Abstract
Corin is a protease that activates atrial natriuretic peptide, a cardiac hormone important in the control of blood pressure and salt-water balance. Here we examined the role of corin in regulating blood pressure and sodium homeostasis upon dietary salt challenge. Radiotelemetry-tracked blood pressure in corin knockout mice on a high salt diet (4% sodium chloride) was significantly increased; however, there was no such change in similarly treated wild type mice. In the knockout mice on the high salt diet there was an impairment of urinary sodium excretion and an increase in body weight, but no elevation of plasma renin or serum aldosterone levels. When the knockout mice on the high salt diet were treated with amiloride, an epithelial sodium channel blocker that inhibits renal sodium reabsorption, the impaired urinary sodium excretion and increased body weight were normalized. Amiloride treatment also reduced high blood pressure caused by the high salt diet in these mice. Thus, the lack of corin in mice impairs their adaptive renal response to high dietary salt, suggesting that corin deficiency may represent an important mechanism underlying salt-sensitive hypertension.
Keywords: ANP, corin, ENaC, salt-sensitive hypertension, sodium reabsorption
Introduction
Hypertension is a major cardiovascular disease. Excessive dietary salt has long been considered a risk factor in hypertension.1 Approximately 50% of essential hypertension are salt-sensitive but the underlying mechanisms are poorly understood.2, 3 Atrial natriuretic peptide (ANP) regulates blood pressure and salt-water balance4. In mice, plasma ANP levels were elevated upon high salt diets5 and deficiency in ANP or its receptor causes hypertension.6, 7 To date, however, it is unclear if a defective ANP pathway alters the response of blood pressure to dietary salts. In ANP-deficient mice, salt-sensitive hypertension was observed initially6 but not confirmed in a later study.8 In ANP receptor knockout mice, elevated blood pressure was unchanged upon dietary salt loading in an early study7 but appeared increased in later studies.9, 10 The reasons for the apparent differences may be due to different experimental protocols (conscious vs. anesthetized conditions)11 and methods for measuring blood pressure, which are notoriously challenging if tail-cuff methods were used.
Corin is a cardiac membrane serine protease.12, 13 It promotes the natriuretic system by activating ANP.14, 15 In mice, corin deficiency prevents pro-ANP to ANP conversion, causing hypertension and cardiac hypertrophy.16, 17 Corin deficiency may also contribute to hypertensive disease in humans. In African Americans, single nucleotide polymorphisms that impaired corin function were found in patients with hypertension, cardiac hypertrophy and heart failure.18–21 Recent studies detected soluble corin in human plasma 22–25 and showed that plasma corin levels were reduced in patients with heart failure.22, 26
In a previous study, hypertension in corin knockout (Cor−/−) mice was exacerbated upon an 8% NaCl diet challenge.16 The same treatment, however, also elevated blood pressure in control wild-type (WT) mice. It is difficult, therefore, to conclude if corin deficiency altered the response of blood pressure to dietary salts. In this study, we examined the effect of 2% and 4% high salt diets on blood pressure in Cor−/− mice and investigated possible mechanisms underlying hypertension in these mice. Our data indicated that corin deficiency impaired renal sodium excretion upon dietary salt challenges, which contributed to salt-sensitive hypertension in these mice.
Results
Salt-Sensitive Hypertension in Cor−/− Mice
We examined the effect of high salt diets on blood pressure in Cor−/− and WT mice. No significant changes were detected when Cor−/− or WT mice were treated with a medium-high (2% NaCl) salt diet for 3 weeks (data not shown). After 1 week on a higher (4% NaCl) salt diet, systolic blood pressure (SBP) increased in Cor−/− mice (from 125.7 ± 3.2 to 133 ± 2.9 mmHg, p<0.01) (Figure 1a). Similar increases in diastolic blood pressure (DBP) and mean arterial blood pressure also were observed (data not shown). In contrast, no significant increase in blood pressure was observed in similarly treated WT mice (Figure 1a). After 3 weeks on 4% NaCl salt diet, Cor−/− mice were switched to a normal salt (0.3% NaCl) diet and their blood pressure remained high for 2 more weeks (Figure 1a). When net changes in SBP were examined, the effect was significantly greater in Cor−/− mice than that in WT controls (Figure 1b), indicating that blood pressure was more sensitive to dietary salt loading in Cor−/− than WT mice.
Figure 1. Salt-sensitive hypertension in Cor−/− mice.
