Abstract
Prior studies showed conflicting results regarding the association between 25-hydroxyvitamin D (25(OH)D) levels and mineral metabolism in end-stage renal disease. In order to determine whether the bioavailable vitamin D (that fraction not bound to vitamin D binding protein) associates more strongly with measures of mineral metabolism than total levels, we identified 94 patients with previously measured 25(OH)D and 1,25-dihydroxyvitamin D (1,25(OH)2D) from a cohort of incident hemodialysis patients. Vitamin D binding protein was measured from stored serum samples. Bioavailable 25(OH)D and 1,25(OH)2D were determined using previously validated formulae. Associations with demographic factors and measures of mineral metabolism were examined. When compared with whites, black patients had lower levels of total, but not bioavailable, 25(OH)D. Bioavailable, but not total, 25(OH)D and 1,25(OH)2D were each significantly correlated with serum calcium. In univariate and multivariate regression analysis, only bioavailable 25(OH)D was significantly associated with parathyroid hormone levels. Hence, bioavailable vitamin D levels are better correlated with measures of mineral metabolism than total levels in patients on hemodialysis.
Keywords: vitamin D, vitamin D binding protein, mineral bone disease, free hormone hypothesis, vitamin D deficiency, chronic kidney disease, dialysis
Introduction
Chronic kidney disease-associated mineral and bone disorder (CKD-MBD) is one of the most appreciated metabolic complications of CKD. As individuals progress toward end-stage renal disease (ESRD), declining renal 1α-hydroxylase activity leads to decreased conversion of 25-hydroxyvitamin D (25(OH)D) to the active 1,25-dihydroxyvitamin D (1,25(OH)2D). These metabolic changes are believed to precipitate the hypocalcemia and secondary hyperparathyroidism that characterize CKD-MBD. Although 1,25(OH)2D is thought to be the biologically active moiety, the majority of vitamin D circulates as 25(OH)D.(1) Low levels of 25(OH)D are common in ESRD; 79% of patients initiating dialysis have 25(OH)D levels below 30 ng/ml, and serum levels below this threshold are nearly universal among black ESRD patients.(2)
The free hormone hypothesis suggests that protein-bound hormones are relatively inactive while those liberated from binding proteins are free to exert biological activity.(3) For some hormones (e.g. testosterone), binding to albumin is considerably weaker than to a specific binding protein. Thus, albumin-bound hormone is often grouped with the free fraction and referred to as the “bioavailable” fraction. The majority (85–90%) of circulating 25(OH)D and 1,25(OH)2D is tightly bound to vitamin D binding protein (DBP), with a smaller amount (10–15%) loosely bound to albumin. Less than 1% of circulating vitamin D exists in a free, unbound form.(4,5) We previously demonstrated that bioavailable 25(OH)D is more tightly associated with bone density than total levels in healthy individuals.(6)
We hypothesized that the relationship between vitamin D and markers of mineral metabolism (e.g. PTH and calcium) in ESRD would be strengthened by use of DBP and albumin to determine bioavailable vitamin D levels. Given the patterns observed in other cohorts, we further hypothesized that the lower total 25(OH)D levels typically seen in black dialysis patients not necessarily be associated with lower bioavailable vitamin D levels in this group.(6,7)
Results
Baseline characteristics of the 94 subjects included in this analysis, which are similar to those of a typical US hemodialysis population, are summarized in Table 1. None of the included subjects were recorded as receiving treatment with activated vitamin D, ergocalciferol, or cholecalciferol before initiating dialysis.
Table 1.
