Abstract
Acute lymphoblastic leukemia (ALL) is the most common malignancy affecting children and a major cause of mortality from hematopoietic malignancies in adults. A substantial number of patients become drug resistant during chemotherapy, necessitating the development of alternative modes of treatment. rGel/BLyS is a toxin-cytokine fusion protein used for selective killing of malignant B-cells expressing receptors for B-cell Activating Factor (BAFF/BLyS) by receptor-targeted delivery of the toxin, Gelonin. Here we demonstrate that rGel/BLyS binds to ALL cells expressing BAFF receptor (BAFF-R) and upon internalization, it induces apoptosis of these cells and causes down-regulation of survival genes even in the presence of stromal protection. Using an immunodeficient transplant model for human ALL, we show that rGel/BLyS prolongs survival of both Philadelphia chromosome-positive and negative ALL-bearing mice. Furthermore, we used AMD3100, a CXCR4 antagonist, to mobilize the leukemic cells protected in the bone marrow microenvironment and the combination with rGel/BLyS resulted in a significant reduction of the tumor load in the bone marrow and complete eradication of ALL cells from the circulation. Thus, a combination treatment with the B-cell-specific fusion toxin rGel/BLyS and the mobilizing agent AMD3100 could be an effective alternative approach to chemotherapy for the treatment of primary and relapsed ALL.
Keywords: BAFF-R, BAFF, Gelonin, AMD3100, stromal co-culture, leukemia cell mobilization
Introduction
Precursor B-lineage acute lymphoblastic leukemia (ALL) is the most common type of leukemia in children up to 9 years of age. In the USA, ALL additionally represents around 1/3 of all cases of adult leukemias.1,2 In such patients, accumulation of blasts at the pre-B stage of differentiation, initially in the bone marrow, is followed by subsequent infiltration at various other peripheral sites.3,4 Treatment usually consists of vincristine, prednisone and anthracycline with or without asparaginase. Those patients that are cured may experience long-term side effects due to the treatment with non-selective cytotoxic drugs. However, relapse during or after treatment remains the most urgent short-term challenge facing patients with ALL.5–7 Unfortunately, there is a limit to what can be accomplished with the addition of non-selective cytotoxic agents due to systemic toxicity.
A frequent site of relapse is the bone marrow 8,9, which may be a particularly natural protective site for such cells. Therefore, we and others are focusing on the bone marrow microenvironment as a possible target for treatment by investigating the interactions between cell surface receptors on the ALL cells that are engaged by factors produced in the bone marrow. Recently, we were the first to report that pre-B ALL cells express the B cell-activating factor receptor (BAFF-R).10 Although this receptor was originally identified only on more mature B-lineage cells, we and others found that many pre-B ALLs including both cell lines and patient samples express cell surface BAFF-R 10–13, but we were unable to detect it on normal bone marrow pre-B cells.
The BAFF-R has a single ligand called BAFF (B-cell activating factor), known also as BLyS, (B-lymphocyte stimulator), which is produced by dendritic cells, monocytes, bone marrow stromal cells and macrophages. In macrophages, expression is induced by pro-inflammatory stimuli such as interferon-γ, but also by interleukin-10 (IL-10). Besides the BAFF-R, there are two other receptors that bind BAFF: BCMA (B-cell maturation antigen), and TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor)14–19. Expression of all three is mainly found on B-lineage hematopoietic cells, and the best characterized functions are in plasma cells and naïve and memory B-cells, 20–22 where they co-stimulate proliferation and promote survival of these mature B cells in vitro and in vivo.14, 15
The BAFF-R expression on normal mature B-cells is maintained in B-lineage malignancies such as mantle cell lymphoma, chronic lymphocytic leukemia (CLL) and diffuse large-B cell lymphoma (DLBL)23, 24, 25 Lyu et al recently generated a recombinant fusion protein between Gelonin and BAFF (BLyS) for the specific delivery of Gelonin to malignant mature B cells expressing BAFF receptors.25 Gelonin is a plant toxin which inhibits protein synthesis by inactivating ribosomes. It cannot enter the cells by itself since it lacks the ability to bind to the cell surface.26 Thus, although recombinant Gel (rGel) alone is ineffective in killing cells, fusion constructs and immunotoxins made with rGel were reported to successfully kill malignant cells.27–33 Interestingly, rGel/BLyS inhibited the growth of DLBL in vitro and in vivo and induced apoptosis in CLL cells and. 31, 33,
The expression of the BAFF-R on pre-B ALL (80%–99%, as detected by FACS analysis)10 prompted us to investigate if this targeted construct could be utilized as a basis to eradicate them. We here report that rGel/BLyS is a very promising therapeutic agent with selective cytotoxicity mediated by its fusion to the ligand for the BAFF-R. Moreover, by combining this selective but toxic fusion protein with a non-toxic ALL mobilizing agent, we were able to significantly deplete the pool of malignant lymphoblasts in vivo that could form the basis for relapse in the bone marrow.
Methods
Reagents and antibodies
AMD3100 octahydrochloridehydrate (1,1’-[1,4-phenylene bis (methylene) bis-1, 4,8,11 Tetra azacyclotetradecane octahydrochloride) was purchased from Sigma-Aldrich, St. Louis, USA. The rGel/BLyS protein, consisting of Gelonin fused to the N-terminus of human BLyS, was expressed in E.coli as previously described.25 Antibodies used are described in Supplementary Methods.
Evaluation of binding of rGel/BLyS to ALL cells
ALL cells used here originate from primary human isolates that have been passaged in NOD/SCID/IL2rγ−/− (NSG) mice and were described previously.10,34 In brief, US7 and US7R were from one patient before and after the development of resistance against treatment; BLQ-1, P-2 and TXL2 are Ph-positive ALLs with and without the T315I mutation in Bcr/Abl. All ALLs were grown on irradiated OP9 feeder layers as previously described.10 For evaluation of their ability to bind to rGel/BLyS, they were incubated with 400 nM rGel/BLyS (recombinant Gelonin-BLyS fusion protein) or rGel (recombinant Gelonin) for 2 hours at 37°C, washed with PBS, fixed, permeabilized and incubated with a polyclonal rabbit anti-Gelonin antibody followed by a FITC conjugated secondary antibody and analyzed by FACS (Accuri flow cytometers Inc, MI, USA). Immunohistochemistry using an anti-Gelonin antibody was performed on permeabilized US.7 cells. For competition assays, US7 ALL cells were pre-incubated with recombinant human BAFF or anti BAFF-R antibody for 2 hours, followed by incubation for 2 hours with 100 nM rGel/BLyS. BAFF-R Fc and rGel/BLyS were added together for the two-hour incubation. Cells were next washed with PBS and detection of binding of the rGel/BLyS fusion protein was done as described above. To detect intracellular survival proteins by FACS, cells were fixed, permeabilized using fixation and permeabilization buffers according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA), incubated with specific antibodies (45 minutes, room temperature) and washed with PBS before analysis.
In vitro treatment
For CXCR4 detection, ALL cells were incubated with 1 µM AMD3100 for 24 hours. Cells were collected, washed with PBS, incubated with anti-human CXCR4 antibody for 30 minutes, washed with PBS and analyzed by flow cytometry. Appropriate isotype antibodies and cells without AMD3100 treatment served as controls. For migration assays, SDF-1α (200 ng/ml) or OP9 stromal cells were added to the lower wells of a 5 µm pore Transwell. After 24 hours, ALL cells were treated with AMD3100 (10 µM) for 30 minutes at 4°C, seeded at 5×104 cells in the upper wells and incubated for 90 minutes. Non-adherent cells were collected from the lower wells and counted using an automated cell counter. Wells without SDF-1α or OP-9 cells served as controls. For adhesion assays, US.7 cells and OP-9 cells were co-cultured for 2 weeks. Floating US.7 cells were removed by gentle washing using medium. At this time, almost all the US.7 cells present were under the stromal layer. AMD3100 (10 µM) together with fresh medium was added to the co-culture plates. Non-adherent cells were counted after 2, 6, 10 and 24 hours of incubation. US.7 cells treated the same way without AMD3100 served as controls. For in vitro combination treatment using rGel/BLyS and AMD3100, drugs were added in different combinations as described in the figure legend. Annexin V staining was done according to the manufacturer’s (BD Pharmingen, San Jose, CA, USA.) instructions. For detection of NF-κB (p65), a nuclear extraction kit (Imgenex, San Diego, CA, USA) was used to separate nuclear and cytoplasmic fractions. 30 µg of nuclear protein was loaded in each well. Western blots were incubated overnight with primary antibody. Cell viability in all experiments was determined by Trypan blue exclusion.