(a) Cor−/− and WT mice on a 0.3% NaCl diet (basal) were switched on a 4% NaCl diet for 3 weeks and back to the 0.3% NaCl diet for 3 weeks. Blood pressure was measured by radiotelemetry. Systolic blood pressure (SBP) in Cor−/− and WT mice was shown. n=6 per group. **p<0.01 vs. WT mice of the same group; †p<0.01 vs. Cor−/− mice of basal group by two-way ANOVA. (b) Changes in SBP from the basal group of the same genotype were calculated. n=6 per group. *p<0.05 vs. WT mice of the same group by two-way ANOVA.
Metabolic and Electrolyte Analyses
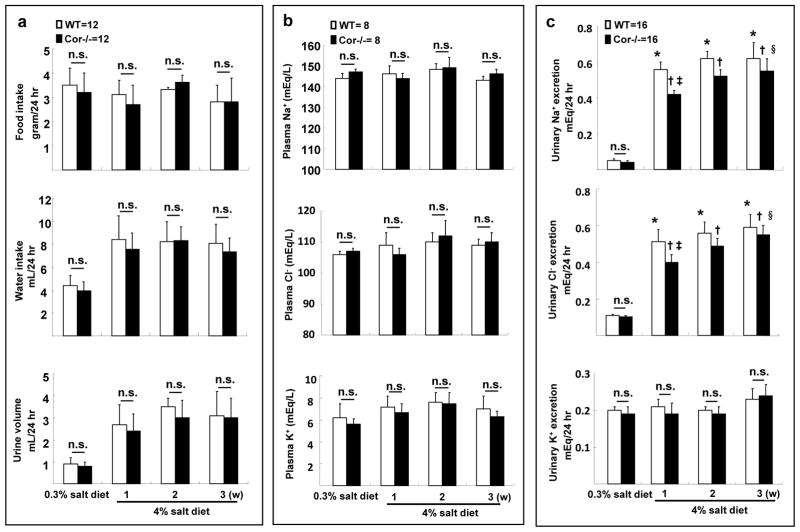
To understand the mechanism underlying the observed salt-sensitive hypertension in Cor−/− mice, we did metabolic studies in WT and Cor−/− mice. Similar food and water intakes were found with the normal salt diet (Figure 2a). On the high salt diet, food intakes remained unchanged, but water intakes and urine volumes increased similarly in WT and Cor−/− mice during the first week and remained at the high levels in weeks 2 and 3. Levels of plasma Na+, Cl−, and K+ were unchanged in these mice on the high-salt diet (Figure 2b). Apparently, plasma electrolyte balance was maintained by increased urinary Na+/Cl− excretion (Figure 2c). Compared to WT mice, however, Cor−/− mice had reduced Na+/Cl− excretion during the first week of high salt diet. The difference in Na+/Cl− excretion between WT and Cor−/− mice narrowed in the following weeks (Figure 2c), indicating that a steady state of salt-water balance was reached gradually in Cor−/− mice. In contrast, levels of urinary K+ excretion were similar in Cor−/− and WT mice (Figure 2c).
Figure 2. Metabolic studies and reduced sodium excretion in Cor−/− mice.
Food and water intake, urine volume (a), plasma eletrolytes (b) and urinary Na+, Cl− and K+ excretion (c) were measured in WT and Cor−/− mice on 0.3% and 4% NaCl diets. n.s., not statistically significant; *p<0.01 vs. WT on 0.3% NaCl diet; †p<0.01 vs. Cor−/− on 0.3% NaCl diet; ‡p<0.01 vs. WT on 4% NaCl diet 1 week; §p<0.05 vs. Cor−/− on 4% NaCl diet 1 week by two-way ANOVA; n=8–16 per group.
