Abstract
Calcifications represent one component of pathology in many brain diseases. With MRI, they are most often detected by exploiting negative contrast in magnitude images. Calcifications are more diamagnetic than tissue, leading to a magnetic field disturbance that can be seen in phase MR images. Most phase imaging studies use gradient recalled echo based pulse sequences. Here, the phase component of SWIFT, a virtually zero acquisition delay sequence, was used to detect calcifications ex vivo and in vivo in rat models of status epilepticus and traumatic brain injury. Calcifications were detected in phase and imaginary SWIFT images based on their dipole like magnetic field disturbances. In magnitude SWIFT images, calcifications were distinguished as hypointense and hyperintense. Hypointense calcifications showed large crystallized granules with few surrounding inflammatory cells, while hyperintense calcifications contained small granules with the presence of more inflammatory cells. The size of the calcifications in SWIFT magnitude images correlated with that in Alizarin stained histological sections. Our data indicate that SWIFT is likely to better preserve signal in the proximity of a calcification or other field perturber in comparison to gradient echo due to its short acquisition delay and broad excitation bandwidth. Furthermore, a quantitative description for the phase contrast near dipole magnetic field inhomogeneities for the SWIFT pulse sequence is given. In vivo detection of calcifications provides a tool to probe the progression of pathology in neurodegenerative diseases. In particular, it appears to provide a surrogate marker for inflammatory cells around the calcifications after brain injury.
Keywords: SWIFT, phase contrast, brain calcification, ultra short echo-time
1 Introduction
Calcifications are found most commonly in metabolic disorders such as atherosclerotic plaques in arteries (Demer et al. 1994, Wallin et al. 2001), but there are also calcifications in brain tissue. In human brain, calcifications have been found to play a role in several pathological conditions causing damage in brain, including infections (Caldemeyer et al. 1997), tumors (Okuchi et al. 1992), epilepsy (Arnold and Kreel. 1991), hypoxic brain tissue (Ansari et al. 1990, Rodríguez et al. 2001), infarction (Parisi et al. 1988) and head trauma (Cervós-Navarro and Lafuente. 1991). Accumulation of calcium phosphate precipitates has also been replicated in many of the animal models of these diseases such as cerebral ischemia (Mäkinen et al. 2007, Shirotani et al. 1994, Watanabe et al. 1998), traumatic brain injury (TBI) (Osteen et al. 2001) and in pilocarpine and kainic acid models of epilepsy (Gayoso et al. 2003, Lafreniere et al. 1992). Though the exact role of the calcifications in brain diseases remains unclear, the most common hypothesis is that calcifications serve as a protective buffer against excess amounts of free calcium ions in the tissue (Mäkinen et al. 2007).
Formation of calcifications has been shown as early as three days after ischemia (Shirotani et al. 1994, Watanabe et al. 1998) and five days after systemic kainic acid injection in an epilepsy model (Gayoso et al. 2003). Calcifications have also been shown to grow over time in the kainic acid model of epilepsy (Gayoso et al. 2003). Therefore, they are more commonly detected in the chronic phase of the disease, that is, several months after TBI (Pierce et al. 1998) or brain ischemic insult (Mäkinen et al. 2007). Hence, if the size of calcifications can be measured non-invasively with an imaging technique, such measurement may provide a surrogate marker for disease progression and treatment effects.
Currently, computerized tomography is considered to be the golden standard for calcification detection in vivo (Avrahami et al. 1994, Wu et al. 2009). However, more recently there has been an increasing interest in using MRI for imaging of calcifications as it offers obvious advantages, namely the absence of ionizing radiation and superior contrast in the surrounding soft tissue of the calcification (Wu et al. 2009).
Calcifications are diamagnetic and usually induce negative contrast in MRI magnitude images through the locally altered magnetic field and intravoxel dephasing. Negative contrast, although easily visible, can be nonspecific in the sense that the exact shape of the calcification is left unseen and it does not differentiate between diamagnetic and paramagnetic lesions.
Phase imaging and its variant susceptibility weighted imaging (SWI) (Haacke et al. 2004) using gradient recalled echo (GRE) sequences have become relatively common approaches to detect local magnetic field variations caused by materials with different magnetic susceptibilities in high magnetic fields. GRE phase contrast and SWI have also been used for calcification detection in human brain (Gupta et al. 2001, Schweser et al. 2010, Wu et al. 2009, Zhu et al. 2008). To date, no studies have been published using GRE phase contrast or SWI to detect calcifications in animals, although one study reported promising results using a standard fast spin-echo MRI methodology (Sandner et al. 2010). A potential drawback of SWI is the need for extensive image post-processing, which typically involves phase unwrapping and correcting for magnetic field (B0) distortion to obtain the phase map (Rauscher et al. 2003), and applying a phase mask to the magnitude image to obtain the final susceptibility-weighted image (Haacke et al. 2004, Haacke et al. 2009). Phase unwrapping is often done by using high-pass filtering or with more complicated algorithms. A problem of high-pass filtering is that it often unintentionally removes valuable information from the image, despite attempts to remove only large-scale field fluctuations. Another drawback of these GRE-based methodologies is the loss of phase information that occurs because the signal surrounding the calcification is diminished by its short transverse relaxation time (T2*). This loss can be significant, since a long echo time (TE) is often employed in these GRE techniques.
An alternative MRI method to obtain susceptibility-induced phase contrast has recently been introduced called SWIFT (Sweep Imaging with Fourier Transformation) (Idiyatullin et al. 2006). In SWIFT the radiofrequency (RF) excitation pulse is gapped and signal acquisition occurs inside these gaps which greatly reduces losses due to T2* signal decay. This feature makes SWIFT ideal for retaining signals from the fast relaxing water spins in steep susceptibility-induced field gradients.
In preliminary studies using SWIFT to detect stem cells labeled with superparamagnetic iron-oxide nanoparticles, it was shown that phase contrast images could be created without the need for post-processing (Zhou et al. 2010). Based on that work, we hypothesized that calcifications in brain could also be detected with SWIFT. Here, this hypothesis is tested by acquiring SWIFT images from rats with TBI or pilocarpine induced status epilepticus, and by comparing results with histology. In addition, we provide a theoretical explanation for the accumulation of phase in the k-space of SWIFT resulting from a magnetic dipole field.
2 Theory
Here, it is shown how phase accumulates in SWIFT and how a field disturbance due to a dipole source is seen in k-space. In the standard implementation of SWIFT, time-domain signal is acquired in the gaps of a frequency swept hyperbolic secant pulse. After correlation with the pulse shape in post processing, the resulting signal is equivalent to an FID that is excited by an infinitely short hard pulse (Idiyatullin et al. 2006). Since the acquisition delay is so short, there is no significant phase accumulation between excitation and signal acquisition.
The MRI signal equation including a spatially-dependent field disturbance term Δf(r̄) can be written
| [1] |
where ρ(r̄) is the spin density including relaxation effects and t is the time since excitation. For simplicity, the surroundings of the dipole field are assumed to be uniform and the spin density is set to ρ(r̄) = 1. Thus, Eq. [1] becomes the Fourier transform of the off-resonance exponential.
The dipole field can be written
| [2] |
where Λ(θ) is the term depending on the angle between θ and B0
| [3] |
and Δf0 is the strength of the dipole in Hz at radius r = r0 and θ = 0°.
The MacLaurin series of the off-resonance exponential of Eq. [1] can be written
| [4] |
As a first order approximation, based on Eq. [4], Eq. [1] can now be written
| [5] |
This approximation is valid given that Δf0t(r/r0)−3 ≪ 1. In other words, Eq. [5] is valid for positions r > r0 or when the frequency offset Δf0 is small at r0 close to the center of the dipole.
The k-space of SWIFT due to a dipole field in Eq. [5] can now be solved in spherical coordinates (Corum. 2005, Marques and Bowtell. 2005) to be
| [6] |
and further by substituting t = 2πrκ/γ|G|:
| [7] |
where (rκθκ,φκ) are the spherical coordinates in k-space,γ is gyromagnetic ratio and |G| is the magnitude of the readout gradient.
Since SWIFT acquisition always starts from the center of the k-space (rκ = 0), Eqs. [6] and [7] show that at the center of k-space, there is no accumulated phase due to the dipole field since the second, imaginary term is zero at the center. This is unlike in GRE, where phase accumulates at the center of k-space depending on the echo time and off-resonance frequency. However, in SWIFT, phase will still accumulate center-out, meaning that the higher spatial frequencies will have a higher phase accumulation. This implies that SWIFT acts as a 3D “cone” high-pass filter to the phase, potentially removing the need for phase unwrapping and/or B0 correction. It should be noted that this does apply to arbitrary off-resonance fields other than just a dipole field, since the expansion of Eq. [4] will have similar form for other arbitrary field distributions.
3 Methods
3.1 Animals
All the animals were housed in individual cages in a climate controlled room (22°C ± 1°C, humidity 50% to 60%) with 12 h light/dark cycle with an ad libitum diet. All animal procedures were approved by the Animal Ethics Committee of the Provincial Government of Southern Finland (for TBI 2008-05812, for epilepsy 2010-05651), and conducted in accordance with the guidelines set by the European Community Council Directives 86/609/EEC.