Characteristics of the population (n=94)
| Median (IQR) or n (%) | |
|---|---|
| Age, years | 65 (50–74) |
| Male | 55 (59%) |
| Black race | 48 (51%) |
| Survived at least one year on dialysis | 47 (50%) |
| Body mass index | 25 (22–30) |
| Systolic blood pressure, mm Hg | 140 (123–153) |
| Diastolic blood pressure, mm Hg | 73 (61–81) |
| Total 25(OH)D, ng/ml | 20 (13–28) |
| Total 1,25(OH)2D, pg/ml | 9.5 (5–16) |
| Parathyroid hormone, pg/ml | 190 (96–307) |
| Corrected Calcium, mg/dl | 8.9 (8.5–9.4) |
| Phosphorus, mg/dl | 4.2 (3–5.5) |
| Alkaline phosphatase, mg/dl | 82 (66–112.5) |
| Albumin, g/dl | 3.4 (3.0–3.8) |
| Vitamin D binding protein, μg/ml | 158 (69–217) |
| Bioavailable 25(OH)D, ng/ml | 3.4 (2.2–5.0) |
| Bioavailable 1,25(OH)2D, pg/ml | 2.2 (1.1–3.8) |
Mineral Metabolism and Vitamin D
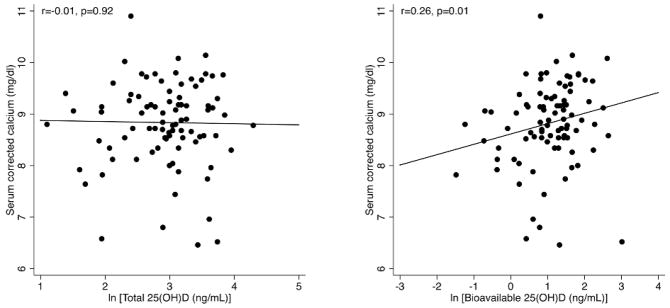
Baseline corrected calcium levels, measured within 14 days of chronic hemodialysis initiation, were not associated with total levels of either 25(OH)D (r=0.01, P=0.92) or 1,25(OH)2D (r=0.08, P=0.44). In contrast, calcium levels correlated positively with both bioavailable 25(OH)D (r=0.26, p=0.01) and bioavailable 1,25(OH)2D (r=0.23, p=0.02). These relationships are plotted in Figure 1. A single individual with the highest bioavailable 25(OH)D and bioavailable 1,25(OH)2D appeared to be an outlier with respect to the observed relationships, with both levels over 4 standard deviations above the mean. To examine the impact of this single data point, we performed a sensitivity analysis by repeating the analysis with this individual excluded. The relationship of calcium with bioavailable 25(OH)D (r=0.30, p=0.003) and bioavailable 1,25(OH)2D (r=0. 27, p=0.008) were both somewhat strengthened.
Figure 1.
Total vs bioavailable 25(OH)D and serum calcium. Total levels of 25(OH)D demonstrated no association with serum calcium levels (corrected for albumin) while bioavailable 25(OH)D levels were positively associated with serum calcium.
Phosphorus levels demonstrated no association with either total levels of 25(OH)D (r=0.14, P=0.19) or 1,25(OH)2D (r=−0.01, P=0.94). Similarly, neither bioavailable 25(OH)D (r=−0.10. P=0.32) nor bioavailable 1,25(OH)2D (r=−0.16, P=0.12) were significantly associated with phosphorus levels.
Alkaline phosphatase was not associated with either total or bioavailable forms of 25(OH)D or 1,25(OH)2D (p>0.05 for all comparisons).
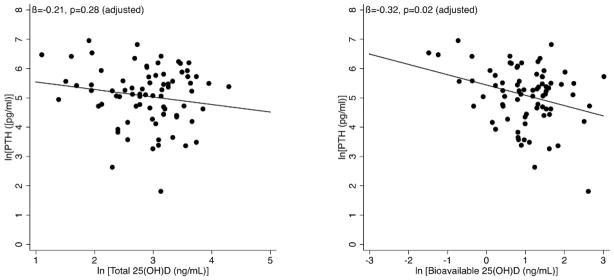
The relationship between PTH and all four forms of vitamin D were examined in univariate and multivariate regression models. In univariate models, only bioavailable 25(OH)D was associated with PTH, with a −0.35 log decrease in PTH for each log increase in bioavailable 25(OH)D (p=0.01). In a multivariate model controlling for age, gender, race, and survival status at one year, this relationship remained unchanged (β= −0.32, p=0.02). A third model adding calcium, phosphorus, and bioavailable 1,25(OH)2D levels demonstrated similar results (Table 3). As with the calcium findings, both the unadjusted and adjusted coefficients became stronger when a single outlier was excluded (unadjusted: β= −0.40, p=0.003; adjusted: β= −0.36, p=0.01). In contrast, there was no significant association between total 25(OH)D and PTH (Figure 2).
Table 3.