Human ALL transplant model
All animal experiments were carried out in concordance with Institutional IACUC and NIH guidelines. Human ALL cells were injected at 1.5×106 cells/animal (US.7) or 3×106 cells/animal (TXL-2) into NSG mice. Cells were allowed to proliferate in vivo for 6 days before start of treatment, at which point all mice had a similar amount of CD10+, CD19+ cells in their PB (Suppl. Figure 2A). US.7 transplanted mice (n = 6/group) were injected i.p. during days 6–25 for a total of 6x with PBS or 6x with rGel/BLyS (3.75 mg/kg). TXL-2 transplanted mice (n = 5/group; n = 6/group for body weight measurements of groups (c) and (d) below) were treated from days 6–33 with (a) 8x PBS (b) 3x PBS followed by 5x AMD3100 (10 mg/kg) (c) 8x with rGel/BLyS (2.75 mg/kg) or (d) 8x with rGel/BLyS plus 5x with AMD3100. Supplementary methods provide additional experimental details.
Statistical analysis
All in vitro experiments were done in triplicate and on triplicate samples. Student’s t-test was performed to assess statistical significance of the results. The significance of survival was analyzed using the log rank test.
Results
rGel/BLyS binds to human pre-B ALL cells
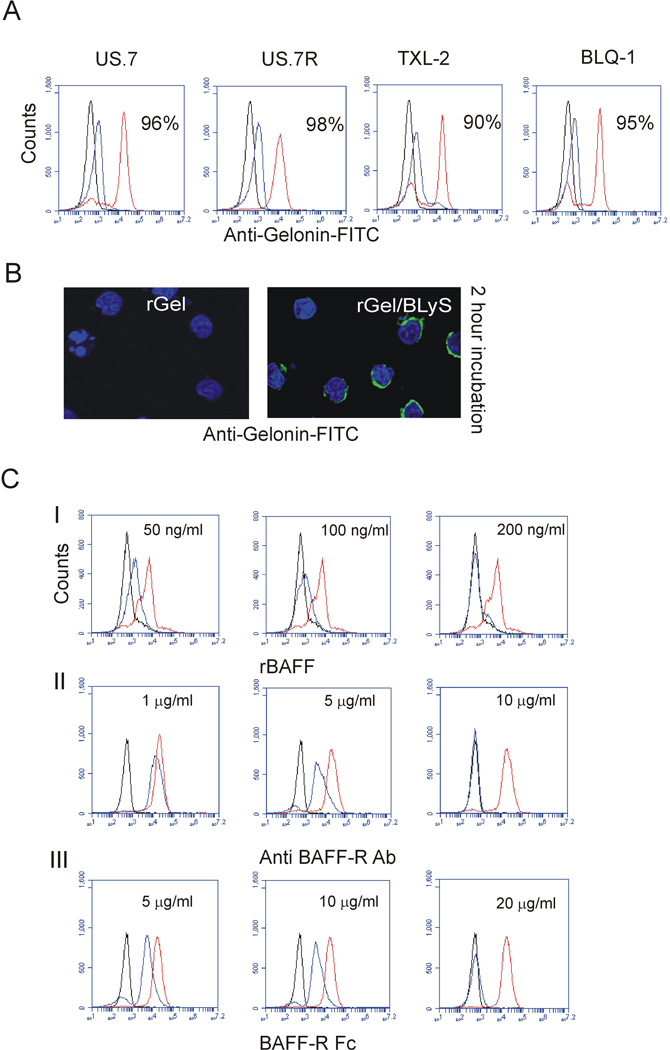
We used both Ph negative (US.7, US.7R) and Ph positive (TXL-2, BLQ-1) ALL cells to examine if the rGel/BLyS fusion protein is able to bind to the BAFF-R expressed by pre-B ALL cells. After a 2-hour incubation of ALL cells with rGel/BLyS fusion protein or with control rGel, we examined the presence of these proteins in and on the ALL cells using a Gelonin-specific antibody. As shown in Figure 1A, rGel-treated cells showed no staining with anti-Gelonin antibodies (black line) and little rGel/BLyS (blue line) was detected on the surface of the cells. However, a clear positive signal in permeabilized cells indicates that rGel/BLyS had been internalized (Figure 1A, red line). This confirmed that Gelonin is unable to cross the plasma membrane without conjugation to BLyS. We also demonstrated the intracellular presence of rGel/BLyS by fluorescent microscopy (Figure 1B). To investigate the specificity of rGel/BLyS binding to the BAFF-R on the pre-B ALL cells, US.7 cells were either pre-treated with recombinant BAFF, with antibodies to BAFF-R (2 hours before addition of rGel/BLyS) or with recombinant BAFF-R Fc (added together with rGel/BLyS). As evidenced by FACS analysis on permeabilized cells (Figure 1C), BAFF, BAFF-R antibody and BAFF-R Fc inhibited rGel/BLyS detection in US.7 cells in a dose- dependent manner. Thus, rGel/BLyS failed to bind to the BAFF-R when these receptors were pre-occupied with recombinant BAFF or BAFF-R antibody.
Figure 1. rGel/BLyS binds to human ALL cells.
(A) FACS analysis using anti-Gelonin antibodies for rGel (black) or cell surface rGel/BLyS (blue) and total rGel/BLyS (red) after incubation with 400 nM rGel/BLyS. Percentages indicate positivity for total rGel/BLyS (B) Immunohistochemistry for rGel or rGel/BLyS in US.7 cells. Note the apparent signal of rGel/BLyS as a perinuclear ring, due to the relatively large nucleus and small volume of cytoplasm in these cells. Green, Gelonin antibodies, blue, DAPI counter-stain for the nucleus. Images were captured using a Leica DIC analyzer, 200x 1.4–0.7, oil. (C) Detection of rGel or rGel/BLyS with anti-Gelonin antibody after pre-incubation of US.7 cells with the indicated concentrations of (I) recombinant human BAFF or (II) BAFF-R antibody; both then followed by incubation with 100 nM rGel/BLyS or (III) when BAFF-R Fc was added together with rGel/BLyS. (n=3). Controls; isotype (black) and positive control (no treatment, red); treated cells (blue).