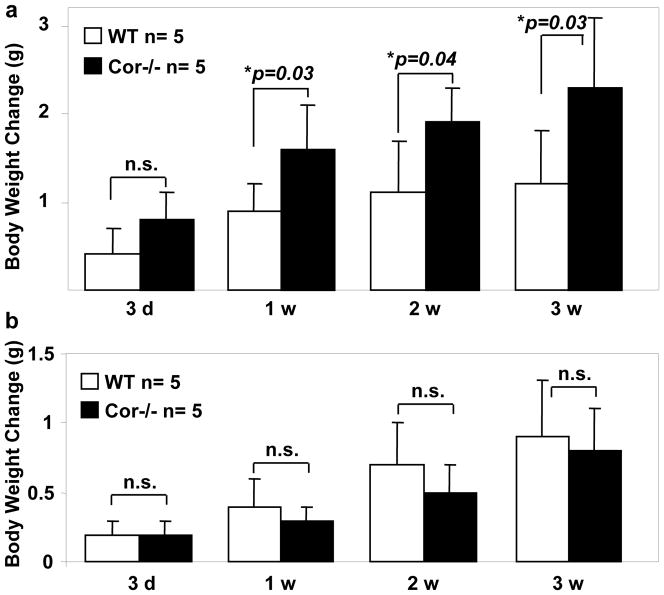
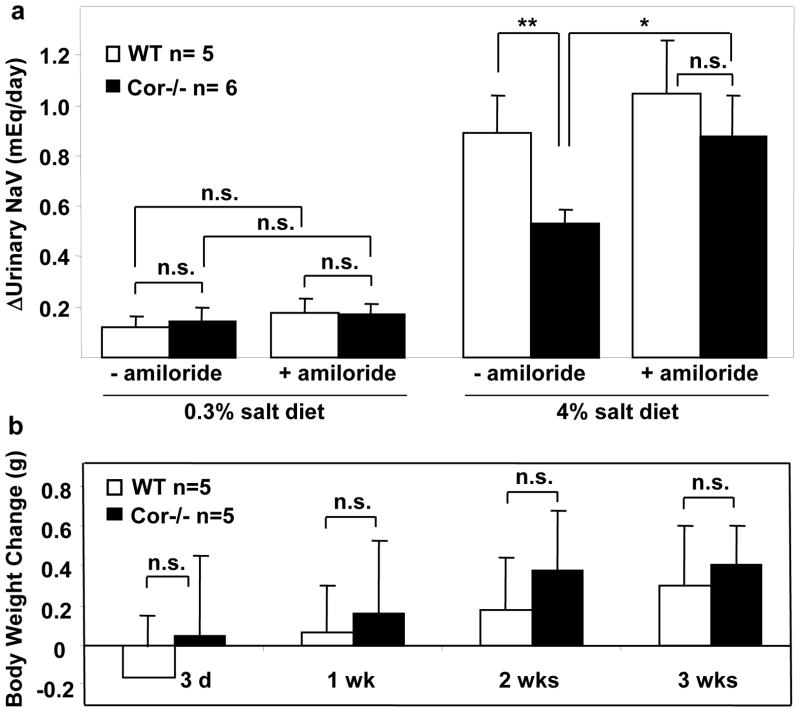
In Cor−/− mice on the high-salt diet, the impaired urinary Na+/Cl− excretion was accompanied with increased body weight compared to that in WT mice (Figure 3a). Such a difference in body weight gain between WT and Cor−/− mice was not observed on the normal salt diet (Figure 3b).
Figure 3. Increased body weight in Cor−/− mice on high salt diet.
WT and Cor−/− mice were on 4% NaCl (a) or 0.3% NaCl (b) diets. Body weight changes up to 3 weeks were examined. n=5 per group. P values vs. WT of the same group by two-way ANOVA were shown. n.s., not statistically significant.
Levels of Plasma Renin and Serum Aldosterone
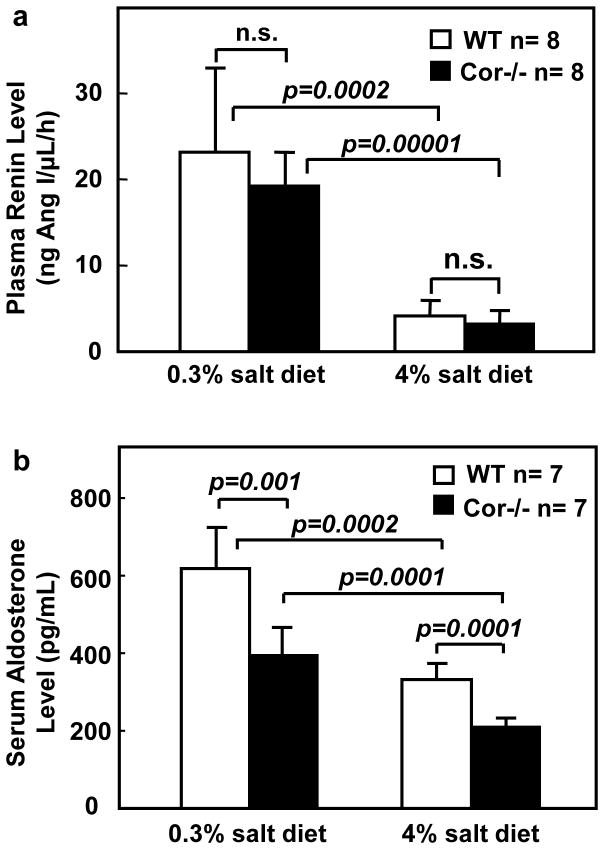
To examine if an enhanced renin-angiotensin-aldosterone system (RAAS) may be responsible for hypertension and sodium-water retention in Cor−/− mice, we measured plasma renin concentration (PRC) and serum aldosterone. Similar plasma renin levels were found in WT and Cor−/− mice on the normal salt diet (Figure 4a). On the high salt diet, both groups had significantly lower PRC, indicating that renin levels were suppressed. There was no significant difference in PRC between the two groups (Figure 4a). Serum aldosterone levels were found to be lower in Cor−/− than that in WT mice on both the normal and high salt diets (Figure 4b). The results indicated that an enhanced RAAS was unlikely to be present in Cor−/− mice.