PTH and bioavailable 25(OH) vitamin D. In univariate and multivariate analyses, bioavailable 25(OH) vitamin D levels were consistently associated with PTH (corresponding p values displayed). PTH and bioavailable vitamin D levels were log transformed prior to analysis, thus β= −0.36 suggests that a 25% increase in bioavailable 25(OH)D is associated with 7.7% decrease in PTH ((1.25−0.36 −1)*100 = −7.7).
| β | p | |
|---|---|---|
| Bioavailable 25(OH)D alone | −0.36 | 0.007 |
| Multivariate model adding age, gender, race | −0.33 | 0.02 |
| Multivariate model with above variables plus survival status at 1 year | −0.32 | 0.02 |
| Multivariate model with above variables plus calcium, phosphorus, bioavailable 1,25(OH)2D | −0.39 | 0.02 |
Figure 2.
Total vs bioavailable 25(OH)D and PTH. After adjustment for age, gender, race, and survival status at one year, bioavailable 25(OH)D was significantly negatively associated with PTH levels, while total 25(OH)D demonstrated no association with PTH.
Patient Factors and Vitamin D
Older individuals had higher total 25(OH)D levels (r=0.31, P=0.003) and bioavailable 25(OH)D (r=0.21, p=0.04). Neither total nor bioavailable 1,25(OH)2D were associated with age. Female gender was associated with lower total 25(OH)D levels (median in men: 22.0 ng/dl, in women: 18.0 ng/dl; p=0.03). While females had numerically lower median total 1,25(OH)2D and bioavailable 25(OH)D and 1,25(OH)2D levels, none of these differences were statistically significant.
Black individuals had lower total 25(OH)D levels (median: 15.2 vs 23.2 ng/ml, p<0.001) but not bioavailable 25(OH)D levels (median: 3.8 vs. 2.8 ng/ml, p=0.21). The contrast in racial differences between these two forms of vitamin D was driven largely by lower DBP levels among blacks. This difference persisted even when examining only individuals who survived for one year on dialysis or those who died in this timeframe (Table 2). PTH levels did not differ significantly by race (median: 201 pg/ml [black] vs. 168 pg/ml [white], p=0.47). Neither total nor bioavailable 1,25(OH)2D levels differed by race (p=0.07 and 0.49, respectively). Of note, we found no racial differences in systolic or diastolic blood pressure, diabetes, or BMI.
Table 2.
Race and vitamin D levels. Black individuals had lower total, but not bioavailable, 25(OH)D levels when compared with their white counterparts. Survivors are patients who survived for at least one year after initiating hemodialysis, while non-survivors died within this year. All values represent group medians.
| Blacks | Whites | p | |
|---|---|---|---|
| Total 25(OH)D (ng/ml) | 15.1 | 23.1 | <0.001 |
| Bioavailable 25(OH)D (ng/ml) | 3.8 | 2.8 | 0.21 |
| Total 1,25(OH)2D (pg/ml) | 8 | 11.5 | 0.07 |
| Bioavailable 1,25(OH)2D (pg/ml) | 2.2 | 2.2 | 0.48 |
| DBP (μg/ml) | 75 | 189 | <0.001 |
| Survivors: DBP (μg/ml) | 88 | 195 | 0.004 |
| Non-survivors: DBP (μg/ml) | 58 | 183 | <0.001 |
| PTH (pg/ml) | 201 | 168 | 0.47 |
Systolic and diastolic blood pressure, BMI, and survival were not associated with any form of vitamin D. Similarly, there was no association between any form of vitamin D and either a diagnosis of diabetic nephropathy or diabetes (data not shown). The study was not specifically powered to address these factors.
Sensitivity Analysis
Sensitivity analyses were performed to address the possibility that uremia might alter DBP’s binding affinity with 25(OH)D or 1,25(OH)2D. With DBP-binding coefficients that were 25% lower than those originally determined by Bikle, et al.,(4,5) bioavailable measures of both 25(OH)D (r=0.26, p=0.01) and 1,25(OH)2D (r=0.22, p=0.03) remained associated with corrected calcium. Similar results were observed with 25% higher coefficients (bioavailable 25(OH)D: r=0.27, p=0.009; bioavailable 1,25(OH)2D (r=0.24, p=0.02). Associations of bioavailable 25(OH)D with PTH remained statistically significant in both cases, with association coefficients changing less than 12% in either univariate or multivariate analyses.