rGel/BLyS induces apoptosis of human ALL cells
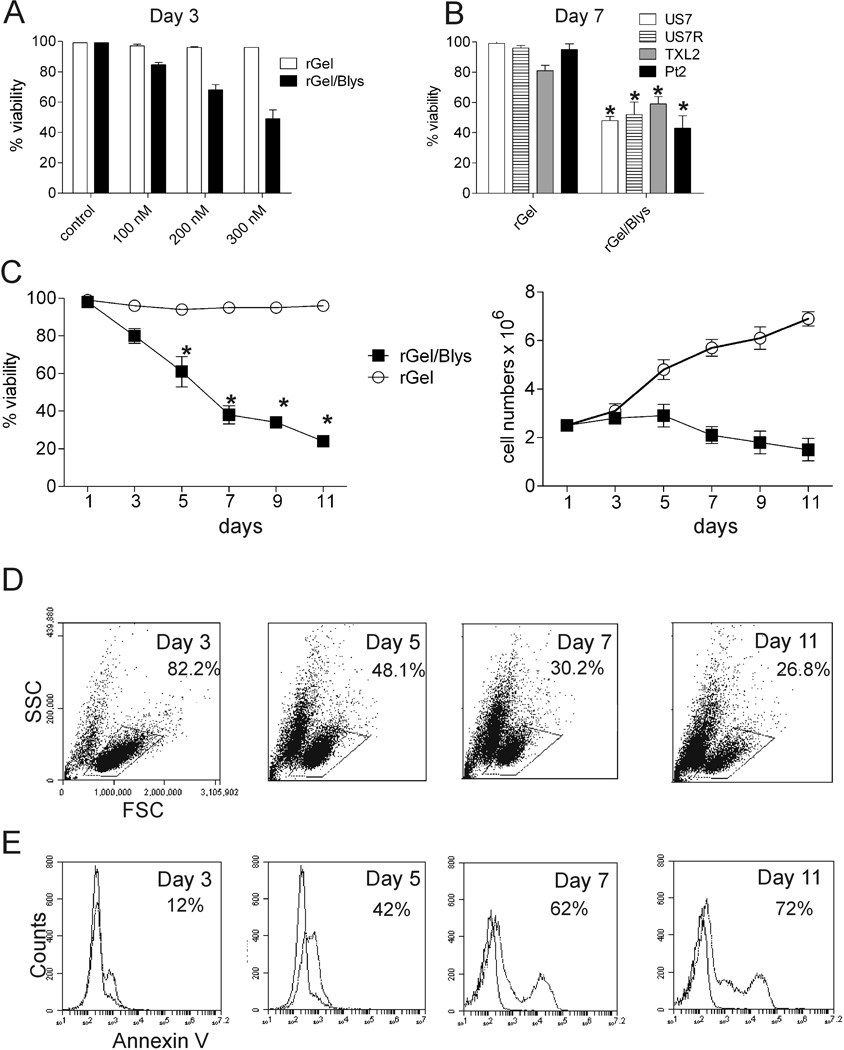
We next examined the effect of rGel/BLyS on the growth of ALL cells. As shown in Figure 2A, rGel/BLyS induced apoptosis in a dose-dependent manner in US.7 cells after 3 days of treatment. Although higher concentrations of rGel/BLyS augmented the killing of US.7 cells, we chose the lowest dose of 100 nM for further studies because it has been shown that Gelonin causes toxicity to mammalian cells over an extended period, killing cells within 3–4 days by inhibiting protein synthesis.33 We also compared the sensitivity of different ALL cells to this toxin-BLyS conjugate. As shown in Figure 2B, 100 nM rGel/BLyS treatment decreased cell viability significantly in all treated cells as measured after 7 days of treatment. Extension of the US7 culture and treatment with rGel/BLyS for 11 days caused a further decline in the percentage of viable cells and inhibited cell proliferation (Figure 2C). The percentage of live gated cells as measured by FSC/SSC (Figure 2D) declined during this period and these cells underwent apoptosis, as confirmed by Annexin V staining (Figure 2E).
Figure 2. Effect of rGel/BLyS on the growth of human ALL cells.
(A) Viability of US.7 cells treated for 3 days with different concentrations of rGel/BLyS or rGel as indicated. rGel/BLyS was added once on day 1. (B) Viability of Ph positive (TXL-2, P-2) or Ph-negative (US.7, US.7R) ALL cells after a 7-day incubation with 100 nM rGel/BLyS. rGel/BLyS was added on alternate days (n = 3). * p < 0.05, rGel/BLyS compared to rGel treated cells. (C–E) US.7 cells incubated with rGel/BLyS for 11 days. rGel/BLyS was added on alternate days with fresh medium (n=3) (C) Viability and viable cell numbers. * p < 0.05, rGel/BLyS compared to rGel-treated cells. (D, E) FACS analysis of rGel/BLyS treated samples for (D, marked area) FSC/SSC on gated live cells and for (E) Annexin V staining. All experiments were done in the presence of irradiated OP9 stroma.
rGel/BLyS treatment down-regulates survival genes and inhibits NF-κB activation in ALL cells
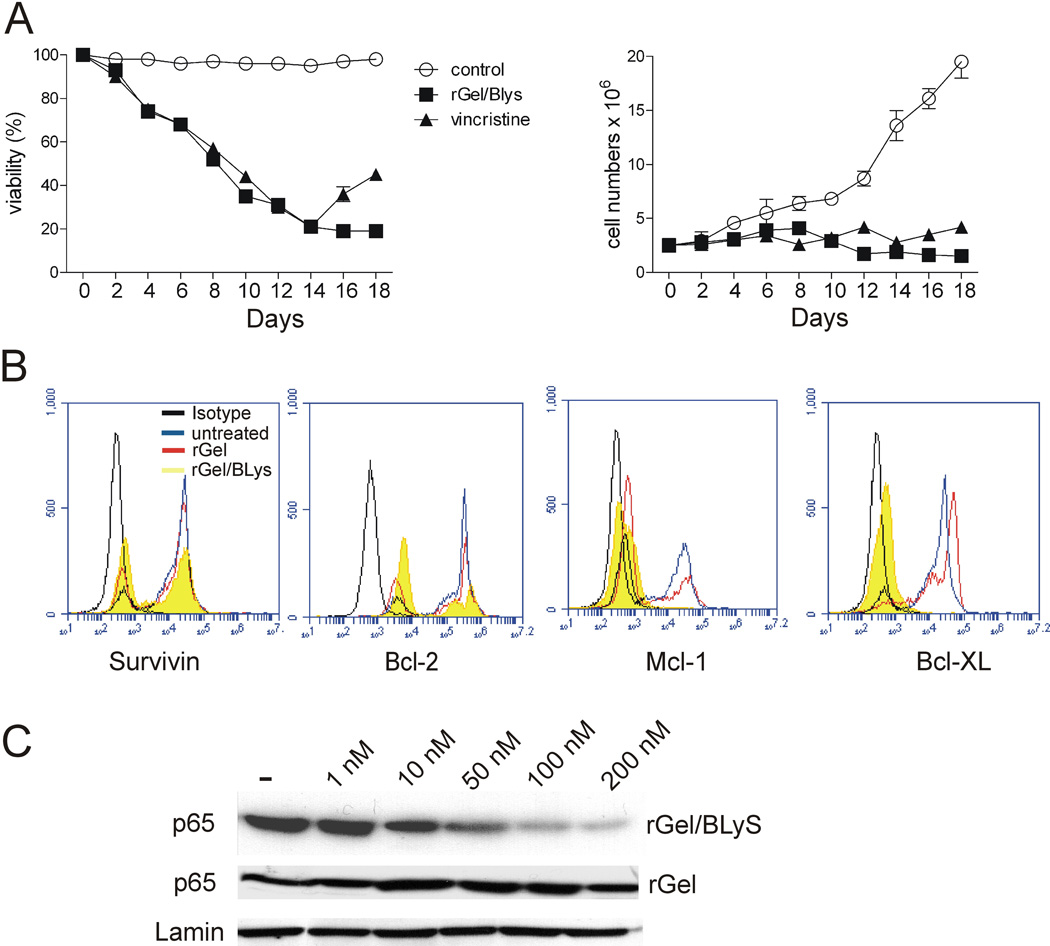
Vincristine is a commonly used toxic chemotherapeutic agent for the treatment of ALL. We therefore further compared the cytotoxic effect of rGel/BLyS to that of vincristine as monotreatment using US.7 cells. As shown in Figure 3A, 100 nM rGel/BLyS reduced viability of US7 cells to a similar extent as 2.5 nM vincristine, and suppressed proliferation of the cells in the presence of stroma (Figure 3A, right panel).
Figure 3. rGel/BLyS treatment down-regulates survival genes and inhibits NF-κB activation in ALL cells.