Figure 4. Levels of plasma renin and serum aldosterone in WT and Cor−/− mice.
Mice were on 0.3% or 4% NaCl diets. Plasma renin (a) and serum aldosterone (b) levels were measured, as described in Methods. n=7–8 per group. P values by two-way ANOVA were shown. n.s., not statistically significant.
Losartan Treatment
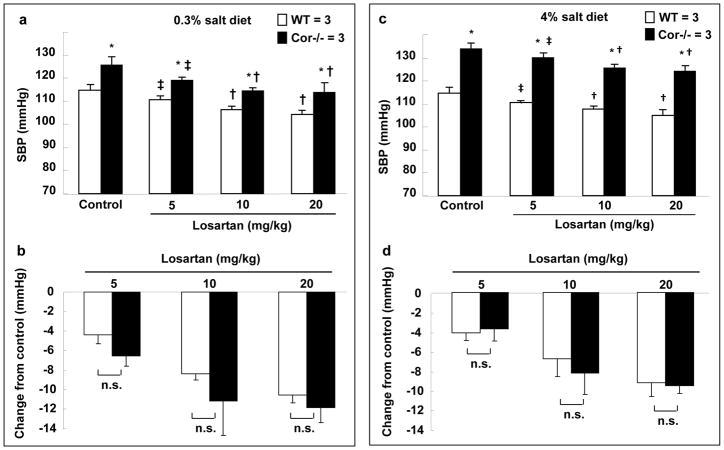
To further examine the potential role of RAAS, we treated Cor−/− mice with losartan, an angiotensin II type 1 receptor blocker. Losartan reduced blood pressure in WT and Cor−/− on both the normal and high salt diets (Figure 5a and c). The extent of reduction was similar in both groups (Figure 5b and d). The treatment did not lower blood pressure in Cor−/− mice to similar levels in WT mice. The results supported that mechanisms other than an enhanced RAAS were likely involved in hypertension in Cor−/− mice.
Figure 5. Losartan did not normalize blood pressure in Cor−/− mice.
WT and Cor−/− mice on 0.3% (a,b) or 4% (c,d) NaCl diet were treated with vehicle (control) or losartan (5, 10 and 20 mg/kg/day, i.p.). SBP was monitored. n=3 per group. *p<0.05 vs. WT of the same group. ‡p<0.05 and †p<0.01 vs. control of the same genotype by two-way ANOVA. Changes in SBP from the control group of the same genotype on 0.3% (b) and 4% (d) NaCl diets were calculated. n.s., not statistically significant by two-way ANOVA.
Amiloride Treatment
Epithelial sodium channel (ENaC) mediates renal Na+ reabsorption, thereby reducing Na+ excretion.27, 28 A recent report showed that the corin/ANP pathway may regulate renal ENaC expression and/or activity.29 To determine if the impaired urinary Na+ excretion in Cor−/− mice on high salt diets was due to an enhanced ENaC activity, we tested the hypothesis that amiloride, an ENaC blocker, might increase urinary Na+ excretion and decrease body weight gain in Cor−/− mice. No significant changes in urinary Na+ excretion were observed in WT and Cor−/− mice on normal salt diet upon amiloride treatment. The treatment, however, corrected impaired Na+ excretion in Cor−/− mice on the high salt diet (Figure 6a). Consistently, Cor−/− mice on the high salt diet and treated with amiloride had body weight gains comparable to that in WT mice (Figure 6b).
Figure 6. Amiloride promoted sodium excretion and reduced body weight gain in Cor−/− mice on high salt diet.
WT and Cor−/− mice on 0.3% or 4% NaCl diet were treated with vehicle (−amiloride) or amiloride (3 mg/kg/day, i.p.). Changes in urinary sodium excretion in one week (a) and body weight up to 3 weeks (b) were shown. n=5–6 per group. *p<0.05; **p<0.01 by two-way ANOVA; n.s., not statistically significant.