Discussion
Using a retrospective cohort of incident dialysis patients, we studied the relationship between measures of mineral metabolism (including serum calcium and PTH) with both total and bioavailable levels of vitamin D. In a prior study, we had assessed the possibility that the free hormone hypothesis could explain discrepant findings in the relationship between bone mineral density (BMD) and 25(OH)D levels in healthy young individuals without CKD. We found that bioavailable 25(OH)D levels were predictive of bone density (as measured by dual X-ray absorptiometry) whereas total 25(OH)D levels did not.(6) We further found that bioavailable levels, which combine free and albumin-bound hormone, were more strongly associated with BMD than free hormone levels alone. In the present study, we similarly found that bioavailable 25(OH)D was associated with both corrected serum calcium levels and PTH, both of which are well-established measures of mineral metabolism in ESRD, while total 25(OH)D demonstrated no such associations. This data builds upon our prior findings: analysis from two separate cohorts now support the hypothesis that bioavailable measures of vitamin D, which take into account binding of vitamin D to albumin and DBP, are more relevant to biological outcomes than are total levels, which are currently the standard measure of vitamin D status.
Some in vitro studies suggest that DBP-binding limits vitamin D activity in multiple target cells.(8,9) Studies of DBP-null mice have shown that these animals display markedly reduced levels of 25(OH)D and 1,25(OH)2D compared with wild-type mice, with a markedly reduced half-life.(10) Despite their low vitamin concentrations, when these mice are provided with a steady source of dietary vitamin D, they show no differences in serum calcium, phosphorus, alkaline phosphatase, and PTH compared to wild-type controls. These studies support the application of the free hormone hypothesis to vitamin D physiology, at least for some biological actions. Despite these findings, uptake of protein-bound hormone in cells expressing megalin appears to be important for some processes, so the biology underlying our findings may be more complex than is immediately apparent and warrants further investigation.(11,12)
We oversampled black patients as our prior data from a separate cohort suggested blacks have lower DBP levels than whites, an observation supported by this study. As previously reported, we and others observed that black race is associated with lower levels of total 25(OH)D.(2,13) As might be expected from these two parallel racial differences (lower total 25(OH)D and lower DBP in blacks vs. whites), we found that levels of bioavailable 25(OH)D are similar. Black individuals are thought to have decreased cutaneous synthesis of vitamin D because the higher concentrations of melanin limit available ultraviolet energy exposure in the skin. Lower DBP levels may be an adaptive response in black individuals to reduce the risk of functional vitamin D deficiency in the setting of decreased production of 25(OH)D. These racial relationships will need to be confirmed in the general population.
Despite similar bioavailable D levels between racial groups, and association between bioavailable 25(OH)D and PTH, black patients had numerically higher PTH levels than their white counterparts. Though this difference was not statistically significant in our sample, larger samples from this cohort have found significantly higher levels of PTH in black individuals.(14) Bioavailable 25(OH)D did not differ by race, yet were negatively associated with PTH, suggesting that racial differences in PTH are not primarily driven by differences in 25(OH)D. Indeed, others have found that PTH levels in blacks are higher than those in whites, even in states of 25(OH)D sufficiency.(13)
Several studies have attempted to assess the metabolic consequences of low 25(OH)D levels in advanced CKD and ESRD, but have yielded conflicting results. Ergocalciferol, a form of nutritional vitamin D that can increase 25(OH)D levels, appears to affect parathyroid hormone (PTH) levels in stage 3, but not in stage 4, CKD.(15,16) Moreover, some studies have demonstrated a significant association between 25(OH)D levels and PTH in ESRD,(17–19) while others have not.(20,21) Associations between 25(OH)D and serum calcium have been similarly mixed.(2,17,22)
This contradictory data has led to confusion about the role that repleting 25(OH)D (e.g. with nutritional forms of vitamin D such as cholecalciferol or ergocalciferol) plays in the management of patients with ERSD.(23,24) In order to study the role of vitamin D deficiency and identify patients who are most likely to benefit from repletion, it is critical to have a biologically relevant measure of vitamin D status. Notably, our study failed to find any significant link between total or bioavailable 1,25(OH)2D and relevant measures of mineral metabolism, echoing the general consensus that circulating serum levels of the active hormone are not useful as a measure of vitamin D status.(1)
We did not find a relationship between survival and vitamin D status, though this sample had considerably less power to detect this relationship than prior studies, which have found that severe vitamin D deficiency (typically defined levels < 10 ng/ml) is associated with increased mortality.(19,25,26) Larger studies will be needed to identify differences in mortality associations between total and bioavailable levels of vitamin D.