TBI was induced using lateral fluid-percussion (LFP) injury model (Kharatishvili et al. 2006) in male Sprague-Dawley rats (10 weeks old, weight 300–350 g, Harlan Netherlands B.V., Horst, Netherlands). Briefly, rats (n = 5) were anesthetized by an injection of a mixture (6 ml/kg, i.p.) containing sodium pentobarbital (58 mg/kg), chloral hydrate (60 mg/kg), magnesium sulfate (127.2 mg/kg), propylene glycol (42.8%), and absolute ethanol (11.6%). A craniectomy of 5 mm in diameter was performed between the bregma and lambda on the left convexity (anterior edge 2.0 mm posterior to the bregma; lateral edge adjacent to the left lateral ridge). LFP injury was induced by a transient (21–23 ms) fluid pulse impact against the exposed dura by using a fluid-percussion device (AmScien Instruments, Richmond, VA). The impact pressure was controlled to 3.2–3.4 atm, to induce severe brain injury. After impact, the dura was checked to ensure it had remained intact. Sham-operated control animals (n = 4) received all surgical procedures except the fluid-percussion impact.
Status epilepticus was induced in male Wistar rats (10 weeks old, weight 300–350 g, National Laboratory Animal Center, Kuopio) (n = 8). Rats were injected with scopolamine (s.c., 2 mg/kg; # S-8502, Sigma, Chemical Co., St. Louis, MO, USA) to reduce the peripheral adverse effects of pilocarpine. Thirty minutes later, status epilepticus was induced by pilocarpine (i.p., 320 mg/kg, # P-6503, Sigma, Chemical Co., St. Louis, MO, USA). The development of status epilepticus was observed visually for 3 h, and only the rats that developed recurrent generalized seizures were included in the study. Finally, diazepam (i.p., 10 mg/kg, Stesolid Novum, Dumex-Alpharma) was administered to reduce mortality. Age- and weight-matched controls received saline (n = 6).
3.2 Tissue preparation
The rats were sacrificed 9 months after status epilepticus or 6 months after TBI for ex vivo imaging and histology. The timing for imaging was based on our previous studies showing that the calcifications should have been developed by the time points chosen in the two models analyzed. The animals were deeply anesthetized with an i.p. injection (7 ml/kg) of solution containing sodium pentobarbital (10 mg/ml), chloral hydrate (10 mg/ml), magnesium sulfate (21.2 mg/ml), propylene glycol (40%), and absolute ethanol (10%) and perfused transcardially with saline for 5 minutes (30 ml/min) followed by 4 % paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (30 ml/min) for 25 minutes. The brains were removed from the skull and post-fixed in 4% paraformaldehyde for 4 h. Prior to ex vivo MRI, the brains were washed and stored in 0.9% NaCl solution for a week.
3.3 MRI
To verify the theoretical predictions, a phantom was composed of a glass bead of diameter 212–300 µm (Sigma-Aldrich, St. Louis, MO) inside agar gel made in saline. The phantom was imaged on a horizontal 9.4 T magnet (Varian Inc, Palo Alto, CA) interfaced with Varian DirectDrive console (Varian Inc, Palo Alto, CA) using a single loop coil. The SWIFT parameters were a TR = 8.1 ms, acquisition bandwidth (SW) = 31.125 kHz, 128000 k-space spokes, nominal flip angle = 7°, field-of-view (FOV) = 4.53 cm3, matrix size = 3843, two averages and a total scan time of 36 min. For comparison, the phantom was also imaged with GRE using TR = 20 ms, TE = 5 ms, SW = 62.5 kHz, flip angle = 11° (500 µs sinc), FOV = 33 cm3, matrix size = 2563, and scan time of 33 min.
The rats with TBI were imaged in vivo 6 months post-injury using 1.0–1.5% isoflurane in 20%/80% oxygen/nitrogen gas mixture for anesthesia. Imaging was conducted using a 9.4 T horizontal magnet (Varian Inc, Palo Alto, CA) interfaced to a Varian DirectDrive console (Varian Inc, Palo Alto, CA). RF transmission and signal reception were performed with a half-volume quadrature coil (Virtumed LLC, Minneapolis, MN) with Teflon® housing. All animals were imaged with SWIFT using TR = 8.1 ms, 49152 k-space spokes, 4 averages, nominal flip angle = 6°, FOV = 4.5 × 4.5 × 6.0 cm3, matrix size = 2563, and a scan time of 40 min. Fat suppression was applied every 16 acquisitions (90°, 6 ms sinc followed by a 1 ms crusher gradient). A relatively low acquisition bandwidth (SW = 31.25 kHz) was used to enhance phase contrast. SWIFT was implemented with a gapped hyperbolic secant pulse (Silver et al. 1985) transmitted in a non-adiabatic manner (Idiyatullin et al. 2006). Ex vivo imaging was conducted in a vertical 9.4 T magnet (Oxford Instruments PLC, Abingdon, UK) interfaced to a Varian DirectDrive console. RF transmission and signal reception were performed with a 19 mm quadrature volume coil (Rapid Biomedical GmbH, Rimpar, Germany). The brains were immersed in perfluoro polyether (Fomblin® LC08, Solvay Solexis, Milan, Italy) for susceptibility matching, to avoid tissue drying and to not have signal outside the brain. All brains were imaged with SWIFT using TR = 5.1 ms, SW = 62.5 kHz, 128000 spokes, 4 averages, flip angle = 5°, FOV = 43 cm3 (pilocarpine) and 3.73 cm3 (TBI), matrix size = 2563, and a scan time of 45 min. The FOV was adjusted relatively large to avoid folding signal from the coil elements. Additionally, for comparison, 3D GRE images were taken from two of the pilocarpine ex vivo brains, using TR = 50 ms, TE = 1.9 ms, SW = 208.3 kHz, 4 averages, flip angle = 25° (500 µs sinc), FOV = 2 × 2 × 4 cm3, matrix size = 128 × 128 × 256, and scan time of 55 min.
SWIFT data were reconstructed using an in-house developed LabVIEW (National Instruments, Austin, TX) program and interpolated to a Cartesian grid using in house developed gridding code utilizing Kaiser-Bessel kernel (Beatty et al. 2005).
3.4 Data analysis
The calcifications were detected and identified by using the SWIFT phase images and imaginary component images, by searching for the dipole field profile expected for calcifications having approximately spherical shape. Additionally, due to the diamagnetic properties of calcifications, they were separated from paramagnetic blood vessels, and in the case of ex vivo imaging, from air bubbles, based on the orientation of the dipole. It should be noted that no post-processing or phase unwrapping was used.
The GRE phase phantom and brain data were high-pass filtered with a 15 and a 10 pixel wide Gaussian kernels, respectively, using complex division (Haacke et al. 2009) to remove low frequency phase fluctuations. The volume of the calcifications in SWIFT magnitude images was manually measured using Aedes (aedes.uku.fi) based on signal intensity. Hypointense and hyperintense calcifications (see below) were separated based on their contrast to the surrounding brain tissue.
3.5 Histology
3.5.1 Processing of tissue
After ex vivo imaging, the brains were washed in 0.9% NaCl for at least for 2 h at 4°C and then placed in a cryoprotective solution containing 20% glycerol in 0.02 M potassium phosphate-buffered saline (pH 7.4) for 36 h. The brains were then blocked, frozen in dry ice, and stored at −70 °C until cutting. Brains from animals with status epilepticus were sectioned in the coronal plane, and brains from rats with TBI in the horizontal plane (30 µm 1-in-5 series) using a sliding microtome. The horizontal sections enabled more direct comparison of the calcification induced dipoles and histology. The first series of sections was stored in 10% formalin and the remaining series in a cryoprotectant tissue-collecting solution (30% ethylene glycol, 25% glycerol in 0.05 M sodium phosphate buffer) at −20°C until processed.
3.5.2 Nissl staining
The first series of sections was stained with thionin to assess the cytoarchitectonic borders, gliosis, and severity of neurodegeneration in various brain areas.
3.5.3 Staining and quantification of calcifications
An adjacent series of sections was stained with Alizarin Red according to (Mäkinen et al. 2007) to detect the occurrence and measure the size of calcifications.
To calculate the volume of calcifications in histologic sections they were viewed under a microscope, photographed, and then outlined from digital images using ImageJ (NIH, Bethesda, MD, version 1.43u). The volume was estimated from successive sections using Cavalieri method (Gundersen and Jensen. 1987). Briefly, the area of the calcification in every fifth section was outlined using the software tool, multiplied by the section thickness (30 µm) and the space between the sections (number of sections, 5). It should be noted that the area outlined as a calcification (in particular the hyperintense calcifications, see below) included some tissue which was unavoidable as calcifications consisted of several foamy-like granular formations closely attached to each other.
3.5.4 Immunohistochemistry
Sections from another series (2–8 sections per animal) were immunohistochemically stained with astroglial and microglial markers to identify the type of glial cells around the calcifications. Sections were washed three times with 0.02 M KPBS (10 min each) and incubated for 15 min in 1% H2O2 in KPBS to remove endogenous peroxidase activity. Then, sections were rinsed 6 times with KPBS (5 min each) and non-specific binding was blocked in a solution containing 10% NHS, 0.4% Triton X-100, and KPBS at room temperature for 2 h. This was followed by incubation at 4°C for 48 h with mouse anti-GFAP (1:4000; #814369; Boehringer Mannheim) or mouse anti-OX42 (1:4000; MCA2756; Serotec) in 1% NHS and 0.4% Triton X-100 in KPBS. Sections were washed with KPBS (3 times 10 min) and incubated for 2 h at room temperature in a secondary antibody solution containing biotinylated horse anti-mouse IgG (1:200; BA-2000; Vector laboratories), 1% NHS, and 0.4% Triton X-100 in KPBS. Sections were washed 3 times with KPBS (10 min each) and moved to 1% avidin-biotin (PK-4000; Vector Laboratories) in KPBS for 1 h at room temperature. After washing, the sections were recycled back to the secondary antibody solution for 45 min, and then to the avidin-biotin solution for 30 min. The secondary antibody was visualized with 0.05% DAB (Pierce Chemical) and 0.04% H2O2 in KPBS. Finally, sections were washed with 0.1 M PB, and mounted on gelatin-coated microscope slides, and then dried overnight at 37°C. The reaction product was intensified with osmium (OsO4; #19170; Electron Microscopy Sciences) and thiocarbohydrazide (#21900; Electron Microscopy Sciences) according to the method of (Lewis et al. 1986).