(A) Comparison of viability (left) and viable cell numbers (right) during long-term vincristine or rGel/BLyS treatment of US.7 cells. Fresh rGel/BLyS or vincristine was added every alternate day (n=2). (B) Expression of Bcl-xL, Mcl-1, Bcl-2, and Survivin in US7 cells, untreated or exposed to rGel/BLyS or rGel (100 nM) for 3 days (n=2). Isotype (black), untreated (blue), rGel (red) and rGel/BLyS (yellow). (C) Western blot for nuclear p65 expression in US.7 cells after 3 days incubation with different doses of rGel/BLyS or rGel, as indicated. Lamin, loading control.
ALL cells express high levels of survival proteins such as Bcl-xL, Bcl-2, Survivin and Mcl-1.35–37 To investigate if the expression of these genes is affected by uptake of rGel/BLyS, we examined their baseline expression by FACS and after rGel/BLyS or rGel treatment. Interestingly, as shown in Figure 3B, Bcl-xL and Mcl-1 levels were very obviously reduced in rGel/BLyS-treated cells, whereas inhibition of Bcl-2 and Survivin expression was much less pronounced. Since these pro-survival genes are under NF-κκB transcriptional regulation 38,39, we also compared NF-κB activation in the rGel/BLyS and rGel treated cells. Figure 3C shows constitutive NF-κB activation in untreated ALL cells as measured by the presence of nuclear p65 and, unlike rGel treatment, rGel/BLyS treatment inhibited the nuclear translocation of NF-κB-p65 in a dose-dependent manner.
rGel/BLyS prolongs the survival of NSG leukemic mice
To investigate to what extent rGel/BLyS binds to non-leukemic B cells, we incubated it with mouse B220+ cells from bone marrow and spleen as well as with human CD19+ cells from peripheral blood (PB) and bone marrow (BM). B cells from human or murine BM, or mouse spleen showed no binding to rGel/BLyS, whereas B cells from human PB showed appreciable binding (Suppl. Figure 1A). We also assessed toxicity of rGel/BLyS on normal mouse B cells in vivo. rGel/BLyS (3.75 mg/kg) was administered biweekly for 3 weeks to C57BL/6 mice, after which B cells from BM and spleen were examined. To confirm the presence of Gelonin in bone marrow of rGel/BLyS injected mice, BM lysates were prepared 5 hours after rGel/BLyS injection. Western blots with anti-Gelonin antibodies confirmed the presence of rGel/BLyS in BM of mice injected with rGel/BLyS but not of controls (Suppl. Figure 1B). B cell populations in both BM and spleen (Suppl. Figure 1C, D) were comparable in treated and control mice based on FACS, indicating that no specific B-lineage populations were ablated. Analysis of serum for LDH activity and histological examination of liver did not provide evidence of tissue damage or liver toxicity in the rGel/BLyS-injected mice compared to controls (Suppl. Figure 1E, F).
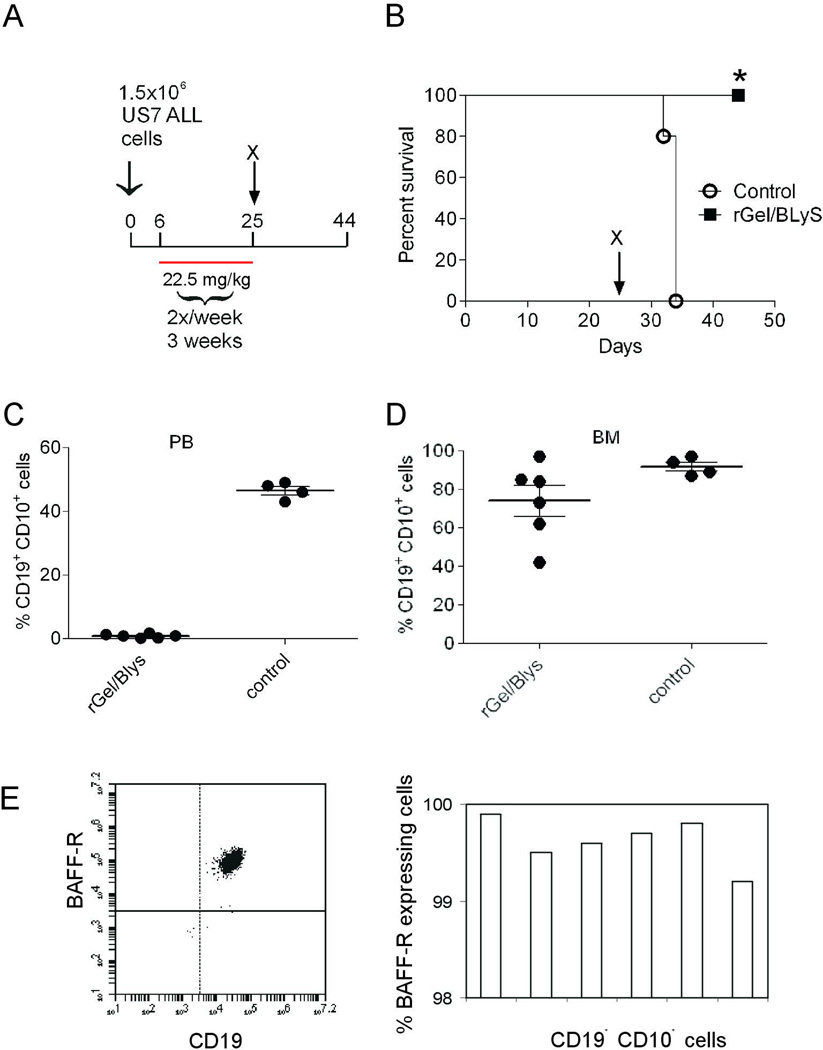
We next examined the ability of rGel/BLyS to suppress pre-B ALL growth in an in vivo NSG mouse model of human ALL. We transplanted US7 cells into NSG mice and allowed the cells to proliferate for 6 days to form an appreciable tumor burden, as detected by FACS analysis of PB (Suppl. Figure 2A). Then 3.75 mg/kg rGel/BLyS was administered bi-weekly for 3 weeks (Figure 4A). Mice not receiving treatment had to be sacrificed at around day 34 whereas mice treated with rGel/BLyS survived significantly (p=0.002) longer (Figure 4B). Remarkably, FACS analysis of PB from mice treated with rGel/BLyS, using human CD19 and CD10 antibodies, showed that the treatment had eliminated virtually all circulating leukemia cells (Figure 4C). However, the BM of these mice clearly contained leukemic cells (Figure 4D). We considered the possibility that the relapsed ALL cells in the BM were selected for loss of BAFF-R expression and thus escaped from rGel/BLyS-induced apoptosis. However, FACS analysis of the ALL cells in the BM of rGel/BLyS treated mice demonstrated that these cells uniformly expressed high levels of the BAFF-R (Figure 4E).
Figure 4. rGel/BLyS monotreatment prolongs survival in an ALL transplant model.
(A) Experimental outline. Treatment with saline or rGel/BLyS (3.75 mg/kg) was started 6 days after transplant. n= 5/group. Total amount of rGel/BLyS injected, 22.5 mg/kg (B) Survival of mice treated with saline (circles), or rGel/BLyS (squares). *p= 0.002, control compared with rGel/BLyS group. (C, D) Human leukemia cells in the peripheral blood (C) or bone marrow (D) of saline-treated mice at sacrifice compared to rGel/BLyS-treated mice, as detected by FACS analysis for human CD19 and CD10. (E) Representative FACS plot (left) and FACS analysis showing the percentage of BAFF-R positive CD19+ CD10+ gated cells in the bone marrow of rGel/BLyS treated group (right). Each bar represents a single mouse BM sample.