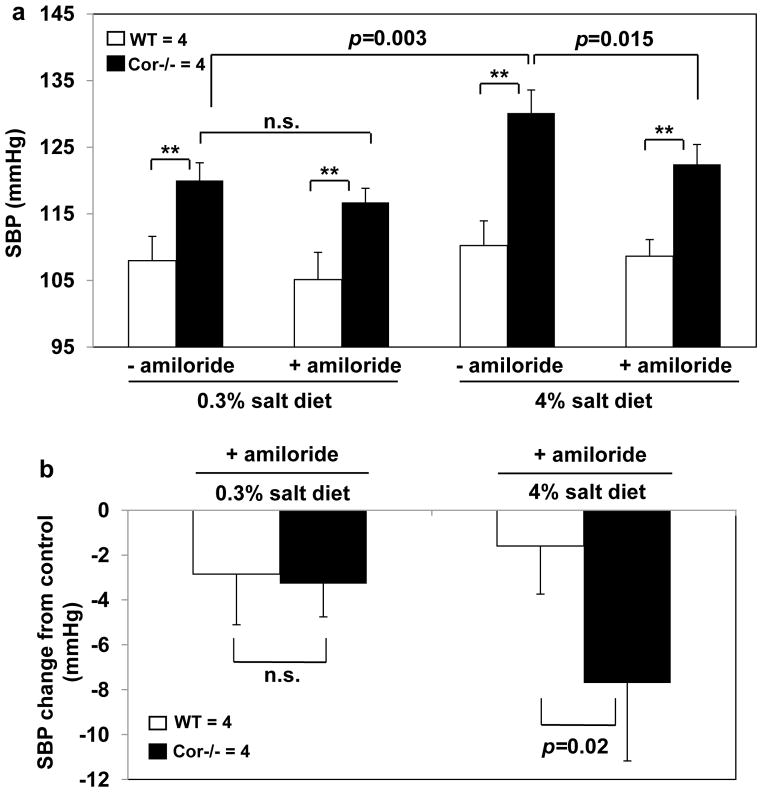
We measured blood pressure in mice treated with amiloride (Figure 7a). No significant changes in blood pressure were observed in Cor−/− mice on the normal salt diet after amiloride treatment. The treatment reduced elevated blood pressure in Cor−/− mice on the high salt diet. In WT mice on the normal or high salt diet, no significant changes in blood pressure were observed after amiloride treatment (Figure 7a). The net effect of amiloride on SBP was significantly greater in Cor−/− mice than that of WT mice on the high salt diet (Figure 7b), indicating that inhibiting ENaC activity increased urinary Na+ excretion and ameliorated hypertension in Cor−/− mice.
Figure 7. Amiloride reduced blood pressure in Cor−/− mice on high salt diet.
(a) WT and Cor−/− mice on 0.3% or 4% NaCl diet were treated with vehicle (−amiloride) or amiloride (3 mg/kg/day, i.p.). SBP was measured. (b) Changes in SBP after amiloride treatment were calculated. **p<0.01 vs. WT of the same group by two-way ANOVA. P values in comparisons between groups were indicated. n=4 per group. n.s., not statistically significant.
Amlodipine Treatment
To understand the mechanism underlying hypertension in Cor−/− mice on the normal salt diet, we examined the effect of amlodipine, a calcium channel blocker, on blood pressure. Amlodipine reduced blood pressure in Cor−/− and WT mice on the normal salt diet (Figure 8a). The reduction was greater in Cor−/− than WT mice when 1.5 mg/kg of amlodipine was used (Figure 8b). Amlodipine also reduced blood pressure in WT and Cor−/− mice on the high salt diet (Figure 8c). However, the reduction was similar in both groups (Figure 8d). Blood pressure in Cor−/− mice remained significantly higher than that in similarly treated WT mice (Figure 8c).
Figure 8. Amlodipine normalized blood pressure in Cor−/− mice on 0.3% but not 4% salt diets.
WT and Cor−/− mice on 0.3% (a,b) or 4% (c,d) NaCl diet were treated with vehicle (control) or amlodipine (0.5 and 1.5 mg/kg/day, i.p.). SBP was measured. *p<0.05; **p<0.01 vs. WT of the same group by two-way ANOVA. Changes in blood pressure from the control group of the same genotype on 0.3% (b) and 4% (d) NaCl diets were calculated. n=4–5 per group, as indicated. *p<0.05 vs. WT of the same group by two-way ANOVA. n.s., not statistically significant.
Discussion
In this study, we used radiotelemetry to monitor blood pressure in Cor−/− mice on normal and high salt diets. We found that blood pressure in Cor−/− mice was more sensitive to dietary salts than that in WT mice, as indicated by exacerbated hypertension in Cor−/− mice on the 4% NaCl diet. We further investigated possible mechanisms underlying the salt-sensitive hypertension in these mice. ANP is known to antagonize the RAAS.30, 31 Elevated plasma renin activities were reported in ANP knockout mice on high salt diets, suggesting that hypertension in these mice may result from an enhanced RAAS.11 This hypothesis, however, was not supported by another study, which showed similar plasma Ang II levels and responses to dietary salts in ANP knockout and WT mice.5 In our study, we did not detect elevated levels of plasma renin or serum aldosterone in Cor−/− mice (Figure 4a and b). Moreover, losartan did not normalize high blood pressure in Cor−/− mice (Figure 5a and c), indicating that an enhanced RAAS is unlikely to be a key determinant for salt-sensitive hypertension in Cor−/− mice.