The presence of glial cells was evaluated in 72 calcifications from GFAP or OX42-immunostained sections. Most of the calcifications were analyzed in both immunostainings in consecutive sections. The scoring in both immunopreparations was as follows: 0 = no glial cells, 1 = a few scattered glial cells around the calcification, 2 = a band of glial cells surrounding the calcification, 3 = a thick and densely populated rim of glial cells around the calcifications. The mean score calculated for each calcification was used for statistical analysis.
4 Results
4.1 MRI
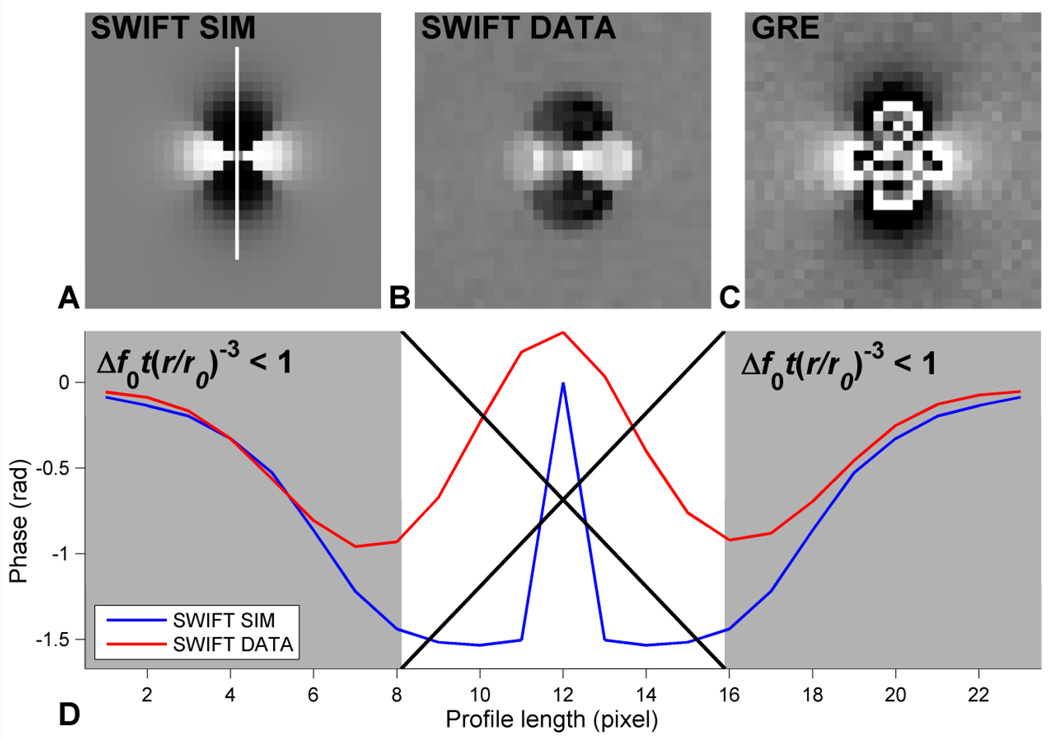
In Fig. 1, the phase of a dipole for SWIFT numerically solved from Eq. [7] and phantom data for SWIFT and GRE phase images are compared. The dipole due to the glass bead is clearly better defined using SWIFT than GRE. There is also no phase wrapping in the simulated and actual SWIFT phase images. Fig. 1D shows that the numerical solution corresponds to the phantom data when the strength of the dipole is set to 81 Hz (= pixel frequency resolution) five pixels away from the center of the dipole given that Δf0t(r/r0)−3 ≪ 1, where t has been set to the acquisition time. It should be noted that since Eq. [7] reaches its maximum at the edges of the k-space, the result has been low-pass filtered with a Gaussian kernel (FWHM = 3 pixels) in Fig. 1A to remove Gibbs ringing due to truncation. For comparison the phantom k-space data have also been low-pass filtered for Fig. 1D.
Fig. 1.
Comparison of simulated and phantom SWIFT data, and GRE phantom data. (A) Numerical inverse Fourier transform of Eq. [7], (B) phase due to a glass bead embedded in agar using (B) SWIFT and (C) GRE (TE = 5 ms). (D) Profiles along B0 indicated in (A) through the phase of the dipole in the simulated and phantom SWIFT data. The area where the approximation of Eq. [7] likely fails is indicated by the large 'X'. t has been set to acquisition time. Since Eq. [7] reaches its' maximum at the edges of the k-space it has to be low-pass filtered. Hence, for a fair comparison, the phantom SWIFT data profile has been filtered in an identical manner. The SWIFT phase due to the dipole field in (B) is clearly sharper than the GRE phase in (C). This is due to the high-pass filtering property of SWIFT shown in Eq. [7].
4.1.1 Status epilepticus
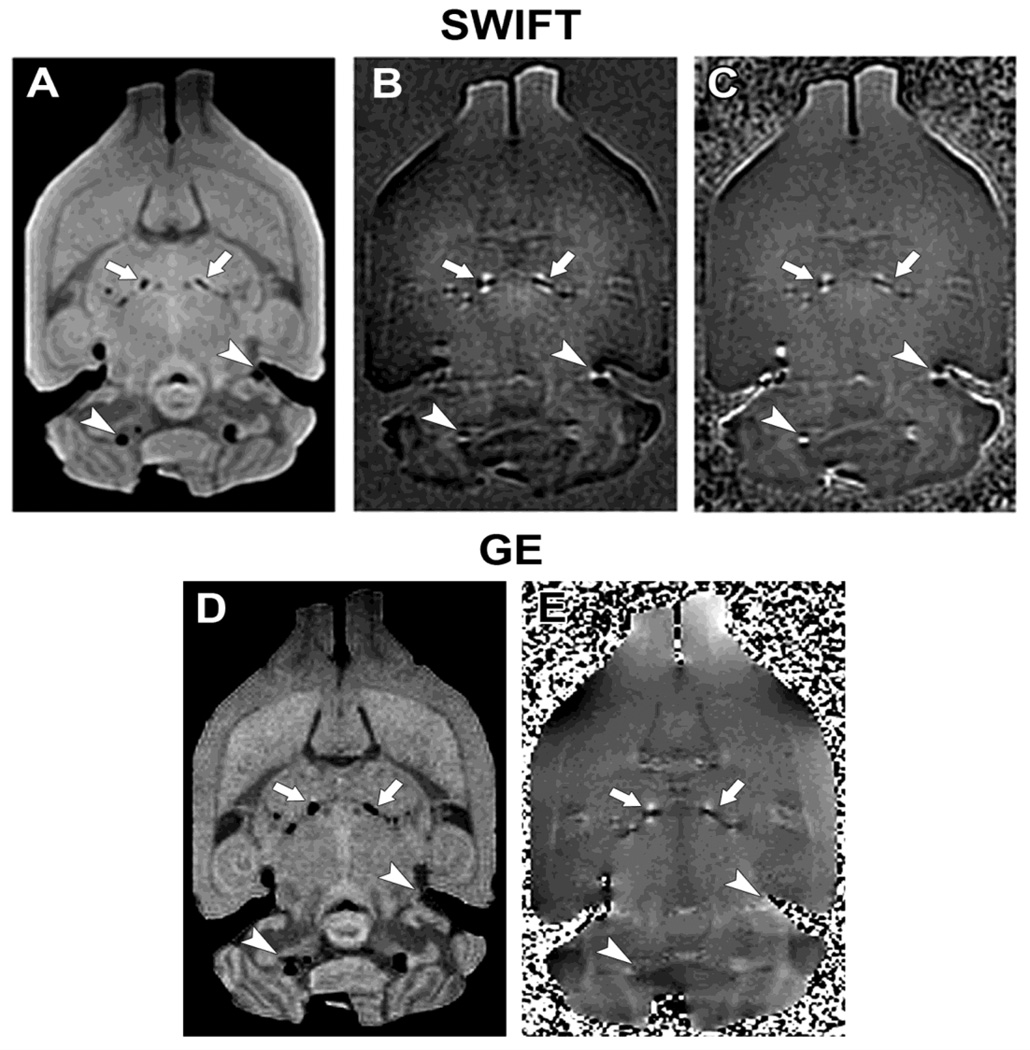
With SWIFT, calcifications appeared bilateral and quite symmetrical in the thalamus of the rats with status epilepticus (Fig. 1). The number of individual calcifications was highly variable ranging from 1 to 25 per brain. The calcifications could easily be separated from air bubbles based on the orientation of the dipole field. The dipole field effect was shown by both SWIFT and GRE, although SWIFT seems to show a stronger contrast in the imaginary component image (Fig. 2B) as compared to GRE phase image (Fig. 2E). SWIFT also detected the dipole field around air bubbles (Fig. 2B, C), whereas GRE was unable to reveal them due to T2* dephasing (Fig. 2E). The magnitude images (Fig. 2A, D) were very similar, although SWIFT showed more signal pile up dislocated by the dipole-like field of the calcifications. This signal was lost in GRE due to a much longer acquisition delay (in this case, TE = 1.9 ms). The SWIFT magnitude images were slightly blurred in comparison to the GRE images due to SWIFT’s radial nature (Lauzon and Rutt. 1996).
Fig. 1.