AMD3100 mobilizes and sensitizes ALL cells to rGel/BLyS treatment
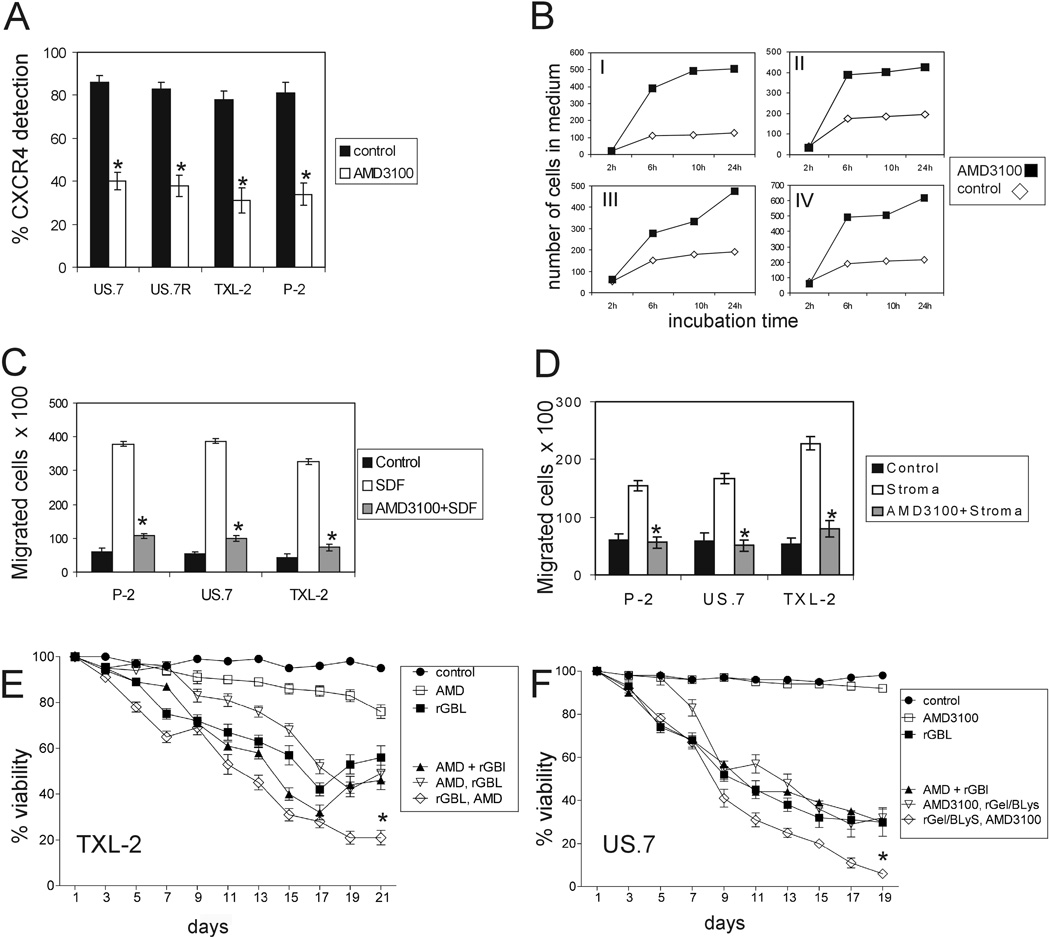
It is thought that leukemic cells are protected by the bone marrow microenvironment against the cytotoxic effects of drugs and we interpreted the bone marrow relapse of BAFF-R-positive ALL cells in rGel/BLyS-treated mice as a typical outcome of chemotherapy. Since the interaction of the chemokine receptor CXCR4 on pre-B ALL cells with SDF-1α produced by bone marrow stroma has been identified as a key factor in retention of ALL cells in the BM 40, 41 we tested the efficacy of AMD3100, a CXCR4 antagonist, in mobilizing ALL cells in vitro. The interaction of AMD3100 with CXCR4 blocks the binding of anti-CXCR4 monoclonal antibodies to the receptor and this correlates directly with SDF-1 inhibition.42, 43 Therefore, to assess the binding of AMD3100 with human ALL cells, CXCR4 expression was analyzed by FACS with or without incubation with AMD3100. As shown in Figure 5A, AMD3100 treatment reduced cell surface CXCR4 detection up to 40% compared to the respective control cells without AMD3100 treatment. To investigate whether AMD3100 is able to disrupt an adhesion that was already established, we co-cultured ALL cells with OP9 feeder layers. Under these conditions, most of the leukemia cells associate with the feeder layer. After 2 weeks of culture, the culture supernatant was removed and replaced with medium containing AMD3100. As shown in Figure 5B, AMD3100 had a marked mobilizing activity in vitro. SDF-1α is a strong chemotactic agent for lymphoblasts and bone marrow stromal cells are good producers of this chemokines.41, 44 We next tested whether AMD3100 could block the migration of leukemic cells towards SDF-1α as well as stroma. As shown in Figure 5C and 5D, pre-incubation of the ALL cells with AMD3100 significantly blocked the SDF-1α-directed (C) as well as stroma-directed (D) chemotaxis.
Figure 5. AMD3100 mobilizes and sensitizes ALL cells to rGel/BLyS treatment.
(A) Human ALL samples as indicated were incubated with AMD3100 (1 µM) and examined for relative surface expression of CXCR4 by FACS compared to untreated cells. Mean values of triplicate samples with standard deviations are shown. (B) After 2 weeks of culture on OP9 stroma, US.7 (I), P-2 (II), TXL-2 (III) or US.7R (IV) cultures were washed to remove the floating cells. AMD3100 (10 µM) was added to the new culture media. Non-adherent cells were counted as indicated. (C, D) Untreated or AMD3100-pretreated human ALL cells were seeded in the upper wells of Transwells. SDF-1α (200 ng/ml) was added to the medium in the bottom wells (C) or stromal cells were plated in the bottom wells (D). Controls, lower wells without SDF-1α (C, medium alone) or stroma (D). *p<0.05, SDF-1α vs. SDF-1α+AMD3100 or stroma vs. stroma+AMD3100. (E) TXL-2 or (F) US.7 cells on OP9 stroma treated with 100 nM rGel/BLyS alone, with 1 µM AMD3100 alone or with a combination of both in three different ways: (1) AMD+RGBL: rGel/BLyS and AMD3100 added together (2) AMD, rGBL: AMD3100 alone added for the first 5 days and then rGel/BLyS plus AMD3100 (3) rGBL, AMD: rGel/BLyS alone added for the first 5 days and then rGel/BLyS plus AMD3100 added together. *p<0.05, for rGel/BLyS followed by AMD3100 + rGel/BLyS treatment, compared to rGel/BLyS alone and rGel/BLyS+AMD3100. The p value is for the final time points of (E) and (F), n=3.
G-CSF is clinically used to mobilize stem cells from the bone marrow and is known to induce the disruption of the CXCR4/SDF-1α chemotactic interaction during hematopoietic stem cell mobilization. 45 However, G-CSF alone did not show an improved mobilizing effect in US.7 cells compared to AMD3100. We therefore also tested the combined effect of G-CSF with AMD3100 to further increase mobilization of these cells, but found no added benefit of the combination beyond AMD3100 alone (Suppl. Figure 3).
We next performed a long-term treatment of TXL-2 and US.7 cells in vitro with rGel/BLyS in combination with AMD3100 in the presence of stroma (Figure 5E and F). Viability of cells treated with AMD3100 alone did not change significantly. Cultures treated only with rGel/BLyS decreased in viability over a period of 17 days (TXL-2) or 14 days (US.7) but, similar to the results shown in Figure 3, even after extended treatment with rGel/BLyS as a single agent, resistance to treatment was evident (Suppl. Figure 4). When we added AMD3100 and rGel/BLyS together at day t =1, viability was very similar to rGel/BLyS alone cultures. (Figure 5E and F, compare black triangles and squares). The best outcome was achieved when rGel/BLyS was added as monotherapy for the first 5 days and then AMD3100 was combined with it (Figure 5E and F and Suppl. Figure 4A and B).