This study utilized a relatively small sample of dialysis patients, and thus it will be important to study these relationships in additional cohorts. None of the individuals in this analysis, who initiated dialysis in 2004 or 2005, had been treated with activated vitamin D analogs prior to initiating dialysis. While this simplified our analysis, the use of these agents in pre-dialysis CKD is increasingly common. It will therefore be important to determine the effects of these analogs on DBP and bioavailable vitamin D levels and on the relationship between bioavailable vitamin D levels and clinically-relevant outcomes. PTH is commonly used as a proxy for metabolic bone disease in dialysis patients, but has an imperfect association with bone disease.(27) Bone biopsies and non-invasive measures of bone density and structure were not available in this study and are potential targets for future analyses. While we previously demonstrated a relationship between bioavailable 25(OH)D and bone density in a healthy population,(6) it is not certain that this relationship will extend to the ESRD population given known alterations in mineral metabolism.
Metabolic changes that accompany ESRD and/or dialysis, as well as genetic variants in DBP or other relevant proteins, have the potential to influence binding of 25(OH)D to DBP. Whereas our sensitivity analysis did not indicate that these factors are likely to affect the fundamental findings of this study, studies that directly measure bound and unbound fractions could the improve upon our initial estimates and the equations used. Lastly, it is possible that measured 25(OH)D levels in this study were influenced by levels of 24,25(OH)D. Confirmation of our findings with assays able to differentiate 25(OH)D, 24,25(OH)D, and 1,24,25(OH)D may further elucidate these biological relationships.
This study provides additional evidence to support the notion that bioavailable, rather than total, levels of vitamin D may be more relevant measures of vitamin D status with respect to its actions on mineral metabolism. While mineral metabolism has been the traditional focus of vitamin D actions, recent data suggest that its actions may be more widespread, with effects on the immune response,(28) hypertension,(29) and insulin sensitivity,(30), among others. Studies assessing the effects of albumin and DBP in modifying these relationships will shed further light on the best way to identify individuals who are most likely to benefit from supplementation.
Methods
Accelerated Mortality on Renal Replacement (ArMORR) is a nationally representative prospective cohort study of incident chronic hemodialysis patients (n=10,044) who began renal replacement between July 1, 2004 and July 30, 2005 at one of 1,056 dialysis centers in the U.S. operated by Fresenius Medical Care, North America (FMC).(31) The ArMORR dataset contains a broad range of demographic and clinical data including co-existing medical conditions, laboratory results, as well as serum and plasma samples. Clinical data were collected prospectively, entered uniformly into a central database by practitioners at the point of care. All clinical data arriving at Fresenius undergo rigorous data quality assurance and quality control (QA/QC) auditing. Blood samples collected for clinical care were shipped to and processed by a central laboratory (Spectra East, Rockland, NJ, USA). After processing for routine clinical testing, remnant samples were shipped on ice to the ArMORR Investigators where the samples were aliquotted and stored in liquid nitrogen. This study was approved by the Institutional Review Board of the Massachusetts General Hospital, which waived the requirement for informed consent, and conducted in accordance with its ethical standards and the Declaration of Helsinki.
Study population
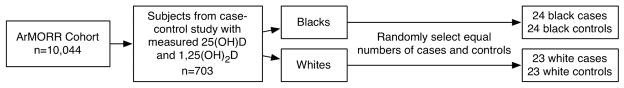
Between July 1, 2004 and June 30, 2005, 10,044 incident hemodialysis patients were prospectively enrolled into ArMORR. We identified subjects who had 25(OH)D and 1,25(OH)2D levels previously measured as part of a case-control survival study.(19) Based on prior results in a healthy population, we set a minimum sample size of 80 subjects. To ensure adequate power for racial comparisons, we randomly selected an approximately equal number of black (n=24) and white (n=23) patients from the controls, and an equal number of race-matched cases. Thus, the total sample size was n=94. Baseline laboratory values were measured from samples collected within 14 days of dialysis initiation.