Representative SWIFT and gradient echo MR images from rat brain, ex vivo, 9 months after inducing status epilepticus with pilocarpine. SWIFT magnitude (A), imaginary (B), and phase (C) images. GRE magnitude (D) and high-pass filtered phase (E) images. Arrows point to groups of calcifications in the thalamus, while arrowheads point to air bubbles. The dipoles created by the calcifications have positive (white) phase shift in front and back lobes, whereas air bubbles create negative (black) phase shift due to the different sign of the magnetic susceptibilities.
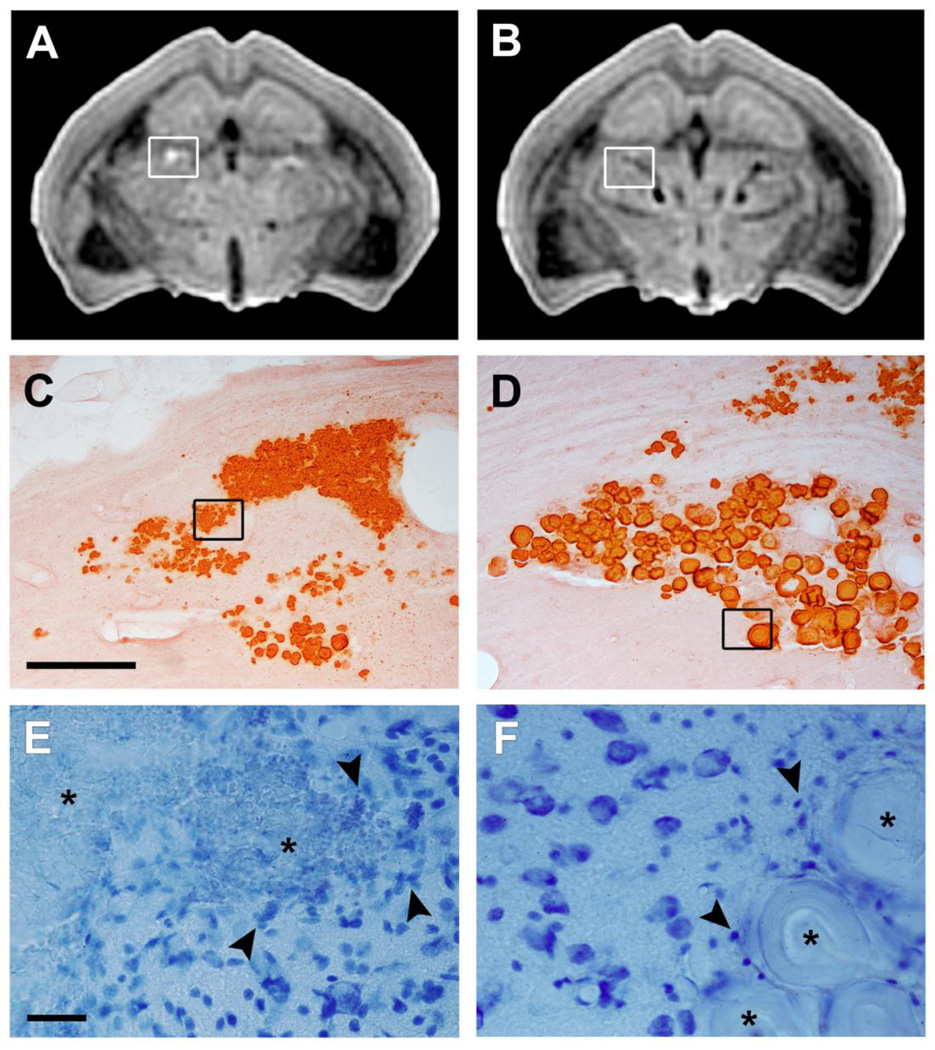
Most of the calcifications of the brains of the rats with status epilepticus appeared hypointense in the SWIFT and GRE magnitude images, but interestingly, we also found hyperintense calcifications (Fig. 3A). The number of individual calcifications was highly variable: overall between 1 and 25, hypointense between 1 and 17, and hyperintense between 0 and 12. Four out of five brains had both types of calcifications.
Fig. 2.
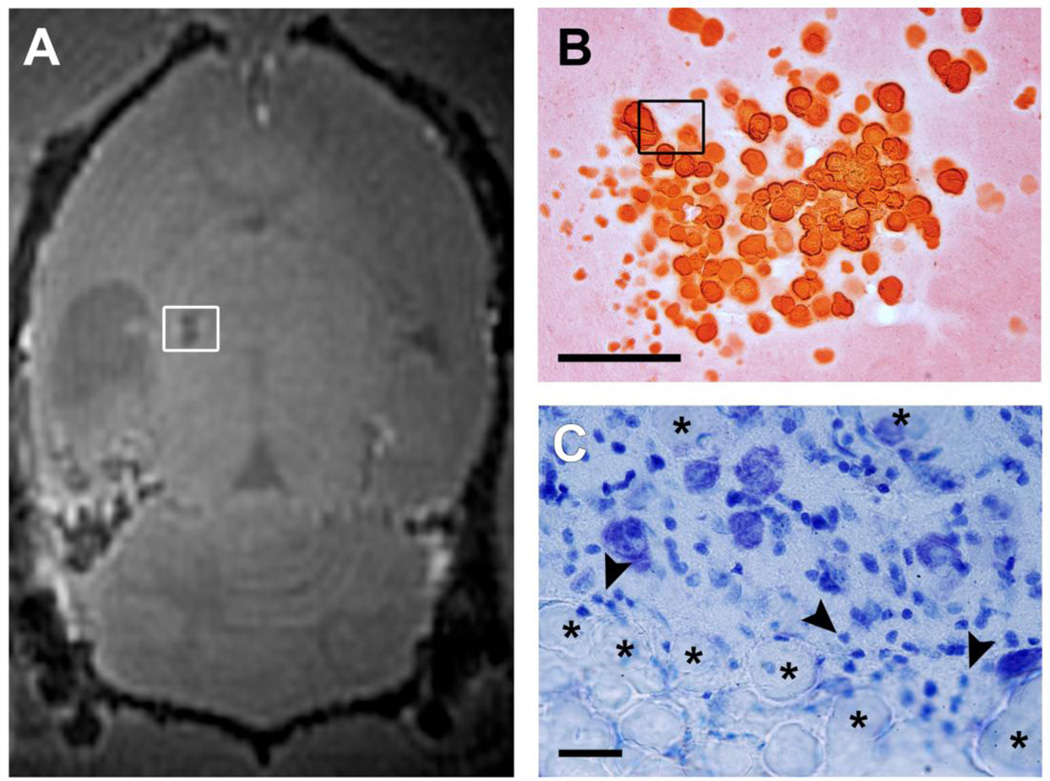
Alizarin stained coronal sections demonstrating the appearance of the two types of calcifications in the thalamus from the same animal that had experienced pilocarpine-induced status epilepticus 9 months earlier. Ex vivo SWIFT magnitude images show both a hyperintense (A) and a hypointense (B) calcification. The hypointense calcification is composed of large crystallized granules, whereas the hyperintense calcification is much finer and diffuse (C, D; scalebar 200 µm). Nissl stained sections (E, F; scalebar 25 µm) demonstrate the presense of glial cells around and within the hyperintense calcification. Note a substantially milder gliosis around the hypointense calcification. Arrowheads point to glial cells and asterisks indicate the calcifications in (E, F).
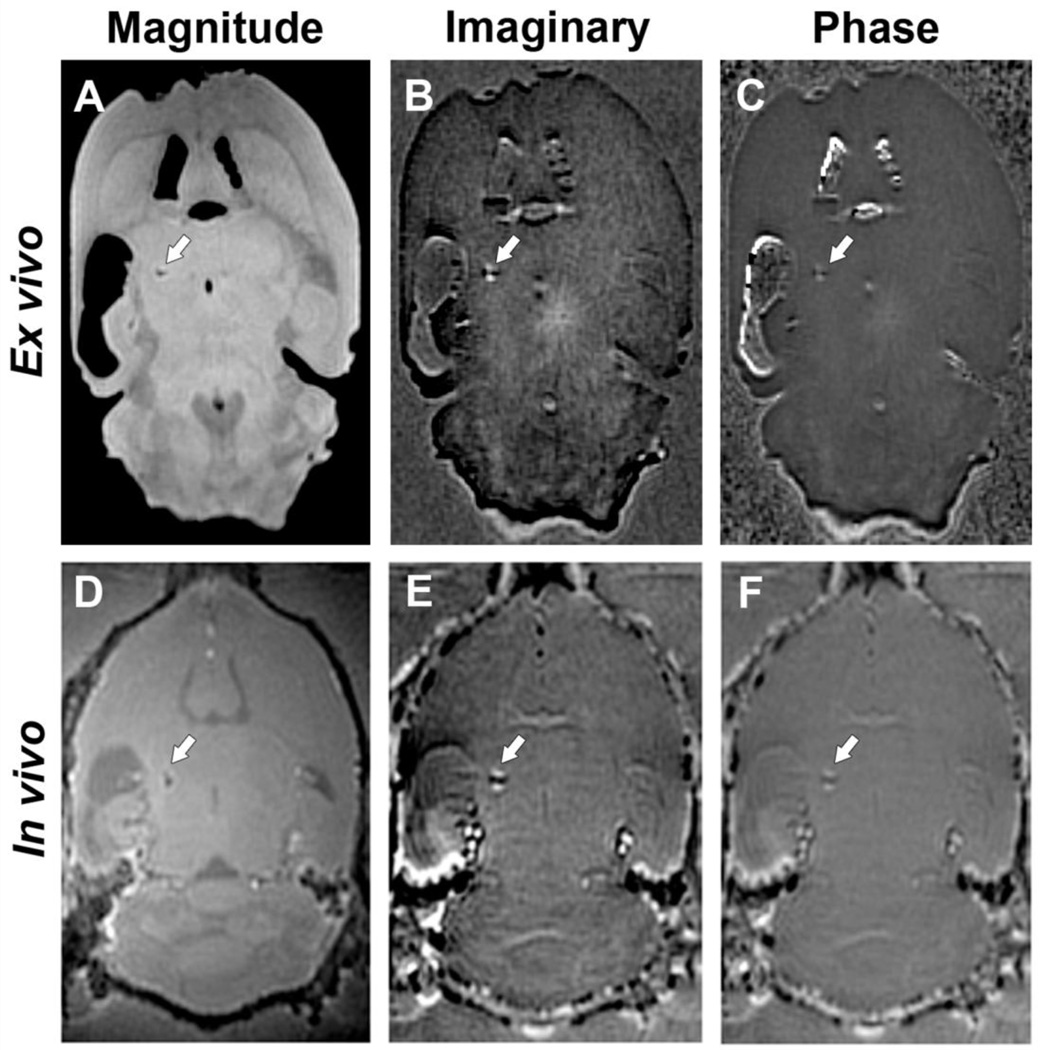
4.1.2 TBI
Fig. 2 shows representative SWIFT images of a TBI brain ex vivo and in vivo. The appearance of calcifications was similar to that in rats with status epilepticus (Fig. 1). However, only a single large calcification was seen in each of the TBI brains in vivo and ex vivo, and it was exclusively located in the thalamus. Unlike after status epilepticus, calcifications were detected only ipsilaterally. The calcifications could be detected in both ex vivo (Fig. 3B, A–C) and in vivo (Fig. 3D–F) images in three out of five animals; one animal did not have any calcifications and the calcification in the other animal was below the MRI detection limit. The imaginary images (Fig. 3B, E) appear to show a slightly stronger contrast for the dipole in comparison to the phase images (Fig. 3C, F).