Combination therapy using rGel/BLyS and AMD3100 results in significantly reduced leukemia burden in bone marrow
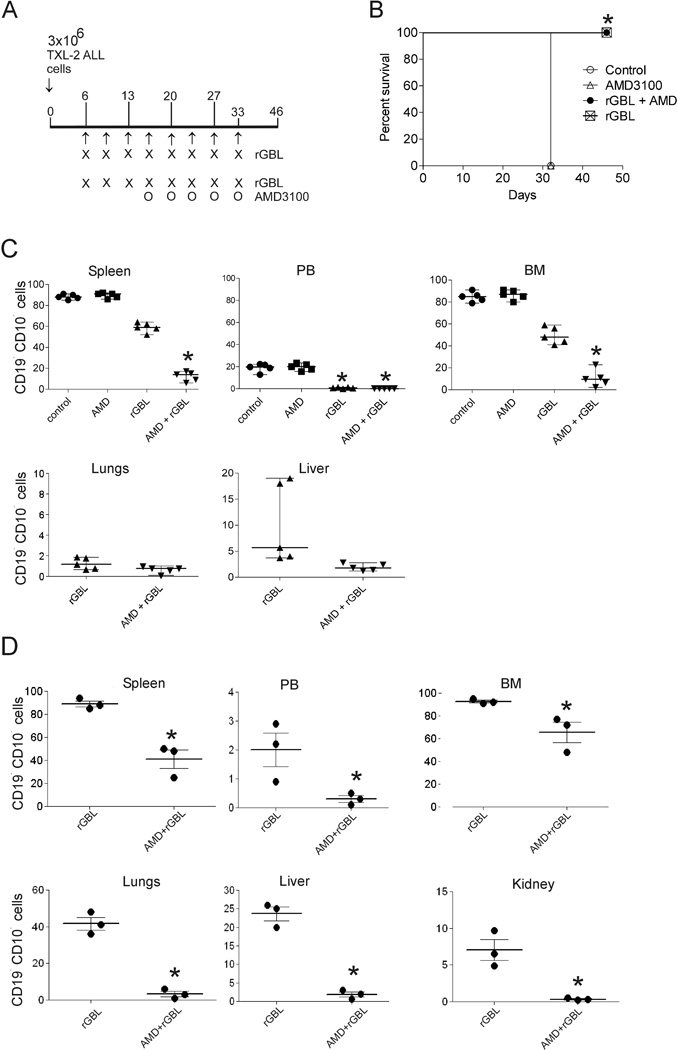
We translated our in vitro observations to an in vivo experiment. For in vivo combination treatments, we used NSG mice transplanted with Ph-positive TXL-2 ALL cells to study the beneficial effect of AMD3100 in combination with rGel/BLyS in killing ALL cells. Mice were treated with PBS, AMD3100 alone, rGel/BLyS alone or rGel/BLyS + AMD3100. In the combination group, mice were first treated with rGel/BLyS alone to reduce the overall leukemia burden. When AMD3100 was added on to this treatment, we injected the AMD3100 5–6 hours before the rGel/BLyS was administered. This strategy was used to ensure the presence of circulating ALL cells at the time when the toxin would also be in the circulation. When the mice in the control and AMD3100 alone treated group lost more than 20% of their initial body weight on day 32 after transplant, they were sacrificed. Leukemic mice treated with the combination of rGel/BLyS+AMD3100 and rGel/BLyS alone did not show any weight loss or other symptoms of the disease at day 32. They were sacrificed on day 46 (Figure 6A, B), when the rGel/BLyS alone treated group had exhibited significant loss of body weight (Suppl. Figure 5A, B). FACS analysis was performed for the presence of human ALL cells in spleen, PB, BM, lungs, liver and kidney. As expected and consistent with the in vivo experiment with US.7 cells, there was a complete eradication of ALL cells from the circulation. However, in contrast to the results with US7-transplanted mice, in which we found high amounts of ALL cells in the bone marrow, mice transplanted with TXL-2 cells and treated with additional applications of rGel/BLyS had a reduction of the leukemia burden in the BM (Figure 6C). Moreover, this was reduced even further in the mice treated with both rGel/BLyS and AMD3100. Human ALL cell numbers in the spleen were also significantly lower in the rGel/BLyS + AMD3100 treated group (Figure 6C). To examine ALL metastasis in other organs, we performed FACS analysis on lungs and liver cells (Figure 6C, bottom row). There was little infiltration of leukemic cells in these peripheral organs. Also, splenomegaly was not present (Suppl. Figure 5C). rGel/BLyS in combination with AMD3100 appeared to be well-tolerated since there was no weight loss in the dual treatment group (Suppl. Figure 5B) or changes in physical appearance.
Figure 6. Combination therapy using rGel/BLyS and AMD3100 results in significantly reduced tumor burden in bone marrow.
(A) Schematic overview of treatment. Mice (n = 5/group) were started on treatment 6 days after transplant with i.p. injections of either saline or rGel/BLyS (2.75 mg/kg) twice a week with the last treatment on day 33. Total amount of rGel/BLyS injected, 22 mg/kg. The combination treatment group additionally was treated with 10 mg/kg AMD3100 as shown. rGel/BLyS was injected 5 hours after administration of AMD3100. (B) Survival of treated mice. *p=0.0047 for AMD3100 + rGel/BLyS or rGel/BLyS versus control or AMD3100. (C) Percentage of TXL-2 ALL cells in rGel/BLyS treated mice, compared to that of rGel/BLyS + AMD3100 treated mice, as detected by FACS for human CD19 and CD10, at the time of sacrifice. Each symbol represents the percentage of CD19+, CD10+ cells in the live-gated population in individual mice. For BM and spleen *p<0.001 (rGel/BLyS compared to AMD3100 + rGel/BLyS) and *p<0.001 for PB samples (rGel/BLyS and AMD3100 + rGel/BLyS group compared to control or AMD3100 alone treated group). (D) Percentage of US.7 ALL cells in rGel/BLyS treated mice compared to rGel/BLyS + AMD3100 treated mice (n=3/group) as detected by FACS analysis for human CD19 and CD10 positive cells. *p<0.001 (rGel/BLyS compared to AMD3100 + rGel/BLyS). Note that different scales are used in the individual panels of (C) and (D).
To confirm the combinatorial effect of rGel/BLyS with AMD3100 in killing ALL cells, we treated mice transplanted with US.7 cells with rGel/BLyS alone or together with AMD3100 as described above. Figure 6D shows that the leukemia burden was reduced significantly in spleen, PB, BM, lungs, liver, and kidney of rGel/BLyS+AMD3100 treated mice, compared to rGel/BLyS-alone treated mice.
Discussion
Because the BAFF-R was only reported on mature B-cell malignancies, the use of rGel/BLyS as an anti-leukemic agent has to date only been evaluated in this type of leukemia. Nimmanapalli et al reported that rGel/BLyS inhibits the production of fast-turnover proteins and that Bcl-2, being a long-lived protein with a half life up to 24 hours, is not affected by rGel/BLyS exposure.33 Our data are in agreement with this, since we found that Bcl-2 expression was not significantly altered in ALL cells after rGel/BLyS treatment. However, levels of other pro-survival proteins including Bcl-xL and Mcl-1 were reduced by this treatment and we found that exposure to rGel/BLyS also inhibited the nuclear translocation of p65-NF-κB, suggesting a correlation with the expression of these pro-survival genes.
To be able to provide a superior activity in comparison with standard cytotoxic drugs, new compounds should exhibit specificity and potency. Our data showing that Gelonin alone does not bind to ALL cells or is internalized is consistent with its lack of effect on the proliferation or viability of ALL cells. The conjugation of BAFF (BLyS) to Gelonin imparts a remarkable specificity to target this toxin to cells that express receptors for BAFF (BLyS). Although BAFF (BLyS) can bind to three distinct receptors, BAFF-R, TACI and BCMA 46, 47 there is very little if any expression of BCMA or TACI on these pre-B ALL cells 10 and thus the entry and cytotoxic effect of rGel/BLyS on pre-B ALL cells is BAFF-R receptor-mediated. Experiments to assess the toxicity of rGel/BLyS in non-leukemic mice did not yield evidence that the toxin had an effect on normal mouse B-lineage cells and is in agreement with earlier data 26–33. However, B cells isolated from human PB clearly showed binding to rGel/BLyS and the toxin would be expected to kill mature normal human B cells.