In the metabolic studies, no major differences were found in food/water intakes, body weight, and plasma electrolyte levels between Cor−/− and WT mice on the normal salt diet. After 1 week of dietary salt challenge, Cor−/− mice had similar levels of plasma electrolytes to that in WT mice but exhibited a significant reduction in urinary Na+/Cl− excretion. This was accompanied with body weight gains, indicating that water was retained to maintain electrolyte homeostasis. Gradually a steady state of renal sodium excretion was reached in Cor−/− mice, indicating that corin deficiency impaired an adaptive renal response to high dietary salts, leading to salt-sensitive hypertension.
Corin is highly expressed in cardiomyocytes.32, 33 Corin mRNA and protein expression was found in the kidney, including the proximal tubule, thick ascending limb, connecting tubule and collecting duct.13, 29 In rat models of proteinuric kidney disease, renal corin expression was markedly reduced and the reduction was associated with sodium retention, indicating that corin defects may impair sodium homeostasis in nephrotic syndrome.29, 34 The result is consistent with our findings of impaired sodium excretion and exacerbated hypertension in Cor−/− mice on high salt diets.
ENaC is essential for renal sodium reabsorption.27, 28 Mutations in the ENaC genes cause hypertension in patients.2 Polzin et al. showed that renal β-ENaC expression was increased in Cor−/− mice, suggesting that corin may down-regulate renal ENaC expression and activity.29, 34 Zhao et al. also showed that ANP-mediated inhibition of sodium reabsorption in distal nephron segments is critical for promoting natriuresis.35 These data suggest that impaired sodium excretion in Cor−/− mice may be due to an increased renal ENaC activity. Consistent with this hypothesis, amiloride treatment prevented sodium retention, normalized body weight gain (Figure 6), and reduced high blood pressure (Figure 7) in Cor−/− mice on high salt diet. These results support the hypothesis that renal corin expression plays an important role in regulating ENaC activity. Because corin is the physiological pro-ANP convertase, it is likely that the renal function of corin is mediated by its activity to activate ANP.
Interestingly, no significant changes in urinary sodium excretion and blood pressure were observed when Cor−/− mice on normal salt diet were treated with amiloride (Figures 6a and 7a). Moreover, no significant differences in urinary sodium excretion were detected between Cor−/− and WT mice on the normal salt diet (Figure 2a), suggesting that impaired sodium excretion may not be a major factor in hypertension in Cor−/− mice on normal salt diet. ANP is known to bind to its receptor on vascular smooth muscles to promote intracellular cGMP production and vasodilation.4 Possibly, lack of corin reduced ANP levels and increased vascular resistance in these mice, contributing to hypertension on normal salt diets. Consistently, treatment of amlodipine, a calcium channel blocker that relaxes vascular smooth muscles, reduced blood pressure in Cor−/− mice to a similar level in WT mice on the normal salt diet (Figure 8a).
Evolutionarily, natriuretic peptides were developed in primitive vertebrates for their adaptation to survive. In salmon and eels, for example, natriuretic peptides are critical for maintaining electrolyte homeostasis when these animals migrate from fresh water to salty water during their life cycles.36, 37 Here we show that corin deficiency prevents mice from adapting to dietary salt challenges, indicating that this ancient molecular mechanism is preserved in mammals for similar purposes. Together, our data provide new insights into the role of corin in regulating sodium homeostasis and the mechanisms underlying salt-sensitive hypertension in Cor−/− mice. Genetic variants that impair corin function have been identified in African Americans, a population known for high prevalence of salt-sensitive hypertension.38 Our results suggest that corin defects may represent an important mechanism underlying hypertension in humans.
Materials And Methods
Mice
Cor−/− mice were generated by deleting the exons coding for corin active sites.16, 39 Male Cor−/− mice and WT littermates, aged 8–12 weeks old and weighed 20–30 grams, were used in this study. Mouse genotypes were not blinded to the persons who performed the experiments. The mice were maintained in a facility with a 12-h light-dark cycle and unrestricted access to food and water. The study was conducted in accordance with the NIH guidelines for the ethical treatment and handling of animals in research and approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Blood Pressure Measurements
Radiotelemetry was used to monitor blood pressure in conscious and unrestrained mice.16 Mice were anesthetized with ketamine/xylazine on a 37°C warming plate. A TA11PA-C10 telemetry device (Data Science International) was inserted into the left common carotid artery under a microscope. After surgery, mice were singly caged and fed with standard diet and water ad libitum. Telemetry receivers (model RPC-1) were used for data acquisition using the Dataquest System (Data Science International). After ~7 days of post-operational recovery, blood pressure was recorded.