All calcifications in the brains of rats with TBI were hypointense in the SWIFT magnitude images (Fig. 4A). Importantly, they had similar structure (Fig. 4B, C) as the hypointense calcifications in the brains of rats with status epilepticus (Fig. 2D, F).
Fig. 3.
Representative SWIFT MR images of a rat brain 6 months after TBI. Ex vivo (A–C) and in vivo (D–F) magnitude, imaginary, and phase images, respectively. The arrow points the calcification in the thalamus. The calcification can be easily identified both ex vivo and in vivo by its dipole field effect.
4.1.3 Histology of calcifications
Histologic analysis of Alizarin stained preparations from the SWIFT imaged brains with status epilepticus (Fig. 2C, D) revealed a clear difference in the composition of the two types of calcifications. The hypointense calcifications consisted of large, well-defined crystallized granules, whereas hyperintense calcifications were composed mainly of small granules.
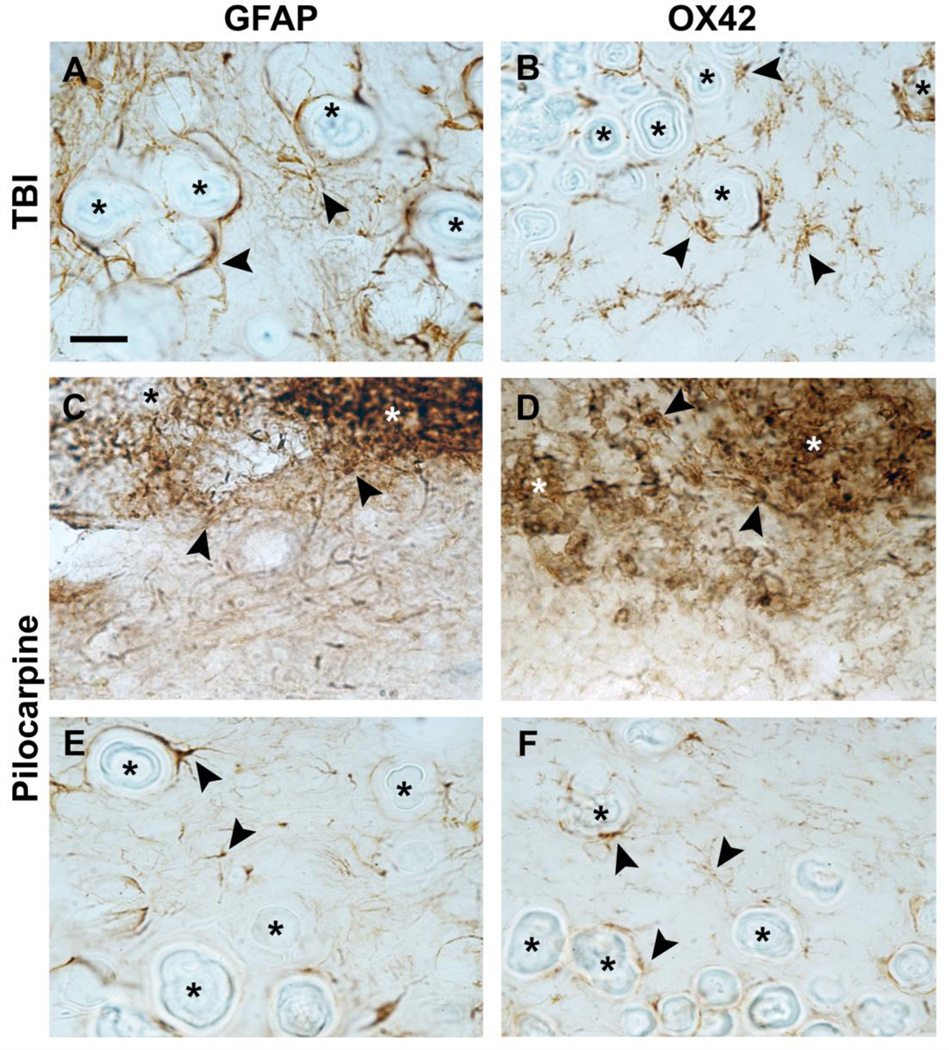
In Nissl-stained sections both hypointense and hyperintense calcifications were typically associated with gliosis which surrounded the larger calcifications and was intermingled between smaller granular calcifications (Fig. 2E, F). Identification of inflammatory cells surrounding the calcifications was done using a marker for astrocytes (GFAP) and a marker for microglia (OX42). Analysis of GFAP and OX42-immunostained sections showed the presence of astrocytes and microglia in both hyperintense and hypointense calcifications even though the density of gliosis varied (Fig. 5). The scores for the astrogliosis and microgliosis around hypointense calcifications in rats with TBI were 1.71 ± 0.49 (GFAP) and 1.57 ± 0.53 (OX42), respectively (Fig. 6A, B). The scores for hypointense calcifications in status epilepticus model were comparable, being 1.73 ± 0.45 (GFAP) and 1.38 ± 0.54 (OX42) (Fig. 5E, F). Hyperintense calcifications were only found in rats that had experienced status epilepticus (Fig. 5C, D). Both hyperintense calcification gliosis scores were higher than those around hypointense calcifications (pGFAP = 0.013 and pOX42 < 0.001, Mann-Whitney U-test), being 2.40 ± 0.97 for astrogliosis and 2.81 ± 0.40 for microgliosis.
Fig. 4.
Horizontal SWIFT MR image and histological sections showing a typical calcification in the thalamus at 6 months post-TBI in the rat. An in vivo SWIFT magnitude image (A) and the calcification in Alizarin (B) and Nissl (C) stained sections (scalebars 200 µm and 25 µm, respectively). Arrowheads point to glial cells and asterisks indicate crystallized calcifications in (C).
Fig. 5.
Photomicrographs of GFAP- and OX42-immunostained sections of TBI (A, B) and status epilepticus (C–F) models. Hypointense calcifications in both animal models (A, B and E, F) showed big crystallized granules (asterisks) with surrounding astrocytes (A, E) and microglia (B, F) (arrowheads). Hyperintense calcifications with small granules (asterisks) displayed an extensive presence of astrocites (C) and microglia (D) (arrowheads). Panels A and B are a representative example of grade 2 in the scoring, panels C and D of grade 3, and panels E and F of grade 1. Scale bar: 25 µm.
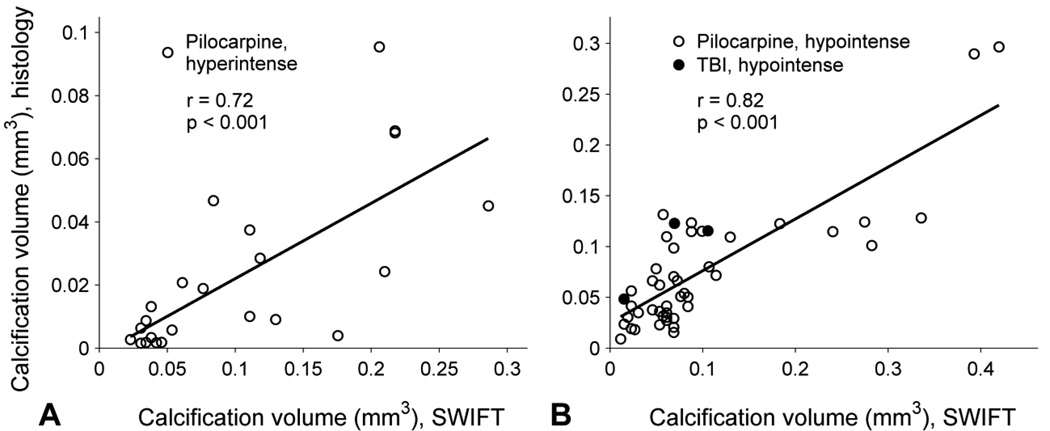
To study the performance of SWIFT in calcification imaging, the size of the calcifications in SWIFT magnitude images were compared to that in histological sections. The hypointense calcifications found in rats with status epilepticus (n = 45) or TBI (n = 3) were pooled together and analyzed separately from the hyperintense calcifications (n = 23) found in rats with status epilepticus. The size of the hyperintense calcifications measured in ex vivo SWIFT magnitude images correlated to that measured in histologic sections (linear regression, r = 0.72, p < 0.001, Fig. 6A). Similar correlation was found in the case of the hypointense calcifications (r = 0.82, p < 0.001, Fig. 6B). The histologic sizes of the smallest hyperintense and hypointense calcifications detected by SWIFT ex vivo were 0.002 mm3 and 0.009 mm3, respectively, when the voxel size was 0.004 mm3 (status epilepticus model). Based on the fits, the size of the hyperintense and hypointense calcifications measured from SWIFT images was approximately quintuple and double compared to that measured histologically, respectively.
