion after the implant is consistent with the increased astrogliosis of the probe track at 24 hrs vs. 4 hrs after implantation (Jaquins-Gerstl and Michael, 2009). But, near the probe track (200 μm), shortly after implantation (2 hrs), a substantial (50%) complement of DAergic activity is present. This justifies a continued search for neuroprotective strategies to offset the progression of the probe-induced DA lesion.
The findings of this study provide additional insight into the matter of how the penetration injury affects the outcome of microdialysis measurements. A question that arises from the suggestion that penetration injury disrupts DA terminals next to the probe is why, in that case, is the dialysate DA concentration responsive to so many pharmacological manipulations (e.g. Hurd and Ungerstedt, 1989; Butcher et al., 1991; Vidal et al., 2005)? Our results show that the probe causes a hypersensitivity to the drugs studied here. This hypersensitivity is especially obvious when the results are normalized with respect to the maximum amplitude of the pre-drug response (Fig 5). In this normalized format, the impact of the drugs on DA as measured by voltammetry is barely perceptible. The impact of the probe on the pharmacological sensitivity of DA in the vicinity of the probe track is not widely recognized. We previously demonstrated this phenomenon in experiments involving only nomifensine (Borland et al., 2005) and the present study demonstrates it for the case of raclopride and nomifensine-after-raclopride.
CONCLUSION
This study lends additional evidence in support of the idea that implantation of a microdialysis probe into the rat striatum triggers a tissue response that, among other things, alters DAergic function at the probe's implantation site. A typical evoked response measured by voltammetry during a 10-s, 45-Hz stimulus decreases in amplitude upon probe implantation at a site 200 μm away. The extent of this signal loss is surprising, however, in light of an ultrastructural analysis (Clapp-Lilly et al., 1999) that found only a partial loss of neuronal terminals at similar distances from the probe. The new insight provided here is that the probe-induced disruption is partially caused by terminal loss and partially caused by an altered regulation of the surviving terminals. The identification of surviving terminals so close to the probe raises the hope of possibly being able to develop neuroprotective strategies against the progressive injury that seems to follow the implantation, despite our recent experience with dexamethasone (Jaquins-Gerstl et al., 2011).
Highlights.
Voltammetry near microdialysis probes quantifies the impact of probe implantation on striatal dopamine activity
Probe implantation causes a 90% loss of evoked DA at microelectrodes near the probe
DA near the probe is hypersensitive to raclopride and nomifensine
The loss of DA function near the probe is due to terminal loss and suppression of surviving terminals
ACKNOWLEDGEMENT
This research was supported by the National Institutes of Health (grant no. MH 075989).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baur JE, Kristensen EW, May LJ, Wiedemann DJ, Wightman RM. Fast-Scan Voltammetry of Biogenic-Amines. Anal Chem. 1988;60:1268–72. doi: 10.1021/ac00164a006. [DOI] [PubMed] [Google Scholar]
- Bert L, Parrot S, Robert F, Desvignes C, Denoroy L, Suaud-Chagny MF, Renaud B. In vivo temporal sequence of rat striatal glutamate, aspartate and dopamine efflux during apomorphine, nomifensine, NMDA and PDC in situ administration. Neuropharmacology. 2002;43:825–35. doi: 10.1016/s0028-3908(02)00170-3. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Diemer NH. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol. (Berl.) 1987;74:234–238. doi: 10.1007/BF00688186. [DOI] [PubMed] [Google Scholar]
- Blaha CD. Evaluation of stearate-graphite paste electrodes for chronic measurements of extracellular dopamine concentrations in the mammalian brain. Pharmacol. Biochem. Behav. 1996;55:351–364. doi: 10.1016/s0091-3057(96)00104-9. [DOI] [PubMed] [Google Scholar]
- Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91:220–9. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods. 2005;146:149–58. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J. Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SP, Liptrot J, Aburthnott GW. Characterisation of methylphenidate and nomifensine induced dopamine release in rat striatum using in vivo brain microdialysis. Neurosci Lett. 1991;122:245–8. doi: 10.1016/0304-3940(91)90869-u. [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999;90:129–42. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- Davies MI, Cooper JD, Desmond SS, Lunte CE, Lunte SM. Analytical considerations for microdialysis sampling. Advanced drug delivery reviews. 2000;45:169–88. doi: 10.1016/s0169-409x(00)00114-9. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol Ther. 2001;91:35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Lovenberg W. In vitro demonstration of dopamine uptake by neostriatal serotonergic neurons of the rat. Neurosci Lett. 1985;59:9–14. doi: 10.1016/0304-3940(85)90207-1. [DOI] [PubMed] [Google Scholar]
- Feng JX, Brazell M, Renner K, Kasser R, Adams RN. Electrochemical pretreatment of carbon fibers for in vivo electrochemistry: effects on sensitivity and response time. Anal Chem. 1987;59:1863–7. doi: 10.1021/ac00141a028. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Ungerstedt U. Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse. 1989;3:48–54. doi: 10.1002/syn.890030107. [DOI] [PubMed] [Google Scholar]
- Jaquins-Gerstl A, Michael AC. Comparison of the brain penetration injury associated with microdialysis and voltammetry. J Neurosci Methods. 2009;183:127–35. doi: 10.1016/j.jneumeth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquins-Gerstl A, Shu Z, Zhang J, Liu Y, Weber SG, Michael AC. Effect of Dexamethasone on Gliosis, Ischemia, and Dopamine Extraction during Microdialysis Sampling in Brain Tissue. Anal Chem. 2011;83:7662–7. doi: 10.1021/ac200782h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM. Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience. 1992;51:55–64. doi: 10.1016/0306-4522(92)90470-m. [DOI] [PubMed] [Google Scholar]