Effect of Dietary Salt on Blood Pressure
Mice were fed with diets containing normal (0.3% NaCl), medium-high (2% NaCl) or high (4% NaCl) salts (Harlan Teklad) for three weeks. Blood pressure was monitored before, during and after the treatment of different salt diets.
Metabolic Studies
Mice were placed singly in metabolic cages (Nalgene Labware) for a 5-day habituation period, during which a normal salt diet was fed. Next, mice were fed with the normal salt diet for 3 days, and then switched to a high salt diet. Body weight, food consumption and water intake were measured daily. Urine samples were collected in a device covered with water-saturated oil to prevent sample evaporation. Urine volume in a 24-h period was recorded. At different time points, mice were euthanized by CO2 inhalation and blood samples were collected in tubes (BD, Franklin Lakes, NJ) containing heparin for electrolyte analysis or EDTA for plasma renin concentration assay.
Plasma and Urinary Electrolytes
Plasma and urine samples were analyzed for sodium, potassium and chloride concentrations using an electrolyte analyzer (Roche AVL9180). Effects of Sodium or Calcium Channel Blockers or Angiotensin II Receptor Antagonist Mice on 0.3% or 4% NaCl diets were treated with amiloride (Alexis Biochemicals, San Diego, CA), an ENaC blocker, or amlodipine (Sigma), a calcium channel blocker, or losartan (U.S. Pharmacopeia, Rockville, MD), an angiotensin II receptor type 1 antagonist, by intraperitoneal injection. The injection volumes were ~0.1 mL and vehicles were used as controls. Blood pressure was monitored by radiotelemetry, starting 7, 5, and 3 days after amiloride, amlodipine and losartan treatment, respectively. Urinary sodium excretion and body weight change after drug treatment were measured.
Plasma Renin and Serum Aldosterone Assays
Active renin concentration in plasma was measured as the amount of angiotensin I (Ang I) generated from angiotensinogen (AGT).40 Plasma samples were diluted and incubated with excess porcine AGT (1 μM, Sigma) in a reaction mixture containing sodium acetate (50 mM, pH 6.5), aminoethylbenzene sulfonyl fluoride (2.5 mM), 8-hydroxyquinoline (1 mM), leupeptin (100 μM), and EDTA (1 mM) at 37°C for 30 min. The reaction was stopped by heating at 100°C. Generated Ang I was determined by an enzyme immunoassay (Peninsula Labs, San Carlos, CA). Plasma renin concentration (PRC) was expressed as the amount of Ang I generated per μL of plasma per hour. As a negative control, the reaction was performed in the presence of pepstatin A (1 μM, Sigma). The value from this reaction was used as a background. Serum aldosterone levels were measured using an ELISA kit from Alpha Diagnostic International (San Antonio, TX), according to the manufacturer’s instructions.
Statistical Analysis
Data were analyzed using the Prism software (Graph-Pad). Comparisons between two groups were made using the Student’s t test. Three or more groups were compared using 2-way ANOVA followed by post hoc least significant difference. All data are presented as means ± SD. A p value of <0.05 was considered to be statistically significant.
Acknowledgments
This work was supported in part by grants from the Bakken Heart-Brain Institute, the Ralph Wilson Medical Foundation and the NIH (R01HL089298, R01HL089298-S1), and by the Priority Academic Program Development of Jiangsu Higher Education Institutions in China.
Footnotes
Disclosures
All the authors declared no competing interests.