Assays
Total 25(OH)D and 1,25(OH)2D were previously measured from thawed samples in duplicate using a commercially available radioimmunoassay (DiaSorin Inc, Stillwater, MN, USA). The interassay coefficients of variation (CVs) for 25(OH)D were <3% at levels <30 ng/ml and for 1,25(OH)2D were <6.5% at levels <32.5 pg/ml. Intact PTH (1–84) was measured using the Nichols Advantage Biointact-PTH assay by the centralized laboratory.
DBP was measured in duplicate in thawed serum samples by commercial enzyme linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, Catalog Number DVDBP0) according to the manufacturer’s instructions. The assay was conducted after diluting serum samples 1 to 2,000 in Calibrator Diluent RD6-11 (R&D Systems Part Number 895489). Inter-assay CV was 8.5% at a concentration of 40 μg/ml. The assay recovered between 93 and 110% of a 100–200 μg/mL dose of exogenous DBP added to human serum samples containing between 25–200 μg/mL of endogenous DBP. There were no differences in the recovery of exogenous DBP in black patients or obese patients. The manufacturer reports no significant cross-reactivity with human albumin or vitamin D3.
DBP levels were below the detection limit in 5 black patients who died within the first year of dialysis. These individuals were assigned a DBP value equal to the lowest detectable level (12.3 μg/dl).
Calculation of Bioavailable Vitamin D
Equilibrium dialysis and centrifugal ultrafiltration dialysis have previously been used by some investigators to indirectly measure free vitamin D levels, allowing estimation of the binding affinity constants for 25(OH)D and 1,25(OH)2D with DBP and albumin.(4,5,32) In these studies, calculated levels of free 25(OH)D and levels measured by centrifugal ultrafiltration were highly correlated (r=0.925).(5) As previously described, the equations developed by Vermeulen et al. and originally applied to testosterone were adapted for determination of bioavailable 25(OH)D and 1,25(OH)2D levels.(6,33) These adapted Vermeulen equations yield free 25(OH)D levels that are nearly identical to those initially determined by Bikle, et al. while also allowing for identification of bioavailable (free+albumin-bound) fractions.(5,6) Bioavailable 1,25(OH)2D levels were determined using the same approach using affinity constants previously derived by centrifugal ultrafiltration dialysis.(4)
These affinity constants were previously validated in both healthy and cirrhotic individuals,(4,5) but have not been directly assessed in hemodialysis patients. We therefore performed a sensitivity analysis of the main findings using DBP binding coefficients for 25(OH)D and 1,252(OH) that were 25% higher or 25% lower than previously measured values.
Statistical Analysis
Prior to analysis, given the role of albumin as a binding protein for both vitamin D and calcium, serum calcium levels were corrected for albumin using the following equation: corrected calcium = total calcium + 0.8*(4-albumin).(34) Spearman correlation analysis was performed to assess linear associations. Group comparisons of vitamin D levels were performed using the Wilcoxon rank sum test. To examine multivariable associations between bioavailable vitamin D and PTH, both variables (because of non-normal distribution) were natural-log transformed and analyzed using multivariate linear regression. All analyses were conducted using STATA Statistical Software (College Station, TX) version 11.
Supplementary Material
Figure 3.
Sample selection. 25(OH)D and 1,25(OH)2D were previously measured as part of a case-control study within the ArMORR cohort. Equal numbers of cases (subjects who died within their first year on dialysis) and controls were randomly selected from each racial group.
Acknowledgments
The authors wish to thank Kathryn Lucchesi, PhD, RPh, for helpful comments and suggestions about the manuscript.
Funding: Dr. Bhan is supported by NIH Young Investigator Grant K23 1K23DK081677 (Bethesda, MD).