5 Discussion
Based on previous results with superparamagnetic iron-oxide nanoparticles (Zhou et al. 2010), we hypothesized that brain calcifications could be detected in SWIFT phase images. Detection was based on the dipole-like magnetic field distortion caused by the diamagnetic nature of calcifications, which could be seen in the phase and imaginary component images. SWIFT was applied to two clinically relevant epileptogenic etiologies, that is, status epilepticus and traumatic brain injury. Our data show that calcifications can be detected by SWIFT in injured brain independent of the injury mechanisms. The size of the calcification measured from the SWIFT magnitude images correlated well with that assessed in Alizarin stained histological sections. Further, the signal obtained depended on the composition of calcification. Moreover, increased signal in magnitude images around hyperintense calcifications indirectly indicates increased gliosis. Even though the role of the calcifications in disease pathology is incompletely understood, it has been reported that their number and size increase with disease progression (Mäkinen et al. 2007). Therefore, detection of calcifications may provide an instrument to non-invasively probe the severity of the disease and/or possible treatment outcome.
5.1 Phase accumulation with almost zero acquisition delay in radial MRI
GRE phase contrast is based on the accumulation of phase due to off-resonance evolution of the magnetization in the acquisition delay (TE) between RF excitation and echo formation. In SWIFT, UTE, and similar sequences, the mechanism is different since the acquisition delay is nearly zero. For SWIFT and back-projection low angle shot (BLAST) (Hafner. 1994) that both excite while the read gradient is on, there is no time for significant phase evolution prior to the start of signal acquisition. UTE, on the other hand, may have enough phase evolution during excitation and gradient ramping to cause phase wrapping (Carl and Chiang. 2011).
A 3D analysis of a dipole in k-space for SWIFT was presented in the Theory section. It shows that a dipole, or other off-resonance fields result in phase accumulation center-out in k-space leading to a phase distribution in image space as shown by the numerical inverse Fourier transform of Eq. [7].
The center out phase accumulation in k-space means that SWIFT acquisition behaves as an intrinsic high-pass filter to the phase data, as demonstrated in Fig. 1 where no phase wrapping is seen in the SWIFT data due to the dipole. As a result, there is no need to remove large spatial phase fluctuations through post-processing, unlike in GRE based phase imaging or in SWI. Additionally, the phase due to the glass bead dipole has a clearly sharper cut off using SWIFT than GRE making the dipole in SWIFT data more localized. It should be noted, though that in this sense, GRE data could benefit from stronger high-pass filtering and shorter TE (here 5 ms).
Good correspondence between this theory and phantom data was demonstrated given that valid range of the approximation is observed. This analysis does not account for a possible phase effect of the HS pulse; the latter is known to create phase dispersion when not used as a 180° pulse (Idiyatullin et al. 2006, Park et al. 2006, Park and Garwood. 2009), as done in SWIFT. However, based on the linearity of the spin system response at small flip angles, all effects should be removed by correlation of the data with the pulse (Idiyatullin et al. 2006).
5.2 Differentiation of calcifications from other sources of magnetic susceptibility
Calcifications are composed of hydroxyapatite (Mäkinen et al. 2007, Yamada et al. 1996), which makes them diamagnetic in comparison to the surrounding tissue. Consequently, they are easily detectable in SWIFT phase and imaginary images. Importantly, the dipole field separates them from paramagnetic substances since the orientation of the dipole changes with the change of the sign of the susceptibility difference. This is of clinical importance as brain microbleeds (BMB) containing paramagnetic iron-protein complex, hemosiderin (37), are a typical example of paramagnetic lesions seen in multiple brain diseases such as TBI (Scheid et al. 2003), ischemia (Cordonnier et al. 2007), and Alzheimer’s disease (Cordonnier and van der Flier. 2011). Furthermore, iron oxide based contrast agents are increasingly used in cellular and molecular imaging emphasizing the need for differentiation between paramagnetic and diamagnetic substances.
All the calcifications visible in SWIFT showed dipole field effects in the phase images that corresponded to more diamagnetic matter than the tissue. Almost all of them were seen as signal voids surrounded by dislocated signal in the magnitude images, but also some hyperintense calcifications were seen in the pilocarpine brains. This is likely due to a T1 shortening in the vicinity of the calcification. That is, translational and rotational frequencies in the liquid layer next to the surface of the calcification are closer to the resonance frequency than in the bulk liquid and this causes T1 shortening (Henkelman et al. 1991). Since calcifications with smaller granules have more surface area, they also have a stronger T1 effect seen as hyperintensities in magnitude images. Based on the Alizarin stained sections, these calcifications are more diffuse and may be closer to liquid state, which is why they do not lead into hypointensity. We postulate that once the calcification evolves toward a more well defined crystal, the T1 effect grows weaker with the decreasing surface area, and decreasing T2* due to the susceptibility difference and decreasing proton density will start to dominate leading to negative contrast.
The water in carotid plaque calcifications (CPCs) has been shown to have a T1 in the range of 100–300 ms and a T2* of 0.4–2 ms when measured with 3 T using UTE (Du et al. 2011). Unlike brain calcifications, CPCs are surrounded by the other components of the atherosclerotic plaque such as lipids and fibrous tissue (Saam et al. 2005). The T1 and T2* of the CPCs are likely of similar order as hyperintense brain calcifications seen with SWIFT at 9.4 T. The fact that short T1 CPCs remain dark using normal UTE (Du et al. 2011), whereas short T1 hyperintense calcifications are seen as bright using SWIFT, is likely due to shorter T2* and much lower water content of the atherosclerotic plaques (~6 – 17 % (Du et al. 2011)) in comparison to brain tissue (~75% (MacDonald et al. 1986)). The T1 of the brain calcifications should be further characterized in the future. Using SWIFT, T1 can be quantified using variable flip angle (Nissi et al. 2010), and inversion or saturation recovery techniques similar to UTE (Du et al. 2011). In comparison to GRE, SWIFT may be able to show T1 hyperintensity longer with decreasing T2* due to its short acquisition delay.
The hyperintense calcifications were surrounded by glia cells that are markers of recent injury and involved in the inflammation process related to the early stage of the formation of the calcification. Once excess calcium has been stored in the calcification, the amount of glia is lowered with the reduced inflammation. Both, hypo- and hyperintense calcifications were surrounded by astrocytes and microglia, but the scoring demonstrated that hyperintense calcifications had more glial cells, indicating different stages in their formation. Inflammatory response seemed to be ongoing in the hyperintense calcifications, while it was attenuated in the hypointense calcifications, in agreement with other studies (Gayoso et al. 2003, Nitsch and Scotti. 1992, Oliveira et al. 2003). In this scenario it is interesting that the brains of the animals with status epilepticus 6 months earlier showed both hypointense and hyperintense granules suggesting ongoing progression in pathology which agrees with other studies demonstrating the progression of brain damage for weeks to months after status epilepticus (Gayoso et al. 2003). The correlation of the measured size of the calcifications between SWIFT magnitude images ex vivo and histology was found to be good even though most calcifications appeared larger in SWIFT than in histology. There are at least four explanations for the differences: 1) partial voluming and dislocation of signal due to off-resonance effects, 2) the hyperintense calcifications may appear even larger in magnitude images due to the surrounding gliosis, 3) histological sections shrink in comparison to an intact brain, and 4) imperfect matching of the calcifications between MRI and histology. The signal dislocation is likely to be a smaller factor for the hyperintense calcifications, since their paramagnetic effect does not dominate the contrast in comparison to the hypointense calcifications. The shrinkage of the histologic sections likely does not affect the calcifications themselves, but the tissue between the granules may be affected.
Partial voluming can be alleviated by increasing the resolution. Signal dislocation causes a calcification to appear larger than it is since signal is “pushed” away from the calcification. This effect can be decreased in SWIFT by increasing the SW. Increasing SW will decrease the relative amount of spatial dislocation of the signal but will also excite signal closer to the calcification since SW is equal to the excitation bandwidth in SWIFT. The localization of the calcifications could possibly be further improved with complex dipole matched filters enhancing the dipoles themselves (Corum et al. 2010) or by adding a counteracting off-resonance term into the reconstruction (Seevinck et al. 2011).
Matching the calcifications seen in MRI to the calcifications in histology may affect the correlation since every fifth 30 µm slice was used for the Alizarine staining in comparison to the slice thickness of 156 µm (pilocarpine) of SWIFT. Hence, although likely more accurate than MRI, the size measurement of the calcifications in histology is also an estimate.
A problem may arise if the calcified area also contains other paramagnetic or ferromagnetic material (Schweser et al. 2010). Since the orientation of the dipole depends on the susceptibility difference of the calcification and tissue, this might erroneously show the calcification as paramagnetic if the dipole orientation is used as a marker. In this situation, a more complex approach is needed (e.g. (Schweser et al. 2010, Shmueli et al. 2009)), where actual susceptibility maps are created by solving an ill-conditioned inverse problem.