- Kennedy RT. In Vivo Peptidomics: Discovery and Monitoring of Neuropeptides Using Microdialysis and Liquid Chromatography with Mass Spectrometry. In: Westerink BHC, Cremers TIFH, editors. Handbook of microdialysis: methods, applications and perspectives. Elsevier Academic Press; 2007. [Google Scholar]
- Kulagina NV, Zigmond MJ, Michael AC. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 2001;102:121–8. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Lowry JP, O'Neill RD, Boutelle MG, Fillenz M. Continuous monitoring of extracellular glucose concentrations in the striatum of freely moving rats with an implanted biosensor. J. Neurochem. 1998;70:391–396. doi: 10.1046/j.1471-4159.1998.70010391.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Peters JL, Michael AC. Direct comparison of the response of voltammetry and microdialysis to electrically evoked release of striatal dopamine. J Neurochem. 1998;70:584–93. doi: 10.1046/j.1471-4159.1998.70020584.x. [DOI] [PubMed] [Google Scholar]
- Mitala CM, Wang Y, Borland LM, Jung M, Shand S, Watkins S, Weber SG, Michael AC. Impact of microdialysis probes on vasculature and dopamine in the rat striatum: a combined fluorescence and voltammetric study. J Neurosci Methods. 2008;174:177–85. doi: 10.1016/j.jneumeth.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. Tonic autoinhibition contributes to the heterogeneity of evoked dopamine release in the rat striatum. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. An inverse correlation between the apparent rate of dopamine clearance and tonic autoinhibition in subdomains of the rat striatum: a possible role of transporter-mediated dopamine efflux. J Neurochem. 2011;117:133–42. doi: 10.1111/j.1471-4159.2011.07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou X, Lennartz MR, Loegering DJ, Stenken JA. Modulation of the foreign body reaction for implants in the subcutaneous space: microdialysis probes as localized drug delivery/sampling devices. Journal of diabetes science and technology. 2011;5:619–31. doi: 10.1177/193229681100500316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill RD, Gonalezmora JL, Boutelle MG, Ormonde DE, Lowry JP, Duff A, Fumero B, Fillenz M, Mas M. Anomalously high concentrations of brain extracellular uric acid detected with chronically implanted probes: implications for in vivo sampling techniques. J. Neurochem. 1991;57:22–29. doi: 10.1111/j.1471-4159.1991.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Elsevier Academic Press; Amsterdam ; Boston: 2005. [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Plenum Press; New York: 1979. [Google Scholar]
- Peters JL, Miner LH, Michael AC, Sesack SR. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. J Neurosci Methods. 2004;137:9–23. doi: 10.1016/j.jneumeth.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Qian F, Wu Y, Yang H, Michael AC. An integrated decoupler for capillary electrophoresis with electrochemical detection: application to analysis of brain microdialysate. Anal. Chem. 1999;71:4486–4492. doi: 10.1021/ac990338f. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–73. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Lovenberg W. In vitro demonstration of dopamine uptake by neostriatal serotonergic neurons of the rat. Neurosci Lett. 1985;59:9–14. doi: 10.1016/0304-3940(85)90207-1. [DOI] [PubMed] [Google Scholar]
- Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J Neurosci Methods. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- Tucci S, Rada P, Sepulveda MJ, Hernandez L. Glutamate measured by 6-s resolution brain microdialysis: capillary electrophoretic and laser-induced fluorescence detection application. J Chromatogr B Biomed Sci Appl. 1997;694:343–9. doi: 10.1016/s0378-4347(96)00488-4. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem. 2003;87:1284–95. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Vidal L, Alfonso M, Campos F, Faro LR, Cervantes RC, Duran R. Effects of manganese on extracellular levels of dopamine in rat striatum: an analysis in vivo by brain microdialysis. Neurochem Res. 2005;30:1147–54. doi: 10.1007/s11064-005-7775-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Moquin KF, Michael AC. Evidence for coupling between steady-state and dynamic extracellular dopamine concentrations in the rat striatum. J Neurochem. 2010;114:150–9. doi: 10.1111/j.1471-4159.2010.06740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH, De Vries JB. Characterization of in vivo dopamine release as determined by brain microdialysis after acute and subchronic implantations: methodological aspects. J Neurochem. 1988;51:683–7. doi: 10.1111/j.1471-4159.1988.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Cremers TIFH. Handbook of microdialysis : methods, applications and perspectives. 1st ed. Elsevier Academic Press; Amsterdam ; Boston: 2007. [Google Scholar]
- Winslow BD, Tresco PA. Quantitative analysis of the tissue response to chronically implanted microwire electrodes in rat cortex. Biomaterials. 2010;31:1558–67. doi: 10.1016/j.biomaterials.2009.11.049. [DOI] [PubMed] [Google Scholar]
- Yang H, Michael AC. In Vivo Fast-Scan Cyclic Voltammetry of Dopamine near Microdialysis Probes. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. CRC Press/Taylor & Francis; 2007. [PubMed] [Google Scholar]
- Zhang MY, Beyer CE. Measurement of neurotransmitters from extracellular fluid in brain by in vivo microdialysis and chromatography-mass spectrometry. J Pharm Biomed Anal. 2006;40:492–9. doi: 10.1016/j.jpba.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL. Hibernation, a model of neuroprotection. Am J Pathol. 2001;158:2145–51. doi: 10.1016/S0002-9440(10)64686-X. [DOI] [PMC free article] [PubMed] [Google Scholar]