The potency of this fusion toxin is likely to be determined by the cytotoxicity of Gelonin once it is internalized, as well as the numbers of BAFF receptors present on the surface of the target cell. Lyu et al reported that the ability of the BLyS component of rGel/BLyS to mediate the delivery of the toxin to the target cell cytoplasm (targeting index) varied from 1 to 187,500 among 17 malignant B cells tested and this was generally correlated with the total number of BAFF receptors expressed by the cell. Of the cells tested, MCL cell lines showing the maximum sensitivity to rGel/BLyS expressed the highest level of BAFF-R, while the HL-60 cell line, being the least sensitive to rGel/BLyS, did not express BAFF-R protein.25 We have previously determined that the pre-B ALL cells express high levels of BAFF-R10 and we indeed found a profound cytotoxic effect of rGel/BLyS on these cells, which compared very favorably with the standard vincristine. In a recent publication, Maia et al showed that all of the 23 primary B-cell precursor ALL samples tested had cell surface expression of the BAFF-R13, which supports the potential therapeutic application of rGel/BLyS for ALL.
The cytotoxicity of rGel/BLyS in vitro was further substantiated by its activity in an NSG ALL transplant model of established leukemia, in which 6 treatments were sufficient to generate a hematological remission. However, analysis of the bone marrow showed that, although ALL cell numbers were reduced, the treatment had not eradicated the ALL cells. This could be caused by reduced or absent expression of the BAFF-R on ALL cells in that location but we found that ALL cells in the bone marrows of the relapsing rGel/BLyS-treated mice all had full expression of the BAFF-R. Because expression of the BAFF-R will result in the specific eradication of those cells through internalization of the rGel/BLyS fusion toxin, it appears that there is strong selective pressure for the maintenance of its expression. Our previous studies, using ALL cells in co-culture with stroma, suggested that the expression of the BAFF-R provides a survival advantage to the cells10 and provide support for this idea.
We therefore considered an alternative explanation for the presence of the ALL cells, namely that the bone marrow presents an unusually challenging environment from the perspective of treatment with a BAFF (BLyS)-conjugate because of the high levels of competing BAFF (BLyS) that is endogenously produced by the bone marrow microenvironment. In support of this, we had noted in our co-culture system that exogenously added recombinant human BAFF (BLyS) is able to inhibit the binding of rGel/BLyS to ALL cells in a dose-dependent manner. In addition, the presence of BAFF-R-expressing ALL cells in the bone marrow could also be explained if the fusion toxin had limited access to the cells at that location.
To circumvent problems associated with localization of leukemic cells in the bone marrow, we applied a strategy we have previously started to explore for ALL, and by others for AML and CML, making use of agents that are able to mobilize leukemic cells out of that location into the circulation.48–51 We used AMD3100, a CXCR4 antagonist, for this purpose. All parameters tested, including CXCR4 cell surface expression, adhesion to stroma, and SDF-1α or stroma-directed chemotaxis could be inhibited by AMD3100 in ALL cells. This observation is in contradiction with a number of earlier publications suggesting that there is a weak adhesion of Bcr/Abl positive leukemic cells to bone marrow cells, which would make Bcr/Abl-positive ALL cells insensitive to CXCR4-mediated signals and chemotaxis towards SDF-1α.52,53 Moreover, we showed that in an in vitro co-culture system, treatment of Ph-positive ALL cells (TXL-2) with rGel/BLyS and AMD3100 significantly reduced viability compared to monotreatment with rGel/BLyS.
These results were replicated in an in vivo transplant model. Although rGel/BLyS monotreatment eliminated circulating ALL cells, the addition of AMD3100 to the treatment significantly further reduced the amount of ALL cells present in the spleen and, importantly, in the bone marrow. Because rGel/BLyS targets only cells expressing the BAFF-R, TACI or BCMA, and because AMD3100 appears to have no cytotoxic effect at all, this treatment could potentially be expanded, if needed, to include a third drug. Moreover, since neither rGel/BLyS nor AMD3100 is currently used to treat patients with ALL, it is unlikely that relapsed patients would have ALL cells that are resistant to these drugs. We conclude that our novel treatment strategy has a very promising clinical application for both Ph-positive and Ph-negative ALLs.
Supplementary Material
Acknowledgements
We thank Donna Foster for excellent care of the mice. This work was supported by PHS grant CA090321 (to NH), by the William Lawrence & Blanche Hughes Foundation (to NH, JG), and a Translational Research Award (LLS 6234-07) from the Leukemia and Lymphoma Society (to MGR) and in part by the Clayton Foundation for Research (MGR).
Footnotes
Authorship contributions- RP designed and performed experiments, analyzed data and wrote the manuscript; MY and ML performed experiments and analyzed data; M-AL, MGR and JG contributed essential reagents and provided input in the design of experiments; NH designed experiments, analyzed data and wrote the manuscript.
Conflict of interest disclosure- M-AL and MGR were supported by the Clayton Foundation.
Supplementary information is available at Leukemia's website.
References
- 1.Pui CH. Recent advances in acute lymphoblastic leukemia. Oncology (Williston Park) 25:341, 346–347. [PubMed] [Google Scholar]
- 2.Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–1411. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- 3.Kreuger A, Garwicz S, Hertz H, Jonmundsson G, Lanning M, Lie SO, et al. Central nervous system disease in childhood acute lymphoblastic leukemia: prognostic factors and results of treatment. Pediatr Hematol Oncol. 1991;8:291–299. doi: 10.3109/08880019109028802. [DOI] [PubMed] [Google Scholar]
- 4.Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84:3122–3133. [PubMed] [Google Scholar]
- 5.Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005;23:7942–7950. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 6.Hardisty RM, McElwain TJ, Darby CW. Vincristine and prednisone for the induction of remissions in acute childhood leukaemia. Br Med J. 1969;2:662–665. doi: 10.1136/bmj.2.5658.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dopfer R, Henze G, Bender-Gotze C, Ebell W, Ehninger G, Friedrich W, et al. Allogeneic bone marrow transplantation for childhood acute lymphoblastic leukemia in second remission after intensive primary and relapse therapy according to the BFM- and CoALL-protocols: results of the German Cooperative Study. Blood. 1991;78:2780–2784. [PubMed] [Google Scholar]
- 8.Henderson MJ, Choi S, Beesley AH, Sutton R, Venn NC, Marshall GM, et al. Mechanism of relapse in pediatric acute lymphoblastic leukemia. Cell Cycle. 2008;7:1315–1320. doi: 10.4161/cc.7.10.5885. [DOI] [PubMed] [Google Scholar]
- 9.Rivera GK, Zhou Y, Hancock ML, Gajjar A, Rubnitz J, Ribeiro RC, et al. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 2005;103:368–376. doi: 10.1002/cncr.20743. [DOI] [PubMed] [Google Scholar]
- 10.Parameswaran R, Muschen M, Kim YM, Groffen J, Heisterkamp N. A functional receptor for B-cell-activating factor is expressed on human acute lymphoblastic leukemias. Cancer Res. 2010;70:4346–4356. doi: 10.1158/0008-5472.CAN-10-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onda K, Iijima K, Katagiri YU, Okita H, Saito M, Shimizu T, et al. Differential effects of BAFF on B cell precursor acute lymphoblastic leukemia and Burkitt lymphoma. Int J Hematol. 2010;91:808–819. doi: 10.1007/s12185-010-0567-z. [DOI] [PubMed] [Google Scholar]