Although the shapes of the entire calcifications were clearly not spherical, they do induce dipole like fields. Likely in other diseases the calcifications are much bigger in relation to the voxel size and far from spherical in shape, and as a result, induce a field distortion that is not recognizable as a dipole. For example, calcifications inside a tumor might not be easily separable (Wu et al. 2009). In these kinds of situations, the tissue surrounding the calcification could still be easily imaged with high SW (bandwidth) SWIFT.
5.3 Comparison to existing methods
In GRE, increased phase contrast can be obtained by increasing the TE. This will also decrease the SNR of the images through increased T2* dephasing. In SWIFT, enhanced phase contrast is achieved by decreasing the SW which has the added advantage of increasing SNR. Hence, in a situation where T2* effects are strong enough to destroy signal in GRE using even a short TE, SWIFT may become the preferred sequence. In both sequences, increasing phase contrast leads to increased scan time.
To our knowledge, UTE phase imaging has not been used for brain calcification detection. It is likely that our analysis of the phase formation for SWIFT will also apply to BLAST (Hafner. 1994) and UTE (Bergin et al. 1991, Rahmer et al. 2006), and that calcifications may be seen in a similar manner. Since in UTE excitation pulse is applied without the readout gradient, in principle, the excitation bandwidth could be lowered in comparison to SWIFT. It is worth mentioning, however, that SWIFT offers a slight advantage over UTE in terms of signal-to-noise ratio when imaging ultrashort-T2* spins. This signal-to-noise ratio advantage arises because the amplitude of the read gradient remains constant during the whole acquisition period. In UTE, the amplitude of the read gradient must be ramped on immediately following RF excitation and this results in loss of signal from ultrashort-T2* spins (Rahmer et al. 2006).
A positive magnitude contrast for a CPC has been achieved with UTE by subtracting a later echo image from the shortest TE image (Chan et al. 2010), or by using adiabatic inversion to null long T2* components surrounding the CPC (Du et al. 2011). These types of techniques may also apply to the hypointense brain calcifications, given a high enough resolution. Collecting an echo after the original SWIFT acquisition may be possible with pseudo-echoes (Park et al. 2006, Park et al. 2011), although it is likely to be more difficult than with UTE. An adiabatic inversion or similar magnetization preparation scheme is easily added to SWIFT. Since SWIFT is inherently a 3D pulse sequence, to save scan time, several spokes are collected after a preparation. While this would not optimally null all long T2 components, it is likely sufficient.
Although possibly limited in in-plane resolution, 2D UTE may offer a scan time advantage. Also, to detect the calcifications in the phase component, a relatively low SW may be required for both UTE and SWIFT. This leads to the calcifications to appear larger due to susceptibility differences. If only magnitude images are desired, a higher SW is advisable. An SW of 125 kHz can be achieved with SWIFT (Idiyatullin et al. 2011), but the maximum SW is restricted by the pulse element spacing (Idiyatullin et al. 2006, Idiyatullin et al. 2008). UTE is not SW limited in this sense due to the pulse and acquire manner of operation. Using GRE or fast spin-echo with high SW may provide a calcification-tissue boundary that appears sharper in comparison to SWIFT and UTE due to not having blurring from short T2* components. However, the signal void will be larger due to T2* loss.
5.4 Imaginary vs. phase in SWIFT
The SWIFT imaginary and phase images are similar. This is due to the short acquisition delay and its high-pass filtering property. The imaginary part has the same origin as the phase, but it is also dependent on the signal magnitude (see Theory). This can lead to a stronger contrast in the imaginary component image relative to the phase image since the signal of the brain tissue affects the visibility of the dipole. Hence, in future studies, the imaginary component may be a better tool for visualization, whereas phase is more useful for quantification.
6 Conclusion
In this study we have demonstrated the use of SWIFT phase images in brain calcification detection in two different models of brain diseases ex vivo and in vivo. The detection was based on the phase effect of the dipole-like field surrounding the calcifications due to their magnetic susceptibility difference in comparison to brain tissue. Since the orientation of the dipole depends on the sign of the susceptibility difference, calcifications can be separated from paramagnetic substances. This work further confirms expectations based on theoretical considerations that, as compared with GRE, SWIFT better retains fast decaying off resonance signals arising close to the calcification. As such, SWIFT may become a useful and robust means to detect diamagnetic cerebral calcifications.
Highlights.
phase contrast without echo-time using sweep imaging with Fourier transformation
calcification detection in two animal models of epileptogenic etiologies
monitoring of disease progression through contrast change of calcifications
Fig. 6.
Correlation between size of calcification obtained from SWIFT magnitude images ex vivo and Alizarin stained histological sections. Calcification data are plotted for (A) hyperintense and (B) hypointense lesions measured in rats with status epilepticus (open circles) or TBI (closed circles). It can be seen that both types of calcifications appeared larger in SWIFT images than in histologic sections, and the discrepancy is greatest for hyperintense calcifications.
Acknowledgments
We thank Maarit Pulkkinen for her invaluable help in handling the histology, Jarmo Hartikainen for his help with the disease models, and Ryan Chamberlain and Rajesh Venkataraman for the use of high performance–gridding code for image reconstruction. We would also like to thank Doctoral Program in Molecular Medicine at University of Eastern Finland, Emil Aaltonen Foundation, Academy of Finland and the strategic funding of the University of Eastern Finland (UEF-Brain). Additional funds were provided by NIH grants, P41 RR008079, P30 NS057091, R21 CA139688, and S10 RR023730.
Glossary
- SWIFT
SWeep Imaging with Fourier Transformation
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Drs. Garwood, Corum and Idiyatullin are entitled to sales royalties from a technology license held by GE Healthcare through the University of Minnesota for products related to the research described in this paper. The University of Minnesota also has a financial interest arising from a right to receive royalty income under the terms of the license agreement. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
References
- Ansari MQ, Chincanchan CA, Armstrong DL. Brain calcification in hypoxic-ischemic lesions: An autopsy review. Pediatr Neurol. 1990;6:94–101. doi: 10.1016/0887-8994(90)90041-x. [DOI] [PubMed] [Google Scholar]
- Arnold MM, Kreel L. Asymptomatic cerebral calcification--a previously unrecognized feature. Postgrad Med J. 1991;67:147. doi: 10.1136/pgmj.67.784.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrahami E, Cohn DF, Feibel M, Tadmor R. MRI demonstration and CT correlation of the brain in patients with idiopathic intracerebral calcification. J Neurol. 1994;241:381–384. doi: 10.1007/BF02033355. [DOI] [PubMed] [Google Scholar]
- Barrett HH, Myers KJ, Rathee S. The dirac delta and other generalized functions. In: Saleh BEA, editor. Foundations of image science. New Jersey: Wiley-Interscience; 2004. pp. 63–94. [Google Scholar]
- Beatty PJ, Nishimura DG, Pauly JM. Rapid gridding reconstruction with a minimal oversampling ratio. IEEE T Med Imaging. 2005;24:799–808. doi: 10.1109/TMI.2005.848376. [DOI] [PubMed] [Google Scholar]
- Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: Projection reconstruction MR imaging. Radiology. 1991;179:777–781. doi: 10.1148/radiology.179.3.2027991. [DOI] [PubMed] [Google Scholar]
- Caldemeyer KS, Mathews VP, Edwards-Brown MK, Smith RR. Central nervous system cryptococcosis: Parenchymal calcification and large gelatinous pseudocysts. Am J Neuroradiol. 1997;18:107–109. [PMC free article] [PubMed] [Google Scholar]
- Carl M, Chiang JA. Investigations of the origin of phase differences seen with ultrashort TE imaging of short T2 meniscal tissue. Magn Reson Med. 2011 doi: 10.1002/mrm.23075. In Press. [DOI] [PubMed] [Google Scholar]
- Cervós-Navarro J, Lafuente JV. Traumatic brain injury: Morphological changes. J Neurol Sci. 1991;103:3–14. doi: 10.1016/0022-510x(91)90002-o. [DOI] [PubMed] [Google Scholar]
- Chan CF, Keenan NG, Nielles-Vallespin S, Gatehouse P, Sheppard MN, Boyle JJ, Pennell DJ, Firmin DN. Ultra-short echo time cardiovascular magnetic resonance of atherosclerotic carotid plaque. J Cardiovasc Magn Reson. 2010;12:17–24. doi: 10.1186/1532-429X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: Systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer’s disease: Innocent observation or key player? Brain. 2011;134:335. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- Corum CA. Nuclear magnetic resonance with the distant dipolar field. Tucson, Arizona: University of Arizona; 2005. 163 pp. Available from: http://arxiv.org/abs/physics/0507103. [Google Scholar]
- Corum CA, Idiyatullin D, Moeller S, Chamberlain R, Garwood M. Dipole matched filter with SWIFT. Proceedings of the 18th Annual Meeting of ISMRM; Stockholm, Sweden. 2010. p. 5113. 2010. [Google Scholar]