References
- 1.Mullins LJ, Bailey MA, Mullins JJ. Hypertension, kidney, and transgenics: a fresh perspective. Physiol Rev. 2006;86(2):709–746. doi: 10.1152/physrev.00016.2005. [DOI] [PubMed] [Google Scholar]
- 2.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27(3 Pt 2):481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 4.McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16(10):469–477. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Angelis E, Tse MY, Pang SC. Interactions between atrial natriuretic peptide and the renin-angiotensin system during salt-sensitivity exhibited by the proANP gene-disrupted mouse. Mol Cell Biochem. 2005;276(1–2):121–131. doi: 10.1007/s11010-005-3672-1. [DOI] [PubMed] [Google Scholar]
- 6.John SW, Krege JH, Oliver PM, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267(5198):679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 7.Lopez MJ, Wong SK, Kishimoto I, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378(6552):65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 8.John SW, Veress AT, Honrath U, et al. Blood pressure and fluid-electrolyte balance in mice with reduced or absent ANP. Am J Physiol. 1996;271(1 Pt 2):R109–114. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- 9.Oliver PM, Fox JE, Kim R, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94(26):14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver PM, John SW, Purdy KE, et al. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci U S A. 1998;95(5):2547–2551. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo LG, Veress AT, Chong CK, et al. Salt-sensitive hypertension in ANP knockout mice: potential role of abnormal plasma renin activity. Am J Physiol. 1998;274(1 Pt 2):R255–261. doi: 10.1152/ajpregu.1998.274.1.R255. [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, Kuo HC, Deng GG. Serine proteases and cardiac function. Biochim Biophys Acta. 2005;1751(1):82–94. doi: 10.1016/j.bbapap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Yan W, Sheng N, Seto M, et al. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274(21):14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 14.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–4190. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Xu-Cai YO, Chen S, et al. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75(2):142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JC, Knudson O, Wu F, et al. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102(3):785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nigrovic PA, Gray DH, Jones T, et al. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173(6):1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dries DL, Victor RG, Rame JE, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112(16):2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 19.Rame JE, Drazner MH, Post W, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49(4):857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 20.Rame JE, Tam SW, McNamara D, et al. Dysfunctional corin I555(P568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail. 2009;2(6):541–548. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Liao X, Fukuda K, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103(5):502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong N, Chen S, Yang J, et al. Plasma Soluble Corin in Patients with Heart Failure. Circ Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong N, Dong J, Liu P, et al. Effects of anticoagulants on human plasma soluble corin levels measured by ELISA. Clin Chim Acta. 2010;411(23–24):1998–2003. doi: 10.1016/j.cca.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang J, Wu S, Wang W, et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286(12):10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peleg A, Jaffe AS, Hasin Y. Enzyme-linked immunoabsorbent assay for detection of human serine protease corin in blood. Clin Chim Acta. 2009;409(1–2):85–89. doi: 10.1016/j.cca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Ibebuogu UN, Gladysheva IP, Houng AK, et al. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic Peptide. Circ Heart Fail. 2011;4(2):114–120. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bubien JK. Epithelial Na+ channel (ENaC), hormones, and hypertension. J Biol Chem. 2010;285(31):23527–23531. doi: 10.1074/jbc.R109.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossier BC, Pradervand S, Schild L, et al. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 29.Polzin D, Kaminski HJ, Kastner C, et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78(7):650–659. doi: 10.1038/ki.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnett JC, Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247(5 Pt 2):F863–866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 31.Granger JP, Meadows E, Sooudi S, et al. Mechanism mediating hypotensive effect of ANF in sodium-depleted dogs. Am J Physiol. 1989;257(2 Pt 2):H502–505. doi: 10.1152/ajpheart.1989.257.2.H502. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Sen S, Young D, et al. Protease corin expression and activity in failing hearts. Am J Physiol Heart Circ Physiol. 2010;299(5):H1687–1692. doi: 10.1152/ajpheart.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran KL, Lu X, Lei M, et al. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am J Physiol Heart Circ Physiol. 2004;287(4):H1625–1631. doi: 10.1152/ajpheart.00298.2004. [DOI] [PubMed] [Google Scholar]
- 34.Klein JD. Corin: an ANP protease that may regulate sodium reabsorption in nephrotic syndrome. Kidney Int. 2010;78(7):635–637. doi: 10.1038/ki.2010.223. [DOI] [PubMed] [Google Scholar]
- 35.Zhao D, Pandey KN, Navar LG. ANP-mediated inhibition of distal nephron fractional sodium reabsorption in wild-type and mice overexpressing natriuretic peptide receptor. Am J Physiol Renal Physiol. 2010;298(1):F103–108. doi: 10.1152/ajprenal.00479.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tervonen V, Arjamaa O, Kokkonen K, et al. A novel cardiac hormone related to A-, B- and C-type natriuretic peptides. Endocrinology Sep. 1998;139(9):4021–4025. doi: 10.1210/endo.139.9.6292. [DOI] [PubMed] [Google Scholar]
- 37.Tsukada T, Takei Y. Integrative approach to osmoregulatory action of atrial natriuretic peptide in seawater eels. Gen Comp Endocrinol. 2006 May 15;147(1):31–38. doi: 10.1016/j.ygcen.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 39.Pan J, Hinzmann B, Yan W, et al. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277(41):38390–38398. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 40.Lantelme P, Rohrwasser A, Gociman B, et al. Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension. 2002;39(5):1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]