- 12.Mihalcik SA, Tschumper RC, Jelinek DF. Transcriptional and post-transcriptional mechanisms of BAFF-receptor dysregulation in human B lineage malignancies. Cell Cycle. 2010;9:4884–4892. doi: 10.4161/cc.9.24.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maia S, Pelletier M, Ding J, Hsu YM, Sallan SE, Rao SP, et al. Abberant Expression of Functional BAFF-system receptors by malignant B-cell precursors impacts leukemia cell survival. PLoS One. 2011;6(6):e20787. doi: 10.1371/journal.pone.0020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 15.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 16.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 17.Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 18.Rennert P, Schneider P, Cachero TG, Thompson J, Trabach L, Hertig S, et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J Exp Med. 2000;192:1677–1684. doi: 10.1084/jem.192.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 20.Do RK, Chen-Kiang S. Mechanism of BLyS action in B cell immunity. Cytokine Growth Factor Rev. 2002;13:19–25. doi: 10.1016/s1359-6101(01)00025-9. [DOI] [PubMed] [Google Scholar]
- 21.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 22.Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194:1691–1697. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–688. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 24.Briones J, Timmerman JM, Hilbert DM, Levy R. BLyS and BLyS receptor expression in non-Hodgkin's lymphoma. Exp Hematol. 2002;30:135–141. doi: 10.1016/s0301-472x(01)00774-3. [DOI] [PubMed] [Google Scholar]
- 25.Lyu MA, Cheung LH, Hittelman WN, Marks JW, Aguiar RC, Rosenblum MG. The rGel/BLyS fusion toxin specifically targets malignant B cells expressing the BLyS receptors BAFF-R, TACI, and BCMA. Mol Cancer Ther. 2007;6:460–470. doi: 10.1158/1535-7163.MCT-06-0254. [DOI] [PubMed] [Google Scholar]
- 26.Stirpe F, Olsnes S, Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980;255:6947–6953. [PubMed] [Google Scholar]
- 27.Rosenblum MG, Cheung LH, Liu Y, Marks JW., 3rd Design, expression, purification, and characterization, in vitro and in vivo, of an antimelanoma single-chain Fv antibody fused to the toxin gelonin. Cancer Res. 2003;63:3995–4002. [PubMed] [Google Scholar]
- 28.Rosenblum MG, Murray JL, Cheung L, Rifkin R, Salmon S, Bartholomew R. A specific and potent immunotoxin composed of antibody ZME-018 and the plant toxin gelonin. Mol Biother. 1991;3:6–13. [PubMed] [Google Scholar]
- 29.Duzkale H, Pagliaro LC, Rosenblum MG, Varan A, Liu B, Reuben J, et al. Bone marrow purging studies in acute myelogenous leukemia using the recombinant anti-CD33 immunotoxin HuM195/rGel. Biol Blood Marrow Transplant. 2003;9:364–372. doi: 10.1016/s1083-8791(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 30.Wen X, Lyu MA, Zhang R, Lu W, Huang Q, Liang D, et al. Biodistribution, Pharmacokinetics, and Nuclear Imaging Studies of (111)In-labeled rGel/BLyS Fusion Toxin in SCID Mice Bearing B Cell Lymphoma. Mol Imaging Biol. 2011;13:721–729. doi: 10.1007/s11307-010-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyu MA, Rai D, Ahn KS, Sung B, Cheung LH, Marks JW, et al. The rGel/BLyS fusion toxin inhibits diffuse large B-cell lymphoma growth in vitro and in vivo. Neoplasia. 2010;12:366–375. doi: 10.1593/neo.91960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyu MA, Sung B, Cheung LH, Marks JW, Aggarwal BB, Aguiar RC, et al. The rGel/BLyS fusion toxin inhibits STAT3 signaling via down-regulation of interleukin-6 receptor in diffuse large B-cell lymphoma. Biochem Pharmacol. 2010;80:1335–1342. doi: 10.1016/j.bcp.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Nimmanapalli R, Lyu MA, Du M, Keating MJ, Rosenblum MG, Gandhi V. The growth factor fusion construct containing B-lymphocyte stimulator (BLyS) and the toxin rGel induces apoptosis specifically in BAFF-R-positive CLL cells. Blood. 2007;109:2557–2564. doi: 10.1182/blood-2006-08-042424. [DOI] [PubMed] [Google Scholar]
- 34.Duy C, Hurtz C, Shojaee S, Cerchietti L, Geng H, Swaminathan S, et al. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature. 2011;473:384–388. doi: 10.1038/nature09883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Addeo R, Caraglia M, Baldi A, D'Angelo V, Casale F, Crisci S, et al. Prognostic role of bcl-xL and p53 in childhood acute lymphoblastic leukemia (ALL) Cancer Biol Ther. 2005;4:32–38. doi: 10.4161/cbt.4.1.1371. [DOI] [PubMed] [Google Scholar]
- 36.Campana D, Coustan-Smith E, Manabe A, Buschle M, Raimondi SC, Behm FG, et al. Prolonged survival of B-lineage acute lymphoblastic leukemia cells is accompanied by overexpression of bcl-2 protein. Blood. 1993;81:1025–1031. [PubMed] [Google Scholar]
- 37.Stam RW, Den Boer ML, Schneider P, de Boer J, Hagelstein J, Valsecchi MG, et al. Association of high-level MCL-1 expression with in vitro and in vivo prednisone resistance in MLL-rearranged infant acute lymphoblastic leukemia. Blood. 2010;115:1018–1025. doi: 10.1182/blood-2009-02-205963. [DOI] [PubMed] [Google Scholar]
- 38.Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol. 2000;165:1743–1754. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 39.Tracey L, Perez-Rosado A, Artiga MJ, Camacho FI, Rodríguez A, Martínez N, et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol. 2005;206:123–134. doi: 10.1002/path.1768. [DOI] [PubMed] [Google Scholar]
- 40.Bradstock KF, Makrynikola V, Bianchi A, Shen W, Hewson J, Gottlieb DJ. Effects of the chemokine stromal cell-derived factor-1 on the migration and localization of precursor-B acute lymphoblastic leukemia cells within bone marrow stromal layers. Leukemia. 2000;14:882–888. doi: 10.1038/sj.leu.2401729. [DOI] [PubMed] [Google Scholar]
- 41.Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF. The chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor-B cells in the bone marrow. Exp Hematol. 2001;29:1439–1447. doi: 10.1016/s0301-472x(01)00741-x. [DOI] [PubMed] [Google Scholar]
- 42.De Clercq E. Inhibition of HIV infection by bicyclams, highly potent and specific CXCR4 antagonists. Mol Pharmacol. 2000;57:833–839. [PubMed] [Google Scholar]
- 43.Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- 44.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192:129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent FB, Morad EF, Mackay F. BAFF and innate immunity: new therapeutic targets for systemic lupus erythematosus. Immunol Cell Biol. 2012 doi: 10.1038/icb.2011.111. [DOI] [PubMed] [Google Scholar]
- 48.Parameswaran R, Yu M, Lim M, Groffen J, Heisterkamp N. Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia. 2011 doi: 10.1038/leu.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;11:6215–24. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu M, Gang EJ, Parameswaran R, Stoddart S, Fei F, Schmidhuber S, et al. AMD3100 sensitizes acute lymphoblastic leukemia cells to chemotherapy in vivo. Blood Cancer Journal. 2011 doi: 10.1038/bcj.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weisberg E, Azab AK, Manley PW, Kung AL, Christie AL, Bronson R, et al. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia. 2011 doi: 10.1038/leu.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geay JF, Buet D, Zhang Y, Foudi A, Jarrier P, Berthebaud M, et al. p210BCR-ABL inhibits SDF-1 chemotactic response via alteration of CXCR4 signaling and down-regulation of CXCR4 expression. Cancer Res. 2005;65:2676–2683. doi: 10.1158/0008-5472.CAN-04-2152. [DOI] [PubMed] [Google Scholar]
- 53.Chen YY, Malik M, Tomkowicz BE, Collman RG, Ptasznik A. BCR-ABL1 alters SDF-1alpha-mediated adhesive responses through the beta2 integrin LFA-1 in leukemia cells. Blood. 2008;111:5182–5186. doi: 10.1182/blood-2007-10-117705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.