- Demer LL, Watson KE, Boström K. Mechanism of calcification in atherosclerosis. Trends Cardiovasc Med. 1994;4:45–49. doi: 10.1016/1050-1738(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Du J, Corbeil J, Znamirowski R, Angle N, Peterson M, Bydder GM, Kahn AM. Direct imaging and quantification of carotid plaque calcification. Magn Reson Med. 2011;65:1013–1020. doi: 10.1002/mrm.22682. [DOI] [PubMed] [Google Scholar]
- Gayoso MJ, Al-Majdalawi A, Garrosa M, Calvo B, Diaz-Flores L. Selective calcification of rat brain lesions caused by systemic administration of kainic acid. Histol Histopathol. 2003;18:855–869. doi: 10.14670/HH-18.855. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Rao SB, Jain R, Pal L, Kumar R, Venkatesh SK, Rathore RKS. Differentiation of calcification from chronic hemorrhage with corrected gradient echo phase imaging. J Comput Assist Tomogr. 2001;25:698–704. doi: 10.1097/00004728-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Xu Y, Cheng YCN, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YCN. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 1. Am J Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner S. Fast imaging in liquids and solids with the back-projection low angle shot (BLAST) technique. Magn Reson Imaging. 1994;12:1047–1051. doi: 10.1016/0730-725x(94)91236-p. [DOI] [PubMed] [Google Scholar]
- Henkelman RM, Watts JF, Kucharczyk W. High signal intensity in MR images of calcified brain tissue. Radiology. 1991;179:199–206. doi: 10.1148/radiology.179.1.1848714. [DOI] [PubMed] [Google Scholar]
- Idiyatullin D, Corum C, Park JY, Garwood M. Fast and quiet MRI using a swept radiofrequency. J Magn Reson. 2006;181:342–349. doi: 10.1016/j.jmr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Idiyatullin D, Corum C, Moeller S, Garwood M. Gapped pulses for frequency-swept MRI. J Magn Reson. 2008;193:267–273. doi: 10.1016/j.jmr.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idiyatullin D, Corum C, Moeller S, Prasad HS, Garwood M, Nixdorf DR. Dental magnetic resonance imaging: Making the invisible visible. J Endod. 2011;37:745–752. doi: 10.1016/j.joen.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Lafreniere GF, Peredery O, Persinger MA. Progressive accumulation of large aggregates of calcium-containing polysaccharides and basophilic debris within specific thalamic nuclei after lithium/pilocarpine-induced seizures. Brain Res Bull. 1992;28:825–830. doi: 10.1016/0361-9230(92)90268-3. [DOI] [PubMed] [Google Scholar]
- Lauzon ML, Rutt BK. Effects of polar sampling in k-space. Magn Reson Med. 1996;36:940–949. doi: 10.1002/mrm.1910360617. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Morrison JH. An immunohistochemical characterization of somatostatin28 and somatostatin-281–12 in monkey prefrontal cortex. J Comp Neurol. 1986;248:1–18. doi: 10.1002/cne.902480102. [DOI] [PubMed] [Google Scholar]
- MacDonald HL, Bell BA, Smith MA, Kean DM, Tocher JL, Douglas RHB, Miller JD, Best JJK. Correlation of human NMR T1 values measured in vivo and brain water content. Br J Radiol. 1986;59:355–357. doi: 10.1259/0007-1285-59-700-355. [DOI] [PubMed] [Google Scholar]
- Mäkinen S, Van Groen T, Clarke J, Thornell A, Corbett D, Hiltunen M, Soininen H, Jolkkonen J. Coaccumulation of calcium and β-amyloid in the thalamus after transient middle cerebral artery occlusion in rats. J Cerebr Blood F Met. 2007;28:263–268. doi: 10.1038/sj.jcbfm.9600529. [DOI] [PubMed] [Google Scholar]
- Marques JP, Bowtell R. Application of a Fourier-based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concept Magn Reson B. 2005;25:65–78. [Google Scholar]
- Nissi M, Lehto LJ, Corum CA, Idiyatullin D, Garwood M. T1 jotain. Proceedings of the 18th Annual Meeting of ISMRM; Stockholm, Sweden. 2010. p. 5113. [Google Scholar]
- Nitsch C, Scotti AL. Ibotenic acid-induced calcium deposits in rat substantia nigra. ultrastructure of their time-dependent formation. Acta Neuropathol. 1992;85:55–70. doi: 10.1007/BF00304634. [DOI] [PubMed] [Google Scholar]
- Okuchi K, Hiramatsu K, Morimoto T, Tsunoda S, Sakaki T, Iwasaki S. Astrocytoma with widespread calcification along axonal fibres. Neuroradiology. 1992;34:328–330. doi: 10.1007/BF00588194. [DOI] [PubMed] [Google Scholar]
- Oliveira A, Hodges H, Rezaie P. Excitotoxic lesioning of the rat basal forebrain with S-AMPA: Consequent mineralization and associated glial response. Exp Neurol. 2003;179:127–138. doi: 10.1016/s0014-4886(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Osteen CL, Moore AH, Prins ML, Hovda DA. Age-dependency of 45calcium accumulation following lateral fluid percussion: Acute and delayed patterns. J Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Parisi J, Place C, Nag S. Calcification in a recent cerebral infarct--radiologic and pathologic correlation. Can J Neurol Sci. 1988;15:152–155. doi: 10.1017/s0317167100027530. [DOI] [PubMed] [Google Scholar]
- Park JY, DelaBarre L, Garwood M. Improved gradient-echo 3D magnetic resonance imaging using pseudo-echoes created by frequency-swept pulses. Magn Reson Med. 2006;55:848–857. doi: 10.1002/mrm.20821. [DOI] [PubMed] [Google Scholar]
- Park JY, Garwood M. Spin-echo MRI using π/2 and π hyperbolic secant pulses. Magn Reson Med. 2009;61:175–187. doi: 10.1002/mrm.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Moeller S, Goerke U, Auerbach E, Chamberlain R, Ellermann J, Garwood M. Short echo-time 3D radial gradient-echo MRI using concurrent dephasing and excitation. Magn Reson Med. 2011 doi: 10.1002/mrm.23026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JES, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Rahmer J, Bornert P, Groen J, Bos C. Three-dimensional radial ultrashort echo-time imaging with T 2 adapted sampling. Magn Reson Med. 2006;55:1075–1082. doi: 10.1002/mrm.20868. [DOI] [PubMed] [Google Scholar]
- Rauscher A, Barth M, Reichenbach JR, Stollberger R, Moser E. Automated unwrapping of MR phase images applied to BOLD MR-venography at 3 tesla. J Magn Reson Im. 2003;18:175–180. doi: 10.1002/jmri.10346. [DOI] [PubMed] [Google Scholar]
- Rodríguez MJ, Ursu G, Bernal F, Cusí V, Mahy N. Perinatal human hypoxia-ischemia vulnerability correlates with brain calcification. Neurobiol Dis. 2001;8:59–68. doi: 10.1006/nbdi.2000.0332. [DOI] [PubMed] [Google Scholar]
- Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–239. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- Sandner G, Angst MJ, Guiberteau T, Guignard B, Brasse D. MRI and X-ray scanning images of the brain of 3-, 6-and 9-month-old rats with bilateral neonatal ventral hippocampus lesions. Neuroimage. 2010;53:44–50. doi: 10.1016/j.neuroimage.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Scheid R, Preul C, Gruber O, Wiggins C, Von Cramon DY. Diffuse axonal injury associated with chronic traumatic brain injury: Evidence from T2*-weighted gradient-echo imaging at 3 T. Am J Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- Schweser F, Deistung A, Lehr BW, Reichenbach JR. Differentiation between diamagnetic and paramagnetic cerebral lesions based on magnetic susceptibility mapping. Med Phys. 2010;37:5165–5178. doi: 10.1118/1.3481505. [DOI] [PubMed] [Google Scholar]
- Seevinck PR, de Leeuw H, Bos C, Bakker CJG. Highly localized positive contrast of small paramagnetic objects using 3D center-out radial sampling with off-resonance reception. Magn Reson Med. 2011;65:146–156. doi: 10.1002/mrm.22594. [DOI] [PubMed] [Google Scholar]
- Shirotani T, Shima K, Iwata M, Kita H, Chigasaki H. Calcium accumulation following middle cerebral artery occlusion in stroke-prone spontaneously hypertensive rats. J Cerebr Blood F Met. 1994;14:831–836. doi: 10.1038/jcbfm.1994.104. [DOI] [PubMed] [Google Scholar]
- Shmueli K, de Zwart JA, van Gelderen P, Li TQ, Dodd SJ, Duyn JH. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med. 2009;62:1510–1522. doi: 10.1002/mrm.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MS, Joseph RI, Hoult DI. Selective spin inversion in nuclear magnetic resonance and coherent optics through an exact solution of the bloch-riccati equation. Phys Rev A. 1985;31:2753–2755. doi: 10.1103/physreva.31.2753. [DOI] [PubMed] [Google Scholar]
- Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: A review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kumon Y, Ohta S, Sakaki S, Matsuda S, Sakanaka M. Changes in protein synthesis and calcium homeostasis in the thalamus of spontaneously hypertensive rats with focal cerebral ischemia. J Cerebr Blood F Met. 1998;18:686–696. doi: 10.1097/00004647-199806000-00011. [DOI] [PubMed] [Google Scholar]
- Wu Z, Mittal S, Kish K, Yu Y, Hu J, Haacke EM. Identification of calcification with MRI using susceptibility-weighted imaging: A case study. J Magn Reson Im. 2009;29:177–182. doi: 10.1002/jmri.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Imakita S, Sakuma T, Takamiya M. Intracranial calcification on gradient-echo phase image: Depiction of diamagnetic susceptibility. Radiology. 1996;198:171. doi: 10.1148/radiology.198.1.8539373. [DOI] [PubMed] [Google Scholar]
- Zhou R, Idiyatullin D, Moeller S, Corum C, Zhang H, Qiao H, Zhong J, Garwood M. SWIFT detection of SPIO-labeled stem cells grafted in the myocardium. Magn Reson Med. 2010;63:1154–1161. doi: 10.1002/mrm.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Qi J, Zhan C, Shu H, Zhang L, Wang C, Xia L, Hu J, Feng D. Magnetic resonance susceptibility weighted imaging in detecting intracranial calcification and hemorrhage. Chin Med J. 2008;121:2021–2025. [PubMed] [Google Scholar]