Footnotes
Disclosure
The authors have no competing financial interests to declare.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Bhan I, Burnett-Bowie S-AM, Ye J, Tonelli M, Thadhani R. Clinical measures identify vitamin D deficiency in dialysis. Clin J Am Soc Nephrol. 2010 Mar;5(3):460–467. doi: 10.2215/CJN.06440909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989 Aug;10(3):232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 4.Bikle DD, Siiteri PK, Ryzen E, Haddad J, Gee E. Serum Protein Binding of 1,25-Dihydroxyvitamin D: A Reevaluation by Direct Measurement of Free Metabolite Levels. J Clin Endocrinol Metab. 1985 Nov 1;61(5):969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 5.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the Free Fraction of 25-Hydroxyvitamin D in Serum and Its Regulation by Albumin and the Vitamin D-Binding Protein. J Clin Endocrinol Metab. 1986 Oct 1;63(4):954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 6.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011 Jul;26(7):1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powe CE, Seely EW, Rana S, Bhan I, Ecker JL, Karumanchi SA, et al. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010 Oct;56(4):758–763. doi: 10.1161/HYPERTENSIONAHA.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010 Jul;95(7):3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikle DD, Gee E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology. 1989 Feb;124(2):649–654. doi: 10.1210/endo-124-2-649. [DOI] [PubMed] [Google Scholar]
- 10.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999 Jan;103(2):239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005 Sep 9;122(5):751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Lundgren S, Carling T, Hjälm G, Juhlin C, Rastad J, Pihlgren U, et al. Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem. 1997 Mar;45(3):383–392. doi: 10.1177/002215549704500306. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporosis Int. 2011 Jun;22(6):1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf M, Betancourt J, Chang Y, Shah A, Teng M, Tamez H, et al. Impact of Activated Vitamin D and Race on Survival among Hemodialysis Patients. J Am Soc Nephrol. 2008 Apr 9;19(7):1379–1388. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of Ergocalciferol Treatment of Vitamin D Deficiency on Serum Parathyroid Hormone Concentrations in Chronic Kidney Disease. Am J Nephrol. 2007;27(1):36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 16.Al-Aly Z, Qazi RA, González EA, Zeringue A, Martin KJ. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007 Jul 1;50(1):59–68. doi: 10.1053/j.ajkd.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Jean G, Charra B, Chazot C. Vitamin D Deficiency and Associated Factors in Hemodialysis Patients. J Ren Nutr. 2008 Sep;18(5):395–399. doi: 10.1053/j.jrn.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009 Jan 1;75(1):88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 19.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007 Oct 1;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 20.González EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004 Aug;24(5):503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 21.London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007 Feb 1;18(2):613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 22.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul M-C. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004 Jul 1;15(7):1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 23.Nigwekar SU, Bhan I, Thadhani R. Nutritional vitamin D in dialysis patients: what to D-iscern? Nephrology Dialysis Transplantation. 2011 Mar;26(3):764–766. doi: 10.1093/ndt/gfq799. [DOI] [PubMed] [Google Scholar]
- 24.Bhan I, Hewison M, Thadhani R. Dietary vitamin D intake in advanced CKD/ESRD. Semin Dial. 2010 Jun;23(4):407–410. doi: 10.1111/j.1525-139X.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 25.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010 Sep;31(18):2253–2261. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2011 Mar;26(3):1024–1032. doi: 10.1093/ndt/gfq606. [DOI] [PubMed] [Google Scholar]
- 27.Ott SM. Review article: Bone density in patients with chronic kidney disease stages 4–5. Nephrology. 2009 Jun;14(4):395–403. doi: 10.1111/j.1440-1797.2009.01159.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhan I, Camargo CA, Wenger J, Ricciardi C, Ye J, Borregaard N, et al. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol. 2011 May;127(5):1302–4.e1. doi: 10.1016/j.jaci.2010.12.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007 May;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 30.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007 Jan 1;71(2):134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 31.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Koeffler HP, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009 Feb 15;48(4):418–424. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hoof HJ, Swinkels LM, Ross HA, Sweep CG, Benraad TJ. Determination of non-protein-bound plasma 1,25-dihydroxyvitamin D by symmetric (rate) dialysis. Anal Biochem. 1998 May 1;258(2):176–183. doi: 10.1006/abio.1998.2586. [DOI] [PubMed] [Google Scholar]
- 33.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999 Oct;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 34.Correcting the calcium. Br Med J. 1977 Mar 5;1(6061):598. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.